Nickel Nanoparticles Promote Lung Adenocarcinoma Progression via CDK1-Mediated Fatty Acid Metabolism Regulation
Abstract
1. Introduction
2. Results
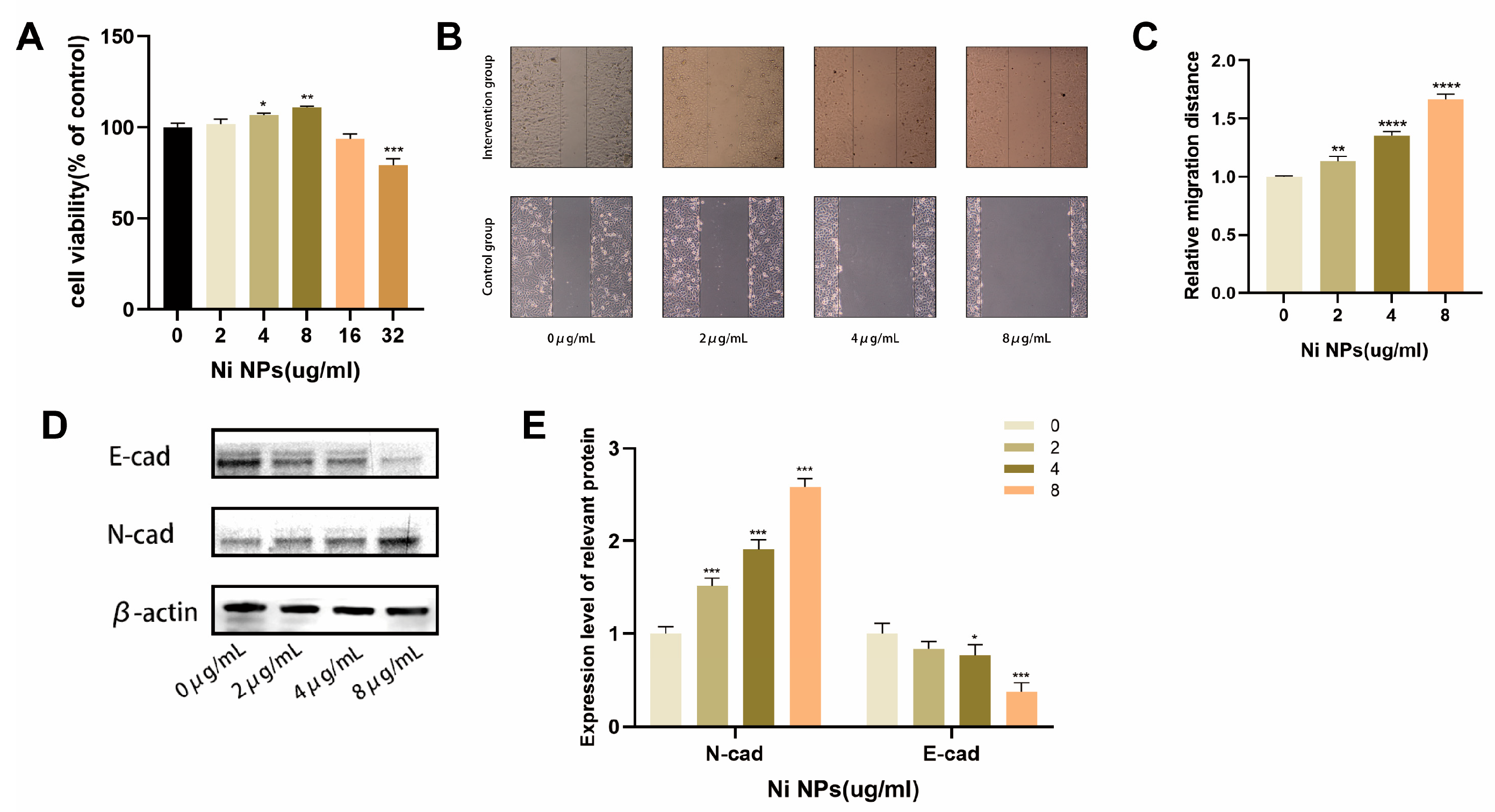
2.1. Effects of NiNPs on LUAD Cell Growth
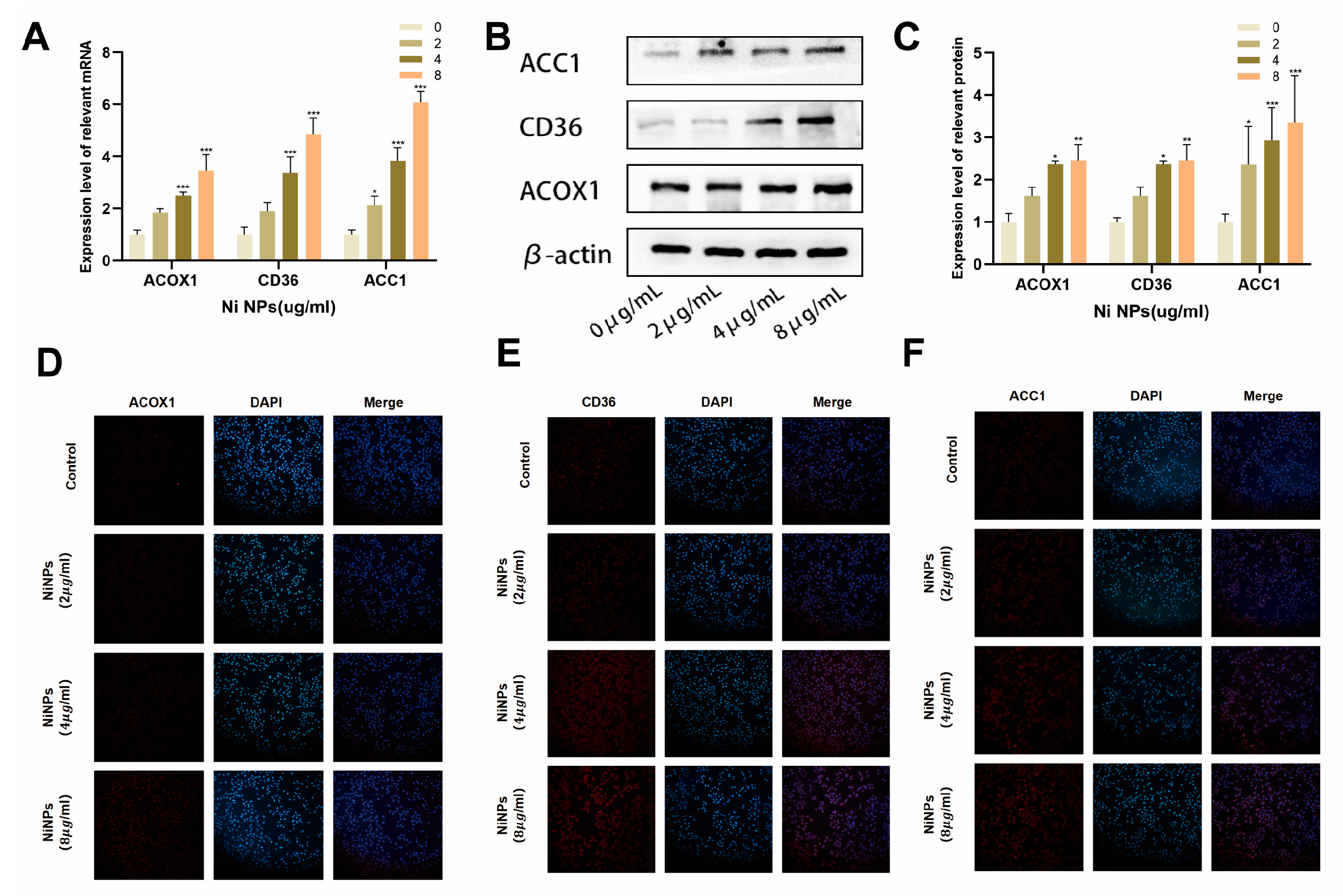
2.2. NiNPs Induce Abnormal FAM in LUAD Cells
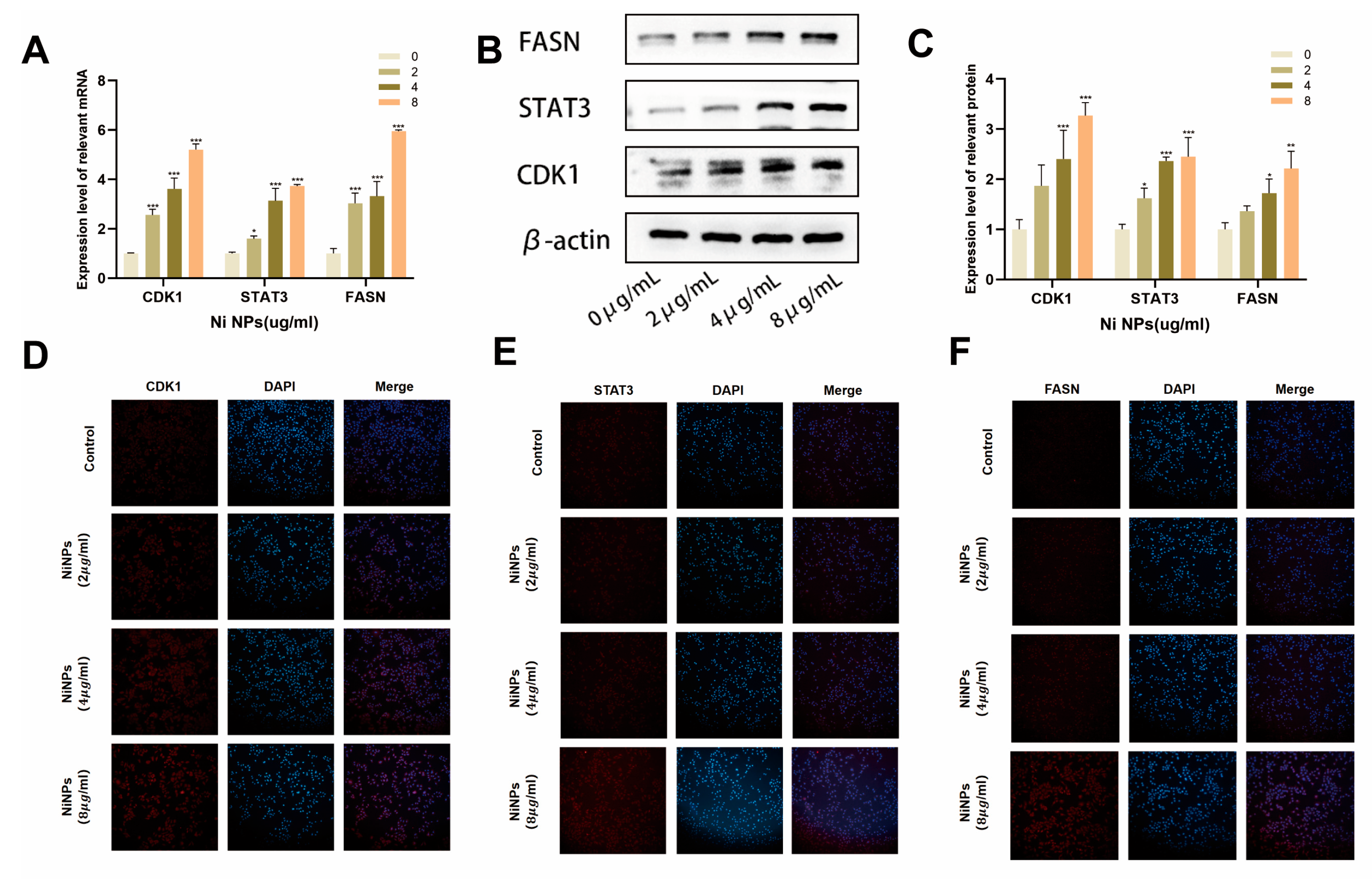
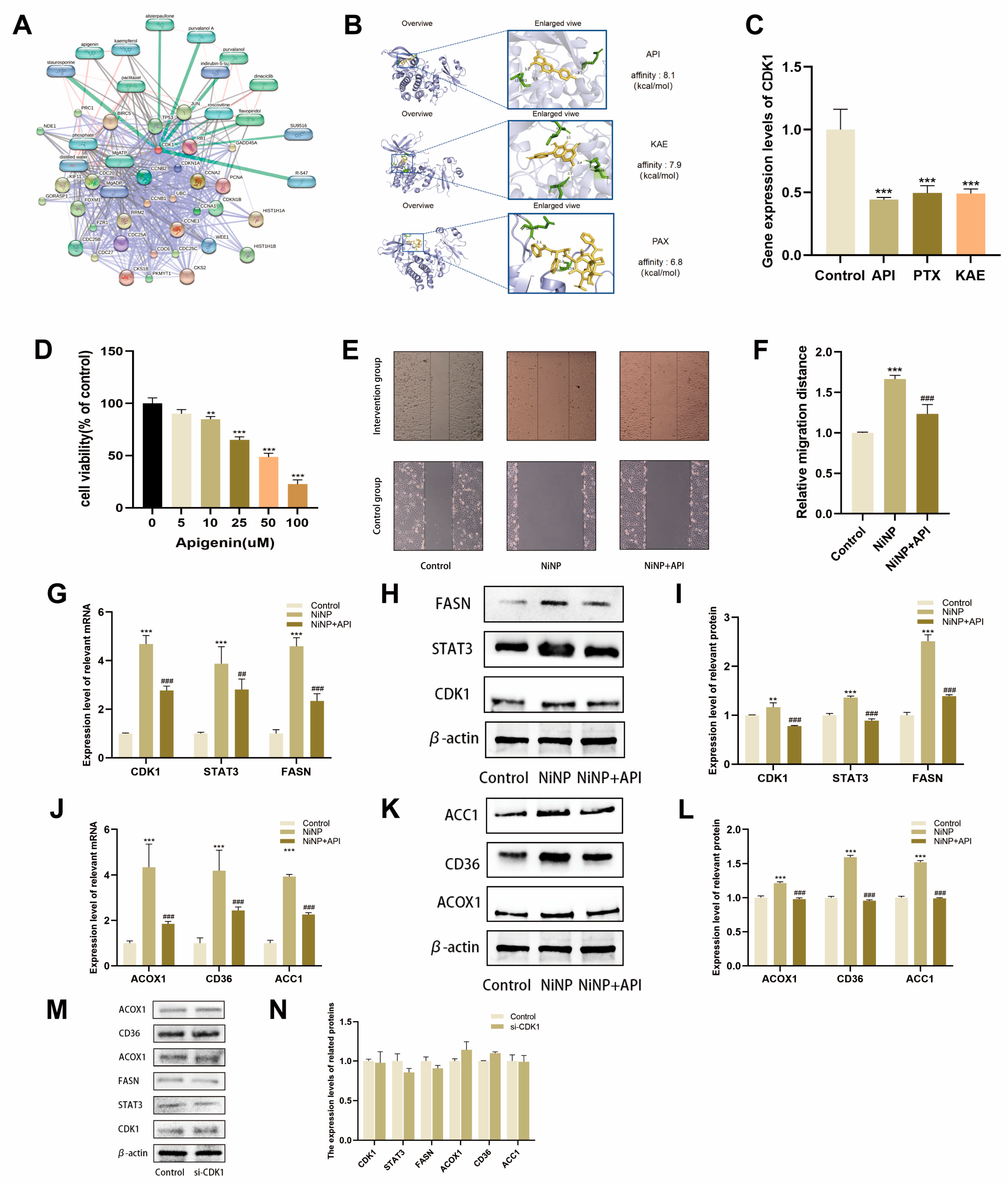
2.3. NiNPs Activate the CDK1 Pathway
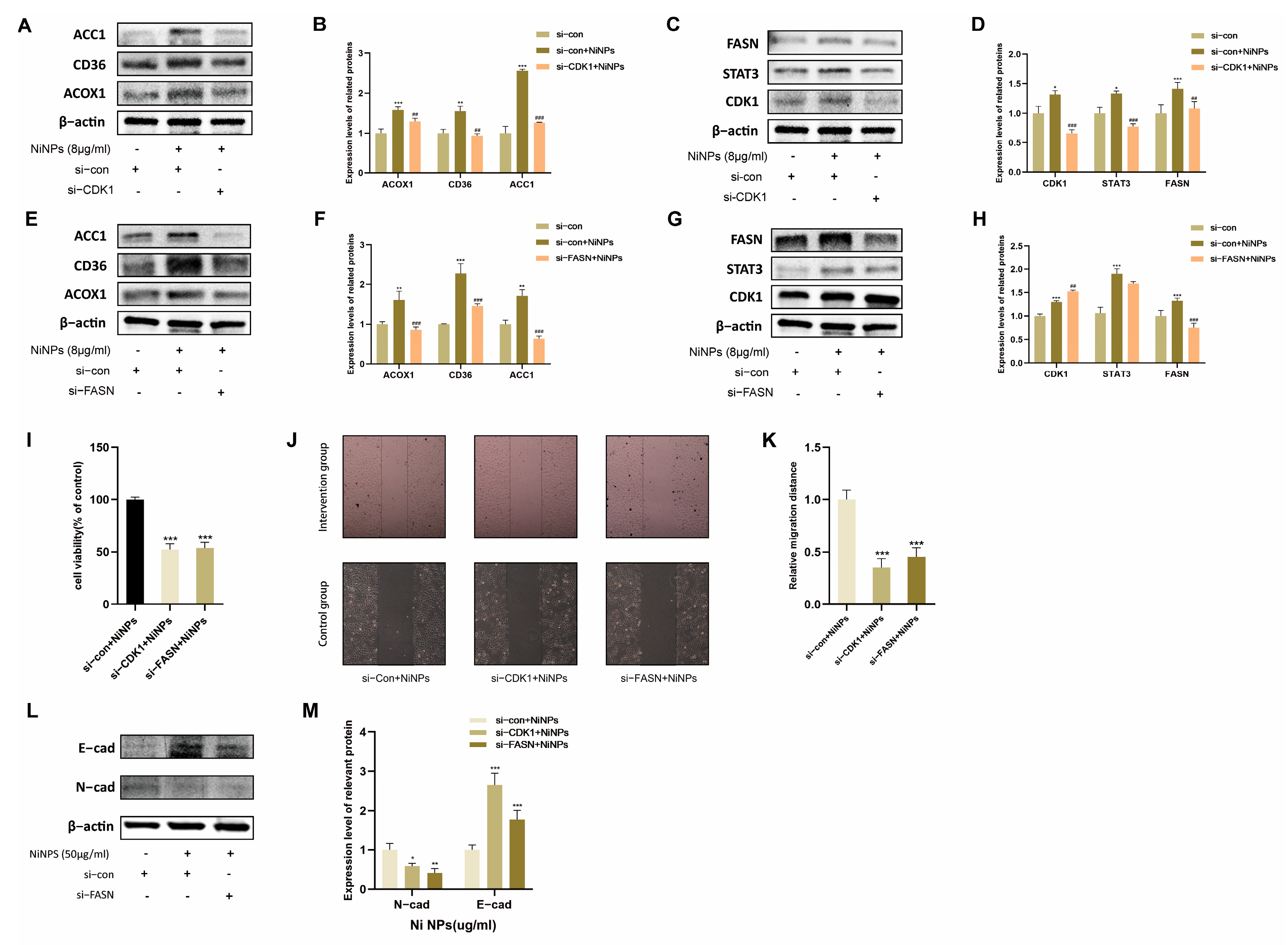
2.4. CDK1 Inhibition Attenuates NiNP-Induced FAM Dysregulation
2.5. CDK1 Promotes LUAD Progression via FAM
2.6. API Reverses NiNP-Induced FAM Dysregulation
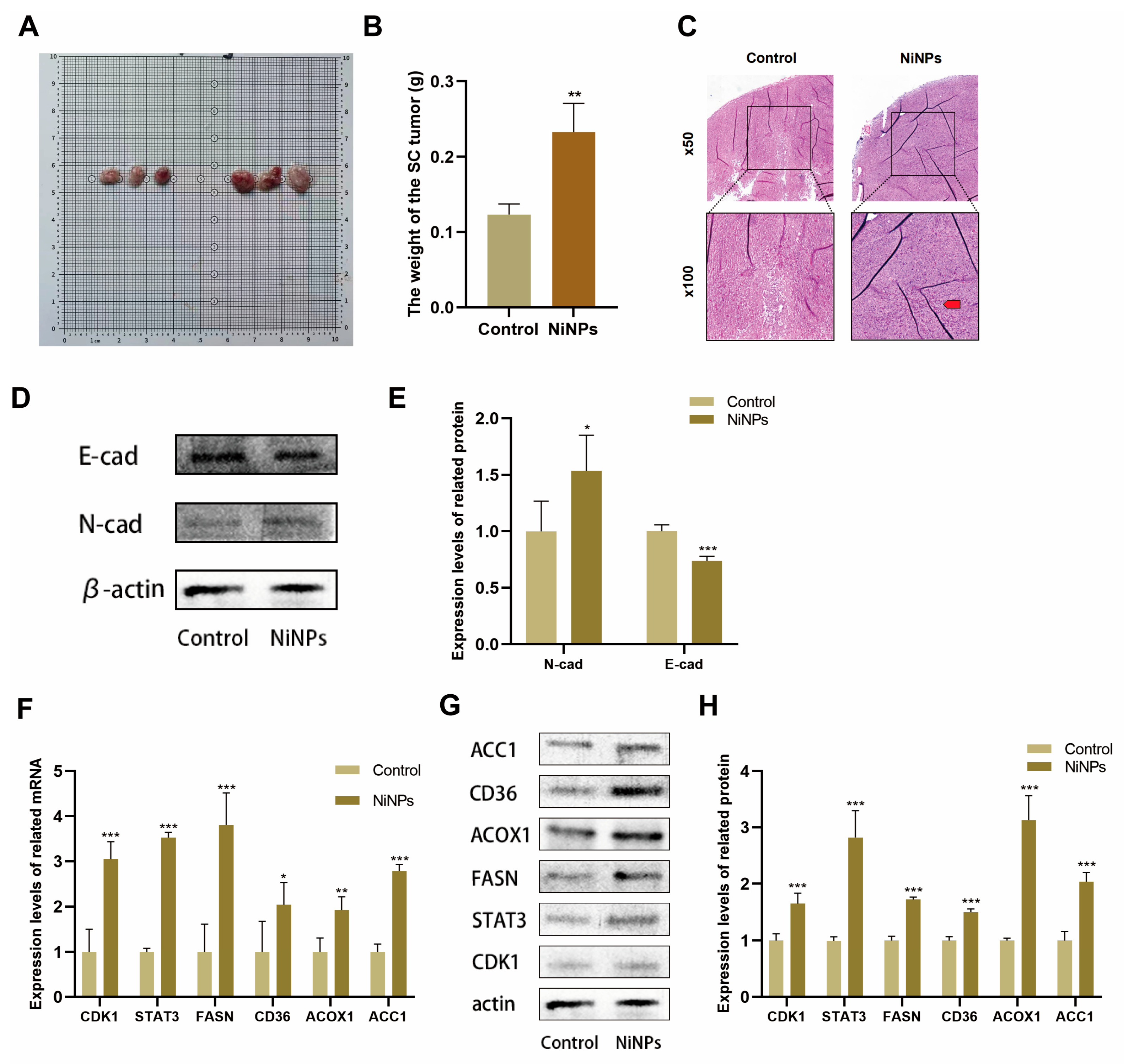
2.7. NiNPs Promote LUAD Progression via CDK1/FAM In Vivo
2.8. API Reverses NiNP-Induced LUAD Progression In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Preparation of NiNPs Suspension
4.3. CCK-8 Assay
