Abstract
Periodontitis is a chronic inflammatory disease of the periodontal tissues primarily caused by dysbiotic bacterial communities. Accumulating evidence suggests that periodontal pathogens not only drive the initiation and progression of periodontitis but also significantly contribute to systemic disorders, including diabetes mellitus, cardiovascular disease, cancer, and adverse pregnancy outcomes, such as preterm birth. The key periodontal pathogens implicated in disease pathogenesis include Porphyromonas gingivalis, Prevotella intermedia, Treponema denticola, Tannerella forsythia, Aggregatibacter actinomycetemcomitans, and Fusobacterium nucleatum. Among the diverse factors governing bacterial colonization and biofilm formation, interspecies interactions, particularly coaggregation, play a critical role in dental plaque maturation and the establishment of pathogenic microbial communities. Coaggregation facilitates the spatial organization of bacteria within biofilms, enhances bacterial survival, and modulates virulence factor expression. This review summarizes current knowledge regarding bacterial interactions involving major periodontal pathogens, with particular emphasis on coaggregation mechanisms, and discusses the implications of this coaggregation for periodontitis pathogenesis and associated systemic diseases.
1. Introduction
The oral cavity harbors one of the most diverse microbial ecosystems in the human body, comparable to that of the gastrointestinal tract. To date, more than 700 microbial species have been identified in the oral environment, of which approximately 500 are bacterial species [1,2]. Oral bacteria adapt to distinct ecological niches, adhering to gingival tissues and tooth surfaces, and thereby facilitating dental plaque formation. Periodontal pathogens are predominantly obligate anaerobes that colonize the subgingival crevice, where oxygen tension is relatively low. In contrast, streptococci, the dominant bacterial group in the oral cavity, exhibit greater tolerance to oxidative stress. Their early colonization of tooth surfaces facilitates the subsequent establishment of late colonizers, including periodontal pathogens [3,4]. Early and late colonizers interact with each other, either directly or indirectly, through mechanisms such as coaggregation [5,6]. Such interspecies interactions are fundamental to plaque maturation and provide a foundation for understanding how key periodontal pathogens establish themselves within the oral microbiome. Moreover, when the ecological balance of these microbial communities is disturbed by factors, such as poor oral hygiene and host immune dysregulation, the resulting dysbiosis promotes the overgrowth of pathogenic species and triggers the chronic inflammation characteristic of periodontitis [7,8]. Understanding these dysbiotic shifts is therefore crucial for elucidating the etiopathogenesis of periodontal disease. Importantly, accumulating evidence suggests that oral dysbiosis not only contributes to periodontal tissue destruction but also influences systemic health, linking periodontitis to several diseases, including cardiovascular disease, Alzheimer’s disease, diabetes, and cancer [9,10].
Several bacterial species have been recognized as major periodontal pathogens. These include Porphyromonas gingivalis, Prevotella intermedia, Treponema denticola, Tannerella forsythia, Aggregatibacter actinomycetemcomitans, and Fusobacterium nucleatum. Each of these organisms exhibits distinct virulence strategies that facilitate colonization of the periodontal niche, disruption and evasion of host defenses, and induction and perpetuation of chronic inflammation [11,12]. In the following sections, we outline the biological characteristics and pathogenic roles of these representative species, beginning with P. gingivalis, which has long been regarded as a keystone pathogen in periodontitis.
2. Porphyromonas gingivalis
P. gingivalis is a Gram-negative, obligate anaerobic, rod-shaped bacterium that derives metabolic energy primarily from protein degradation products, heme, and vitamin K. It is an opportunistic pathogen that colonizes the oral cavity and is prevalent in the human population. As a major etiological agent of chronic periodontitis, P. gingivalis has been extensively studied [13]. Beyond its role in periodontal disease, this bacterium has also been implicated in a variety of systemic disorders, including cardiovascular disease [14,15], Alzheimer’s disease [16,17], and rheumatoid arthritis [18,19].
Among the various virulence factors of P. gingivalis, gingipains represent the most prominent and extensively studied components. Gingipains comprise two types of cysteine proteases: arginine-specific gingipains (RgpA and RgpB) and lysine-specific protease (Kgp) [20,21]. RgpA and Kgp are multidomain proteins consisting of an N-terminal catalytic protease domain and multiple C-terminal adhesin (hemagglutinin/adhesin) domains, whereas RgpB contains only the catalytic domain [22]. The catalytic domains of gingipains cleave a broad range of host extracellular matrix proteins, including fibronectin, fibrinogen, laminin, type I and IV collagen, and junctional adhesion molecule-1 [23,24,25]. The adhesin domains promote attachment to host tissues and acquisition of heme and other nutrients from host proteins [26,27]. Collectively, gingipains contribute to tissue destruction and nutrient acquisition, thereby enhancing bacterial survival within the periodontal niche and contributing significantly to the pathogenesis of periodontitis.
In addition to gingipains, fimbriae represent another major virulence factor of P. gingivalis. Fimbriae mediate critical interactions between the bacterium and host tissues, facilitating adherence to and invasion of target sites. These filamentous structures can bind a wide range of host components, including salivary proteins, and various host cells, such as macrophages, epithelial cells, and fibroblasts [28,29,30,31,32]. Through these interactions, fimbriae contribute not only to colonization of the periodontal niche but also to modulation of host immune responses, thereby promoting bacterial persistence and disease progression.
Besides gingipains and fimbriae, P. gingivalis produces several other virulence factors that function synergistically to promote colonization and immune modulation. These include capsule polysaccharides that protect against phagocytosis [33], lipopolysaccharide (LPS) with atypical lipid A structures capable of modulating host inflammatory signaling [34], and outer membrane vesicles that serve as delivery vehicles for virulence factors into host tissues [35,36].
3. Prevotella intermedia
P. intermedia is a Gram-negative, obligate anaerobic, rod-shaped bacterium that primarily utilizes peptides, heme, and vitamin K as nutrient sources. Along with P. gingivalis, this organism is classified as a black-pigmented bacterium, reflecting its ability to accumulate hemin-derived pigments on blood agar plates. P. intermedia is commonly found in the oral cavity and is frequently detected in periodontal pockets in individuals with periodontitis [37]. As an opportunistic pathogen, it has been recognized as a significant contributor to the initiation and progression of periodontal disease [38,39]. In addition to its established role in periodontitis, this bacterium has been linked to various systemic conditions, including respiratory infections [40,41], preterm birth [42], and other adverse pregnancy outcomes [43].
P. intermedia produces a range of virulence factors, including fimbriae, LPS, and elastase, but proteases are considered particularly important for pathogenesis [44]. These proteolytic enzymes mediate the degradation of host immune components, such as immunoglobulins, CD14, and LPS-binding protein, which may result in enhanced survival of P. intermedia and increased virulence of Gram-negative bacterial species [45,46]. In addition, the enzymatic activity of dipeptidyl peptidase IV is enhanced in the presence of estradiol [47], which can serve as an alternative nutrient source to vitamin K, suggesting a potential link between P. intermedia and estrogen metabolism. Additionally, P. intermedia has been reported to suppress neutrophil function and modulate host cytokine responses, underscoring its involvement in immune evasion and the perpetuation of chronic inflammation [48,49,50].
