Unraveling the Effects of Melissa officinalis L. on Cognition and Sleep Quality: A Narrative Review
Abstract
1. Introduction
2. Methods
2.1. Focused Question
2.2. Language
2.3. Databases
2.4. Study Selection
2.5. Data Extraction
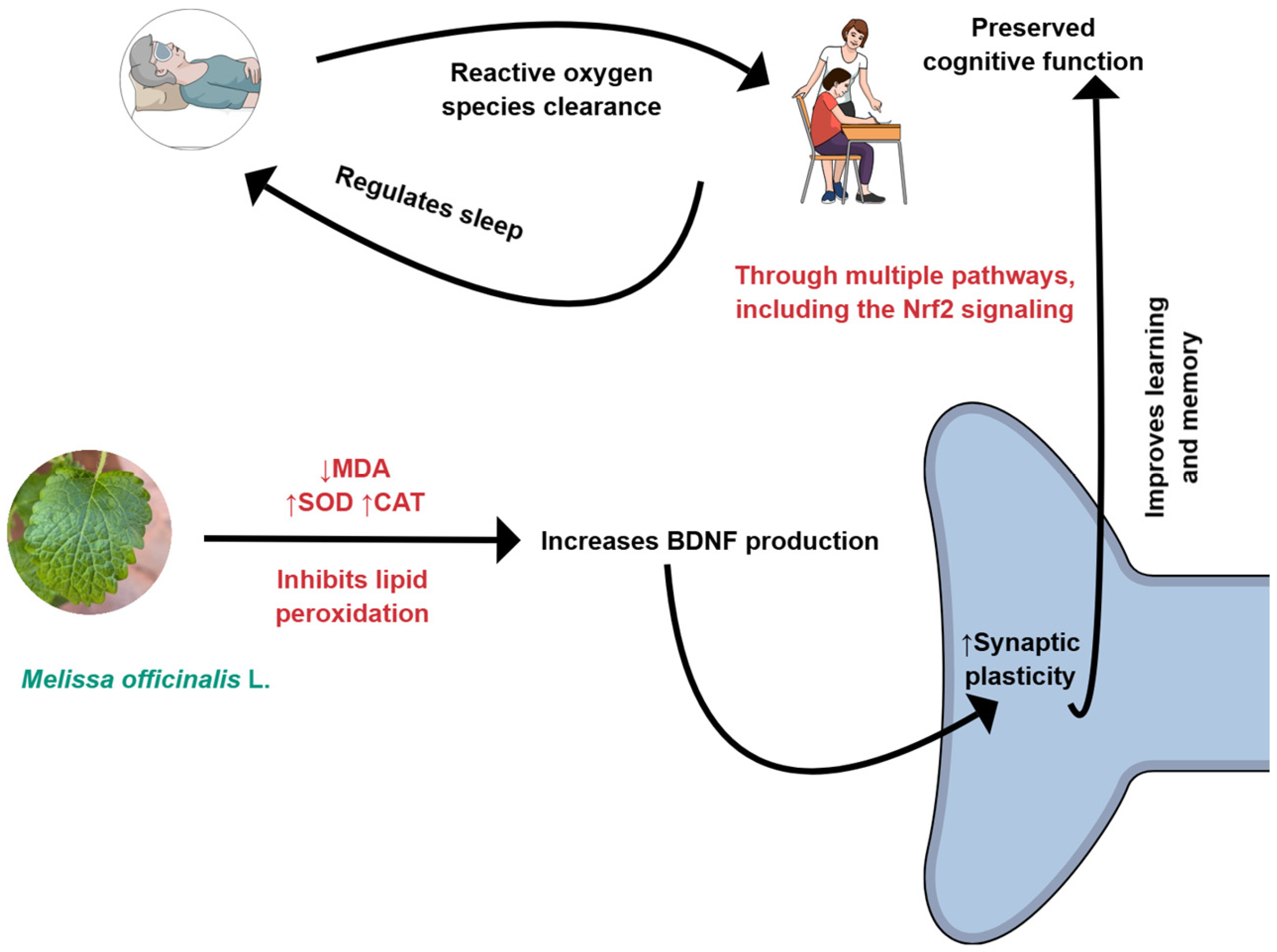
3. Melissa officinalis and Conditions That Can Be Affected by This Plant
3.1. M. officinalis
3.2. Cognition and Neurodegenerative Conditions
3.3. Cognition and Inflammatory Processes
3.4. Cognition and Oxidative Stress
3.5. Sleep Quality
4. Clinical Trials That Evaluated the Effects of M. officinalis on Cognition
5. Clinical Trials That Evaluated the Effects of M. officinalis on Sleep Quality
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacheco, L.M.; da Cunha, B.M.S.d.C.; Queiroz, L.A.Q.; Rocha, S.L.R.; Noleto, T.O.N.; Rodrigues, I.G.; Fernanda Costa Dalla Mutta Resende, C.; Cristian de Souza, H. Doenças cardiovasculares em idosos usuários do SUS: Prevalência e fatores associados: Cardiovascular diseases in elderly sus users: Prevalence and associated factors. Rev. Master Ensino Pesqui. E Extensão 2022, 6, 30–34. [Google Scholar] [CrossRef]
- de Souza, E.; Reis, N.; Reis, S.; Bemvenuto, R.; Ferreira, I.; Rosário, R.; Santos, M.; Reis, S.; Oliveira, A.; Araújo, K. Riscos de quedas em idosos e a COVID-19: Um alerta de saúde e proposta de exercícios funcionais. Rev. Bras. Atividade Física Saúde 2020, 25, 1–7. [Google Scholar] [CrossRef]
- Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13, 1129. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16, 2721. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef]
- Jee, H. Aging world: Mishap or time for revision. J. Exerc. Rehabil. 2024, 20, 91. [Google Scholar] [CrossRef]
- Gonçalves, L.; Oliveira, J.; Guimarães, A.; Guimarães, B.; Melo Soares, C.E.; Gomes, H.; Queiroz, T. A problemática da epidemia de demência vascular no Brasil: Uma revisão bibliográfica/The problem of the epidemic of vascular dementia in Brazil: A bibliographic review. Braz. J. Health Rev. 2020, 3, 15451–15459. [Google Scholar] [CrossRef]
- Kron, J.O.Z.J.; Keenan, R.J.; Hoyer, D.; Jacobson, L.H. Orexin Receptor Antagonism: Normalizing Sleep Architecture in Old Age and Disease. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 359–386. [Google Scholar] [CrossRef]
- Rigillo, G.; Blom, J.M.C.; Cocchi, A.; Martinucci, V.; Favaro, F.; Baini, G.; Cappellucci, G.; Tascedda, F.; Biagi, M. Medicinal Plants for Child Mental Health: Clinical Insights, Active Compounds, and Perspectives for Rational Use. Children 2025, 12, 1142. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Guo, Y.; Liu, Y.; Dong, X. Sleep structure assessed by objective measurement in patients with mild cognitive impairment: A meta-analysis. Sleep Med. 2024, 113, 397–405. [Google Scholar] [CrossRef]
- Murray, M.E.; Smith, C.; Menon, V.; Keene, C.D.; Lein, E.; Hawrylycz, M.; Aguzzi, A.; Benedetti, B.; Brose, K.; Caetano-Anolles, K.; et al. Accelerating biomedical discoveries in brain health through transformative neuropathology of aging and neurodegeneration. Neuron, 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Yang, H.; Xu, L.; Qin, W.; Hu, F.; Li, L.; Chen, C.; Tang, W. Gender differences in the modifying effect of living arrangements on the association of sleep quality with cognitive function among community-dwelling older adults: A cross-sectional study. Front. Public Health 2023, 11, 1142362. [Google Scholar] [CrossRef]
- Hu, Y.; Zuo, L.; Pan, Y.; Yan, H.; Wang, Y.; Zhao, X. Persisting Short or Long Sleep Duration Predicts Post-Stroke Depression One year After Stroke and Transient Ischemic Attack. Nat. Sci. Sleep 2025, 17, 1507–1519. [Google Scholar] [CrossRef]
- Awlqadr, F.H.; Altemimi, A.B.; Qadir, S.A.; Mohammed, O.A.; Saeed, M.N.; Hesarinejad, M.A.; Lakhssassi, N. Bioactive Compounds, Medicinal Benefits, and Contemporary Extraction Methods for Lemon Balm (Melissa officinalis). Food Sci. Nutr. 2025, 13, e70864. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, L.C.; O’Shea, B.Q.; Joseph, C.; Finlay, J.M. Acute relationships between mental health and cognitive function during the COVID-19 pandemic: Longitudinal evidence from middle-aged and older US adults. SSM Ment. Health 2022, 2, 100097. [Google Scholar] [CrossRef] [PubMed]
- Ayyıldız, M.; Kalafat, Ş. The Relationship between Perceived Stress, Sleep Quality and the Everyday Memory of the Senior Middle School, High School and College Students in Turkey. PsyArXiv 2022. [Google Scholar] [CrossRef]
- Jiahao, W.; Kameyama, J.; Udono, M.; Katakura, Y. Metabolic changes in the one-carbon metabolism-related amino acids during etoposide-induced cellular senescence of neuronal cells. Cytotechnology 2025, 77, 131. [Google Scholar] [CrossRef]
- Piełunowicz, M.; Kotuła, J.; Kotuła, K.; Więckiewicz, M.; Lis, J.; Kawala, B.; Kuc, A.E.; Sarul, M. Effects of rapid maxillary expansion and functional orthodontic treatment in children with sleep disordered breathing: A systematic review. BMC Oral Health 2025, 25, 1059. [Google Scholar] [CrossRef]
- Mandel, N.; Agarwal, N. Role of SUMOylation in Neurodegenerative Diseases. Cells 2022, 11, 3395. [Google Scholar] [CrossRef]
- Pan, X.; Dutta, D.; Lu, S.; Bellen, H.J. Sphingolipids in neurodegenerative diseases. Front. Neurosci. 2023, 17, 1137893. [Google Scholar] [CrossRef]
- Yıldırım, İ.G.; Doğan, F.; Şanlıer, N. Nörodejeneratif Hastalıklar ve Fitokimyasallar. Beslenme Ve Diyet Derg. 2023, 51, 111–118. [Google Scholar] [CrossRef]
