Functional Characterization of HGD Gene Variants by Minigene Splicing Assay
Abstract
1. Introduction
2. Results
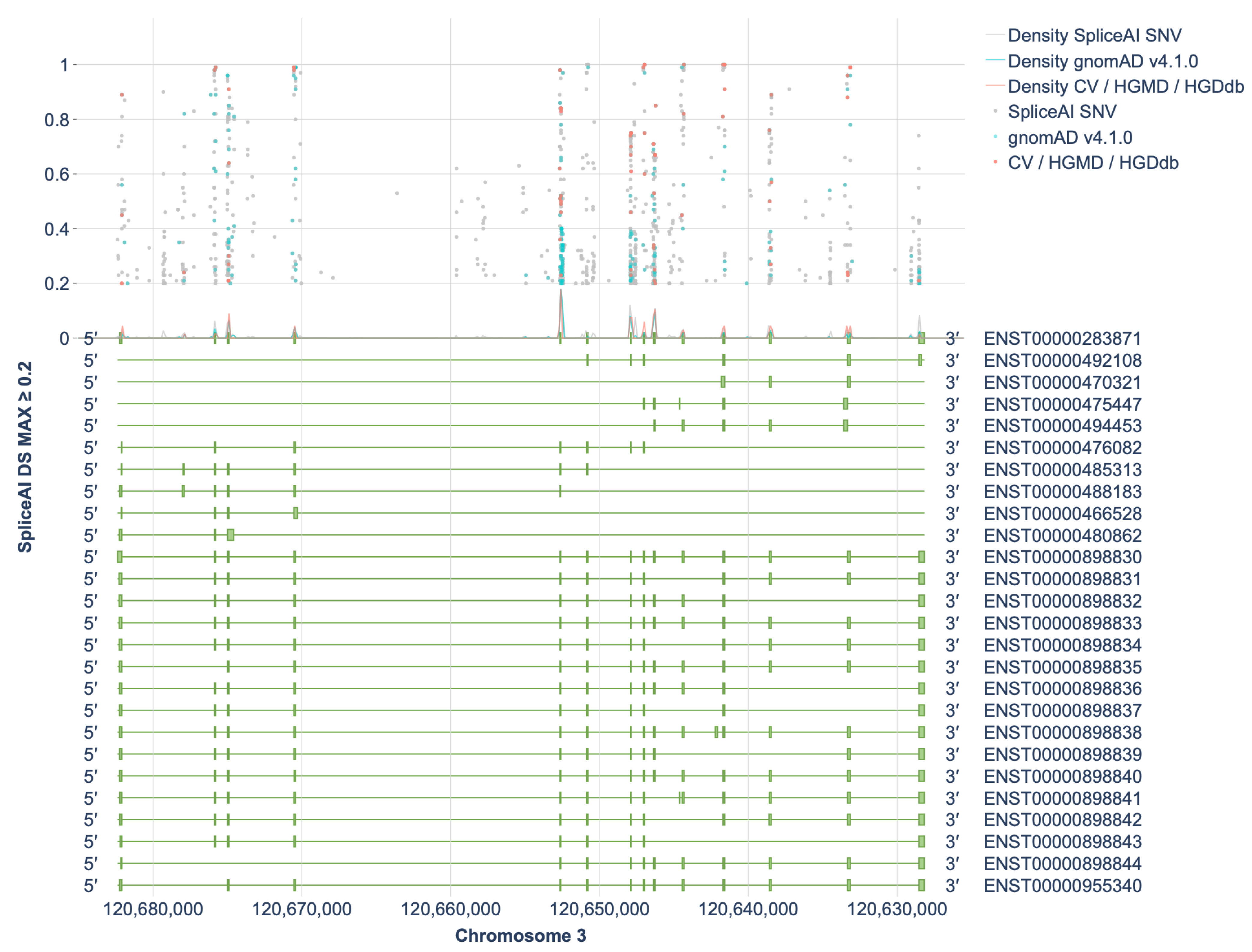
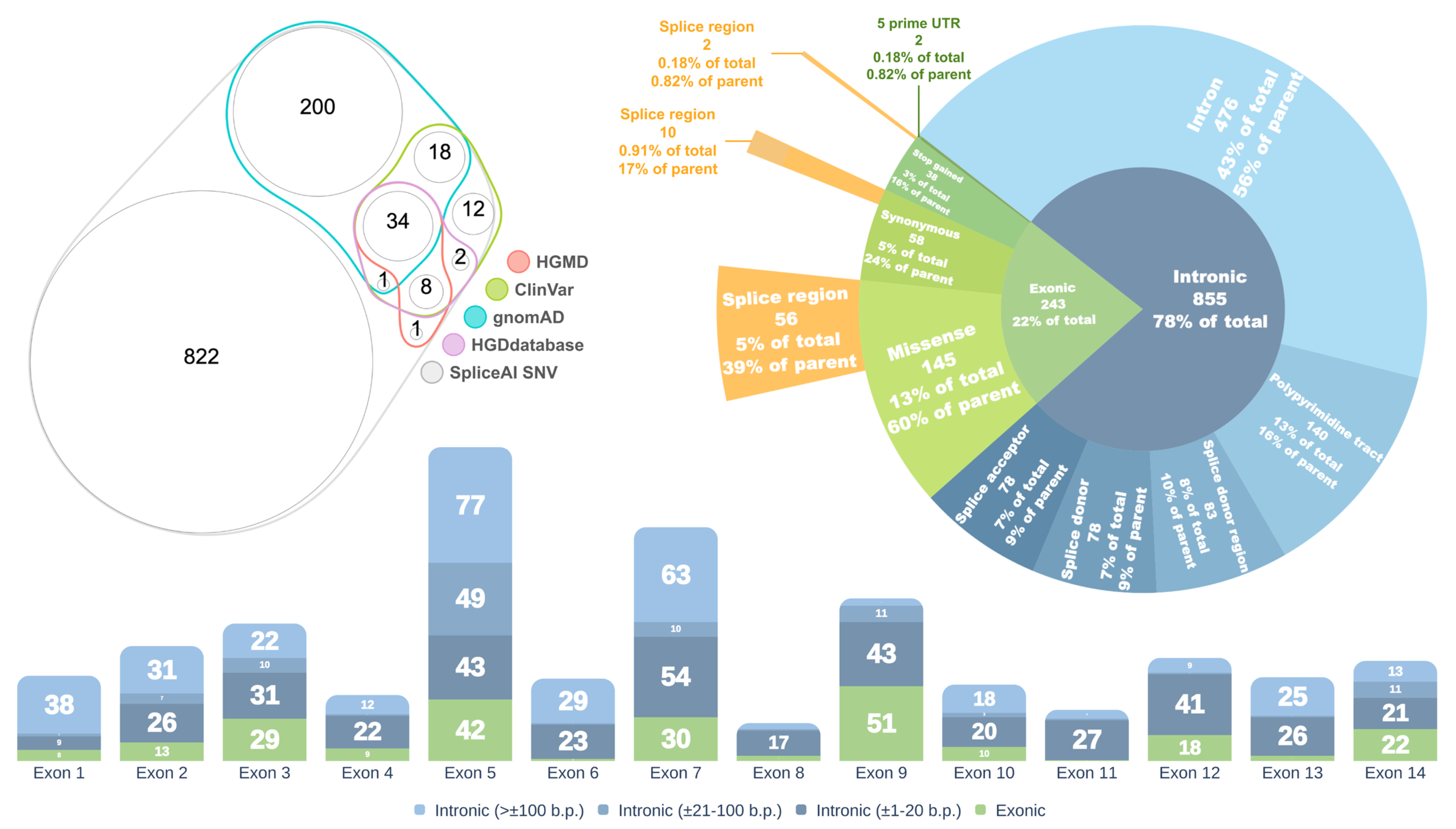
2.1. Assessment of the Spliceogenic Potential of HGD Gene Variants
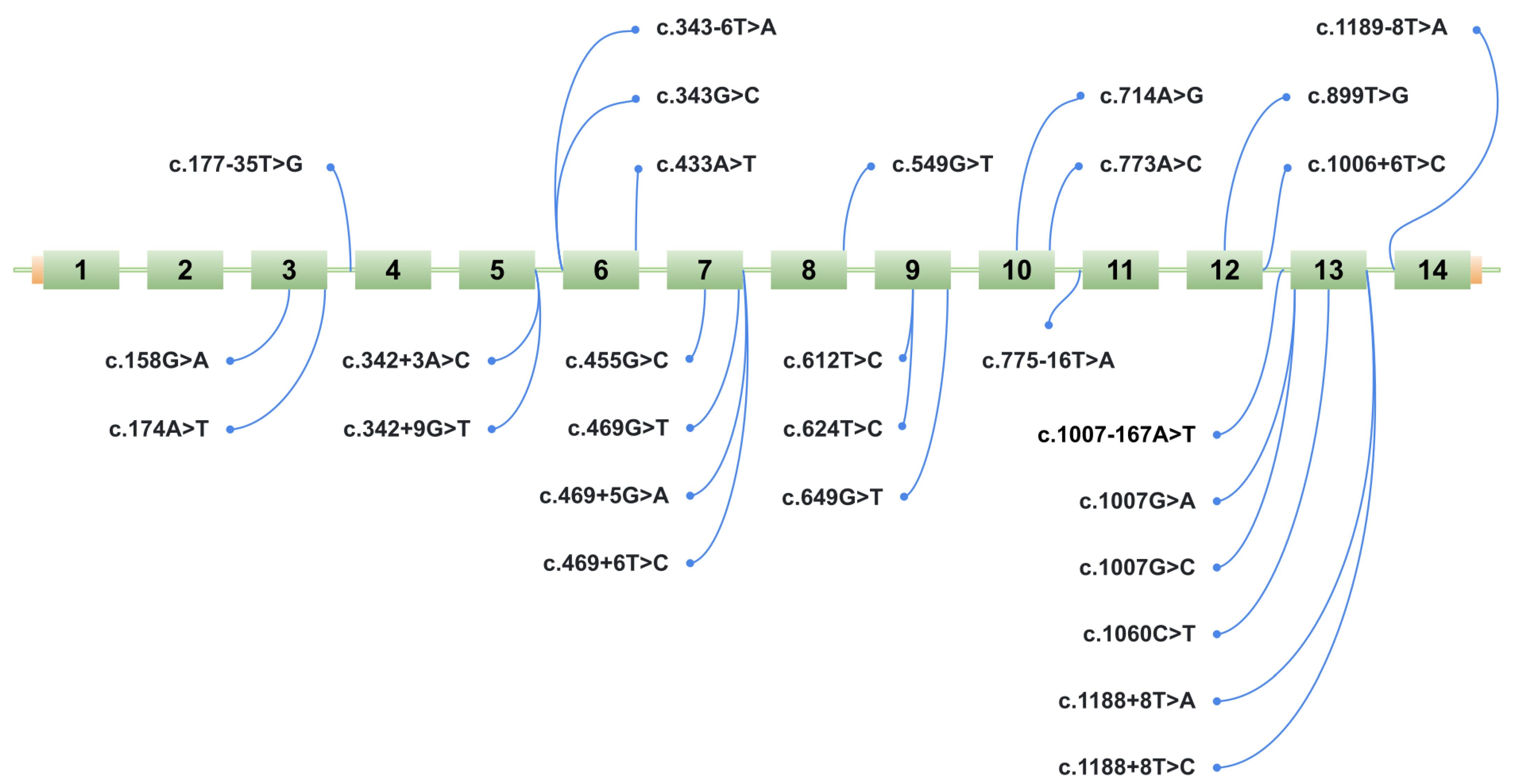
2.2. Selection of Variants for Functional Validation Using a Minigene Assay
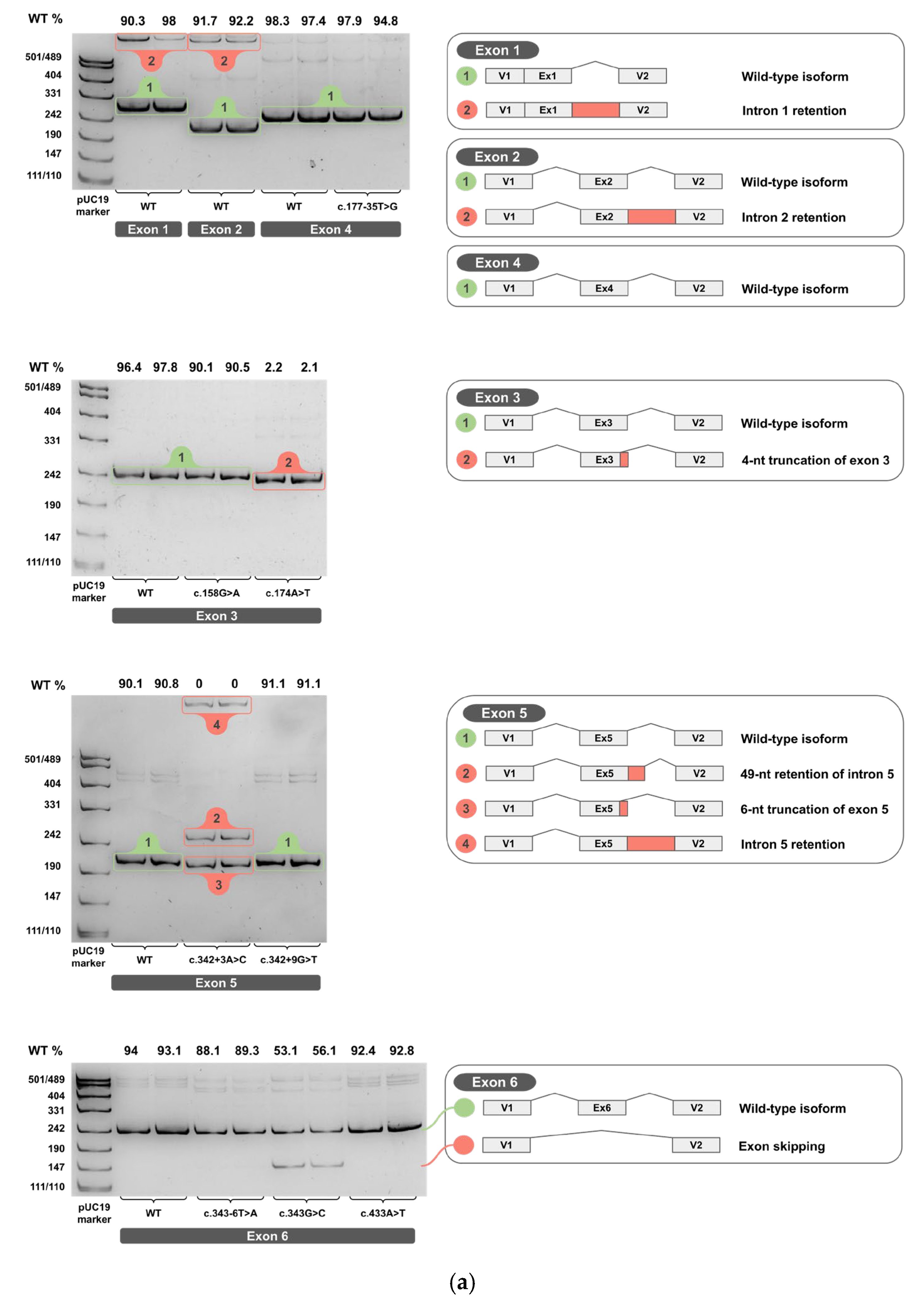
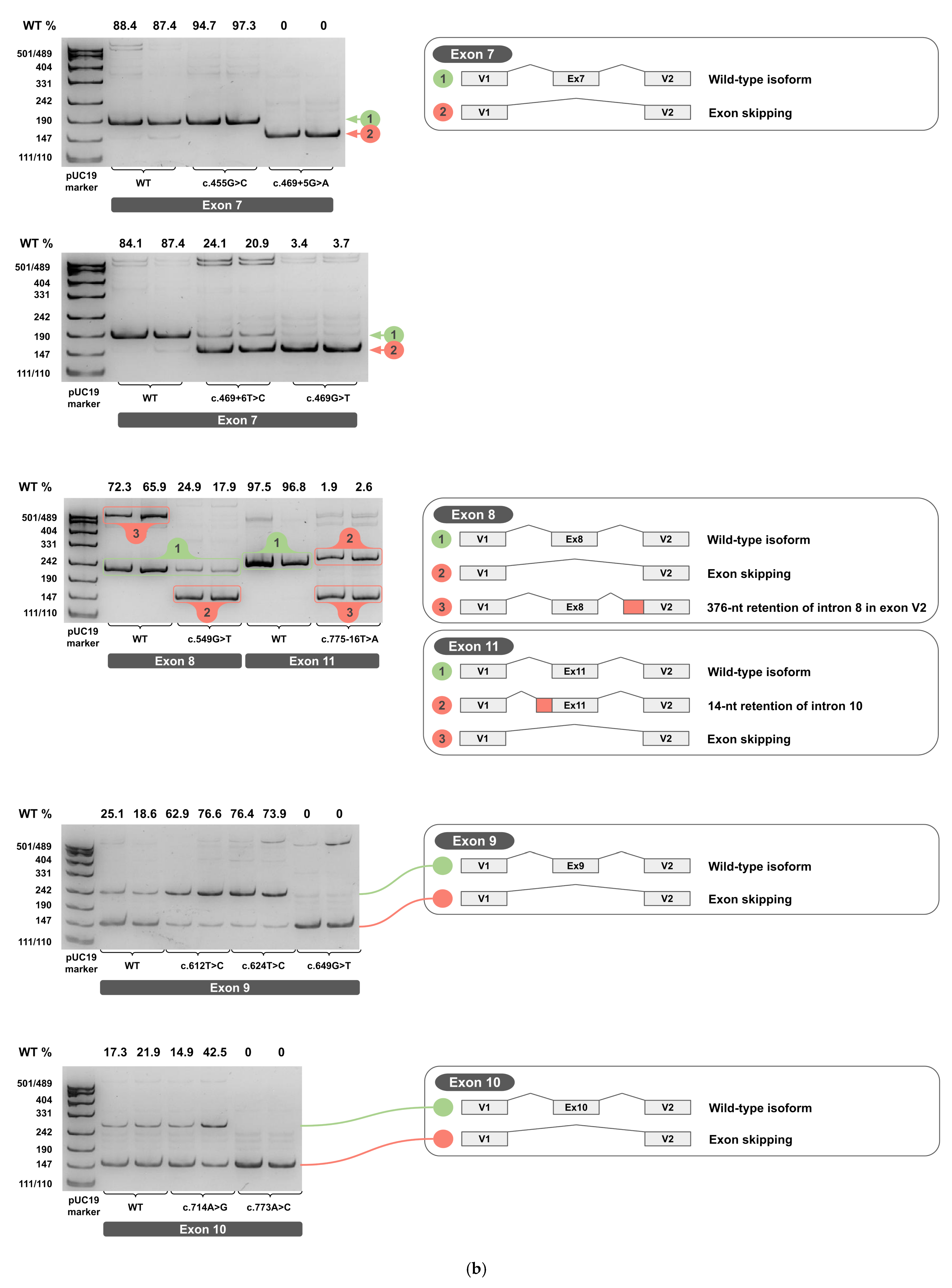
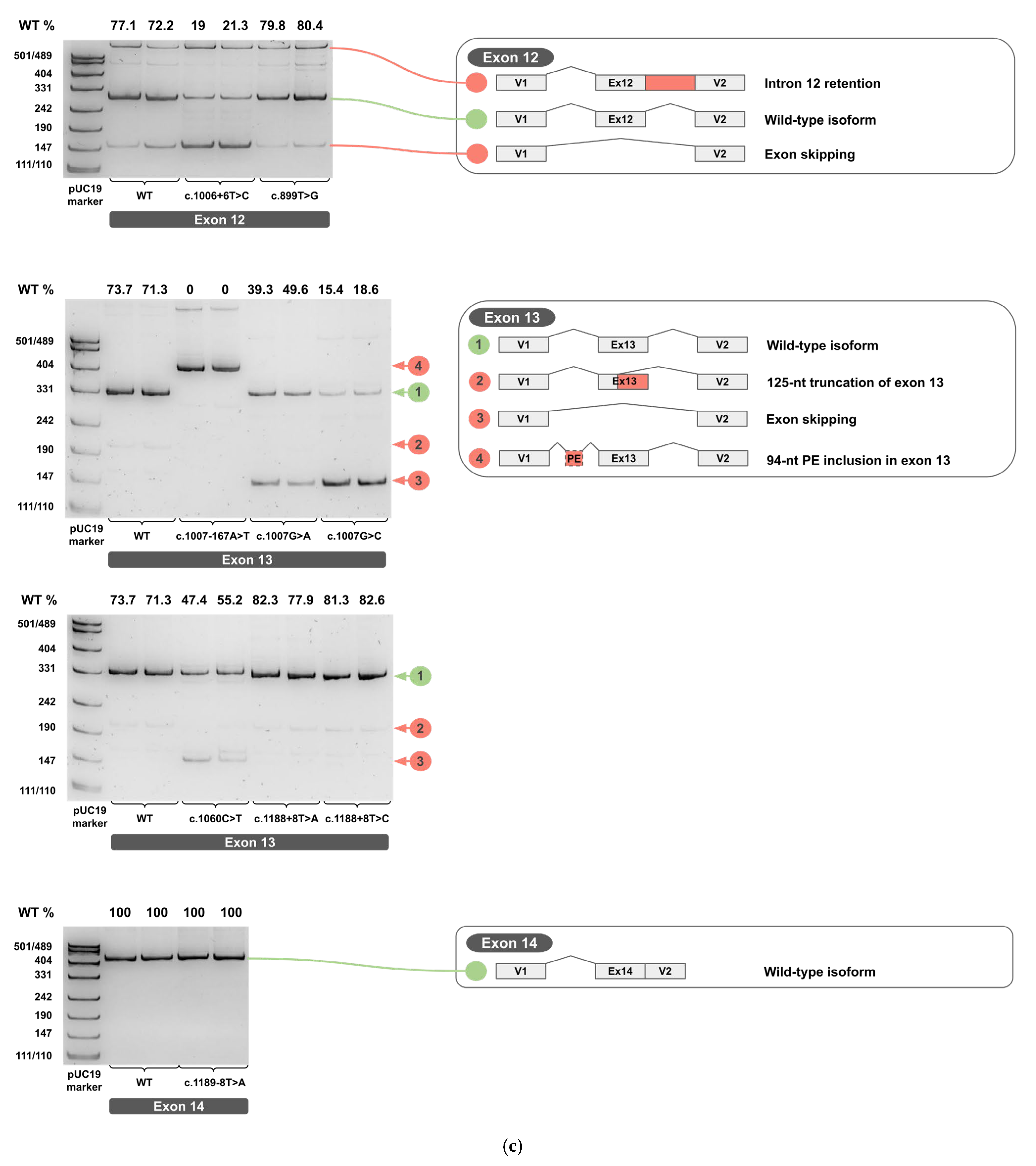
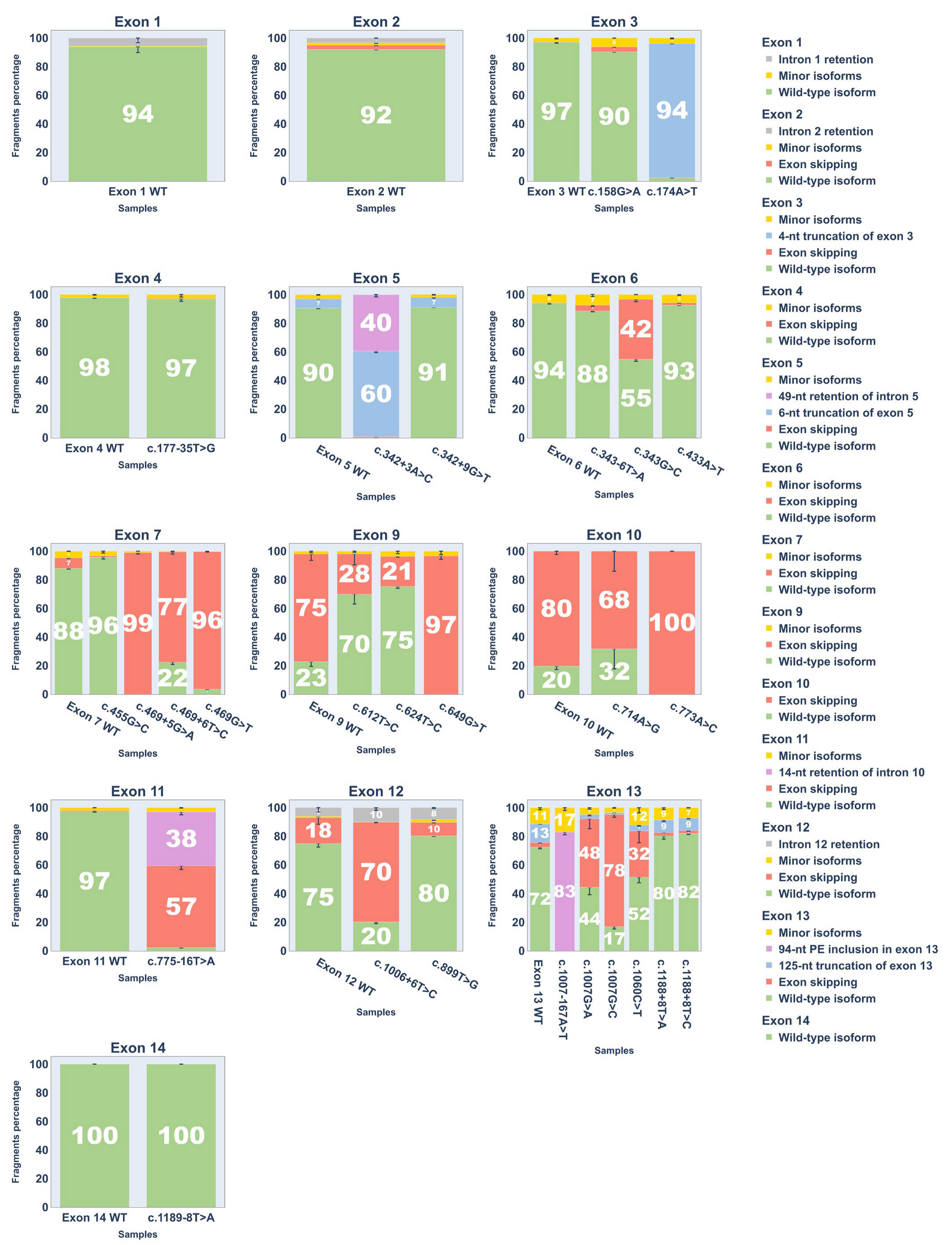
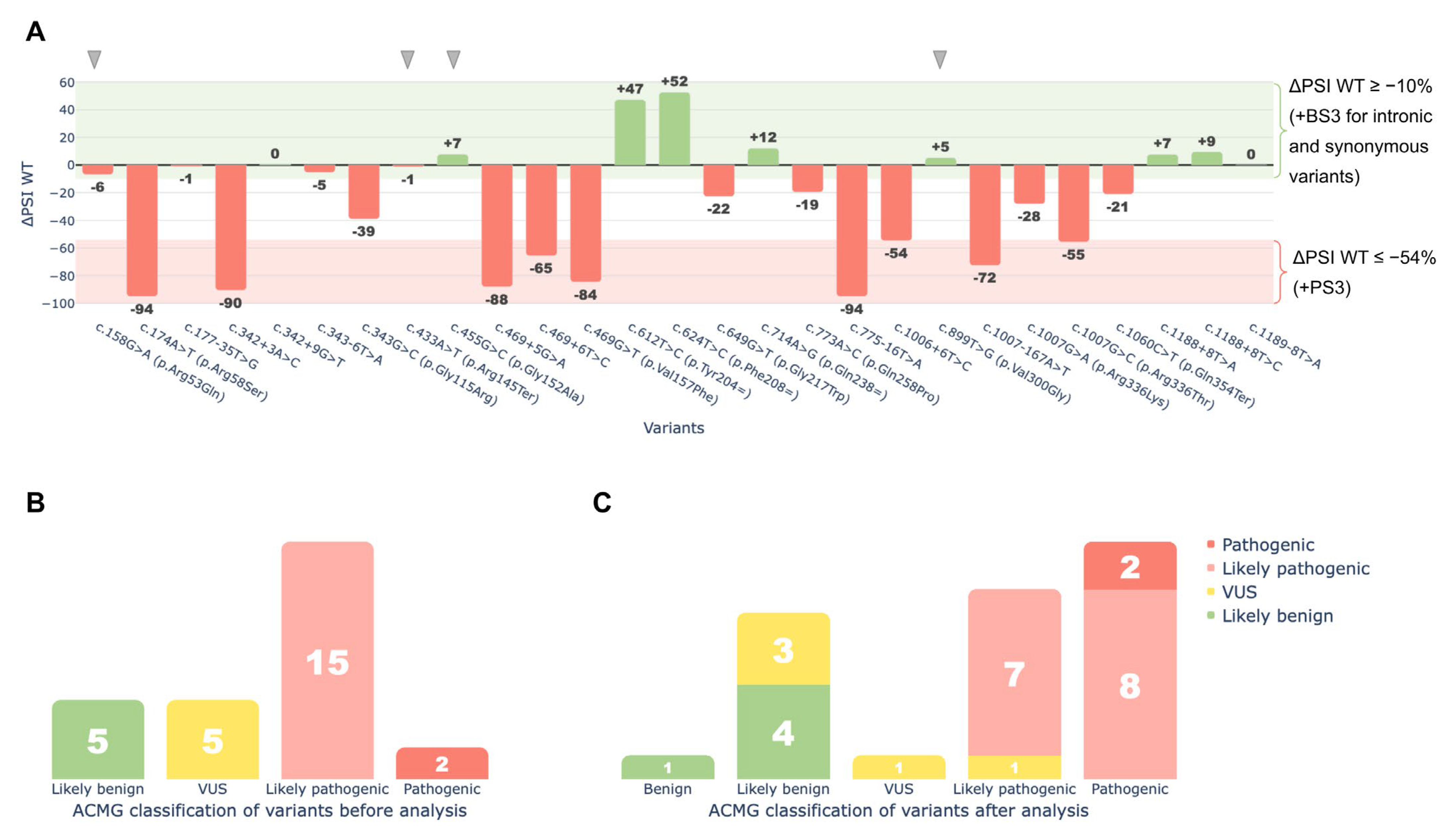
2.3. Minigene Analysis
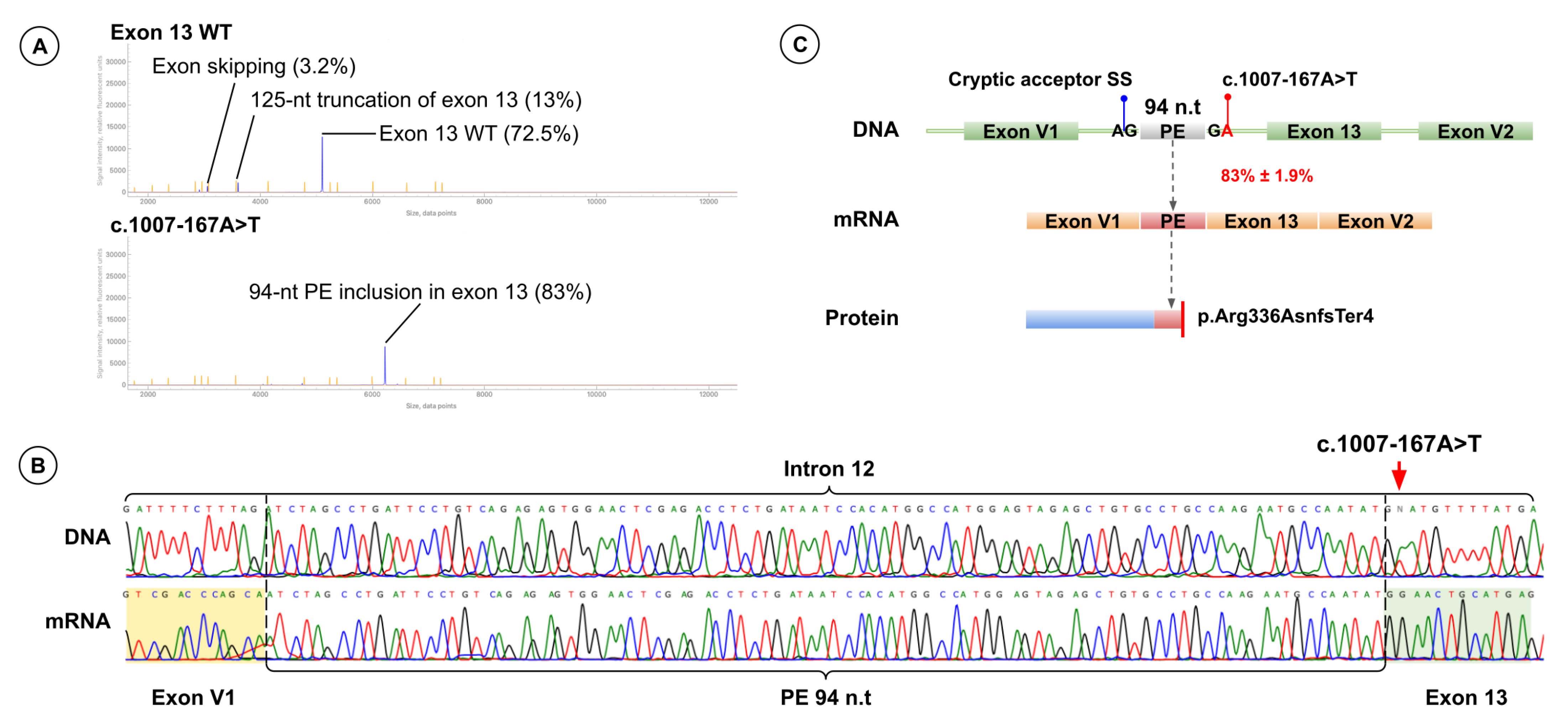
2.4. A Novel Deep Intronic Variant
2.5. ACMG/AMP Reclassification Results
3. Discussion
3.1. Dual Effects of Exonic Variants
3.2. Regulation of Exon 9 Splicing
3.3. Aberrant 3′-End Deletion of Exon 13
3.4. Cloning Artifact Revealed by Exon 8 Analysis
3.5. Aberrant Splicing Isoforms of Exon 5
3.6. Limitations of the Minigene Splicing Assay
3.7. Classification of Variants Affecting Splicing
4. Materials and Methods
4.1. Assessment of the Spliceogenic Potential of HGD Gene Variants
4.2. Selection of Variants for Functional Validation Using a Minigene Assay
4.3. Construction of Minigenes
4.4. Minigene Splicing Assay
4.4.1. Transfection
4.4.2. RNA Extraction, cDNA Synthesis, and Analysis
4.4.3. Fragment Analysis
4.5. Variant Nomenclature and Accession
4.6. Clinical Classification of Variants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKU | Alkaptonuria |
| HGD | Homogentisate 1, 2-dioxygenase |
| BQA | Benzoquinone acetic acid |
| RNA | Ribonucleic acid |
| cDNA | Complementary DNA |
| PAGE | Polyacrylamide Gel Electrophoresis |
| PCR | Polymerase Chain Reaction |
