In Vitro Detoxification of Fumonisin B1 (FB1) into Hydrolyzed Fumonisin B1 (HFB1) by Lactobacillus spp. Isolated from Pig Caecum
Abstract
1. Introduction
2. Results
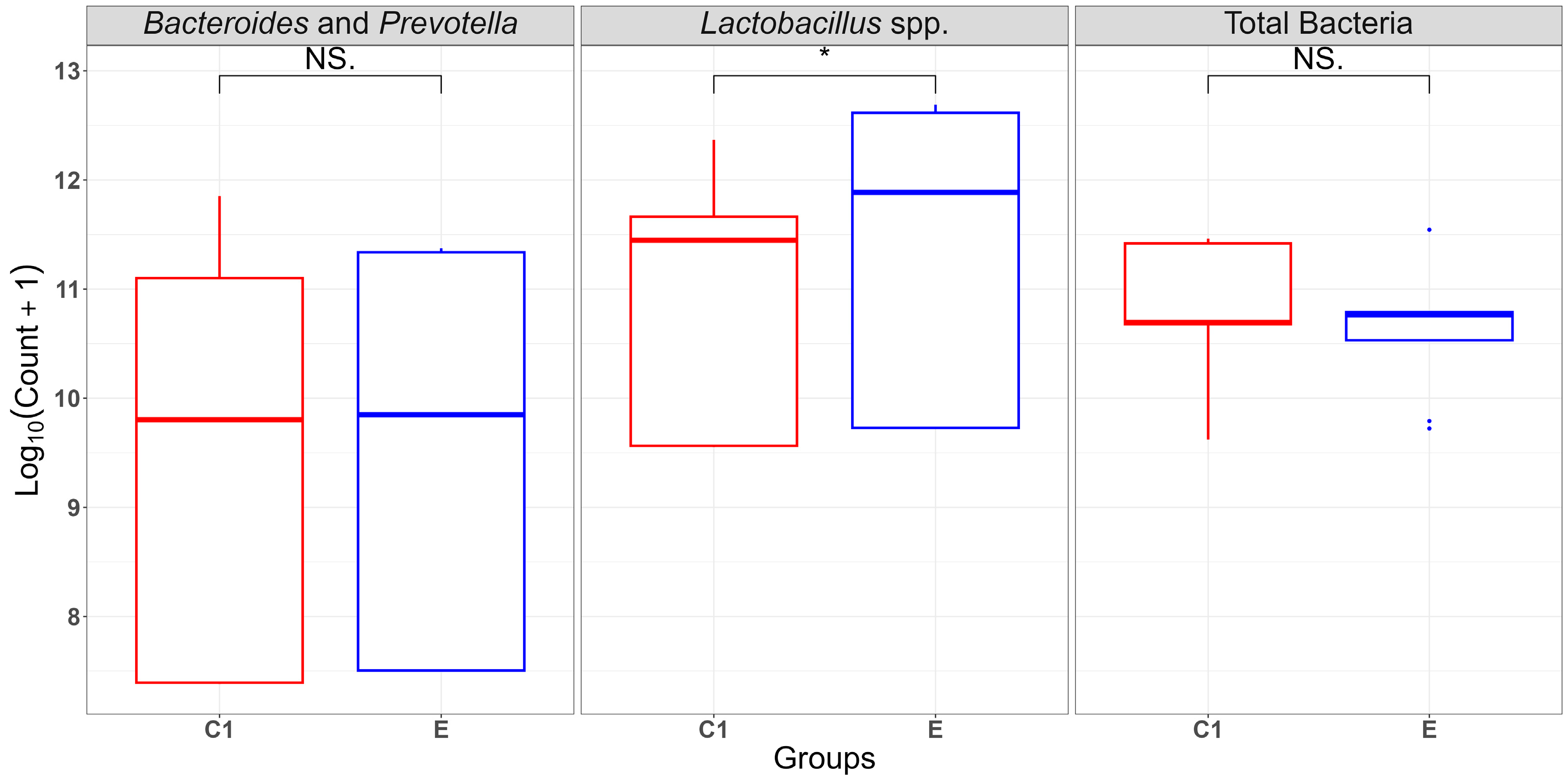
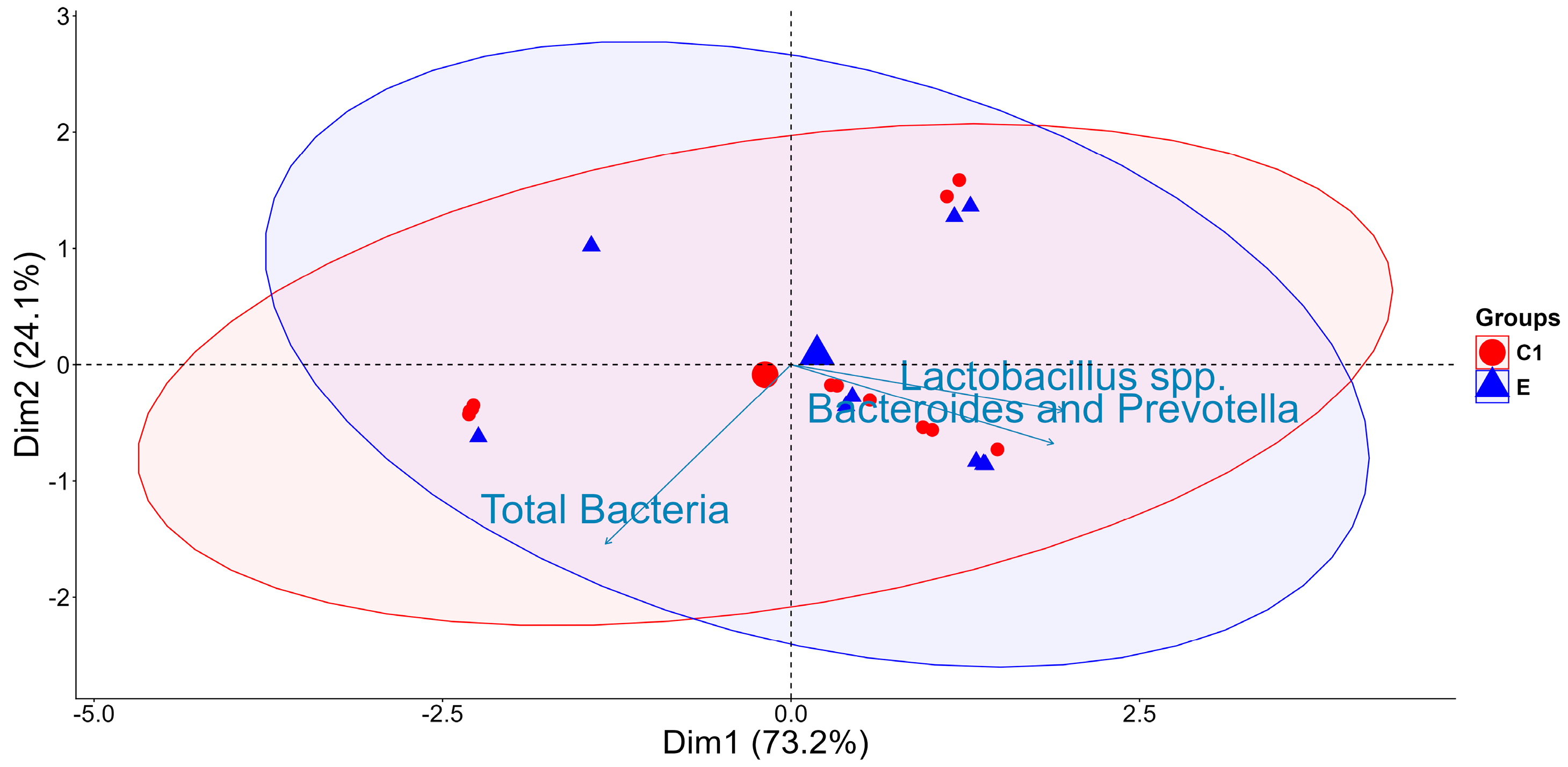
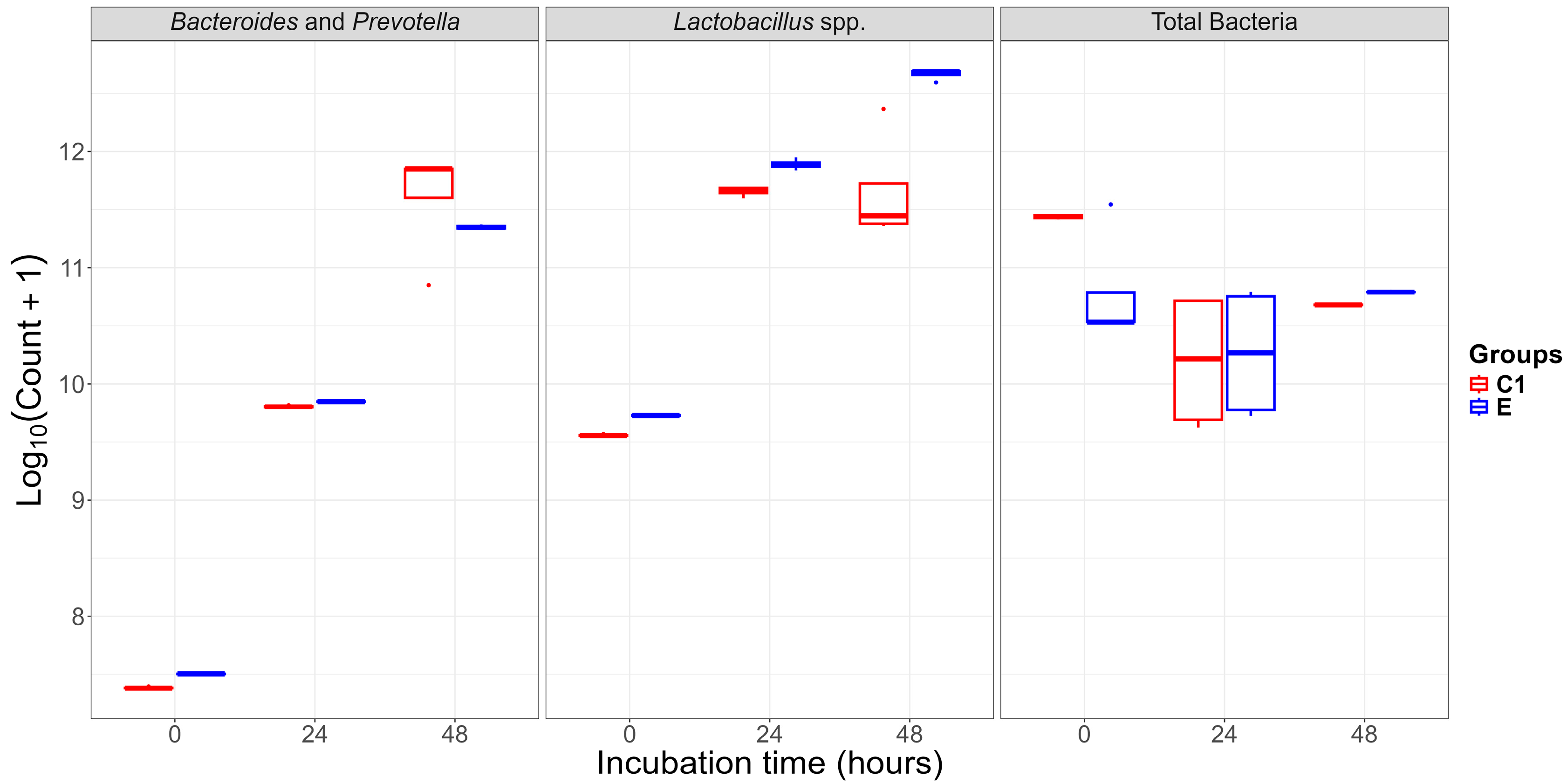
2.1. Bacterial Abundance and Temporal Dynamics of Gut Microbiota Incubated Without (C1: Buffer + Caecal Chyme) and with FB1 (E: Buffer + Caecal Chyme + FB1)
2.2. Effect of Gut Microbiota on FB1
2.3. Efficiency of Detoxification of FB1 to HFB1 (EFB1, %)
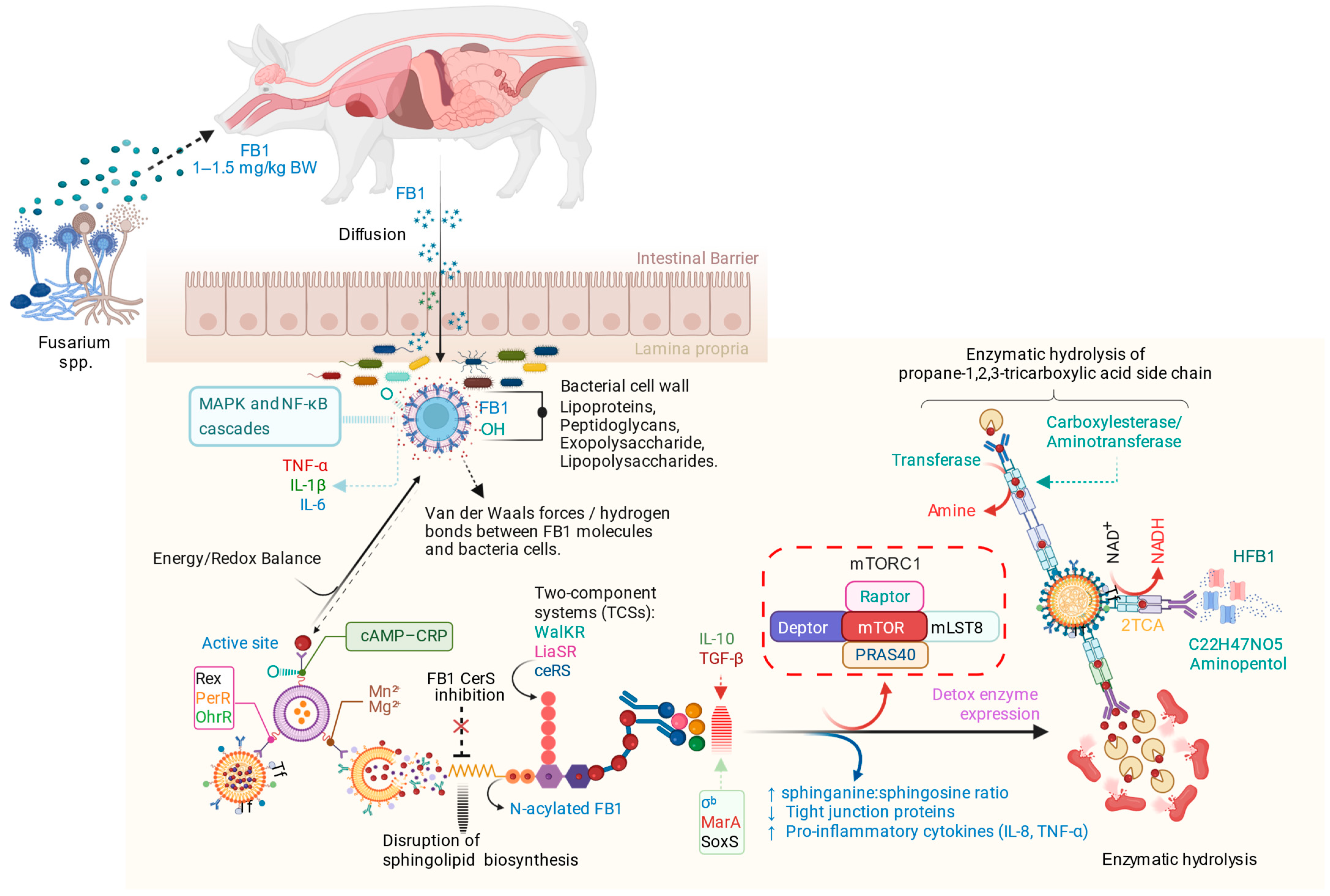
3. Discussion
- Adsorption: The first step involves physical interactions in which FB1 rapidly binds to microbial cell walls through non-covalent forces such as Van der Waals interactions and hydrogen bonds. This step occurs within minutes, concentrating the toxins on microbial surfaces and preventing their reabsorption into the gut lumen.
- Stabilization and Mass Transfer: After binding, the toxins are stabilized on microbial surfaces, reducing their dissociation back into the intestinal lumen. The bound toxins are distributed among microbial communities through diffusion and convective flow, influenced by gut dynamics over several hours to days.
