Phylogenetic and Molecular Characterization of a Novel Reassortant High-Pathogenicity Avian Influenza A (H7N6) Virus Detected in New Zealand Poultry
Abstract
1. Introduction
2. Results
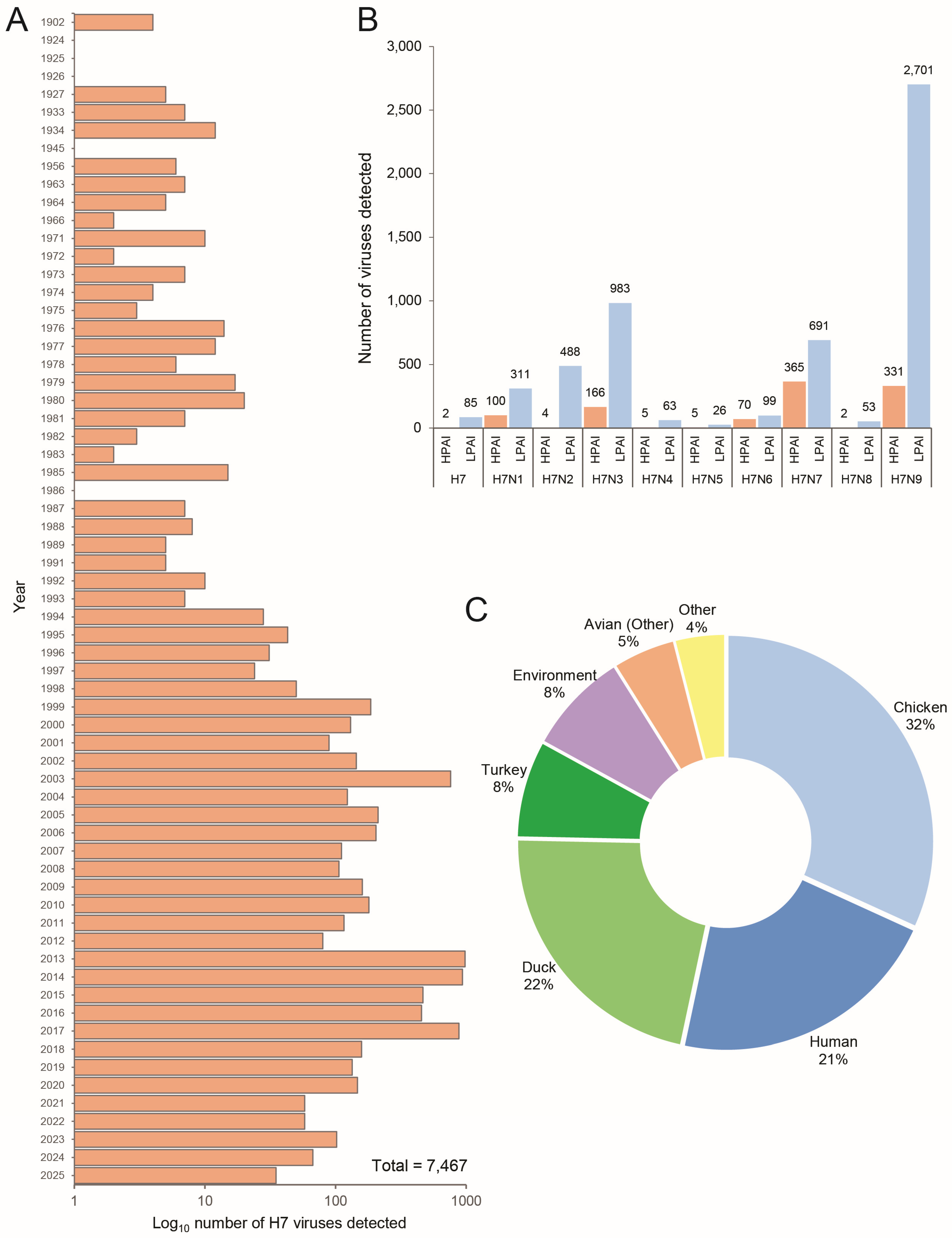
2.1. Genomic Analysis of Global H7 Viruses Indicates a Pathogenic Shift from a Domestic Strain of H7 LPAI
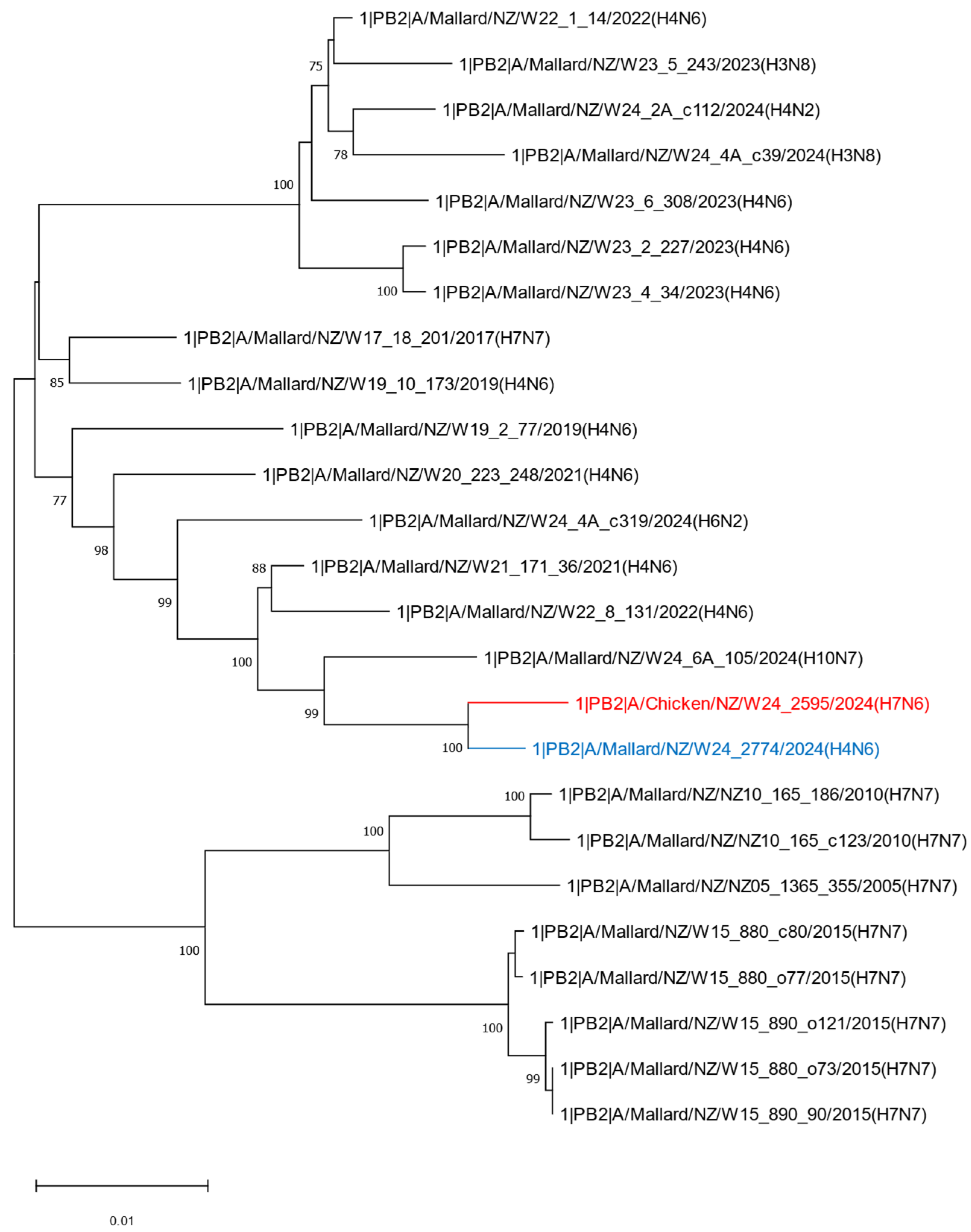
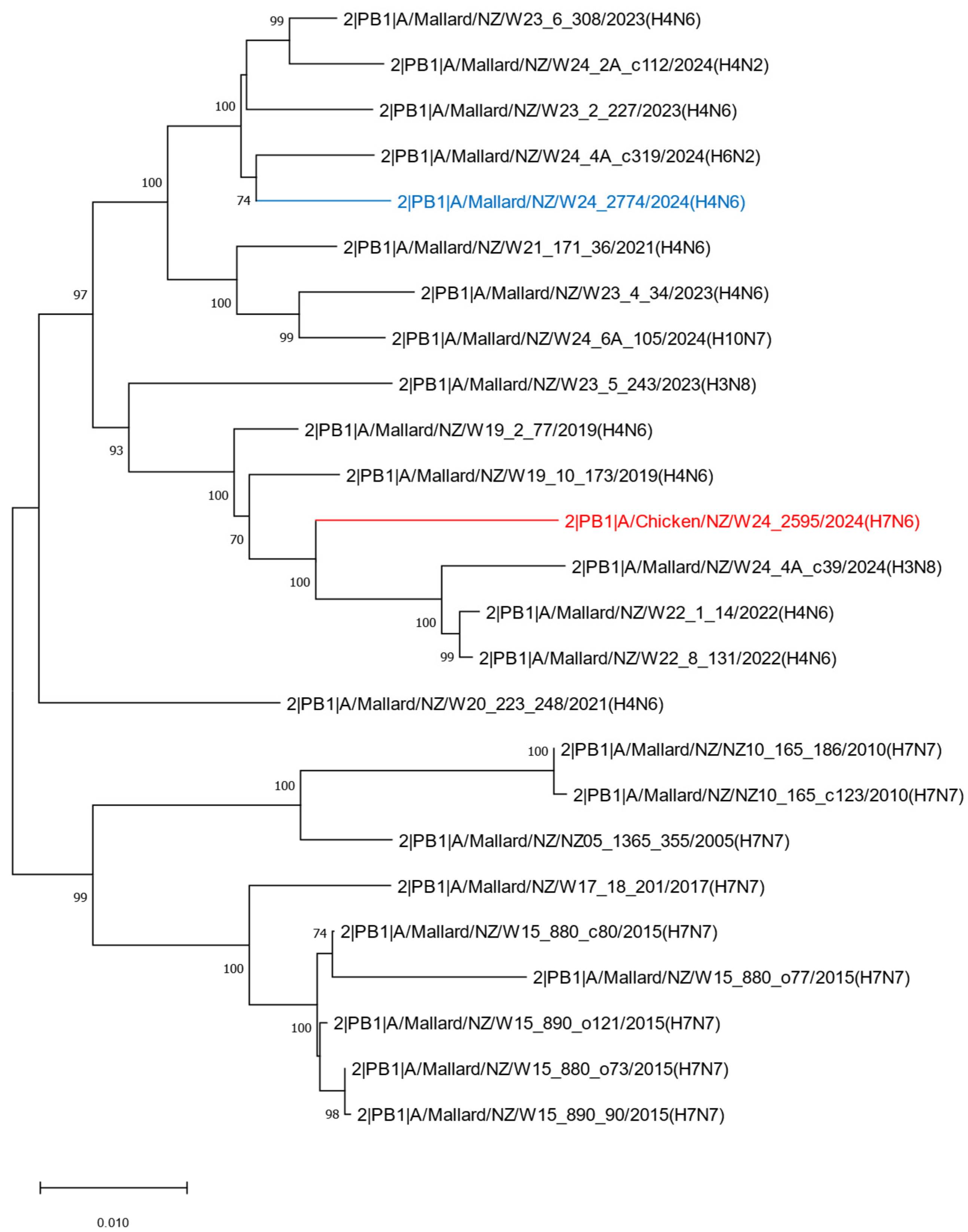
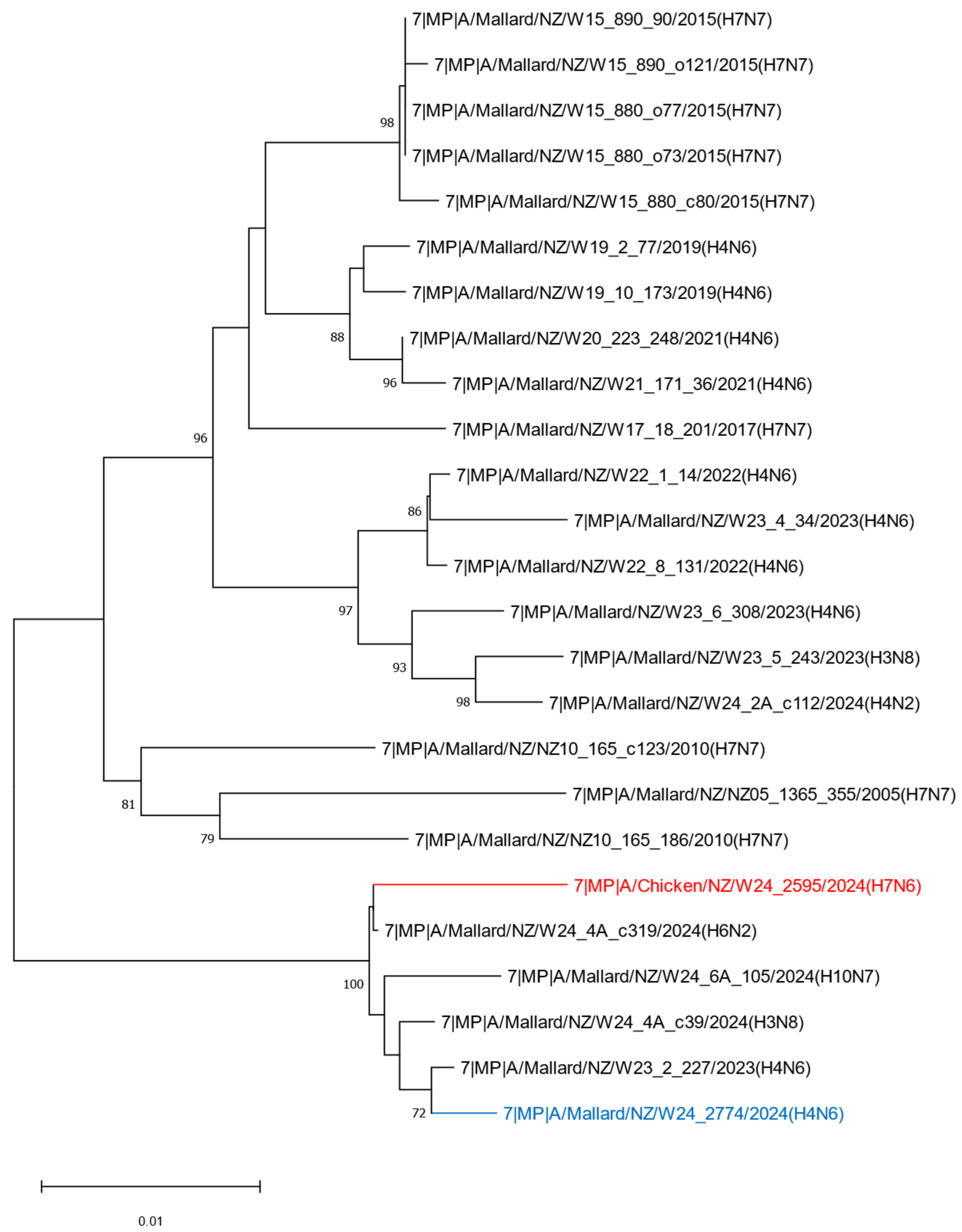
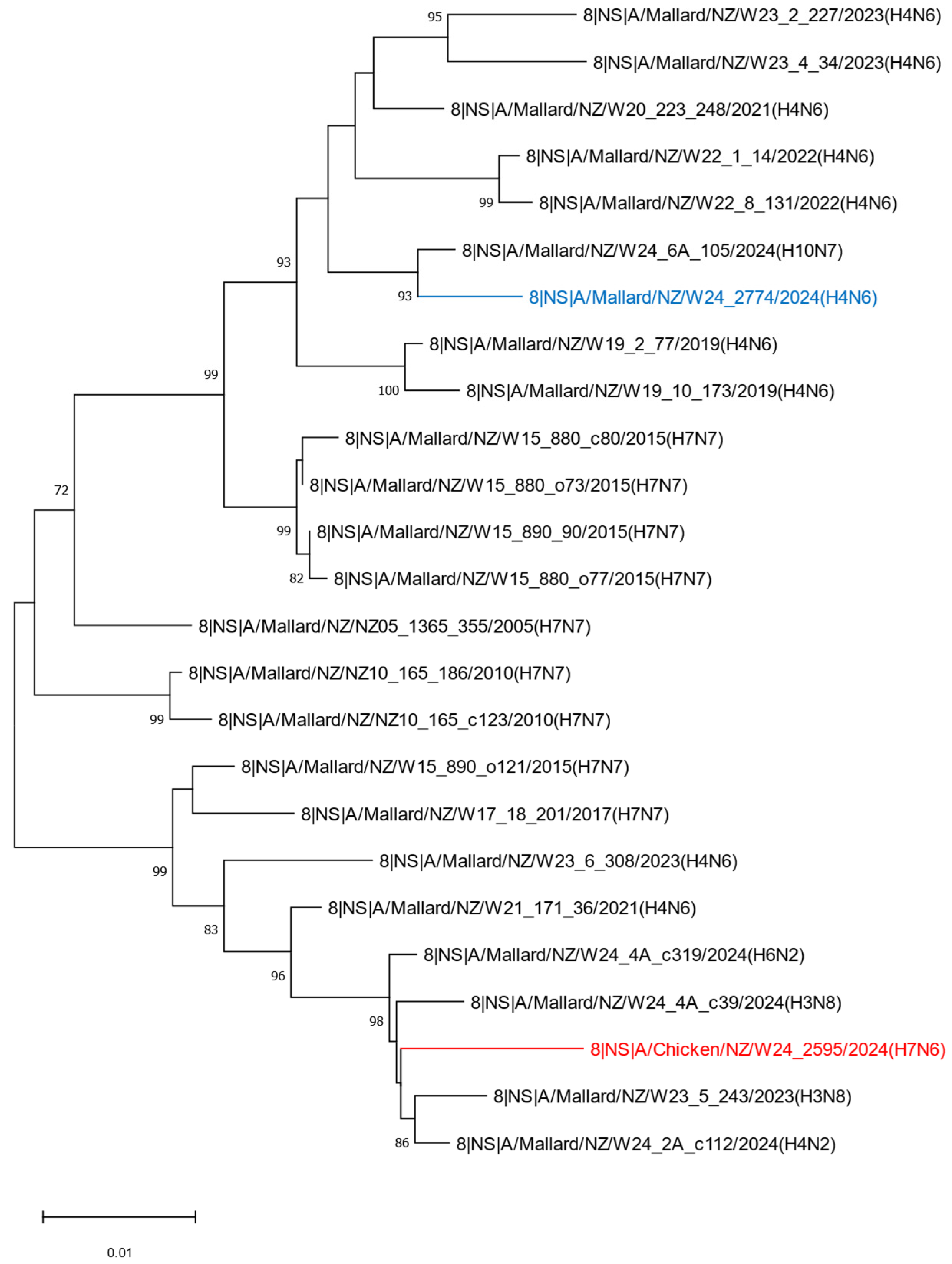
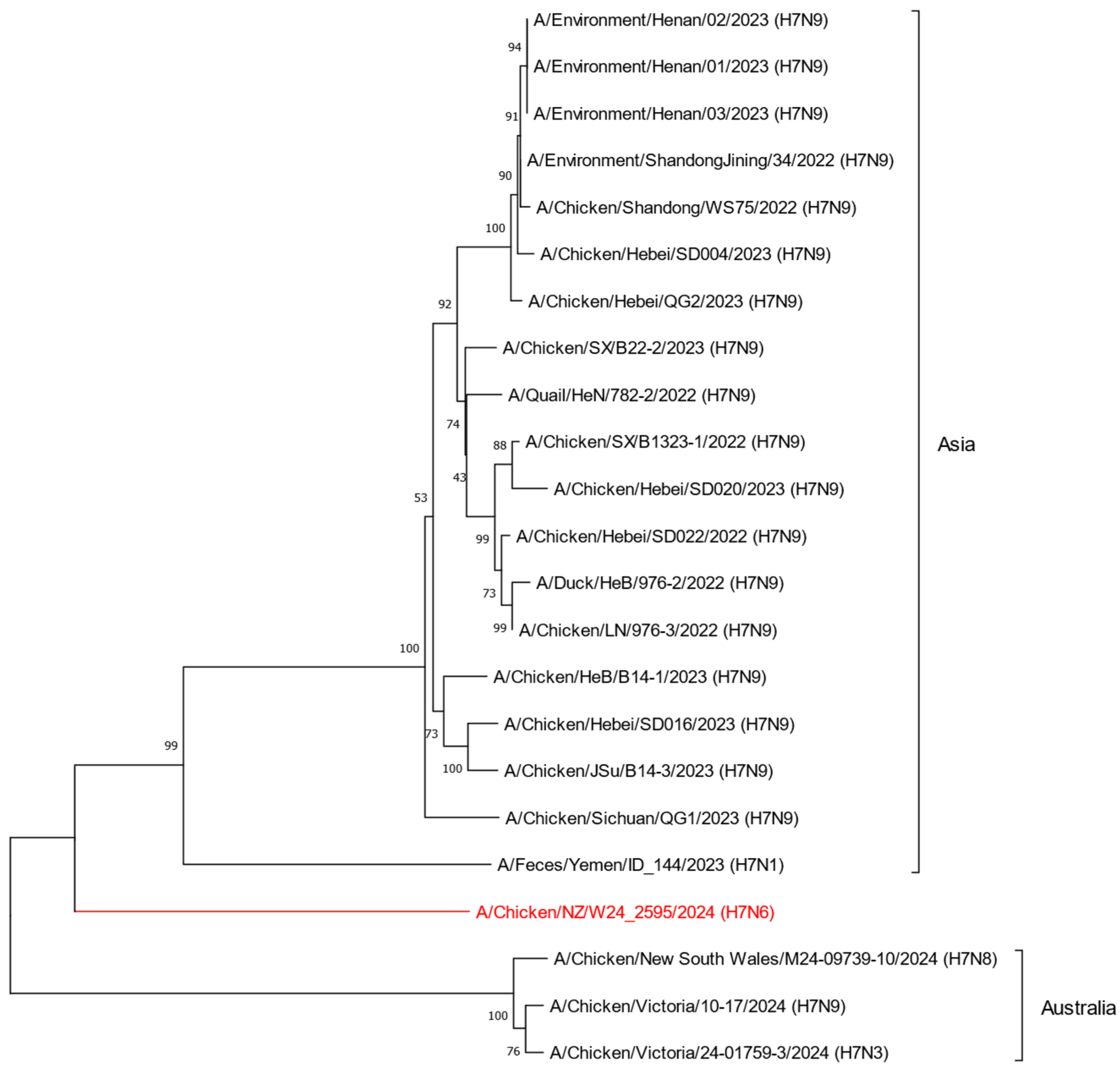
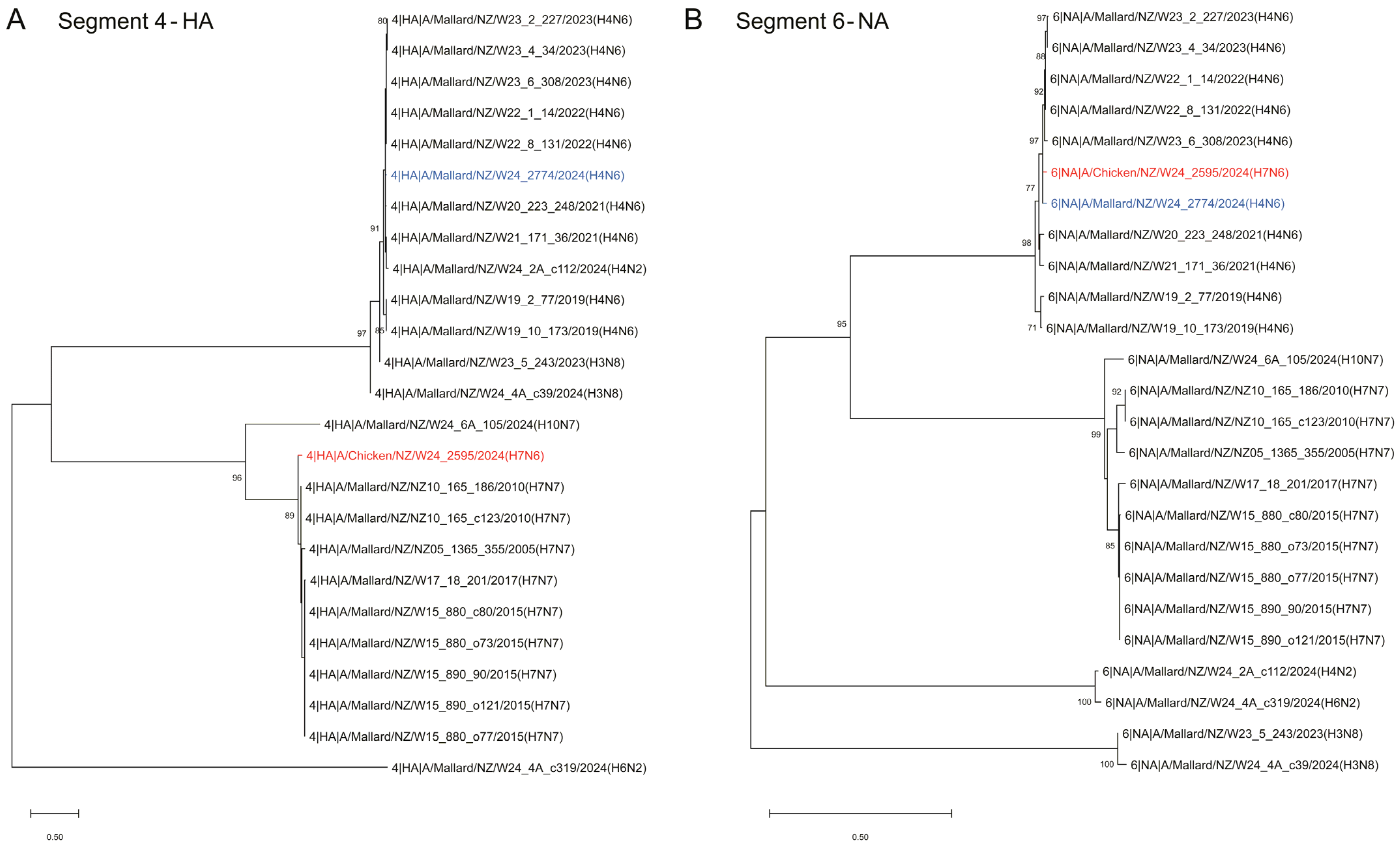
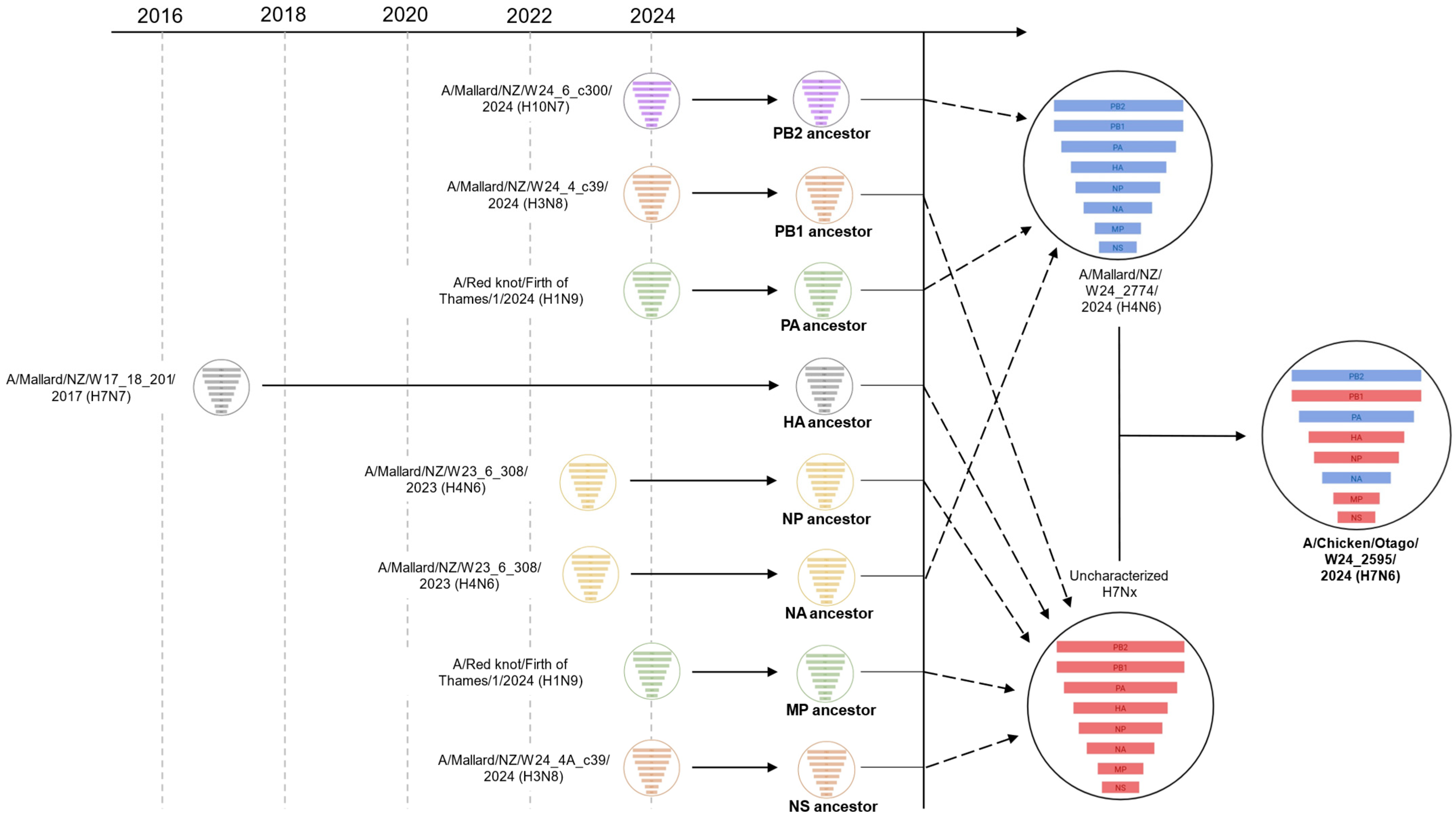
2.2. Phylogenetic Analysis of the H7N6 HPAI Virus Provides Strong Evidence of Reassortment Between Endemic LPAI Viruses
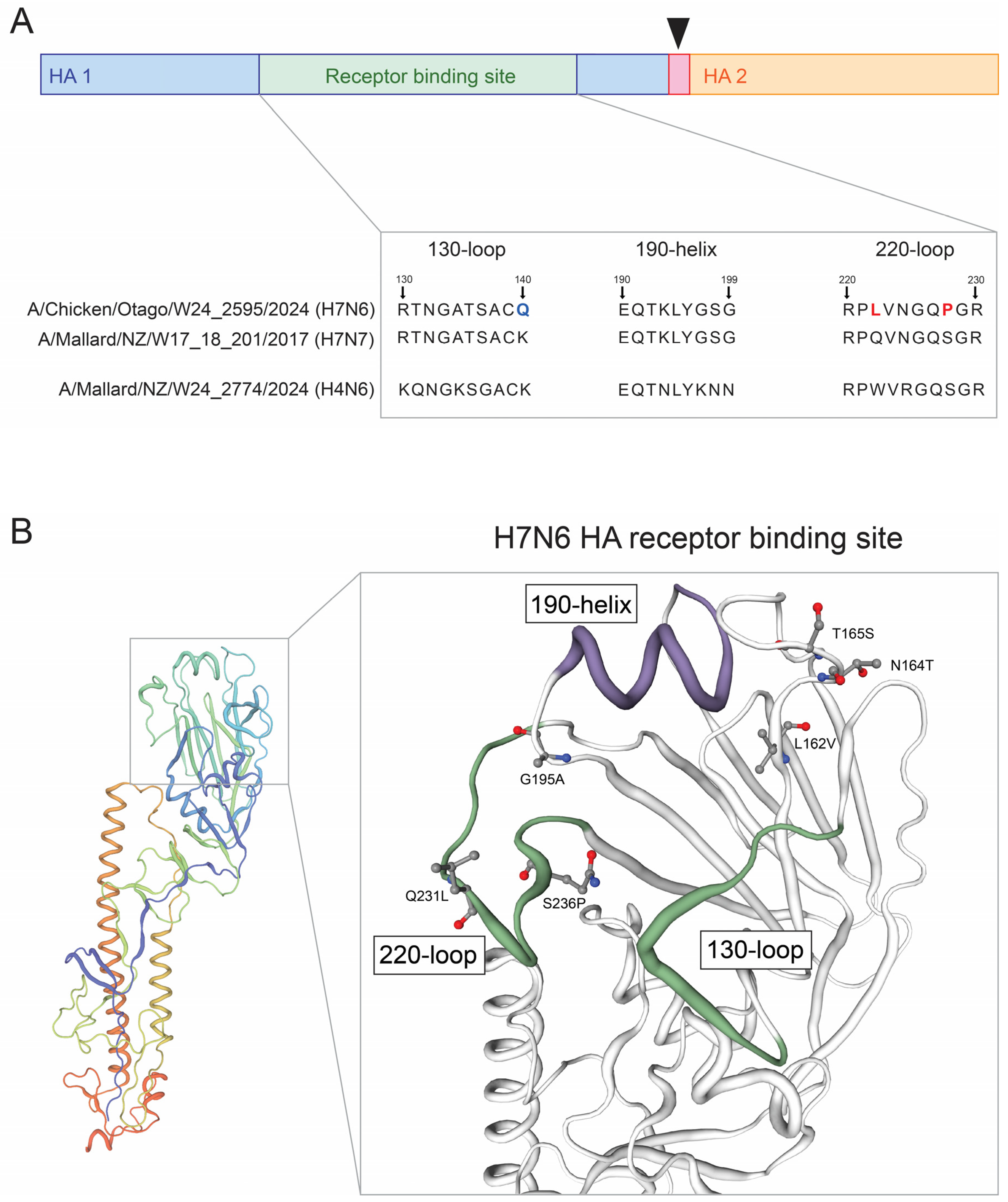
2.3. Molecular Analysis of the H7N6 HPAI Virus Reveals Mutational Events Associated with Changes in Host Specificity and Host Cell Receptor Binding
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Nucleic Acid Extraction
4.2. Whole Viral Genome Amplification and Sequencing
4.3. Genome Assembly
4.4. Avian Influenza Subtype Analysis
4.5. Phylogenetic Analysis
4.6. Mutation Analysis and Protein Structure Modelling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Spackman, E. A Brief Introduction to Avian Influenza Virus. Methods Mol. Biol. 2020, 2123, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Azeem, S.; Guo, B.; Sun, D.; Killian, M.L.; Baroch, J.A.; Yoon, K.-J. Evaluation of PCR-based hemagglutinin subtyping as a tool to aid in surveillance of avian influenza viruses in migratory wild birds. J. Virol. Methods 2022, 308, 114594. [Google Scholar] [CrossRef] [PubMed]
- Luczo, J.M.; Stambas, J.; Durr, P.A.; Michalski, W.P.; Bingham, J. Molecular pathogenesis of H5 highly pathogenic avian influenza: The role of the haemagglutinin cleavage site motif. Rev. Med. Virol. 2015, 25, 406–430. [Google Scholar] [CrossRef]
- Straus, M.R.; Whittaker, G.R. A peptide-based approach to evaluate the adaptability of influenza A virus to humans based on its hemagglutinin proteolytic cleavage site. PLoS ONE 2017, 12, e0174827. [Google Scholar] [CrossRef]
- de Bruin, A.C.M.; Funk, M.; Spronken, M.I.; Gultyaev, A.P.; Fouchier, R.A.M.; Richard, M. Hemagglutinin Subtype Specificity and Mechanisms of Highly Pathogenic Avian Influenza Virus Genesis. Viruses 2022, 14, 1566. [Google Scholar] [CrossRef]
