Monitoring and Evaluation of Corrosion at the Interface of Zirconium Alloy Biomaterials Under Simulated Oxidative Biological Environment
Abstract
1. Introduction
2. Results and Discussion
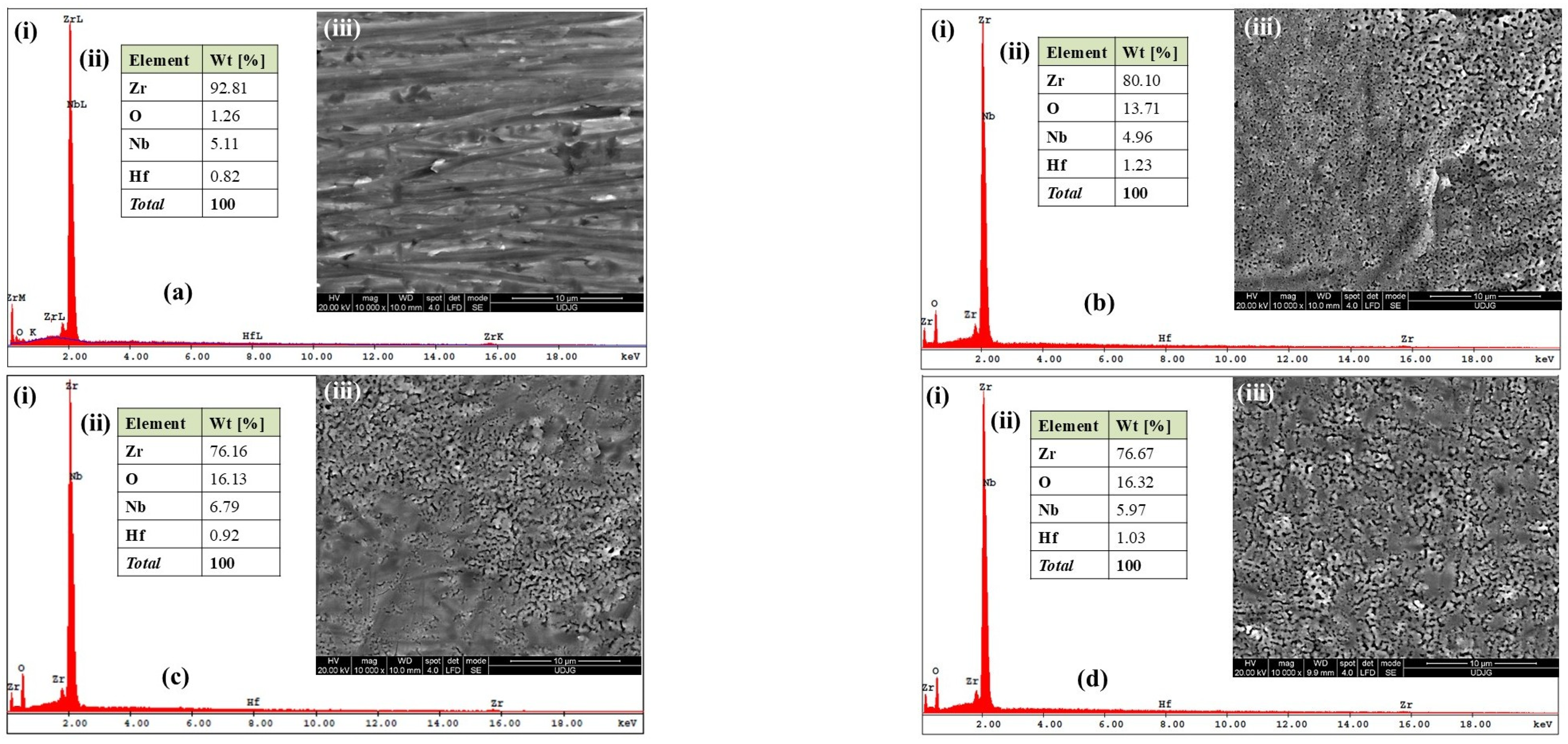
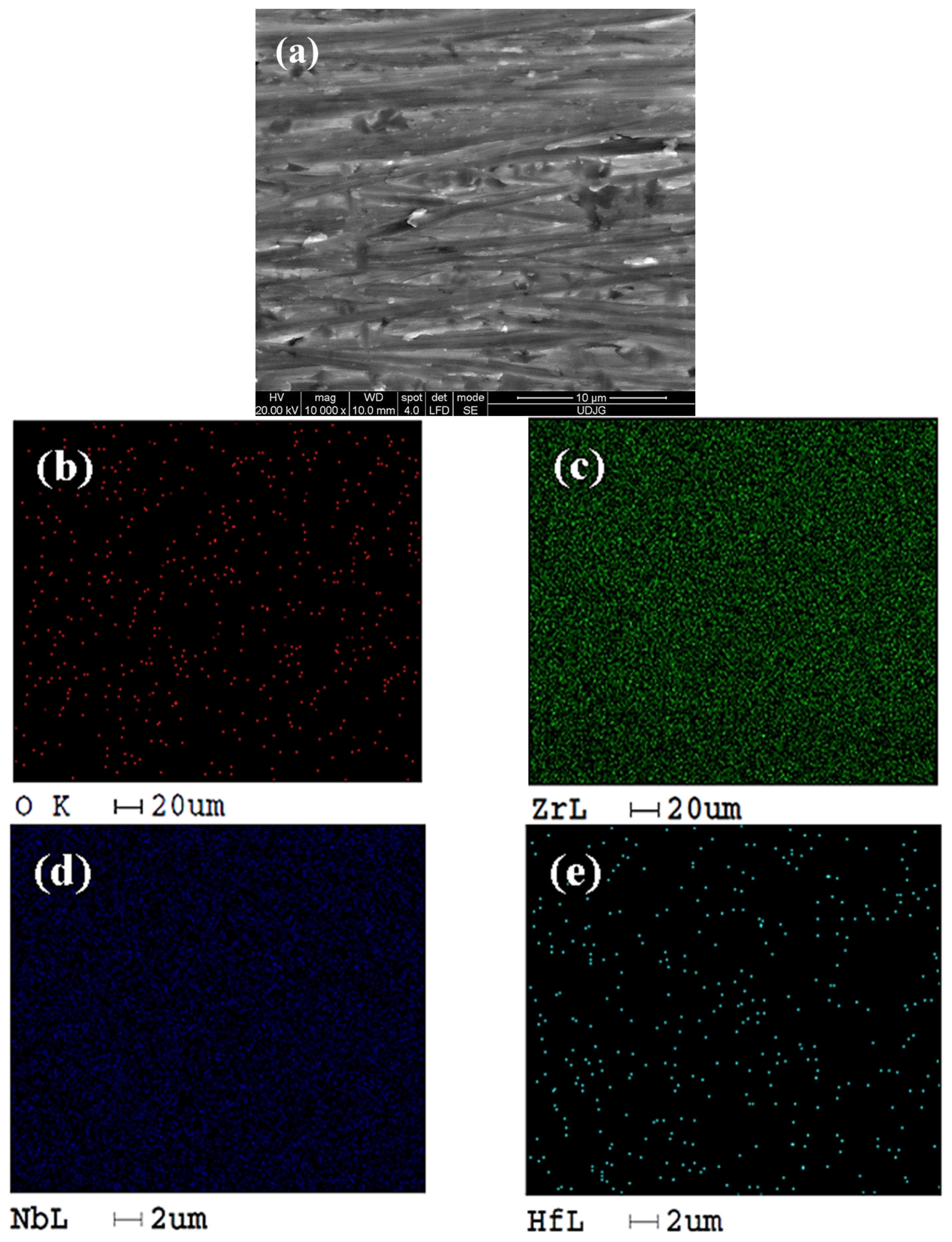
2.1. Morphological and Compositional Analysis of the Surfaces of the Zr2.5Nb Alloy and the Obtained Oxide Films, Using Scanning Electron Microscopy (SEM–EDX)
SEM–EDX Analysis of Untreated and Electrochemically Modified Zr2.5Nb Alloy
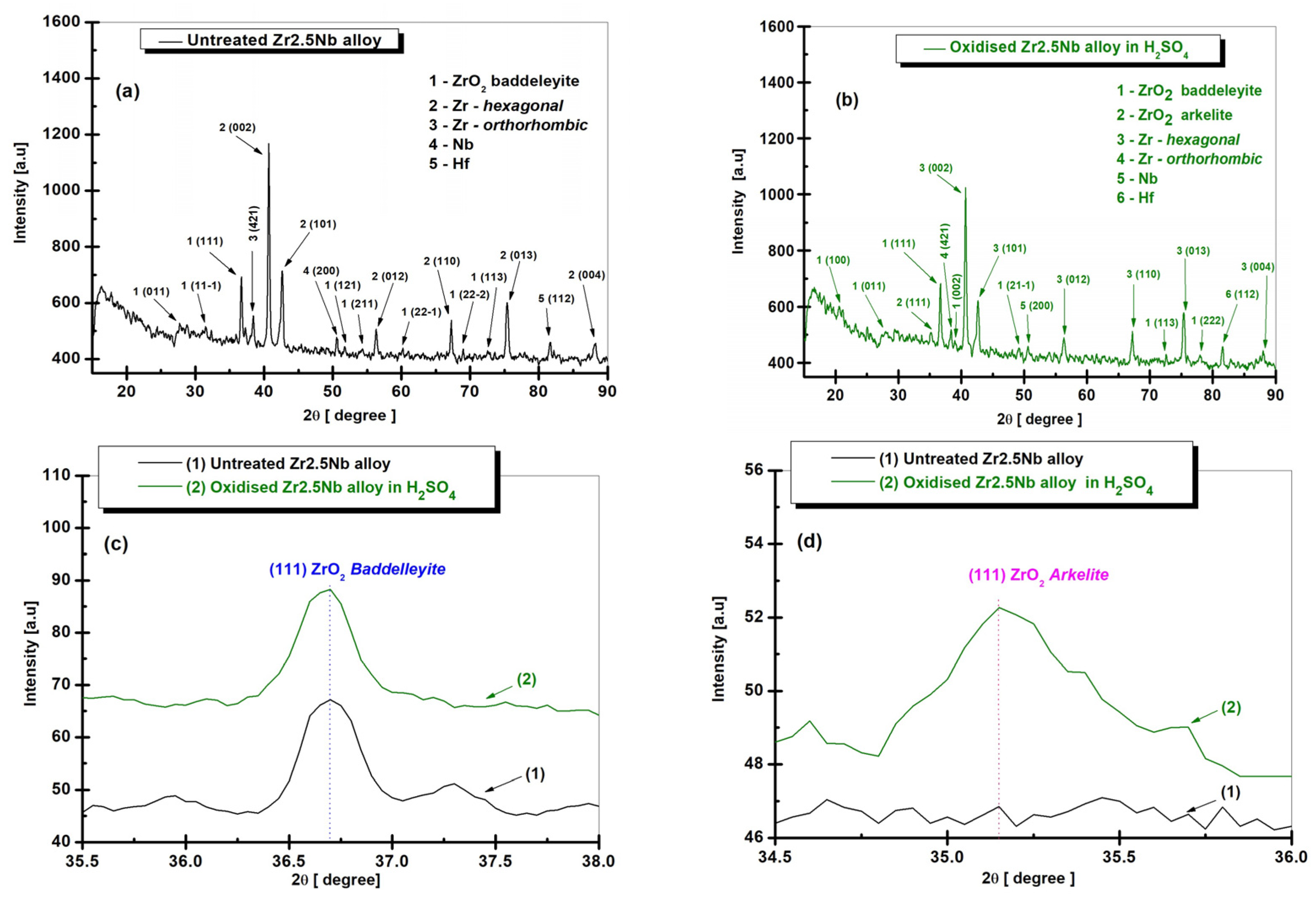
2.2. Structural Characterization by X-Ray Diffraction (XRD) of Untreated and Electrochemically Modified Zr2.5Nb Alloy
2.3. Corrosion Resistance Assessment of Untreated and Electrochemically Modified Zr2.5Nb Alloy in Both Physiological and Pathological Artificial Solutions
2.3.1. Evolution of the Open Circuit Potential (OCP) for Untreated and Electrochemically Modified Zr2.5Nb Alloy After Immersion in Physiological and Pathological Environments
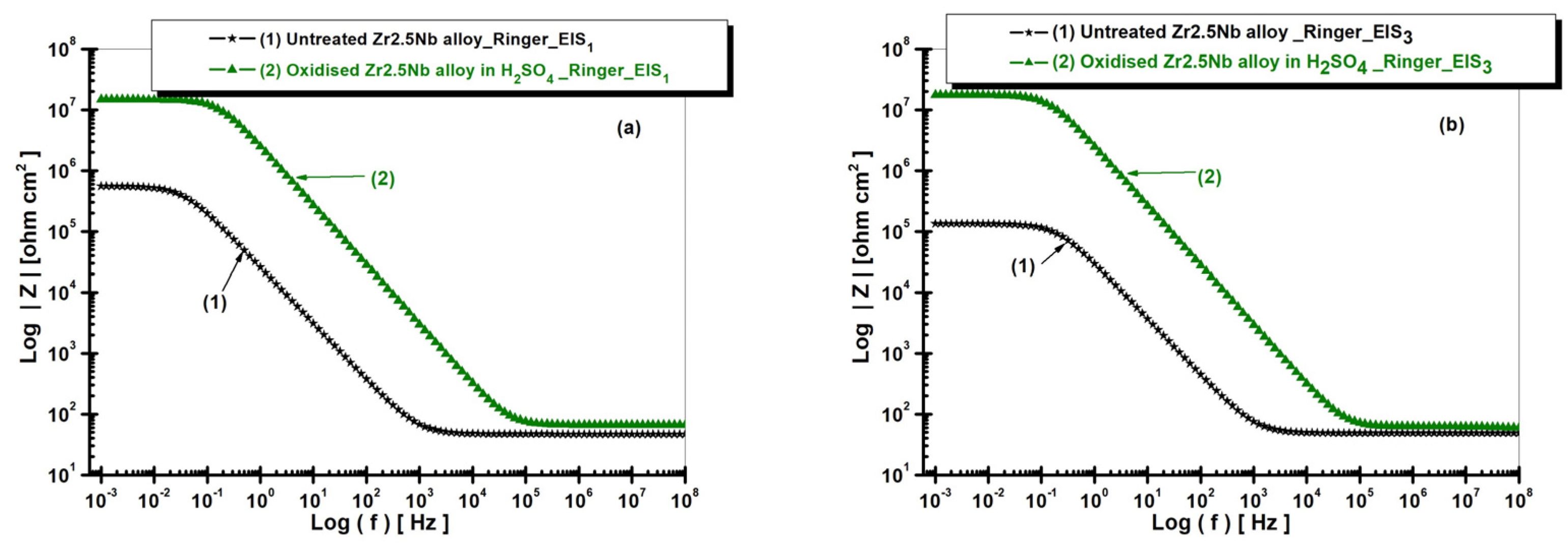
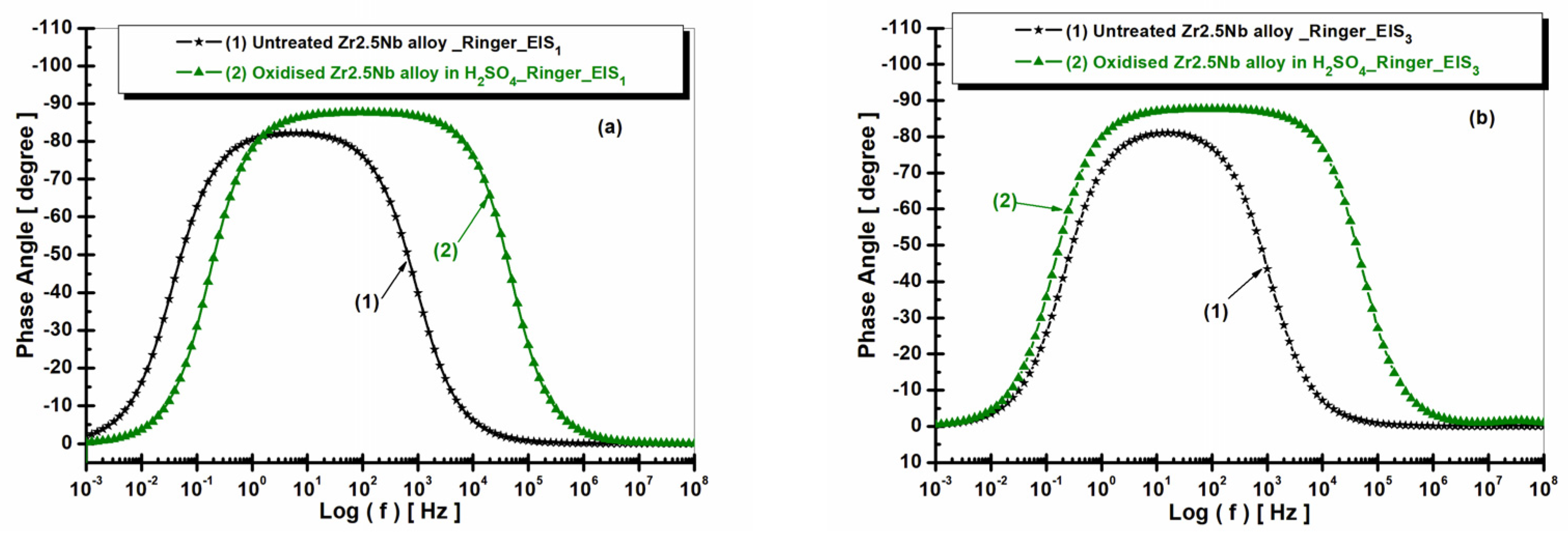
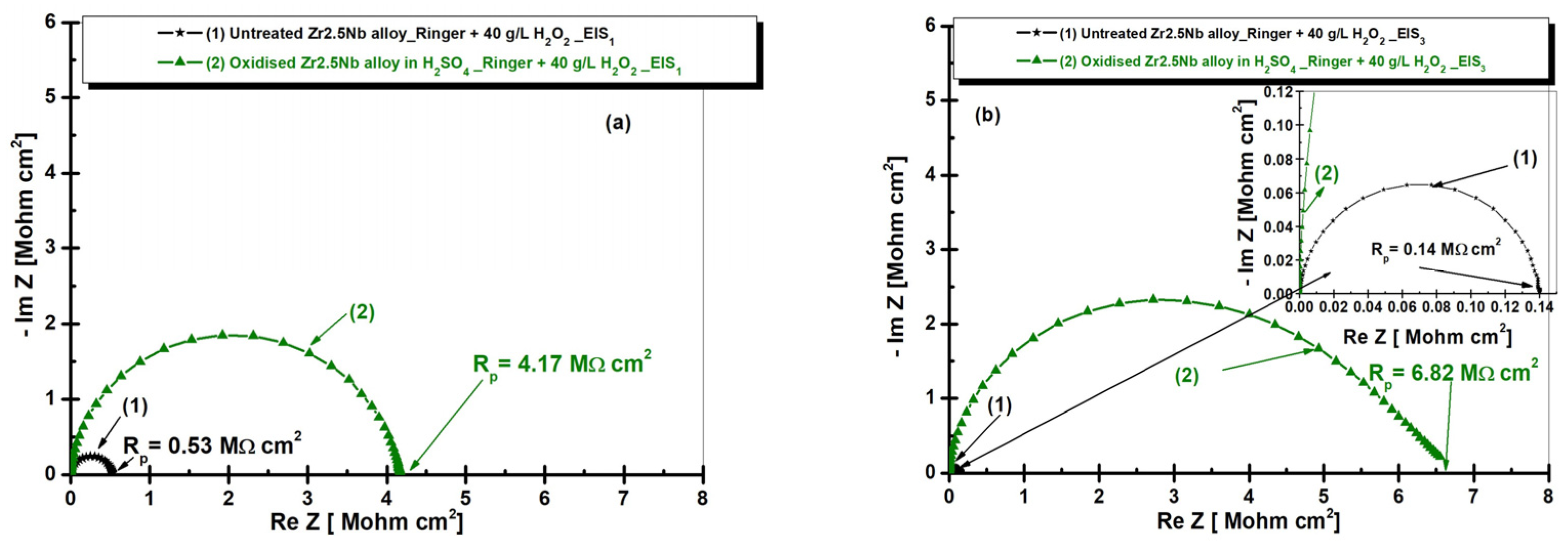
2.3.2. Electrochemical Impedance Spectroscopy (EIS) of Untreated and Electrochemically Modified Zr2.5Nb Alloy After Immersion in Physiological and Pathological Environment
3. Materials and Methods
3.1. Zr2.5Nb Alloy
3.2. Electrochemical Oxidation Process
3.3. Description of the Corrosion Test Method
3.4. Morphological and Compositional Characterization (SEM–EDX)
