Establishment and Characterization of OS-MET-R-092: A Novel Patient-Derived Cell Culture from an Osteosarcoma Bone Metastasis
Abstract
1. Introduction
2. Results
2.1. Clinical History
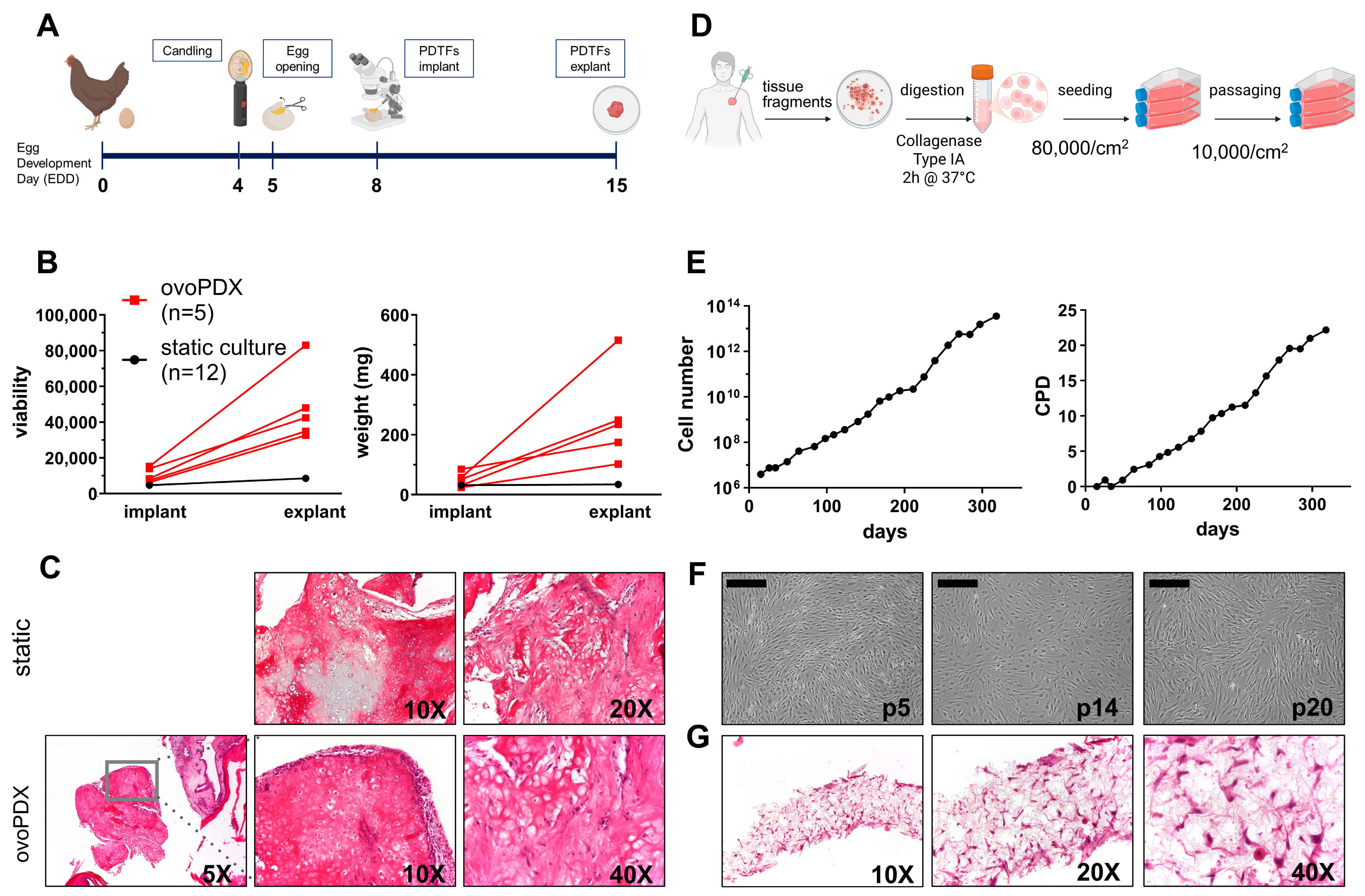
2.2. Establishment of OS-MET-092 Models
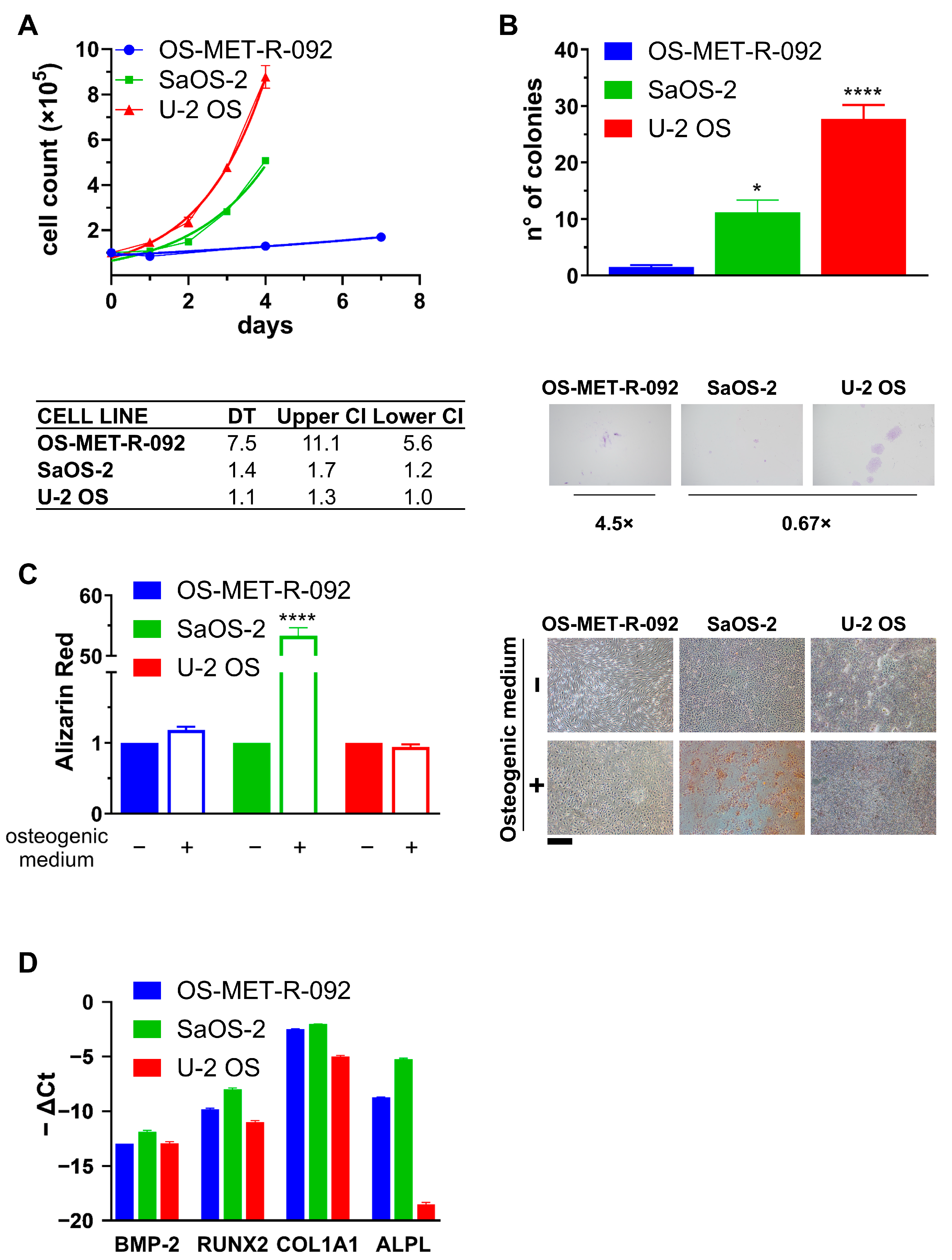
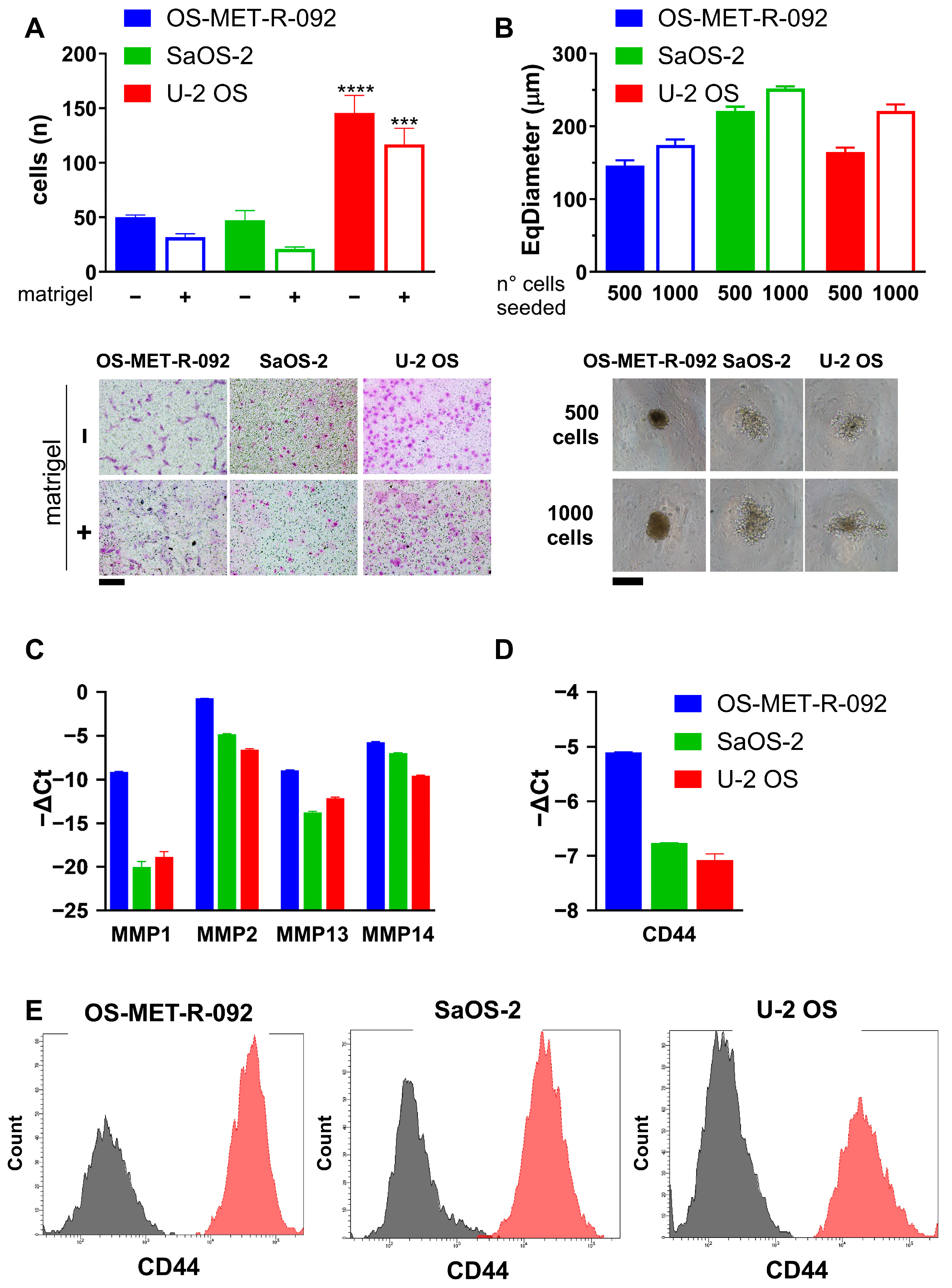
2.3. Characterization of OS-MET-R-092 Cells
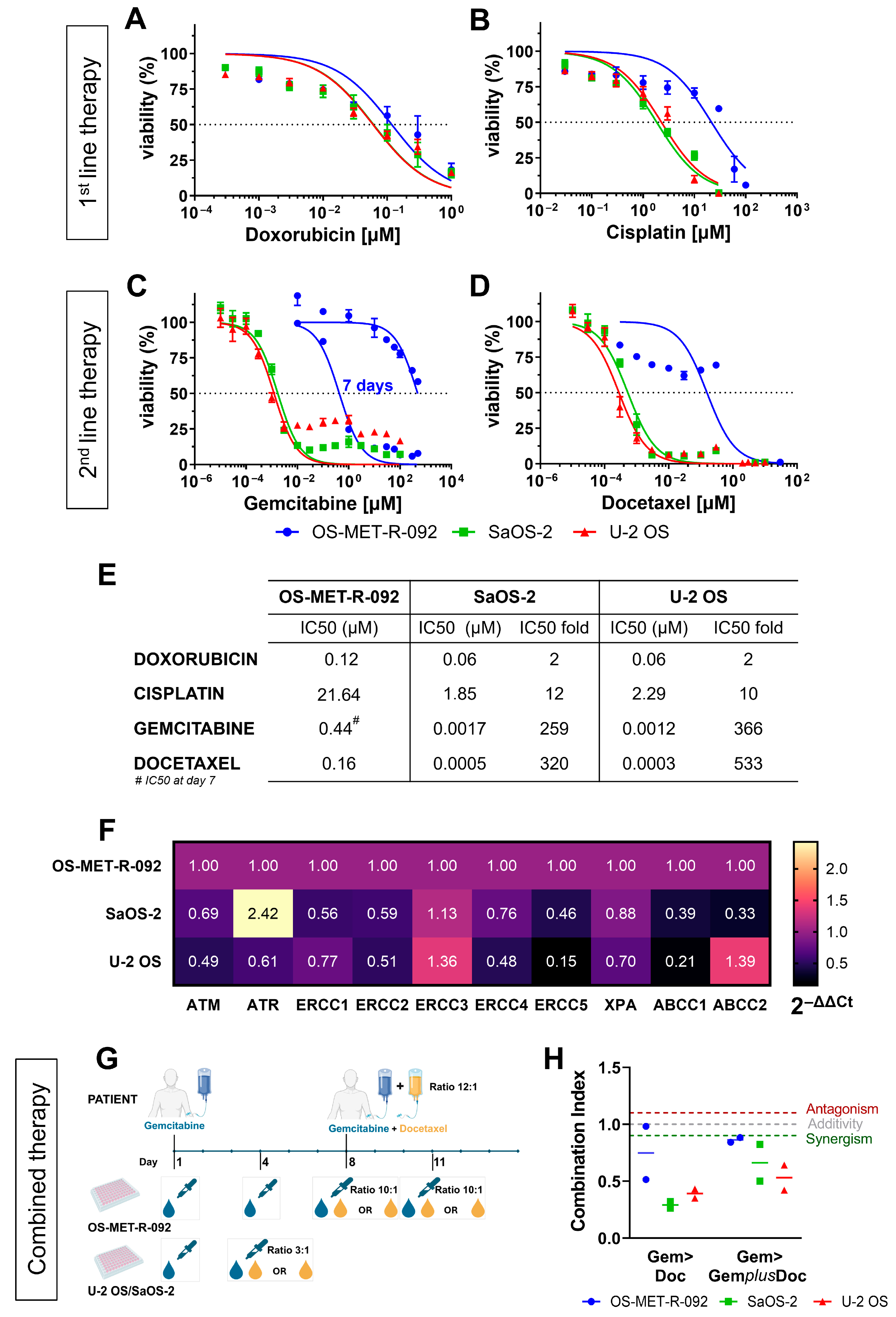
2.4. OS-MET-R-092 Cells Display a Multi-Drug Resistant Phenotype
3. Discussion
4. Materials and Methods
4.1. Patient’s History
4.2. Establishment of the Cell Line and Authentication
4.3. 3D Culture on Commercial Scaffolds
4.4. Two-Dimensional Clonogenic Assay
4.5. Osteogenic Differentiation Assay
4.6. Invasion and Migration Assays
4.7. Spheroid Formation Assay
4.8. Gene Expression Analyses
4.9. Drug Treatment
4.10. Flow Cytometry
4.11. Establishment of OvoPDXs
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-MEM | Alpha-Modified Eagle’s Minimum Essential Medium |
| ABCB1 | ATP Binding Cassette Subfamily B Member 1 |
| ABCC | ATP Binding Cassette Subfamily C Member |
| ALPL | Alkaline phosphatase |
| ATM | ATM Serine/Threonine kinase |
| ATR | ATR Checkpoint kinase |
| BMP | bone morphogenetic protein |
| CAM | chorioallantoic membrane |
| CD31 | Cluster of differentiation 31 |
| CD44 | Cluster of differentiation 44 |
| CDDP | cisplatin |
| CECT | contrast-enhanced computed tomography |
| CI | combination index |
| COL1A1 | Collagen type I α1 |
| CPD | cumulative population doubling |
| DNA | Deoxyribonucleic Acid |
| DMEM HG | Dulbecco’s Modified Eagle Medium high glucose |
| DOC | docetaxel |
| DT | doubling time |
| DOXO | doxorubicin |
| ECM | extracellular matrix |
| EDD | egg development day |
| ERCC | ERCC Excision Repair |
| FBS HI | heat-inactivated fetal bovine serum |
| GEM | gemcitabine |
| HGF | Hepatocyte growth factor |
| H/E | hematoxylin/eosin |
| IFO | ifosfamide |
| MAP | methotrexate adriamicin cisplatin |
| MMP | matrix metalloproteinase |
| MTX | methotrexate |
| NER | Nucleotide excixion repair |
| OS | osteosarcoma |
| ovoPDX | Patient-derived xenograft in ovo |
| PBS | phosphate-buffered saline |
| PD | population doubling |
| PDCC | patient-derived cell culture |
| PDTF | patient-derived tissue fragment |
| qRT-PCR | Quantitative real time polymerase chain reaction |
| RNA | ribonucleic acid |
| RUNX2 | RUNX Family Transcription Factor 2 |
| SPF | specific pathogen-free |
| STR | short tandem repeat |
| ULA | ultra-low attachment |