- Beerens, N.; Heutink, R.; Harders, F.; Bossers, A.; Koch, G.; Peeters, B. Emergence and Selection of a Highly Pathogenic Avian Influenza H7N3 Virus. J. Virol. 2020, 94, e01818-19. [Google Scholar] [CrossRef]
- Graziosi, G.; Lupini, C.; Catelli, E.; Carnaccini, S. Highly Pathogenic Avian Influenza (HPAI) H5 Clade 2.3.4.4b Virus Infection in Birds and Mammals. Animals 2024, 14, 1372. [Google Scholar] [CrossRef]
- de Graaf, M. and Fouchier, R.A.M. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014, 33, 823–841, Erratum in EMBO J. 2014, 33, 1614. [Google Scholar] [CrossRef]
- Tzarum, N.; de Vries, R.P.; Zhu, X.; Yu, W.; McBride, R.; Paulson, J.C.; Wilson, I.A. Structure and Receptor Binding of the Hemagglutinin from a Human H6N1 Influenza Virus. Cell Host Microbe 2015, 17, 369–376. [Google Scholar] [CrossRef]
- Sheikh, M.O.B.; Rashid, P.M.A.; Rahim, Z.H.; Marouf, A.S.; Saeed, S.S. Molecular characterization and genetic analysis of highly pathogenic H5N1 clade 2.3.4.4b in seagulls from Dukan Lake, Iraq. Virus Genes 2025, 61, 193–203. [Google Scholar] [CrossRef]
- Zou, S.; Zhang, Y.; Li, X.; Bo, H.; Wei, H.; Dong, L.; Yang, L.; Dong, J.; Liu, J.; Shu, Y.; et al. Molecular characterization and receptor binding specificity of H9N2 avian influenza viruses based on poultry-related environmental surveillance in China between 2013 and 2016. Virology 2019, 529, 135–143. [Google Scholar] [CrossRef]
- Di, H.; Thor, S.W.; Trujillo, A.A.; Stark, T.J.; Marinova-Petkova, A.; Jones, J.; Wentworth, D.E.; Barnes, J.R.; Davis, C.T. Comparison of nucleic acid extraction methods for next-generation sequencing of avian influenza A virus from ferret respiratory samples. J. Virol. Methods 2019, 270, 95–105. [Google Scholar] [CrossRef]
- Lu, L.; Lycett, S.J.; Brown, A.J.L. Reassortment patterns of avian influenza virus internal segments among different subtypes. BMC Evol. Biol. 2014, 14, 16. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Si, K.; Yu, C.; Shang, K.; Yu, Z.; Wei, Y.; Ding, C.; Sarker, S.; Chen, S. Evidence of an emerging triple-reassortant H3N3 avian influenza virus in China. BMC Genom. 2024, 25, 1249. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Brock, N.; Belser, J.A.; Sun, X.; Pappas, C.; Kieran, T.J.; Thakur, P.B.; Zeng, H.; Cui, D.; Frederick, J.; et al. Highly pathogenic avian influenza A(H5N1) virus of clade 2.3.4.4b isolated from a human case in Chile causes fatal disease and transmits between co-housed ferrets. Emerg. Microbes Infect. 2024, 13, 2332667. [Google Scholar] [CrossRef]
