Combinative Treatment of the PARP Inhibitor Olaparib and Antimetastasis Ruthenium(II)–Arene Compound RAPTA-T for Triple-Negative BRCA1 Wild-Type Breast Cancer Cells
Abstract
1. Introduction
2. Results
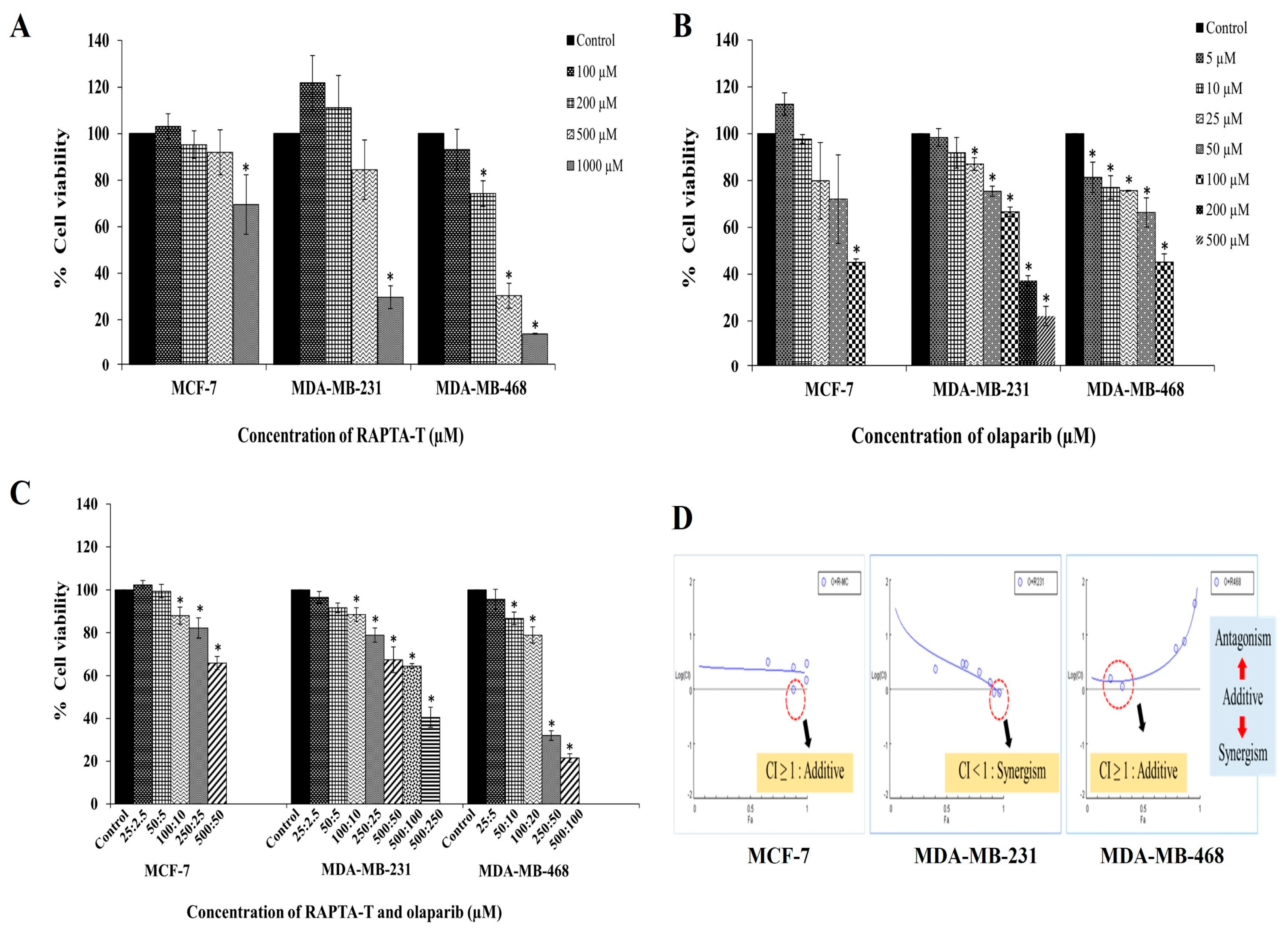
2.1. Cytotoxic Effects of RAPTA-T or Olaparib as a Single Agent and Combination Compound on Human Breast Cancer Cells
2.2. Combination Index (CI)
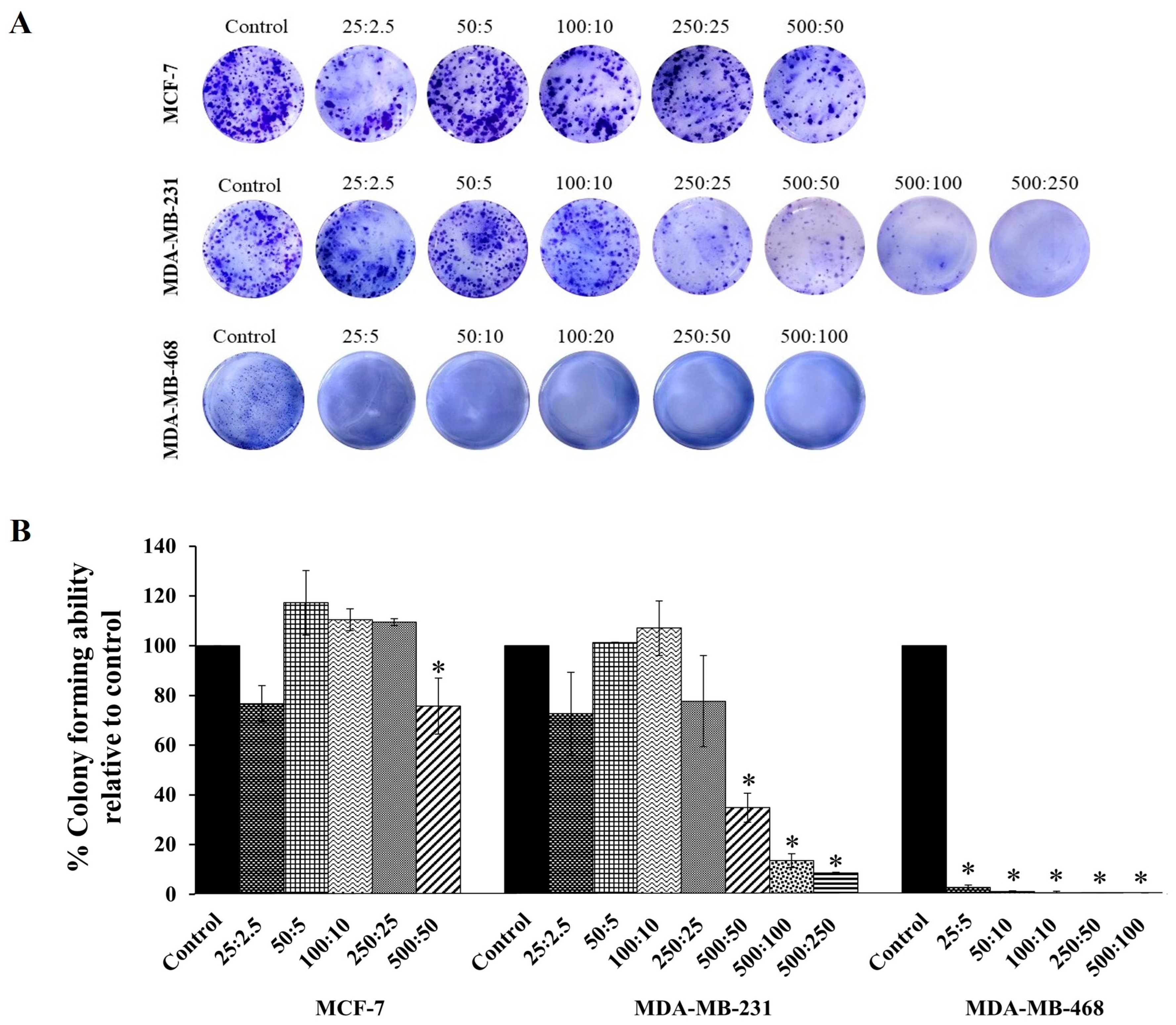
2.3. Clonogenic Assay
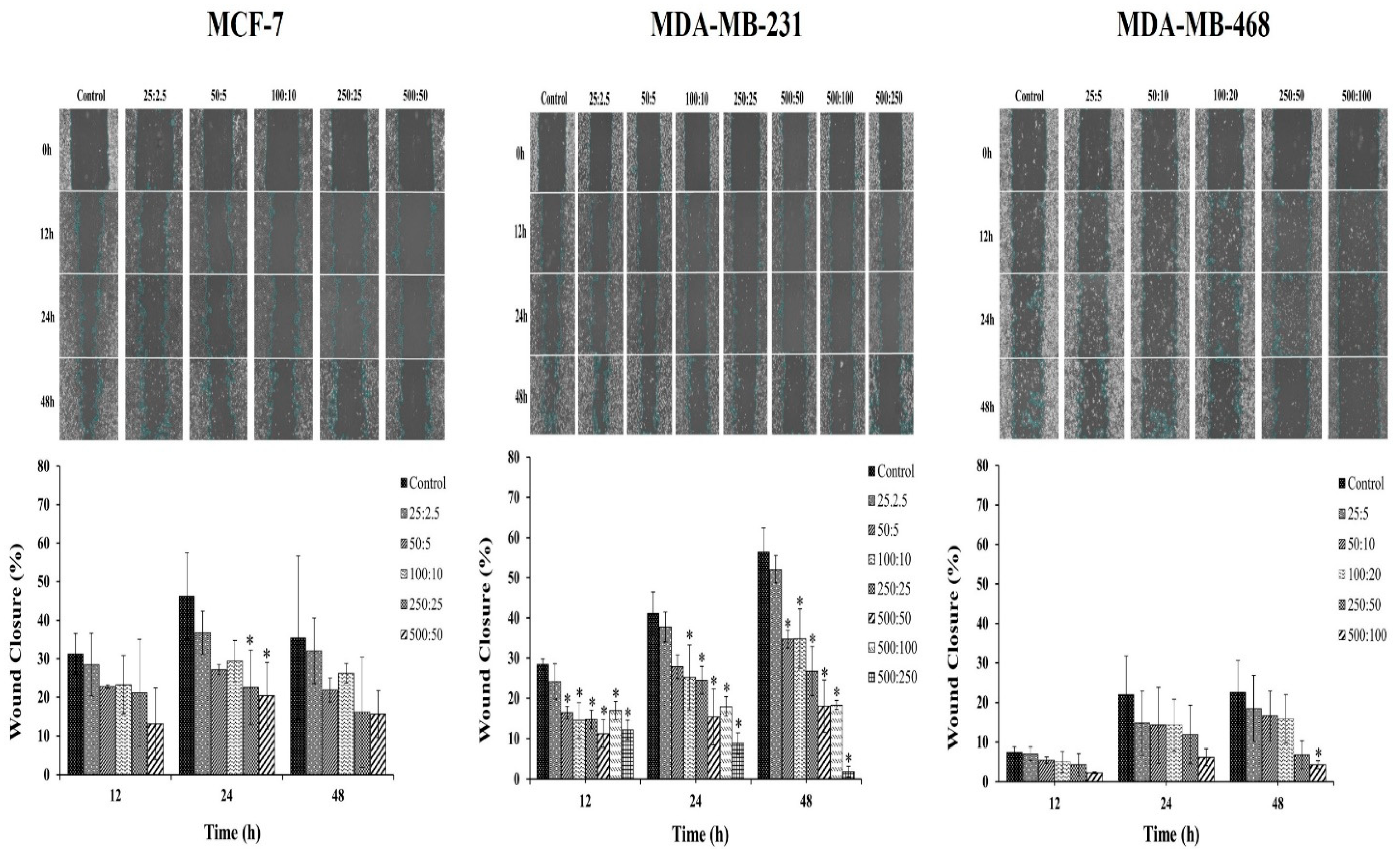
2.4. Scratch-Wound Assay
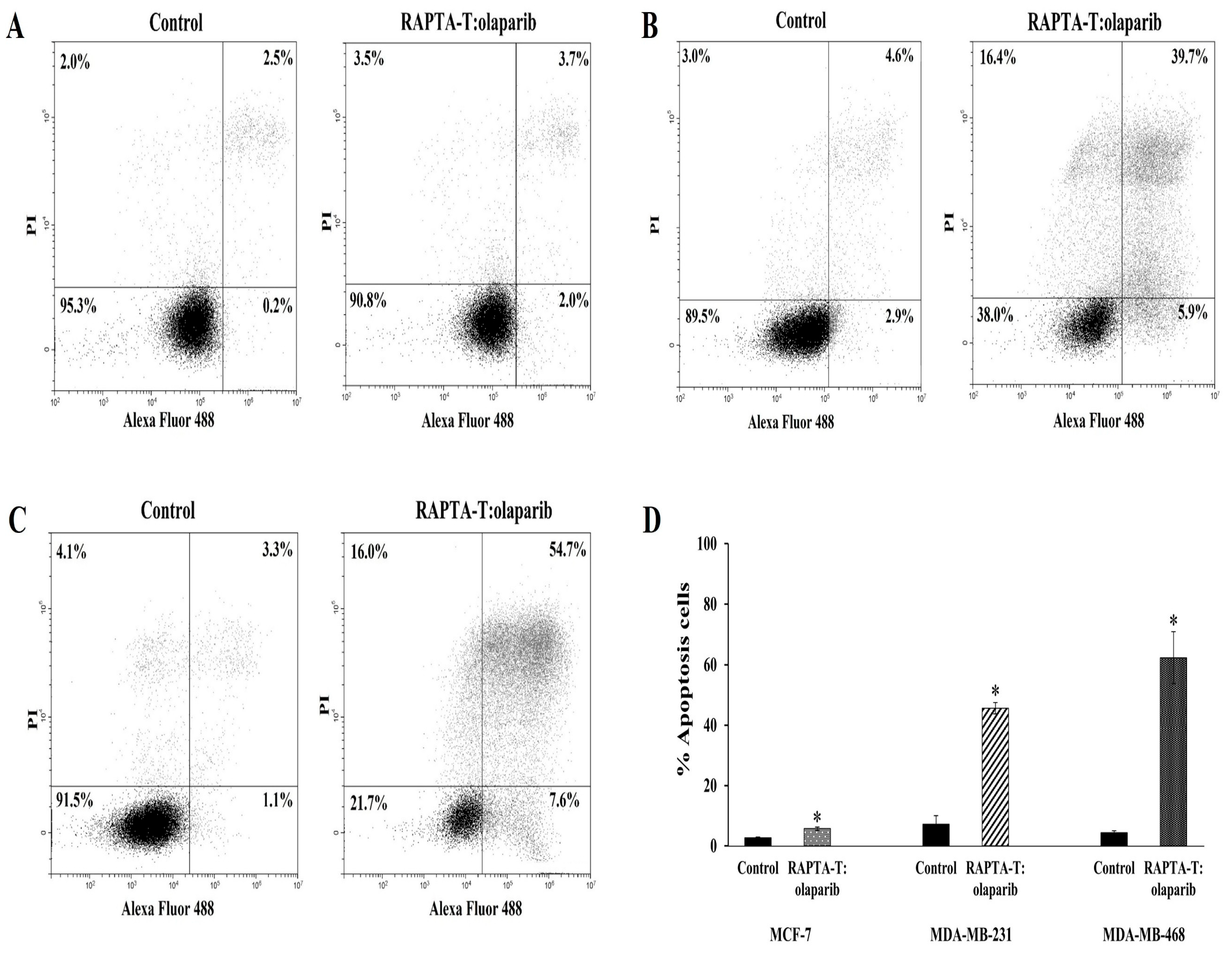
2.5. Apoptosis Annexin V/FITC Assay
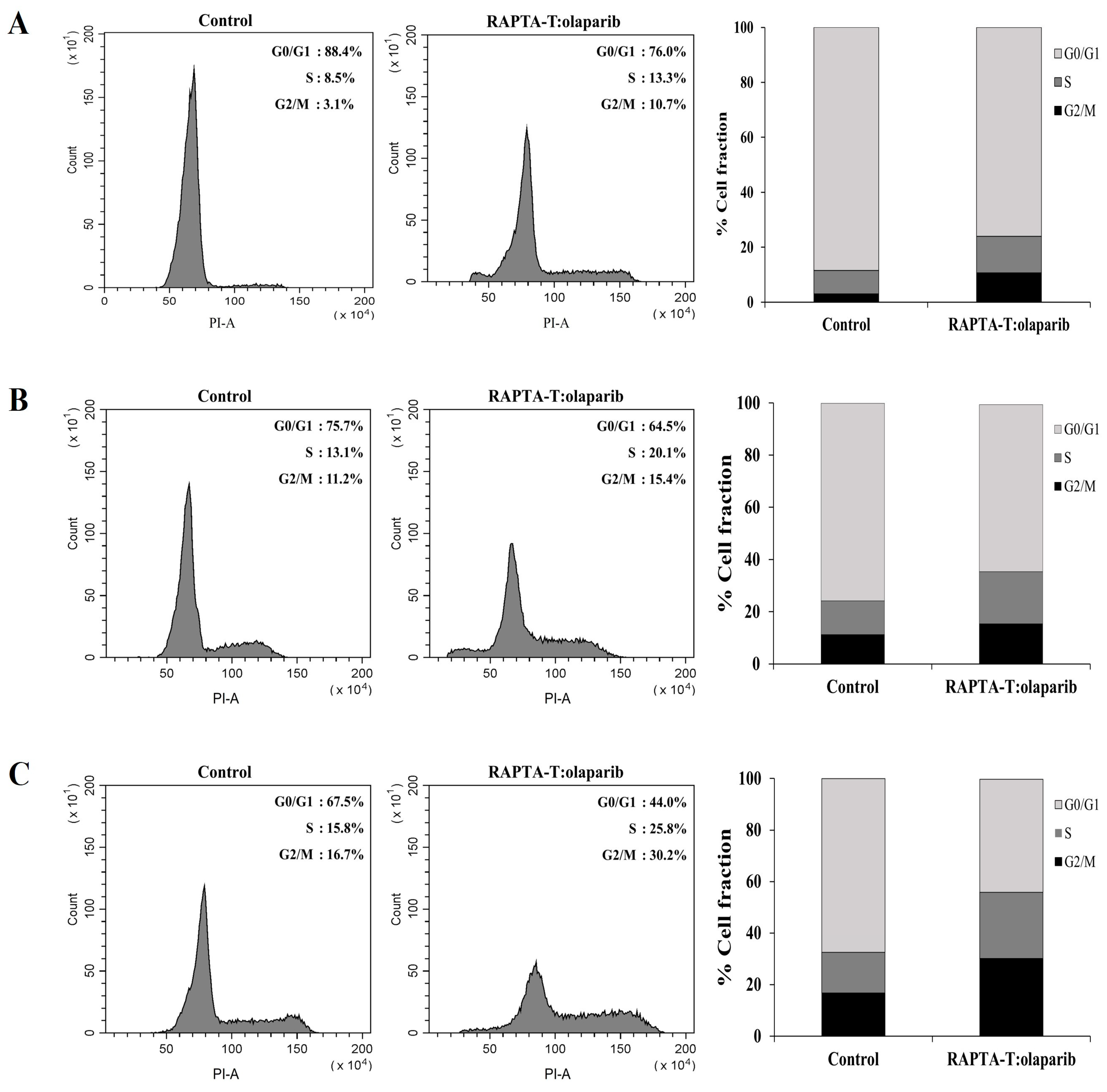
2.6. Cell Cycle Analysis
2.7. Immunofluorescence Assay
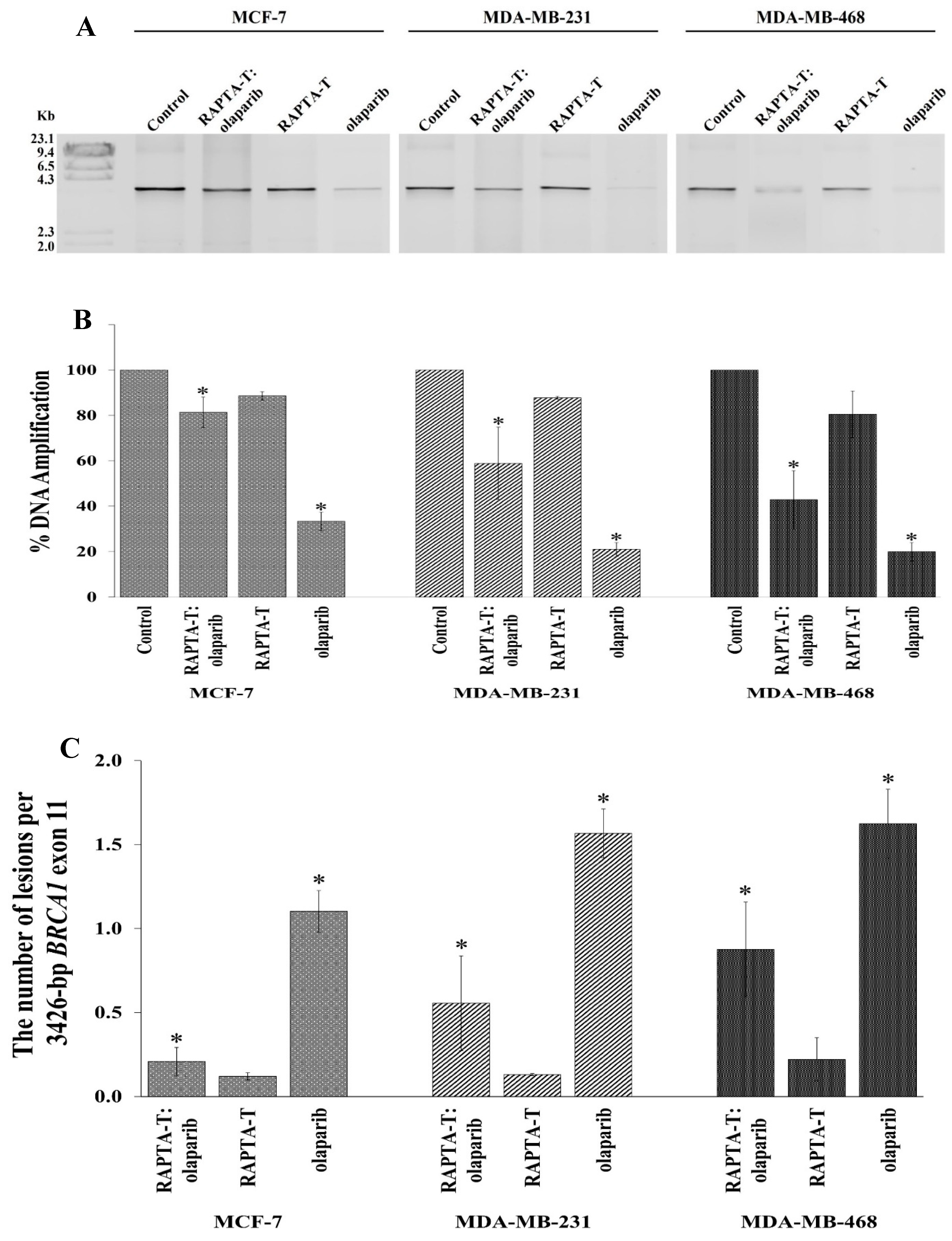
2.8. Quantitative PCR (QPCR) for Cellular BRCA1 Damage
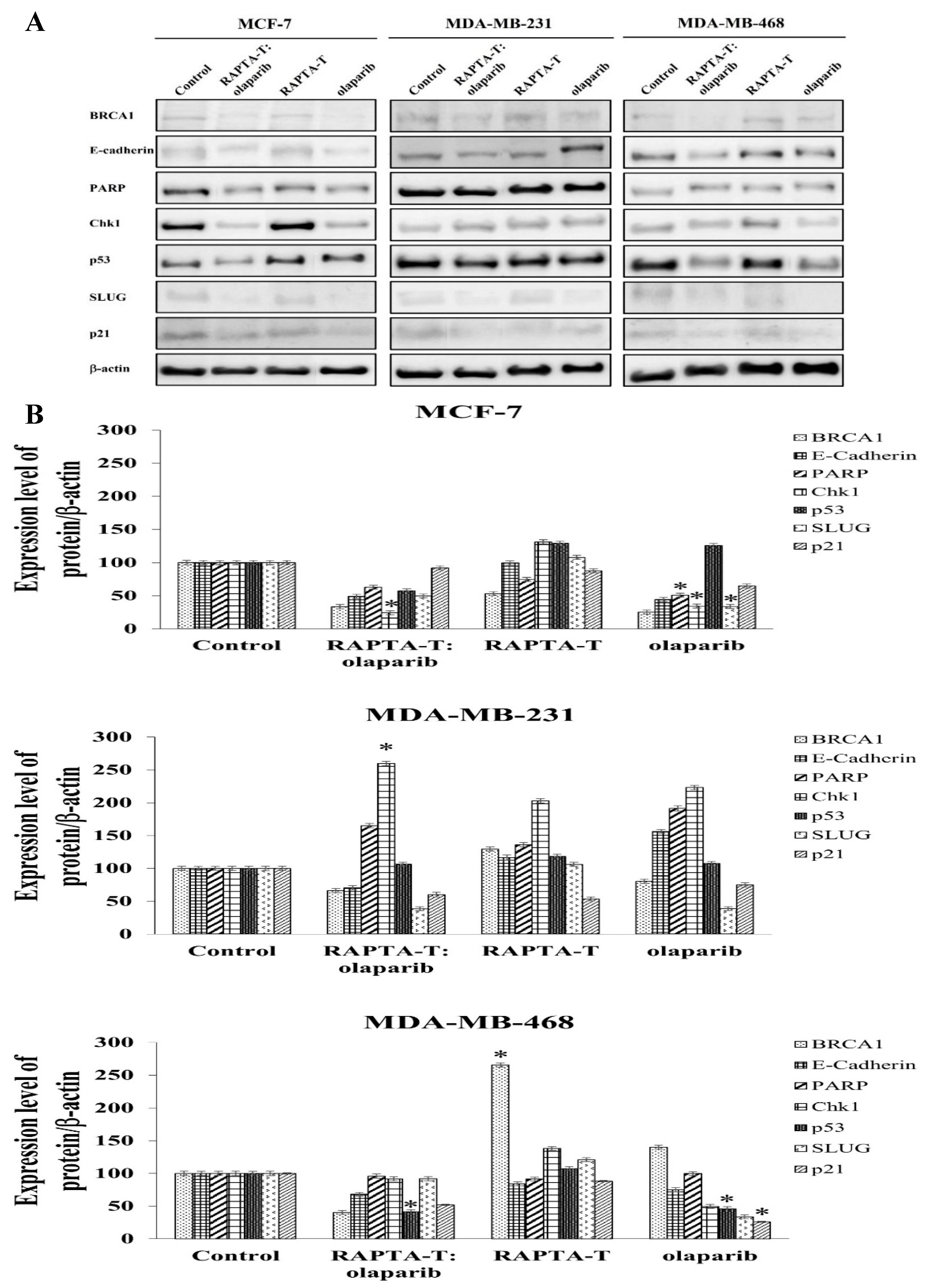
2.9. Protein Extraction and Western Blotting Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Lines
4.3. MTT Proliferation Assay
4.4. Drug Interaction Analysis
4.5. Clonogenic Survival Assay
4.6. Scratch-Wound Assay
4.7. Apoptosis Annexin V/FITC Assay
4.8. Cell Cycle Analysis
4.9. Immunofluorescence Assay
4.10. Quantitative PCR (QPCR) for Cellular BRCA1 Damage
4.11. Protein Extraction and Western Blotting Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijin, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumors. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijin, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Sorlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef]
- Hu, Z.; Fan, C.; Oh, D.S.; Marron, J.S.; He, X.; Qaqish, B.F.; Livasy, C.; Carey, L.A.; Reynolds, E.; Dressler, L.; et al. The molecular portraits of breast tumors are conserved across microarray platform. BMC Genom. 2006, 7, 96. [Google Scholar] [CrossRef]
- Sorlie, T.; Wang, Y.; Xiao, C.; Johnsen, H.; Naume, B.; Samaha, R.R.; Borresen-Dale, A.L. Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: Gene expression analyses across three different platforms. BMC Genom. 2006, 7, 127. [Google Scholar] [CrossRef]