| XPA | XPA, DNA Damage Recognition And Repair Factor |
References
- Kim, C.; Davis, L.E.; Albert, C.M.; Samuels, B.; Roberts, J.L.; Wagner, M.J. Osteosarcoma in Pediatric and Adult Populations: Are Adults Just Big Kids? Cancers 2023, 15, 5044. [Google Scholar] [CrossRef]
- Sheng, G.; Gao, Y.; Yang, Y.; Wu, H. Osteosarcoma and Metastasis. Front. Oncol. 2021, 11, 780264. [Google Scholar] [CrossRef]
- Collier, D.A.H.; Busam, K.; Salob, S. Cutaneous Metastasis of Osteosarcoma. J. Am. Acad. Dermatol. 2003, 49, 757–760. [Google Scholar] [CrossRef]
- Danziger, J.; Wallace, S.; Handel, S.F.; Desantos, L.A. Metastatic Osteogenic Sarcoma to the Brain. Cancer 1979, 43, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Sajadi, K.R.; Heck, R.K.; Neel, M.D.; Rao, B.N.; Daw, N.; Rodriguez-Galindo, C.; Hoffer, F.A.; Stacy, G.S.; Peabody, T.D.; Simon, M.A. The Incidence and Prognosis of Osteosarcoma Skip Metastases. Clin. Orthop. Relat. Res. 2004, 426, 92–96. [Google Scholar] [CrossRef]
- Lockshin, M.D.; Higgins, I.T. Bone Metastasis in Osteogenic Sarcoma. Arch. Intern. Med. 1966, 118, 203–204. [Google Scholar] [CrossRef]
- Jeffree, G.M.; Price, C.H.G.; Sissons, H.A. The Metastatic Patterns Of Osteosarcoma. Br. J. Cancer 1975, 32, 87–107. [Google Scholar] [CrossRef]
- Price, C.H.G.; Jeffree, G.M. Metastatic spread of osteosarcoma. Br. J. Cancer 1973, 28, 515–524, Erratum in Br. J. Cancer 1974, 29, vi. [Google Scholar]
- Bacci, G.; Longhi, A.; Bertoni, F.; Briccoli, A.; Versari, M.; Pignotti, E.; Picci, P. Bone Metastases in Osteosarcoma Patients Treated with Neoadjuvant or Adjuvant Chemotherapy: The Rizzoli Experience in 52 Patients. Acta Orthop. 2006, 77, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Avella, M.; Picci, P.; Briccoli, A.; Dallari, D.; Campanacci, M. Metastatic Patterns in Osteosarcoma. Tumori J. 1988, 74, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Scanagatta, P. Metastatic Osteosarcoma: A Challenging Multidisciplinary Treatment. Expert Rev. Anticancer Ther. 2016, 16, 543–556. [Google Scholar] [CrossRef]
- Hattori, E.; Oyama, R.; Kondo, T. Systematic Review of the Current Status of Human Sarcoma Cell Lines. Cells 2019, 8, 157. [Google Scholar] [CrossRef]
- Chow, T.; Wutami, I.; Lucarelli, E.; Choong, P.F.; Duchi, S.; Di Bella, C. Creating in Vitro Three-Dimensional Tumor Models: A Guide for the Biofabrication of a Primary Osteosarcoma Model. Tissue Eng. Part B Rev. 2021, 27, 514–529. [Google Scholar] [CrossRef]