4. Treponema denticola
T. denticola is a motile, Gram-negative, obligate anaerobic spirochete that is frequently detected in subgingival plaque and is strongly linked to advanced periodontal lesions, including necrotizing periodontal diseases [51,52]. Through its characteristic motility and diverse virulence mechanisms, T. denticola contributes to periodontal tissue destruction and chronic inflammation [53,54]. Beyond its role in periodontal disease, T. denticola has also been implicated in systemic conditions, including cardiovascular disorders, likely through its ability to induce inflammatory responses in host tissues [55].
Among the various virulence factors of T. denticola, the surface-expressed protease dentilisin represents the most extensively characterized component. Dentilisin is a trypsin-like serine protease that degrades various host proteins, such as fibronectin, laminin, and fibrinogen, thereby facilitating bacterial colonization and modulating hemostasis in periodontal tissues [56,57]. In addition to dentilisin, the major sheath protein (Msp) forms filamentous outer membrane structures and exhibits pore-forming activity against epithelial cells, as well as hemolytic and hemagglutinating activities. Moreover, Msp forms a hetero-oligomeric complex with dentilisin, which may enhance bacterial colonization [58,59,60]. The characteristic motility of T. denticola, driven by periplasmic flagella, further facilitates both penetration into gingival tissues and movement within the subgingival environment [61]. Collectively, these virulence factors facilitate tissue destruction in periodontal lesions.
5. Tannerella forsythia
T. forsythia is a Gram-negative, obligate anaerobic, rod-shaped bacterium that colonizes subgingival plaque, particularly among periodontitis patients [62]. Along with P. gingivalis and T. denticola, this organism is classified as part of the “Red complex”, a group of bacteria strongly associated with chronic periodontitis progression [63]. T. forsythia possesses multiple virulence factors [64], and the S-layer, a glycosylated crystalline surface layer, represents the most distinctive feature among them. The S-layer mediates complement resistance, modulates immune recognition, and contributes to multispecies biofilm formation, thereby facilitating bacterial persistence in the periodontal niche [65,66,67]. Although primarily an oral pathogen, T. forsythia may contribute to systemic inflammation, and its involvement in cardiovascular disease and metabolic disorders has been suggested in recent studies [68].
T. forsythia produces several distinct proteases, including karilysin, a matrix metalloprotease-like enzyme that degrades elastin, fibrinogen, and fibronectin [69], and mirolase, a subtilisin-like serine protease that degrades fibrinogen, hemoglobin, and the antimicrobial peptide LL-37 [70]. These proteases contribute to tissue destruction and immune evasion [69,70,71]. The bacterium also expresses BspA, an outer membrane leucine-rich repeat protein that mediates adhesion to epithelial cells and extracellular matrix components while triggering host inflammatory responses [72,73]. Furthermore, its LPS possesses immunomodulatory properties that contribute to bacterial survival and chronic inflammation.
6. Aggregatibacter actinomycetemcomitans
A. actinomycetemcomitans is a Gram-negative, facultative anaerobe implicated in periodontitis, particularly aggressive forms of the disease [74,75]. In addition to its role in periodontal disease, this organism has been implicated in various systemic disorders, including endocarditis [76], Alzheimer’s disease [77], and brain abscess [78].
Its major virulence factors include LPS [79], pili [80,81], leukotoxin [82], cytolethal distending toxin (CDT) [83], outer membrane proteins, such as Omp100 (ApiA) [84,85] and EmaA [86,87], and outer membrane vesicles [88]. LPS contributes to the modulation of host immune responses and induction of inflammatory signaling. Pili mediate adhesion to host cells and extracellular matrix components, facilitating colonization of the periodontal niche. Leukotoxin selectively targets polymorphonuclear leukocytes, lymphocytes, and monocytes/macrophages, impairing host immune defenses and promoting bacterial persistence. CDT induces cell cycle arrest and apoptosis in host cells, further disrupting tissue homeostasis and promoting periodontal tissue destruction. Omp100 (ApiA) mediates adhesion to epithelial cells and extracellular matrix proteins, while EmaA facilitates collagen binding and enhances biofilm formation.
7. Fusobacterium nucleatum
F. nucleatum is a Gram-negative, obligate anaerobic, fusiform bacterium commonly found in the human oral cavity. This organism contributes to the progression of periodontitis through multiple mechanisms, including adherence to and invasion of gingival epithelial cells [89], thereby triggering inflammatory cytokine production [90]. While its direct contribution to periodontitis is less clearly defined compared to the aforementioned periodontal pathogens, F. nucleatum plays a crucial role in facilitating coaggregation between early and late colonizing bacteria and promoting biofilm maturation [91,92]. Beyond its oral pathogenic potential, F. nucleatum has also been implicated in systemic diseases, including atherosclerotic cardiovascular disease, adverse pregnancy outcomes, inflammatory bowel disease, and cancer, with particular attention given to its strong association with colorectal cancer, which has been extensively investigated in recent years [93,94].
Key virulence factors of F. nucleatum include the cell surface adhesin FadA and the outer membrane autotransporter protein Fap2, which mediate both interbacterial interactions and adhesion to host cells. FadA binds to E-cadherin on epithelial cells, facilitating bacterial invasion and activation of β-catenin signaling, which promotes pro-inflammatory responses and may contribute to colorectal tumorigenesis [95,96]. Fap2 interacts with host immune cells and other bacteria, enhancing coaggregation and biofilm maturation while inhibiting natural killer cell activity, thereby modulating host immune defenses [97,98,99,100]. Through these virulence factors, F. nucleatum functions as a bridging organism in dental plaque, promotes persistent inflammation in the oral cavity, and supports colonization and progression of colorectal cancer, illustrating its dual role in both local and systemic pathogenesis.
8. Coaggregation Between F. nucleatum and Oral-Bacterial Species
F. nucleatum actively mediates the bridging of early and late colonizers within the oral cavity by coaggregating with a wide range of bacterial species, thereby facilitating dental plaque maturation [101]. In this section, we highlight representative examples of oral-bacterial species that coaggregate with F. nucleatum and summarize the molecular determinants underlying these interactions. Table 1 summarizes oral bacterial species that coaggregate with F. nucleatum, along with the molecules involved in these interactions and the molecules that inhibit them.
The coaggregation between F. nucleatum and A. actinomycetemcomitans has long been characterized, and this interaction is known to be serotype-dependent. A. actinomycetemcomitans is classified into seven serotypes (a, b, c, d, e, f, and g) based on the antigenicity of the O-polysaccharide (O-PS) regions of its LPS [102,103,104,105]. Strains reported to coaggregate with F. nucleatum include Y4 (serotype b) [91], SA269 (serotype d) [106], and CU1060N (serotype f) [107]. More recently, we demonstrated that coaggregation between A. actinomycetemcomitans strains HK1651 (serotype b) and IDH781 (serotype d) and F. nucleatum is mediated by serotype-specific recognition of O-PS, with F. nucleatum utilizing the autotransporter proteins Fap2 and CmpA, respectively [108] (Figure 1). These findings highlight that bacterial coaggregation can vary not only between species but also among strains within the same species, underscoring the complexity of interbacterial interactions in the oral cavity. Additionally, the autotransporter protein RadD exhibits coaggregation activity to A. actinomycetemcomitans JP2 (serotype b) [109].