- Basiri, R.; Rajanala, Y. Effects of Individualized Nutrition Therapy and Continuous Glucose Monitoring on Dietary and Sleep Quality in Individuals with Prediabetes and Overweight or Obesity. Nutrients 2025, 17, 1507. [Google Scholar] [CrossRef]
- Merchant, A.M.; Gray, S.R.; Gray, C.M.; Finlayson, G.; Manyara, A.M.; Gabler Trisotti, M.F.; Gill, J.M.R. Effect of a cognitive behavioural therapy intervention to improve sleep on food preferences: A randomized controlled trial in adults with overweight and obesity. Appetite 2025, 212, 108022. [Google Scholar] [CrossRef] [PubMed]
- Mathews, I.M.; Eastwood, J.; Lamport, D.J.; Cozannet, R.L.; Fanca-Berthon, P.; Williams, C.M. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients 2024, 16, 3545. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Oh, J. Factors Affecting Sleep Quality of College Students during the Coronavirus Disease 2019 Pandemic: A Cross-Sectional Study. Medicina 2023, 59, 416. [Google Scholar] [CrossRef] [PubMed]
- Zakiah, N.I.; Syahirah, N. Sleep Disruption and Its Impact on Academic Performance in Medical Students: A Systematic Review. Univers. J. Public Health 2023, 11, 1–7. [Google Scholar]
- Jyothi, R.; Mathangi, D.; Chellaiyan, V. Lifestyle Behaviour and Obstructive Sleep Apnea (OSA): An Association Study Among Young Adults. Natl. J. Community Med. 2022, 13, 400–403. [Google Scholar] [CrossRef]
- Zam, W.; Quispe, C.; Sharifi-Rad, J.; López, M.D.; Schoebitz, M.; Martorell, M.; Sharopov, F.; Fokou, P.V.T.; Mishra, A.P.; Chandran, D.; et al. An Updated Review on The Properties of Melissa officinalis L.: Not Exclusively Anti-anxiety. Front. Biosci. 2022, 14, 16. [Google Scholar] [CrossRef]
- Agustiyaningsih, T.; Ririn, H.; Lilis, S. Factors Affecting the Quality of Sleep in Patients with Chronic Obstructive Pulmonary Disease (COPD). Formosa J. Sci. Technol. 2023, 2, 1105–1114. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H. Factors Affecting the Quality of Sleep and Social Participation of Stroke Patients. Brain Sci. 2023, 13, 1068. [Google Scholar] [CrossRef]
- Wei, W.X.; Meng, L.; Mao, Z.F.; Mo, Z.H.; Yang, L.; Qin, Y.; Huang, J.Y. The association between the incident risk of Parkinson’s disease and depression in middle-aged and older adults, and the moderating role of lifestyle: Evidence from the CHARLS. Front. Psychol. 2025, 16, 1590931. [Google Scholar] [CrossRef]
- Burigo, F.E.T.; Salviano, F.d.N.S.; Pincerati, M.R.; Silva, I.S.d. Benefícios das plantas medicinais sobre eventos moleculares associados ao tratamento da Doença de Alzheimer. Rev. DELOS 2024, 17, e1509. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Simili, O.A.G.; Araújo, A.C.; Guiguer, E.L.; Direito, R.; Valenti, V.E.; de Oliveira, V.; de Oliveira, J.S.; Yanaguizawa Junior, J.L.; Dias, J.A.; et al. Melatonin from Plants: Going Beyond Traditional Central Nervous System Targeting-A Comprehensive Review of Its Unusual Health Benefits. Biology 2025, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, G.L.O.; Santos, L.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.; Goulart, R.A.; Catharin, V.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef]
- Safari, M.; Asadi, A.; Aryaeian, N.; Huseini, H.F.; Shidfar, F.; Jazayeri, S.; Malek, M.; Hosseini, A.F.; Hamidi, Z. The effects of melissa officinalis on depression and anxiety in type 2 diabetes patients with depression: A randomized double-blinded placebo-controlled clinical trial. BMC Complement. Med. Ther. 2023, 23, 140. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef]
- Arshad Ullah, M.; Hassan, A. Medicinal benefits of lemon balm (Melissa officinalis) for human health. World J. Chem. Pharm. Sci. 2022, 1, 028–033. [Google Scholar] [CrossRef]
- Alsahafi, T.; Bouback, T.; Albeshri, A.; Alnhhas, S.; Ali, M.; Moatasim, Y.; Kutkat, O.; Gaballah, M.; Alfasi, F.; Mater, E.H.; et al. Author Correction: Antiviral potential of Melissa officinalis extracts against influenza and emerging coronaviruses. Sci. Rep. 2025, 15, 20076. [Google Scholar] [CrossRef]
- Gutiérrez-Pacheco, M.M.; Torres-Moreno, H.; Flores-Lopez, M.L.; Velázquez Guadarrama, N.; Ayala-Zavala, J.F.; Ortega-Ramírez, L.A.; López-Romero, J.C. Mechanisms and Applications of Citral’s Antimicrobial Properties in Food Preservation and Pharmaceuticals Formulations. Antibiotics 2023, 12, 1608. [Google Scholar] [CrossRef]
- Li, Y.; Mei, J.; Xie, J. Citral: Bioactivity, Metabolism, Delivery Systems, and Food Preservation Applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70168. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, D.; Xiang, Y.; Jiang, X.; Liu, J.; Bi, K.; Dong, X.; Wu, T.; Zhang, Y. Antifungal activity of essential oils and their potential synergistic effect with amphotericin B. Sci. Rep. 2024, 14, 31125. [Google Scholar] [CrossRef]
- Rokonuzzman, M.; Bhuia, M.S.; Al-Qaaneh, A.M.; El-Nashar, H.A.S.; Islam, T.; Chowdhury, R.; Hasan Shanto, H.; Al Hasan, M.S.; El-Shazly, M.; Torequl Islam, M. Biomedical Perspectives of Citronellal: Biological Activities, Toxicological Profile and Molecular Mechanisms. Chem. Biodivers. 2025, 22, e202401973. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wei, Y.; Yang, Z.; Zhang, L.; Shan, A. Synergism between nisin and citronellal against Fusarium graminearum and their application in maize preservation. Int. J. Food Microbiol. 2025, 441, 111331. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, H.; Krishnatreyya, H. Technological Advancements in Mosquito Repellents: Challenges and Opportunities in Plant-Based Repellents. Acta Parasitol. 2025, 70, 117. [Google Scholar] [CrossRef] [PubMed]