| VUS | Variant of Uncertain Significance |
| ACMG/AMP | American College of Medical Genetics and Genomics/Association for Molecular Pathology |
| DS MAX | Delta Score Maximum |
| SS | Splice site |
| BP | Branch point |
| ESR | Exonic splicing regulator |
| PSI | Percent-Spliced-In |
| ΔPSI | Absolute change in Percent-Spliced-In |
| δPSI | Relative change in Percent-Spliced-In |
| SNV | Single Nucleotide Variant |
| UTR | Untranslated Region |
| MCS | Multiple Cloning Site |
| WT | Wild Type |
| PE | Pseudoexon |
| HGMD | Human Gene Mutation Database |
| VEP | Variant Effect Predictor |
| HExoSplice | Human Exonic Splicing Regulatory Element Predictor |
| CMV | Cytomegalovirus |
| HSV-TK | Herpes Simplex Virus Thymidine Kinase |
| FAM | Fluorescein Amidite (fluorescent dye label) |
| NCBI | National Center for Biotechnology Information |
| RefSeq | Reference Sequence |
| GRCh38 | Genome Reference Consortium Human Build 38 |
| HEK293T | Human Embryonic Kidney 293T cells |
| AA | Amyloid A |
| NTR | Nothing to Report |
| TPM | Transcripts Per Million |
| SD | Standard Deviation |
References
- Garrod, A.E. The incidence of alkaptonuria: A study in chemical individuality. Mol. Med. 1996, 2, 274–282. [Google Scholar] [CrossRef]
- Zannoni, V.G.; Lomtevas, N.; Goldfinger, S. Oxidation of homogentisic acid to ochronotic pigment in connective tissue. Biochim. Biophys. Acta 1969, 177, 94–105. [Google Scholar] [CrossRef]
- Mistry, J.B.; Bukhari, M.; Taylor, A.M. Alkaptonuria. Rare Dis. 2013, 1, e27475. [Google Scholar] [CrossRef]