3.5. Structural Analysis with X-Ray Diffractometer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erwin, A.V. Surface Modification for Biocompatibility. In Engineered Biomimicry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 189–220. [Google Scholar] [CrossRef]
- Abd El-Ghany, O.S.; Sherief, A.H. Zirconia based ceramics, some clinical and biological aspects: Review. Future Dent. J. 2016, 2, 55–64. [Google Scholar] [CrossRef]
- Nie, L.; Zhan, Y.; Liu, H.; Tang, C. Novel β-type Zr–Mo–Ti alloys for biological hard tissue replacements. Mater. Des. 2014, 53, 8–12. [Google Scholar] [CrossRef]
- Li, C.; Zhan, Y.; Jiang, W. Zr–Si biomaterials with high strength and low elastic modulus. Mater. Des. 2011, 32, 4598–4602. [Google Scholar] [CrossRef]
- Hua, N.; Huang, L.; Wang, J.; Cao, Y.; He, W.; Pang, S.; Zhang, T. Corrosion behavior and in vitro biocompatibility of Zr–Al–Co–Ag bulk metallic glasses: An experimental case study. J. Non-Cryst. Solids 2012, 358, 1599–1604. [Google Scholar] [CrossRef]
- Lu, X.; Huang, L.; Pang, S.; Zhang, T. Formation and biocorrosion behavior of Zr-Al-Co-Nb bulk metallic glasses. Chin. Sci. Bull. 2012, 57, 1723–1727. [Google Scholar] [CrossRef]
- Mehjabeen, A.; Song, T.; Xu, W.; Tang, H.P.; Qian, M. Zirconium alloys for orthopaedic and dental applications. Adv. Eng. Mater. 2018, 20, 1800207. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Z.; Zhao, X.; Xu, J. MRI-compatible Nb–60Ta–2Zr alloy for vascular stents: Electrochemical corrosion behavior in simulated plasma solution. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 205–214. [Google Scholar] [CrossRef]