- Godad, A.; Sawant, R.; Pahelkar, A.R.; Pereira, G.; Sathaye, S. Exploring the Potential Mechanism of Action of Ursolic Acid for Parkinson’s Disease: An Integrative Network Pharmacology, Docking and Molecular Dynamics Study. Mol. Neurobiol. 2025, 62, 13636–13649. [Google Scholar] [CrossRef]
- Fajardo, J.B.; Vianna, M.H.; Ferreira, T.G.; de O.Lemos, A.S.; Souza, T.F.; Campos, L.M.; Paula, P.L.; Andrade, N.B.; Gamarano, L.R.; Queiroz, L.S.; et al. Enhanced Antitumor and Antibacterial Activities of Ursolic Acid through β-Cyclodextrin Inclusion Complexation. ACS Omega 2025, 10, 12906–12916. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, F.; Li, C.; Guo, L.; Chen, B.; Wang, F.; Liu, S.; Han, S. Ursolic acid improves growth performance, intestinal health, and antioxidant status in broilers by regulating lipid metabolism and the KEAP1-NRF2 pathway. J. Anim. Sci. 2025, 103, skaf333. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Günther, A.; Sip, S.; Bednarek-Rajewska, K.; Zalewski, P. Enhancing the Pharmacological Properties of Triterpenes Through Acetylation: An Anticancer and Antioxidant Perspective. Molecules 2025, 30, 2661. [Google Scholar] [CrossRef]
- Günther, A.; Bednarczyk-Cwynar, B. Oleanolic Acid: A Promising Antioxidant-Sources, Mechanisms of Action, Therapeutic Potential, and Enhancement of Bioactivity. Antioxidants 2025, 14, 598. [Google Scholar] [CrossRef]
- Qin, K.; Sun, X.; Liu, J.; Wang, R.; Huang, X.; Wang, Y.; Wang, H.; Yang, J.; Wang, S. A rosmarinic acid-fish skin protein-chitosan hybrid nano-delivery system with excellent sustained-release and antioxidant performances. Food Chem. 2025, 491, 145316. [Google Scholar] [CrossRef]
- Uçar-Ekin, C.; Aşır, F.; Şahin, F.; Kaplan, Ş. Rosmarinic acid alleviate hepatotoxicity induced by cyclophosphamide in rats. Cir. Cir. 2025, 93, 546–555. [Google Scholar] [CrossRef]
- Hassler, B.; Mohamed Hizam, V.; Pisani, A.; Sisto, R.; Paciello, F.; Grassi, C.; Fetoni, A.R. Targeting NLRP3 inflammasome activation in styrene-induced ototoxicity: Comparative efficacy of rosmarinic acid and anakinra in mitigating oxidative and inflammatory damage. Int. J. Audiol. 2025; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Al-Hunaiti, A.; Thiab, T.A.; Zihlif, M.; Abdul Majid, A.M.S.; Imraish, A.; Batarseh, Y.; Al Shhab, M. Rosmarinic acid attenuates doxorubicin-induced cardiotoxicity: Bio-nanocarrier system development and an in vitro study using H9c2 rat cardiomyocytes. Nanoscale Adv. 2025. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xu, Z.; Zhu, L.; Zhu, M.; Zhang, W.; Gong, M.; Liu, M.; Wang, M.; Xu, E.; Dai, L. Standardized aqueous extract of Abutilon theophrasti Medic. ameliorates oxidative stress and inflammatory responses against hydrochloric acid/ethanol-induced gastric ulcer in rats. Front. Pharmacol. 2025, 16, 1599810. [Google Scholar] [CrossRef] [PubMed]
- Dinh-Hung, N.; Khang, L.T.P.; Wisetkaeo, S.; Tran, N.T.; Po-Tsang, L.; Brown, C.L.; Sangsawad, P.; Dwinanti, S.H.; Permpoonpattana, P.; Linh, N.V. Caffeic Acid as a Promising Natural Feed Additive: Advancing Sustainable Aquaculture. Biology 2025, 14, 1160. [Google Scholar] [CrossRef]
- Draginic, N.D.; Jakovljevic, V.L.; Jeremic, J.N.; Srejovic, I.M.; Andjic, M.M.; Rankovic, M.R.; Sretenovic, J.Z.; Zivkovic, V.I.; Ljujic, B.T.; Mitrovic, S.L.; et al. Melissa officinalis L. Supplementation Provides Cardioprotection in a Rat Model of Experimental Autoimmune Myocarditis. Oxidative Med. Cell. Longev. 2022, 2022, 1344946. [Google Scholar] [CrossRef]
- Gan, X.; Li, J.; Li, S.; Wang, X.; Wang, Q.; Chen, X.; Huang, Y.; Nie, M.; Kang, H.; Dai, H. Integrating superlubricative nanomaterials with precision drug delivery for advanced osteoarthritis therapy. Mater. Today Bio 2025, 35, 102359. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Coleman, D.T.; Bigelow, R.; Cardelli, J.A. Inhibition of fatty acid synthase by luteolin post-transcriptionally down-regulates c-Met expression independent of proteosomal/lysosomal degradation. Mol. Cancer Ther. 2009, 8, 214–224. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Yu, T.; Song, R.; Nie, H.; Ding, Y. Anti-Oxidant, Anti-Inflammatory and Antiviral Properties of Luteolin Against SARS-CoV-2: Based on Network Pharmacology. Pharmaceuticals 2025, 18, 1329. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Sisti, D.; Rocchi, M.; Belli, A.; Bertuccioli, A.; Cazzaniga, M.; Palazzi, C.M.; Tanda, M.L.; Zerbinati, N. Effects of Melissa officinalis Phytosome on Sleep Quality: Results of a Prospective, Double-Blind, Placebo-Controlled, and Cross-Over Study. Nutrients 2024, 16, 4199. [Google Scholar] [CrossRef] [PubMed]
- Abbasnia, V.; Foadoddini, M.; Khazdair, M.R. Protective Effect of Melissa officinalis (Lemon Balm) Extract on Cytokine Levels and Oxidative Stress in Ovalbumin Induced Lung Toxicity in Rats. Basic. Clin. Pharmacol. Toxicol. 2025, 137, e70068. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ, Ü.; Macar, O.; Kalefetoğlu Macar, T.; Yalçın, E.; Çavuşoğlu, K. Effect of Melissa officinalis L. leaf extract on manganese-induced cyto-genotoxicity on Allium cepa L. Sci. Rep. 2023, 13, 22110. [Google Scholar] [CrossRef]