- Fan, C.; Oh, D.S.; Wessels, L.; Weigelt, B.; Nuyten, D.S.; Nobel, A.B.; van’t Veer, L.J.; Perou, C.M. Concordance among gene-expression-based predictors for breast cancer. N. Eng. J. Med. 2006, 355, 560–569. [Google Scholar] [CrossRef]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California Cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef]
- Cleator, S.; Heller, W.; Coombes, R.C. Triple-negative breast cancer: Therapeutic options. Lancet Oncol. 2007, 8, 235–244. [Google Scholar] [CrossRef]
- Cleere, D.W. Triple-negative breast cancer: A clinical update. Commun. Oncol. 2010, 7, 203–211. [Google Scholar] [CrossRef]
- Irvin, W.J., Jr.; Carey, L.A. What is triple-negative breast cancer? Eur. J. Cancer 2008, 44, 2799–2805. [Google Scholar] [CrossRef]
- Isakoff, S.J. Triple-negative breast cancer: Role of specific chemotherapy agents. Cancer J. 2010, 16, 53–61. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Hugh, J.; Hanson, J.; Cheang, M.C.U.; Nielsen, T.O.; Perou, C.M.; Dumontet, C.; Reed, J.; Krajewska, M.; Treilleux, I.; Rupin, M.; et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: Use of immunohistochemical definition in the BCIRG 001 trial. J. Clin. Oncol. 2009, 27, 1168–1176. [Google Scholar] [CrossRef]
- Ellis, P.; Barrett-Lee, P.; Johnson, L.; Cameron, D.; Wardley, A.; O’ Reilly, S.; Verrli, M.; Smith, I.; Yarnold, J.; Coleman, R.; et al. The TACT trial management group and the TACT trialists. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): An open-label, phase III, randomized controlled trial. Lancet 2009, 373, 1681–1692. [Google Scholar] [CrossRef]
- Hayes, D.; Thor, A.D.; Dressler, L.G.; Weaver, D.; Ederton, S.; Cowan, D.; Broadwater, G.; Goldstein, L.J.; Martino, S.; Ingle, J.; et al. The cancer and leukemia group B (CALGB) investigator. HER2 and response to paclitaxel in node-positive breast cancer. N. Engl. J. Med. 2007, 357, 1496–1506. [Google Scholar] [CrossRef]
- Sikov, W.M.; Dizon, D.S.; Strenger, R.; Legare, R.D.; Theall, K.P.; Graves, T.A.; Gass, J.S. Frequent pathologic complete responses in aggressive stage II to III breast cancers with every-4-week-carboplatin and weekly paclitaxel with or without trastuzumab: A Brown University Oncology Group study. J. Clin. Oncol. 2008, 927, 4693–4700. [Google Scholar] [CrossRef]
