Transcriptomic Insights into Late-Life Depression and the Role of Environmental Drinking Water Composition: A Study on 18-Month-Old Mice
Abstract
1. Introduction
2. Results
2.1. Deuterium and Mineral Analysis of the Samples
2.2. A Comparison of Brain Gene Expression Between 18-Month-Old and 3-Month-Old Mice
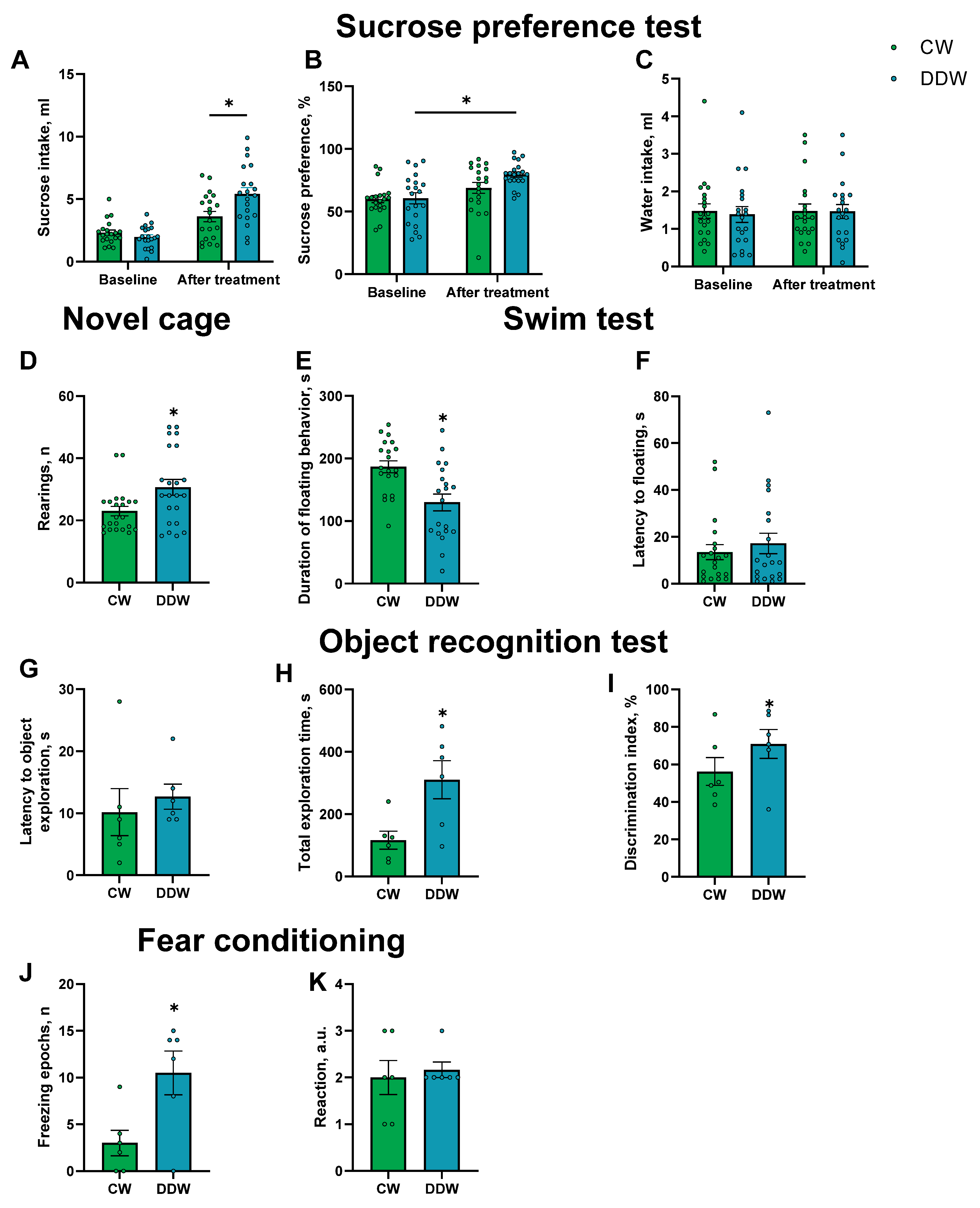
2.3. Effects of DDW Exposure on Parameters of Emotionality
2.4. Illumina Gene Expression Profiling of Old Mice Exposed to DDW or CW
2.5. Pathway Analysis and Functional Roles of Differentially Expressed Genes
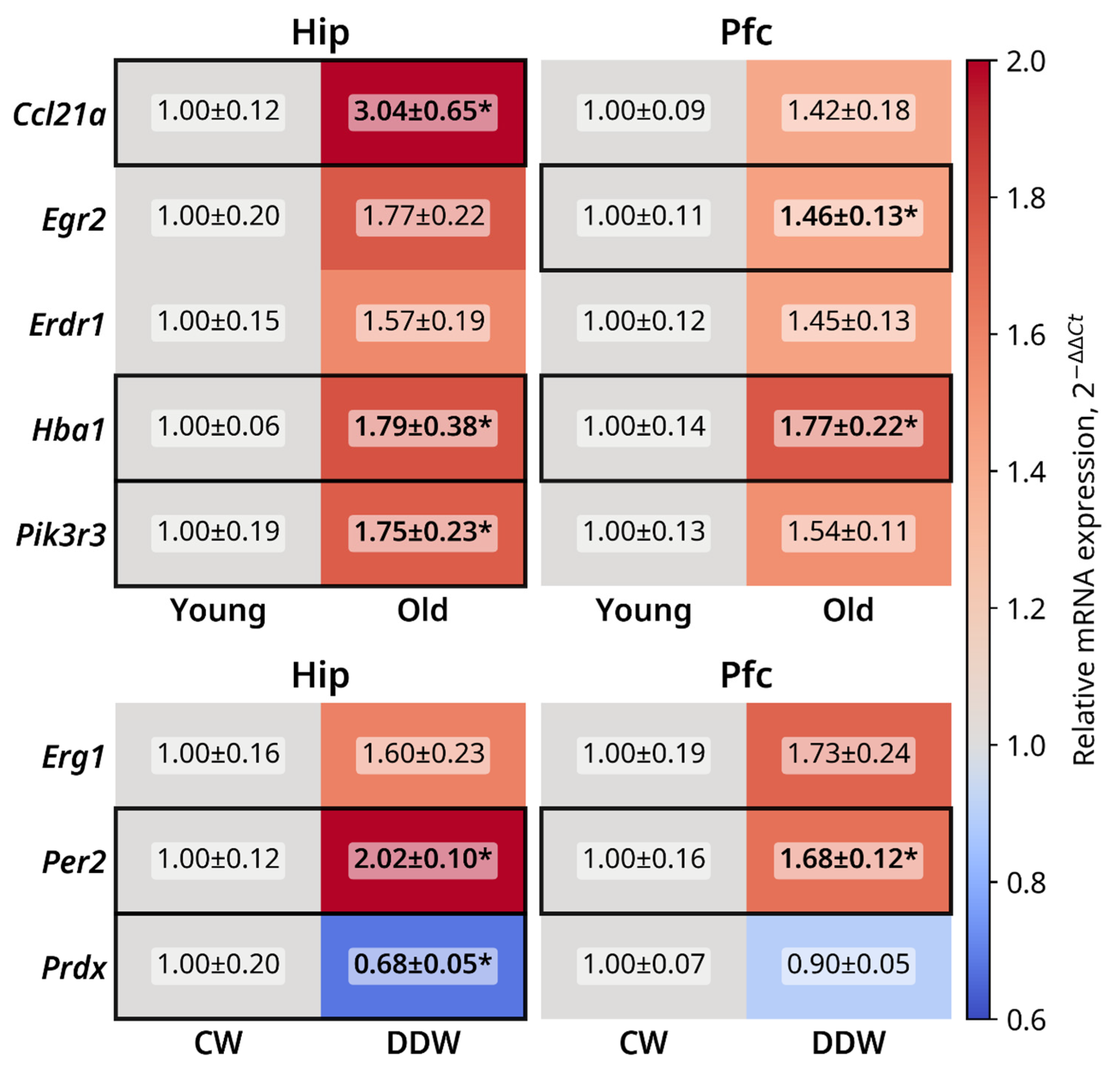
2.6. qRT-PCR Gene Expression Profiling of Selected Genes
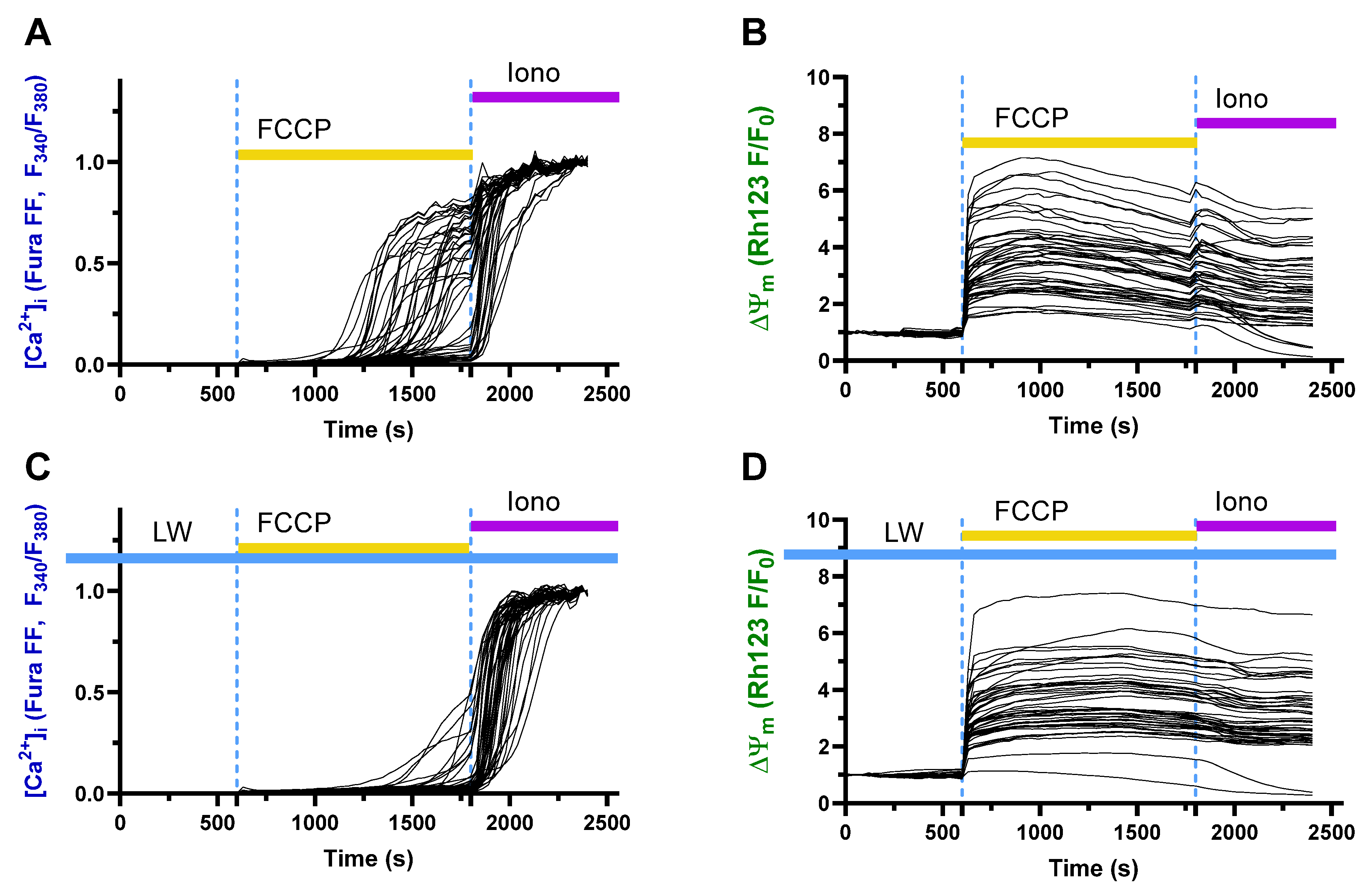
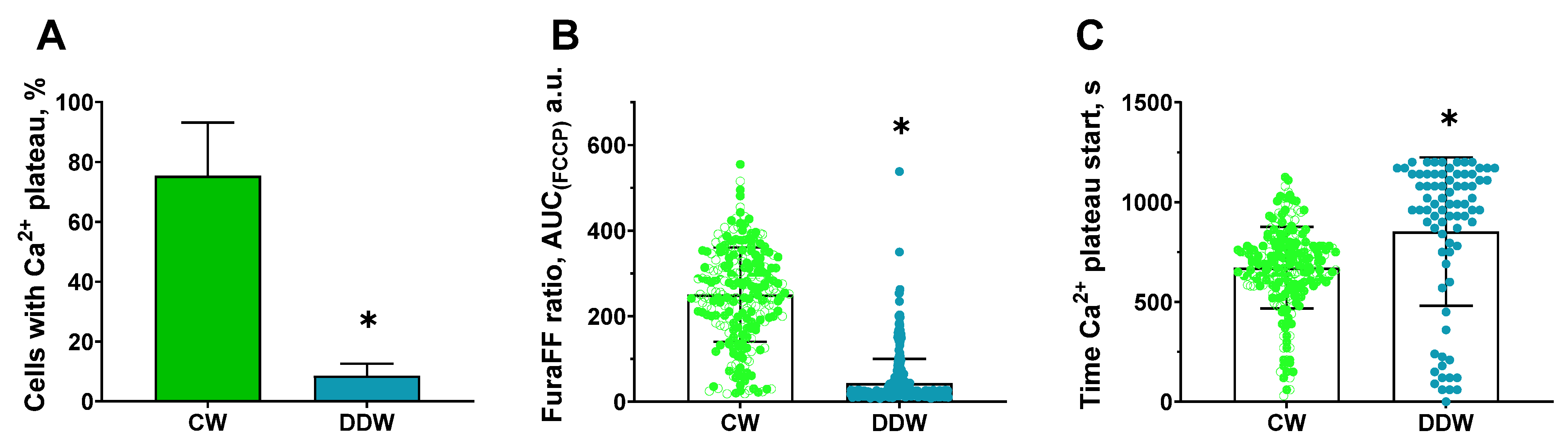
2.7. Protective Effects of Decreased Deuterium Levels on the Protonophore FCCP-Induced Ca2+ Influx in a Model of Neurotoxicity and Neuronal Damage in a Rat Neuronal Culture
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Study Flow
4.3. Culling and Brain Dissection
4.4. Illumina Gene Expression Profiling
4.5. Pathway Analysis
4.6. Behavioral Tests
4.6.1. Sucrose Preference Test
4.6.2. Novel Cage Test
4.6.3. The O-Maze
4.6.4. Swim Test
4.6.5. The Dark–Light Box
4.6.6. Object Recognition Test
4.6.7. Fear Conditioning
4.7. Quantitative Real-Time qPCR
4.8. A Study of the Effects DDW on the Protonophore FCCP-Induced Ca2+ Influx, a Model of Neurotoxicity and Neuronal Damage, in a Rat Neuronal Culture
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katon, W.J.; Schoenbaum, M.; Fan, M.-Y.; Callahan, C.M.; Williams, J.; Hunkeler, E.; Harpole, L.; Zhou, X.-H.A.; Langston, C.; Unützer, J. Cost-Effectiveness of Improving Primary Care Treatment of Late-Life Depression. Arch. Gen. Psychiatry 2005, 62, 1313–1320. [Google Scholar] [CrossRef]
- Smit, F.; Ederveen, A.; Cuijpers, P.; Deeg, D.; Beekman, A. Opportunities for Cost-Effective Prevention of Late-Life Depression: An Epidemiological Approach. Arch. Gen. Psychiatry 2006, 63, 290–296. [Google Scholar] [CrossRef]
- Luppa, M.; Sikorski, C.; Motzek, T.; Konnopka, A.; König, H.-H.; Riedel-Heller, S.G. Health Service Utilization and Costs of Depressive Symptoms in Late Life—A Systematic Review. Curr. Pharm. Des. 2012, 18, 5936–5957. [Google Scholar] [CrossRef]
- Bock, J.-O.; Brettschneider, C.; Weyerer, S.; Werle, J.; Wagner, M.; Maier, W.; Scherer, M.; Kaduszkiewicz, H.; Wiese, B.; Moor, L.; et al. Excess Health Care Costs of Late-Life Depression—Results of the AgeMooDe Study. J. Affect. Disord. 2016, 199, 139–147. [Google Scholar] [CrossRef]
- Holvast, F.; Massoudi, B.; Oude Voshaar, R.C.; Verhaak, P.F.M. Non-Pharmacological Treatment for Depressed Older Patients in Primary Care: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0184666. [Google Scholar] [CrossRef]
- Naismith, S.L.; Norrie, L.M.; Mowszowski, L.; Hickie, I.B. The Neurobiology of Depression in Later-Life: Clinical, Neuropsychological, Neuroimaging and Pathophysiological Features. Prog. Neurobiol. 2012, 98, 99–143. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lyness, J.M.; Tang, W.; Tu, X.; Conwell, Y. Outcomes and Predictors of Late-Life Depression Trajectories in Older Primary Care Patients. Am. J. Geriatr. Psychiatry 2008, 16, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Butters, M.A.; Whyte, E.M.; Nebes, R.D.; Begley, A.E.; Dew, M.A.; Mulsant, B.H.; Zmuda, M.D.; Bhalla, R.; Meltzer, C.C.; Pollock, B.G.; et al. The Nature and Determinants of Neuropsychological Functioning in Late-Life Depression. Arch. Gen. Psychiatry 2004, 61, 587–595. [Google Scholar] [CrossRef]
- Alexopoulos, G.S. Depression in the Elderly. Lancet 2005, 365, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Hegeman, J.M.; Kok, R.M.; van der Mast, R.C.; Giltay, E.J. Phenomenology of Depression in Older Compared with Younger Adults: Meta-Analysis. Br. J. Psychiatry 2012, 200, 275–281. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Dumas, J.A. Further Evidence of Peripheral Inflammatory Markers Involvement in Late-Onset Depression and Cognitive Decline. Am. J. Geriatr. Psychiatry 2022, 30, 701–702. [Google Scholar] [CrossRef]
- Diniz, B.S.; Teixeira, A.L.; Talib, L.L.; Mendonça, V.A.; Gattaz, W.F.; Forlenza, O.V. Serum Brain-Derived Neurotrophic Factor Level Is Reduced in Antidepressant-Free Patients with Late-Life Depression. World J. Biol. Psychiatry 2010, 11, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.D.; Aizenstein, H.J.; Alexopoulos, G.S. The Vascular Depression Hypothesis: Mechanisms Linking Vascular Disease with Depression. Mol. Psychiatry 2013, 18, 963–974. [Google Scholar] [CrossRef]
- Bruno, D.; Reichert Plaska, C.; Clark, D.P.A.; Zetterberg, H.; Blennow, K.; Verbeek, M.M.; Pomara, N. CSF α-Synuclein Correlates with CSF Neurogranin in Late-Life Depression. Int. J. Neurosci. 2021, 131, 357–361. [Google Scholar] [CrossRef]
- Blaveri, E.; Kelly, F.; Mallei, A.; Harris, K.; Taylor, A.; Reid, J.; Razzoli, M.; Carboni, L.; Piubelli, C.; Musazzi, L.; et al. Expression Profiling of a Genetic Animal Model of Depression Reveals Novel Molecular Pathways Underlying Depressive-like Behaviours. PLoS ONE 2010, 5, e12596. [Google Scholar] [CrossRef]
- Marchetti, L.; Lauria, M.; Caberlotto, L.; Musazzi, L.; Popoli, M.; Mathé, A.A.; Domenici, E.; Carboni, L. Gene Expression Signature of Antidepressant Treatment Response/Non-Response in Flinders Sensitive Line Rats Subjected to Maternal Separation. Eur. Neuropsychopharmacol. 2020, 31, 69–85. [Google Scholar] [CrossRef]
- Hamilton, M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967, 4, 278–296. [Google Scholar] [CrossRef]
- Mangold, C.A.; Wronowski, B.; Du, M.; Masser, D.R.; Hadad, N.; Bixler, G.V.; Brucklacher, R.M.; Ford, M.M.; Sonntag, W.E.; Freeman, W.M. Sexually Divergent Induction of Microglial-Associated Neuroinflammation with Hippocampal Aging. J. Neuroinflamm. 2017, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Su, S.; Cai, W.; Cao, J.; Miao, X.; Zang, W.; Gao, S.; Xu, Y.; Yang, J.; Tao, Y.-X.; et al. Differentially Expressed Genes in the Brain of Aging Mice with Cognitive Alteration and Depression- and Anxiety-Like Behaviors. Front. Cell Dev. Biol. 2020, 8, 814. [Google Scholar] [CrossRef]
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.A.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased Expression of Synapse-Related Genes and Loss of Synapses in Major Depressive Disorder. Nat. Med. 2012, 18, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Verwer, R.W.H.; Gao, S.-F.; Qi, X.-R.; Lucassen, P.J.; Kessels, H.W.; Swaab, D.F. Prefrontal Alterations in GABAergic and Glutamatergic Gene Expression in Relation to Depression and Suicide. J. Psychiatr. Res. 2018, 102, 261–274. [Google Scholar] [CrossRef]
- Zeng, L.; Fujita, M.; Gao, Z.; White, C.C.; Green, G.S.; Habib, N.; Menon, V.; Bennett, D.A.; Boyle, P.; Klein, H.-U.; et al. A Single-Nucleus Transcriptome-Wide Association Study Implicates Novel Genes in Depression Pathogenesis. Biol. Psychiatry 2024, 96, 34–43. [Google Scholar] [CrossRef]
- Nagy, C.; Schwabe, D.; Jones, W.; Brown, A.; Shupe, M.; Mancone, A.; Camillocci, J. “Time to Talk About It: Physician Depression and Suicide” Video/Discussion Session for Interns, Residents, and Fellows. MedEdPORTAL 2016, 12, 10508. [Google Scholar] [CrossRef]
- Primiani, C.T.; Ryan, V.H.; Rao, J.S.; Cam, M.C.; Ahn, K.; Modi, H.R.; Rapoport, S.I. Coordinated Gene Expression of Neuroinflammatory and Cell Signaling Markers in Dorsolateral Prefrontal Cortex during Human Brain Development and Aging. PLoS ONE 2014, 9, e110972. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Rocha-Dias, I.; de Oliveira, L.R.S.; Improta-Caria, A.C.; Monteiro, R.S., Jr.; Cassilhas, R.C. Molecular mechanisms of physical exercise on depression in the elderly: A systematic review. Mol. Biol. Rep. 2021, 48, 3853–3862. [Google Scholar] [CrossRef]
- Cardona, D.; Segura, A.; Segura, Á.; Garzón, M.O. Contextual effects associated with depression risk variability in the elderly, Antioquia, Colombia, 2012. Biomedica 2015, 35, 73–80. [Google Scholar] [CrossRef]
- Saravanakumar, P.; Muhammad, T.; Paul, R.; Srivastava, S. Explaining the Urban-Rural Difference in Late-Life Depression in India: Evidence from a Multivariate Decomposition Analysis Based on Longitudinal Aging Study in India, Wave 2017–2018. Clin. Gerontol. 2024, 47, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Behlen, M.; Henriques, A.; Severo, M.; Santos, C.J.; Ribeiro, A.I. Exposure to Green and Blue Spaces and Depression in Older Adults: Findings from the EPIPorto Cohort. Eur. J. Public Health 2023, 33, ckad160.1195. [Google Scholar] [CrossRef]
