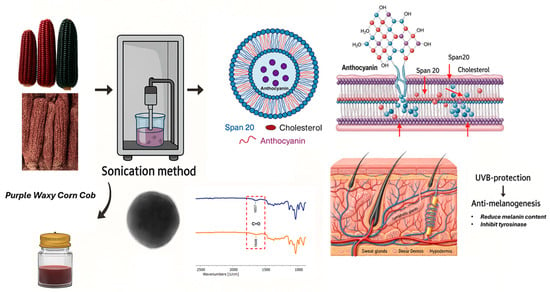
Development of Niosome-Entrapped Purple Waxy Corn Cobs (Zea mays L.) Extracts to Enhance UVB-Protection and Anti-Melanogenesis Activities
Abstract
1. Introduction
2. Result
2.1. Extraction Results of Anthocyanin and the Composition from Purple Waxy Corn Cobs
2.2. The Characterization of Niosome Formulation
2.2.1. Entrapment Efficiency, Particle Size, Polydispersity (PDI), and Zeta Potential
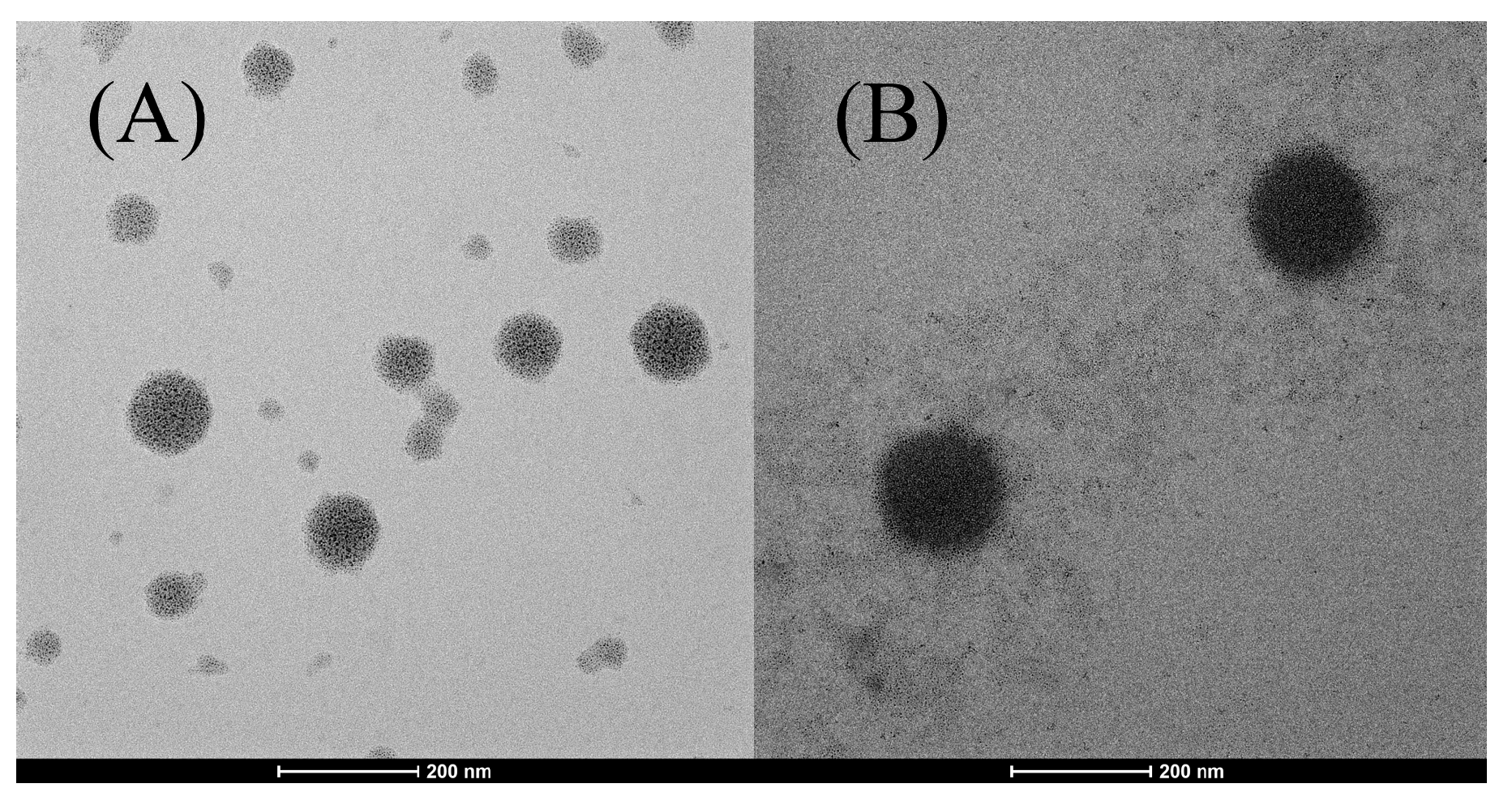
2.2.2. Transmission Electron Microscopy (TEM) Measurement
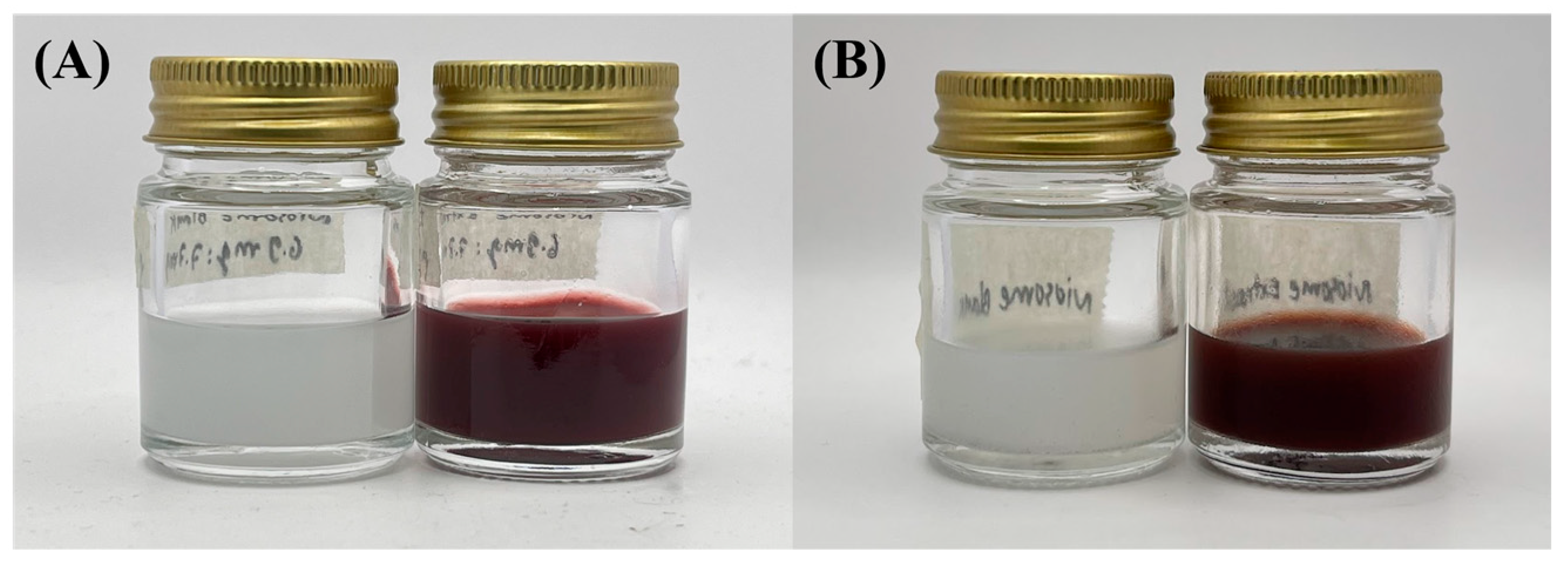
2.2.3. Stability Evaluation
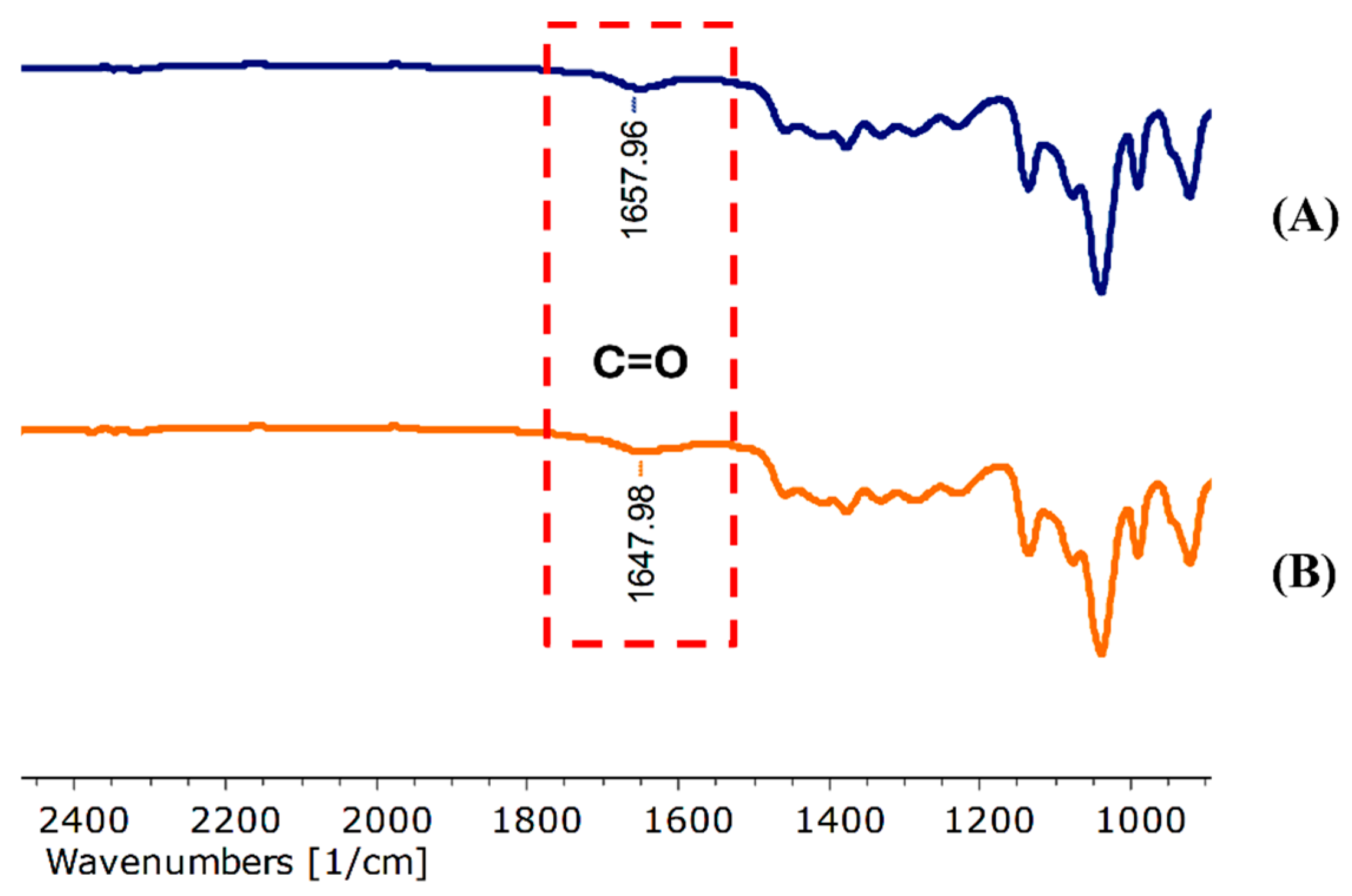
2.2.4. FT-IR Spectroscopy
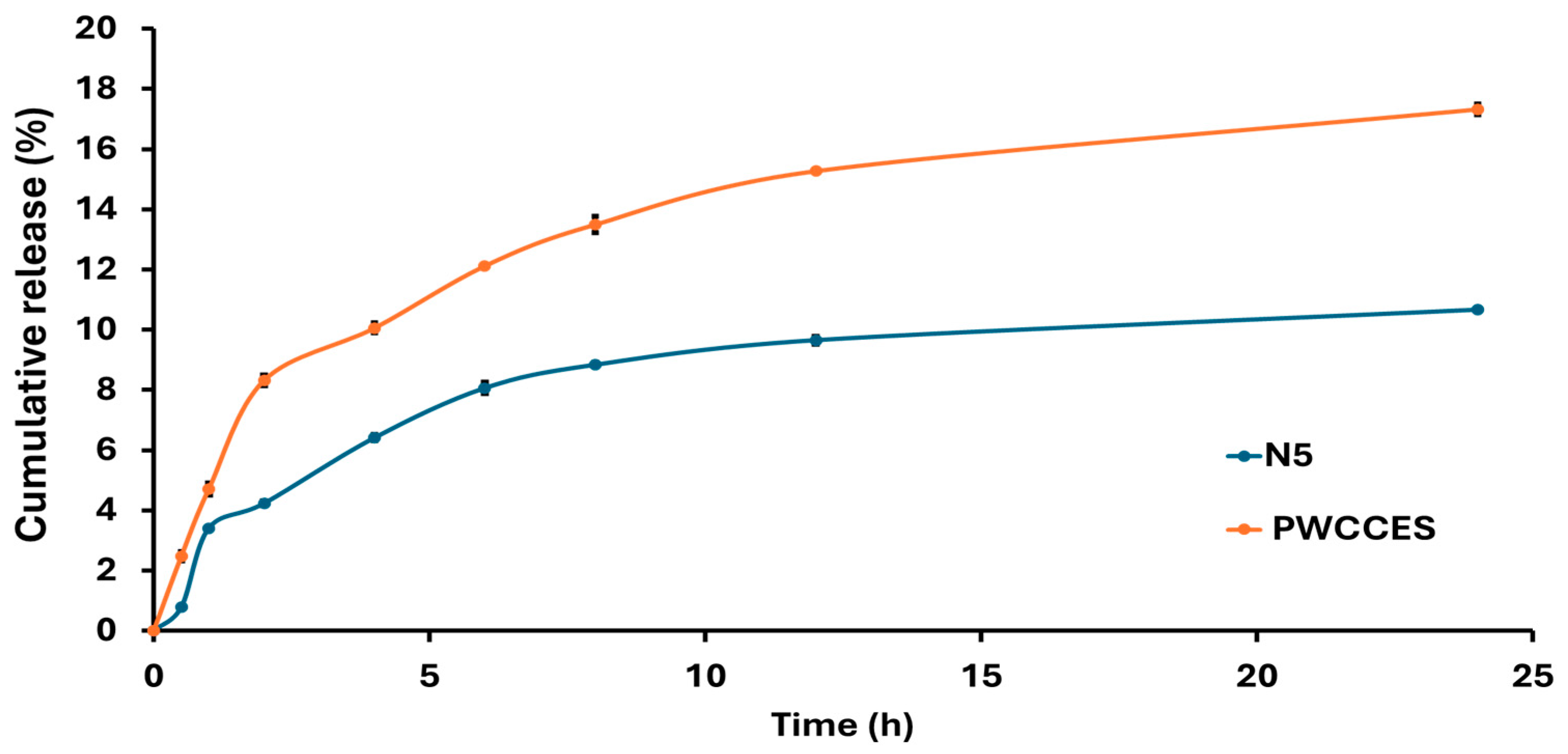
2.2.5. In Vitro Release Study
2.2.6. The Biological Activities of Niosome Formulation
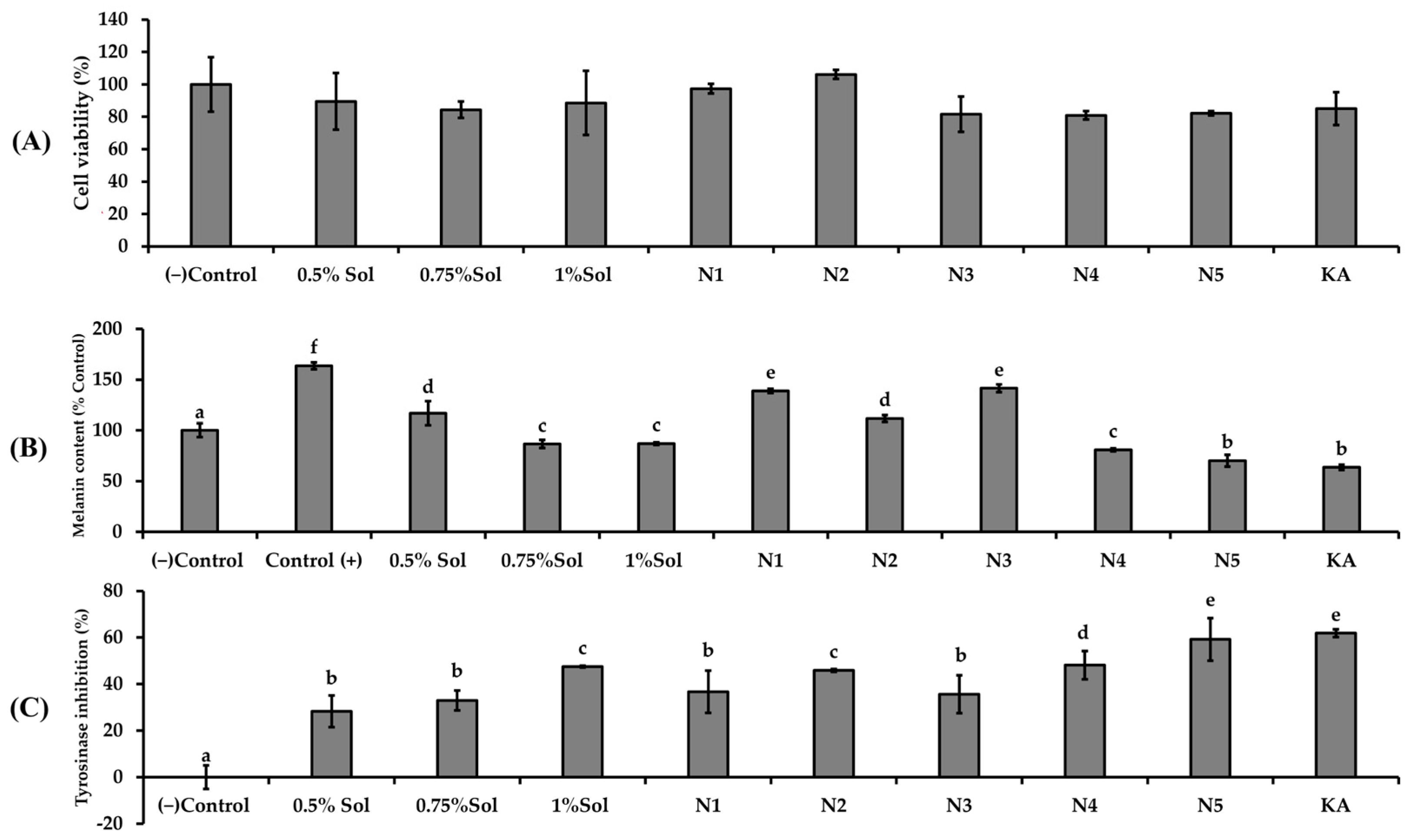
UVB Protection
Anti-Melanogenesis
3. Discussion
3.1. The Extraction of Anthocyanin and the Composition from Purple Waxy Corn Cobs
3.2. The Characterization of Niosome Formulation
Entrapment Efficiency, Particle Size, Polydispersity (PDI), and Zeta Potential
3.3. Stability Evaluation
3.4. In Vitro Release Study
3.5. The Biological Activities of Niosome Formulation
4. Materials and Methods
4.1. Materials
4.2. Preparation of Extracts
4.2.1. The Cyanidin-3-Glucoside Content by HPLC Determination
4.2.2. Extraction of Anthocyanin from Purple Waxy Corn Cobs
- A = (Abs520nm-Abs700nm) pH1 − (Abs520nm-Abs700nm) pH4.5,
- MW = molecular weight (449.2 g/mol for cyanidin-3-glucoside)
- DF = dilution factor, 1000 = factor for conversion from g to mg
- ƹ = molar extinction coefficient (26,900) in L mol−1 cm−1
- 1 = path length in cm.
4.3. Preparation of Niosome Formulation
4.4. The Characterization of Niosome Properties
4.4.1. Percent Entrapment Efficiency (%EE)
4.4.2. Dynamic Light Scattering (DLS)