- Foulkes, W.D. Traffic control for BRCA1. N. Engl. J. Med. 2010, 326, 755–756. [Google Scholar] [CrossRef]
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.O.; Calogrias, D.; Buraimoh, A.; et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J. Clin. Oncol. 2010, 28, 1145–1153. [Google Scholar] [CrossRef]
- Koshy, N.; Quispe, D.; Shi, R.; Mansour, R.; Burton, G.V. Cisplatin-gemcitabine therapy in metastatic breast cancer: Improved outcome in triple-negative breast cancer patients compared to non-triple negative patients. Breast 2010, 19, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.O.; Vidnovic, N.; DeYoung, M.P.; Sgroi, D.; Ellisen, L.W. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J. Clin. Investig. 2007, 117, 1370–1380. [Google Scholar] [CrossRef]
- Wong, S.W.; Tiong, K.H.; Kong, W.Y.; Yue, Y.C.; Chua, C.H.; Lim, J.Y.; Lee, C.Y.; Quah, S.I.; Fow, C.; Chung, C.; et al. Rapamycin synergizes cisplatin sensitivity in basal-like breast cancer through up-regulation of p73. Breast Cancer Res. Treat. 2011, 128, 301–313. [Google Scholar] [CrossRef]
- Gong, J.G.; Costanzo, A.; Yang, H.-Q.; Melinos, G.; Kaelin, W.G.; Levrero, M.; Wang, J.Y.J. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 1999, 399, 806–809. [Google Scholar] [CrossRef]
- Hastak, K.; Elizabeth, A.; Ford, J.M. Synergistic chemosensitivity of triple-negative breast cancer cell line to poly (ADP-ribose) polymerase inhibition, gemcitabine, and cisplatin. Cancer Res. 2010, 70, 7970–7980. [Google Scholar] [CrossRef]
- Santana-Davila, R.; Perez, E.A. Treatment options for patients with triple-negative breast cancer. J. Hematol. Oncol. 2010, 3, 42. [Google Scholar] [CrossRef]
- Byrski, T.; Huzarski, T.; Dent, R.; Gronwald, J.; Zuziak, D.; Cybulski, C.; Kladny, J.; Gorski, B.; Lubinski, J.; Narod, S.A. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res. Treat. 2009, 115, 359–363. [Google Scholar] [CrossRef]