- D’Souza, J.; Bergmans, R.; Fossa, A.; Szpiro, A.A.; Mendes de Leon, C.; Kaufman, J.; Hirth, R.; Faul, J.; Adar, S. Physical Environmental Hazards and Major Depression in Older U.S. Adults: The Health and Retirement Study. In Proceedings of the ISEE 2022: 34th Annual Conference of the International Society of Environmental Epidemiology, Athens, Greece, 18–21 September 2022; Volume 2022. [Google Scholar] [CrossRef]
- Bauer, M.; Glenn, T.; Alda, M.; Andreassen, O.A.; Ardau, R.; Bellivier, F.; Berk, M.; Bjella, T.D.; Bossini, L.; Del Zompo, M.; et al. Impact of Sunlight on the Age of Onset of Bipolar Disorder. Bipolar Disord. 2012, 14, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, H.; Xia, Y.; Zhao, Y. The Association between Water Source and Depressive Symptoms in China: A Cross-Sectional and Longitudinal Study. J. Affect. Disord. 2021, 295, 56–62. [Google Scholar] [CrossRef]
- Ljubicić, D.; Stipcević, T.; Pivac, N.; Jakovljević, M.; Mück-Seler, D. The Influence of Daylight Exposure on Platelet 5-HT Levels in Patients with Major Depression and Schizophrenia. J. Photochem. Photobiol. B 2007, 89, 63–69. [Google Scholar] [CrossRef]
- Komulainen, K.; Hakulinen, C.; Lipsanen, J.; Partonen, T.; Pulkki-Råback, L.; Kähönen, M.; Virtanen, M.; Ruuhela, R.; Raitakari, O.; Elovainio, M. Associations of Long-Term Solar Insolation with Specific Depressive Symptoms: Evidence from a Prospective Cohort Study. J. Psychiatr. Res. 2022, 151, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; De Meyer, F.; Thompson, P.; Peeters, D.; Cosyns, P. Synchronized Annual Rhythms in Violent Suicide Rate, Ambient Temperature and the Light-Dark Span. Acta Psychiatr. Scand. 1994, 90, 391–396. [Google Scholar] [CrossRef]
- Brazienė, A.; Venclovienė, J.; Vaičiulis, V.; Lukšienė, D.; Tamošiūnas, A.; Milvidaitė, I.; Radišauskas, R.; Bobak, M. Relationship between Depressive Symptoms and Weather Conditions. Int. J. Environ. Res. Public Health 2022, 19, 5069. [Google Scholar] [CrossRef]
- Flaten, T.P. Aluminium as a Risk Factor in Alzheimer’s Disease, with Emphasis on Drinking Water. Brain Res. Bull. 2001, 55, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S. Role of Selenium and Other Trace Elements in the Geography of Schizophrenia. Schizophr. Bull. 1994, 20, 387–398. [Google Scholar] [CrossRef][Green Version]
- Baj, J.; Bargieł, J.; Cabaj, J.; Skierkowski, B.; Hunek, G.; Portincasa, P.; Flieger, J.; Smoleń, A. Trace Elements Levels in Major Depressive Disorder-Evaluation of Potential Threats and Possible Therapeutic Approaches. Int. Mol. Sci. 2023, 24, 15071. [Google Scholar] [CrossRef]
- Heidari, H.; Lawrence, D.A. Climate Stressors and Physiological Dysregulations: Mechanistic Connections to Pathologies. Int. J. Environ. Res. Public Health 2023, 21, 28. [Google Scholar] [CrossRef]
- Sundas, A.; Contreras, I.; Mujahid, O.; Beneyto, A.; Vehi, J. The Effects of Environmental Factors on General Human Health: A Scoping Review. Healthcare 2024, 12, 2123. [Google Scholar] [CrossRef] [PubMed]
- Alum, E.U. Climate Change and Its Impact on the Bioactive Compound Profile of Medicinal Plants: Implications for Global Health. Plant Signal. Behav. 2024, 19, 2419683. [Google Scholar] [CrossRef]
- Dutton, A.; Wilkinson, B.H.; Welker, J.M.; Bowen, G.J.; Lohmann, K.C. Spatial Distribution and Seasonal Variation in18O/16O of Modern Precipitation and River Water across the Conterminous USA. Hydrol. Process. 2005, 19, 4121–4146. [Google Scholar] [CrossRef]
- Kendall, C.; Coplen, T.B. Distribution of Oxygen--18 and Deuterium in River Waters across the United States. Hydrol. Process. 2001, 15, 1363–1393. [Google Scholar] [CrossRef]
- Friedman, I.; Redfield, A.C.; Schoen, B.; Harris, J. The Variation of the Deuterium Content of Natural Waters in the Hydrologic Cycle. Rev. Geophys. 1964, 2, 177–224. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Reference Sheet for VSMOW2 and SLAP2; International Measurement Standards; International Atomic Energy Agency: Vienna, Austria, 2017. [Google Scholar]
- Strekalova, T.; Evans, M.; Chernopiatko, A.; Couch, Y.; Costa-Nunes, J.; Cespuglio, R.; Chesson, L.; Vignisse, J.; Steinbusch, H.W.; Anthony, D.C.; et al. Deuterium Content of Water Increases Depression Susceptibility: The Potential Role of a Serotonin-Related Mechanism. Behav. Brain Res. 2015, 277, 237–244. [Google Scholar] [CrossRef]
- Bowen, G.J.; Ehleringer, J.R.; Chesson, L.A.; Stange, E.; Cerling, T.E. Stable Isotope Ratios of Tap Water in the Contiguous United States. Water Resour. Res. 2007, 43, 2006WR005186. [Google Scholar] [CrossRef]
- Goncharuk, V.V.; Lapshin, V.B.; Burdeinaya, T.N.; Pleteneva, T.V.; Chernopyatko, A.S.; Atamanenko, I.D.; Ul’Yantsev, A.S.; Uspenskaya, E.V.; Samsoni-Todorov, A.O.; Taranov, V.V.; et al. Physicochemical properties and biological activity of the water depleted of heavy isotopes. J. Water Chem. Technol. 2011, 33, 8–13. [Google Scholar]
- Gat, J.R.; Magaritz, M. Climatic Variations in the Eastern Mediterranean Sea Area. Naturwissenschaften 1980, 67, 80–87. [Google Scholar] [CrossRef]
- Qu, J.; Xu, Y.; Zhao, S.; Xiong, L.; Jing, J.; Lui, S.; Huang, J.; Shi, H. The Biological Impact of Deuterium and Therapeutic Potential of Deuterium-Depleted Water. Front. Pharmacol. 2024, 15, 1431204. [Google Scholar] [CrossRef] [PubMed]
- Kirkina, A.A.; Lobyshev, V.I.; Lopina, O.D.; Doronin, Y.-K.; Burdeinaya, T.N.; Chernopyatko, A.S. Isotopic effects of low concentration of deuterium in water on biological systems. Biofizika 2014, 59, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, V.; Katona, E. Deuteration as a Tool in Investigating the Role of Water in the Structure and Function of Excitable Membranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1986; Volume 127, pp. 662–678. ISBN 978-0-12-182027-5. [Google Scholar]
- Kravtsov, A.; Kozin, S.; Basov, A.; Butina, E.; Baryshev, M.; Malyshko, V.; Moiseev, A.; Elkina, A.; Dzhimak, S. Reduction of Deuterium Level Supports Resistance of Neurons to Glucose Deprivation and Hypoxia: Study in Cultures of Neurons and on Animals. Molecules 2021, 27, 243. [Google Scholar] [CrossRef]
- Pomytkin, I.A.; Kolesova, O.E. Relationship between Natural Concentration of Heavy Water Isotopologs and Rate of H2O2 Generation by Mitochondria. Bull. Exp. Biol. Med. 2006, 142, 570–572. [Google Scholar] [CrossRef]
- Zhang, X.; Gaetani, M.; Chernobrovkin, A.; Zubarev, R.A. Anticancer Effect of Deuterium Depleted Water-Redox Disbalance Leads to Oxidative Stress. Mol. Cell. Proteomics 2019, 18, 2373–2387. [Google Scholar] [CrossRef]
- Olgun, A. Biological Effects of Deuteronation: ATP Synthase as an Example. Theor. Biol. Med. Model. 2007, 4, 9. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Timokhina, E.P.; Obernikhin, S.S.; Yaglov, V.V. Emerging Role of Deuterium/Protium Disbalance in Cell Cycle and Apoptosis. Int. J. Mol. Sci. 2023, 24, 3107. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, D.; Yang, H.; Wang, W.; Xiao, J.; Zhou, L.; Fu, H. Neuroprotective Effects of Deuterium-Depleted Water (DDW) Against H2O2-Induced Oxidative Stress in Differentiated PC12 Cells Through the PI3K/Akt Signaling Pathway. Neurochem. Res. 2020, 45, 1034–1044. [Google Scholar] [CrossRef]
- Bayrak, B.B.; Kulak, G.Y.; Yanardag, R.; Yarat, A. Short Term Deuterium Depletion in Drinking Water Reduced Tumor Induced Oxidative Stress in Mice Liver. Pathol.-Res. Pract. 2022, 240, 154186. [Google Scholar] [CrossRef]
- Korchinsky, N.; Davis, A.M.; Boros, L.G. Nutritional Deuterium Depletion and Health: A Scoping Review. Metabolomics 2024, 20, 117. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, H. Deuterium-Depleted Water in Cancer Therapy: A Systematic Review of Clinical and Experimental Trials. Nutrients 2024, 16, 1397. [Google Scholar] [CrossRef] [PubMed]
- Molnár, M.; Horváth, K.; Dankó, T.; Somlyai, I.; Kovács, B.Z.; Somlyai, G. Deuterium-Depleted Water Stimulates GLUT4 Translocation in the Presence of Insulin, Which Leads to Decreased Blood Glucose Concentration. Mol. Cell. Biochem. 2021, 476, 4507–4516. [Google Scholar] [CrossRef] [PubMed]