- Millucci, L.; Spreafico, A.; Tinti, L.; Braconi, D.; Ghezzi, L.; Paccagnini, E.; Bernardini, G.; Amato, L.; Laschi, M.; Selvi, E.; et al. Alkaptonuria is a novel human secondary amyloidogenic disease. Biochim. Biophys. Acta 2012, 1822, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Phornphutkul, C.; Introne, W.J.; Perry, M.B.; Bernardini, I.; Murphey, M.D.; Fitzpatrick, D.L.; Anderson, P.D.; Huizing, M.; Anikster, Y.; Gerber, L.H.; et al. Natural history of alkaptonuria. N. Engl. J. Med. 2002, 347, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, G.; Braconi, D.; Zatkova, A.; Sireau, N.; Kujawa, M.J.; Introne, W.J.; Spiga, O.; Geminiani, M.; Gallagher, J.A.; Ranganath, L.R.; et al. Alkaptonuria. Nat. Rev. Dis. Primers 2024, 10, 16. [Google Scholar] [CrossRef]
- Yépez, V.A.; Gusic, M.; Kopajtich, R.; Mertes, C.; Smith, N.H.; Alston, C.L.; Ban, R.; Beblo, S.; Berutti, R.; Blessing, H.; et al. Clinical implementation of RNA sequencing for Mendelian disease diagnostics. Genome Med. 2022, 14, 38. [Google Scholar] [CrossRef]
- Ha, C.; Kim, J.W.; Jang, J.H. Performance Evaluation of SpliceAI for the Prediction of Splicing of NF1 Variants. Genes 2021, 12, 1308. [Google Scholar] [CrossRef]
- Love, S.L.; Emerson, J.D.; Koide, K.; Hoskins, A.A. Pre-mRNA splicing-associated diseases and therapies. RNA Biol. 2023, 20, 525–538. [Google Scholar] [CrossRef]
- Ward, A.J.; Cooper, T.A. The pathobiology of splicing. J. Pathol. 2010, 220, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Martínez, E.; Sanoguera-Miralles, L.; Valenzuela-Palomo, A.; Esteban-Sánchez, A.; Lorca, V.; Llinares-Burguet, I.; Allen, J.; García-Álvarez, A.; Pérez-Segura, P.; Durán, M.; et al. Minigene-based splicing analysis and ACMG/AMP-based tentative classification of 56 ATM variants. J. Pathol. 2022, 258, 83–101. [Google Scholar] [CrossRef]