- Caray, L.; Winer, E.; Viale, G.; Cameron, D.; Gianni, L. Triple-negative breast cancer: Disease entity or title of convenience? Nat. Rev. Clin. Oncol. 2010, 7, 683–692. [Google Scholar] [CrossRef]
- Frasci, G.; Comella, P.; Rinaldo, M.; Iodice, G.; Di Bonito, M.; D’Aiuto, M.; Lastoria, S.; Sini, C.; Comella, G.; D’Aiuto, G. Preoperative weekly cisplatin-epirubicin-paclitaxel with G-CSF support in triple-negative large operable breast cancer. Ann. Oncol. 2009, 20, 1185–1192. [Google Scholar] [CrossRef]
- Ciarimboli, G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 2014, 34, 547–550. [Google Scholar] [CrossRef]
- Dorcier, A.; Dyson, P.J.; Gossens, C.; Rothlisberger, U.; Scopelliti, R.; Tavernelli, I. Binding of organometallic ruthenium(II) and osmium(II) complexes to an oligonucleotide: A combined mass spectrometric and theoretical study. Organometallics 2015, 24, 2114–2123. [Google Scholar] [CrossRef]
- Scolaro, C.; Bergamo, A.; Brescacin, L.; Delfino, R.; Cocchietto, M.; Laurenczy, G.; Geldbach, T.J.; Sava, G.; Dyson, P.J. In vitro and in vivo evaluation of ruthenium(II)-arene PTA complexes. J. Med. Chem. 2005, 48, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Süss-Fink, G. Arene ruthenium complexes as anticancer agents. Dalton Trans. 2010, 39, 1673–1688. [Google Scholar] [CrossRef]
- Battistin, F.; Scaletti, F.; Balducci, G.; Pillozzi, S.; Arcangeli, A.; Messori, L.; Alessio, E. Water-soluble Ru(II)- and Ru(III)-halide-PTA complexes (PTA=1,3,5-triaza-7-phosphaadamantane): Chemical and biological properties. J. Inorg. Biochem. 2016, 160, 180–188. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kundu, S.; Bhattacharyya, A.; Hartinger, C.G.; Dyson, P.J. The ruthenium(II)-arene compound RAPTA-C induces apoptosis in ECA cells through mitochondrial and p53-JNK pathway. J. Biol. Inorg. Chem. 2018, 13, 149–1155. [Google Scholar]
- Temboot, P.; Lee, R.F.S.; Menin, L.; Patiny, L.; Dyson, P.J.; Ratanaphan, A. Biochemical and biophysical characterization of ruthenation of BRCA1 RING protein by RAPTA complexes and its E3 ubiquitin ligase activity. Biochem. Biophys. Res. Commun. 2017, 488, 355–361. [Google Scholar] [CrossRef]