- Borgonetti, V.; Pressi, G.; Bertaiola, O.; Guarnerio, C.; Mandrone, M.; Chiocchio, I.; Galeotti, N. Attenuation of neuroinflammation in microglia cells by extracts with high content of rosmarinic acid from in vitro cultured Melissa officinalis L. cells. J. Pharm. Biomed. Anal. 2022, 220, 114969. [Google Scholar] [CrossRef]
- Tokatly Latzer, I.; Pearl, P.L. Update on inherited disorders of GABA metabolism. Eur. J. Paediatr. Neurol. 2025, 56, 10–16. [Google Scholar] [CrossRef]
- Anheyer, M.; Cramer, H.; Ostermann, T.; Längler, A.; Anheyer, D. Herbal Medicine for Treating Herpes Labialis: A Systematic Review. J. Integr. Complement. Med. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Thapa, K.; Kanojia, N.; Khan, H.; Kumar, J. Unveiling the anti-cancer potential of Melissa officinalis. Pharmacol. Res. Nat. Prod. 2025, 9, 100384. [Google Scholar] [CrossRef]
- Silva, B.; Cadavez, V.; Caleja, C.; Pereira, E.; Calhelha, R.; Añibarro Ortega, M.; Finimundy, T.; Kostic, M.; Soković, M.; Teixeira, J.; et al. Phytochemical Composition and Bioactive Potential of Melissa officinalis L., Salvia officinalis L. and Mentha spicata L. Extracts. Foods 2023, 12, 947. [Google Scholar] [CrossRef]
- Mabotja, M.B.; Aremu, A.O.; Doležal, K.; Ruzvidzo, O.; Amoo, S.O. Enhancing in vitro propagation of Melissa officinalis L.: Assessing the role of plant growth regulators as a pathway to sustainable production. Ind. Crops Prod. 2025, 233, 121377. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation. Foods 2013, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Taherianrad, F.; Dehghan, H.; Abbasabadi, N.; Padash, A.; Tehrani, H.J.; Tat, M.; Dayani, A.; Salimi, A. Melissa officinalis extract nanoemulsion, Caffeic acid and Quercetin as a novel inducer for investigating neural differentiation of human Wharton’s jelly mesenchymal stem cells. Tissue Cell 2025, 95, 102815. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, C.; Hiemstra, I.S.A.; Kazbar, A.; Costantino, G.; Righetti, L. Towards eco-metabolomics: NADES-guided extraction enables semi-quantitative metabolomics for Melissa officinalis. Adv. Sample Prep. 2025, 13, 100154. [Google Scholar] [CrossRef]
- Draginic, N.; Jakovljevic, V.; Andjic, M.; Jeremic, J.; Srejovic, I.; Rankovic, M.; Tomovic, M.; Nikolic Turnic, T.; Svistunov, A.; Bolevich, S.; et al. Melissa officinalis L. as a Nutritional Strategy for Cardioprotection. Front. Physiol. 2021, 12, 661778. [Google Scholar] [CrossRef]
- Posłuszny, M.A.; Chłopecka-Słomińska, M.; Cherer, S.S.; Cisse, S.; Benarbia, M.E.A.; Mendel, M. Verification of the Utility of the Standardized Melissa officinalis Extract to Control Gut Contractility in Sheep-Ex Vivo Study. Animals 2025, 15, 626. [Google Scholar] [CrossRef]
- Catalano, L.; Sagliano, L.; Visciglio, A.; Russo, P.; Miniello, S.; Trojano, L.; Panico, F. An integrated multifocal tDCS-EEG protocol for reducing cognitive and affective symptoms in mild cognitive impairment and early stages of dementia: A crossover double-blind randomized controlled trial. Front. Neurol. 2025, 16, 1605970. [Google Scholar] [CrossRef]
- Gavarić, N.; Radovanović, K.; Kladar, N.; Hitl, M.; Čonić, B.S.; Jovin, V.M.; Samojlik, I. Can we use Melissa officinalis (lemon balm) postdistillation waste extracts in pharmacy? In vivo pharmacodynamic studies. S. Afr. J. Bot. 2024, 172, 396–406. [Google Scholar] [CrossRef]
- Srinivas, N.S.; Vimalan, V.; Padmanabhan, P.; Gulyás, B. An Overview on Cognitive Function Enhancement through Physical Exercises. Brain Sci. 2021, 11, 1289. [Google Scholar] [CrossRef]
- Parker, T.D.; Hain, J.A.; Rooney, E.J.; Zimmerman, K.A.; Lee, Y.; Del Giovane, M.; Graham, N.S.N.; Patel, M.; Hampshire, A.; Wilson, M.G.; et al. Brain health concerns in former rugby players: Clinical and cognitive phenotypes. Brain 2025, 148, 2698–2713. [Google Scholar] [CrossRef]
- Lobach, A.R.; Schmidt, F.; Fedrizzi, D.; Müller, S. Toxicological safety evaluation of an aqueous lemon balm (Melissa officinalis) extract. Food Chem. Toxicol. 2024, 187, 114565. [Google Scholar] [CrossRef]
- Agnello, L.; Ciaccio, M. Neurodegenerative Diseases: From Molecular Basis to Therapy. Int. J. Mol. Sci. 2022, 23, 12854. [Google Scholar] [CrossRef]
- Souza-Talarico, J.N.; Perkhounkova, Y.; Hein, M.; Lee, J.; Hefti, M.; Sindi, S. Allostatic load dynamics, Alzheimer’s disease biomarkers, and progression in individuals with mild cognitive impairment: Findings from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 2025, 17, e70140. [Google Scholar] [CrossRef] [PubMed]
- Temple, S. Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell 2023, 30, 512–529. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Pan, G.; Zhang, M.; He, X.; Yao, M.; Yang, Y. The relationship between ageism, loneliness, and anxiety in widowed older adults: Cognitive function as a moderator in the mediated model. Front. Psychol. 2025, 16, 1624197. [Google Scholar] [CrossRef] [PubMed]
- Rommer, P.S.; Fuchs, D.; Leblhuber, F.; Schroth, R.; Greilberger, M.; Tafeit, E.; Greilberger, J. Lowered Levels of Carbonyl Proteins after Vitamin B Supplementation in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Neurodegener. Dis. 2016, 16, 284–289. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals 2025, 18, 133. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Liu, X.; Baxley, S.; Hebron, M.; Turner, R.S.; Moussa, C. Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5044. [Google Scholar] [CrossRef]