- Cooper, T.A. Use of minigene systems to dissect alternative splicing elements. Methods 2005, 37, 331–340. [Google Scholar] [CrossRef]
- Bychkov, I.; Kamenets, E.; Kurkina, M.; Rychkov, G.; Ilyushkina, A.; Filatova, A.; Guseva, D.; Baydakova, G.; Nekrasov, A.; Cheblokov, A.; et al. Alkaptonuria in Russia: Mutational spectrum and novel variants. Eur. J. Med. Genet. 2021, 64, 104165. [Google Scholar] [CrossRef]
- Tao, L.; Deng, C.; Ma, M.; Zhang, Y.; Duan, J.; Li, Y.; Fang, L.; Zhou, Y.; He, X.; Wang, Y.; et al. A novel mutation in the homogentisate 1,2 dioxygenase gene identified in Chinese Hani pediatric patients with Alkaptonuria. Clin. Chim. Acta 2022, 532, 164–171. [Google Scholar] [CrossRef]
- Lai, C.Y.; Tsai, I.J.; Chiu, P.C.; Ascher, D.B.; Chien, Y.H.; Huang, Y.H.; Lin, Y.L.; Hwu, W.L.; Lee, N.C. A novel deep intronic variant strongly associates with Alkaptonuria. npj Genom. Med. 2021, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Ascher, D.B.; Spiga, O.; Sekelska, M.; Pires, D.E.V.; Bernini, A.; Tiezzi, M.; Kralovicova, J.; Borovska, I.; Soltysova, A.; Olsson, B.; et al. Homogentisate 1,2-dioxygenase (HGD) gene variants, their analysis and genotype-phenotype correlations in the largest cohort of patients with AKU. Eur. J. Hum. Genet. EJHG 2019, 27, 888–902. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Shaw, K.; Phillips, A.; Cooper, D.N. The Human Gene Mutation Database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum. Genet. 2014, 133, 1–9. [Google Scholar] [CrossRef]
- Nissim-Rafinia, M.; Kerem, B. Splicing regulation as a potential genetic modifier. Trends Genet. TIG 2002, 18, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Dawes, R.; Bournazos, A.M.; Bryen, S.J.; Bommireddipalli, S.; Marchant, R.G.; Joshi, H.; Cooper, S.T. SpliceVault predicts the precise nature of variant-associated mis-splicing. Nat. Genet. 2023, 55, 324–332. [Google Scholar] [CrossRef]