- Groessl, M.; Tsybin, Y.O.; Hartinger, C.G.; Keppler, B.K.; Dyson, P.J. Ruthenium versus platinum: Interactions of anticancer metallodrugs with duplex oligonucleotides characterised by electrospray ionisation mass spectrometry. J. Biol. Inorg. Chem. 2010, 15, 677–688. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; van Beijnum, J.R.; Casini, A.; Nazarov, A.A.; Wagnieres, G.; van den Bergh, H.; Dyson, P.J.; Griffioen, A.W. Organometallic ruthenium(II) arene compounds with antiangiogenic activity. J. Med. Chem. 2011, 54, 3895–3902. [Google Scholar] [CrossRef]
- Nhukeaw, T.; Hongthong, K.; Dyson, P.J.; Ratanaphan, A. Cellular responses of BRCA1 defective HCC1937 breast cancer cell induced by the antimetastasis ruthenium(II) arene compound RAPTA T. Apoptosis 2019, 24, 612–622. [Google Scholar] [CrossRef]
- Yang, Y.-G.; Cortes, U.; Patnaik, S.; Jasin, M.; Wang, Z.-Q. Ablation of PARP-1 does not interfere with the repair of DNA double-strand breaks, but compromises the reactivation of stalled replication forks. Oncogene 2004, 23, 3872–3882. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Mateo, J.; Lord, C.J.; Serra, V.; Tutt, A.; Balmaña, J.; Castroviejo-Bermejo, M.; Cruz, C.; Oaknin, A.; Kaye, S.B.; de Bono, J.S. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019, 30, 1437–1447. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017, 377, 523–533, Erratum in N. Engl. J. Med. 2017, 377, 1700. [Google Scholar] [CrossRef]
- Yap, T.A.; Plummer, R.; Azad, N.S.; Helleday, T. The DNA damaging revolution: PARP inhibitors and beyond. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 185–195. [Google Scholar] [CrossRef]
- Narod, S.A. BRCA mutations in the management of breast cancer: The state of the art. Nat. Rev. Clin. Oncol. 2010, 7, 702–707. [Google Scholar] [CrossRef]
- Pilie, P.; Gay, C.M.; Byers, L.A.; O’Connor, M.J.; Yap, T.A. PARP inhibitors: Extending benefit beyond BRCA mutant cancers. Clin. Cancer Res. 2019, 25, 3759–3771. [Google Scholar] [CrossRef]