- Virtanen, S.; Milošev, I.; Gomez-Barrena, E.; Trebše, R.; Salo, J.; Konttinen, Y.T. Special modes of corrosion under physiological and simulated physiological conditions. Acta Biomater. 2008, 4, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Nadaraia, K.V.; Mashtalyar, D.V.; Piatkova, M.A.; Pleshkova, A.I.; Imshinetskiy, I.M.; Gerasimenko, M.S.; Belov, E.A.; Kumeiko, V.V.; Kozyrev, D.N.; Fomenko, K.A.; et al. Antibacterial HA-coatings on bioresorbable Mg alloy. J. Magnes. Alloys 2024, 12, 1965–1985. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Mali, S.A. Medical Implant Corrosion: Electrochemistry at Metallic Biomaterial Surfaces. In Degradation of Implant Materials; Eliaz, N., Ed.; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar] [CrossRef]
- Shilong, L.; Lei, S.; Yingjie, G.; Jingyang, W.; Di, L.; Shenlong, Z. Selective oxygen reduction reaction: Mechanism understanding, catalyst design and practical application. Chem. Sci. 2024, 15, 11188–11228. [Google Scholar] [CrossRef]
- Katunar, M.R.; Gomez Sanchez, A.; Santos Coquillat, A.; Civantos, A.; Martinez Campos, E.; Ballarre, J.; Vico, T.; Baca, M.; Ramos, V.; Cere, S. In vitro and in vivo characterization of anodised zirconium as a potential material for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 957–968. [Google Scholar] [CrossRef]
- Sanchez, A.G.; Ballarre, J.; Orellano, J.C.; Duffó, G.; Cere, S. Surface modification of zirconium by anodisation as material for permanent implants: In vitro and in vivo study. J. Mater. Sci. Mater. Med. 2013, 24, 161–169. [Google Scholar] [CrossRef]