- i.
- Transport: Mycotoxins migrate through the intestinal lumen toward microbial surfaces via diffusion and convective flow, influenced by gut motility and luminal content dynamics.
- ii.
- Surface Interaction: Mycotoxins associate with microbial surface polymers, such as peptidoglycans, lipopolysaccharides, exopolysaccharides, and lipoproteins, which facilitate toxin binding to the bacterial cell walls.
- iii.
- Adsorption: Mycotoxins bind non-covalently to microbial cell walls, creating stable associations that reduce their availability for absorption in the gut.
- iv.
- Biotransformation: Microbial enzymes hydrolyze FB1 into less toxic metabolites, such as hydrolyzed HFB1 and aminopentol, thereby reducing its toxicity in the gut.
4. Materials and Methods
4.1. Experimental Design, Sampling and Isolating
4.2. DNA Sequencing: Extraction, Quantification, and qPCR
4.3. Extraction of FB1, HFB1 and LC-MS Analysis Protocol
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| C1 | Control group 1 |
| C2 | Control group 2 |
| E | Experimental group |
| FB1 | Fumonisin B1 |
| HFB1 | Hydrolyzed fumonisin B1 |
| LC-MS | Liquid chromatography–mass spectrometry |
| MRS | de Man, Rogosa, and Sharpe |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| qPCR | Quantitative Polymerase Chain Reaction |
References
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed]
- Niderkorn, V.; Morgavi, D.P.; Aboab, B.; Lemaire, M.; Boudra, H. Cell wall component and mycotoxin moieties involved in the binding of fumonisin B1 and B2 by lactic acid bacteria. J. Appl. Microbiol. 2009, 106, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Zeebone, Y.Y.; Bota, B.; Halas, V.; Libisch, B.; Olasz, F.; Papp, P.; Kereszteny, T.; Gerocs, A.; Ali, O.; Kovacs, M.; et al. Gut-Faecal Microbial and Health-Marker Response to Dietary Fumonisins in Weaned Pigs. Toxins 2023, 15, 328. [Google Scholar] [CrossRef]
- Gelineau-van Waes, J.; Starr, L.; Maddox, J.; Aleman, F.; Voss, K.A.; Wilberding, J.; Riley, R.T. Maternal fumonisin exposure and risk for neural tube defects: Mechanisms in an in vivo mouse model. Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 487–497. [Google Scholar] [CrossRef]
- Vanara, F.; Scarpino, V.; Blandino, M. Fumonisin Distribution in Maize Dry-Milling Products and By-Products: Impact of Two Industrial Degermination Systems. Toxins 2018, 10, 357. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Cheng, Y.; Gao, C.; Guo, L.; Wang, T.; Xu, J. Sphinganine-Analog Mycotoxins (SAMs): Chemical Structures, Bioactivities, and Genetic Controls. J. Fungi 2020, 6, 312. [Google Scholar] [CrossRef]
- Rheeder, J.P.; Marasas, W.F.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef]
- Voss, K.A.; Smith, G.W.; Haschek, W.M. Fumonisins: Toxicokinetics, Mechanism of Action and Toxicity. Anim. Feed Sci. Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Szabo, A.; Szabo-Fodor, J.; Kachlek, M.; Mezes, M.; Balogh, K.; Glavits, R.; Ali, O.; Zeebone, Y.Y.; Kovacs, M. Dose and Exposure Time-Dependent Renal and Hepatic Effects of Intraperitoneally Administered Fumonisin B1 in Rats. Toxins 2018, 10, 465. [Google Scholar] [CrossRef]
- European_Union On official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0625&from=EN (accessed on 31 March 2025).