- Giusti, V.; Miserocchi, G.; Sbanchi, G.; Pannella, M.; Hattinger, C.M.; Cesari, M.; Fantoni, L.; Guerrieri, A.N.; Bellotti, C.; De Vita, A.; et al. Xenografting Human Musculoskeletal Sarcomas in Mice, Chick Embryo, and Zebrafish: How to Boost Translational Research. Biomedicines 2024, 12, 1921. [Google Scholar] [CrossRef]
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Doppelt-Flikshtain, O.; Asbi, T.; Younis, A.; Ginesin, O.; Cohen, Z.; Tamari, T.; Berg, T.; Yanovich, C.; Aran, D.; Zohar, Y.; et al. Inhibition of Osteosarcoma Metastasis in Vivo by Targeted Downregulation of MMP1 and MMP9. Matrix Biol. 2024, 134, 48–58. [Google Scholar] [CrossRef]
- Fernández-Tabanera, E.; Melero-Fernández de Mera, R.M.; Alonso, J. CD44 In Sarcomas: A Comprehensive Review and Future Perspectives. Front. Oncol. 2022, 12, 909450. [Google Scholar] [CrossRef]
- Mayr, L.; Pirker, C.; Lötsch, D.; Van Schoonhoven, S.; Windhager, R.; Englinger, B.; Berger, W.; Kubista, B. CD44 Drives Aggressiveness and Chemoresistance of a Metastatic Human Osteosarcoma Xenograft Model. Oncotarget 2017, 8, 114095–114108. [Google Scholar] [CrossRef] [PubMed]
- Chow, T.; Humble, W.; Lucarelli, E.; Onofrillo, C.; Choong, P.F.; Di Bella, C.; Duchi, S. Feasibility and Barriers to Rapid Establishment of Patient-Derived Primary Osteosarcoma Cell Lines in Clinical Management. iScience 2024, 27, 110251. [Google Scholar] [CrossRef] [PubMed]
- Arihiro, K.; Inai, K. Expression of CD31, Met/Hepatocyte Growth Factor Receptor and Bone Morphogenetic Protein in Bone Metastasis of Osteosarcoma. Pathol. Int. 2001, 51, 100–106. [Google Scholar] [CrossRef]
- Mohseny, A.B.; MacHado, I.; Cai, Y.; Schaefer, K.L.; Serra, M.; Hogendoorn, P.C.W.; Llombart-Bosch, A.; Cleton-Jansen, A.M. Functional Characterization of Osteosarcoma Cell Lines Provides Representative Models to Study the Human Disease. Lab. Investig. 2011, 91, 1195–1205. [Google Scholar] [CrossRef]
- Lauvrak, S.U.; Munthe, E.; Kresse, S.H.; Stratford, E.W.; Namløs, H.M.; Meza-Zepeda, L.A.; Myklebost, O. Functional Characterisation of Osteosarcoma Cell Lines and Identification of MRNAs and MiRNAs Associated with Aggressive Cancer Phenotypes. Br. J. Cancer 2013, 109, 2228–2236. [Google Scholar] [CrossRef]
- Sys, G.; Van Bockstal, M.; Forsyth, R.; Balke, M.; Poffyn, B.; Uyttendaele, D.; Bracke, M.; De Wever, O. Tumor Grafts Derived from Sarcoma Patients Retain Tumor Morphology, Viability, and Invasion Potential and Indicate Disease Outcomes in the Chick Chorioallantoic Membrane Model. Cancer Lett. 2012, 326, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Landuzzi, L.; Manara, M.C.; Lollini, P.L.; Scotlandi, K. Patient Derived Xenografts for Genome-Driven Therapy of Osteosarcoma. Cells 2021, 10, 416. [Google Scholar] [CrossRef]