4.4. Wound Healing Assay
4.5. siRNA Transfection for Gene Silencing
4.6. Real-Time qPCR
4.7. Western Blot Analysis
4.8. Chemical–Protein Interaction Analysis
4.9. Molecular Docking Prediction
4.10. Animal Experiment Design
4.11. Hematoxylin and Eosin (HE) Staining
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NiNPs | Nickel Nanoparticles |
| LUAD | Lung Adenocarcinoma |
| FAM | Fatty Acid Metabolism |
| CDK1 | Cyclin-Dependent Kinase 1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| FASN | Fatty Acid Synthase |
| API | Apigenin |
| IARC | International Agency for Research on Cancer |
| ROS | Reactive Oxygen Species |
| PPARγ | Peroxisome Proliferator-Activated Receptor γ |
| NF-κB | Nuclear Factor Kappa-B |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| XO | Xanthine Oxidase |
| NLRP3 | NOD-Like Receptor Pyrin Domain-Containing 3 |
| ACOX1 | Acyl-CoA Oxidase 1 |
| ACC1 | Acetyl-CoA Carboxylase 1 |
| CD36 | Cluster of Differentiation 36 |
| EMT | Epithelial–Mesenchymal Transition |
| PC-TWA | Permissible Concentration–Time Weighted Average |
| ISO | International Organization for Standardization |
| MAK | Maximale Arbeitsplatzkonzentration |
| GBZ | Guobiao Zhi (Chinese National Occupational Health Standard) |
Appendix A
| CDK1 | AAACTACAGGTCAAGTGGTAGCC | 23 |
| TCCTGCATAAGCACATCCTGA | 21 | |
| STAT3 | ACCAGCAGTATAGCCGCTTC | 20 |
| GCCACAATCCGGGCAATCT | 19 | |
| FASN | AAGGACCTGTCTAGGTTTGATGC | 23 |
| TGGCTTCATAGGTGACTTCCA | 21 | |
| ACOX1 | AATCGGGACCCATAAGCCTTT | 21 |
| GGGAATACGATGGTTGTCCATTT | 23 | |
| ACC1 | ATGTCTGGCTTGCACCTAGTA | 21 |
| CCCCAAAGCGAGTAACAAATTCT | 23 | |
| CD36 | TACTCTTGCCTTGCGGACTAA | 21 |
| CCGAAGCAGTTCACCGATATAC | 22 |
References
