Comparative Preclinical Evaluation of the Tumor-Targeting Properties of Radioiodine and Technetium-Labeled Designed Ankyrin Repeat Proteins for Imaging of Epidermal Growth Factor Receptor Expression in Malignant Tumors
Abstract
1. Introduction
2. Results
2.1. Protein Production and Characterization
2.2. Radiolabeling
2.3. In Vitro Studies
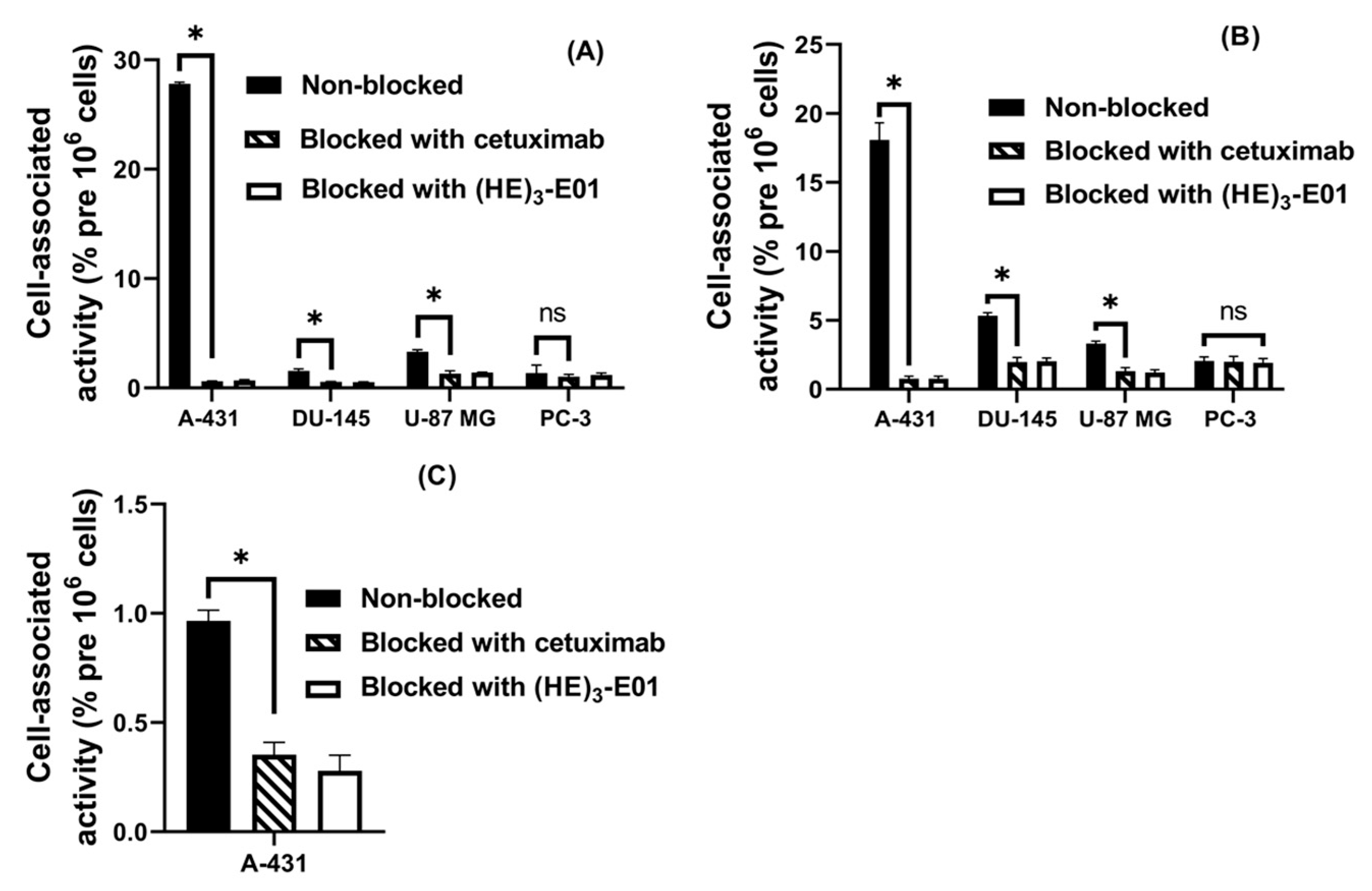
2.3.1. Specificity Test
2.3.2. Internalization
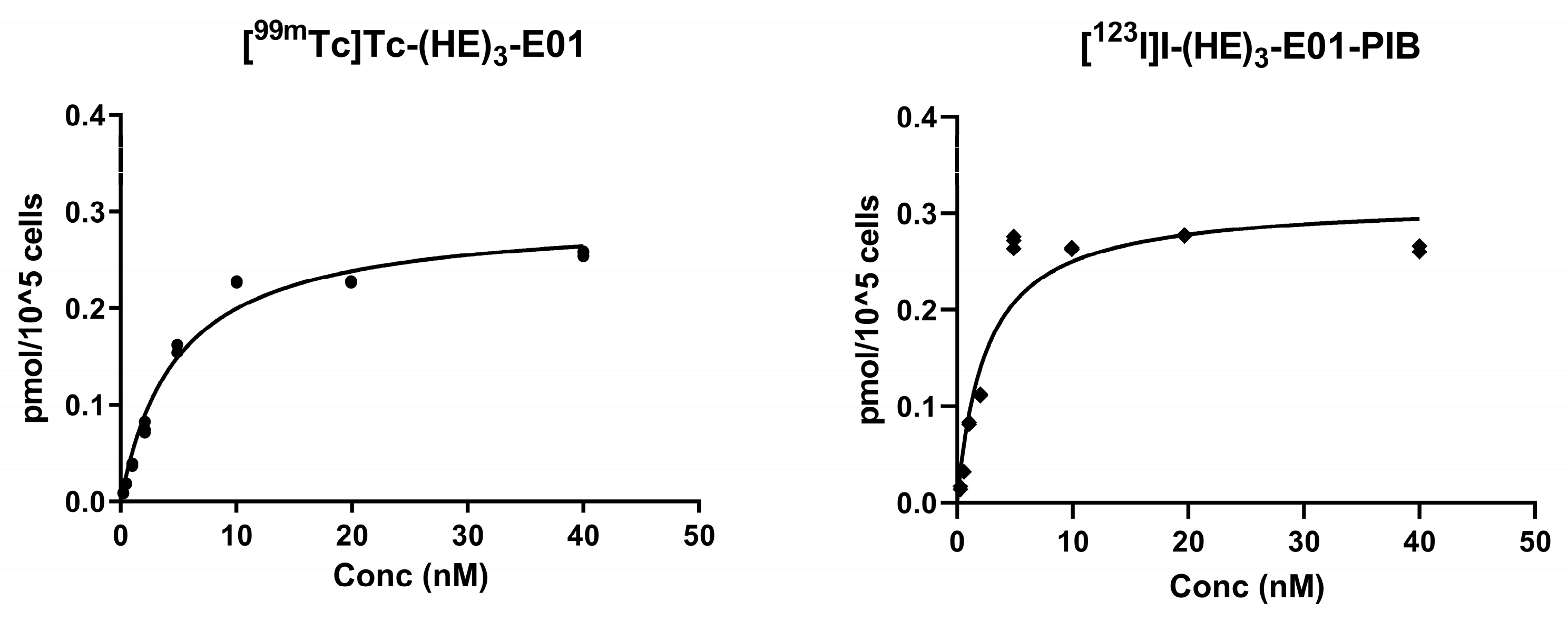
2.3.3. Saturation Assay
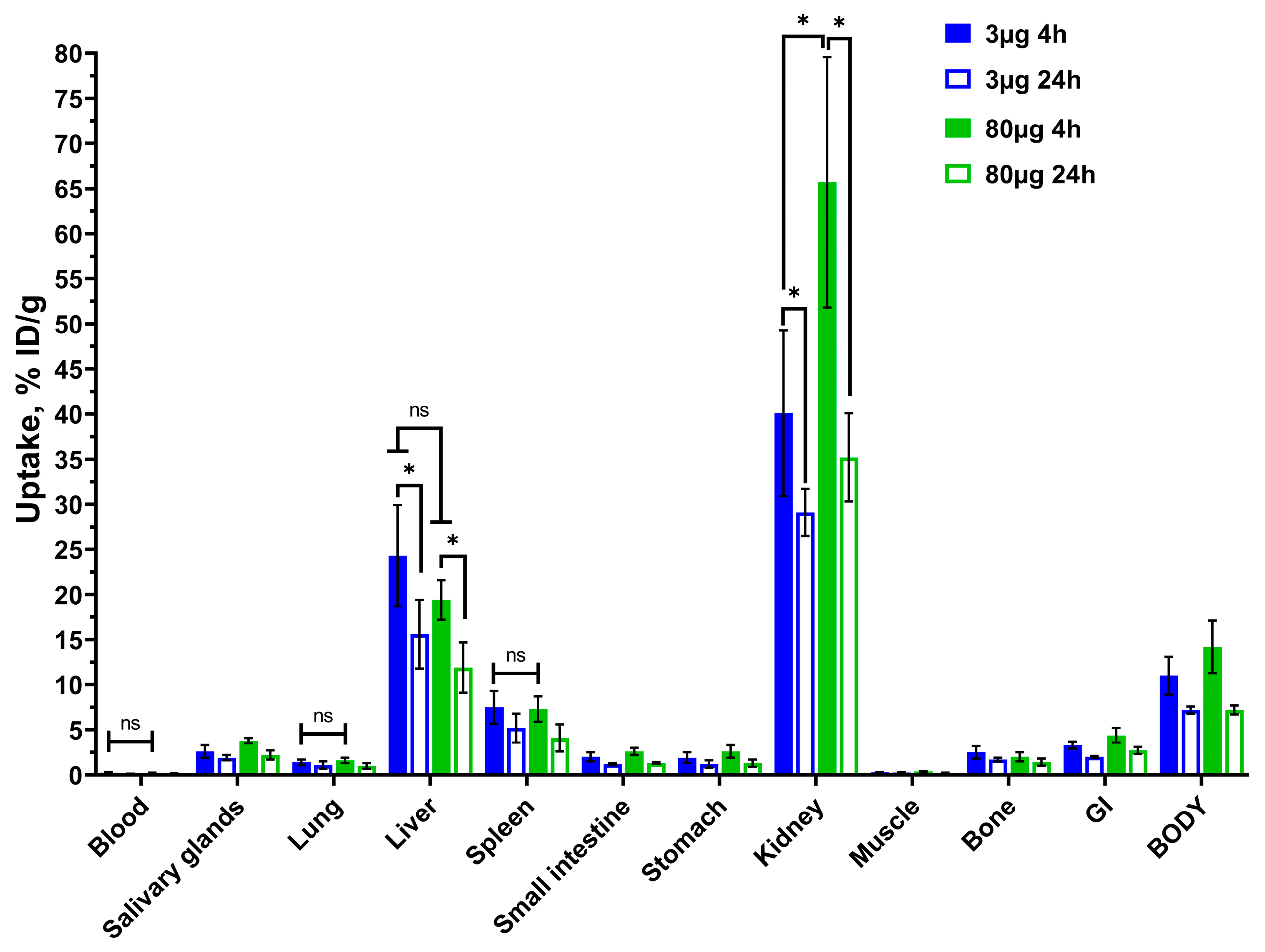
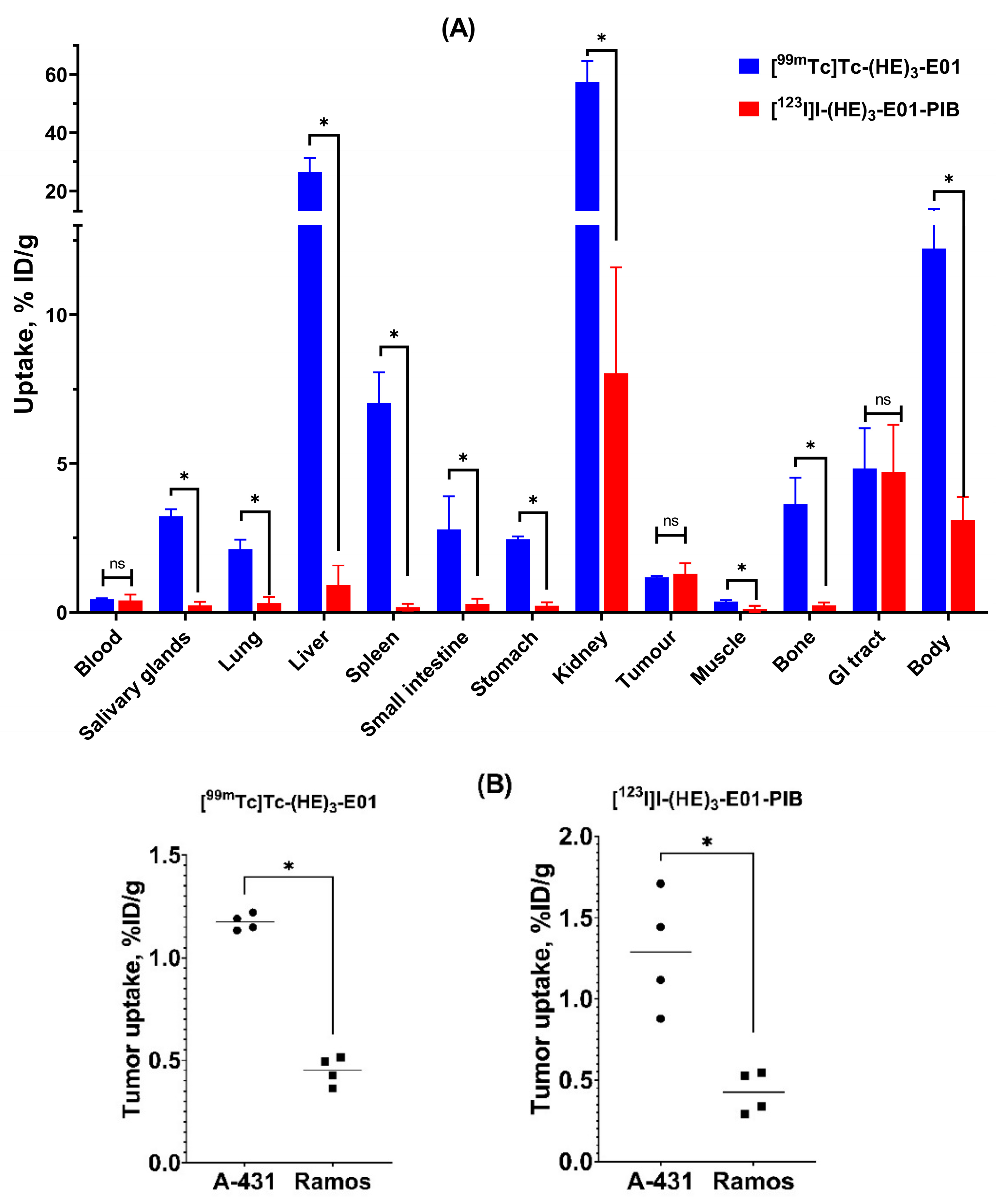
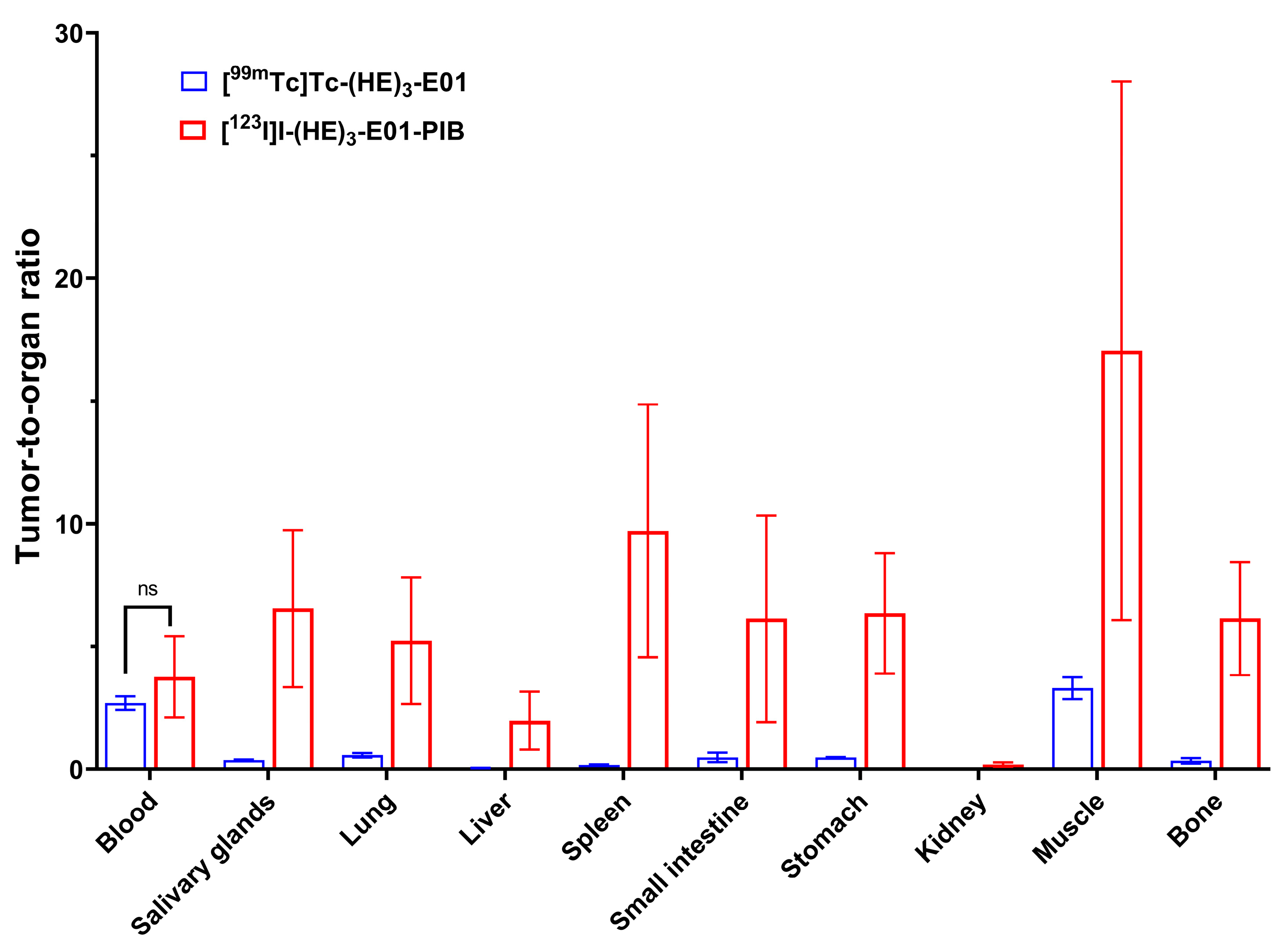
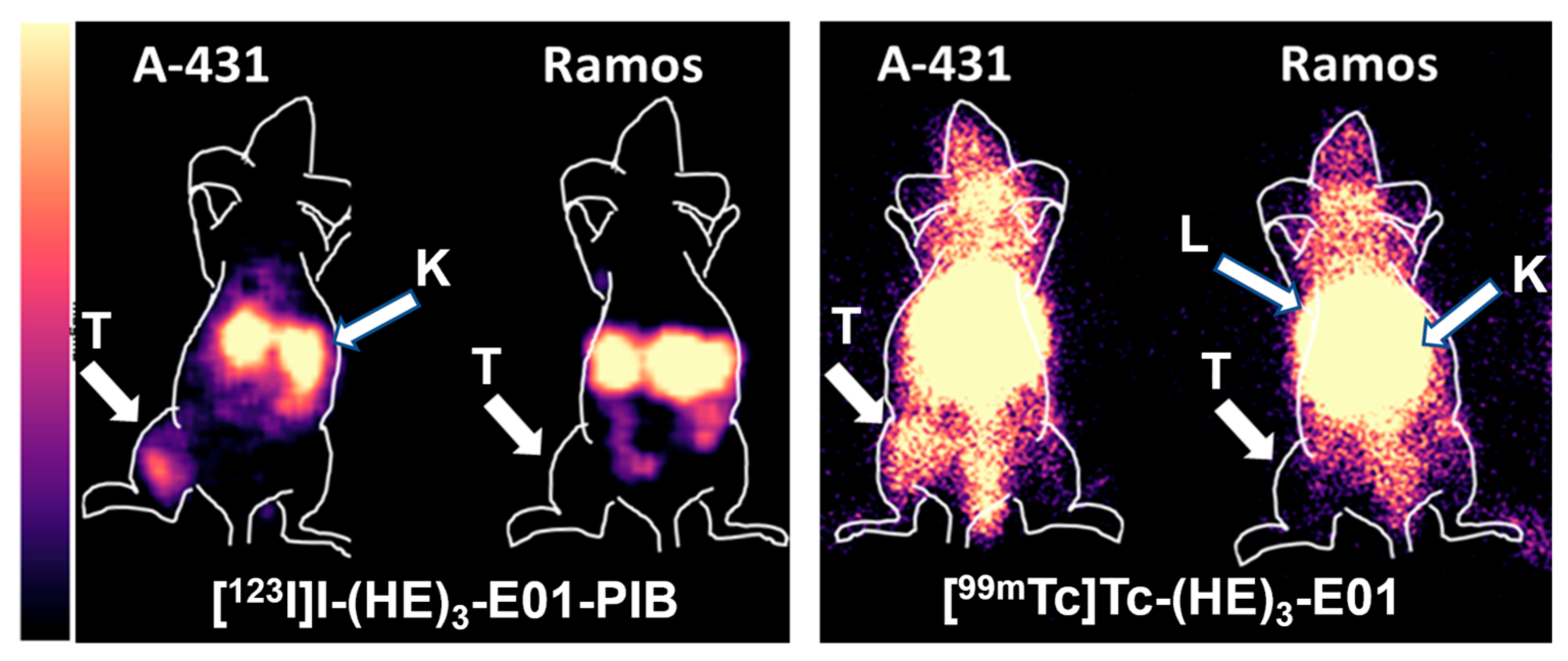
2.4. In Vivo Studies
3. Discussion
4. Materials and Methods
4.1. General Materials and Instruments
4.2. Cell Lines
4.3. Protein Production and Characterization
4.4. Synthesis of N-Succinimidyl-p-(Trimethylstannyl)Benzoate
4.5. Radiolabeling
4.6. In Vitro Studies
4.7. Animal Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, J.-W.; Qiu, S.-Q.; Zhang, G.-J. Molecular and Functional Imaging in Cancer-Targeted Therapy: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2023, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Slobbe, P.; Poot, A.J.; Windhorst, A.D.; Van Dongen, G.A.M.S. PET Imaging with Small-Molecule Tyrosine Kinase Inhibitors: TKI-PET. Drug Discov. Today 2012, 17, 1175–1187. [Google Scholar] [CrossRef]
- Sebastian, S.; Settleman, J.; Reshkin, S.J.; Azzariti, A.; Bellizzi, A.; Paradiso, A. The Complexity of Targeting EGFR Signalling in Cancer: From Expression to Turnover. Biochim. Biophys. Acta 2006, 1766, 120–139. [Google Scholar] [CrossRef]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus Cetuximab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: An Open-Label, Multi-Arm, Non-Randomised, Multicentre, Phase 2 Trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef]
- Shyamsunder, S.; Lu, Z.; Takiar, V.; Waltz, S.E. Challenges and resistance mechanisms to EGFR targeted therapies in head and neck cancers and breast cancer: Insights into RTK dependent and independent mechanisms. Oncotarget 2025, 16, 508–530. [Google Scholar] [CrossRef]
- Rikimaru, K.; Tadokoro, K.; Yamamoto, T.; Enomoto, S.; Tsuchida, N. Gene Amplification and Overexpression of Epidermal Growth Factor Receptor in Squamous Cell Carcinoma of the Head and Neck. Head Neck 1992, 14, 8–13. [Google Scholar] [CrossRef]
- Cívico-Ortega, J.L.; González-Ruiz, I.; Ramos-García, P.; Cruz-Granados, D.; Samayoa-Descamps, V.; González-Moles, M.Á. Prognostic and Clinicopathological Significance of Epidermal Growth Factor Receptor (EGFR) Expression in Oral Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 11888. [Google Scholar] [CrossRef]