- Somlyai, G.; Somlyai, I.; Fórizs, I.; Czuppon, G.; Papp, A.; Molnár, M. Effect of Systemic Subnormal Deuterium Level on Metabolic Syndrome Related and Other Blood Parameters in Humans: A Preliminary Study. Molecules 2020, 25, 1376. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Somlyai, I.; Kovács, B.Z.; Puskás, L.G.; Nagy, L.I.; Dux, L.; Farkas, G.; Somlyai, G. Deuterium Depletion Inhibits Cell Proliferation, RNA and Nuclear Membrane Turnover to Enhance Survival in Pancreatic Cancer. Cancer Control 2021, 28, 1073274821999655. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L.; Gabrielli, F.; Pregnolato, M. Depression, Osteoporosis, Serotonin and Cell Membrane Viscosity Between Biology and Philosophical Anthropology. Ann. Gen. Psychiatry 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Cespuglio, R.; Rousset, C.; Debilly, G.; Rochat, C.; Millan, M.J. Acute Administration of the Novel Serotonin and Noradrenaline Reuptake Inhibitor, S33005, Markedly Modifies Sleep-Wake Cycle Architecture in the Rat. Psychopharmacology 2005, 181, 639–652. [Google Scholar] [CrossRef]
- Newberg, A.B.; Amsterdam, J.D.; Wintering, N.; Shults, J. Low Brain Serotonin Transporter Binding in Major Depressive Disorder. Psychiatry Res. Neuroimaging 2012, 202, 161–167. [Google Scholar] [CrossRef]
- Zia, A.; Pourbagher-Shahri, A.M.; Farkhondeh, T.; Samarghandian, S. Molecular and Cellular Pathways Contributing to Brain Aging. Behav. Brain Funct. 2021, 17, 6. [Google Scholar] [CrossRef]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef]
- Mastrobattista, E.; Lenze, E.J.; Reynolds, C.F.; Mulsant, B.H.; Wetherell, J.; Wu, G.F.; Blumberger, D.M.; Karp, J.F.; Butters, M.A.; Mendes-Silva, A.P.; et al. Late-Life Depression Is Associated with Increased Levels of GDF-15, a Pro-Aging Mitokine. Am. J. Geriatr. Psychiatry 2023, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Barrientos, F.A.; Luque-Campos, N.; Araya, M.J.; Lara-Barba, E.; Solminihac, J.; Pradenas, C.; Molina, L.; Herrera-Luna, Y.; Utreras-Mendoza, Y.; Elizondo-Vega, R.; et al. Mitochondrial Dysfunction in Neurodegenerative Disorders: Potential Therapeutic Application of Mitochondrial Transfer to Central Nervous System-Residing Cells. J. Transl. Med. 2023, 21, 613. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Tian, Y. Neuron-Periphery Mitochondrial Stress Communication in Aging and Diseases. Life Med. 2022, 1, 168–178. [Google Scholar] [CrossRef]
- Glaesmer, H.; Riedel-Heller, S.; Braehler, E.; Spangenberg, L.; Luppa, M. Age- and Gender-Specific Prevalence and Risk Factors for Depressive Symptoms in the Elderly: A Population-Based Study. Int. Psychogeriatr. 2011, 23, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Zanni, G.R.; Wick, J.Y. Understanding Suicide in the Elderly. In The Consultant Pharmacist; American Society of Consultant Pharmacists: Alexandria, VA, USA, 2010; Volume 25, pp. 93–102. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yu, S.-C.; McQuoid, D.R.; Messer, D.F.; Taylor, W.D.; Singh, K.; Boyd, B.D.; Krishnan, K.R.R.; MacFall, J.R.; Steffens, D.C.; et al. Reduction of Dorsolateral Prefrontal Cortex Gray Matter in Late-Life Depression. Psychiatry Res. 2011, 193, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I. Depression and the Hippocampus: Cause or Effect? Biol. Psychiatry 2011, 70, 308–309. [Google Scholar] [CrossRef]
- Zhao, Z.; Taylor, W.D.; Styner, M.; Steffens, D.C.; Krishnan, K.R.R.; MacFall, J.R. Hippocampus Shape Analysis and Late-Life Depression. PLoS ONE 2008, 3, e1837. [Google Scholar] [CrossRef]
- Malatynska, E.; Steinbusch, H.W.M.; Redkozubova, O.; Bolkunov, A.; Kubatiev, A.; Yeritsyan, N.B.; Vignisse, J.; Bachurin, S.; Strekalova, T. Anhedonic-like Traits and Lack of Affective Deficits in 18-Month-Old C57BL/6 Mice: Implications for Modeling Elderly Depression. Exp. Gerontol. 2012, 47, 552–564. [Google Scholar] [CrossRef]
- Lawton, M.P.; Parmelee, P.A.; Katz, I.R.; Nesselroade, J. Affective States in Normal and Depressed Older People. J. Gerontol. B Psychol. Sci. Soc. Sci. 1996, 51, P309–P316. [Google Scholar] [CrossRef][Green Version]
- Ngan, J.S.T.; Chan, W.C.; Wong, S.T.; Wong, C.S.M.; Cheng, C.P.W. Reward System in Late-Life Depression: A Cross-Sectional Case-Control Study. East. Asian Arch. Psychiatry 2023, 33, 71–76. [Google Scholar] [CrossRef]
- Strekalova, T.; Bahzenova, N.; Trofimov, A.; Schmitt-Böhrer, A.G.; Markova, N.; Grigoriev, V.; Zamoyski, V.; Serkova, T.; Redkozubova, O.; Vinogradova, D.; et al. Pro-Neurogenic, Memory-Enhancing and Anti-Stress Effects of DF302, a Novel Fluorine Gamma-Carboline Derivative with Multi-Target Mechanism of Action. Mol. Neurobiol. 2018, 55, 335–349. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zubarev, R.A. Slight Deuterium Enrichment in Water Acts as an Antioxidant: Is Deuterium a Cell Growth Regulator? Mol. Cell. Proteomics 2020, 19, 1790–1804. [Google Scholar] [CrossRef]
- Shikama, K.; Matsuoka, A. Human Haemoglobin: A New Paradigm for Oxygen Binding Involving Two Types of Alphabeta Contacts. Eur. J. Biochem. 2003, 270, 4041–4051. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.J.; Zhou, S.; Feng, L.; Gell, D.A.; Mackay, J.P.; Shi, Y.; Gow, A.J. Role of Alpha-Hemoglobin-Stabilizing Protein in Normal Erythropoiesis and Beta-Thalassemia. Ann. N. Y. Acad. Sci. 2005, 1054, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Chin, R.K.; Lee, Y.; Kang, H.-S.; Wang, Y.; Weinstock, J.V.; Banks, T.; Ware, C.F.; Franzoso, G.; Fu, Y.-X. Differential Regulation of CCL21 in Lymphoid/Nonlymphoid Tissues for Effectively Attracting T Cells to Peripheral Tissues. J. Clin. Investig. 2003, 112, 1495–1505. [Google Scholar] [CrossRef]
- Bhadhprasit, W.; Kinoshita, C.; Matsumura, N.; Aoyama, K. Erythroid Differentiation Regulator 1 as a Regulator of Neuronal GSH Synthesis. Antioxidants 2024, 13, 771. [Google Scholar] [CrossRef]
- Wang, Y. Erdr1 Drives Macrophage Programming via Dynamic Interplay with YAP1 and Mid1. Immunohorizons 2024, 8, 198–213. [Google Scholar] [CrossRef]
- Di Segni, M.; D’ADdario, S.L.; Babicola, L.; Ielpo, D.; Iacono, L.L.; Andolina, D.; Accoto, A.; Luchetti, A.; Mancini, C.; Parisi, C.; et al. Xlr4 as a New Candidate Gene Underlying Vulnerability to Cocaine Effects. Neuropharmacology 2020, 168, 108019. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, X.; Luo, X.; Wang, J.; Hu, J.; Feng, Y. PIK3R3 Inhibits Cell Senescence through P53/P21 Signaling. Cell Death Dis. 2020, 11, 798. [Google Scholar] [CrossRef]
- Yang, X.; Fu, Y.; Hu, F.; Luo, X.; Hu, J.; Wang, J. PIK3R3 Regulates PPARα Expression to Stimulate Fatty Acid β-Oxidation and Decrease Hepatosteatosis. Exp. Mol. Med. 2018, 50, e431. [Google Scholar] [CrossRef]
- Lujan, D.A.; Ochoa, J.L.; Hartley, R.S. Cold-Inducible RNA Binding Protein in Cancer and Inflammation. Wiley Interdiscip. Rev. RNA 2018, 9, e1462. [Google Scholar] [CrossRef]
- Zhong, P.; Peng, J.; Bian, Z.; Huang, H. The Role of Cold Inducible RNA-Binding Protein in Cardiac Physiology and Diseases. Front. Pharmacol. 2021, 12, 610792. [Google Scholar] [CrossRef]
- Al-Kandari, W.; Koneni, R.; Navalgund, V.; Aleksandrova, A.; Jambunathan, S.; Fontes, J.D. The Zinc Finger Proteins ZXDA and ZXDC Form a Complex That Binds CIITA and Regulates MHC II Gene Transcription. J. Mol. Biol. 2007, 369, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.L.; Wu, Y.T.; Lin, H.Y.; Hsu, F.C.; Liu, S.K.; Chang, B.I.; Chen, W.S.; Lai, C.H.; Shi, G.Y.; Wu, H.L. Functions of Rhomboid Family Protease RHBDL2 and Thrombomodulin in Wound Healing. J. Investig. Dermatol. 2011, 131, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, K.; Zheng, D.; Liu, Y.; Li, L.; He, Z.; Sun, C.; Yu, C. RHBDL2 Promotes the Proliferation, Migration, and Invasion of Pancreatic Cancer by Stabilizing the N1ICD via the OTUD7B and Activating the Notch Signaling Pathway. Cell Death Dis. 2022, 13, 945. [Google Scholar] [CrossRef]
- Pipkin, M.E.; Rao, A.; Lichtenheld, M.G. The Transcriptional Control of the Perforin Locus. Immunol. Rev. 2010, 235, 55–72. [Google Scholar] [CrossRef]
- Revelo, X.S.; Tsai, S.; Lei, H.; Luck, H.; Ghazarian, M.; Tsui, H.; Shi, S.Y.; Schroer, S.; Luk, C.T.; Lin, G.H.Y.; et al. Perforin Is a Novel Immune Regulator of Obesity-Related Insulin Resistance. Diabetes 2015, 64, 90–103. [Google Scholar] [CrossRef]