4.4.3. Transmission Electron Microscopy (TEM)
4.4.4. Thermal Stability Testing
4.4.5. In Vitro Release Study
4.4.6. Fourier Transform Infrared Spectroscopy FT-IR Spectroscopy
4.5. UVB-Protection
4.5.1. Assessing Cytotoxicity in HaCaT Cells with the MTT Assay
4.5.2. The UVB Irradiation on HaCaT Cells
4.5.3. Effective UVB Protection for HaCaT Cells
4.6. Anti-Melanogenesis
4.6.1. Cytotoxicity on the B16F10 Cells by the MTT Assay
4.6.2. Melanin Contents
4.6.3. Anti-Tyrosinase
4.7. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Thapphasaraphong, S. Crops of Waxy Purple Corn: A Valuable Source of Antioxidative Phytochemicals. Int. J. Adv. Agric. Environ. Eng. 2016, 3, 73–77. [Google Scholar] [CrossRef]
- Chuntakaruk, H.; Kongtawelert, P.; Pothacharoen, P. Chondroprotective Effects of Purple Corn Anthocyanins on Advanced Glycation End Products Induction through Suppression of NF-κB and MAPK Signaling. Sci. Rep. 2021, 11, 1895. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Hucl, P.; Rabalski, I. Compositional and Antioxidant Properties of Anthocyanin-Rich Products Prepared from Purple Wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and Cherry Anthocyanin Consumption Prevents Oxidative Stress and Inflammation in Diet-induced Obese Mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Sevimli-Gur, C.; Cetin, B.; Akay, S.; Gulce-Iz, S.; Yesil-Celiktas, O. Extracts from Black Carrot Tissue Culture as Potent Anticancer Agents. Plant Foods Hum. Nutr. 2013, 68, 293–298. [Google Scholar] [CrossRef]
- Kanpipit, N.; Nualkaew, N.; Thapphasaraphong, S. The Potential of Purple Waxy Corn Cob (Zea mays L.) Extract Loaded-Sericin Hydrogel for Anti-Hyperpigmentation, UV Protection and Anti-Aging Properties as Topical Product Applications. Pharmaceuticals 2022, 16, 35. [Google Scholar] [CrossRef]