- Marchenko, V.Y.; Panova, A.S.; Kolosova, N.P.; Gudymo, A.S.; Svyatchenko, S.V.; Danilenko, A.V.; Vasiltsova, N.N.; Egorova, M.L.; Onkhonova, G.S.; Zhestkov, P.D.; et al. Characterization of H5N1 avian influenza virus isolated from bird in Russia with the E627K mutation in the PB2 protein. Sci. Rep. 2024, 14, 26490. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Avian Influenza (Including Infection with High Pathogenicity Avian Influenza Viruses), in Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2024. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.03.04_AI.pdf. (accessed on 22 August 2025).
- McDuie, F.; Matchett, E.L.; Prosser, D.J.; Takekawa, J.Y.; Pitesky, M.E.; Lorenz, A.A.; McCuen, M.M.; T, O.C.; Ackerman, J.T.; De La Cruz, S.E.W.; et al. Pathways for avian influenza virus spread: GPS reveals wild waterfowl in commercial livestock facilities and connectivity with the natural wetland landscape. Transbound. Emerg. Dis. 2022, 69, 2898–2912. [Google Scholar] [CrossRef]
- Lebarbenchon, C.; Feare, C.J.; Renaud, F.; Thomas, F.; Gauthier-Clerc, M. Persistence of Highly Pathogenic Avian Influenza Viruses in Natural Ecosystems. Emerg. Infect. Dis. 2010, 16, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.J.; Bishop, M.A.; Trovão, N.S.; Ineson, K.M.; Schaefer, A.L.; Puryear, W.B.; Zhou, K.; Foss, A.D.; Clark, D.E.; MacKenzie, K.G.; et al. Ecological divergence of wild birds drives avian influenza spillover and global spread. PLOS Pathog. 2022, 18, e1010062. [Google Scholar] [CrossRef] [PubMed]
- Stanislawek, W.L.; Tana, T.; Rawdon, T.G.; Cork, S.C.; Chen, K.; Fatoyinbo, H.; Cogger, N.; Webby, R.J.; Webster, R.G.; Joyce, M.; et al. Avian influenza viruses in New Zealand wild birds, with an emphasis on subtypes H5 and H7: Their distinctive epidemiology and genomic properties. PLoS ONE 2024, 19, e0303756. [Google Scholar] [CrossRef] [PubMed]
- Stanislawek, W.L.; Wilks, C.R.; Meers, J.; Horner, G.W.; Alexander, D.J.; Manvell, R.J.; Kattenbelt, J.A.; Gould, A.R. Avian paramyxoviruses and influenza viruses isolated from mallard ducks (Anas platyrhynchos) in New Zealand. Arch. Virol. 2002, 147, 1287–1302. [Google Scholar] [CrossRef]