- Xu, Q.; Modrek, B.; Lee, C. Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res. 2002, 30, 3754–3766. [Google Scholar] [CrossRef] [PubMed]
- van der Klift, H.M.; Jansen, A.M.L.; van der Steenstraten, N.; Bik, E.C.; Tops, C.M.J.; Devilee, P.; Wijnen, J.T. Splicing analysis for exonic and intronic mismatch repair gene variants associated with Lynch syndrome confirms high concordance between minigene assays and patient RNA analyses. Mol. Genet. Genom. Med. 2015, 3, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kim, J.H. Identification of HGD mutations in an alkaptonuria patient: Using the Internet to seek rare diseases. J. Genet. Med. 2018, 15, 17–19. [Google Scholar] [CrossRef]
- Davydenko, K.; Filatova, A.; Skoblov, M. Assessing Splicing Variants in the PAX6 Gene: A Comprehensive Minigene Approach. J. Cell. Mol. Med. 2025, 29, e70459. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Wada, Y.; Hall, L.D.; Mitchell, D.W.; Glazer, A.M.; Roden, D.M. Functional Assays Reclassify Suspected Splice-Altering Variants of Uncertain Significance in Mendelian Channelopathies. Circ. Genom. Precis. Med. 2022, 15, e003782. [Google Scholar] [CrossRef]
- Fraile-Bethencourt, E.; Valenzuela-Palomo, A.; Díez-Gómez, B.; Acedo, A.; Velasco, E.A. Identification of Eight Spliceogenic Variants in BRCA2 Exon 16 by Minigene Assays. Front. Genet. 2018, 9, 188. [Google Scholar] [CrossRef]
- Fraile-Bethencourt, E.; Díez-Gómez, B.; Velásquez-Zapata, V.; Acedo, A.; Sanz, D.J.; Velasco, E.A. Functional classification of DNA variants by hybrid minigenes: Identification of 30 spliceogenic variants of BRCA2 exons 17 and 18. PLoS Genet. 2017, 13, e1006691. [Google Scholar] [CrossRef]