- Ohnishi, A.; Senda, M.; Yamane, T.; Sasaki, M.; Mikami, T.; Nishio, T.; Ikari, Y.; Nishida, H.; Shukuri, M.; Takashima, T.; et al. Human whole-body biodistribution and dosimetry of a new PET tracer, [(11)C]ketoprofen methyl ester, for imagings of neuroinflammation. Nucl. Med. Biol. 2014, 41, 594–599. [Google Scholar] [CrossRef]
- Moon, S.; Sarmento, C.V.M.; Smirnova, I.V.; Colgrove, Y.; Lai, S.M.; Lyons, K.E.; Liu, W. A pilot randomized clinical trial examining the effects of Qigong on inflammatory status and sleep quality in people with Parkinson’s disease. J. Bodyw. Mov. Ther. 2024, 40, 1002–1007. [Google Scholar] [CrossRef]
- Qin, H.; Hussain, L.; Liu, Z.; Yan, X.; Awwad, F.A.; Butt, F.M.; Salaria, U.A.; Ismail, E.A.A. Optimizing deep learning models to combat amyotrophic lateral sclerosis (ALS) disease progression. Digit. Health 2025, 11, 20552076251349719. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, C.H.; Ikawa, M.; Liow, J.S.; Zoghbi, S.S.; Morse, C.L.; Pike, V.W.; Fujita, M.; Innis, R.B.; Kreisl, W.C. Cerebellum Can Serve As a Pseudo-Reference Region in Alzheimer Disease to Detect Neuroinflammation Measured with PET Radioligand Binding to Translocator Protein. J. Nucl. Med. 2015, 56, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- De Wel, B.; Mobach, T.; Pfeffer, G.; Jewett, G. Tofersen treatment normalizes neurofilament levels in autosomal recessive SOD1 Amyotrophic Lateral Sclerosis. Can. J. Neurol. Sci. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Dodd, G.T. Hypothalamic neuronal-glial crosstalk in metabolic disease. NPJ Metab. Health Dis. 2024, 2, 27. [Google Scholar] [CrossRef]
- Fu, X.; Cai, H.; Quan, S.; Ren, Z.; Xu, Y.; Jia, L. Immune cells in Alzheimer’s disease: Insights into pathogenesis and potential therapeutic targets. Med. Rev. 2025, 5, 179–202. [Google Scholar] [CrossRef]
- Rohden, F.; Ferreira, P.C.L.; Bellaver, B.; Ferrari-Souza, J.P.; Aguzzoli, C.S.; Soares, C.; Abbas, S.; Zalzale, H.; Povala, G.; Lussier, F.Z.; et al. Glial reactivity correlates with synaptic dysfunction across aging and Alzheimer’s disease. Nat. Commun. 2025, 16, 5653. [Google Scholar] [CrossRef]
- Arnold, D.L.; Elliott, C.; Martin, E.C.; Hyvert, Y.; Tomic, D.; Montalban, X. Effect of Evobrutinib on Slowly Expanding Lesion Volume in Relapsing Multiple Sclerosis: A Post Hoc Analysis of a Phase 2 Trial. Neurology 2024, 102, e208058. [Google Scholar] [CrossRef]
- Koch, M.W.; Kaur, S.; Sage, K.; Kim, J.; Levesque-Roy, M.; Cerchiaro, G.; Yong, V.W.; Cutter, G.R.; Metz, L.M. Hydroxychloroquine for Primary Progressive Multiple Sclerosis. Ann. Neurol. 2021, 90, 940–948. [Google Scholar] [CrossRef]
- Xiao, M.; Gao, G.; Mu, J.; Sun, Q.; Zhao, Y.; Fan, X. MLKL Modulates Necroptosis and Neuroinflammation in a Mouse Model of MS. Inflammation, 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, A.M.; Hu, T.; Yang, J.; Wang, M.; Xu, Y.; Cao, W.Y. Peripheral administration of the NAT10 inhibitor Remodelin prevents against Lipopolysaccharide induced depression in male mice. Eur. J. Pharmacol. 2025, 1003, 177884. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Kinuthia, U.M.; Möhle, C.; Adams, R.H.; Langmann, T. Immunomodulation of inflammatory responses preserves retinal integrity in murine models of pericyte-depletion retinopathy. JCI Insight 2025, 10, e184465. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, O.; Seghier, M.L. The validity of studying healthy aging with cognitive tests measuring different constructs. Sci. Rep. 2024, 14, 23880. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.Y.; Liang, J.; Chen, Y.J.; Wu, C.C.; Shyu, Y.L. Influences of social support following hip-fracture surgery for older adults with cognitive impairment and their family caregivers. BMC Geriatr. 2025, 25, 469. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Baker, L.D.; Carrillo, M.C.; Snyder, H.M.; Cleveland, M.L.; Gitelman, D.R.; Kivipelto, M.; Leng, X.I.; Lovato, L.; Papp, K.V.; et al. Baseline characteristics of the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER): Successful enrollment of a diverse clinical trial cohort at risk for cognitive decline. Alzheimers Dement. 2025, 21, e70351. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Alcalá-Lozano, R.; Carmona-Hernández, R.; Ocampo-Romero, A.G.; Sosa-Millán, A.L.; Morelos-Santana, E.D.; Abarca, D.Z.; Castro-de-Aquino, D.V.; Cabrera-Muñoz, E.A.; Ramírez-Rodríguez, G.B.; Sosa Ortiz, A.L.; et al. Predicting the Beneficial Effects of Cognitive Stimulation and Transcranial Direct Current Stimulation in Amnestic Mild Cognitive Impairment with Clinical, Inflammation, and Human Microglia Exposed to Serum as Potential Markers: A Double-Blind Placebo-Controlled Randomized Clinical Trial. Int. J. Mol. Sci. 2025, 26, 1754. [Google Scholar] [CrossRef]
- Lavisse, S.; Goutal, S.; Wimberley, C.; Tonietto, M.; Bottlaender, M.; Gervais, P.; Kuhnast, B.; Peyronneau, M.A.; Barret, O.; Lagarde, J.; et al. Increased microglial activation in patients with Parkinson disease using [(18)F]-DPA714 TSPO PET imaging. Park. Relat. Disord. 2021, 82, 29–36. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Akimbekov, N.S.; Grant, W.B.; Dean, C.; Fang, X.; Razzaque, M.S. Neuroprotective effects of magnesium: Implications for neuroinflammation and cognitive decline. Front. Endocrinol. 2024, 15, 1406455. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease—A double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Franklin, E.E.; Li, Y.; Joseph-Mathurin, N.; Burns, A.L.; Hobbs, D.A.; McCullough, A.A.; Schultz, S.A.; Xiong, C.; Wang, G.; et al. Immunohistochemical evaluation of a trial of gantenerumab or solanezumab in dominantly inherited Alzheimer disease. Acta Neuropathol. 2025, 149, 57. [Google Scholar] [CrossRef]