- McCulley, M.; Wilson, A.D.; Jauregui, R.; Gias, E.; Low, Y.S.; Ohneiser, S.; Stanislawek, W.; Bryant, L.; Chernyavtseva, A.; O’KEefe, J. High pathogenicity avian influenza (HPAI) H7N6 virus detected in New Zealand poultry. Genome Announc. 2025, 14, e0008825. [Google Scholar] [CrossRef] [PubMed]
- Duong, B.T.; Bal, J.; Sung, H.W.; Yeo, S.-J.; Park, H. Molecular Analysis of the Avian H7 Influenza Viruses Circulating in South Korea during 2018–2019: Evolutionary Significance and Associated Zoonotic Threats. Viruses 2021, 13, 2260. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Gao, W.; Zu, Z.; Liu, J.; Song, J.; Wang, X.; Wang, C.; Liu, L.; Tong, Q.; Wang, M.; Sun, H.; et al. Prevailing I292V PB2 mutation in avian influenza H9N2 virus increases viral polymerase function and attenuates IFN-β induction in human cells. J. Gen. Virol. 2019, 100, 1273–1281. [Google Scholar] [CrossRef]
- Miotto, O.; Heiny, A.T.; Tan, T.W.; August, J.T.; Brusic, V. Identification of human-to-human transmissibility factors in PB2 proteins of influenza A by large-scale mutual information analysis. BMC Bioinform. 2008, 9, S18. [Google Scholar] [CrossRef]
- Ha, Y.; Stevens, D.J.; Skehel, J.J.; Wiley, D.C. X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc. Natl. Acad. Sci. USA 2001, 98, 11181–11186. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ibrahim, M.S.; Ellakany, H.F.; Kawashita, N.; Mizuike, R.; Hiramatsu, H.; Sriwilaijaroen, N.; Takagi, T.; Suzuki, Y.; Ikuta, K. Acquisition of Human-Type Receptor Binding Specificity by New H5N1 Influenza Virus Sublineages during Their Emergence in Birds in Egypt. PLOS Pathog. 2011, 7, e1002068. [Google Scholar] [CrossRef]
- Luoh, S.M.; McGregor, M.W.; Hinshaw, V.S. Hemagglutinin mutations related to antigenic variation in H1 swine influenza viruses. J. Virol. 1992, 66, 1066–1073. [Google Scholar] [CrossRef]
- Kaverin, N.V.; Rudneva, I.A.; Ilyushina, N.A.; Lipatov, A.S.; Krauss, S.; Webster, R.G. Structural Differences among Hemagglutinins of Influenza A Virus Subtypes Are Reflected in Their Antigenic Architecture: Analysis of H9 Escape Mutants. J. Virol. 2004, 78, 240–249. [Google Scholar] [CrossRef]