- Hu, L.; Wang, R.-Y.; Cai, J.; Feng, D.; Yang, G.-Z.; Xu, Q.-G.; Zhai, Y.-X.; Zhang, Y.; Zhou, W.-P.; Cai, Q.-P. Overexpression of CHKA Contributes to Tumor Progression and Metastasis and Predicts Poor Prognosis in Colorectal Carcinoma. Oncotarget 2016, 7, 66660–66678. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, J.; Wei, J.; Meng, W.; Wang, Y.; Shi, M.; Zhang, Y.; Liu, Y.; Wang, H.; Wang, Y. LncRNA SNHG11 Promotes Gastric Cancer Progression by Activating the Wnt/β-Catenin Pathway and Oncogenic Autophagy. Front. Oncol. 2020, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Abbonante, V.; Malara, A.; Chrisam, M.; Metti, S.; Soprano, P.; Semplicini, C.; Bello, L.; Bozzi, V.; Battiston, M.; Pecci, A.; et al. Lack of COL6/collagen VI causes megakaryocyte dysfunction by impairing autophagy and inducing apoptosis. Autophagy 2022, 19, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4, 1964. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.B.; Xu, C.; Wang, Y.; Zhang, L.W. Activating transcription factor 3-activated long noncoding RNA forkhead box P4-antisense RNA 1 aggravates colorectal cancer progression by regulating microRNA-423-5p/nucleus accumbens associated 1 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4901–4908. [Google Scholar]
- Wei, Z.Y.; Wang, L.P.; Gao, D.; Zhu, L.; Wu, J.F.; Shi, J.; Li, Y.N.; Tang, X.D.; Feng, Y.M.; Pan, X.B.; et al. Bulk and single-cell RNA-seq analyses reveal canonical RNA editing associated with microglia homeostasis and its role in sepsis-associated encephalopathy. Neuroscience 2024, 560, 167–180. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Tsakali, S.S.; Whitehead, M.; Chennell, G.; Wu, M.Y.; Molenaar, C.; Kutikhin, A.; Chen, Y.; Ahmad, S.; Bogdanov, L.; et al. Matrix-associated extracellular vesicles modulate human smooth muscle cell adhesion and directionality by presenting collagen VI. Elife 2025, 12, RP90375. [Google Scholar] [CrossRef] [PubMed]
- Manon-Jensen, T.; Kjeld, N.G.; Karsdal, M.A. Collagen-mediated hemostasis. J. Thromb. Haemost. 2016, 14, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Maffucci, P.; Filion, C.A.; Boisson, B.; Itan, Y.; Shang, L.; Casanova, J.L.; Puel, A.; Revy, P.; Cunningham-Rundles, C. Genetic Diagnosis Using Whole Exome Sequencing in Common Variable Immunodeficiency. Front. Immunol. 2016, 7, 220. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, X.; Zhang, M.; Tang, Y.; Wu, J.; Wang, T.; Wang, X.; Zhang, Y.; Wang, Z.; Wang, Y.; et al. Bi-Allelic Mutations in STXBP2 Reveal a Complementary Role for STXBP1 in Cytotoxic Lymphocyte Killing. Front. Immunol. 2018, 9, 529. [Google Scholar] [CrossRef]
- Cwetsch, A.W.; Pinto, B.; Savardi, A.; Cancedda, L. In vivo methods for acute modulation of gene expression in the central nervous system. Prog. Neurobiol. 2018, 170, 69–85. [Google Scholar] [CrossRef]
- Sungur, A.O.; Schwarting, R.K.W. The role of the lateral septum in anxiety and locomotion. Behav. Brain Res. 2012, 233, 61–68. [Google Scholar] [CrossRef]
- Jiang, H.; Shukla, A.; Wang, X.; Chen, W.Y.; Bernstein, B.E.; Roeder, R.G. Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell 2011, 144, 513–525, Erratum in Cell 2011, 144, 825. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chang, F.C.; Ross, A.E.; Lee, J.; Nakayama, K.; Nakayama, K.; Desiderio, S. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J recombinase to the cell cycle. Mol. Cell 2005, 18, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, A.; Cancedda, L.; Reid, S.W.; Tessarollo, L.; Porciatti, V.; Pizzorusso, T.; Maffei, L. Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation but normal critical period for monocular deprivation. J. Neurosci. 2002, 22, 10072–10077. [Google Scholar] [CrossRef]
- Matsuo, T.; Ohtsuki, T.; Ohtsuki, T.; Koga, M.; Kato, N.; Kato, T. Functional polymorphisms in the upstream region of the GRIK4 gene are associated with increased risk of schizophrenia. Am. J. Hum. Genet. 2007, 80, 812–813. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Murray, D.T.; Kato, M.; Lin, Y.; Thurber, K.R.; Hung, I.; McKnight, S.L.; Tycko, R. Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell 2017, 171, 615–627.e16. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A.; Neumann, M. Fused in Sarcoma Neuropathology in Neurodegenerative Disease. Cold Spring Harb. Perspect. Med. 2017, 7, a024299. [Google Scholar] [CrossRef]
- Toropova, K.; Zalyte, R.; Woolfson, D.N.; Roberts, A.J. Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nat. Struct. Mol. Biol. 2019, 26, 823–829. [Google Scholar] [CrossRef]
- Hou, Y.; Qin, H.; Follit, J.A.; Pazour, G.J.; Rosenbaum, J.L.; Witman, G.B. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 2007, 176, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Martel, G.; Uchida, S.; Hevi, C.; Chevere-Torres, I.; Fuentes, I.; Park, Y.J.; Hafeez, H.; Yamagata, H.; Watanabe, Y.; Shumyatsky, G.P. Genetic demonstration of a role for stathmin in adult hippocampal neurogenesis, spinogenesis, and NMDA receptor-dependent memory. J. Neurosci. 2016, 36, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Leman, E.E.; Fyffe, R.E.W.; Raine, C.S.; Schubart, U.K. Stathmin-deficient mice develop an age-dependent axonopathy of the central and peripheral nervous systems. Am. J. Pathol. 2002, 160, 469–480. [Google Scholar] [CrossRef]
- Liu, A.; Muggironi, M.; Marin-Husstege, M.; Casaccia-Bonnefil, P. Oligodendrocyte process outgrowth in G1 phase is regulated by Cdc42 activation and cyclin-dependent kinase inhibition. J. Neurosci. 2005, 25, 8315–8326. [Google Scholar] [CrossRef]
- Panza, P.; Sitko, A.A.; Maischein, H.M.; Koch, I.; Flötenmeyer, M.; Wright, G.J.; Mandai, K.; Mason, C.A.; Söllner, C. The LRR receptor Islr2 is required for retinal axon routing at the vertebrate optic chiasm. Development 2015, 142, 1167–1177. [Google Scholar] [CrossRef]
- Abudureyimu, S.; Asai, N.; Enomoto, A.; Weng, L.; Kobayashi, H.; Wang, X.; Chen, C.; Mii, S.; Takahashi, M. Essential role of Linx/Islr2 in the development of the forebrain commissural system. Cell Rep. 2018, 23, 2524–2537. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; Yang, C.; Su, R.; Long, M.; Liu, J.; Shi, L.; Xue, Y.; Su, Y.Q. LSM14B is an oocyte-specific RNA-binding protein indispensable for maternal mRNA metabolism and oocyte development in mice. Adv. Sci. 2023, 10, e2300043. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Zheng, Q. The RNA-binding protein LSM family regulating reproductive development via different RNA metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167808. [Google Scholar] [CrossRef]
- Wang, J.; Choi, J.M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174, 688–699.e16. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Z.; Yin, L.; Li, Q.; He, S.; Li, H.; Li, J.; Sheng, L.; Wu, H.; Chen, H.; et al. BAX-mediated ammonia-driven cell death: A novel prognostic and therapeutic target in clear cell renal cell carcinoma. Hum. Genom. 2025, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Liu, W.; Gao, G.; Yang, H. Serine-129 phosphorylated α-synuclein drives mitochondrial dysfunction and calcium dysregulation in Parkinson’s disease model. Front. Aging Neurosci. 2025, 17, 1538166. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Yu, G.; Gu, L.; Zhu, H.; Feng, S.; Xiong, X.; Jian, Z. Mitochondria-associated endoplasmic reticulum membranes tethering protein VAPB-PTPIP51 protects against ischemic stroke through inhibiting the activation of autophagy. CNS Neurosci. Ther. 2024, 4, e14707. [Google Scholar] [CrossRef]
- Schwieger, J.; Wei, C.; Munro, G.; Petersen, K.A.; Lenarz, T.; Scheper, V. Concentration Dependent Effects of Human Cometin on Spiral Ganglion Neuron Survival and Neurite Outgrowth. Audiol. Neurootol 2025, 4, 355–373. [Google Scholar] [CrossRef]
- Zhao, P.; Li, C.; Chen, B.; Sun, G.; Chao, H.; Tu, Y.; Bao, Z.; Fan, L.; Du, X.; Ji, J. Up-regulation of CHMP4B alleviates microglial necroptosis induced by traumatic brain injury. J. Cell Mol. Med. 2020, 15, 8466–8479. [Google Scholar] [CrossRef]
- Zhou, Y.; Bennett, T.M.; Shiels, A. A charged multivesicular body protein (CHMP4B) is required for lens growth and differentiation. Differentiation 2019, 109, 16–27. [Google Scholar] [CrossRef]
- Imamura, M.; Matsumoto, H.; Mannen, H.; Takeda, S.; Aoki, Y. The R436Q missense mutation in WWP1 disrupts autoinhibition of its E3 ubiquitin ligase activity, leading to self-degradation and loss of function. In In Vitro Cellular & Developmental Biology—Animal; Springer: Berlin/Heidelberg, Germany, 2024; Volume 7, pp. 771–780. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, S.M.; Zhong, P.; Kim, H.T.; Kim, D.I.; Kim, J.M.; Heo, W.D.; Kim, D.W.; Yeo, C.Y.; Kim, C.H.; et al. Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes. Nat. Commun. 2018, 9, 3434. [Google Scholar] [CrossRef] [PubMed]