- Armerinayanti, N.W.; Lestari, D.P.O.; Wiguna, I.G.W.W. High Cytoplasmic and Membranous Epidermal Growth Factor Receptor (EGFR) Expression as a Risk Factor for High-Grade Ovarian Carcinoma (HGOC). Biomed. Pharmacol. J. 2025, 18, 1645–1652. [Google Scholar] [CrossRef]
- Hashmi, A.A.; Hussain, Z.F.; Irfan, M.; Khan, E.Y.; Faridi, N.; Naqvi, H.; Khan, A.; Edhi, M.M. Prognostic significance of epidermal growth factor receptor (EGFR) over expression in urothelial carcinoma of urinary bladder. BMC Urol. 2018, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Gårdmark, T.; Wester, K.; De La Torre, M.; Carlsson, J.; Malmström, P.U. Analysis of HER2 Expression in Primary Urinary Bladder Carcinoma and Corresponding Metastases. BJU Int. 2005, 95, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Miao, Y.; Wang, Z.; Zhang, Q.; Zhou, P.; Zhang, F. Prognostic significance of epidermal growth factor receptor and programmed cell death-ligand 1 co-expression in esophageal squamous cell carcinoma. Aging 2023, 15, 1107–1129. [Google Scholar] [CrossRef]
- Nieto, Y.; Nawaz, F.; Jones, R.B.; Shpall, E.J.; Nawaz, S. Prognostic Significance of Overexpression and Phosphorylation of Epidermal Growth Factor Receptor (EGFR) and the Presence of Truncated EGFRvIII in Locoregionally Advanced Breast Cancer. J. Clin. Oncol. 2007, 25, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Vuong, T.; Hayashi, S.; Haegert, D.; Tornillo, L.; Terracciano, L.; Lugli, A.; Jass, J. A Simple and Reproducible Scoring System for EGFR in Colorectal Cancer: Application to Prognosis and Prediction of Response to Preoperative Brachytherapy. Br. J. Cancer 2007, 96, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hopper-Borge, E.A.; Nasto, R.E.; Ratushny, V.; Weiner, L.M.; Golemis, E.A.; Astsaturov, I. Mechanisms of tumor resistance to EGFR-targeted therapies. Expert. Opin. Ther. Targets 2009, 13, 339–362. [Google Scholar] [CrossRef]
- Haixian, L.; Shu, P.; Zhao, L.; Chunfeng, L.; Lun, L. Machine learning approaches for EGFR mutation status prediction in NSCLC: An updated systematic review. Front. Oncol. 2025, 15, 1576461. [Google Scholar] [CrossRef]
- Tolmachev, V.; Rosik, D.; Wållberg, H.; Sjöberg, A.; Sandström, M.; Hansson, M.; Wennborg, A.; Orlova, A. Imaging of EGFR Expression in Murine Xenografts Using Site-Specifically Labelled Anti-EGFR 111In-DOTA-ZEGFR:2377 Affibody Molecule: Aspect of the Injected Tracer Amount. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 613–622. [Google Scholar] [CrossRef]