- Wu, H.; Wang, H.; Lv, Y.; Wu, Y.; Wang, Y.; Luo, Q.; Hui, Y.; Liu, L.; Zhang, M.; Hou, K.; et al. Ultra-small Metallic Nickel Nanoparticles on Dealuminated Zeolite for Active and Durable Catalytic Dehydrogenation. Angew. Chem. Int. Ed. 2025, 64, e202420306. (In English) [Google Scholar] [CrossRef]
- Su, Y.; Yuan, G.; Hu, J.; Zhang, G.; Tang, Y.; Chen, Y.; Tian, Y.; Wang, S.; Shakouri, M.; Pang, H. Thiosalicylic-Acid-Mediated Coordination Structure of Nickel Center via Thermodynamic Modulation for Aqueous Ni-Zn Batteries. Adv. Mater. 2024, 36, e2406094. [Google Scholar] [CrossRef]
- Xu, H.; Lei, X.; Pan, Y.; Cao, X.; Deng, J.; Yang, J. Bubble-based electrochemical chip integrated with cobalt-nickel bimetallic hybrid for enhanced detection of dopamine. Biosens. Bioelectron. 2025, 268, 116881. [Google Scholar] [CrossRef]
- Mu, L.; Yao, Y.; Liu, Q.; Li, J.; Wang, Y.; Sun, C.L.; He, J.; Qu, M. Superhydrophilic and Underwater Superaerophobic Dual-Function Peony-Shaped Selenide Micro-nano Array Self-Supported Electrodes for High-Efficiency Overall Water Splitting Driven by Renewable Energy. ACS Appl. Mater. Interfaces 2024, 16, 49349–49361. [Google Scholar] [CrossRef]
- Song, P.; Jin, S.; Cao, Y.; Zhang, S.; Yin, N.; Zhang, H.; Wang, D. Multifunctional biocompatible Ni/Ni-P nanospheres for anti-tumor “neoadjuvant phototherapy” combining photothermal therapy and photodynamic therapy. J. Mater. Chem. B 2023, 11, 10019–10028. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, Y.; Zhang, Q. The pulmonary effects of nickel-containing nanoparticles: Cytotoxicity, genotoxicity, carcinogenicity, and their underlying mechanisms. Environ. Sci. Nano 2024, 11, 1817–1846. [Google Scholar] [CrossRef]
- ISO/TS 10303-1309:2018; Industrial Automation Systems and Integration—Product Data Representation and Exchange. ISO: Geneva, Switzerland, 2018.