- Kravchick, D.O.; Karpova, A.; Hrdinka, M.; Lopez-Rojas, J.; Iacobas, S.; Carbonell, A.U.; Iacobas, D.A.; Kreutz, M.R.; Jordan, B.A. Synaptonuclear messenger PRR7 inhibits c-Jun ubiquitination and regulates NMDA-mediated excitotoxicity. EMBO J. 2016, 35, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.O.; Colameo, D.; Ammann, I.; Winterer, J.; Schratt, G. miR-329- and miR-495-mediated Prr7 down-regulation is required for homeostatic synaptic depression in rat hippocampal neurons. Life Sci. Alliance. 2022, 5, e202201520. [Google Scholar] [CrossRef]
- Han, S.H.; Cho, J.G.; Park, S.J.; Shin, Y.K.; Hong, Y.B.; Han, J.Y.; Park, H.T.; Park, J.I. Transcription Factors and Coregulators in Schwann Cell Differentiation, Myelination, and Remyelination: Implications for Peripheral Neuropathy. J. Neurosci. Res. 2025, 103, e70053. [Google Scholar] [CrossRef]
- Jouaud, M.; Gonnaud, P.M.; Richard, L.; Latour, P.; Ollagnon-Roman, E.; Sturtz, F.; Mathis, S.; Magy, L.; Vallat, J.M. A de novo EGR2 variant, c.1232A>G (p.Asp411Gly), causes a severe early-onset Charcot-Marie-Tooth disease type 1D. Neuromuscul. Disord. 2020, 30, 73–76. [Google Scholar] [CrossRef]
- Topilko, P.; Schneider-Maunoury, S.; Levi, G.; Baron-Van Evercooren, A.; Chennoufi, A.B.; Seitanidou, T.; Babinet, C.; Charnay, P. Krox-20 controls myelination in the peripheral nervous system. Nature 1994, 371, 796–799. [Google Scholar] [CrossRef]
- Migaud, M.; Charlesworth, P.; Dempster, M.; Webster, L.C.; Watabe, A.M.; Makhinson, M.; He, Y.; Ramsay, M.F.; Morris, R.G.; Morrison, J.H.; et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 1998, 396, 433–439. [Google Scholar] [CrossRef]
- He, J.; Bellini, M.; Xu, J.; Castleberry, A.M.; Hall, R.A. PDZ domain-containing protein PIST regulates the intracellular trafficking and function of the metabotropic glutamate receptor 5. J. Biol. Chem. 2004, 279, 50190–50196. [Google Scholar] [CrossRef]
- Freudenberg, F. Quantitative analysis of Gria1, Gria2, Dlg1 and Dlg4 expression levels in hippocampus following forced swim stress in mice. Sci. Rep. 2019, 9, 14060. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.L.; Van Steenwinckel, J.; Schang, A.L.; Yan, J.; Arnadottir, J.; Le Charpentier, T.; Csaba, Z.; Dournaud, P.; Cipriani, S.; Auvynet, C.; et al. Integrative genomics of microglia implicates DLG4 (PSD95) in the white matter development of preterm infants. Nat. Commun. 2017, 8, 428. [Google Scholar] [CrossRef]
- Friedrich, T.; Tavraz, N.N.; Junghans, C.; Schmitz, B.; Döring, F.; Koenderink, J.B.; Bamberg, E.; Jentsch, T.J.; Pusch, M.; Decher, N. A de novo mutation in ATP1A2 causes familial hemiplegic migraine type 2 in a child with alternating hemiplegia. Front. Physiol. 2016, 7, 239. [Google Scholar] [CrossRef]
- Bozon, B.; Davis, S.; Laroche, S. A Requirement for the Immediate Early Gene Zif268 in Reconsolidation of Recognition Memory after Retrieval. Neuron 2003, 40, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.W.; Errington, M.L.; French, P.J.; Fine, A.; Bliss, T.V.P.; Garel, S.; Charnay, P.; Bozon, B.; Laroche, S.; Davis, S. A Requirement for the Immediate Early Gene Zif268 in the Expression of Late LTP and Long-Term Memories. Nat. Neurosci. 2001, 4, 289–296. [Google Scholar] [CrossRef]
- Bristot, G.; Feiten, J.G.; Pfaffenseller, B.; Hizo, G.H.; Possebon, G.M.P.; Valiati, F.E.; Pinto, J.V.; Caldieraro, M.A.; Fleck, M.P.D.A.; Gama, C.S.; et al. Early Growth Response 1 (EGR1) Is Downregulated in Peripheral Blood from Patients with Major Psychiatric Disorders. Trends Psychiatry Psychother. 2024, 11, 35. [Google Scholar] [CrossRef]
- Duclot, F.; Kabbaj, M. The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders. Front. Behav. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.T.; Katche, C.; Morici, J.F.; Medina, J.H.; Weisstaub, N.V. Immediate Early Genes, Memory and Psychiatric Disorders: Focus on c-Fos, Egr1 and Arc. Front. Behav. Neurosci. 2018, 12, 79. [Google Scholar] [CrossRef]
- Kammermeier, P.J.; Worley, P.F. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc. Natl. Acad. Sci. USA 2007, 104, 14. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.V.; Hartmann, J.; Mangold, K.; Wang, X.-D.; Labermaier, C.; Liebl, C.; Wolf, M.; Gassen, N.C.; Holsboer, F.; Rein, T.; et al. Homer1 Mediates Acute Stress-Induced Cognitive Deficits in the Dorsal Hippocampus. J. Neurosci. 2013, 33, 3857–3864. [Google Scholar] [CrossRef]
- Rietschel, M.; Mattheisen, M.; Frank, J.; Treutlein, J.; Degenhardt, F.; Breuer, R.; Steffens, M.; Mier, D.; Esslinger, C.; Walter, H.; et al. Genome-Wide Association-, Replication-, and Neuroimaging Study Implicates HOMER1 in the Etiology of Major Depression. Biol. Psychiatry 2010, 68, 578–585. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, L.; Zhong, J.; Zhong, L.; Zhang, R.; Kang, T.; Wu, Y. RNA-Binding Protein RBM28 Can Translocate from the Nucleolus to the Nucleoplasm to Inhibit the Transcriptional Activity of P53. J. Biol. Chem. 2022, 298, 101524. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Jarne-Ferrer, J.; Bellver-Sanchis, A.; Ribalta-Vilella, M.; Barroso, E.; Salvador, J.M.; Jurado-Aguilar, J.; Palomer, X.; Vázquez-Carrera, M.; Pallàs, M. Deletion of Gadd45a Expression in Mice Leads to Cognitive and Synaptic Impairment Associated with Alzheimer’s Disease Hallmarks. Int. J. Mol. Sci. 2024, 25, 2595. [Google Scholar] [CrossRef]
- You, W.; Xu, Z.; Sun, Y.; Valencak, T.G.; Wang, Y.; Shan, T. GADD45α Drives Brown Adipose Tissue Formation Through Upregulating PPARγ in Mice. Cell Death Dis. 2020, 11, 585. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, Z.; Yao, X.; Pang, S.; Liu, M.; Jiang, W.; Jiang, J.; Zhang, Q. Capping Enzyme mRNA-Cap/RNGTT Regulates Hedgehog Pathway Activity by Antagonizing Protein Kinase A. Sci. Rep. 2017, 7, 2891. [Google Scholar] [CrossRef] [PubMed]
- Majd, S.; Power, J.H.T. Oxidative Stress and Decreased Mitochondrial Superoxide Dismutase 2 and Peroxiredoxins 1 and 4 Based Mechanism of Concurrent Activation of AMPK and mTOR in Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 764–776. [Google Scholar] [CrossRef]
- Yan, Y.; Wladyka, C.; Fujii, J.; Sockanathan, S. Prdx4 Is a Compartment-Specific H2O2 Sensor That Regulates Neurogenesis by Controlling Surface Expression of GDE2. Nat. Commun. 2015, 6, 7006. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Chen, P.; Cheng, Y. PRDX4 and Its Roles in Various Cancers. Technol. Cancer Res. Treat. 2019, 18, 1533033819864313. [Google Scholar] [CrossRef]
- Liu, Y.; Song, H.; Zhou, Y.; Ma, X.; Xu, J.; Yu, Z.; Chen, L. The Oncogenic Role of Protein Kinase D3 in Cancer. J. Cancer 2021, 12, 735–739. [Google Scholar] [CrossRef]
- Ueda, Y.; Ooshio, I.; Fusamae, Y.; Kitae, K.; Kawaguchi, M.; Jingushi, K.; Hase, H.; Harada, K.; Hirata, K.; Tsujikawa, K. AlkB Homolog 3-Mediated tRNA Demethylation Promotes Protein Synthesis in Cancer Cells. Sci. Rep. 2017, 7, 42271. [Google Scholar] [CrossRef]
- Liefke, R.; Windhof-Jaidhauser, I.M.; Gaedcke, J.; Salinas-Riester, G.; Wu, F.; Ghadimi, M.; Dango, S. The Oxidative Demethylase ALKBH3 Marks Hyperactive Gene Promoters in Human Cancer Cells. Genome Med. 2015, 7, 66. [Google Scholar] [CrossRef]
- Tasaki, M.; Shimada, K.; Kimura, H.; Tsujikawa, K.; Konishi, N. ALKBH3, a Human AlkB Homologue, Contributes to Cell Survival in Human Non-Small-Cell Lung Cancer. Br. J. Cancer 2011, 104, 700–706. [Google Scholar] [CrossRef]
- Funk, M.C.; Bera, A.N.; Menchen, T.; Kuales, G.; Thriene, K.; Lienkamp, S.S.; Dengjel, J.; Omran, H.; Frank, M.; Arnold, S.J. Cyclin O. (Ccno) Functions during Deuterosome–mediated Centriole Amplification of Multiciliated Cells. EMBO J. 2015, 34, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, J.; Sun, L.; Zheng, Q.; Cao, C.; Ding, W.; Yang, S.; Ma, L.; Zhang, W. GALNT9 Enrichment Attenuates MPP+-Induced Cytotoxicity by Ameliorating Protein Aggregations Containing α-Synuclein and Mitochondrial Dysfunction. Biol. Direct 2024, 19, 77. [Google Scholar] [CrossRef]