- Aerts, H.J.W.L.; Dubois, L.; Perk, L.; Vermaelen, P.; Van Dongen, G.A.M.S.; Wouters, B.G.; Lambin, P. Disparity between in Vivo EGFR Expression and 89Zr-Labeled Cetuximab Uptake Assessed with PET. J. Nucl. Med. 2009, 50, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Even, A.J.G.; Hamming-Vrieze, O.; van Elmpt, W.; Winnepenninckx, V.J.L.; Heukelom, J.; Tesselaar, M.E.T.; Vogel, W.V.; Hoeben, A.; Zegers, C.M.L.; Vugts, D.J.; et al. Quantitative Assessment of Zirconium-89 Labeled Cetuximab Using PET/CT Imaging in Patients with Advanced Head and Neck Cancer: A Theragnostic Approach. Oncotarget 2017, 8, 3870–3880. [Google Scholar] [CrossRef]
- Huhtala, T.; Laakkonen, P.; Sallinen, H.; Ylä-Herttuala, S.; Närvänen, A. In Vivo SPECT/CT Imaging of Human Orthotopic Ovarian Carcinoma Xenografts with 111In-Labeled Monoclonal Antibodies. Nucl. Med. Biol. 2010, 37, 957–964. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Wittrup, K.D. A Modeling Analysis of the Effects of Molecular Size and Binding Affinity on Tumor Targeting. Mol. Cancer Ther. 2009, 8, 2861–2871. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Garousi, J.; Orlova, A.; Frejd, F.Y.; Tolmachev, V. Imaging Using Radiolabelled Targeted Proteins: Radioimmunodetection and Beyond. EJNMMI Radiopharm. Chem. 2020, 5, 16. [Google Scholar] [CrossRef]
- Lub-De Hooge, M.N.; Kosterink, J.G.W.; Perik, P.J.; Nijnuis, H.; Tran, L.; Bart, J.; Suurmeijer, A.J.H.; De Jong, S.; Jager, P.L.; De Vries, E.G.E. Preclinical Characterisation of 111In-DTPA-Trastuzumab. Br. J. Pharmacol. 2004, 143, 99–106. [Google Scholar] [CrossRef]
- Van Terwisscha Scheltinga, A.G.T.; Ogasawara, A.; Pacheco, G.; Vanderbilt, A.N.; Tinianow, J.N.; Gupta, N.; Li, D.; Firestein, R.; Marik, J.; Scales, S.J.; et al. Preclinical Efficacy of an Antibody-Drug Conjugate Targeting Mesothelin Correlates with Quantitative 89 Zr-Immuno PET. Mol. Cancer Ther. 2017, 16, 134–142. [Google Scholar] [CrossRef]
- Van Dijk, L.K.; Hoeben, B.A.W.; Kaanders, J.H.A.M.; Franssen, G.M.; Boerman, O.C.; Bussink, J. Imaging of Epidermal Growth Factor Receptor Expression in Head and Neck Cancer with SPECT/CT and 111In-Labeled Cetuximab-F(Ab′) 2. J. Nucl. Med. 2013, 54, 2118–2124. [Google Scholar] [CrossRef]
- Van Dijk, L.K.; Yim, C.B.; Franssen, G.M.; Kaanders, J.H.A.M.; Rajander, J.; Solin, O.; Grönroos, T.J.; Boerman, O.C.; Bussink, J. PET of EGFR with 64Cu-Cetuximab-F(Ab′)2 in Mice with Head and Neck Squamous Cell Carcinoma Xenografts. Contrast Media Mol. Imaging 2016, 11, 65–70. [Google Scholar] [CrossRef]