- Vayupharp, B.; Laksanalamai, V. Antioxidant Properties and Color Stability of Anthocyanin Purified Extracts from Thai Waxy Purple Corn Cob. J. Food Nutr. Res. 2015, 3, 629–636. [Google Scholar]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D.; Viskelis, P. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 288. [Google Scholar] [CrossRef]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red Cabbage Anthocyanins: Stability, Extraction, Biological Activities and Applications in Food Systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Fidan-Yardimci, M.; Akay, S.; Sharifi, F.; Sevimli-Gur, C.; Ongen, G.; Yesil-Celiktas, O. A Novel Niosome Formulation for Encapsulation of Anthocyanins and Modelling Intestinal Transport. Food Chem. 2019, 293, 57–65. [Google Scholar] [CrossRef]
- Colorado, D.; Fernandez, M.; Orozco, J.; Lopera, Y.; Muñoz, D.L.; Acín, S.; Balcazar, N. Metabolic Activity of Anthocyanin Extracts Loaded into Non-Ionic Niosomes in Diet-Induced Obese Mice. Pharm. Res. 2020, 37, 152. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, C.; Nigro, F.; Campos, V.E.B.; Rossi, A.; Santos-Oliveira, R.; Cardoso, V.; Vermelho, A.B.; Dos Santos, E.P.; Mansur, C.R.E. Nanovesicle-Based Formulations for Photoprotection: A Safety and Efficacy Approach. Nanotechnology 2019, 30, 345102. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Chu, B.-S.; Yaakob, H. Niosomal Drug Delivery Systems: Formulation, Preparation and Applications. World Appl. Sci. J. 2014, 32, 1671–1685. [Google Scholar]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current Advances in Specialised Niosomal Drug Delivery: Manufacture, Characterization and Drug Delivery Applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.; Rodrigo-García, J.; Martínez-Ruiz, N.; Cárdenas-Robles, A.; Mendoza-Díaz, S.; Álvarez-Parrilla, E.; González-Aguilar, G.; De La Rosa, L.