- Dubbelman, M.A.; Liu, A.; Donohue, M.C.; Langford, O.; Raman, R.; Rentz, D.M.; Amariglio, R.; Sperling, R.A.; Aisen, P.S.; Marshall, G.A. Changes in Daily Functioning in Association With Tau and Amyloid Among Unimpaired Older Adults With and Without Elevated Amyloid. Neurology 2025, 104, e213775. [Google Scholar] [CrossRef]
- Safety and efficacy of trehalose in amyotrophic lateral sclerosis (HEALEY ALS Platform Trial): An adaptive, phase 2/3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2025, 24, 500–511. [CrossRef]
- Fleisher, A.S.; Munsie, L.M.; Perahia, D.G.S.; Andersen, S.W.; Higgins, I.A.; Hauck, P.M.; Lo, A.C.; Sims, J.R.; Brys, M.; Mintun, M. Assessment of Efficacy and Safety of Zagotenemab: Results From PERISCOPE-ALZ, a Phase 2 Study in Early Symptomatic Alzheimer Disease. Neurology 2024, 102, e208061. [Google Scholar] [CrossRef]
- Risen, S.J.; Boland, S.W.; Sharma, S.; Weisman, G.M.; Shirley, P.M.; Latham, A.S.; Hay, A.J.D.; Gilberto, V.S.; Hines, A.D.; Brindley, S.; et al. Targeting Neuroinflammation by Pharmacologic Downregulation of Inflammatory Pathways Is Neuroprotective in Protein Misfolding Disorders. ACS Chem. Neurosci. 2024, 15, 1533–1547. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, Z.; Zheng, J.; Deng, C.; Liu, D.; Chen, Y.; Zhou, R.; Zou, J.; Huang, G.; Zeng, Q.; et al. Electroacupuncture Attenuates High-Fat Diet-Exacerbated Alzheimer’s Pathology by Enhancing TFEB/TFE3-Mediated Autophagic Clearance of Tau and NLRP3 Inflammasome in 3xTg Mice. CNS Neurosci. Ther. 2025, 31, e70497. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Behrouz, V.; Zahroodi, M.; Clark, C.C.T.; Mir, E.; Atashi, N.; Rivaz, R. Effects of Garlic Supplementation on Cardiovascular Risk Factors in Adults: A Comprehensive Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. Oxidative damage in neurodegeneration: Roles in the pathogenesis and progression of Alzheimer disease. Physiol. Rev. 2023, 104, 103–197. [Google Scholar] [CrossRef]
- De Falco, A.; Cukierman, D.; Hauser-Davis, R.; Rey, N.A. Alzheimer’s disease: Etiological hypotheses and treatment perspectives. Química Nova 2015, 39. [Google Scholar] [CrossRef]
- Qin, Y.; Qiu, Y.; Zhong, J.; Ouyang, Z.; Jin, L.; Wu, H.; Zeng, Y. Neuroprotective Effects of Anisodine Hydromide in a Rat Model of Vascular Dementia and the Antioxidative Stress Mechanisms Involved. Sichuan Da Xue Xue Bao Yi Xue Ban 2025, 56, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Mulè, S.; Ferrari, S.; Rosso, G.; Galla, R.; Battaglia, S.; Curti, V.; Molinari, C.; Uberti, F. The Combined Effect of Green Tea, Saffron, Resveratrol, and Citicoline against Neurodegeneration Induced by Oxidative Stress in an In Vitro Model of Cognitive Decline. Oxidative Med. Cell. Longev. 2024, 2024, 7465045. [Google Scholar] [CrossRef] [PubMed]
- Piñar-Morales, R.; Durán, R.; Bautista-García, A.; García-Mansilla, M.J.; Aliaga-Gaspar, P.; Vives-Montero, F.; Barrero-Hernández, F.J. The impact of oxidative stress on symptoms associated with multiple sclerosis. Sci. Rep. 2025, 15, 22983. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, A.R.; Lee, J.Y.; Kim, H.S.; Yang, C.; Kim, J.K.; Go, Y.; Jung, I.C. Herbal Medicine for Patients with Cognitive Impairment: An Observational Study. Neuropsychiatr. Dis. Treat. 2021, 17, 3183–3194. [Google Scholar] [CrossRef]
- Kim, C.; Lee, Y.; Kang, S.G.; Lee, S.H. Effectiveness of Information and Communication Technology-Based Cognitive Behavioral Therapy Using the Smart Sleep App on Insomnia in Older Adults: Randomized Controlled Trial. J. Med. Internet Res. 2025, 27, e67751. [Google Scholar] [CrossRef]
- Shi, L.; Chen, S.J.; Ma, M.Y.; Bao, Y.P.; Han, Y.; Wang, Y.M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep. Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef]
- Pavlova, M.K.; Latreille, V. Sleep Disorders. Am. J. Med. 2019, 132, 292–299. [Google Scholar] [CrossRef]
- Kim, J.; Ben-Umeh, K.C.; Weir, R.; Manotas, K.; Kleinschmit, K.; Fischer, A.; Weir, P.; Wilson, F. Evaluating the risk of sleep disorders in subjects with a prior COVID-19 infection. PLoS ONE 2024, 19, e0311929. [Google Scholar] [CrossRef]
- Te, T.T.; Boland, M.R.; Ghadimi, S.; Dzierzewski, J.M.; Alessi, C.; Martin, J.L.; Kremen, S.; Bui, A.A.T.; Naeim, A.; Fung, C.H. Predicting subjective sleepiness during auditory cognitive testing using voice signaling analysis. Sleep. Sci. Pract. 2025, 9, 19. [Google Scholar] [CrossRef]
- Rowe, R.K.; Schulz, P.; He, P.; Mannino, G.S.; Opp, M.R.; Sierks, M.R. Acute sleep deprivation in mice generates protein pathology consistent with neurodegenerative diseases. Front. Neurosci. 2024, 18, 1436966. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xiao, L.D.; Guo, X.; Hu, Y.; Wang, N.; Wang, Y. The impact of social support and social constraints on sleep disturbances in patients with lung cancer undergoing chemotherapy: Serial mediators of sleep cognition and anxiety-depression. Asia Pac. J. Oncol. Nurs. 2025, 12, 100740. [Google Scholar] [CrossRef] [PubMed]