- Huang, L.; Dong, Y.; Li, J.; Yang, X.; Li, X.; Wu, J.; Huang, J.; Zhang, Q.; Wan, Z.; Hu, S.; et al. Epidermal Growth Factor Receptor (EGFR)-Targeting Peptides and Their Applications in Tumor Imaging Probe Construction: Current Advances and Future Perspectives. Biology 2025, 14, 1011. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, M.; Skerra, A. Engineered Protein Scaffolds as Next-Generation Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 391–415. [Google Scholar] [CrossRef]
- Krasniqi, A.; D’Huyvetter, M.; Devoogdt, N.; Frejd, F.Y.; Sörensen, J.; Orlova, A.; Keyaerts, M.; Tolmachev, V. Same-Day Imaging Using Small Proteins: Clinical Experience and Translational Prospects in Oncology. J. Nucl. Med. 2018, 59, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Plückthun, A. Designed Ankyrin Repeat Proteins (DARPins): Binding Proteins for Research, Diagnostics, and Therapy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Kobe, B.; Kajava, A.V. When Protein Folding Is Simplified by Protein Coiling Solenoid Structures. Trend Biochem. Sci. 2000, 25, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Interlandi, G.; Wetzel, S.K.; Settanni, G.; Plückthun, A.; Caflisch, A. Characterization and Further Stabilization of Designed Ankyrin Repeat Proteins by Combining Molecular Dynamics Simulations and Experiments. J. Mol. Biol. 2008, 375, 837–854. [Google Scholar] [CrossRef] [PubMed]
- Karsten, L.; Janson, N.; Le Joncour, V.; Alam, S.; Müller, B.; Ramanathan, J.T.; Laakkonen, P.; Sewald, N.; Müller, K.M. Bivalent EGFR-Targeting DARPin-MMAE Conjugates. Int. J. Mol. Sci. 2022, 23, 2468. [Google Scholar] [CrossRef]
- Stumpp, M.T.; Kirkin, V.; Sanderson, M.P.; Dawson, K.M.; Legenne, P. A Decade of Clinical Experience with DARPins in Oncology Virology: A Systematic Review. medRxiv 2025. [Google Scholar] [CrossRef]
- Gabriele, F.; Palerma, M.; Ippoliti, R.; Angelucci, F.; Pitari, G.; Ardini, M. Recent Advances on Affibody- and DARPin-Conjugated Nanomaterials in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 8680. [Google Scholar] [CrossRef]
- Brandl, F.; Busslinger, S.; Zangemeister-Wittke, U.; Plückthun, A. Optimizing the anti-tumor efficacy of protein-drug conjugates by engineering the molecular size and half-life. J. Control. Release 2020, 327, 186–197. [Google Scholar] [CrossRef]
- Caputi, A.P.; Navarra, P. Beyond antibodies: Ankyrins and DARPins. From basic research to drug approval. Curr. Opin. Pharmacol. 2020, 51, 93–101. [Google Scholar] [CrossRef]