- Chutinimitkul, S.; Van Riel, D.; Munster, V.J.; van den Brand, J.M.A.; Rimmelzwaan, G.F.; Kuiken, T.; Osterhaus, A.D.M.E.; Fouchier, R.A.M.; De Wit, E. In Vitro Assessment of Attachment Pattern and Replication Efficiency of H5N1 Influenza A Viruses with Altered Receptor Specificity. J. Virol. 2010, 84, 6825–6833. [Google Scholar] [CrossRef] [PubMed]
- Mänz, B.; Matrosovich, M.; Bovin, N.; Schwemmle, M. A Polymorphism in the Hemagglutinin of the Human Isolate of a Highly Pathogenic H5N1 Influenza Virus Determines Organ Tropism in Mice. J. Virol. 2010, 84, 8316–8321. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, A.; Tuzikov, A.; Pazynina, G.; Bovin, N.; Balish, A.; Klimov, A. Evolution of the receptor binding phenotype of influenza A (H5) viruses. Virology 2006, 344, 432–438. [Google Scholar] [CrossRef]
- Chothe, S.K.; Srinivas, S.; Misra, S.; Nallipogu, N.C.; Gilbride, E.; LaBella, L.; Mukherjee, S.; Gauthier, C.H.; Pecoraro, H.L.; Webb, B.T.; et al. Marked neurotropism and potential adaptation of H5N1 clade 2.3.4.4.b virus in naturally infected domestic cats. Emerg. Microbes Infect. 2024, 14, 2440498. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Seekings, A.H.; Howard, W.A.; Nuñéz, A.; Slomka, M.J.; Banyard, A.C.; Hicks, D.; Ellis, R.J.; Nuñéz-García, J.; Hartgroves, L.C.; Barclay, W.S.; et al. The Emergence of H7N7 Highly Pathogenic Avian Influenza Virus from Low Pathogenicity Avian Influenza Virus Using an In Ovo Embryo Culture Model. Viruses 2020, 12, 920. [Google Scholar] [CrossRef]
- Dietze, K.; Graaf, A.; Homeier-Bachmann, T.; Grund, C.; Forth, L.; Pohlmann, A.; Jeske, C.; Wintermann, M.; Beer, M.; Conraths, F.J.; et al. From low to high pathogenicity-Characterization of H7N7 avian influenza viruses in two epidemiologically linked outbreaks. Transbound. Emerg. Dis. 2018, 65, 1576–1587. [Google Scholar] [CrossRef]
- Ahrens, A.K.; Pohlmann, A.; Grund, C.; Beer, M.; Harder, T.C. Out of the blue: Detection of a unique highly pathogenic avian influenza virus of subtype H7N5 in Germany. Emerg. Microbes Infect. 2024, 13, 2420723. [Google Scholar] [CrossRef]
- Seekings, A.; Slomka, M.; Russell, C.; Howard, W.; Choudhury, B.; Nuñéz, A.; Löndt, B.; Cox, W.; Ceeraz, V.; Thorén, P.; et al. Direct evidence of H7N7 avian influenza virus mutation from low to high virulence on a single poultry premises during an outbreak in free range chickens in the UK. Infect. Genet. Evol. 2018, 64, 13–31. [Google Scholar] [CrossRef]