- Versace, S.; Pellitteri, G.; Sperotto, R.; Tartaglia, S.; Da Porto, A.; Catena, C.; Gigli, G.L.; Cavarape, A.; Valente, M. A State-of-Art Review of the Vicious Circle of Sleep Disorders, Diabetes and Neurodegeneration Involving Metabolism and Microbiota Alterations. Int. J. Mol. Sci. 2023, 24, 10615. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Xu, Y.; Hu, L.; Wei, Y.; Wang, Z. Machine learning-based brain magnetic resonance imaging radiomics for identifying rapid eye movement sleep behavior disorder in Parkinson’s disease patients. BMC Med. Imaging 2025, 25, 227. [Google Scholar] [CrossRef]
- Seiger, A.N.; Penzel, T.; Fietze, I. Chronic pain management and sleep disorders. Cell Rep. Med. 2024, 5, 101761. [Google Scholar] [CrossRef]
- Qiu, Y.; Duan, X.; Zhang, Z.; Zhao, L.; Yuan, Q.; Wang, M.; Xiao, S.; Sun, L. Sleep disturbances and language function impairment in the elderly: Evidence of limbic and prefrontal tracts involvement. J. Alzheimer’s Dis. JAD 2025, 106, 1261–1271. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wake, G.; Savelev, S.; Tildesley, N.T.J.; Perry, E.K.; Wesnes, K.A.; Scholey, A.B. Modulation of Mood and Cognitive Performance Following Acute Administration of Single Doses of Melissa Officinalis (Lemon Balm) with Human CNS Nicotinic and Muscarinic Receptor-Binding Properties. Neuropsychopharmacology 2003, 28, 1871–1881. [Google Scholar] [CrossRef]
- Watson, K.; Hatcher, D.; Good, A. A randomised controlled trial of Lavender (Lavandula Angustifolia) and Lemon Balm (Melissa Officinalis) essential oils for the treatment of agitated behaviour in older people with and without dementia. Complement. Ther. Med. 2019, 42, 366–373. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Nagai, T.; Kobayashi, S.; Komatsu, J.; Samuraki-Yokohama, M.; Iwasa, K.; Yokoyama, K.; Nakamura, H.; et al. Safety and efficacy of Melissa officinalis extract containing rosmarinic acid in the prevention of Alzheimer’s disease progression. Sci. Rep. 2020, 10, 18627. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Hamaguchi, T.; Sakai, K.; Komatsu, J.; Iwasa, K.; Horimoto, M.; Nakamura, H.; Yamada, M.; Ono, K. Effects of Melissa officinalis Extract Containing Rosmarinic Acid on Cognition in Older Adults Without Dementia: A Randomized Controlled Trial. J. Alzheimers Dis. 2023, 91, 805–814. [Google Scholar] [CrossRef]
- Taavoni, S.; Nazem Ekbatani, N.; Haghani, H. Valerian/lemon balm use for sleep disorders during menopause. Complement. Ther. Clin. Pract. 2013, 19, 193–196. [Google Scholar] [CrossRef]
- Haybar, H.; Javid, A.; Haghighizadeh, M.H.; Valizadeh, E.; Mohaghegh, S.M.; Mohammadzadeh, A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clin. Nutr. ESPEN 2018, 26, 47–52. [Google Scholar] [CrossRef]
- Shirazi, M.; Jalalian, M.N.; Abed, M.; Ghaemi, M. The Effectiveness of Melissa Officinalis L. versus Citalopram on Quality of Life of Menopausal Women with Sleep Disorder: A Randomized Double-Blind Clinical Trial. Rev. Bras. Ginecol. Obs. 2021, 43, 126–130. [Google Scholar] [CrossRef]


| Bioactive Compound | Structure | Functions | Reference |
|---|---|---|---|
| Volatile Compounds | |||
| Geranial (Citral A) |  | Antifungal, antibacterial | [37,40] |
| Neral (Citral B) | 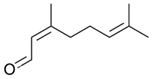 | Antifungal, antibacterial | [37,41,42] |
| Citronellal |  | Antimicrobial, insecticidal | [37,43,44,45] |
| Triterpenes | |||
| Ursolic acid |  | Antibacterial, antioxidant | [37,46,47,48] |
| Oleanolic acid | 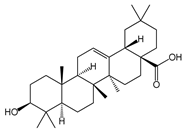 | Antiviral, hepatoprotective, antioxidant | [37,49,50] |
| Phenolic Compounds | |||
| Rosmarinic acid | 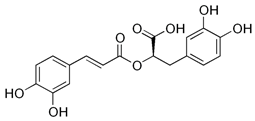 | Antioxidant, anti-inflammatory | [37,51,52,53,54] |
| Caffeic acid | 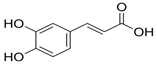 | Antioxidant, anti-inflammatory | [37,55,56] |
| Flavonoids | |||
| Quercetin | 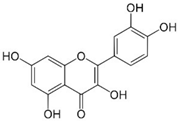 | Anti-inflammatory, antioxidant | [37,57,58,59] |
| Luteolin | 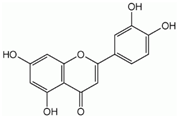 | Antioxidant, anti-inflammatory, immune system modulator, fatty acid synthase inhibitor | [60,61,62] |
| Model/Country | Population | Intervention/Comparison | Outcomes | Side Effects | Reference |
|---|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled, crossover study/United Kingdom | 20 healthy young volunteers. | Participants received acute single doses of 600 mg, 1000 mg, and 1600 mg of encapsulated dried leaf M. officinalis, compared to a placebo. | Improvements in cognitive performance and positive mood effects were observed, particularly with the dose of 1600 mg of M. officinalis. | NR by patients | [139] |
| Randomized, placebo-controlled trial/Australia | 49 older adults, with and without dementia, exhibiting agitated behaviors. | Participants were randomized to receive aromatherapy treatment with either lavender (Lavandula angustifolia) essential oil, lemon balm (M. officinalis) essential oil, or a placebo (sunflower oil) once daily for 2 weeks. | The study found no statistically significant differences in agitation levels between the treatment groups and the placebo group, independent of cognitive groups. | NR by patients | [140] |