- Sulaiman, A.; Wang, L. Bridging the Divide: Preclinical Research Discrepancies between Triple-Negative Breast Cancer Cell Lines and Patient Tumors. Oncotarget 2017, 8, 113269–113281. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Schröder, S.K.; Buhl, E.M.; Weiskirchen, R. A Beginner’s Guide to Cell Culture: Practical Advice for Preventing Needless Problems. Cells 2023, 12, 682. [Google Scholar] [CrossRef]
- Luo, X.; Chen, J.; Song, W.X.; Tang, N.; Luo, J.; Deng, Z.L.; Sharff, K.A.; He, G.; Bi, Y.; He, B.C.; et al. Osteogenic BMPs Promote Tumor Growth of Human Osteosarcomas That Harbor Differentiation Defects. Lab. Investig. 2008, 88, 1264–1277. [Google Scholar] [CrossRef]
- Xu, C.; Wang, M.; Zandieh-Doulabi, B.; Sun, W.; Wei, L.; Liu, Y. To B (Bone Morphogenic Protein-2) or Not to B (Bone Morphogenic Protein-2): Mesenchymal Stem Cells May Explain the Protein’s Role in Osteosarcomagenesis. Front. Cell Dev. Biol. 2021, 9, 740783. [Google Scholar] [CrossRef]
- Piazzi, M.; Bavelloni, A.; Greco, S.; Focaccia, E.; Orsini, A.; Benini, S.; Gambarotti, M.; Faenza, I.; Blalock, W.L. Expression of the Double-Stranded RNA-Dependent Kinase PKR Influences Osteosarcoma Attachment Independent Growth, Migration, and Invasion. J. Cell. Physiol. 2020, 235, 1103–1119. [Google Scholar] [CrossRef]
- Serra, M.; Hattinger, C.M.; Pasello, M.; Casotti, C.; Fantoni, L.; Riganti, C.; Manara, M.C. Impact of ABC Transporters in Osteosarcoma and Ewing’s Sarcoma: Which Are Involved in Chemoresistance and Which Are Not? Cells 2021, 10, 2461. [Google Scholar] [CrossRef]
- Gerardo-Ramírez, M.; Keggenhoff, F.L.; Giam, V.; Becker, D.; Groth, M.; Hartmann, N.; Straub, B.K.; Morrison, H.; Galle, P.R.; Marquardt, J.U.; et al. CD44 Contributes to the Regulation of MDR1 Protein and Doxorubicin Chemoresistance in Osteosarcoma. Int. J. Mol. Sci. 2022, 23, 8616. [Google Scholar] [CrossRef]
- Boichuk, S.; Bikinieva, F.; Valeeva, E.; Dunaev, P.; Vasileva, M.; Kopnin, P.; Mikheeva, E.; Ivoilova, T.; Mustafin, I.; Galembikova, A. Establishment and Characterization of Multi-Drug Resistant p53-Negative Osteosarcoma SaOS-2 Subline. Diagnostics 2023, 13, 2646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gazouli, I.; Kyriazoglou, A.; Kotsantis, I.; Anastasiou, M.; Pantazopoulos, A.; Prevezanou, M.; Chatzidakis, I.; Kavourakis, G.; Economopoulou, P.; Kontogeorgakos, V.; et al. Systematic Review of Recurrent Osteosarcoma Systemic Therapy. Cancers 2021, 13, 1757. [Google Scholar] [CrossRef]