- Yang, H.; Sasaki, T.; Minoshima, S.; Shimizu, N. Identification of Three Novel Proteins (SGSM1, 2, 3) Which Modulate Small G Protein (RAP and RAB)-Mediated Signaling Pathway. Genomics 2007, 90, 249–260. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Ding, Y.; Zhao, J.; Wang, W. Prognostic Biomarker SGSM1 and Its Correlation with Immune Infiltration in Gliomas. BMC Cancer 2022, 22, 466. [Google Scholar] [CrossRef]
- Kim, M.; de la Peña, J.B.; Cheong, J.H.; Kim, H.J. Neurobiological Functions of the Period Circadian Clock 2 Gene, Per2. Biomol. Ther. 2018, 26, 358–367. [Google Scholar] [CrossRef]
- Lavebratt, C.; Sjöholm, L.K.; Partonen, T.; Schalling, M.; Forsell, Y. PER2 Variantion Is Associated with Depression Vulnerability. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2010, 153B, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Sudic Hukic, D.; Forsell, Y.; Schalling, M.; Ösby, U.; Lavebratt, C. Depression-Associated ARNTL and PER2 Genetic Variants in Psychotic Disorders. Chronobiol. Int. 2015, 32, 579–584. [Google Scholar] [CrossRef]
- Nakamura, F.; Ohshima, T.; Goshima, Y. Collapsin Response Mediator Proteins: Their Biological Functions and Pathophysiology in Neuronal Development and Regeneration. Front. Cell. Neurosci. 2020, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, E.; Arashiki, N.; Becker, L.-L.; Takizawa, K.; Lévy, J.; Rambaud, T.; Makridis, K.L.; Goshima, Y.; Li, N.; Vreeburg, M.; et al. Monoallelic CRMP1 Gene Variants Cause Neurodevelopmental Disorder. eLife 2022, 11, e80793. [Google Scholar] [CrossRef]
- Yoshitane, H.; Asano, Y.; Sagami, A.; Sakai, S.; Suzuki, Y.; Okamura, H.; Iwasaki, W.; Ozaki, H.; Fukada, Y. Functional D-Box Sequences Reset the Circadian Clock and Drive mRNA Rhythms. Commun. Biol. 2019, 2, 300. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional Architecture of the Mammalian Circadian Clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Ideker, T.; Thorsson, V.; Ranish, J.A.; Christmas, R.; Buhler, J.; Eng, J.K.; Bumgarner, R.; Goodlett, D.R.; Aebersold, R.; Hood, L. Integrated Genomic and Proteomic Analyses of a Systematically Perturbed Metabolic Network. Science 2001, 292, 929–934. [Google Scholar] [CrossRef]
- Oleksiak, M.F.; Roach, J.L.; Crawford, D.L. Natural Variation in Cardiac Metabolism and Gene Expression in Fundulus Heteroclitus. Nat. Genet. 2005, 37, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Bigler, J.; Rand, H.A.; Kerkof, K.; Timour, M.; Russell, C.B. Cross-Study Homogeneity of Psoriasis Gene Expression in Skin across a Large Expression Range. PLoS ONE 2013, 8, e52242. [Google Scholar] [CrossRef]
- Keren, L.; Hausser, J.; Lotan-Pompan, M.; Vainberg Slutskin, I.; Alisar, H.; Kaminski, S.; Weinberger, A.; Alon, U.; Milo, R.; Segal, E. Massively Parallel Interrogation of the Effects of Gene Expression Levels on Fitness. Cell 2016, 166, 1282–1294.e18. [Google Scholar] [CrossRef]
- Strekalova, T.; Moskvin, O.; Jain, A.Y.; Gorbunov, N.; Gorlova, A.; Sadovnik, D.; Umriukhin, A.; Cespuglio, R.; Yu, W.S.; Tse, A.C.K.; et al. Molecular signature of excessive female aggression: Study of stressed mice with genetic inactivation of neuronal serotonin synthesis. J. Neural Transm. 2023, 130, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Svirin, E.; Gorlova, A.; Sheveleva, E.; Burova, A.; Khairetdinova, A.; Sitdikova, K.; Zakharova, E.; Dudchenko, A.M.; Lyundup, A.; et al. Resilience and Vulnerability to Stress-Induced Anhedonia: Unveiling Brain Gene Expression and Mitochondrial Dynamics in a Mouse Chronic Stress Depression Model. Biomolecules 2023, 13, 1782. [Google Scholar] [CrossRef]
- Lu, J.; Xie, L.; Sylvester, J.; Wang, J.; Bai, J.; Baybutt, R.; Wang, W. Different Gene Expression of Skin Tissues between Mice with Weight Controlled by Either Calorie Restriction or Physical Exercise. Exp. Biol. Med. 2007, 232, 473–480. [Google Scholar]
- Magri, C.; Giacopuzzi, E.; Sacco, C.; Bocchio-Chiavetto, L.; Minelli, A.; Gennarelli, M. Alterations observed in the interferon α and β signaling pathway in MDD patients are marginally influenced by cis-acting alleles. Sci. Rep. 2021, 11, 1179. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Cao, L.; He, Y.; Ji, X.; Lin, H.; Geng, C.L.; Liu, L.; Qu, P. Cold-inducible RNA-binding protein CIRBP promotes neuroinflammation via activating NF-κB and inducing IL-6 in microglial cells. Neurosci. Bull. 2017, 33, 273–282. [Google Scholar] [CrossRef]
- Milenkovic, V.M.; Stanton, E.H.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. The Role of Chemokines in the Pathophysiology of Major Depressive Disorder. Int. J. Mol. Sci. 2019, 20, 2283. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.H.; Kim, D.G.; Ahn, Y.S. Microarray analysis of differentially expressed genes in the brains of tubby mice. Korean J. Physiol. Pharmacol. 2009, 91, 7. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Cai, X.; Yang, Z.; Li, H.; Su, X.; Song, M.; Zhou, D.-S.; Li, X.; Zhang, C.; et al. Identification of a functional human-unique 351-bp Alu insertion polymorphism associated with major depressive disorder in the 1p31.1 GWAS risk loci. Neuropsychopharmacology 2020, 45, 580–588. [Google Scholar] [CrossRef]
- Cline, B.H.; Steinbusch, H.W.M.; Malin, D.; Revishchin, A.V.; Pavlova, G.V.; Cespuglio, R.; Strekalova, T. The neuronal insulin sensitizer dicholine succinate reduces stress-induced depressive traits and memory deficit: Possible role of insulin-like growth factor 2. BMC Neurosci. 2012, 13, 110. [Google Scholar] [CrossRef]
- Guarch, J.; Marcos, T.; Salamero, M.; Gastó, C.; Blesa, R. Mild cognitive impairment: A risk indicator of later dementia, or a preclinical phase of the disease? Int. J. Geriatr. Psychiatry 2008, 23, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Zhang, X.; Shu, H.; Wang, Z.; Liu, D.; Zhang, Z. The characteristic of cognitive dysfunction in remitted late life depression and amnestic mild cognitive impairment. Psychiatry Res. 2017, 251, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ono, K.; Ouchi, H.; Tsushima, R.; Murakami, Y. Social isolation stress down-regulates cortical early growth response 1 (Egr-1) expression in mice. Neurosci. Res. 2012, 73, 257–262. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, J.; Sun, J.; Ding, L.; Ruan, L.; Reed, M.; Yu, X.; Klabnik, J.; Lin, D.; Li, J.; et al. Inhibition of phosphodiesterase 2 reverses impaired cognition and neuronal remodeling caused by chronic stress. Neurobiol. Aging 2015, 36, 955–970. [Google Scholar] [CrossRef]
- Besnard, A.; Caboche, J.; Laroche, S. Recall and reconsolidation of contextual fear memory: Differential control by ERK and Zif268 expression dosage. PLoS ONE 2013, 8, e72006. [Google Scholar] [CrossRef]
- Wei, J.; Wu, X.; Luo, P.; Yue, K.; Yu, Y.; Pu, J.; Zhang, L.; Dai, S.; Han, D.; Fei, Z. Homer1a attenuates endoplasmic reticulum stress-induced mitochondrial stress after ischemic reperfusion injury by inhibiting the PERK pathway. Front. Cell Neurosci. 2019, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, P.; Rao, W.; Dai, S.; Zhang, L.; Ma, W.; Pu, J.; Yu, Y.; Wang, J.; Fei, Z. Homer1a attenuates hydrogen peroxide-induced oxidative damage in HT-22 cells through AMPK-dependent autophagy. Front. Neurosci. 2018, 12, 51. [Google Scholar] [CrossRef]
- Lominac, K.D.; Oleson, E.B.; Pava, M.; Klugmann, M.; Schwarz, M.K.; Seeburg, P.H.; During, M.J.; Worley, P.F.; Kalivas, P.W.; Szumlinski, K.K. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J. Neurosci. 2005, 25, 11586–11594. [Google Scholar] [CrossRef]
- Szumlinski, K.K.; Lominac, K.D.; Kleschen, M.J.; Oleson, E.B.; Dehoff, M.H.; Schwartz, M.K.; Seeburg, P.H.; Worley, P.F.; Kalivas, P.W. Behavioral and neurochemical phenotyping of Homer1 mutant mice: Possible relevance to schizophrenia. Genes Brain Behav. 2005, 4, 273–288. [Google Scholar] [CrossRef]
- Grassi, D.; Franz, H.; Vezzali, R.; Bovio, P.; Heidrich, S.; Dehghanian, F.; Lagunas, N.; Belzung, C.; Krieglstein, K.; Vogel, T. Neuronal activity, TGFβ-signaling and unpredictable chronic stress modulate transcription of Gadd45 family members and DNA methylation in the hippocampus. Cereb Cortex 2017, 27, 4166–4181. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Myung, S.K.; Cho, J.J.; Jung, Y.J.; Yoon, J.L.; Kim, M.Y. Night shift work and risk of depression: Meta-analysis of observational studies. J. Korean Med. Sci. 2017, 32, 1091–1096. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Li, J.; Cai, X.; Sun, Y.; Zhang, B.; Zhao, H. Depression-like behavior is associated with lower Per2 mRNA expression in the lateral habenula of rats. Genes Brain Behav. 2021, 20, e12702. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Ji, Y.B.; Li, S.X.; Serchov, T. The crosstalk between CREB and PER2 mediates the transition between mania- and depression-like behavior. Neuropsychopharmacology 2025, 50, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Somlyai, G.; Jancsó, G.; Jákli, G.; Vass, K.; Barna, B.; Lakic, V.; Gaál, T. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993, 317, 1–4. [Google Scholar] [CrossRef]