- Kristensen, C.; Larsen, L.E.; Trebbien, R.; Jensen, H.E. The avian influenza A virus receptor SA-α2,3-Gal is expressed in the porcine nasal mucosa sustaining the pig as a mixing vessel for new influenza viruses. Virus Res. 2024, 340, 199304. [Google Scholar] [CrossRef] [PubMed]
- MacCosham, A.; Vasiliu, A.G.; Atchessi, N. A rapid review of the avian influenza PB2 E627K mutation in human infection studies. Can. Commun. Dis. Rep. 2025, 51, 137–144. [Google Scholar] [CrossRef]
- Rawdon, T.; Tana, T.; Thornton, R.; McKenzie, J.; Stanislawek, W.; Kittelberger, R.; Geale, D.; Stevenson, M.; Gerber, N.; Cork, S. Surveillance for avian influenza virus subtypes H5 and H7 in chickens and turkeys farmed commercially in New Zealand. N. Z. Veter. J. 2010, 58, 292–298. [Google Scholar] [CrossRef]
- El-Zoghby, E.F.; Aly, M.M.; Nasef, S.A.; Hassan, M.K.; Arafa, A.-S.; Selim, A.A.; Kholousy, S.G.; Kilany, W.H.; Safwat, M.; Abdelwhab, E.M.; et al. Surveillance on A/H5N1 virus in domestic poultry and wild birds in Egypt. Virol. J. 2013, 10, 203. [Google Scholar] [CrossRef]
- Vredenberg, I.; van Schaik, G.; Velkers, F.C.; Fabri, T.; Spierenburg, M.A.H.; Germeraad, E.A.; van der Poel, W.H.M.; Stegeman, A. Assessing the Use of Different Surveillance Components to Detect Highly Pathogenic Avian Influenza Outbreaks in Poultry in the Netherlands in Low- and High-Risk Years. Transbound. Emerg. Dis. 2025, 2025, 7441785. [Google Scholar] [CrossRef] [PubMed]
- Waller, S.J.; Wierenga, J.R.; Heremia, L.; Darnley, J.A.; de Vries, I.; Dubrulle, J.; Robinson, Z.; Miller, A.K.; Niebuhr, C.N.; Melville, D.S.; et al. Avian Influenza Virus Surveillance Across New Zealand and Its Subantarctic Islands Detects H1N9 in Migratory Shorebirds, but Not 2.3.4.4b HPAI H5N1. Influ. Other Respir. Viruses 2025, 19, e70099. [Google Scholar] [CrossRef] [PubMed]
- McCulley, M.; Dewar, M.L.; Low, Y.S.; Wilson, A.; Jauregui, R.; Chernyavtseva, A.; O’KEefe, J. High pathogenicity avian influenza (HPAI) H5N1 virus detected in brown Skua using portable laboratory while at sea in Antarctica. Genome Announc. 2025, 14, e0004125. [Google Scholar] [CrossRef]
- Spackman, E.; Senne, D.A.; Bulaga, L.L.; Myers, T.J.; Perdue, M.L.; Garber, L.P.; Lohman, K.; Daum, L.T.; Suarez, D.L. Development of Real-Time RT-PCR for the Detection of Avian Influenza Virus. Avian Dis. 2003, 47, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Hoffmann, D.; Henritzi, D.; Beer, M.; Harder, T.C. Riems influenza a typing array (RITA): An RT-qPCR-based low density array for subtyping avian and mammalian influenza a viruses. Sci. Rep. 2016, 6, 27211. [Google Scholar] [CrossRef]