- Guder, W.K.; Hartmann, W.; Buhles, C.; Burdack, M.; Busch, M.; Dünker, N.; Hardes, J.; Dirksen, U.; Bauer, S.; Streitbürger, A. 5-ALA-Mediated Fluorescence of Musculoskeletal Tumors in a Chick Chorio-Allantoic Membrane Model: Preclinical in Vivo Qualification Analysis as a Fluorescence-Guided Surgery Agent in Orthopedic Oncology. J. Orthop. Surg. Res. 2022, 17, 34. [Google Scholar] [CrossRef]
- Sys, G.M.L.; Lapeire, L.; Stevens, N.; Favoreel, H.; Forsyth, R.; Bracke, M.; De Wever, O. The In Ovo CAM-Assay as a Xenograft Model for Sarcoma. J. Vis. Exp. 2013, 77, e50522. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane Patient-Derived Xenograft (PDX) Model. Pathol. Res. Pract. 2023, 243, 154367. [Google Scholar] [CrossRef]
- De Cock, L.; Palubeckaitė, I.; Bersani, F.; Faehling, T.; Pasquali, S.; Umbaugh, S.; Meister, M.T.; Danks, M.R.; Manasterski, P.; Miallot, R.; et al. Establishment of Patient-Derived 3D in Vitro Models of Sarcomas: Literature Review and Guidelines on Behalf of the FORTRESS Working Group. Neoplasia 2025, 65, 101171. [Google Scholar] [CrossRef]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front. Cell Dev. Biol. 2021, 9, 711381. [Google Scholar] [CrossRef]
- Prasad, C.P.; Tripathi, S.C.; Kumar, M.; Mohapatra, P. Passage Number of Cancer Cell Lines: Importance, Intricacies, and Way-Forward. Biotechnol. Bioeng. 2023, 120, 2049–2055. [Google Scholar] [CrossRef]
- Hoppock, G.A.; Buettmann, E.G.; Denisco, J.A.; Goldscheitter, G.M.; Condyles, S.N.; Juhl IV, O.J.; Friedman, M.A.; Zhang, Y.; Donahue, H.J. Connexin 43 and Cell Culture Substrate Differentially Regulate OCY454 Osteocytic Differentiation and Signaling to Primary Bone Cells. Am. J. Physiol. Cell Physiol. 2023, 325, C907–C920. [Google Scholar] [CrossRef]
- Cortini, M.; Macchi, F.; Reggiani, F.; Vitale, E.; Lipreri, M.V.; Perut, F.; Ciarrocchi, A.; Baldini, N.; Avnet, S. Endogenous Extracellular Matrix Regulates the Response of Osteosarcoma 3D Spheroids to Doxorubicin. Cancers 2023, 15, 1221. [Google Scholar] [CrossRef]
- Tang, H.; Tang, Z.; Jiang, Y.; Wei, W.; Lu, J.; Ding, L.; Liu, T.; Qu, Y.; Kang, Z.; Guo, L.; et al. Pathological and Therapeutic Aspects of Matrix Metalloproteinases: Implications in Osteosarcoma. Exp. Biol. Med. 2024, 26, 787–797. [Google Scholar] [CrossRef]