- Mousavi-Semnani, S.Z.; Yousefpour, M.; Zareidoost, A. Enhancing the biocompatibility of ZrO2 thin film on Zr-2.5Nb alloy by anodizing treatment using an electrolyte containing biofunctional groups. Thin Solid Films 2022, 753, 139279. [Google Scholar] [CrossRef]
- Song, K.Y.; Zhang, H.; Zhang, W.J.; Teixeira, A. Enhancement of the surface free energy of PDMS for reversible and leakage-free bonding of PDMS–PS microfluidic cell-culture systems. Microfluid. Nanofluidics 2018, 22, 135. [Google Scholar] [CrossRef]
- bin Anwar Fadzil, A.F.; Pramanik, A.; Basak, A.K.; Prakash, C.; Shankar, S. Role of surface quality on biocompatibility of implants—A review. Ann. 3D Print. Med. 2022, 8, 100082. [Google Scholar] [CrossRef]
- Pralhad, P.; Shivprakash, B. Surface modification of titanium and titanium alloy by plasmaelectrolytic oxidation process for biomedical applications: A review. Mater. Today Proc. 2021, 46, 594–602. [Google Scholar] [CrossRef]
- Mori, Y.; Masahashi, N.; Aizawa, T. A Review of anodized TiNbSn alloys for improvement in layer quality and application to orthopedic implants. Materials 2022, 15, 5116. [Google Scholar] [CrossRef]
- Lestari, F.P.; Sari, Y.R.; Rokhmanto, F.; Asmaria, T.; Pramono, A.W. Surface modification of Ti-6Al-4V alloy by anodization technique at low potential to produce oxide layer. J. Electron. Electromed. Eng. Med. Inform. 2020, 2, 93–102. [Google Scholar] [CrossRef]
- Benea, L.; Ravoiu, A.; Neaga, V.; Axente, E.R. Using Applied Electrochemistry to Obtain Nanoporous TiO2 Films on Ti6Al4V Implant Alloys and Their Preclinical In Vitro Characterization in Biological Solutions. Coatings 2023, 13, 614. [Google Scholar] [CrossRef]