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Jo, K.; Bae, G.Y.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K.-B. An Anthocyanin-Enriched Extract from Vaccinium uliginosum Improves Signs of Skin Aging in UVB-Induced Photodamage. Antioxidants 2020, 9, 844. [Google Scholar] [CrossRef]
- Sangkaew, O.; Yompakdee, C. Fermented Unpolished Black Rice (Oryza sativa L.) Inhibits Melanogenesis via ERK, P38, and AKT Phosphorylation in B16F10 Melanoma Cells. J. Microbiol. Biotechnol. 2020, 30, 1184–1194. [Google Scholar] [CrossRef]
- Song, Y.-R.; Lim, W.-C.; Han, A.; Lee, M.; Shin, E.J.; Lee, K.-M.; Nam, T.-G.; Lim, T.-G. Rose Petal Extract (Rosa gallica) Exerts Skin Whitening and Anti-Skin Wrinkle Effects. J. Med. Food 2020, 23, 870–878. [Google Scholar] [CrossRef]
- Rimdusit, T.; Thapphasaraphong, S.; Puthongking, P.; Priprem, A. Effects of Anthocyanins and Melatonin from Purple Waxy Corn By-Products on Collagen Production by Cultured Human Fibroblasts. Nat. Prod. Commun. 2019, 14, 1934578X19863510. [Google Scholar] [CrossRef]
- Limsitthichaikoon, S.; Priprem, A.; Damrongrungruang, T. Niosomes Encapsulated Anthocyanins Complex Loaded in a Topical Oral Gel. Key Eng. Mater. 2020, 859, 232–238. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.; Wang, Y.; Shi, Z.; Xie, K.; Liao, X.; Tan, J. Factors Affecting the Stability of Anthocyanins and Strategies for Improving Their Stability: A Review. Food Chem. X 2024, 24, 101883. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Yuksel, N. Investigation of Formulation Variables and Excipient Interaction on the Production of Niosomes. AAPS Pharm. Sci. Tech. 2012, 13, 826–835. [Google Scholar] [CrossRef]