- Jaganathan, K.; Kyriazopoulou Panagiotopoulou, S.; McRae, J.F.; Darbandi, S.F.; Knowles, D.; Li, Y.I.; Kosmicki, J.A.; Arbelaez, J.; Cui, W.; Schwartz, G.B.; et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell 2019, 176, 535–548.e24. [Google Scholar] [CrossRef]
- Cheng, J.; Nguyen, T.Y.D.; Cygan, K.J.; Çelik, M.H.; Fairbrother, W.G.; Avsec, Ž.; Gagneur, J. MMSplice: Modular modeling improves the predictions of genetic variant effects on splicing. Genome Biol. 2019, 20, 48. [Google Scholar] [CrossRef]
- Leman, R.; Parfait, B.; Vidaud, D.; Girodon, E.; Pacot, L.; Le Gac, G.; Ka, C.; Ferec, C.; Fichou, Y.; Quesnelle, C.; et al. SPiP: Splicing Prediction Pipeline, a machine learning tool for massive detection of exonic and intronic variant effects on mRNA splicing. Hum. Mutat. 2022, 43, 2308–2323. [Google Scholar] [CrossRef]
- Bychkov, I.; Kuznetsova, A.; Baydakova, G.; Gorobets, L.; Kenis, V.; Dimitrieva, A.; Filatova, A.; Tabakov, V.; Skoblov, M.; Zakharova, E. Processed pseudogene insertion in GLB1 causes Morquio B disease by altering intronic splicing regulatory landscape. npj Genom. Med. 2022, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Filatova, A.Y.; Vasilyeva, T.A.; Marakhonov, A.V.; Voskresenskaya, A.A.; Zinchenko, R.A.; Skoblov, M.Y. Functional reassessment of PAX6 single nucleotide variants by in vitro splicing assay. Eur. J. Hum. Genet. EJHG 2019, 27, 488–493. [Google Scholar] [CrossRef]
- 4363115; GeneScan™ 500 LIZ™ Size Standard for SeqStudio™ Flex, SeqStudio™, 3500, 3730, and 3130 Series Instruments. Thermo Fisher Scientific: Warrington, UK, 2022.