- Gomez Sanchez, A.; Katunar, M.; Ceré, S. Chapter ten—Structural characteristics and barrier properties of anodic zirconium oxides for biomedical applications. In Micro and Nano Technologies Nanostructured Anodic Metal Oxides; Grzegorz, D.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 321–347. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Qiu, K.J.; Bian, D.; Zheng, Y.F.; Lin, J.P. A Comparative in vitro study on biomedical Zr-2.5X (X = Nb, Sn) alloys. J. Mater. Sci. Technol. 2014, 30, 299–306. [Google Scholar] [CrossRef]
- Romonti, D.E.; Gomez Sanchez, A.V.; Milošev, I.; Demetrescu, I.; Ceré, S. Effect of anodization on the surface characteristics and electrochemical behaviour of zirconium in artificial saliva. Mater. Sci. Eng. C 2016, 62, 458–466. [Google Scholar] [CrossRef]
- Cox, B.; Gascoin, F.; Wong, Y.M. Properties of thin anodic oxide films on zirconium alloys. J. Nucl. Mater. 1995, 218, 113–128. [Google Scholar] [CrossRef]
- Epp, J. 4—X-ray diffraction (XRD) techniques for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Hübschen, G., Altpeter, I., Tschuncky, R., Herrmann, H.G., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 81–124. [Google Scholar] [CrossRef]
- Khan, H.; Yerramilli, A.S.; D’Oliveira, A.; Alford, T.L.; Boffito, D.C.; Patience, G.S. Experimental methods in chemical engineering: X-ray diffraction spectroscopy—XRD. Can. J. Chem. Eng. 2020, 98, 1255–1266. [Google Scholar] [CrossRef]
- Motta, A.; Yilmazbayham, A.; Comstock, R.; Partezana, J.; Sabol, G.P.; Lai, B.; Cai, Z. Microstructure and Growth Mechanism of Oxide Layers Formed on Zr Alloys Studied with Micro-Beam Synchrotron Radiation. J. ASTM Int. 2005, 2, JAI12375. [Google Scholar] [CrossRef]