- Silva, H.R.D.; Assis, D.D.C.D.; Prada, A.L.; Silva, J.O.C.; Sousa, M.B.D.; Ferreira, A.M.; Amado, J.R.R.; Carvalho, H.D.O.; Santos, A.V.T.D.L.T.D.; Carvalho, J.C.T. Obtaining and Characterization of Anthocyanins from Euterpe oleracea (Açaí) Dry Extract for Nutraceutical and Food Preparations. Rev. Bras. Farmacogn. 2019, 29, 677–685. [Google Scholar] [CrossRef]
- Swer, T.L.; Mukhim, C.; Bashir, K.; Chauhan, K. Optimization of Enzyme Aided Extraction of Anthocyanins from Prunus nepalensis L. LWT 2018, 91, 382–390. [Google Scholar] [CrossRef]
- Nasirian, H.; TarvijEslami, S.; Ghourchian, H.; Ebrahimi, M.; Piri-Gharaghie, T.; Ghajari, G. Niosomes Containing Enciprazine Hydrochloride Have Been Shown to Efficiently Inhibit the Proliferation and Induce Apoptosis in Colorectal Cancer Cells. Adv. Cancer Biol. Metastasis 2024, 12, 100128. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Enayatifard, R.; Morteza-Semnani, K.; Hassan Hashemi, S.M.; Babaei, A.; Rahimnia, S.M.; Rostamkalaei, S.S.; Nokhodchi, A. Curcumin niosomes (Curcusomes) as an Alternative to Conventional Vehicles: A Potential for Efficient Dermal Delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 102035. [Google Scholar] [CrossRef]
- Sangkana, S.; Eawsakul, K.; Ongtanasup, T.; Boonhok, R.; Mitsuwan, W.; Chimplee, S.; Paul, A.K.; Saravanabhavan, S.S.; Mahboob, T.; Nawaz, M.; et al. Preparation and Evaluation of a Niosomal Delivery System Containing G. Mangostana Extract and Study of Its Anti-Acanthamoeba Activity. Nanoscale Adv. 2024, 6, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, R.; Tavano, L. Niosomal Drug Delivery for Transdermal Targeting: Recent Advances. Res. Rep. Transdermal Drug Deliv. 2015, 23, 23–33. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Abtahi, M.S.; Hejabi, F.; Ren, Q. Current Advances in Niosomes Applications for Drug Delivery and Cancer Treatment. Mater. Today Bio 2023, 23, 100837. [Google Scholar] [CrossRef] [PubMed]