- Evers, B.; Drost, R.; Schut, E.; de Bruin, M.; van der Burg, E.; Derksen, P.W.B.; Holstege, H.; Liu, X.; van Drunen, E.; Beverloo, H.B.; et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin. Cancer Res. 2008, 14, 3916–3925. [Google Scholar] [CrossRef]
- Shen, Y.T.; Evans, J.C.; Zafarana, G.; Allen, C.; Piquette-Miller, M. BRCA status does not predict synergism of a carboplatin and olaparib combination in high-grade serous ovarian cancer cell lines. Mol. Pharm. 2018, 15, 2742–2753. [Google Scholar] [CrossRef]
- Eetezadi, S.; Evans, J.C.; Shen, Y.-T.; De Souza, R.; Piquette-Miller, M.; Allen, C. Ratio-dependent synergism of a doxorubicin and olaparib combination in 2D and spheroid models of ovarian cancer. Mol. Pharm. 2018, 15, 472–485. [Google Scholar] [CrossRef]
- Oza, A.M.; Cibula, D.; Benzaquen, A.O.; Poole, C.; Mathijssen, R.H.J.; Sonke, G.S.; Colombo, N.; Špacek, J.; Vuylsteke, P.; Hirte, H.; et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: A randomised phase 2 trial. Lancet Oncol. 2015, 16, 87–97, Correction in Lancet Oncol. 2015, 16, e55. [Google Scholar] [CrossRef]
- Balmaña, J.; Tung, N.M.; Isakoff, S.J.; Graña, B.; Ryan, P.D.; Saura, C.; Lowe, E.S.; Frewer, P.; Winer, E.; Baselga, J.; et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann. Oncol. 2014, 25, 1656–1663. [Google Scholar] [CrossRef]
- Yusoh, N.A.; Leong, S.W.; Chia, S.L.; Harun, S.N.; Abdul Rahman, M.B.; Vallis, K.A.; Gill, M.R.; Ahmad, H. Metallointercalator [Ru(dppz)2(PIP)]2+ Renders BRCA Wild-Type Triple-Negative Breast Cancer Cells Hypersensitive to PARP Inhibition. ACS Chem. Biol. 2020, 15, 378–387. [Google Scholar] [CrossRef]
- Hongthong, K.; Nhukeaw, T.; Temboot, P.; Dyson, P.J.; Ratanaphan, A. Anticancer activity of RAPTA-EA1 in triple-negative BRCA1 proficient breast cancer cells: Single and combined treatment with the PARP inhibitor olaparib. Heliyon 2021, 7, e07749. [Google Scholar] [CrossRef]
- Ashworth, A.A. Synthetic lethal therapeutic approach: Poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Bailey, C.K.; Mittal, M.K.; Misra, S.; Chaudhuri, G. High motility of triple-negative breast cancer cells is due to repression of plakoglobin gene by metastasis modulator protein SLUG. J. Biol. Chem. 2012, 287, 19472–19486. [Google Scholar] [CrossRef]