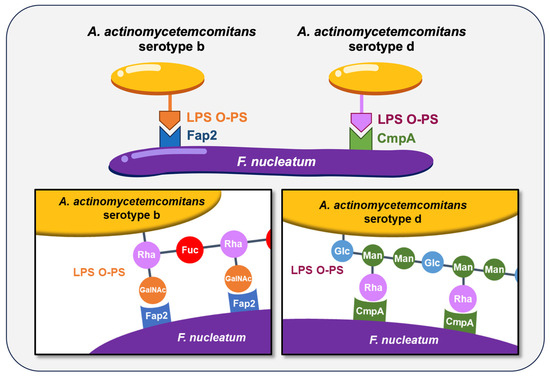
Figure 1.
The coaggregation of F. nucleatum with A. actinomycetemcomitans serotypes b and d. These coaggregations are mediated by specific surface proteins and O-polysaccharide (O-PS) regions of lipopolysaccharide (LPS). Rha, L-rhamnose; Fuc, D-fucose; GalNAc, N-acetyl-D-galactosamine; Glc, D-glucose; Man, D-mannose.
P. gingivalis has been shown to coaggregate with F. nucleatum. The coaggregation between F. nucleatum PK1594 and P. gingivalis PK1924 has been reported to be inhibited by lactose, N-acetyl-D-galactosamine, and D-galactose [110,111]. Galactose-dependent coaggregation was also observed in F. nucleatum ATCC 23726, and screening of mutants defective in this phenotype identified Fap2 as a coaggregation factor of F. nucleatum [109,112]. Additionally, deletion of the porin protein FomA in P. gingivalis ATCC 33277 reduced its coaggregation with F. nucleatum ATCC 10953 [113].
Coaggregation between F. nucleatum ATCC 25586 and P. intermedia ATCC 25611 is inhibited by EDTA and N-acetyl-D-galactosamine [114]. These findings suggest a coaggregation mechanism similar to that observed with P. gingivalis, although the specific coaggregation factors have not yet been identified. This interaction was suppressed by heat or protease treatment of F. nucleatum, but not by the same treatments applied to P. intermedia [114], suggesting that the coaggregation factor on the P. intermedia side is likely a galactose-containing surface polysaccharide.
The molecular mechanisms underlying coaggregation between T. forsythia and F. nucleatum have not been extensively characterized. Coaggregation between F. nucleatum ATCC 10953 and T. forsythia ATCC 43037 was attenuated by deletion of the surface adhesin BspA in T. forsythia [115]. We also reported that F. nucleatum ATCC 25586 strongly coaggregates with T. forsythia ATCC 43037, and that coaggregation was enhanced when T. forsythia lacked the S-layer [65]. Since the S-layer of T. forsythia is glycosylated [116], inhibition assays were performed using sugars associated with S-layer glycosylation; however, these sugars did not suppress coaggregation between F. nucleatum and wild-type T. forsythia [65]. These findings suggest that surface proteins, such as BspA, rather than the S-layer itself, may be responsible for mediating the coaggregation.
F. nucleatum PK1594 coaggregates with T. denticola strains ATCC 35404, ATCC 33520, and GM-1. This coaggregation is inhibited by the addition of EDTA and galactose. Msp of T. denticola is modified with galactose-containing glycans, and purified Msp inhibited the binding between F. nucleatum and T. denticola in a concentration-dependent manner [117]. However, deletion of Msp did not reduce interbacterial binding, suggesting that multiple coaggregation factors may mediate this interaction in T. denticola [117].
In addition to periodontal pathogens, it has also been reported that F. nucleatum coaggregates with the oral commensals Actinomyces oris, Actinomyces naeslundii, Streptococcus gordonii, Streptococcus oralis, and Streptococcus sanguinis through its autotransporter proteins RadD and/or CmpA [118,119,120]. Many of these coaggregation interactions are inhibited by the addition of arginine [118]. Wu et al. performed a genome-wide screening of F. nucleatum to identify genes involved in coaggregation with S. gordonii, and identified genes within the rad operon, carS encoding a histidine kinase, and those related to the lysine degradation pathway [109]. Furthermore, they found that radD and genes involved in lysine metabolism are regulated by the CarRS two-component system, suggesting that lysine metabolism induced by CarRS regulation may contribute to coaggregation with S. gordonii [109]. Disruption of CarRS-regulated genes involved in lysine metabolism led to increased lysine concentrations in the culture supernatant [109], which suggests that RadD-mediated coaggregation may be modulated by both arginine and lysine.

Table 1.
Coaggregation partners of F. nucleatum.
Table 1.
Coaggregation partners of F. nucleatum.
| Partner Species | Coaggregation Factor of F. nucleatum | Coaggregation Factor of Partner 1 | Inhibitor 2 | Reference |
|---|---|---|---|---|
| A. actinomycetemcomitans HK1651 | Fap2 | Serotype b O-PS | GalNac, Rha | [108] |
| A. actinomycetemcomitans IDH781 | CmpA | Serotype d O-PS | Rha | [108] |
| A. actinomycetemcomitans JP2 | RadD | Unidentified | - | [109] |
| P. gingivalis PK1924 | Unidentified | Unidentified | Lac, Gal, GalNac | [110] |
| P. gingivalis PK1924 | Unidentified | CPS, LPS | EDTA, Gal | [111] |
| P. gingivalis PK1924 | Fap2 | Unidentified | Gal | [112] |
| P. gingivalis PK1924 | Fap2 | Unidentified | - | [109] |
| P. gingivalis ATCC 33277 | FomA | Unidentified | - | [113] |
| P. intermedia ATCC 25611 | Unidentified | Unidentified | EDTA, GalNac | [114] |
| T. forsythia ATCC 43037 | Unidentified | BspA | - | [115] |
| T. forsythia ATCC 43037 | Unidentified | Unidentified | - | [65] |
| T. denticola ATCC 35404, ATCC 33520, GM-1 | Unidentified | Msp | EDTA, Gal | [117] |
| A. oris MG-1 | RadD | Unidentified | - | [119] |
| A. naeslundii ATCC 12104 | RadD | Unidentified | L-arginine | [118] |
| S. gordonii ATCC 10558 | RadD | Unidentified | L-arginine | [118] |
| S. gordonii ATCC 10558, ATCC 51656,DL1 | RadD, CmpA | Unidentified | - | [120] |
| S. gordonii DL1 | RadD | Unidentified | - | [109] |
| S. oralis ATCC 10557 | RadD | Unidentified | L-arginine | [118] |
| S. sanguinis ATCC 10556 | RadD | Unidentified | L-arginine | [118] |
1 O-PS, O-polysaccharide; CPS, capsule polysaccharide; LPS, lipopolysaccharide. 2 GalNac, N-acetyl-D-galactosamine; Rha, rhamnose; Lac, lactose; Gal, galactose.
9. Coaggregation Between Red Complex Species
In chronic periodontitis lesions, the so-called “Red complex” species—P. gingivalis, T. denticola, and T. forsythia—are frequently co-isolated and are considered the major periodontal pathogens. In this section, we summarize reports describing coaggregation among these bacterial species (Table 2).

Table 2.
Coaggregations between Red complex species.
Regarding the coaggregation between P. gingivalis and T. denticola, several studies have identified molecular factors involved in this interaction. Hashimoto et al. demonstrated that dentilisin of T. denticola can bind to fimbrial proteins of P. gingivalis, suggesting its potential role in coaggregation [121]. Yamada et al. reported that P. gingivalis strain FDC381 and T. denticola strain ATCC 35405 coaggregate strongly, and that this interaction is reduced when T. denticola mutant strains lacking flgE (flagellar gene) or cfpA (cytoplasmic filament gene) are used [122]. Furthermore, Yoshikawa et al. showed that the Hgp44 domain of RgpA in P. gingivalis is critically involved in coaggregation between P. gingivalis ATCC 33277 and T. denticola ATCC 35405 [123].