- Silva, C.; Leonard, K.; Trammel, M.; Bryan, C. Characterization of different forms of Zr-2.5Nb samples before and after neutron irradiation. Mater. Sci. Eng. A 2018, 716, 296–307. [Google Scholar] [CrossRef]
- Mangla, O.; Roy, S. Monoclinic Zirconium Oxide Nanostructures Having Tunable Band Gap Synthesized under Extremely Non-Equilibrium Plasma Conditions. Proceedings 2019, 3, 10. [Google Scholar] [CrossRef]
- Mordyuk, B.N.; Karasevskaya, O.P.; Prokopenko, G.I. Structurally induced enhancement in corrosion resistance of Zr–2.5%Nb alloy in saline solution by applying ultrasonic impact peening. Mater. Sci. Eng. A 2013, 559, 453–461. [Google Scholar] [CrossRef]
- Straumal, B.; Gornakova, A.; Fabrichnaya, O.; Kriegel, M.; Mazilkin, A.; Baretzky, B.; Gusak, A.; Dobatkin, S. Effective temperature of high pressure torsion in Zr-Nb alloys. High Temp. Mater. Process. 2012, 31, 339–350. [Google Scholar] [CrossRef]
- Branzoi, I.V.; Iordoc, M.; Codescu, M. Electrochemical studies on the stability and corrosion resistance of new zirconium-based alloys for biomedical applications. Surf. Interface Anal. 2008, 40, 167–173. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles—Synthesized via sol–gel route. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu-Bogatu, N. Reactivity and Corrosion Behaviors of Ti6Al4V Alloy Implant Biomaterial under Metabolic Perturbation Conditions in Physiological Solutions. Materials 2021, 14, 7404. [Google Scholar] [CrossRef]