- Chlebicz, A.; Slizewska, K. In Vitro Detoxification of Aflatoxin B1, Deoxynivalenol, Fumonisins, T-2 Toxin and Zearalenone by Probiotic Bacteria from Genus Lactobacillus and Saccharomyces cerevisiae Yeast. Probiotics Antimicrob. Proteins 2020, 12, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, R.; Nazzi, F.; Locci, R.; Firrao, G. Degradation of fumonisin B1 by a bacterial strain isolated from soil. Biodegradation 2006, 17, 31–38. [Google Scholar] [CrossRef]
- Heinl, S.; Hartinger, D.; Thamhesl, M.; Vekiru, E.; Krska, R.; Schatzmayr, G.; Moll, W.D.; Grabherr, R. Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 2010, 145, 120–129. [Google Scholar] [CrossRef]
- Niderkorn, V.; Morgavi, D.P.; Pujos, E.; Tissandier, A.; Boudra, H. Screening of fermentative bacteria for their ability to bind and biotransform deoxynivalenol, zearalenone and fumonisins in an in vitro simulated corn silage model. Food Addit. Contam. 2007, 24, 406–415. [Google Scholar] [CrossRef]
- Qu, L.; Wang, L.; Ji, H.; Fang, Y.; Lei, P.; Zhang, X.; Jin, L.; Sun, D.; Dong, H. Toxic Mechanism and Biological Detoxification of Fumonisins. Toxins 2022, 14, 182. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, S.; Yu, S.; Zhao, Y.; Wu, Y.; Wu, A. LC-MS/MS Analysis of Fumonisin B1, B2, B3, and Their Hydrolyzed Metabolites in Broiler Chicken Feed and Excreta. Toxins 2022, 14, 131. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; Wei, D. Biological Detoxification of Mycotoxins: Current Status and Future Advances. Int. J. Mol. Sci. 2022, 23, 1064. [Google Scholar] [CrossRef]
- Szabo, C.; Kachungwa Lugata, J.; Ortega, A. Gut Health and Influencing Factors in Pigs. Animals 2023, 13, 1350. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Bernardes, T.F.; Copani, G.; Fortunati, P.; Giuberti, G.; Bruschi, S.; Bryan, K.A.; Nielsen, N.G.; Witt, K.L.; Masoero, F. Effect of Inoculation with Lactobacillus Buchneri LB1819 and Lactococcus Lactis O224 on Fermentation and Mycotoxin Production in Maize Silage Compacted at Different Densities. Anim. Feed Sci. Technol. 2018, 246, 36–45. [Google Scholar] [CrossRef]
- Szabo, A.; Szabo-Fodor, J.; Febel, H.; Romvari, R.; Kovacs, M. Individual and combined haematotoxic effects of fumonisin B1 and T-2 mycotoxins in rabbits. Food Chem. Toxicol. 2014, 72, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, X.; Zhang, J.; Zhang, J.; Zhang, B. The mechanism of Lactobacillus strains for their ability to remove fumonisins B1 and B2. Food Chem. Toxicol. 2016, 97, 40–46. [Google Scholar] [CrossRef]
- De Angelis, M.; Siragusa, S.; Berloco, M.; Caputo, L.; Settanni, L.; Alfonsi, G.; Amerio, M.; Grandi, A.; Ragni, A.; Gobbetti, M. Selection of potential probiotic lactobacilli from pig feces to be used as additives in pelleted feeding. Res. Microbiol. 2006, 157, 792–801. [Google Scholar] [CrossRef]
- Valeriano, V.D.; Balolong, M.P.; Kang, D.K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Maidana, L.; de Souza, M.; Bracarense, A. Lactobacillus plantarum and Deoxynivalenol Detoxification: A Concise Review. J. Food Prot. 2022, 85, 1815–1823. [Google Scholar] [CrossRef]
- Mischler, S.; Andre, A.; Chetschik, I.; Miescher Schwenninger, S. Potential for the Bio-Detoxification of the Mycotoxins Enniatin B and Deoxynivalenol by Lactic Acid Bacteria and Bacillus spp. Microorganisms 2024, 12, 1892. [Google Scholar] [CrossRef] [PubMed]