- Bragina, O.; Chernov, V.; Schulga, A.; Konovalova, E.; Garbukov, E.; Vorobyeva, A.; Orlova, A.; Tashireva, L.; Sörensen, J.; Zelchan, R.; et al. Phase I Trial of 99mTc-(HE)3-G3, a DARPin-Based Probe for Imaging of HER2 Expression in Breast Cancer. J. Nucl. Med. 2022, 63, 528–535. [Google Scholar] [CrossRef]
- Zelchan, R.; Chernov, V.; Medvedeva, A.; Rybina, A.; Bragina, O.; Mishina, E.; Larkina, M.; Varvashenya, R.; Fominykh, A.; Schulga, A.; et al. Phase I Clinical Evaluation of Designed Ankyrin Repeat Protein [99mTc]Tc(CO)3-(HE)3-Ec1 for Visualization of EpCAM-Expressing Lung Cancer. Cancers 2024, 16, 2815. [Google Scholar] [CrossRef]
- Knop, S.; Szarejko, M.; Grząśko, N.; Bringhen, S.; Trautmann-Grill, K.; Jurczyszyn, A.; Vacca, A.; Khandanpour, C.; Gamberi, B.; Pour, L.; et al. A phase 1b/2 study evaluating efficacy and safety of MP0250, a designed ankyrin repeat protein (DARPin) simultaneously targeting vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF), in combination with bortezomib and dexamethasone, in patients with relapsed or refractory multiple myeloma. EJHaem 2024, 5, 940–950. [Google Scholar] [PubMed]
- Steiner, D.; Forrer, P.; Plückthun, A. Efficient Selection of DARPins with Sub-Nanomolar Affinities Using SRP Phage Display. J. Mol. Biol. 2008, 382, 1211–1227. [Google Scholar] [CrossRef]
- Boersma, Y.L.; Chao, G.; Steiner, D.; Wittrup, K.D.; Plückthun, A. Bispecific Designed Ankyrin Repeat Proteins (DARPins) Targeting Epidermal Growth Factor Receptor Inhibit A431 Cell Proliferation and Receptor Recycling. J. Biol. Chem. 2011, 286, 41273–41285. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, N.E.M.; Dhingra, S.; Jois, S.D.; Da Vicente, M.G.H. Molecular Targeting of Epidermal Growth Factor Receptor (Egfr) and Vascular Endothelial Growth Factor Receptor (Vegfr). Molecules 2021, 26, 1076. [Google Scholar] [CrossRef]
- Divgi, C.R.; Welt, S.; Kris, M.; Real, F.X.; Yeh, S.D.J.; Gralla, R.; Merchant, B.; Schweighart, S.; Unger, M.; Larson, S.M.; et al. Phase I and Imaging Trial of Indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J. Natl. Cancer Inst. 1991, 83, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Friedman, M.; Sandström, M.; Eriksson, T.L.; Rosik, D.; Hodik, M.; Ståhl, S.; Frejd, F.Y.; Orlova, A. Affibody Molecules for Epidermal Growth Factor Receptor Targeting In Vivo: Aspects of Dimerization and Labeling Chemistry. J. Nucl. Med. 2009, 50, 274–283. [Google Scholar] [CrossRef]