- den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.-F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E.; et al. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]

| Variant | Exon | gnomAD v4.1.0 Total | SpliceAI | SPiP | MMSplice | AlphaMissense | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DS_AG | DS_AL | DS_DG | DS_DL | Interpretation | BP | InterConfident | Delta Logit Psi | Score | |||
| c.158G>A (p.Arg53Gln) | 3 | 0.00003107 | 0.01 | 0.18 | 0 | 0.27 | Alter by create New splice site + Alter ESR | No | 85.91 | −0.41 | 0.77 |
| c.174A>T (p.Arg58Ser) | 3 | 0 | 0 | 0.02 | 0.91 | 0.71 | Alter by SPiCE + Alter ESR | No | 98.11 | −2.62 | 0.97 |
| c.177-35T>G | 4 | 0 | 0 | 0.02 | 0 | 0.02 | Alter BP | Yes | 10.53 | −0.40 | - |
| c.342+3A>C | 5 | 0.000003728 | 0 | 0.01 | 0.32 | 0.84 | Alter by SPiCE | No | 98.41 | −3.85 | - |
| c.342+9G>T | 5 | 6.227 × 107 | 0.01 | 0.01 | 0.08 | 0.23 | NTR | No | 0.72 | 0.33 | - |
| c.343-6T>A | 6 | 0.00002737 | 0.01 | 0.1 | 0 | 0.05 | Alter by SPiCE | No | 54 | −1.48 | - |
| c.343G>C (p.Gly115Arg) | 6 | 0.00004528 | 0.02 | 0.08 | 0.01 | 0.02 | Alter by SPiCE | No | 69.33 | −0.79 | 0.89 |
| c.433A>T (p.Arg145Ter) | 6 | 0 | 0.01 | 0.03 | 0.01 | 0.01 | Alter by SPiCE | No | 54 | −1.10 | - |
| c.455G>C (p.Gly152Ala) | 7 | 0.000003101 | 0.23 | 0 | 0.18 | 0 | NTR | No | 8.04 | 1.10 | 0.51 |
| c.469+5G>A | 7 | 0 | 0 | 0.6 | 0 | 0.75 | Alter by SPiCE | No | 98.41 | −3.82 | - |
| c.469+6T>C | 7 | 0 | 0 | 0.49 | 0 | 0.61 | Alter by SPiCE | No | 85.91 | −0.97 | - |
| c.469G>T (p.Val157Phe) | 7 | 0.000001862 | 0 | 0.58 | 0 | 0.7 | Alter by SPiCE | No | 98.41 | −2.48 | 0.87 |
| c.549G>T (p.Gln183His) | 8 | 0.000003100 | 0.01 | 0.42 | 0.11 | 0.75 | Alter by SPiCE | No | 98.41 | −4.09 | 0.88 |
| c.612T>C (p.Tyr204=) | 9 | 0.000003099 | 0.2 | 0 | 0.21 | 0 | Alter by complex event | No | 26.62 | 0.34 | - |
| c.624T>C (p.Phe208=) | 9 | 0.000002480 | 0.21 | 0 | 0.25 | 0 | NTR | No | 7.62 | 0.51 | - |
| c.649G>T (p.Gly217Trp) | 9 | 0 | 0 | 0.66 | 0 | 0.67 | Alter by SPiCE | No | 98.41 | −3.87 | 1.00 |
| c.714A>G (p.Gln238=) | 10 | 0 | 0 | 0 | 0.02 | 0.01 | Alter by creating a new splice site + Alter ESR | No | 47.89 | −0.19 | - |
| c.773A>C (p.Gln258Pro) | 10 | 0.000002478 | 0.01 | 0.04 | 0.09 | 0.05 | Alter by SPiCE | No | 98.41 | −0.45 | 0.88 |
| c.775-16T>A | 11 | 6.433 × 107 | 0.81 | 0.35 | 0 | 0.05 | Alter by MES (Poly TC) | No | 98.41 | −2.56 | - |
| c.1006+6T>C | 12 | 0 | 0 | 0.45 | 0 | 0.57 | Alter by SPiCE | No | 96.71 | −1.04 | - |
| c.899T>G (p.Val300Gly) | 12 | 0.00004151 | 0.5 | 0.01 | 0.07 | 0 | NTR | No | 9.76 | 0.37 | 0.95 |
| c.1007-167A>T | 13 | 0 | 0.47 | 0 | 0.66 | 0.01 | Alter by creating de Novo Exon | No | 2.66 | −0.17 | - |
| c.1007G>A (p.Arg336Lys) | 13 | 0 | 0.09 | 0.15 | 0 | 0.03 | Alter by SPiCE | No | 43.04 | −0.95 | 0.79 |
| c.1007G>C (p.Arg336Thr) | 13 | 0 | 0.11 | 0.24 | 0 | 0.04 | Alter by SPiCE | No | 30.67 | −0.79 | 0.98 |
| c.1060C>T (p.Gln354Ter) | 13 | 0 | 0.02 | 0.06 | 0 | 0.02 | Alter by creating a new splice site + Alter ESR | No | 26.62 | −0.19 | - |
| c.1188+8T>A | 13 | 0.000001239 | 0 | 0.01 | 0.15 | 0 | Alter by creating a new splice site | No | 30.67 | −0.57 | - |
| c.1188+8T>C | 13 | 0 | 0 | 0 | 0.02 | 0 | Alter by creating a new splice site | No | 13.87 | −0.06 | - |
| c.1189-8T>A | 14 | 0 | 0 | 0.21 | 0 | 0.06 | Alter by SPiCE | No | 43.04 | −0.70 | - |
| Variant | Exon | Clinvar | HGDdatabase | Absolute WT ΔPSI (%) | Pathogenicity Criteria Before Analysis | Pathogenicity Criteria After Analysis | Pathogenicity Class Before Analysis | Pathogenicity Class After Analysis |
|---|---|---|---|---|---|---|---|---|
| c.158G>A (p.Arg53Gln) | 3 | Pathogenic/Likely pathogenic | Pathogenic | −6.8 | PM3, PP3, PM2, PM5, PP2, PP5 | - | Likely pathogenic | - |
| c.174A>T (p.Arg58Ser) | 3 | Pathogenic | Pathogenic | −94.9 | PM3, PP3, PM2, PM5, PP2, PP5 | PM3, PP3, PM2, PP5, PS3 | Likely pathogenic | Pathogenic |
| c.177-35T>G | 4 | - | Probably no pathogenicity | −1.0 | PM2, BP7 | PM2, BP7, BS3 | VUS | Likely benign |