- Mateos, I.; Combes, S.; Pascal, G.; Cauquil, L.; Barilly, C.; Cossalter, A.M.; Laffitte, J.; Botti, S.; Pinton, P.; Oswald, I.P. Fumonisin-Exposure Impairs Age-Related Ecological Succession of Bacterial Species in Weaned Pig Gut Microbiota. Toxins 2018, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Isaacson, R.E.; et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 54. [Google Scholar] [CrossRef]
- Sanca, F.M.M.; Blanco, I.R.; Dias, M.; Moreno, A.M.; Martins, S.; Stephano, M.A.; Mendes, M.A.; Mendonca, C.M.N.; Pereira, W.A.; Azevedo, P.O.S.; et al. Antimicrobial Activity of Peptides Produced by Lactococcus lactis subsp. lactis on Swine Pathogens. Animals 2023, 13, 2442. [Google Scholar] [CrossRef]
- Su, Y.; Yao, W.; Perez-Gutierrez, O.N.; Smidt, H.; Zhu, W.Y. Changes in abundance of Lactobacillus spp. and Streptococcus suis in the stomach, jejunum and ileum of piglets after weaning. FEMS Microbiol. Ecol. 2008, 66, 546–555. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalban-Lopez, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Callewaert, R.; De Vuyst, L. Bacteriocin production with Lactobacillus amylovorus DCE 471 is improved and stabilized by fed-batch fermentation. Appl. Environ. Microbiol. 2000, 66, 606–613. [Google Scholar] [CrossRef]
- Lei, X.; Liu, Q.; Li, W.; Li, Y.; Zhao, L.; Liu, W. Comparative Genomics of Limosilactobacillus pontis Strains: Niche-Specific Variations and Adaptations. Diversity 2024, 16, 380. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.A.; Jang, H.J.; Kim, D.H.; Kim, Y. Complete genome sequence of functional probiotic candidate Lactobacillus amylovorus CACC736. J. Anim. Sci. Technol. 2023, 65, 473–477. [Google Scholar] [CrossRef]
- Padmavathi, T.; Bhargavi, R.; Priyanka, P.R.; Niranjan, N.R.; Pavitra, P.V. Screening of potential probiotic lactic acid bacteria and production of amylase and its partial purification. J. Genet. Eng. Biotechnol. 2018, 16, 357–362. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Zhao, Y.; Lin, Z.; Ji, L.; Ma, X. Tibetan Pig-Derived Probiotic Lactobacillus amylovorus SLZX20-1 Improved Intestinal Function via Producing Enzymes and Regulating Intestinal Microflora. Front. Nutr. 2022, 9, 846991. [Google Scholar] [CrossRef] [PubMed]
- Wylensek, D.; Hitch, T.C.A.; Riedel, T.; Afrizal, A.; Kumar, N.; Wortmann, E.; Liu, T.; Devendran, S.; Lesker, T.R.; Hernandez, S.B.; et al. A collection of bacterial isolates from the pig intestine reveals functional and taxonomic diversity. Nat. Commun. 2020, 11, 6389. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.J.K.; Yang, Q.; Brandao Melo, A.D.; Marcal, D.A.; Korth, N.; Pavlovikj, N.; Benson, A.K.; Htoo, J.K.K.; Brand, H.G.; Hauschild, L.; et al. Fecal microbiome of pigs fed diets differing in protein and amino acid content raised in thermoneutral or cyclical heat stress conditions. Front. Microbiol. 2025, 16, 1585374. [Google Scholar] [CrossRef]
- Zang, J.; Lin, T.; Xu, C.; Lin, Y.; Ma, K.; Zhang, C.; Rui, X.; Gan, D.; Li, W. Advances in Lactic Acid Bacteria and Their Metabolites in Fermented Foods: Mechanisms of Food Quality Enhancement, Gut Microbiota modulation, and Future Prospects. Food Rev. Int. 2025, 35, 1–35. [Google Scholar] [CrossRef]
- Fodor, J.; Balogh, K.; Weber, M.; Miklos, M.; Kametler, L.; Posa, R.; Mamet, R.; Bauer, J.; Horn, P.; Kovacs, F.; et al. Absorption, distribution and elimination of fumonisin B1 metabolites in weaned piglets. Food Addit. Contam. Part. A 2008, 25, 88–96. [Google Scholar] [CrossRef]
- Masching, S.; Naehrer, K.; Schwartz-Zimmermann, H.E.; Sarandan, M.; Schaumberger, S.; Dohnal, I.; Nagl, V.; Schatzmayr, D. Gastrointestinal Degradation of Fumonisin B1 by Carboxylesterase FumD Prevents Fumonisin Induced Alteration of Sphingolipid Metabolism in Turkey and Swine. Toxins 2016, 8, 84. [Google Scholar] [CrossRef]