Concerning the coaggregation between P. gingivalis and T. forsythia, Jung et al. reported that the interaction between P. gingivalis ATCC 33277 and T. forsythia ATCC 43037 is strongly inhibited by L-arginine and L-lysine, and that a gingipain-null mutant of P. gingivalis (kgp−, rgpA−, rgpB−) completely lost its ability to coaggregate with T. forsythia [124]. Additionally, Śmiga et al. investigated the role of the redox-sensing protein (PgRsp) in P. gingivalis and found that a pgRsp-deficient mutant of strain A7436 displayed enhanced coaggregation with T. forsythia ATCC 43037. This phenotype was attributed to downregulated fimbrial gene expression in the pgRsp mutant, which may increase the exposure of other P. gingivalis surface factors and thereby promote coaggregation [125].
In the case of coaggregation between T. denticola and T. forsythia, Ikegami et al. demonstrated that the leucine-rich repeat protein LrrA of T. denticola ATCC 35405 mediates coaggregation with T. forsythia ATCC 43037 by interacting with the surface protein BspA of T. forsythia [126]. Furthermore, Sano et al. confirmed the coaggregation between T. denticola ATCC 35405 and T. forsythia ATCC 43037 and showed that the surface protease dentilisin of T. denticola contributes to this interaction [127].
10. Conclusions and Perspectives
Oral bacteria engage in a wide range of interspecies coaggregation events, with Fusobacterium nucleatum serving as a central bridging organism. Major periodontal pathogens coaggregate not only through F. nucleatum but also directly with each other, and such microbial colocalization is thought to promote the progression and exacerbation of periodontitis. Importantly, F. nucleatum also interacts with diverse bacterial species outside the oral cavity, including Staphylococcus aureus on the skin, Helicobacter pylori in the stomach, and Clostridioides difficile and Limosilactobacillus reuteri in the intestine [128,129,130,131]. These broad coaggregation capabilities may facilitate the dissemination of periodontal pathogens beyond the oral environment and provide a mechanistic link to systemic diseases. Future research should focus on elucidating the detailed molecular mechanisms underlying these coaggregation events and exploring their potential as therapeutic targets. Additionally, the role of coaggregation in establishing dysbiotic microbial communities warrants further investigation.
Author Contributions
Conceptualization, Y.O. and M.N.; writing—original draft preparation, Y.O., Y.T.; writing—review and editing, Y.O. and M.N.; funding acquisition, Y.O. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by JSPS KAKENHI grant numbers 23K09141 and 22K19633.
Institutional Review Board Statement
This is a review article and does not report on original research involving human or animal subjects.
Informed Consent Statement
This is a review article and not a report on original research, so informed consent does not apply.
Data Availability Statement
This review article relies exclusively on previously published data, fully cited in the bibliography. The original datasets used in the cited studies were not accessed or analyzed by the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial diversity in human subgingival plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef]
- Loesche, W.J.; Gusberti, F.; Mettraux, G.; Higgins, T.; Syed, S. Relationship between oxygen tension and subgingival bacterial flora in untreated human periodontal pockets. Infect. Immun. 1983, 42, 659–667. [Google Scholar] [CrossRef]
- Nyvad, B.; Kilian, M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand. J. Dent. Res. 1987, 95, 369–380. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Allison, C. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect. Immun. 1998, 66, 4729–4732. [Google Scholar] [CrossRef]
- Periasamy, S.; Kolenbrander, P.E. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J. Bacteriol. 2009, 191, 6804–6811. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.W.; Low, Z.Y.; Tan, W.Q.; Azman, A.S. Microbiota-host interactions: Exploring their dynamics and contributions to human diseases. Microbiologyopen 2025, 14, e70043. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, R.; Vudathaneni, V.K.P.; Bodduru, R.; Todima, J.; Dasari, A.B.; Chintala, L. Exploring the link between periodontal pathogens and systemic inflammatory markers in patients with metabolic syndrome. J. Pharm. Bioallied Sci. 2025, 17 (Suppl. 2), S1808–S1810. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Coffman, J.A.; Garcia-Godoy, F. Oral pathogens’ substantial burden on cancer, cardiovascular diseases, Alzheimer’s, diabetes, and other systemic diseases: A public health crisis-A comprehensive review. Pathogens 2024, 13, 1084. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontology 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Polishchuk, H.; Synowiec, A.; Zubrzycka, N.; Kantyka, T. Porphyromonas gingivalis: Multiple tools of an inflammatory damage. Mol. Oral Microbiol. 2025, 40, 159–176. [Google Scholar] [CrossRef]
- Pavlic, V.; Peric, D.; Kalezic, I.S.; Madi, M.; Bhat, S.G.; Brkic, Z.; Staletovic, D. Identification of periopathogens in atheromatous plaques obtained from carotid and coronary arteries. Biomed. Res. Int. 2021, 2021, 9986375. [Google Scholar] [CrossRef]
- Ruan, Q.; Guan, P.; Qi, W.; Li, J.; Xi, M.; Xiao, L.; Zhong, S.; Ma, D.; Ni, J. Porphyromonas gingivalis regulates atherosclerosis through an immune pathway. Front. Immunol. 2023, 14, 1103592. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Díaz-Zúñiga, J.; More, J.; Melgar-Rodríguez, S.; Jiménez-Unión, M.; Villalobos-Orchard, F.; Muñoz-Manríquez, C.; Monasterio, G.; Valdés, J.L.; Vernal, R.; Paula-Lima, A. Alzheimer’s disease-like pathology triggered by Porphyromonas gingivalis in wild type rats is serotype dependent. Front. Immunol. 2020, 11, 588036. [Google Scholar] [CrossRef] [PubMed]
- Maresz, K.J.; Hellvard, A.; Sroka, A.; Adamowicz, K.; Bielecka, E.; Koziel, J.; Gawron, K.; Mizgalska, D.; Marcinska, K.A.; Benedyk, M.; et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 2013, 9, e1003627. [Google Scholar] [CrossRef]