- Mawazi, S.M.; Ann, T.J.; Widodo, R.T. Application of Niosomes in Cosmetics: A Systematic Review. Cosmetics 2022, 9, 127. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Li, Y.; Yuan, K.; Zhang, W.; Cai, D.; Peng, Z.; Hu, Y.; Sun, J.; Bai, W. Bioactivity and Application of Anthocyanins in Skin Protection and Cosmetics: An Extension as a Functional Pigment. Phytochem. Rev. 2023, 22, 1441–1467. [Google Scholar] [CrossRef]
- Li, K.; Zhang, M.; Chen, H.; Peng, J.; Jiang, F.; Shi, X.; Bai, Y.; Jian, M.; Jia, Y. Anthocyanins from Black Peanut Skin Protect against UV-B Induced Keratinocyte Cell and Skin Oxidative Damage through Activating Nrf 2 Signaling. Food Funct. 2019, 10, 6815–6828. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications. In Measurement of Antioxidant Activity & Capacity; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 77–106. ISBN 978-1-119-13538-8. [Google Scholar]
- Damrongrungruang, T.; Paphangkorakit, J.; Limsitthichaikoon, S.; Khampaenjiraroch, B.; Davies, M.J.; Sungthong, B.; Priprem, A. Anthocyanin Complex Niosome Gel Accelerates Oral Wound Healing: In Vitro and Clinical Studies. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102423. [Google Scholar] [CrossRef]
- Wu, P.-S.; Li, Y.-S.; Kuo, Y.-C.; Tsai, S.-J.J.; Lin, C.-C. Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules 2019, 24, 600. [Google Scholar] [CrossRef]
- Negi, P.; Singh, B.; Sharma, G.; Beg, S.; Katare, O.P. Biocompatible Lidocaine and Prilocaine Loaded-Nanoemulsion System for Enhanced Percutaneous Absorption: QbD-Based Optimisation, Dermatokinetics and In Vivo Evaluation. J. Microencapsul. 2015, 32, 419–431. [Google Scholar] [CrossRef]