- van de Guchte, M.; Serror, P.; Chervaux, C.; Smokvina, T.; Ehrlich, S.D.; Maguin, E. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 2002, 82, 187–216. [Google Scholar] [CrossRef] [PubMed]
- Martinez Tuppia, C.; Atanasova-Penichon, V.; Chereau, S.; Ferrer, N.; Marchegay, G.; Savoie, J.M.; Richard-Forget, F. Yeast and bacteria from ensiled high moisture maize grains as potential mitigation agents of fumonisin B1. J. Sci. Food Agric. 2017, 97, 2443–2452. [Google Scholar] [CrossRef]
- Dang, H.A.; Zsolnai, A.; Kovacs, M.; Bors, I.; Bonai, A.; Bota, B.; Szabo Fodor, J. In vitro Interaction between Fumonisin B1 and the Intestinal Microflora of Pigs. Pol. J. Microbiol. 2017, 66, 245–250. [Google Scholar] [CrossRef]
- Fodor, J.; Meyer, K.; Gottschalk, C.; Mamet, R.; Kametler, L.; Bauer, J.; Horn, P.; Kovacs, F.; Kovacs, M. In vitro microbial metabolism of fumonisin B1. Food Addit. Contam. 2007, 24, 416–420. [Google Scholar] [CrossRef]
- Malgwi, I.H. Proposed Mechanism of Fumonisin B1 (FB1) Biodegradation in the Gut. Available online: https://BioRender.com/4lrg2dp (accessed on 9 October 2025).
- Wang, Z.; Lv, Z.; Czabany, T.; Nagl, V.; Krska, R.; Wang, X.; Han, B.; Tao, H.; Liu, J.; Wang, J. Comparison Study of Two Fumonisin-Degrading Enzymes for Detoxification in Piglets. Toxins 2023, 16, 3. [Google Scholar] [CrossRef]
- Grenier, B.; Oswald, I. Mycotoxin Co-Contamination of Food and Feed: Meta-Analysis of Publications Describing Toxicological Interactions. World Mycotoxin J. 2011, 4, 28. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaed, M.; Haghdoust, S. Chapter 1—Fundamentals of Adsorption Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, p. 70. [Google Scholar]
- Antonissen, G.; De Baere, S.; Novak, B.; Schatzmayr, D.; den Hollander, D.; Devreese, M.; Croubels, S. Toxicokinetics of Hydrolyzed Fumonisin B1 after Single Oral or Intravenous Bolus to Broiler Chickens Fed a Control or a Fumonisins-Contaminated Diet. Toxins 2020, 12, 413. [Google Scholar] [CrossRef]
- Ndiaye, S.; Zhang, M.; Fall, M.; Ayessou, N.M.; Zhang, Q.; Li, P. Current Review of Mycotoxin Biodegradation and Bioadsorption: Microorganisms, Mechanisms, and Main Important Applications. Toxins 2022, 14, 729. [Google Scholar] [CrossRef]
- Castillo, M.; Martin-Orue, S.M.; Manzanilla, E.G.; Badiola, I.; Martin, M.; Gasa, J. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Vet. Microbiol. 2006, 114, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Dymock, D.; Wade, W.G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 1998, 64, 795–799, Erratum in Appl. Environ. Microbiol. 1998, 64, 2333. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Morrison, M.; Yu, Z. Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. 2011, 76, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Heilig, H.G.; Zoetendal, E.G.; Vaughan, E.E.; Marteau, P.; Akkermans, A.D.; de Vos, W.M. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 2002, 68, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Fujita, K.; Maeno, S.; Shiwa, Y.; Endo, A.; Yokota, K.; Igimi, S.; Kajikawa, A. PCR-based screening, isolation, and partial characterization of motile lactobacilli from various animal feces. BMC Microbiol. 2020, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing, Version 4.4.1. Computer Software. Available online: https://www.R-project.org/ (accessed on 1 October 2025).