- Mitchell, P.K.; Cronk, B.D.; Voorhees, I.E.H.; Rothenheber, D.; Anderson, R.R.; Chan, T.H.; Wasik, B.R.; Dubovi, E.J.; Parrish, C.R.; Goodman, L.B. Method comparison of targeted influenza A virus typing and whole-genome sequencing from respiratory specimens of companion animals. J. Veter. Diagn. Investig. 2020, 33, 191–201. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
| Segment | Length (bp) | GenBank ID |
|---|---|---|
| 1—PB2 | 2277 | PX270996 |
| 2—PB1 | 2271 | PX270997 |
| 3—PA | 2209 | PX270998 |
| 4—HA | 1695 | PX270999 |
| 5—NP | 1494 | PX271000 |
| 6—NA | 1446 | PX271001 |
| 7—MP | 1002 | PX271002 |
| 8—NS | 849 | PX271003 |
| Segment | Viruses with Greatest Homology | Pairwise ID (%) | GenBank ID |
|---|---|---|---|
| 1—PB2 | A/Mallard/NZ/W24_2774/2024 (H4N6) | 99.1 | PX270996.1 |
| A/Mallard/NZ/W24_6A_c300/2024 (H10N7) | 97.7 | PX312466.1 | |
| 2—PB1 | A/Mallard/NZ/W24_4A_c39/2024 (H3N8) | 96.7 | PX271014.1 |
| A/Red knot/Firth of Thames/1/2024 (H1N9) | 94.9 | PQ358077.1 | |
| 3—PA | A/Mallard/NZ/W24_2774/2024 (H4N6) | 99.2 | PX270998.1 |
| A/Red knot/Firth of Thames/1/2024 (H1N9) | 98.4 | PQ358078.1 | |
| 4—HA | A/Mallard/NZ/W17_18_201/2017 (H7N7) | 94.7 | PX271007.1 |
| A/Mallard/NZ/15.880.80.121/2015 (H7N7) | 94.1 | MH276960.1 | |
| 5—NP | A/Mallard/NZ/W23_6_308/2023 (H4N6) | 98.9 | PX271025.1 |
| A/Mallard/NZ/1365-350/2005 (H6N9) | 94.5 | CY077587.1 | |
| 6—NA | A/Mallard/NZ/W24_2774/2024 (H4N6) | 98.8 | PX271001.1 |
| A/Mallard/NZ/W23_6_308/2023 (H4N6) | 98.7 | PX271026.1 | |
| 7—MP | A/Red knot/Firth of Thames/1/2024 (H1N9) | 98.8 | PQ358082.1 |
| A/Mallard/NZ/479-8/2005 (H6N2) | 96.0 | CY039368.2 | |
| 8—NS | A/Mallard/NZ/W24_4A_c39/2024 (H3N8) | 98.3 | PX271020.1 |
| A/Mallard/NZ/449-68/2004 (H1N2) | 96.0 | CY077517.1 |
| Sequence | Mutation | Predicted Effects and/or Structural Interactions | Reference |
|---|---|---|---|
| 1|PB2|A/Chicken/NZ/W24_2595/2024(H7N6) | I292V | Host specificity shift | [27] |
| 4|HA|A/Chicken/NZ/W24_2595/2024(H7N6) | L162V | Antigenic drift, escape mutant, host specificity shift, host cell receptor binding, binding small ligand(s), antibody recognition sites | [28,29] |