- De Silva, L.; Fu, J.-Y.; Htar, T.T.; Muniyandy, S.; Kasbollah, A.; Wan Kamal, W.H.B.; Chuah, L.-H. Characterization, Optimization, and in Vitro Evaluation of Technetium-99m-Labeled Niosomes. Int. J. Nanomed. 2019, 14, 1101–1117. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Conceição, J.; Amaral, M.H.; Santos, D.; Silva, J.B.; Lobo, J.M.S. Characterization and Stability Studies of Emulsion Systems Containing Pumice. Braz. J. Pharm. Sci. 2014, 50, 361–369. [Google Scholar] [CrossRef]
- Kanpipit, N.; Mattariganont, S.; Janphuang, P.; Rongsak, J.; Daduang, S.; Chulikhit, Y.; Thapphasaraphong, S. Comparative Study of Lycopene-Loaded Niosomes Prepared by Microfluidic and Thin-Film Hydration Techniques for UVB Protection and Anti-Hyperpigmentation Activity. Int. J. Mol. Sci. 2024, 25, 11717. [Google Scholar] [CrossRef]

| Assay | Yield | TAC | Antioxidant Activities | ||
|---|---|---|---|---|---|
| Unit | (%) | mg C3GE/g DW | DPPH (IC50 μg/mL) | ABTS (IC50 μg/mL) | FRAP (mg Fe(II)/g) |
| PWCC Extract | 6.34 | 3.02 ± 0.81 | 72.32 ± 9.71 | 17.78 ± 0.57 | 4.21 ± 0.10 |
| Vit C/Trolox | 17.11 ± 0.18 | 1.01 ± 0.34 | |||
| Formulation | %EE | Size (nm) | PDI | Zeta (mV) |
|---|---|---|---|---|
| N1 | - a | 285.70 ± 17.35 d | 0.16 ± 0.04 a | −29.30 ± 4.63 a |
| N2 | 84.88 ± 2.11 c | 221.80 ± 3.43 b | 0.16 ± 0.04 a | −30.70 ± 0.91 a |
| N3 | - a | 185.60 ± 20.96 a | 0.42 ± 0.12 b | −30.70 ± 0.73 a |
| N4 | 83.68 ± 3.18 b | 296.50 ± 2.34 d | 0.16 ± 0.05 a | −32.10 ± 0.11 a |
| N5 | 85.74 ± 1.19 c | 248.10 ± 9.72 c | 0.14 ± 0.06 a | −30.90 ± 2.89 a |
| (%EE) | Size (nm) | PDI | Zeta (mV) | |||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| N3 | - | - | 164.60 ± 32.96 * | 257.80 ± 4.86 * | 0.28 ± 0.01 | 0.24 ± 0.03 | −30.43 ± 0.42 * | −44.40 ± 0.36 * |
| N5 | 82.65 ± 3.65 | 80.71 ± 188 | 265.23 ± 9.03 * | 344.03 ± 9.65 * | 0.33 ± 0.01 * | 0.19 ± 0.02 * | −30.10 ± 0.26 * | −40.30 ± 0.17 * |
| Sample | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | K0 | R2 | K1 | R2 | KH | R2 | n | Kp | |