- Ding, L.; Liu, T.; Qu, Y.; Kang, Z.; Guo, L.; Zhang, H.; Jiang, J.; Qu, F.; Ge, W.; Zhang, S. LncRNA MELTF-AS1 Facilitates Osteosarcoma Metastasis by Modulating MMP14 Expression. Mol. Ther. Nucleic Acids 2021, 26, 787–797. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Yi, Z.; Ma, B.; Wang, H.; Pu, Y.; Wang, J.; Wang, S. WNT5A Promotes Migration and Invasion of Human Osteosarcoma Cells via SRC/ERK/MMP-14 Pathway. Cell Biol. Int. 2018, 42, 598–607. [Google Scholar] [CrossRef]
- Liang, S.; Li, L.; Guo, Z.; Sun, H.; Yang, Y. Co-Expression of CD44v6 and MMP2 Predicts Lung Metastasis and Unfavorable Prognosis in Osteosarcoma. Future Oncol. 2024, 20, 1799–1806. [Google Scholar] [CrossRef]
- Hirahata, M.; Osaki, M.; Kanda, Y.; Sugimoto, Y.; Yoshioka, Y.; Kosaka, N.; Takeshita, F.; Fujiwara, T.; Kawai, A.; Ito, H.; et al. PAI-1, a Target Gene of MiR-143, Regulates Invasion and Metastasis by Upregulating MMP-13 Expression of Human Osteosarcoma. Cancer Med. 2016, 5, 892–902. [Google Scholar] [CrossRef]
- Poudel, B.H.; Koks, S. The Whole Transcriptome Analysis Using FFPE and Fresh Tissue Samples Identifies the Molecular Fingerprint of Osteosarcoma. Exp. Biol. Med. 2024, 249, 10161. [Google Scholar] [CrossRef]
- Ho, X.D.; Phung, P.; Le, V.Q.; Nguyen, V.H.; Reimann, E.; Prans, E.; Kõks, G.; Maasalu, K.; Le, N.T.N.; Trinh, L.H.; et al. Whole Transcriptome Analysis Identifies Differentially Regulated Networks between Osteosarcoma and Normal Bone Samples. Exp. Biol. Med. 2017, 242, 1802–1811. [Google Scholar] [CrossRef]
- Vaezi, M.A.; Eghtedari, A.R.; Safizadeh, B.; Ghasempour, G.; Salimi, V.; Nourbakhsh, M.; Nazem, S.; Tavakoli-Yaraki, M. Up-Regulation of Matrix Metalloproteinase-9 in Primary Bone Tumors and Its Association with Tumor Aggressiveness. Mol. Biol. Rep. 2022, 49, 9409–9427. [Google Scholar] [CrossRef]
- Kong, L.; Ji, H.; Gan, X.; Cao, S.; Li, Z.; Jin, Y. Knockdown of CD44 Inhibits Proliferation, Migration and Invasion of Osteosarcoma Cells Accompanied by Downregulation of Cathepsin S. J. Orthop. Surg. Res. 2022, 17, 154. [Google Scholar] [CrossRef]
- He, A.; Yang, X.; Huang, Y.; Feng, T.; Wang, Y.; Sun, Y.; Shen, Z.; Yao, Y. CD133+CD44+ Cells Mediate in the Lung Metastasis of Osteosarcoma. J. Cell. Biochem. 2015, 116, 1719–1729. [Google Scholar] [CrossRef]
- Zhang, Y.; Lun, L.; Zhu, B.; Wang, Q.; Ding, C.; Hu, Y.; Huang, W.; Zhou, L.; Chen, X.; Huang, H. Diagnostic Accuracy of CD44V6 for Osteosarcoma: A Meta-Analysis. J. Orthop. Surg. Res. 2016, 11, 133. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, Y.; Shen, J.K.; Lin, M.; Choy, E.; Cote, G.M.; Harmon, D.C.; Mankin, H.J.; Hornicek, F.J.; Duan, Z. CD44 Is a Direct Target of MiR-199a-3p and Contributes to Aggressive Progression in Osteosarcoma. Sci. Rep. 2015, 5, 11365. [Google Scholar] [CrossRef]