- Bergamo, A.; Masi, A.; Dyson, P.J.; Sava, G. Modulation of the metastatic progression of breast cancer with an organometallic ruthenium compound. Int. J. Oncol. 2008, 33, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.A.P.; Romano-deGea, J.; Barbosa, A.I.; Lima, S.A.C.; Dyson, P.J.; Saraiva, M.L.M.F.S. Fine-tuning the cytotoxicity of ruthenium(II) arene compounds to enhance selectivity against breast cancers. Dalton Trans. 2023, 52, 11679–11690. [Google Scholar] [CrossRef]
- Adhireksan, Z.; Davey, G.E.; Campomanes, P.; Groessl, M.; Clavel, C.M.; Yu, H.; Nazarov, A.A.; Yeo, C.H.F.; Ang, W.H.; Dröge, P.; et al. Ligand substitutions between ruthenium-cymene compounds can control protein versus DNA targeting and anticancer activity. Nat. Commun. 2014, 5, 3462–3475. [Google Scholar] [CrossRef]
- Babak, M.V.; Meier, S.M.; Huber, K.V.M.; Reynisson, J.; Legin, A.A.; Jakupec, M.A.; Roller, A.; Stukalov, A.; Gridling, M.; Bennett, K.L.; et al. Target profling of an antimetastatic RAPTA agent by chemical proteomics: Relevance to the mode of action. Chem. Sci. 2015, 6, 2449–2456. [Google Scholar] [CrossRef]
- Chakree, K.; Ovatlarnporn, C.; Dyson, P.J.; Ratanaphan, A. Altered DNA binding and amplifcation of human breast cancer suppressor gene BRCA1 induced by a novel antitumor compound, [Ru(η6-p-phenylethacrynate)Cl2(pta)]. Int. J. Mol. Sci. 2012, 13, 13183–13202. [Google Scholar] [CrossRef]
- Ratanaphan, A.; Temboot, P.; Dyson, P.J. In vitro ruthenation of human breast cancer suppress or gene 1 (BRCA1) by the antimetastasis compound RAPTA-C and its analogue CarboRAPTA-C. Chem. Biodivers. 2010, 7, 1290–1302. [Google Scholar] [CrossRef]
- Groessl, M.; Terenghi, M.; Casini, A.; Elviri, L.; Lobinski, R.; Dyson, P.J. Reactivity of anticancer metallodrugs with serum proteins: New insights from size exclusion chromatography-ICP-MS and ESI-MS. J. Anal. At. Spectrom. 2010, 25, 305–313. [Google Scholar] [CrossRef]