| N5 | 0.9742 | 0.1866 | 0.9385 | 0.0605 | 0.9901 | 3.1461 | 0.9864 | 0.4838 | 3.2636 |
| PWCCES | 0.919 | 1.149 | 0.8177 | 0.0569 | 0.9662 | 4.5362 | 0.9556 | 0.4800 | 5.1713 |
| Formulation | PWCC Extract (%) | Span 20 (%) | Cholesterol (%) | Propylene Glycol (%) | PBS PH 5.5 (%) | Total (%) |
|---|---|---|---|---|---|---|
| N1 | 0.000 | 0.035 | 0.039 | 10.000 | 89.926 | 100.000 |
| N2 | 0.500 | 0.035 | 0.039 | 10.000 | 89.426 | 100.000 |
| N3 | 0.000 | 0.070 | 0.078 | 10.000 | 89.852 | 100.000 |
| N4 | 0.750 | 0.070 | 0.078 | 10.000 | 89.102 | 100.000 |
| N5 | 1.000 | 0.070 | 0.078 | 10.000 | 88.852 | 100.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongphachanh, I.; Kanpipit, N.; Thapphasaraphong, S. Development of Niosome-Entrapped Purple Waxy Corn Cobs (Zea mays L.) Extracts to Enhance UVB-Protection and Anti-Melanogenesis Activities. Int. J. Mol. Sci. 2025, 26, 10586. https://doi.org/10.3390/ijms262110586
Thongphachanh I, Kanpipit N, Thapphasaraphong S. Development of Niosome-Entrapped Purple Waxy Corn Cobs (Zea mays L.) Extracts to Enhance UVB-Protection and Anti-Melanogenesis Activities. International Journal of Molecular Sciences. 2025; 26(21):10586. https://doi.org/10.3390/ijms262110586
Chicago/Turabian StyleThongphachanh, Inpakob, Nattawadee Kanpipit, and Suthasinee Thapphasaraphong. 2025. "Development of Niosome-Entrapped Purple Waxy Corn Cobs (Zea mays L.) Extracts to Enhance UVB-Protection and Anti-Melanogenesis Activities" International Journal of Molecular Sciences 26, no. 21: 10586. https://doi.org/10.3390/ijms262110586
APA StyleThongphachanh, I., Kanpipit, N., & Thapphasaraphong, S. (2025). Development of Niosome-Entrapped Purple Waxy Corn Cobs (Zea mays L.) Extracts to Enhance UVB-Protection and Anti-Melanogenesis Activities. International Journal of Molecular Sciences, 26(21), 10586. https://doi.org/10.3390/ijms262110586