| N164T | Virulence, antigenic drift, escape mutant, viral oligomerization, antibody recognition sites, binding small ligand(s) | [30] | |
| T165S | Virulence, antigenic drift, escape mutant, viral oligomerization, antibody recognition sites, binding small ligand(s) | [31] | |
| G195A | Virulence, antigenic drift, escape mutant, host specificity shift, host cell receptor binding, binding small ligand(s), viral oligomerization, antibody recognition sites | [28,32] | |
| Q231L | Virulence, viral oligomerization, binding small ligand(s), host cell receptor binding, antibody recognition sites | [33] | |
| S236P | Host specificity shift, viral oligomerization, binding small ligand(s), antibody recognition sites, host cell receptor binding | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, A.; Jauregui, R.; Gias, E.; Low, Y.S.; Little, A.; Johnston, H.; Stanislawek, W.; Chernyavtseva, A.; McCulley, M. Phylogenetic and Molecular Characterization of a Novel Reassortant High-Pathogenicity Avian Influenza A (H7N6) Virus Detected in New Zealand Poultry. Int. J. Mol. Sci. 2025, 26, 10623. https://doi.org/10.3390/ijms262110623
Wilson A, Jauregui R, Gias E, Low YS, Little A, Johnston H, Stanislawek W, Chernyavtseva A, McCulley M. Phylogenetic and Molecular Characterization of a Novel Reassortant High-Pathogenicity Avian Influenza A (H7N6) Virus Detected in New Zealand Poultry. International Journal of Molecular Sciences. 2025; 26(21):10623. https://doi.org/10.3390/ijms262110623
Chicago/Turabian StyleWilson, Andrew, Ruy Jauregui, Edna Gias, Yee Syuen Low, Alvey Little, Helen Johnston, Wlodek Stanislawek, Anastasia Chernyavtseva, and Michelle McCulley. 2025. "Phylogenetic and Molecular Characterization of a Novel Reassortant High-Pathogenicity Avian Influenza A (H7N6) Virus Detected in New Zealand Poultry" International Journal of Molecular Sciences 26, no. 21: 10623. https://doi.org/10.3390/ijms262110623
APA StyleWilson, A., Jauregui, R., Gias, E., Low, Y. S., Little, A., Johnston, H., Stanislawek, W., Chernyavtseva, A., & McCulley, M. (2025). Phylogenetic and Molecular Characterization of a Novel Reassortant High-Pathogenicity Avian Influenza A (H7N6) Virus Detected in New Zealand Poultry. International Journal of Molecular Sciences, 26(21), 10623. https://doi.org/10.3390/ijms262110623