- Deyev, S.M.; Vorobyeva, A.; Schulga, A.; Abouzayed, A.; Günther, T.; Garousi, J.; Konovalova, E.; Ding, H.; Gräslund, T.; Orlova, A.; et al. Effect of a Radiolabel Biochemical Nature on Tumor-Targeting Properties of EpCAM-Binding Engineered Scaffold Protein DARPin Ec1. Int. J. Biol. Macromol. 2020, 145, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, A.; Schulga, A.; Konovalova, E.; Güler, R.; Löfblom, J.; Sandström, M.; Garousi, J.; Chernov, V.; Bragina, O.; Orlova, A.; et al. Optimal Composition and Position of Histidine-Containing Tags Improves Biodistribution of 99mTc-Labeled DARPin G3. Sci. Rep. 2019, 9, 9405. [Google Scholar] [CrossRef]
- Deyev, S.; Vorobyeva, A.; Schulga, A.; Proshkina, G.; Güler, R.; Löfblom, J.; Mitran, B.; Garousi, J.; Altai, M.; Buijs, J.; et al. Comparative Evaluation of Two DARPin Variants: Effect of Affinity, Size, and Label on Tumor Targeting Properties. Mol. Pharm. 2019, 16, 995–1008. [Google Scholar] [CrossRef]
- Koziorowski, J.; Henssen, C.; Weinreich, R. A New Convenient Route to Radioiodinated N-Succinimidyl 3- and 4- Iodobenzoate, Two Reagents for Radioiodination of Proteins. Appl. Radiat. Isot. 1998, 49, 955–959. [Google Scholar] [CrossRef]
- Eisenhut, M.; Mier, W. Radioiodination Chemistry and Radioiodinated Compounds. In Handbook of Nuclear Chemistry; Vértes, A., Nagy, S., Klencsár, Z., Lovas, R.G., Rösch, F., Eds.; Springer: Boston, MA, USA, 2011. [Google Scholar] [CrossRef]
- Deyev, S.M.; Xu, T.; Liu, Y.; Schulga, A.; Konovalova, E.; Garousi, J.; Rinne, S.S.; Larkina, M.; Ding, H.; Gräslund, T.; et al. Influence of the Position and Composition of Radiometals and Radioiodine Labels on Imaging of Epcam Expression in Prostate Cancer Model Using the DARPin Ec1. Cancers 2021, 13, 3589. [Google Scholar] [CrossRef]
- Riihimäki, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Clinical Landscape of Cancer Metastases. Cancer Med. 2018, 7, 5534–5542. [Google Scholar] [CrossRef]
- Hofström, C.; Altai, M.; Honarvar, H.; Strand, J.; Malmberg, J.; Hosseinimehr, S.J.; Orlova, A.; Gräslund, T.; Tolmachev, V. HAHAHA, HEHEHE, HIHIHI, or HKHKHK: Influence of Position and Composition of Histidine Containing Tags on Biodistribution of [99mTc(CO)3]+-Labeled Affibody Molecules. J. Med. Chem. 2013, 56, 4966–4974. [Google Scholar] [CrossRef]
- Lindbo, S.; Garousi, J.; Åstrand, M.; Honarvar, H.; Orlova, A.; Hober, S.; Tolmachev, V. Influence of Histidine-Containing Tags on the Biodistribution of ADAPT Scaffold Proteins. Bioconjug. Chem. 2016, 27, 716–726. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; Vos, J.; Caveliers, V.; Vaneycken, I.; Heemskerk, J.; Duhoux, F.P.; Fontaine, C.; Vanhoeij, M.; Windhorst, A.D.; Aa, F.V.; et al. Phase I Trial of 131I-GMIB-Anti-HER2-VHH1, a New Promising Candidate for HER2-Targeted Radionuclide Therapy in Breast Cancer Patients. J. Nucl. Med. 2021, 62, 1097–1105. [Google Scholar] [CrossRef]