- Benea, L.; Mardare-Danaila, E.; Mardare, M.; Celis, J.P. Preparation of titanium oxide and hydroxyapatite on Ti–6Al–4V alloy surface and electrochemical behaviour in bio-simulated fluid solution. Corros. Sci. 2014, 80, 331–338. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu, N. Impact of hydrogen peroxide and albumin on the corrosion behavior of titanium alloy (Ti6Al4V) in saline solution. Int. J. Electrochem. Sci. 2021, 16, 210244. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Wang, B.L.; Qiu, K.J.; Lin, W.J.; Li, L.; Wang, Y.B.; Nie, F.L.; Zheng, Y.F. Microstructure, corrosion behavior and cytotoxicity of Zr–Nb alloys for biomedical application. Mater. Sci. Eng. C 2012, 32, 851–857. [Google Scholar] [CrossRef]
- Sowa, M.; Dercz, G.; Suchanek, K.; Simka, W. Investigation of anodic oxide coatings on zirconium after heat treatment. Appl. Surf. Sci. 2015, 346, 534–542. [Google Scholar] [CrossRef]
- Sowa, M.; Łastówka, D.; Kukharenko, A.I.; Korotin, D.M.; Kurmaev, E.Z.; Cholakh, S.O.; Simka, W. Characterisation of anodic oxide films on zirconium formed in sulphuric acid: XPS and corrosion resistance investigations. J. Solid State Electrochem. 2017, 21, 203–210. [Google Scholar] [CrossRef]
- Neaga, V.; Benea, L.; Axente, E.R. Corrosion Assessment of Zr2.5Nb Alloy in Ringer’s Solution by Electrochemical Methods. Appl. Sci. 2022, 12, 7976. [Google Scholar] [CrossRef]
- Benea, L.; Ravoiu Lupu, A.; Bounegru, I.; Vizureanu, P. Effect of functional nanoporous TiO2 film obtained on Ti6Al4V implant alloy to improve resistance in biological solution for inflammatory conditions. Int. J. Mol. Sci. 2023, 24, 8529. [Google Scholar] [CrossRef]