- Gvozdenovic, A.; Arlt, M.J.E.; Campanile, C.; Brennecke, P.; Husmann, K.; Li, Y.; Born, W.; Muff, R.; Fuchs, B. CD44 Enhances Tumor Formation and Lung Metastasis in Experimental Osteosarcoma and Is an Additional Predictor for Poor Patient Outcome. J. Bone Miner. Res. 2013, 28, 838–847. [Google Scholar] [CrossRef]
- Sabaawy, H.E.; Broggini, M.; Gupta, S.K. Editorial: Patient-Derived Tumor Models for Drug Development. Front. Oncol. 2023, 13, 1243534. [Google Scholar] [CrossRef]
- Pasello, M.; Michelacci, F.; Scionti, I.; Hattinger, C.M.; Zuntini, M.; Caccuri, A.M.; Scotlandi, K.; Picci, P.; Serra, M. Overcoming Glutathione S-Transferase P1-Related Cisplatin Resistance in Osteosarcoma. Cancer Res. 2008, 68, 6661–6668. [Google Scholar] [CrossRef]
- Fanelli, M.; Hattinger, C.M.; Vella, S.; Tavanti, E.; Michelacci, F.; Gudeman, B.; Barnett, D.; Picci, P.; Serra, M. Targeting ABCB1 and ABCC1 with Their Specific Inhibitor CBT-1® Can Overcome Drug Resistance in Osteosarcoma. Curr. Cancer Drug Targets 2016, 16, 261–274. [Google Scholar] [CrossRef]
- Fanelli, M.; Tavanti, E.; Patrizio, M.P.; Vella, S.; Fernandez-Ramos, A.; Magagnoli, F.; Luppi, S.; Hattinger, C.M.; Serra, M. Cisplatin Resistance in Osteosarcoma: In Vitro Validation of Candidate DNA Repair-Related Therapeutic Targets and Drugs for Tailored Treatments. Front. Oncol. 2020, 10, 331. [Google Scholar] [CrossRef]
- Dussault, A.A.; Pouliot, M. Rapid and Simple Comparison of Messenger RNA Levels Using Real-Time PCR. Biol. Proced. Online 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681, Erratum in Pharmacol. Rev. 2007, 59, 124. [Google Scholar] [CrossRef]

| Locus | Patient Tumor Sample | OS-MET-R-092 p6 |
|---|---|---|
| D8S1179 | 13, 14 | 13, 14 |
| D21S11 | 29, 30, 31.2 | 29, 30, 31.2 |
| D7S820 | 11, 11 | 11, 11 |
| CSF1PO | 11, 12 | 11, 12 |
| D3S1358 | 17, 18 | 17, 18 |
| TH01 § | 6, 7 | 7, 7 |
| D13S317 | 9, 9 | 9, 9 |
| D16S539 | 11, 11 | 11, 11 |
| D2S1338 | 20, 21 | 20, 21 |
| D19S433 | 14, 14.2 | 14, 14.2 |
| vWa § | 17, 18, 19 | 17, 18 |
| TPOX | 8, 10 | 8, 10 |
| D18S51 § | 15, 16, 17 | 16, 17 |
| AMEL | X, Y | X, Y |
| D5S818 | 12, 13 | 12, 13 |
| FGA | 20, 26 | 20, 26 |
| Target Name | Forward Primer Seq. 5′-3′ | Reverse Primer Seq. 5′-3′ | Assay Number |
|---|---|---|---|
| β-actin | ATCGTCCACCGCAAATGCTTCTA | AGCCATGCCAATCTCATCTTGTT | |
| CD44 | CTGCCGCTTTGCAGGTGTA | CATTGTGGGCAAGGTGCTATT | |
| COL1A1 | GCTATGATGAGAAATCAACCG | CATTGTGGGCAAGGTGCTATT | |