- Yamakawa, M.; Ouhara, K.; Kajiya, M.; Munenaga, S.; Kittaka, M.; Yamasaki, S.; Takeda, K.; Takeshita, K.; Mizuno, N.; Fujita, T.; et al. Porphyromonas gingivalis infection exacerbates the onset of rheumatoid arthritis in SKG mice. Clin. Exp. Immunol. 2016, 186, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Kadowaki, T.; Okamoto, K.; Yamamoto, K. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis. Evidence for significant contribution of Arg-gingipain to virulence. J. Biol. Chem. 1995, 270, 23619–23626. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Ma, Q.; Zhou, X.; Li, Y. The secretion and maturation journey of gingipains. Mol. Oral Microbiol. 2025, 40, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Collyer, C.A. Gingipains from Porphyromonas gingivalis—Complex domain structures confer diverse functions. Eur. J. Microbiol. Immunol. 2011, 1, 41–58. [Google Scholar] [CrossRef]
- Baba, A.; Abe, N.; Kadowaki, T.; Nakanishi, H.; Ohishi, M.; Asao, T.; Yamamoto, K. Arg-gingipain is responsible for the degradation of cell adhesion molecules of human gingival fibroblasts and their death induced by Porphyromonas gingivalis. Biol. Chem. 2001, 382, 817–824. [Google Scholar] [CrossRef]
- Potempa, J.; Banbula, A.; Travis, J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontology 2000 2000, 24, 153–192. [Google Scholar] [CrossRef]
- Takeuchi, H.; Sasaki, N.; Yamaga, S.; Kuboniwa, M.; Matsusaki, M.; Amano, A. Porphyromonas gingivalis induces penetration of lipopolysaccharide and peptidoglycan through the gingival epithelium via degradation of junctional adhesion molecule 1. PLoS Pathog. 2019, 15, e1008124. [Google Scholar] [CrossRef]
- Shi, Y.; Ratnayake, D.B.; Okamoto, K.; Abe, N.; Yamamoto, K.; Nakayama, K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J. Biol. Chem. 1999, 274, 17955–17960. [Google Scholar] [CrossRef]
- DeCarlo, A.A.; Paramaesvaran, M.; Yun, P.L.; Collyer, C.; Hunter, N. Porphyrin-mediated binding to hemoglobin by the HA2 domain of cysteine proteinases (gingipains) and hemagglutinins from the periodontal pathogen Porphyromonas gingivalis. J. Bacteriol. 1999, 181, 3784–3791. [Google Scholar] [CrossRef]
- Nakamura, T.; Amano, A.; Nakagawa, I.; Hamada, S. Specific interactions between Porphyromonas gingivalis fimbriae and human extracellular matrix proteins. FEMS Microbiol. Lett. 1999, 175, 267–272. [Google Scholar] [CrossRef][Green Version]
- Amano, A.; Nakamura, T.; Kimura, S.; Morisaki, I.; Nakagawa, I.; Kawabata, S.; Hamada, S. Molecular interactions of Porphyromonas gingivalis fimbriae with host proteins: Kinetic analyses based on surface plasmon resonance. Infect. Immun. 1999, 67, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Watanabe, K.; Lamont, R.J. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol. 2002, 4, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, A.; Murakami, Y.; Yamashita, Y.; Ishida, M.; Fujisawa, S.; Kitano, S.; Hanazawa, S. Porphyromonas gingivalis fimbriae use β2 integrin (CD11/CD18) on mouse peritoneal macrophages as a cellular receptor, and the CD18 β chain plays a functional role in fimbrial signaling. Infect. Immun. 1998, 66, 4056–4060. [Google Scholar] [CrossRef]
- Nakagawa, I.; Amano, A.; Kuboniwa, M.; Nakamura, T.; Kawabata, S.; Hamada, S. Functional differences among FimA variants of Porphyromonas gingivalis and their effects on adhesion to and invasion of human epithelial cells. Infect. Immun. 2002, 70, 277–285. [Google Scholar] [CrossRef]
- Singh, A.; Wyant, T.; Anaya-Bergman, C.; Aduse-Opoku, J.; Brunner, J.; Laine, M.L.; Curtis, M.A.; Lewis, J.P. The capsule of Porphyromonas gingivalis leads to a reduction in the host inflammatory response, evasion of phagocytosis, and increase in virulence. Infect. Immun. 2011, 79, 4533–4542. [Google Scholar] [CrossRef]
- Darveau, R.P.; Pham, T.T.; Lemley, K.; Reife, R.A.; Bainbridge, B.W.; Coats, S.R.; Howald, W.N.; Way, S.S.; Hajjar, A.M. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both Toll-like receptors 2 and 4. Infect. Immun. 2004, 72, 5041–5051. [Google Scholar] [CrossRef]
- Furuta, N.; Takeuchi, H.; Amano, A. Entry of Porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment. Infect. Immun. 2009, 77, 4761–4770. [Google Scholar] [CrossRef]
- Wu, Z.; Long, W.; Yin, Y.; Tan, B.; Liu, C.; Li, H.; Ge, S. Outer membrane vesicles of Porphyromonas gingivalis: Recent advances in pathogenicity and associated mechanisms. Front. Microbiol. 2025, 16, 1555868. [Google Scholar] [CrossRef] [PubMed]
- Ashimoto, A.; Chen, C.; Bakker, I.; Slots, J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol. Immunol. 1996, 11, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.Y.; Zhang, Q.; Li, J.L.; Yang, S.H.; Shi, Q. Progression of periodontal inflammation in adolescents is associated with increased number of Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythensis, and Fusobacterium nucleatum. Int. J. Paediatr. Dent. 2014, 24, 226–233. [Google Scholar] [CrossRef]
- Maeda, N.; Okamoto, M.; Kondo, K.; Ishikawa, H.; Osada, R.; Tsurumoto, A.; Fujita, H. Incidence of Prevotella intermedia and Prevotella nigrescens in periodontal health and disease. Microbiol. Immunol. 1998, 42, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, K.; Yanagihara, K.; Morinaga, Y.; Nakamura, S.; Harada, T.; Hasegawa, H.; Izumikawa, K.; Ishimatsu, Y.; Kakeya, H.; Nishimura, M.; et al. Prevotella intermedia induces severe bacteremic pneumococcal pneumonia in mice with upregulated platelet-activating factor receptor expression. Infect. Immun. 2014, 82, 587–593. [Google Scholar] [CrossRef]
- Ashizawa, H.; Iwanaga, N.; Nemoto, K.; Hirayama, T.; Yoshida, M.; Takeda, K.; Ide, S.; Tashiro, M.; Hosogaya, N.; Takazono, T.; et al. Prevotella intermedia synergistically exacerbates pneumonia induced by oral streptococci. J. Infect. Dis. 2025, 232, e280–e289. [Google Scholar] [CrossRef]
- Jang, H.; Patoine, A.; Wu, T.T.; Castillo, D.A.; Xiao, J. Oral microflora and pregnancy: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16870. [Google Scholar] [CrossRef]
- Shira Davenport, E. Preterm low birthweight and the role of oral bacteria. J. Oral. Microbiol. 2010, 2, 5779. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Lalsiamthara, J.; Peng, Y.; Qi, L.; Deng, S.; Wang, Q. Current research progress on Prevotella intermedia and associated diseases. Crit. Rev. Microbiol. 2025, 51, 545–562. [Google Scholar] [CrossRef]
- Deschner, J.; Singhal, A.; Long, P.; Liu, C.C.; Piesco, N.; Agarwal, S. Cleavage of CD14 and LBP by a protease from Prevotella intermedia. Arch. Microbiol. 2003, 179, 430–436. [Google Scholar] [CrossRef][Green Version]