- DFG. MAK- und BAT-Werte 2021; ZB MED-Publikationsportal Lebenswissenschaften: Düsseldorf, Germany, 2021. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Mo, Y.; Jiang, M.; Zhang, Y.; Wan, R.; Li, J.; Zhong, C.J.; Li, H.; Tang, S.; Zhang, Q. Comparative mouse lung injury by nickel nanoparticles with differential surface modification. J. Nanobiotechnol. 2019, 17, 2. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, Y.; Zhang, Y.; Yuan, J.; Mo, L.; Zhang, Q. Nickel nanoparticle-induced cell transformation: Involvement of DNA damage and DNA repair defect through HIF-1α/miR-210/Rad52 pathway. J. Nanobiotechnol. 2021, 19, 370. [Google Scholar] [CrossRef]
- Capasso, L.; Camatini, M.; Gualtieri, M. Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. Toxicol. Lett. 2014, 226, 28–34. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, Y.; Wan, R.; Jiang, M.; Xu, Y.; Zhang, Q. miR-21 mediates nickel nanoparticle-induced pulmonary injury and fibrosis. Nanotoxicology 2020, 14, 1175–1197. [Google Scholar] [CrossRef]
- Magaye, R.; Zhou, Q.; Bowman, L.; Zou, B.; Mao, G.; Xu, J.; Castranova, V.; Zhao, J.; Ding, M. Metallic nickel nanoparticles may exhibit higher carcinogenic potential than fine particles in JB6 cells. PLoS ONE 2014, 9, e92418. [Google Scholar] [CrossRef]
- Iqbal, S.; Jabeen, F.; Peng, C.; Shah, M.A.; Ijaz, M.U.; Rasul, A.; Ali, S.; Rauf, A.; Batiha, G.E.; Kłodzińska, E. Nickel nanoparticles induce hepatotoxicity via oxidative and nitrative stress-mediated apoptosis and inflammation. Toxicol. Ind. Health 2021, 37, 619–634. [Google Scholar] [CrossRef]
- Wedan, R.J.; Longenecker, J.Z.; Nowinski, S.M. Mitochondrial fatty acid synthesis is an emergent central regulator of mammalian oxidative metabolism. Cell Metab. 2024, 36, 36–47. [Google Scholar] [CrossRef]
- Jin, H.R.; Wang, J.; Wang, Z.J.; Xi, M.J.; Xia, B.H.; Deng, K.; Yang, J.L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef]
- Munir, R.; Lisec, J.; Swinnen, J.V.; Zaidi, N. Too complex to fail? Targeting fatty acid metabolism for cancer therapy. Prog. Lipid Res. 2022, 85, 101143. [Google Scholar] [CrossRef]
- Bartolacci, C.; Andreani, C.; Vale, G.; Berto, S.; Melegari, M.; Crouch, A.C.; Baluya, D.L.; Kemble, G.; Hodges, K.; Starrett, J.; et al. Targeting de novo lipogenesis and the Lands cycle induces ferroptosis in KRAS-mutant lung cancer. Nat. Commun. 2022, 13, 4327, Correction in Nat. Commun. 2022, 13, 4640. https://doi.org/10.1002/advs.202510217. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, C.; Nguyen, P.G.; Metts, J.M.; Collins, L.C.; Jain, K.; Mills, C.A.; Vlach, L.; Li, K.; Brademeyer, A.L.; et al. A SETD2-CDK1-lamin axis maintains nuclear morphology and genome stability. Nat. Cell Biol. 2025, 27, 1327–1341. [Google Scholar] [CrossRef]
- Wang, Q.; Bode, A.M.; Zhang, T. Targeting CDK1 in cancer: Mechanisms and implications. npj Precis. Oncol. 2023, 7, 58. [Google Scholar] [CrossRef]
- Ow, J.R.; Caldez, M.J.; Zafer, G.; Foo, J.C.; Li, H.Y.; Ghosh, S.; Wollmann, H.; Cazenave-Gassiot, A.; Ong, C.B.; Wenk, M.R.; et al. Remodeling of whole-body lipid metabolism and a diabetic-like phenotype caused by loss of CDK1 and hepatocyte division. eLife 2020, 9, e63835. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, Q.; Liang, J.; Li, Y.; Li, M.; Zhang, Y.; Wang, X.; Zeng, Y.; Jiao, Y. A CRISPR knockout negative screen reveals synergy between CDKs inhibitor and metformin in the treatment of human cancer in vitro and in vivo. Signal Transduct. Target. Ther. 2020, 5, 152. [Google Scholar] [CrossRef]
- Kuang, Y.; Guo, W.; Ling, J.; Xu, D.; Liao, Y.; Zhao, H.; Du, X.; Wang, H.; Xu, M.; Song, H.; et al. Iron-dependent CDK1 activity promotes lung carcinogenesis via activation of the GP130/STAT3 signaling pathway. Cell Death Dis. 2019, 10, 297. [Google Scholar] [CrossRef]
- Gao, P.; Wang, L.L.; Liu, J.; Dong, F.; Song, W.; Liao, L.; Wang, B.; Zhang, W.; Zhou, X.; Xie, Q.; et al. Dihydroartemisinin inhibits endothelial cell tube formation by suppression of the STAT3 signaling pathway. Life Sci. 2020, 242, 117221. [Google Scholar] [CrossRef]
- Yang, T.; Qiao, S.; Zhu, X. High-dose radiation-resistant lung cancer cells stored many functional lipid drops through JAK2/p-STAT3/FASN pathway. J. Cancer Res. Clin. Oncol. 2023, 149, 14169–14183. [Google Scholar] [CrossRef]