- Nayak, T.K.; Garmestani, K.; Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. Preparation, biological evaluation, and pharmacokinetics of the human anti-HER1 monoclonal antibody panitumumab labeled with 86Y for quantitative PET of carcinoma. J. Nucl. Med. 2010, 51, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.H.; Lee, S.H.; Koh, Y.J.; Kim, M.-K.; Hwang, J.W.; Cho, M.Y.; Lee, J.W.; Lin, P. Bispecific Chimeric Proteins with DARPin-Molecules. European Patent Office EP2829552A1, 28 January 2015. [Google Scholar]
- Kovalchuk, S.I.; Jensen, O.N.; Rogowska-Wrzesinska, A. FlashPack: Fast and Simple Preparation of Ultrahigh-Performance Capillary Columns for LC-MS. Mol. Cell. Proteom. 2019, 18, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Larkina, M.; Plotnikov, E.; Bezverkhniaia, E.; Shabanova, Y.; Tretyakova, M.; Yuldasheva, F.; Zelchan, R.; Schulga, A.; Konovalova, E.; Vorobyeva, A.; et al. Comparative Preclinical Evaluation of Peptide-Based Chelators for the Labeling of DARPin G3 with 99mTc for Radionuclide Imaging of HER2 Expression in Cancer. Int. J. Mol. Sci. 2022, 23, 13443. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, A.; Schulga, A.; Rinne, S.S.; Günther, T.; Orlova, A.; Deyev, S.; Tolmachev, V. Indirect Radioiodination of Darpin G3 Using N-Succinimidyl-Para-Iodobenzoate Improves the Contrast of Her2 Molecular Imaging. Int. J. Mol. Sci. 2019, 20, 3047. [Google Scholar] [CrossRef]
- Larkina, M.; Varvashenya, R.; Yuldasheva, F.; Plotnikov, E.; Bezverkhniaia, E.; Tretyakova, M.; Zelchan, R.; Schulga, A.; Konovalova, E.; Vorobyeva, A.; et al. Comparative Preclinical Evaluation of HYNIC-Modified Designed Ankyrin Repeat Proteins G3 for the 99mTc-Based Imaging of HER2-Expressing Malignant Tumors. Mol. Pharm. 2024, 21, 1919–1932. [Google Scholar] [CrossRef]
- Yang, X.D.; Jia, X.C.; Corvalan, J.R.F.; Wang, P.; Davis, C.G. Development of ABX-EGF, a Fully Human Anti-EGF Receptor Monoclonal Antibody, for Cancer Therapy. Crit. Rev. Oncol. Hematol. 2001, 38, 17–23. [Google Scholar] [CrossRef]
- Wållberg, H.; Orlova, A. Slow internalization of anti-HER2 synthetic affibody monomer 111In-DOTA-ZHER2:342-pep2: Implications for development of labeled tracers. Cancer Biother. Radiopharm. 2008, 23, 435–442. [Google Scholar] [PubMed]
- Malmberg, J.; Tolmachev, V.; Orlova, A. Imaging Agents for in Vivo Molecular Profiling of Disseminated Prostate Cancer—Targeting EGFR Receptors in Prostate Cancer: Comparison of Cellular Processing of [111In]-Labeled Affibody Molecule ZEGFR:2377 and Cetuximab. Int. J. Oncol. 2011, 38, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