- Wolters, D.A.; Stefanopoulou, M.; Dyson, P.J.; Groessl, M. Combination of metallomics and proteomics to study the effects of the metallodrug RAPTA-T on human cancer cells. Metallomics 2012, 4, 1185–1196. [Google Scholar] [CrossRef]
- Tang, J.; Ahmad, A.; Sarkar, F.H. The role of microRNAs in breast cancer migration, invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 13414–13437. [Google Scholar] [CrossRef]
- Ratanaphan, A. Therapeutic potential of metal-based and PARP inhibitors chemotherapy for BRCA1-associated triple-negative breast cancer. Int. J. Mol. Sci. 2025, 26, 9881. [Google Scholar] [CrossRef]


| Compound | IC50 (µM) | ||
|---|---|---|---|
| MCF-7 | MDA-MB-231 | MDA-MB-468 | |
| RAPTA-T | >1000 | 819.0 ± 6.9 | 484.9 ± 50.3 |
| olaparib | 82.7 ± 8.2 | 160.0 ± 26.5 | 86.5 ± 9.0 |
| RAPTA-T:olaparib (Final concentration) | 500:42 | 410:80 | 242:43 |
| Ratio (RAPTA-T:olaparib) | 12:1 | 5:1 | 6:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratanaphan, A. Combinative Treatment of the PARP Inhibitor Olaparib and Antimetastasis Ruthenium(II)–Arene Compound RAPTA-T for Triple-Negative BRCA1 Wild-Type Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 10613. https://doi.org/10.3390/ijms262110613
Ratanaphan A. Combinative Treatment of the PARP Inhibitor Olaparib and Antimetastasis Ruthenium(II)–Arene Compound RAPTA-T for Triple-Negative BRCA1 Wild-Type Breast Cancer Cells. International Journal of Molecular Sciences. 2025; 26(21):10613. https://doi.org/10.3390/ijms262110613
Chicago/Turabian StyleRatanaphan, Adisorn. 2025. "Combinative Treatment of the PARP Inhibitor Olaparib and Antimetastasis Ruthenium(II)–Arene Compound RAPTA-T for Triple-Negative BRCA1 Wild-Type Breast Cancer Cells" International Journal of Molecular Sciences 26, no. 21: 10613. https://doi.org/10.3390/ijms262110613
APA StyleRatanaphan, A. (2025). Combinative Treatment of the PARP Inhibitor Olaparib and Antimetastasis Ruthenium(II)–Arene Compound RAPTA-T for Triple-Negative BRCA1 Wild-Type Breast Cancer Cells. International Journal of Molecular Sciences, 26(21), 10613. https://doi.org/10.3390/ijms262110613


_Kim.png)