| 0 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
| Log10 Copy Number/g | ||||||
| Bacteria Groups | C1 | E | C1 | E | C1 | E |
| Total Bacteria | 11.44 ± 0.02 aA | 10.78 ± 0.51 aB | 10.19 ± 0.61 bA | 10.26 ± 0.58 bA | 10.68 ± 0.00 bA | 10.79 ± 0.00 bA |
| Bacteroides and Prevotella | 7.39 ± 0.01 Ab | 7.5 ± 0.00 aA | 9.8 ± 0.01 bA | 9.85 ± 0.00 bA | 11.6 ± 0.50 cA | 11.35 ± 0.02 cA |
| Lactobacillus spp. | 9.56 ± 0.01 aB | 9.73 ± 0.00 aA | 11.66 ± 0.04 bA | 11.89 ± 0.05 bA | 11.66 ± 0.48 bB | 12.66 ± 0.05 cA |
| Total Bacteria | |||||
|---|---|---|---|---|---|
| Source of Variation | df | Sum of Squares | Mean Square | F-Value | p-Value |
| Groups | 1 | 0.15 | 0.15 | 0.934 | 0.35 |
| Time (h) | 2 | 3.162 | 1.581 | 9.849 | 0.001 |
| Groups: Time (h) | 2 | 0.741 | 0.37 | 2.307 | 0.13 |
| Residuals | 18 | 2.89 | 0.161 | ||
| Bacteroides and Prevotella | |||||
| Groups | 1 | 0 | 0 | 0.119 | 0.73 |
| Time_hours | 2 | 65.73 | 32.86 | 786.751 | <0.001 |
| Groups: Time (h) | 2 | 0.15 | 0.08 | 1.816 | 0.19 |
| Residuals | 18 | 0.75 | 0.04 | ||
| Lactobacillus spp. | |||||
| Groups | 1 | 1.327 | 1.327 | 33.79 | <0.001 |
| Time (h) | 2 | 29.418 | 14.709 | 374.46 | <0.001 |
| Groups: Time (h) | 2 | 0.871 | 0.435 | 11.08 | <0.001 |
| Residuals | 18 | 0.707 | 0.039 |
| Item | Sampling Time, Hours | HFB1 Concentrations, µg/mL | EFB1, % | |
|---|---|---|---|---|
| C2 | E | |||
| 0 | 0 | 0 | ||
| HFB1 | 24 | 0 | 1.238 ± 0.339 | 47.14 |
| 48 | 0 | 1.483 ± 0.079 | 56.47 | |
| Item | Treatments | ||
|---|---|---|---|
| C1 | C2 | E | |
| Buffer, mL | 5.67 | 5.67 | 5.67 |
| Caecal chyme, g | 3.33 | - | 3.33 |
| Distilled H2O, mL | 1 | - | - |
| FB1 Concentrations, µg/mL | - | 5 | 5 |
| Samples per treatment and incubation time | 4 | 4 | 4 |
| Incubation time, hours | 0, 24, 48 | 0, 24, 48 | 0, 24, 48 |
| Bacteria | Primer Sequences | Amplicon Size (bp) | References | |
|---|---|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | |||
| Total Bacteria | GCAGGCCTAACACATGCAAGTC | CTGCTGCCTCCCGTAGGAGT | 292 | [51,52] |
| Bacteroides and Prevotella | GAAGGTCCCCCACATTG | CAATCGGAGTTCTTCGTG | 418 | [53] |
| Lactobacillus spp. | AGCAGTAGGGAATCTTCCA | CACCGCTACACATGGAG | 340 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, H.A.; Zsolnai, A.; Kachlek, M.; Halas, V.; Giannuzzi, D.; Schiavon, S.; Malgwi, I.H. In Vitro Detoxification of Fumonisin B1 (FB1) into Hydrolyzed Fumonisin B1 (HFB1) by Lactobacillus spp. Isolated from Pig Caecum. Int. J. Mol. Sci. 2025, 26, 10557. https://doi.org/10.3390/ijms262110557
Dang HA, Zsolnai A, Kachlek M, Halas V, Giannuzzi D, Schiavon S, Malgwi IH. In Vitro Detoxification of Fumonisin B1 (FB1) into Hydrolyzed Fumonisin B1 (HFB1) by Lactobacillus spp. Isolated from Pig Caecum. International Journal of Molecular Sciences. 2025; 26(21):10557. https://doi.org/10.3390/ijms262110557
Chicago/Turabian StyleDang, Huu Anh, Attila Zsolnai, Mariam Kachlek, Veronika Halas, Diana Giannuzzi, Stefano Schiavon, and Isaac Hyeladi Malgwi. 2025. "In Vitro Detoxification of Fumonisin B1 (FB1) into Hydrolyzed Fumonisin B1 (HFB1) by Lactobacillus spp. Isolated from Pig Caecum" International Journal of Molecular Sciences 26, no. 21: 10557. https://doi.org/10.3390/ijms262110557
APA StyleDang, H. A., Zsolnai, A., Kachlek, M., Halas, V., Giannuzzi, D., Schiavon, S., & Malgwi, I. H. (2025). In Vitro Detoxification of Fumonisin B1 (FB1) into Hydrolyzed Fumonisin B1 (HFB1) by Lactobacillus spp. Isolated from Pig Caecum. International Journal of Molecular Sciences, 26(21), 10557. https://doi.org/10.3390/ijms262110557