- Zhai, H.; Zheng, T.; Fan, L. Unveiling the STAT3-ACC1 axis: A key driver of lipid metabolism and tumor progression in non-small cell lung cancer. J. Cancer 2024, 15, 2340–2353. [Google Scholar] [CrossRef]
- Xiu, C.; Zhang, L.; Zhang, C.; Zhang, Y.; Luo, X.; Zhang, Z.; Zhao, H.; Ji, K.; Chen, Z.; He, G.; et al. Pharmacologically targeting fatty acid synthase-mediated de novo lipogenesis alleviates osteolytic bone loss by directly inhibiting osteoclastogenesis through suppression of STAT3 palmitoylation and ROS signaling. Metab. Clin. Exp. 2025, 167, 156186. [Google Scholar] [CrossRef]
- Menendez, J.A.; Cuyàs, E.; Encinar, J.A.; Vander Steen, T.; Verdura, S.; Llop-Hernández, À.; López, J.; Serrano-Hervás, E.; Osuna, S.; Martin-Castillo, B.; et al. Fatty acid synthase (FASN) signalome: A molecular guide for precision oncology. Mol. Oncol. 2024, 18, 479–516. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Q.; Jiang, Z.; Sun, R.; Wang, Q.; Liu, S.; Luan, X.; Campisi, J.; Kirkland, J.L.; Zhang, W.; et al. Targeting Senescence with Apigenin Improves Chemotherapeutic Efficacy and Ameliorates Age-Related Conditions in Mice. Adv. Sci. 2025, 12, e2412950, Erratum in Adv. Sci. 2025, 12, e10217. https://doi.org/10.1002/advs.202510217. [Google Scholar] [CrossRef]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Casagrande, F.; Darbon, J.M. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem. Pharmacol. 2001, 61, 1205–1215. [Google Scholar] [CrossRef]
- Kong, L.; Gao, X.; Zhu, J.; Cheng, K.; Tang, M. Mechanisms involved in reproductive toxicity caused by nickel nanoparticle in female rats. Environ. Toxicol. 2016, 31, 1674–1683. [Google Scholar] [CrossRef]
- Boran, H.; Şaffak, S. Comparison of Dissolved Nickel and Nickel Nanoparticles Toxicity in Larval Zebrafish in Terms of Gene Expression and DNA Damage. Arch. Environ. Contam. Toxicol. 2018, 74, 193–202. [Google Scholar] [CrossRef]
- Han, Z.; Yan, Z.; Ma, Z.; Wang, Y.; Beus, M.; Lu, J.; Weidenhammer, L.B.; Lakhani, K.; Lee, J.; Civils, J.D.; et al. Targeting ABCD1-ACOX1-MET/IGF1R axis suppresses multiple myeloma. Leukemia 2025, 39, 720–733. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, D.; Shao, Y.; Shan, Z.; Liu, Q.; Weng, J.; He, W.; Zhang, R.; Li, Q.; Wang, Z.; et al. circCAPRIN1 interacts with STAT2 to promote tumor progression and lipid synthesis via upregulating ACC1 expression in colorectal cancer. Cancer Commun. 2023, 43, 100–122. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Yang, D.; Li, L.; Li, S.; Lu, S.; Li, N. Interplay of CD36, autophagy, and lipid metabolism: Insights into cancer progression. Metab. Clin. Exp. 2024, 155, 155905. [Google Scholar] [CrossRef]
- Jiang, T.; Qi, J.; Xue, Z.; Liu, B.; Liu, J.; Hu, Q.; Li, Y.; Ren, J.; Song, H.; Xu, Y.; et al. The m(6)A modification mediated-lncRNA POU6F2-AS1 reprograms fatty acid metabolism and facilitates the growth of colorectal cancer via upregulation of FASN. Mol. Cancer 2024, 23, 55. [Google Scholar] [CrossRef]
- Chen, J.; Zou, L.; Lu, G.; Grinchuk, O.; Fang, L.; Ong, D.S.T.; Taneja, R.; Ong, C.N.; Shen, H.M. PFKP alleviates glucose starvation-induced metabolic stress in lung cancer cells via AMPK-ACC2 dependent fatty acid oxidation. Cell Discov. 2022, 8, 52. [Google Scholar] [CrossRef]
- Ma, L.; Chen, C.; Zhao, C.; Li, T.; Ma, L.; Jiang, J.; Duan, Z.; Si, Q.; Chuang, T.H.; Xiang, R.; et al. Targeting carnitine palmitoyl transferase 1A (CPT1A) induces ferroptosis and synergizes with immunotherapy in lung cancer. Signal Transduct. Target. Ther. 2024, 9, 64. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Wu, R.Z.; Sun, Q.Q.; Fu, Y.; Yu, H.N.; Liu, W.Y.; Wu, Y.H.; Zhang, H.; Pan, Y.L.; Rui, X. Fatty acid metabolism-derived prognostic model for lung adenocarcinoma: Unraveling the link to survival and immune response. Front. Immunol. 2025, 16, 1507845. [Google Scholar] [CrossRef]
- Chen, N.P.; Aretz, J.; Fässler, R. CDK1-cyclin-B1-induced kindlin degradation drives focal adhesion disassembly at mitotic entry. Nat. Cell Biol. 2022, 24, 723–736. [Google Scholar] [CrossRef]