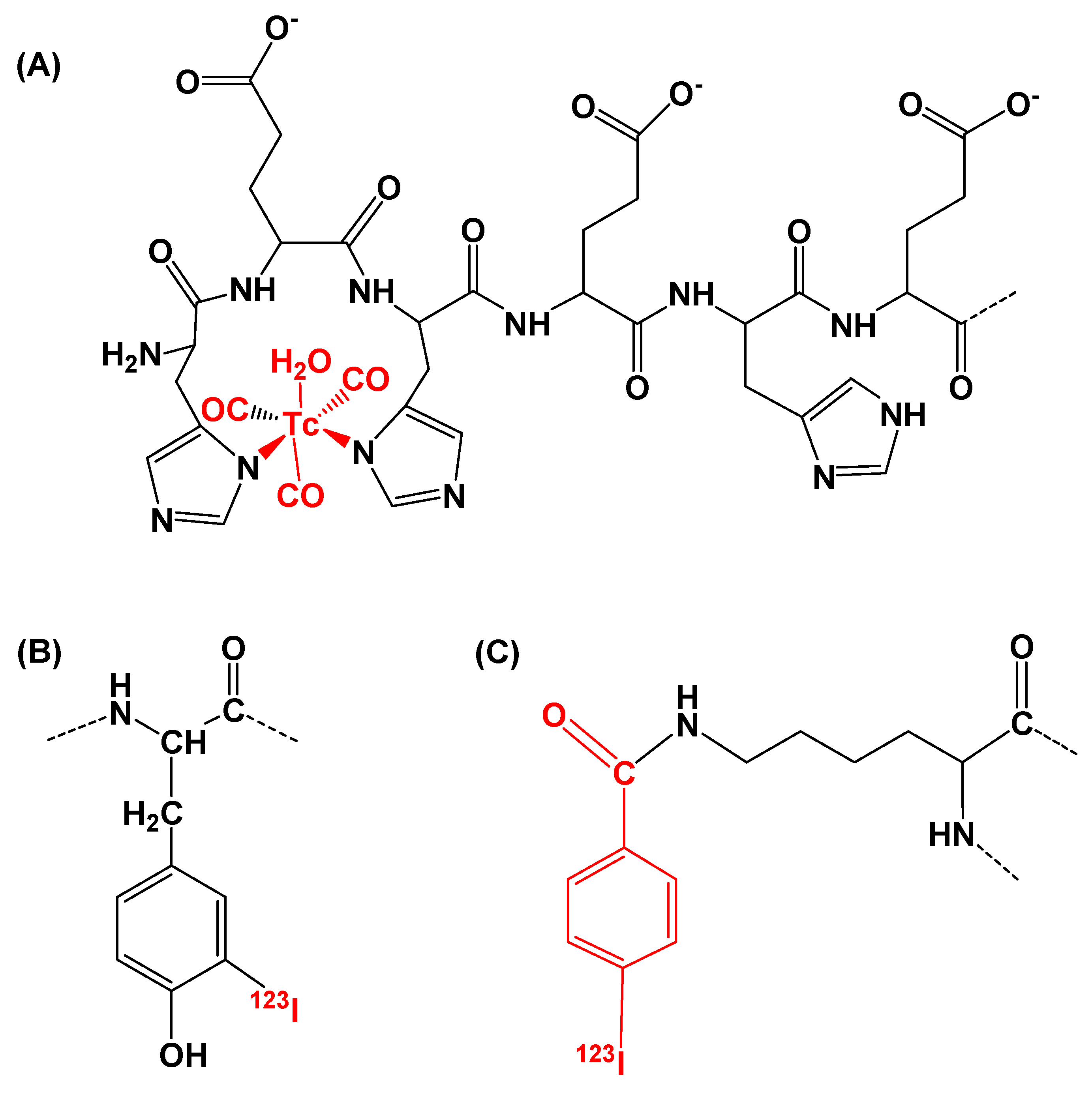

| Variant | Radiochemical Yield, % | Isolated Yield, % | Radiochemical Purity, % | Molar Activity, MBq/nmol |
|---|---|---|---|---|
| [99mTc]Tc-(HE)3-E01 | 90 ± 2 | 86 ± 2 | 99 ± 1 | 18.0 |
| [123I]I-PIB | 85 ± 6 | n/a | n/a | 21.3 |
| [123I]I-(HE)3-E01-PIB | 15 ± 6 | 12 ± 3 | 98 ± 2 | 5.5 |
| [123I]I-(HE)3-E01 | 83 ± 1 | 74 ± 3 | 100 ± 0 | 9.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larkina, M.; Yanovich, G.; Hasnowo, L.A.; Varvashenya, R.; Yuldasheva, F.; Tretyakova, M.; Plotnikov, E.; Zelchan, R.; Schulga, A.; Konovalova, E.; et al. Comparative Preclinical Evaluation of the Tumor-Targeting Properties of Radioiodine and Technetium-Labeled Designed Ankyrin Repeat Proteins for Imaging of Epidermal Growth Factor Receptor Expression in Malignant Tumors. Int. J. Mol. Sci. 2025, 26, 10609. https://doi.org/10.3390/ijms262110609
Larkina M, Yanovich G, Hasnowo LA, Varvashenya R, Yuldasheva F, Tretyakova M, Plotnikov E, Zelchan R, Schulga A, Konovalova E, et al. Comparative Preclinical Evaluation of the Tumor-Targeting Properties of Radioiodine and Technetium-Labeled Designed Ankyrin Repeat Proteins for Imaging of Epidermal Growth Factor Receptor Expression in Malignant Tumors. International Journal of Molecular Sciences. 2025; 26(21):10609. https://doi.org/10.3390/ijms262110609
Chicago/Turabian StyleLarkina, Mariia, Gleb Yanovich, Lutfi Aditya Hasnowo, Ruslan Varvashenya, Feruza Yuldasheva, Maria Tretyakova, Evgenii Plotnikov, Roman Zelchan, Alexey Schulga, Elena Konovalova, and et al. 2025. "Comparative Preclinical Evaluation of the Tumor-Targeting Properties of Radioiodine and Technetium-Labeled Designed Ankyrin Repeat Proteins for Imaging of Epidermal Growth Factor Receptor Expression in Malignant Tumors" International Journal of Molecular Sciences 26, no. 21: 10609. https://doi.org/10.3390/ijms262110609
APA StyleLarkina, M., Yanovich, G., Hasnowo, L. A., Varvashenya, R., Yuldasheva, F., Tretyakova, M., Plotnikov, E., Zelchan, R., Schulga, A., Konovalova, E., Ziganshin, R., Belousov, M. V., Tolmachev, V., & Deyev, S. M. (2025). Comparative Preclinical Evaluation of the Tumor-Targeting Properties of Radioiodine and Technetium-Labeled Designed Ankyrin Repeat Proteins for Imaging of Epidermal Growth Factor Receptor Expression in Malignant Tumors. International Journal of Molecular Sciences, 26(21), 10609. https://doi.org/10.3390/ijms262110609