- Jansen, H.J.; Grenier, D.; Van der Hoeven, J.S. Characterization of immunoglobulin G-degrading proteases of Prevotella intermedia and Prevotella nigrescens. Oral. Microbiol. Immunol. 1995, 10, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Fteita, D.; Könönen, E.; Gürsoy, M.; Söderling, E.; Gürsoy, U.K. Does estradiol have an impact on the dipeptidyl peptidase IV enzyme activity of the Prevotella intermedia group bacteria? Anaerobe 2015, 36, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Doke, M.; Fukamachi, H.; Morisaki, H.; Arimoto, T.; Kataoka, H.; Kuwata, H. Nucleases from Prevotella intermedia can degrade neutrophil extracellular traps. Mol. Oral Microbiol. 2017, 32, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Yang, S.; Iki, K.; Hatakeyama, J.; Tamai, R.; Takeuchi, O.; Akashi, S.; Espevik, T.; Akira, S.; Takada, H. Monocytic cell activation by nonendotoxic glycoprotein from Prevotella intermedia ATCC 25611 is mediated by Toll-like receptor 2. Infect Immun 2001, 69, 4951–4957. [Google Scholar] [CrossRef]
- Guan, S.M.; Shu, L.; Fu, S.M.; Liu, B.; Xu, X.L.; Wu, J.Z. Prevotella intermedia induces matrix metalloproteinase-9 expression in human periodontal ligament cells. FEMS Microbiol. Lett. 2008, 283, 47–53. [Google Scholar] [CrossRef]
- Asai, Y.; Jinno, T.; Igarashi, H.; Ohyama, Y.; Ogawa, T. Detection and quantification of oral treponemes in subgingival plaque by real-time PCR. J. Clin. Microbiol. 2002, 40, 3334–3340. [Google Scholar] [CrossRef]
- Sela, M.N. Role of Treponema denticola in periodontal diseases. Crit. Rev. Oral Biol. Med. 2001, 12, 399–413. [Google Scholar] [CrossRef]
- Rosen, G.; Sela, M.N.; Naor, R.; Halabi, A.; Barak, V.; Shapira, L. Activation of murine macrophages by lipoprotein and lipooligosaccharide of Treponema denticola. Infect. Immun. 1999, 67, 1180–1186. [Google Scholar] [CrossRef]
- Malone, E.T.; Ganther, S.; Mena, N.; Radaic, A.; Shariati, K.; Kindberg, A.; Tafolla, C.; Kamarajan, P.; Fenno, J.C.; Zhan, L.; et al. Treponema denticola-Induced RASA4 upregulation mediates cytoskeletal dysfunction and MMP-2 activity in periodontal fibroblasts. Front. Cell Infect. Microbiol. 2021, 11, 671968. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Kimizuka, R.; Miyamoto, M.; Kato, T.; Yamada, S.; Okuda, K.; Ishihara, K. Treponema denticola induces interleukin-8 and macrophage chemoattractant protein 1 production in human umbilical vein epithelial cells. Microbes Infect. 2007, 9, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Uitto, V.J.; Pan, Y.M.; Leung, W.K.; Larjava, H.; Ellen, R.P.; Finlay, B.B.; McBride, B.C. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect. Immun. 1995, 63, 3401–3410. [Google Scholar] [CrossRef]
- Bamford, C.V.; Fenno, J.C.; Jenkinson, H.F.; Dymock, D. The chymotrypsin-like protease complex of Treponema denticola ATCC 35405 mediates fibrinogen adherence and degradation. Infect. Immun. 2007, 75, 4364–4372. [Google Scholar] [CrossRef]
- Egli, C.; Leung, W.K.; Müller, K.H.; Hancock, R.E.; McBride, B.C. Pore-forming properties of the major 53-kilodalton surface antigen from the outer sheath of Treponema denticola. Infect. Immun. 1993, 61, 1694–1699. [Google Scholar] [CrossRef]
- Fenno, J.C.; Hannam, P.M.; Leung, W.K.; Tamura, M.; Uitto, V.J.; McBride, B.C. Cytopathic effects of the major surface protein and the chymotrypsinlike protease of Treponema denticola. Infect. Immun. 1998, 66, 1869–1877. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Tian, Y.; Huang, H.; Zhao, F.; Deng, X. Treponema denticola major surface protein (Msp): A key player in periodontal pathogenicity and immune evasion. Arch. Microbiol. 2025, 207, 36. [Google Scholar] [CrossRef]
- Ruby, J.D.; Li, H.; Kuramitsu, H.; Norris, S.J.; Goldstein, S.F.; Buttle, K.F.; Charon, N.W. Relationship of Treponema denticola periplasmic flagella to irregular cell morphology. J. Bacteriol. 1997, 179, 1628–1635. [Google Scholar] [CrossRef][Green Version]
- Tanner, A.C.R.; Listgarten, M.A.; Ebersole, J.L.; Strzempko, M.N. Bacteroides forsythus sp. nov. a slow-growing, fusiform Bacteroides sp. from the human oral cavity. Int. J. Syst. Evol. Microbiol. 1986, 36, 213–221. [Google Scholar] [CrossRef]
- Mohanty, R.; Asopa, S.J.; Joseph, M.D.; Singh, B.; Rajguru, J.P.; Saidath, K.; Sharma, U. Red complex: Polymicrobial conglomerate in oral flora: A review. J. Family Med. Prim. Care 2019, 8, 3480–3486. [Google Scholar]
- Schäffer, C.; Andrukhov, O. The intriguing strategies of Tannerella forsythia’s host interaction. Front. Oral Health 2024, 5, 1434217. [Google Scholar] [CrossRef]
- Shimotahira, N.; Oogai, Y.; Kawada-Matsuo, M.; Yamada, S.; Fukutsuji, K.; Nagano, K.; Yoshimura, F.; Noguchi, K.; Komatsuzawa, H. The surface layer of Tannerella forsythia contributes to serum resistance and oral bacterial coaggregation. Infect. Immun. 2013, 81, 1198–1206. [Google Scholar] [CrossRef]
- Settem, R.P.; Honma, K.; Nakajima, T.; Phansopa, C.; Roy, S.; Stafford, G.P.; Sharma, A. A bacterial glycan core linked to surface (S)-layer proteins modulates host immunity through Th17 suppression. Mucosal Immunol. 2013, 6, 415–426. [Google Scholar] [CrossRef]
- Bloch, S.; Thurnheer, T.; Murakami, Y.; Belibasakis, G.N.; Schäffer, C. Behavior of two Tannerella forsythia strains and their cell surface mutants in multispecies oral biofilms. Mol. Oral Microbiol. 2017, 32, 404–418. [Google Scholar] [CrossRef]
- Lee, H.R.; Jun, H.K.; Choi, B.K. Tannerella forsythia BspA increases the risk factors for atherosclerosis in ApoE−/− mice. Oral Dis. 2014, 20, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.Y.; Kulczycka, M.; Kantyka, T.; Dubin, G.; Jabaiah, A.; Daugherty, P.S.; Thogersen, I.B.; Enghild, J.J.; Nguyen, K.A.; Potempa, J. A novel matrix metalloprotease-like enzyme (karilysin) of the periodontal pathogen Tannerella forsythia ATCC 43037. Biol. Chem. 2010, 391, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, M.; Karim, A.Y.; Bryzek, D.; Enghild, J.J.; Thøgersen, I.B.; Koziel, J.; Potempa, J. Mirolase, a novel subtilisin-like serine protease from the periodontopathogen Tannerella forsythia. Biol. Chem. 2015, 396, 261–275. [Google Scholar] [CrossRef]
- Jusko, M.; Potempa, J.; Karim, A.Y.; Ksiazek, M.; Riesbeck, K.; Garred, P.; Eick, S.; Blom, A.M. A metalloproteinase karilysin present in the majority of Tannerella forsythia isolates inhibits all pathways of the complement system. J. Immunol. 2012, 188, 2338–2349. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sojar, H.T.; Glurich, I.; Honma, K.; Kuramitsu, H.K.; Genco, R.J. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect. Immun. 1998, 66, 5703–5710. [Google Scholar] [CrossRef]