- Chang, J.H.; Cheng, C.W.; Yang, Y.C.; Chen, W.S.; Hung, W.Y.; Chow, J.M.; Chen, P.S.; Hsiao, M.; Lee, W.J.; Chien, M.H. Downregulating CD26/DPPIV by apigenin modulates the interplay between Akt and Snail/Slug signaling to restrain metastasis of lung cancer with multiple EGFR statuses. J. Exp. Clin. Cancer Res. Cancer Res. 2018, 37, 199. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Navarrete, T.G.; Mejía-Guerra, K.; Mukundi, E.; Eubank, T.D.; Grotewold, E.; Arango, D.; Doseff, A.I. Transcriptome reprogramming through alternative splicing triggered by apigenin drives cell death in triple-negative breast cancer. Cell Death Dis. 2023, 14, 824. [Google Scholar] [CrossRef]
- Ganai, S.A. Plant-derived flavone Apigenin: The small-molecule with promising activity against therapeutically resistant prostate cancer. Biomed. Pharmacother. 2017, 85, 47–56. [Google Scholar] [CrossRef]
- Yang, C.; Song, J.; Hwang, S.; Choi, J.; Song, G.; Lim, W. Apigenin enhances apoptosis induction by 5-fluorouracil through regulation of thymidylate synthase in colorectal cancer cells. Redox Biol. 2021, 47, 102144. [Google Scholar] [CrossRef]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef]
- Li, J.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Cui, S. Protective Effects of Naringenin and Apigenin in Ameliorating Skin Damage via Mediating the Nrf2 and NF-κB Pathways in Mice. Foods 2023, 12, 2120. [Google Scholar] [CrossRef]
- Lv, Y.; Gao, X.; Luo, Y.; Fan, W.; Shen, T.; Ding, C.; Yao, M.; Song, S.; Yan, L. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J. Nutr. Biochem. 2019, 71, 110–121. [Google Scholar] [CrossRef]
- Lü, W.D.; Liu, Y.Z.; Yang, Y.Q.; Liu, Z.G.; Zhao, K.; Lu, J.R.; Lei, G.Y.; Wang, Y.Y.; Cai, L.; Sun, R.F. Effect of naturally derived surgical hemostatic materials on the proliferation of A549 human lung adenocarcinoma cells. Mater. Today Bio 2022, 14, 100233. [Google Scholar] [CrossRef]
- Fitzgerald, J.E.; Byrd, B.K.; Patil, R.A.; Strawbridge, R.R.; Davis, S.C.; Bellini, C.; Niedre, M. Heterogeneity of circulating tumor cell dissemination and lung metastases in a subcutaneous Lewis lung carcinoma model. Biomed. Opt. Express 2020, 11, 3633–3647. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Liu, Y.; Liang, J.; Liu, Q.; Xu, Z.; Chen, Z.; Zhang, X.; Zhang, K.; Xu, C. Blockading a new NSCLC immunosuppressive target by pluripotent autologous tumor vaccines magnifies sequential immunotherapy. Bioact. Mater. 2022, 13, 223–238. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, Y.; Li, T.; Zhong, H.; Shan, L.; Yu, P.; Xia, C.; Xu, L. Apigenin promotes apoptosis of 4T1 cells through PI3K/AKT/Nrf2 pathway and improves tumor immune microenvironment in vivo. Toxicol. Res. 2024, 13, tfae011. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; He, Y.; Wu, J.; Liu, Y.; Li, X.; Li, Z.; Yuan, Q.; Li, J.; Zhang, X.; et al. Stereo-seq V2: Spatial mapping of total RNA on FFPE sections with high resolution. Cell 2025. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Head, T.; Clarke, P.B.S. Analyzing lognormal data: A nonmathematical practical guide. Pharmacol. Rev. 2025, 77, 100049. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.-Z.; Zhang, B.; Yu, H.-N.; Sun, Q.-Q.; Yao, W.-X.; Liu, W.-Y.; Lv, J.-J.; Xu, Z.-W.; Qi, H.-Q.; Fu, Y.; et al. Nickel Nanoparticles Promote Lung Adenocarcinoma Progression via CDK1-Mediated Fatty Acid Metabolism Regulation. Int. J. Mol. Sci. 2025, 26, 10624. https://doi.org/10.3390/ijms262110624
Wu R-Z, Zhang B, Yu H-N, Sun Q-Q, Yao W-X, Liu W-Y, Lv J-J, Xu Z-W, Qi H-Q, Fu Y, et al. Nickel Nanoparticles Promote Lung Adenocarcinoma Progression via CDK1-Mediated Fatty Acid Metabolism Regulation. International Journal of Molecular Sciences. 2025; 26(21):10624. https://doi.org/10.3390/ijms262110624
Chicago/Turabian StyleWu, Rui-Ze, Bo Zhang, Han-Nong Yu, Qian-Qian Sun, Wen-Xue Yao, Wei-Yang Liu, Jun-Jie Lv, Zhi-Wei Xu, Hong-Qing Qi, Yao Fu, and et al. 2025. "Nickel Nanoparticles Promote Lung Adenocarcinoma Progression via CDK1-Mediated Fatty Acid Metabolism Regulation" International Journal of Molecular Sciences 26, no. 21: 10624. https://doi.org/10.3390/ijms262110624
APA StyleWu, R.-Z., Zhang, B., Yu, H.-N., Sun, Q.-Q., Yao, W.-X., Liu, W.-Y., Lv, J.-J., Xu, Z.-W., Qi, H.-Q., Fu, Y., Zhao, A.-Y., Pan, Y.-L., Wu, Y.-H., & Xin, R. (2025). Nickel Nanoparticles Promote Lung Adenocarcinoma Progression via CDK1-Mediated Fatty Acid Metabolism Regulation. International Journal of Molecular Sciences, 26(21), 10624. https://doi.org/10.3390/ijms262110624