| BMP-2 | NM_001200.2 | ||
| ALP | GGGCTCCAGAAGCTCAACAC | GTGGAGCTGACCCTTGAGCAT | |
| RUNX-2 | TGATGACACTGCCACCTCTGA | GCACCTGCCTGGCTCTTCT | |
| MMP1 | CAAATGGGCTTGAAGCTGCTTACG | GTGTAGCACATTCTGTCCCTGAACA | |
| MMP2 | GCTCCACCACCTACAACTTTGAGAA | TGTCATAGGATGTGCCCTGGAA | |
| MMP3 | AGTCTTCCAATCCTACTGTTGCT | TCCCCGTCACCTCCAATCC | |
| MMP9 | CTTTGAGTCCGGTGGACGAT | TCGCCAGTACTTCCCATCCT | |
| MMP13 | GACTTCCCAGGAATTGGTGA | TGACGCGAACAATACGGTTA | |
| MMP14 | GGCTACAGCAATATGGCTACC | GATGGCCGCTGAGAGTGAC | |
| ABCC1 | Hs00219905_m1 | ||
| ABCC2 | Hs00166123_m1 | ||
| ACTB | Hs99999903_m1 | ||
| ATM | Hs00175892_m1 | ||
| ATR | Hs00992123_m1 | ||
| ERCC1 | Hs01012158_m1 | ||
| ERCC2 | Hs00361161_m1 | ||
| ERCC3 | Hs01554457_m1 | ||
| ERCC4 | Hs00193342_m1 | ||
| ERCC5 | Hs01557031_m1 | ||
| XPA | Hs00166045_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giusti, V.; Fantoni, L.; Torsello, M.; Frega, G.; Martinuzzi, A.; Sbanchi, G.; Dalrio, C.; Lucarelli, E.; Bellotti, C.; Casotti, C.; et al. Establishment and Characterization of OS-MET-R-092: A Novel Patient-Derived Cell Culture from an Osteosarcoma Bone Metastasis. Int. J. Mol. Sci. 2025, 26, 10540. https://doi.org/10.3390/ijms262110540
Giusti V, Fantoni L, Torsello M, Frega G, Martinuzzi A, Sbanchi G, Dalrio C, Lucarelli E, Bellotti C, Casotti C, et al. Establishment and Characterization of OS-MET-R-092: A Novel Patient-Derived Cell Culture from an Osteosarcoma Bone Metastasis. International Journal of Molecular Sciences. 2025; 26(21):10540. https://doi.org/10.3390/ijms262110540
Chicago/Turabian StyleGiusti, Veronica, Leonardo Fantoni, Monica Torsello, Giorgio Frega, Arianna Martinuzzi, Giulia Sbanchi, Caterina Dalrio, Enrico Lucarelli, Chiara Bellotti, Chiara Casotti, and et al. 2025. "Establishment and Characterization of OS-MET-R-092: A Novel Patient-Derived Cell Culture from an Osteosarcoma Bone Metastasis" International Journal of Molecular Sciences 26, no. 21: 10540. https://doi.org/10.3390/ijms262110540
APA StyleGiusti, V., Fantoni, L., Torsello, M., Frega, G., Martinuzzi, A., Sbanchi, G., Dalrio, C., Lucarelli, E., Bellotti, C., Casotti, C., Caddeo, E., Guerrieri, A. N., Paglia, S., Hattinger, C. M., Serra, M., Maioli, M., Gambarotti, M., Benini, S., Cattini, L., ... Mercatali, L. (2025). Establishment and Characterization of OS-MET-R-092: A Novel Patient-Derived Cell Culture from an Osteosarcoma Bone Metastasis. International Journal of Molecular Sciences, 26(21), 10540. https://doi.org/10.3390/ijms262110540