- Inagaki, S.; Onishi, S.; Kuramitsu, H.K.; Sharma, A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by “Tannerella forsythia”. Infect. Immun. 2006, 74, 5023–5028. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter actinomycetemcomitans (Aa) under the radar: Myths and misunderstandings of Aa and its role in aggressive periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Meštrović, T.; Matijević, T.; Mesarić, D.; Katalinić, D.; Erić, S.; Milostić-Srb, A.; Flam, J.; Škrlec, I. Aggregatibacter actinomycetemcomitans: From the oral cavity to the heart valves. Microorganisms 2024, 12, 1451. [Google Scholar] [CrossRef]
- Nakano, K.; Inaba, H.; Nomura, R.; Nemoto, H.; Tamura, K.; Miyamoto, E.; Yoshioka, H.; Taniguchi, K.; Amano, A.; Ooshima, T. Detection and serotype distribution of Actinobacillus actinomycetemcomitans in cardiovascular specimens from Japanese patients. Oral Microbiol. Immunol. 2007, 22, 136–139. [Google Scholar] [CrossRef]
- Díaz-Zúñiga, J.; Muñoz, Y.; Melgar-Rodríguez, S.; More, J.; Bruna, B.; Lobos, P.; Monasterio, G.; Vernal, R.; Paula-Lima, A. Serotype b of Aggregatibacter actinomycetemcomitans triggers pro-inflammatory responses and amyloid beta secretion in hippocampal cells: A novel link between periodontitis and Alzheimer’s disease? J. Oral Microbiol. 2019, 11, 1586423. [Google Scholar] [CrossRef]
- Zijlstra, E.E.; Swart, G.R.; Godfroy, F.J.; Degener, J.E. Pericarditis, pneumonia and brain abscess due to a combined Actinomyces—Actinobacillus actinomycetemcomitans infection. J. Infect. 1992, 25, 83–87. [Google Scholar] [CrossRef]
- Ahlstrand, T.; Kovesjoki, L.; Maula, T.; Oscarsson, J.; Ihalin, R. Aggregatibacter actinomycetemcomitans LPS binds human interleukin-8. J. Oral Microbiol. 2019, 11, 1549931. [Google Scholar] [CrossRef]
- Kachlany, S.C.; Planet, P.J.; Desalle, R.; Fine, D.H.; Figurski, D.H.; Kaplan, J.B. flp-1, the first representative of a new pilin gene subfamily, is required for non-specific adherence of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 2001, 40, 542–554. [Google Scholar] [CrossRef]
- Bhattacharjee, M.K.; Kachlany, S.C.; Fine, D.H.; Figurski, D.H. Nonspecific adherence and fibril biogenesis by Actinobacillus actinomycetemcomitans: TadA protein is an ATPase. J. Bacteriol. 2001, 183, 5927–5936. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 2011, 3, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Sugai, M.; Kawamoto, T.; Pérès, S.Y.; Ueno, Y.; Komatsuzawa, H.; Fujiwara, T.; Kurihara, H.; Suginaka, H.; Oswald, E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 1998, 66, 5008–5019. [Google Scholar] [CrossRef]
- Asakawa, R.; Komatsuzawa, H.; Kawai, T.; Yamada, S.; Goncalves, R.B.; Izumi, S.; Fujiwara, T.; Nakano, Y.; Suzuki, N.; Uchida, Y.; et al. Outer membrane protein 100, a versatile virulence factor of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 2003, 50, 1125–1139. [Google Scholar] [CrossRef]
- Yue, G.; Kaplan, J.B.; Furgang, D.; Mansfield, K.G.; Fine, D.H. A second Aggregatibacter actinomycetemcomitans autotransporter adhesin exhibits specificity for buccal epithelial cells in humans and old world primates. Infect. Immun. 2007, 75, 4440–4448. [Google Scholar] [CrossRef]
- Mintz, K.P. Identification of an extracellular matrix protein adhesin, EmaA, which mediates the adhesion of Actinobacillus actinomycetemcomitans to collagen. Microbiology 2004, 150, 2677–2688. [Google Scholar] [CrossRef]
- Ruiz, T.; Lenox, C.; Radermacher, M.; Mintz, K.P. Novel surface structures are associated with the adhesion of Actinobacillus actinomycetemcomitans to collagen. Infect. Immun. 2006, 74, 6163–6170. [Google Scholar] [CrossRef]
- Fu, Y.; Trautwein-Schult, A.; Piersma, S.; Sun, C.; Westra, J.; de Jong, A.; Becher, D.; van Dijl, J.M. Characterization of outer membrane vesicles of Aggregatibacter actinomycetemcomitans serotypes a, b and c and their interactions with human neutrophils. Int. J. Med. Microbiol. 2025, 319, 151655. [Google Scholar] [CrossRef]
- Han, Y.W.; Shi, W.; Huang, G.T.; Kinder Haake, S.; Park, N.H.; Kuramitsu, H.; Genco, R.J. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect. Immun. 2000, 68, 3140–3146. [Google Scholar] [CrossRef]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Moore, L.V. Coaggregation of Fusobacterium nucleatum, Selenomonas flueggei, Selenomonas infelix, Selenomonas noxia, and Selenomonas sputigena with strains from 11 genera of oral bacteria. Infect. Immun. 1989, 57, 3194–3203. [Google Scholar] [CrossRef]
- Guo, L.; He, X.; Shi, W. Intercellular communications in multispecies oral microbial communities. Front. Microbiol. 2014, 5, 328. [Google Scholar] [CrossRef]
- Jiang, S.S.; Chen, Y.X.; Fang, J.Y. Fusobacterium nucleatum: Ecology, pathogenesis and clinical implications. Nat. Rev. Microbiol. 2025. [Google Scholar] [CrossRef]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium nucleatum and its associated systemic diseases: Epidemiologic studies and possible mechanisms. J. Oral Microbiol. 2023, 15, 2145729. [Google Scholar] [CrossRef]
- Ikegami, A.; Chung, P.; Han, Y.W. Complementation of the fadA mutation in Fusobacterium nucleatum demonstrates that the surface-exposed adhesin promotes cellular invasion and placental colonization. Infect. Immun. 2009, 77, 3075–3079. [Google Scholar] [CrossRef] [PubMed]
- Fardini, Y.; Wang, X.; Témoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Emgård, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef]
- Parhi, L.; Abed, J.; Shhadeh, A.; Alon-Maimon, T.; Udi, S.; Ben-Arye, S.L.; Tam, J.; Parnas, O.; Padler-Karavani, V.; Goldman-Wohl, D.; et al. Placental colonization by Fusobacterium nucleatum is mediated by binding of the Fap2 lectin to placentally displayed Gal-GalNAc. Cell Rep. 2022, 38, 110537. [Google Scholar] [CrossRef]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef] [PubMed]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef]
- Perry, M.B.; MacLean, L.M.; Brisson, J.R.; Wilson, M.E. Structures of the antigenic O-polysaccharides of lipopolysaccharides produced by Actinobacillus actinomycetemcomitans serotypes a, c, d and e. Eur. J. Biochem. 1996, 242, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Perry, M.B.; MacLean, L.L.; Furgang, D.; Wilson, M.E.; Fine, D.H. Structural and genetic analyses of O polysaccharide from Actinobacillus actinomycetemcomitans serotype f. Infect. Immun. 2001, 69, 5375–5384. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Saito, M.; Tsuzukibashi, O.; Kawashima, Y.; Ishida, S.; Hirasawa, M. Characterization of a new serotype g isolate of Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2010, 25, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.B.; MacLean, L.L.; Gmür, R.; Wilson, M.E. Characterization of the O-polysaccharide structure of lipopolysaccharide from Actinobacillus actinomycetemcomitans serotype b. Infect. Immun. 1996, 64, 1215–1219. [Google Scholar] [CrossRef]