| Randomized, double-blind, placebo-controlled, 24-week trial, with an open-label extension to 48 weeks/Japan | 23 patients (12 were in the M. officinalis extract group and 11 in the placebo group) with mild dementia, due to AD. | Patients were randomly assigned in a 1:1 ratio to receive the intervention (M. officinalis extract containing 500 mg of RA daily) or placebo, based on age and sex, for 24 weeks. In the extension period (an additional 24 weeks), all participants were allocated to the M. officinalis extract group. | The results suggested that RA may help prevent the worsening of AD-related neuropsychiatric symptoms. | The side effects reported during the extension period were bruising, headache, and hematuria. | [141] |
| Randomized, placebo-controlled, double-blind study/Japan | 323 individuals diagnosed with subjective or mild cognitive impairment aged between 65 and 79 years. | In phase 1, participants were randomized into M. officinalis group (n = 162, 185 mg of M. officinalis extract along with 50 mg of RA, 60 mg of lactose and 5 mg of calcium stearate) and placebo (n = 161, 210 mg of lactose, 35 mg of caramel and 5 mg of calcium stearate) for 96 weeks. Phase 2 was a washout period of 24 weeks for all individuals. | The results showed no significant differences in cognitive measures between the placebo and M. officinalis groups from baseline to 96 weeks. However, based on the analysis of CDR-SB scores in participants without hypertension, the score increased by 0.006 and decreased by 0.085 in the M. officinalis and placebo groups, respectively (p = 0.036). | 116 participants in the M. officinalis group and 114 in the placebo group reported adverse effects such as trauma, skin disease, orthopedic, cardiovascular disease, etc. | [142] |
| Reference | Model/Country | Population | Intervention/Comparison | Outcomes | Side Effects |
|---|---|---|---|---|---|
| [63] | Prospective, double-blind, placebo-controlled, and crossover study/Italy | 30 individuals (13 men and 17 women); 18–65 years, and perception of fatigue upon waking and unrefreshing sleep. | Patients received two tablets of M. officinalis highly standardized extract, formulated as Phytosome (MOP) or a placebo, every evening, 30 min before bedtime. | There was a significant reduction in the ISI score in the treated group, with 87% of the treated group having improvement in sleep quality, compared to 30% in the placebo group. | NR by patients |
| [143] | Randomized, triple-blind, placebo-controlled trial/Iran | 100 menopausal women aged 50–60 years experiencing sleep disturbances. | Intervention group (n = 50) received two capsules daily containing 160 mg of essence of Valeriana officinalis and 80 mg of Melissa officinalis (lemon balm). Placebo group (n = 50) received capsules containing 50 mg starch. | Significant improvement in sleep quality was observed in the treatment group, as measured by the PSQI. | NR by patients |
| [144] | Randomized, double-blind, placebo-controlled trial/Iran | 80 patients aged 40–75 years with chronic stable angina. | Participants received 3 g per day of M. officinalis (n = 40) or a placebo (n = 40) for 8 weeks. | Significant reductions in depression, anxiety, stress, and sleep disorders were observed in the M. officinalis group. | NR by patients |
| [145] | Randomized, double-blind clinical trial/Iran | 60 postmenopausal women (average age: 51.9 years) with menopause confirmation tests (FSH > 40 mIU/mL, and estradiol < 20 pg/mL), who slept poorly according to the PSQI. | Participants were randomized into three groups of 20 people, with one group receiving 30 mg of citalopram, another receiving one capsule of 500 mg M. officinalis, and the third group a 500 mg capsule of starch (placebo) once daily for 8 weeks. | All MENQOL domain scores improved significantly in the M. officinalis group compared to citalopram and placebo (p < 0.001). | Two participants in the citalopram group reported nausea, and one reported headache; no reports of adverse effects in the M. officinalis and placebo groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.V.B.; Garguerra, J.A.; Lamas, C.B.; Laurindo, L.F.; Rodrigues, V.D.; Sloan, K.P.; Sloan, L.A.; Chagas, E.F.B.; Guiguer, E.L.; Detregiachi, C.R.P.; et al. Unraveling the Effects of Melissa officinalis L. on Cognition and Sleep Quality: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 10566. https://doi.org/10.3390/ijms262110566
Oliveira MVB, Garguerra JA, Lamas CB, Laurindo LF, Rodrigues VD, Sloan KP, Sloan LA, Chagas EFB, Guiguer EL, Detregiachi CRP, et al. Unraveling the Effects of Melissa officinalis L. on Cognition and Sleep Quality: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(21):10566. https://doi.org/10.3390/ijms262110566
Chicago/Turabian StyleOliveira, Maria Vitória Barroso, Júlia Aparecida Garguerra, Caroline Barbalho Lamas, Lucas Fornari Laurindo, Victória Dogani Rodrigues, Kátia Portero Sloan, Lance Alan Sloan, Eduardo Federighi Baisi Chagas, Elen Landgraf Guiguer, Claudia Rucco Penteado Detregiachi, and et al. 2025. "Unraveling the Effects of Melissa officinalis L. on Cognition and Sleep Quality: A Narrative Review" International Journal of Molecular Sciences 26, no. 21: 10566. https://doi.org/10.3390/ijms262110566
APA StyleOliveira, M. V. B., Garguerra, J. A., Lamas, C. B., Laurindo, L. F., Rodrigues, V. D., Sloan, K. P., Sloan, L. A., Chagas, E. F. B., Guiguer, E. L., Detregiachi, C. R. P., Miglino, M. A., Pereira, E. d. S. B. M., Valenti, V. E., Silva, L. R., & Barbalho, S. M. (2025). Unraveling the Effects of Melissa officinalis L. on Cognition and Sleep Quality: A Narrative Review. International Journal of Molecular Sciences, 26(21), 10566. https://doi.org/10.3390/ijms262110566