- Somlyai, G.; Nagy, L.I.; Puskás, L.G.; Papp, A.; Kovács, B.Z.; Fórizs, I.; Czuppon, G.; Somlyai, I. Deuterium content of the organic compounds in food has an impact on tumor growth in mice. Curr. Issues Mol. Biol. 2023, 45, 66–77. [Google Scholar] [CrossRef]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.; Ponomarev, E.D.; Strekalova, T. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci. Ther. 2018, 24, 763–774. [Google Scholar] [CrossRef]
- van Varsseveld, N.; van Bunderen, C.; Sohl, E.; Comijs, H.; Penninx, B.; Lips, P.; Drent, M. Serum insulin-like growth factor 1 and late-life depression: A population-based study. Psychoneuroendocrinology 2015, 54, 31–40. [Google Scholar] [CrossRef]
- Strekalova, T.; Steinbusch, H.W.M. Measuring behavior in mice with chronic stress depression paradigm. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 348–361. [Google Scholar] [CrossRef]
- Schroeter, C.A.; Gorlova, A.; Sicker, M.; Umriukhin, A.; Burova, A.; Shulgin, B.; Morozov, S.; Costa-Nunes, J.P.; Strekalova, T. Unveiling the Mechanisms of a Remission in Major Depressive Disorder (MDD)-like Syndrome: The Role of Hippocampal Palmitoyltransferase Expression and Stress Susceptibility. Biomolecules 2025, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wang, J.; Zhang, B. RefFinder: A web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genom. 2023, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- de Munter, J.; Babaevskaya, D.; Wolters, E.C.; Pavlov, D.; Lysikova, E.; Kalueff, A.V.; Gorlova, A.; Oplatchikova, M.; Pomytkin, I.A.; Proshin, A.; et al. Molecular and behavioural abnormalities in the FUS-tg mice mimic frontotemporal lobar degeneration: Effects of old and new anti-inflammatory therapies. J. Cell Mol. Med. 2020, 24, 10251–10257. [Google Scholar] [CrossRef]
- Veniaminova, E.; Cespuglio, R.; Chernukha, I.; Schmitt-Boehrer, A.G.; Morozov, S.; Kalueff, A.V.; Kuznetsova, O.; Anthony, D.C.; Lesch, K.-P.; Strekalova, T. Metabolic, molecular, and behavioral effects of Western diet in serotonin transporter-deficient mice: Rescue by heterozygosity? Front. Neurosci. 2020, 14, 24. [Google Scholar] [CrossRef]
- Vignisse, J.; Steinbusch, H.W.M.; Grigoriev, V.; Bolkunov, A.; Proshin, A.; Bettendorff, L.; Bachurin, S.; Strekalova, T. Concomitant Manipulation of Murine NMDA- and AMPA-Receptors to Produce pro-Cognitive Drug Effects in Mice. Eur. Neuropsychopharmacol. 2014, 24, 309–320. [Google Scholar] [CrossRef]
- Vignisse, J.; Sambon, M.; Gorlova, A.; Pavlov, D.; Caron, N.; Malgrange, B.; Shevtsova, E.; Svistunov, A.; Anthony, D.C.; Markova, N.; et al. Thiamine and Benfotiamine Prevent Stress-Induced Suppression of Hippocampal Neurogenesis in Mice Exposed to Predation without Affecting Brain Thiamine Diphosphate Levels. Mol. Cell Neurosci. 2017, 82, 126–136. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 22 September 2025).
- Tenenbaum, D.; Maintainer, B. KEGGREST: Client-Side REST Access to the Kyoto Encyclopedia of Genes and Genomes (KEGG). 2025. Available online: https://bioconductor.org/packages/KEGGREST (accessed on 22 September 2025).
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Korotkevich, G.; Sukhov, V.; Budin, N.; Shpak, B.; Artyomov, M.N.; Sergushichev, A. Fast gene set enrichment analysis. bioRxiv 2021, 060012. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Costa-Nunes, J.P.; Gorlova, A.; Pavlov, D.; Cespuglio, R.; Gorovaya, A.; Proshin, A.; Umriukhin, A.; Ponomarev, E.D.; Kalueff, A.V.; Strekalova, T.; et al. Ultrasound Stress Compromises the Correlates of Emotional-like States and Brain AMPAR Expression in Mice: Effects of Antioxidant and Anti-Inflammatory Herbal Treatment. Stress 2020, 23, 481–495. [Google Scholar] [CrossRef]
- Strekalova, T.; Anthony, D.C.; Dolgov, O.; Anokhin, K.; Kubatiev, A.; Steinbusch, H.M.; Schroeter, C. The differential effects of chronic imipramine or citalopram administration on physiological and behavioral outcomes in naïve mice. Behav. Brain Res. 2013, 245, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Radford-Smith, D.; Dunstan, I.K.; Gorlova, A.; Svirin, E.; Sheveleva, E.; Burova, A.; Morozov, S.; Lyundup, A.; Berger, G.; et al. Omega-3 alleviates behavioral and molecular changes in a mouse model of stress-induced juvenile depression. Neurobiol. Stress 2024, 31, 100646. [Google Scholar] [CrossRef]
- Wang, G.J.; Thayer, S.A. Sequestration of glutamate-induced Ca2+ loads by mitochondria in cultured rat hippocampal neurons. J. Neurophysiol. 1996, 76, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
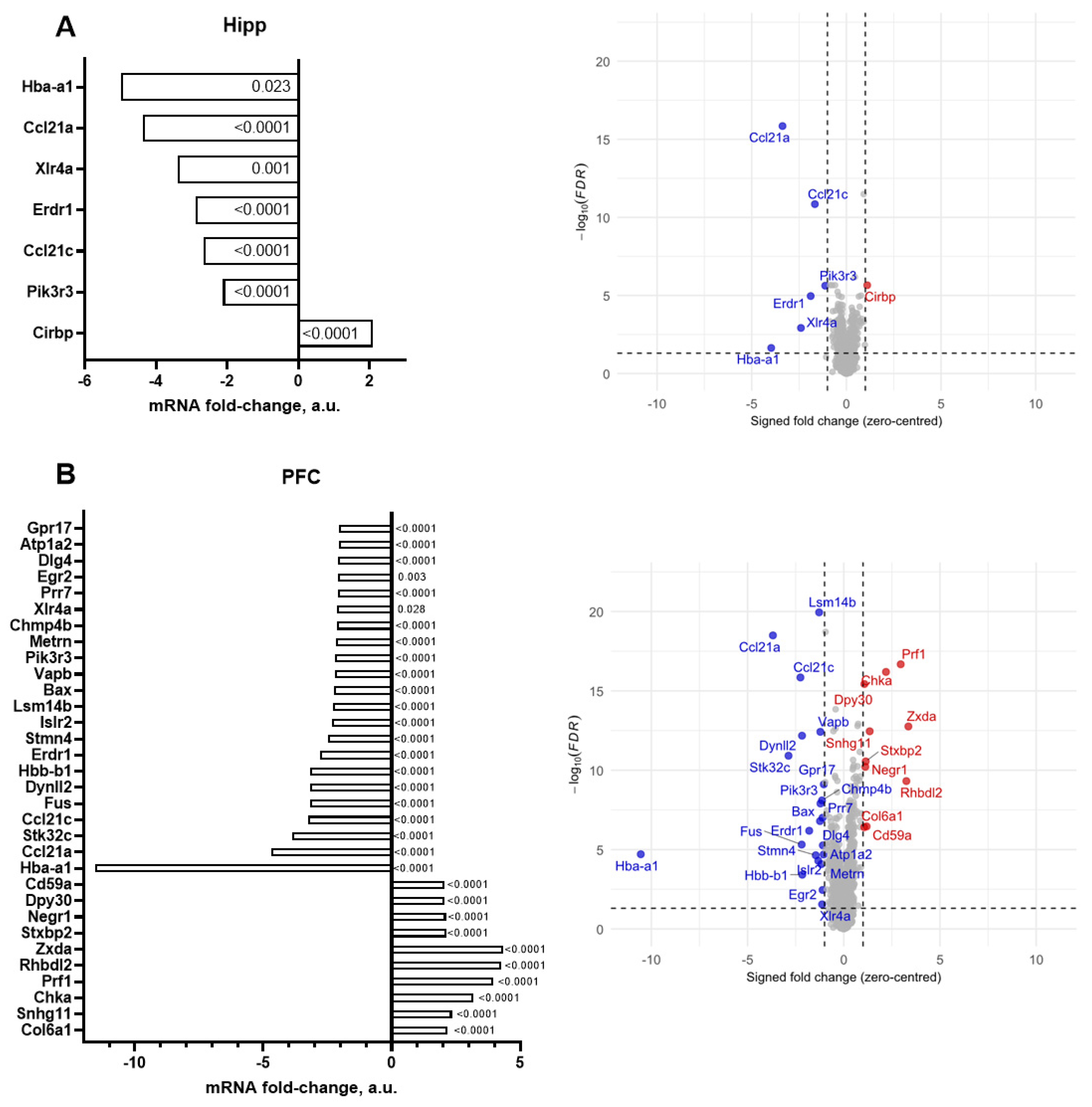


| |Fold Change| ≥ 2.0 | |Fold Change| ≥ 2.5 | |
|---|---|---|
| Hippocampus | Hba-a1, Ccl21a, Xlr4a, Erdr1, Ccl21c, Pik3r3, Cirbp | Hba-a1, Ccl21a, Xlr4a, Erdr1, Ccl21c |
| Prefrontal cortex | Hba-a1, Ccl21a, Stk32c, Ccl21c, Fus, Dynll2, Hbb-b1, Erdr1, Stmn4, Islr2, Lsm14b, Bax, Vapb, Pik3r3, Metrn, Chmp4b, Xlr4a, Prr7, Egr2, Dlg4, Atp1a2, Gpr17, Zxda, Rhbdl2, Prf1, Chka, Snhg11, Col6a1, Stxbp2, Negr1, Dpy30, Cd59a | Hba-a1, Ccl21a, Stk32c, Ccl21c, Fus, Dynll2, Hbb-b1, Erdr1, Zxda, Rhbdl2, Prf1, Chka, Snhg11 |
| ID of Gene | Gene Name | Old vs. Young Mice, Fold Change | Functional Role of Encoded Protein |
|---|---|---|---|
| Hba-a1 | Hemoglobin subunit alpha-1 | −4.97 | Oxygen transport and oxidative metabolism [84]; beta-thalassemia [85]. |
| Ccl21a | C-C motif chemokine 21a | −4.37 | Immune cell trafficking, initial recruitment of T cells, balance of immune responses in lymphoid and nonlymphoid tissues [86]. |
| Ccl21c | C-C motif chemokine 21c | −2.66 | |
| Erdr1 | Erythroid differentiation regulator 1 | −2.89 | Cell survival under stress, cellular growth, activation of immune system [87]; anti-inflammatory cytokine production/inhibition in macrophages [88]. |
| Xlr4a | X-linked lymphocyte-regulated 4A | −3.40 | Chromatin remodeling and dendritic spine morphology [89]. |
| Pik3r3 | Phosphoinositide-3-kinase regulatory subunit 3 | −2.12 | Cellular growth and proliferation in cancer [90], PPARα-mediated hepatic lipid metabolism [91]. |
| Cirbp | Cold-inducible RNA binding protein | 2.10 | RNA binding, stress response [92,93]. |
| ID of Gene | Gene Name | Old vs. Young Mice, Fold Change | Functional Role of Encoded Protein |
|---|---|---|---|
| Zxda | Zinc finger X-linked duplicated A | 4.36 | Histocompatibility complex class I and class II regulation [94]. |
| Rhbdl2 | Rhomboid-like 2 | 4.25 | Cleavage of thrombomodulin; wound healing [95], proliferation, migration, and invasion of pancreatic cancer [96]. |
| Prf1 | Perforin-1 | 3.96 | Cytotoxic granule-mediated killing of infected or malignant cells [97], T-cell proliferation and cytokine production [98]. |
| Chka | Choline kinase alpha | 3.19 | Tumor growth, cell proliferation mediated via EGFR/PI3K/AKT signaling [99]. |
| Snhg11 | Small nucleolar RNA host gene 11 | 2.35 | Cancer cell proliferation, invasion and migration (glioma, lung cancer, and gastric cancer) [100,101]. |
| Col6a1 | Collagen type VI alpha 1 chain | 2.19 | Component of type VI collagen, autophagy, and apoptosis in megakaryocytes and neurons [102,103,104,105]; muscle regeneration [106]. |
| Stxbp2 | Syntaxin-binding protein 2 | 2.14 | Synaptic transmission, neurodevelopment [107], NK-cell and T-cell functions [108]. |
| Negr1 | Neuronal growth regulator 1 | 2.12 | Neuronal growth, synapse formation, and neurotransmission [109,110]. |
| Dpy30 | Protein dpy-30 homolog | 2.07 | Methylation of histone H3, early embryonic development, and cell differentiation [111]. |
| Cd59a | CD59a glycoprotein | 2.07 | Complement system, T-cell activity, and inflammation [112,113]. |
| Hba-a1 | Hemoglobin subunit alpha-1 | −11.55 | See Table 2 |
| Hbb-b1 | Hemoglobin subunit beta-1 | −3.16 | Oxygen transport [84], beta-thalassemia [85]. |
| Ccl21a | C-C motif chemokine 21a | −4.68 | See Table 2 |