- Karched, M.; Bhardwaj, R.G.; Asikainen, S.E. Coaggregation and biofilm growth of Granulicatella spp. with Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans. BMC Microbiol. 2015, 15, 114. [Google Scholar] [CrossRef]
- Rupani, D.; Izano, E.A.; Schreiner, H.C.; Fine, D.H.; Kaplan, J.B. Aggregatibacter actinomycetemcomitans serotype f O-polysaccharide mediates coaggregation with Fusobacterium nucleatum. Oral Microbiol. Immunol. 2008, 23, 127–130. [Google Scholar] [CrossRef]
- Tanaka, Y.; Oogai, Y.; Matsumoto, A.; Noguchi, K.; Nakata, M. The outer membrane autotransporters Fap2 and CmpA facilitate specific coaggregation between Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans serotypes b and d. Appl. Environ. Microbiol. 2025, e01132-25. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Y.W.; Scheible, M.; Chang, C.; Wittchen, M.; Lee, J.H.; Luong, T.T.; Tiner, B.L.; Tauch, A.; Das, A.; et al. Genetic and molecular determinants of polymicrobial interactions in Fusobacterium nucleatum. Proc. Natl. Acad. Sci. USA 2021, 118, e2006482118. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N. Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect. Immun. 1989, 57, 3204–3209. [Google Scholar] [CrossRef]
- Rosen, G.; Sela, M.N. Coaggregation of Porphyromonas gingivalis and Fusobacterium nucleatum PK 1594 is mediated by capsular polysaccharide and lipopolysaccharide. FEMS Microbiol. Lett. 2006, 256, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Coppenhagen-Glazer, S.; Sol, A.; Abed, J.; Naor, R.; Zhang, X.; Han, Y.W.; Bachrach, G. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect. Immun. 2015, 83, 1104–1113. [Google Scholar] [CrossRef]
- Liu, P.F.; Shi, W.; Zhu, W.; Smith, J.W.; Hsieh, S.L.; Gallo, R.L.; Huang, C.M. Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: Implication for treatment of periodontal infection and halitosis. Vaccine 2010, 28, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Kokubu, E.; Kawana, T.; Saito, A.; Okuda, K.; Ishihara, K. Synergy in biofilm formation between Fusobacterium nucleatum and Prevotella species. Anaerobe 2012, 18, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Inagaki, S.; Sigurdson, W.; Kuramitsu, H.K. Synergy between Tannerella forsythia and Fusobacterium nucleatum in biofilm formation. Oral Microbiol. Immunol. 2005, 20, 39–42. [Google Scholar] [CrossRef]
- Posch, G.; Pabst, M.; Brecker, L.; Altmann, F.; Messner, P.; Schäffer, C. Characterization and scope of S-layer protein O-glycosylation in Tannerella forsythia. J. Biol. Chem. 2011, 286, 38714–38724. [Google Scholar] [CrossRef]
- Rosen, G.; Genzler, T.; Sela, M.N. Coaggregation of Treponema denticola with Porphyromonas gingivalis and Fusobacterium nucleatum is mediated by the major outer sheath protein of Treponema denticola. FEMS Microbiol. Lett. 2008, 289, 59–66. [Google Scholar] [CrossRef][Green Version]
- Kaplan, C.W.; Lux, R.; Haake, S.K.; Shi, W. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol. Microbiol. 2009, 71, 35–47. [Google Scholar] [CrossRef]
- Bibek, G.C.; Wu, C. The CarSR two-component system directly controls radD expression as a global regulator that senses bacterial coaggregation in Fusobacterium nucleatum. J. Bacteriol. 2025, 207, e00529-24. [Google Scholar]
- Lima, B.P.; Shi, W.; Lux, R. Identification and characterization of a novel Fusobacterium nucleatum adhesin involved in physical interaction and biofilm formation with Streptococcus gordonii. Microbiologyopen 2017, 6, e00444. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ogawa, S.; Asai, Y.; Takai, Y.; Ogawa, T. Binding of Porphyromonas gingivalis fimbriae to Treponema denticola dentilisin. FEMS Microbiol. Lett. 2003, 226, 267–271. [Google Scholar] [CrossRef]
- Yamada, M.; Ikegami, A.; Kuramitsu, H.K. Synergistic biofilm formation by Treponema denticola and Porphyromonas gingivalis. FEMS Microbiol. Lett. 2005, 250, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Kikuchi, Y.; Kokubu, E.; Imamura, K.; Saito, A.; Ishihara, K. Identification of a specific domain of Porphyromonas gingivalis Hgp44 responsible for adhesion to Treponema denticola. Pathog. Dis. 2018, 76, fty047. [Google Scholar] [CrossRef]
- Jung, Y.J.; Jun, H.K.; Choi, B.K. Gingipain-dependent augmentation by Porphyromonas gingivalis of phagocytosis of Tannerella forsythia. Mol. Oral Microbiol. 2016, 31, 457–471. [Google Scholar] [CrossRef]
- Śmiga, M.; Olczak, T. PgRsp is a novel redox-sensing transcription regulator essential for Porphyromonas gingivalis virulence. Microorganisms 2019, 7, 623. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, A.; Honma, K.; Sharma, A.; Kuramitsu, H.K. Multiple functions of the leucine-rich repeat protein LrrA of Treponema denticola. Infect. Immun. 2004, 72, 4619–4627. [Google Scholar] [CrossRef]
- Sano, Y.; Okamoto-Shibayama, K.; Tanaka, K.; Ito, R.; Shintani, S.; Yakushiji, M.; Ishihara, K. Dentilisin involvement in coaggregation between Treponema denticola and Tannerella forsythia. Anaerobe 2014, 30, 45–50. [Google Scholar] [CrossRef]
- Lima, B.P.; Hu, L.I.; Vreeman, G.W.; Weibel, D.B.; Lux, R. The Oral Bacterium Fusobacterium nucleatum binds Staphylococcus aureus and alters expression of the staphylococcal accessory regulator sarA. Microb. Ecol. 2019, 78, 336–347. [Google Scholar] [CrossRef]
- Andersen, R.N.; Ganeshkumar, N.; Kolenbrander, P.E. Helicobacter pylori adheres selectively to Fusobacterium spp. Oral Microbiol. Immunol. 1998, 13, 51–54. [Google Scholar]
- Engevik, M.A.; Danhof, H.A.; Auchtung, J.; Endres, B.T.; Ruan, W.; Bassères, E.; Engevik, A.C.; Wu, Q.; Nicholson, M.; Luna, R.A.; et al. Fusobacterium nucleatum adheres to Clostridioides difficile via the RadD adhesin to enhance biofilm formation in intestinal mucus. Gastroenterology 2021, 160, 1301–1314.e8. [Google Scholar] [CrossRef]
- Yang, L.; Sriram, G.; Chew, R.J.J.; Tan, K.S. Limosilactobacillus reuteri-Fusobacterium nucleatum interactions modulate biofilm composition and immunogenicity. J. Periodontal Res. 2025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).