| c.342+3A>C | 5 | Conflicting classifications of pathogenicity Pathogenic(1); Uncertain significance(1) | Pathogenic | −90.5 | PM3, PP3, PM2, PP5 | PM3, PP3, PM2, PP5, PS3 | Likely pathogenic | Pathogenic |
| c.342+9G>T | 5 | Likely benign | - | +0.6 | PM2, BP4, BP6 | PM2, BP4, BP6, BS3 | Likely benign | Likely benign |
| c.343-6T>A | 6 | Likely benign | - | −5.3 | PM2, BP6 | PM2, BP6, BS3 | VUS | Likely benign |
| c.343G>C (p.Gly115Arg) | 6 | Likely pathogenic | Pathogenic | −39.0 | PM3, PP3, PM2, PM1, PP2, PP5 | - | Likely pathogenic | - |
| c.433A>T (p.Arg145Ter) | 6 | Pathogenic | Pathogenic | −1.2 | PVS1, PM2, PM3, PP5 | - | Pathogenic | - |
| c.455G>C (p.Gly152Ala) | 7 | Uncertain significance | Pathogenic | +7.8 | PP3, PM2, PM1, PM5, PP2 | - | Likely pathogenic | - |
| c.469+5G>A | 7 | Pathogenic | Pathogenic | −88.0 | PP3, PM2, PM3, PP5 | PP3, PM2, PM3, PP5, PS3 | Likely pathogenic | Pathogenic |
| c.469+6T>C | 7 | Pathogenic | Pathogenic | −65.6 | PP3, PM2, PM3, PP5 | PP3, PM2, PM3, PP5, PS3 | Likely pathogenic | Pathogenic |
| c.469G>T (p.Val157Phe) | 7 | Pathogenic | Pathogenic | −84.5 | PP3, PM2, PP2, PM3, PP5 | PP3, PM2, PM3, PP5, PS3 | Likely pathogenic | Pathogenic |
| c.549G>T (p.Gln183His) | 8 | Uncertain significance | Pathogenic | −48.1 | PP3, PM2, PM5, PP2 | - | Likely pathogenic | - |
| c.612T>C (p.Tyr204=) | 9 | Likely benign | - | +47.2 | PM2, BP7, BP6 | PM2, BP7, BP6, BS3 | Likely benign | Likely benign |
| c.624T>C (p.Phe208=) | 9 | Likely benign | - | +52.6 | PM2, BS2, BP7, BP6 | PM2, BS2, BP7, BP6, BS3 | Likely benign | Benign |
| c.649G>T (p.Gly217Trp) | 9 | Pathogenic | Pathogenic | −22.7 | PP3, PM2, PP2, PM3, PP5 | - | Likely pathogenic | - |
| c.714A>G (p.Gln238=) | 10 | Likely benign | - | +12.1 | PM2, BP7, BP6 | PM2, BP7, BP6, BS3 | Likely benign | Likely benign |
| c.773A>C (p.Gln258Pro) | 10 | Pathogenic | Pathogenic | −19.6 | PM2, PM1, PP3, PP2, PM3, PP5 | - | Likely pathogenic | - |
| c.775-16T>A | 11 | Pathogenic | Pathogenic | −94.9 | PM2, PM3, PP5, PP3 | PM2, PM3, PP5, PP3, PS3 | Likely pathogenic | Pathogenic |
| c.1006+6T>C | 12 | Pathogenic | Pathogenic | −54.7 | PP3, PM2, PP5 | PP3, PM2, PP5, PS3 | VUS | Likely pathogenic |
| c.899T>G (p.Val300Gly) | 12 | Pathogenic/Likely pathogenic | Pathogenic | +5.3 | PM3, PP3, PM2, PM5, PP2, PP5 | - | Likely pathogenic | - |
| c.1007-167A>T | 13 | - | - | −72.5 | PM2, PM3, PP3, PP4 | PM2, PM3, PP3, PP4, PS3 | Likely pathogenic | Pathogenic |
| c.1007G>A (p.Arg336Lys) | 13 | Pathogenic | Pathogenic | −28.1 | PM2, PM1, PP3, PM5, PP2, PM3, PP5 | - | Likely pathogenic | - |
| c.1007G>C (p.Arg336Thr) | 13 | Pathogenic | Pathogenic | −55.6 | PP3, PM2, PM1, PM5, PP2, PM3, PP5 | PP3, PM2, PM3, PP5, PS3 | Likely pathogenic | Pathogenic |
| c.1060C>T (p.Gln354Ter) | 13 | Pathogenic | Pathogenic | −21.0 | PVS1, PM2, PM3, PP5 | - | Pathogenic | - |
| c.1188+8T>A | 13 | Pathogenic | Pathogenic | +7.7 | PM2, PM3, PP5 | PM2, PM3, PP5, BS3 | VUS | VUS |
| c.1188+8T>C | 13 | Likely benign | - | +9.5 | PM2, BP4, BP6 | PM2, BP4, BP6, BS3 | Likely benign | Likely benign |
| c.1189-8T>A | 14 | Likely benign | - | 0 | PM2, BP6 | PM2, BP6, BS3 | VUS | Likely benign |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekrasov, A.; Shchukina, E.; Rimskaya, B.; Zakharova, E. Functional Characterization of HGD Gene Variants by Minigene Splicing Assay. Int. J. Mol. Sci. 2025, 26, 10639. https://doi.org/10.3390/ijms262110639
Nekrasov A, Shchukina E, Rimskaya B, Zakharova E. Functional Characterization of HGD Gene Variants by Minigene Splicing Assay. International Journal of Molecular Sciences. 2025; 26(21):10639. https://doi.org/10.3390/ijms262110639
Chicago/Turabian StyleNekrasov, Andrey, Elza Shchukina, Beatrisa Rimskaya, and Ekaterina Zakharova. 2025. "Functional Characterization of HGD Gene Variants by Minigene Splicing Assay" International Journal of Molecular Sciences 26, no. 21: 10639. https://doi.org/10.3390/ijms262110639
APA StyleNekrasov, A., Shchukina, E., Rimskaya, B., & Zakharova, E. (2025). Functional Characterization of HGD Gene Variants by Minigene Splicing Assay. International Journal of Molecular Sciences, 26(21), 10639. https://doi.org/10.3390/ijms262110639