| Ccl21c | C-C motif chemokine 21c | −3.25 | |
| Stk32c | Serine/threonine kinase 32C | −3.87 | Bladder and breast cancer, tumor cell proliferation, migration, and invasion [114,115]. |
| Fus | RNA-binding protein fused in sarcoma | −3.19 | DNA/RNA-binding, RNA transcription, splicing and transport, DNA repair, amyotrophic lateral sclerosis [116,117]. |
| Dynll2 | Dynein light chain 2 | −3.17 | Intracellular retrograde transport of vesicles and organelles [118,119]. |
| Erdr1 | Erythroid differentiation regulator 1 | −2.80 | See Table 2 |
| Stmn4 | Stathmin-4 | −2.46 | Adult hippocampal neurogenesis and spinogenesis [120,121]; demyelinating disorders, myelin repair [122]. |
| Islr2 | Immunoglobulin superfamily containing leucine-rich repeat 2 | −2.33 | Axon guidance, brain development [123]; peripheral nervous system and forebrain connectivity [124]. |
| Lsm14b | LSM14 homolog B | −2.28 | RNA-binding, oocyte maturation and early development; storage, stability, and translation of maternal mRNAs [125,126,127]. |
| Bax | BCL2-associated X protein | −2.24 | Programmed cell death, activation of the intrinsic apoptotic pathway, development and response to cellular stress [128]. |
| Vapb | Vesicle-associated membrane protein-associated protein B | −2.22 | Membrane trafficking, lipid transport, and membrane contacts; cellular lipid homeostasis [129,130]. |
| Pik3r3 | Phosphoinositide-3-kinase regulatory subunit 3 | −2.21 | See Table 2 |
| Metrn | Meteorin | −2.17 | Glial cell specialization and axonal development, neurogenesis [131]. |
| Chmp4b | Charged multivesicular body protein 4B | −2.13 | Component of the endosomal sorting complex transport-III, endosomal sorting, neuronal apoptosis [132], cell proliferation in hepatocellular carcinoma [133]. |
| Xlr4a | X-linked lymphocyte-regulated 4A | −2.13 | See Table 2 |
| Prr7 | Proline-rich 7 | −2.11 | T-cell receptor signaling and apoptosis [134,135], T-cell development [136]; synaptic depression [137]. |
| Egr2 | Early growth response 2 | −2.10 | DNA-binding transcription factor [138]; myelination in the peripheral nervous system and Schwann cell differentiation [139,140]. |
| Dlg4 | Disks large homolog 4 | −2.09 | Synaptogenesis and synaptic plasticity [141,142,143]; microglia development and inflammatory responses [144]. |
| Atp1a2 | ATPase Na+/K+ transporting subunit alpha 2 | −2.05 | Catalytic component of ATPase Na+/K+, brain development [145,146]. |
| |Fold Change| ≥ 1.25 | |
|---|---|
| Hippocampus | Prdx4, Prkd3, Alkbh3, Ccno, Egr1, Homer1, Rbm28, Gadd45a, Rngtt |
| Prefrontal cortex | Crmp1, Dbp, Galnt9, Egr1, Sgsm1, Per2 |
| ID of Gene | Gene Name | DDW Group Fold- Change | Functional Role of Encoded Protein |
|---|---|---|---|
| Egr1 | Early Growth Response 1 | 1.52 | Memory consolidation and reconsolidation [147,148,149,150]. |
| Homer1 | Homer Scaffold Protein 1 | 1.43 | Postsynaptic density and mGluR signaling [151,152], clinical depression [153]. |
| Rbm28 | RNA-binding protein 28 | 1.27 | DNA repair, cellular stress response [154]. |
| Gadd45a | Growth Arrest And DNA Damage Inducible Alpha | 1.26 | DNA repair, neuronal survival [155]; mitogenesis [156]. |
| Rngtt | RNA Guanylyltransferase And 5′-Phosphatase | 1.26 | mRNA protection and transport [157]. |
| Prdx4 | Peroxiredoxin 4 | −1.21 | Oxidative damage [158,159], neurogenesis and neuronal differentiation [160]. |
| Prkd3 | Protein Kinase D3 | −1.27 | Cell signaling and growth, protein transport and transcription; cancer cell proliferation, growth, migration and invasion [161] |
| Alkbh3 | AlkB homolog 3 | −1.27 | DNA and RNA repair, cell survival and cancer [162,163,164]. |
| Ccno | Cyclin O | −1.32 | Deuterosome formation and centriole amplification in multiciliated cells [165]. |
| ID of Gene | Gene Name | DDW Group Fold- Change | Functional Role of Encoded Protein |
|---|---|---|---|
| Galnt9 | Polypeptide N-Acetylgalactos-aminyltransferase 9 | 1.66 | O-linked oligosaccharide biosynthesis, mitoprotective effects [166]. |
| Egr1 | Early Growth Response 1 | 1.61 | See Table 1 |
| Sgsm1 | Small G Protein Signaling Modulator 1 | 1.35 | Brain functions [167]; neuro-oncological disorders [168]. |
| Per2 | Period Circadian Regulator 2 | −1.33 | Circadian regulation [169]; major depression [170]; other psychotic disorders [171]. |
| Crmp1 | Collapsin Response Mediator Protein 1 | −1.28 | Neuronal development and axonal guidance [172]; neurodevelopmental disorders [173]. |
| Dbp | Albumin D-site-Binding Protein | 1.30 | Circadian regulation [174,175]. |
| GO-BP Term | Pathway Description | FDR-Corrected p-Value | NES |
|---|---|---|---|
| GO:0099645 | Neurotransmitter receptor localization to postsynaptic specialization membrane | 0.001 | 0.001 |
| GO:0007268 | Chemical synaptic transmission | 0.023 | 0.023 |
| GO:0007214 | Gamma-aminobutyric acid signaling pathway | 0.029 | 0.029 |
| GO:0007616 | Long-term memory | 0.029 | 0.029 |
| GO:0050773 | Regulation of dendrite development | 0.029 | 0.029 |
| GO:0007399 | Nervous system development | 0.034 | 0.034 |
| GO:0099175 | Regulation of postsynapse organization | 0.039 | 0.039 |
| GO:0016477 | Cell migration | 0.039 | 0.039 |
| GO-BP Term | Pathway Description | FDR-Corrected p-Value | NES |
|---|---|---|---|
| mmu04810 | Regulation of actin cytoskeleton | 0.004 | 1.76 |
| mmu04921 | Oxytocin signaling pathway | 0.004 | 1.83 |
| mmu04024 | cAMP signaling pathway | 0.005 | 1.70 |
| mmu04022 | cGMP-PKG signaling pathway | 0.005 | 1.75 |
| mmu04148 | Efferocytosis | 0.005 | 1.75 |
| mmu04961 | Endocrine and other factor-regulated calcium reabsorption | 0.007 | 1.91 |
| mmu04142 | Lysosome | 0.009 | −1.73 |
| mmu04010 | MAPK signaling pathway | 0.009 | 1.57 |
| mmu04724 | Glutamatergic synapse | 0.009 | 1.78 |
| mmu05202 | Transcriptional misregulation in cancer | 0.009 | 1.66 |
| mmu05031 | Amphetamine addiction | 0.009 | 1.85 |
| mmu04261 | Adrenergic signaling in cardiomyocytes | 0.011 | 1.66 |
| mmu05030 | Cocaine addiction | 0.012 | 1.88 |
| mmu04144 | Endocytosis | 0.015 | 1.57 |
| mmu05032 | Morphine addiction | 0.015 | 1.76 |
| mmu04510 | Focal adhesion | 0.023 | 1.57 |
| mmu04068 | FoxO signaling pathway | 0.031 | 1.61 |
| mmu04928 | Parathyroid hormone synthesis, secretion, and action | 0.031 | 1.64 |
| mmu00970 | Aminoacyl-tRNA biosynthesis | 0.042 | −1.81 |
| mmu04020 | Calcium signaling pathway | 0.042 | 1.47 |
| mmu04977 | Vitamin digestion and absorption | 0.042 | −1.81 |
| mmu04713 | Circadian entrainment | 0.042 | 1.62 |
| mmu04666 | Fc gamma R-mediated phagocytosis | 0.048 | 1.61 |
| mmu04962 | Vasopressin-regulated water reabsorption | 0.048 | 1.73 |
| mmu05135 | Yersinia infection | 0.048 | 1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-Nunes, J.P.; Sitdikova, K.; Svirin, E.; de Munter, J.; Somlyai, G.; Gorlova, A.; Litavrin, A.; Arajyan, G.M.; Nefedova, Z.; Lyundup, A.; et al. Transcriptomic Insights into Late-Life Depression and the Role of Environmental Drinking Water Composition: A Study on 18-Month-Old Mice. Int. J. Mol. Sci. 2025, 26, 10626. https://doi.org/10.3390/ijms262110626
Costa-Nunes JP, Sitdikova K, Svirin E, de Munter J, Somlyai G, Gorlova A, Litavrin A, Arajyan GM, Nefedova Z, Lyundup A, et al. Transcriptomic Insights into Late-Life Depression and the Role of Environmental Drinking Water Composition: A Study on 18-Month-Old Mice. International Journal of Molecular Sciences. 2025; 26(21):10626. https://doi.org/10.3390/ijms262110626
Chicago/Turabian StyleCosta-Nunes, João Pedro, Kseniia Sitdikova, Evgeniy Svirin, Johannes de Munter, Gabor Somlyai, Anna Gorlova, Alexandr Litavrin, Gohar M. Arajyan, Zlata Nefedova, Alexei Lyundup, and et al. 2025. "Transcriptomic Insights into Late-Life Depression and the Role of Environmental Drinking Water Composition: A Study on 18-Month-Old Mice" International Journal of Molecular Sciences 26, no. 21: 10626. https://doi.org/10.3390/ijms262110626
APA StyleCosta-Nunes, J. P., Sitdikova, K., Svirin, E., de Munter, J., Somlyai, G., Gorlova, A., Litavrin, A., Arajyan, G. M., Nefedova, Z., Lyundup, A., Morozov, S., Umriukhin, A., Iliynskaya, S., Chernopiatko, A., & Strekalova, T. (2025). Transcriptomic Insights into Late-Life Depression and the Role of Environmental Drinking Water Composition: A Study on 18-Month-Old Mice. International Journal of Molecular Sciences, 26(21), 10626. https://doi.org/10.3390/ijms262110626