- Zhang, Y.; Addison, O.; Yu, F.; Troconis, B.C.R.; Scully, J.R.; Davenport, A.J. Time-dependent enhanced corrosion of Ti6Al4V in the presence of H2O2 and albumin. Sci. Rep. 2018, 8, 3185. [Google Scholar] [CrossRef] [PubMed]
- Ravoiu, A. Effect of Surface Modification of Ti6Al4V Alloys on Their Behavior in the Biological Implant Environment Under Inflammatory Conditions. PhD Thesis, Dunarea de Jos University of Galati, Galați, Romania, 2023. [Google Scholar]
- Uttiya, S.; Contarino, D.; Prandi, S.; Carnasciali, M.M.; Gemme, G.; Mattera, L.; Rolandi, R.; Canepa, M.; Cavalleri, O. Anodic oxidation of titanium in sulphuric acid and phosphoric acid electrolytes. J. Mater. Sci. Nanotechnol. 2014, 1, S106. [Google Scholar] [CrossRef]
- Trentin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galusek, D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers 2022, 14, 2306. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, D.; Masia, N. An insight on corrosion resistance ability of biocompatible dental implants through electrochemical impedance spectroscopy. In Corrosion—Fundamentals and Protection Mechanisms; Fahmina, Z., Anujit, G., Eram, S., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Okazaki, Y.; Nagata, H. Comparisons of immersion and electrochemical properties of highly biocompatible Ti–15Zr–4Nb–4Ta alloy and other implantable metals for orthopedic implants. Sci. Technol. Adv. Mater. 2012, 13, 064216. [Google Scholar] [CrossRef]



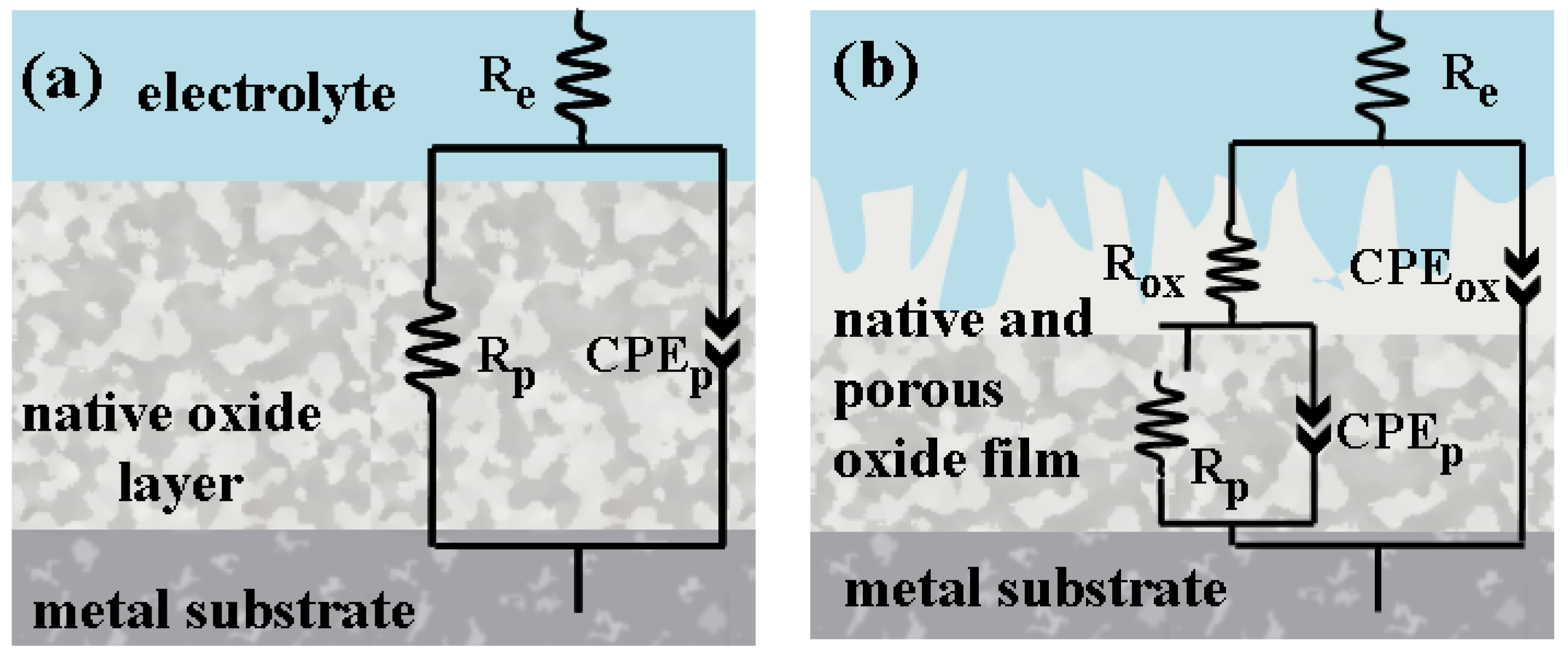
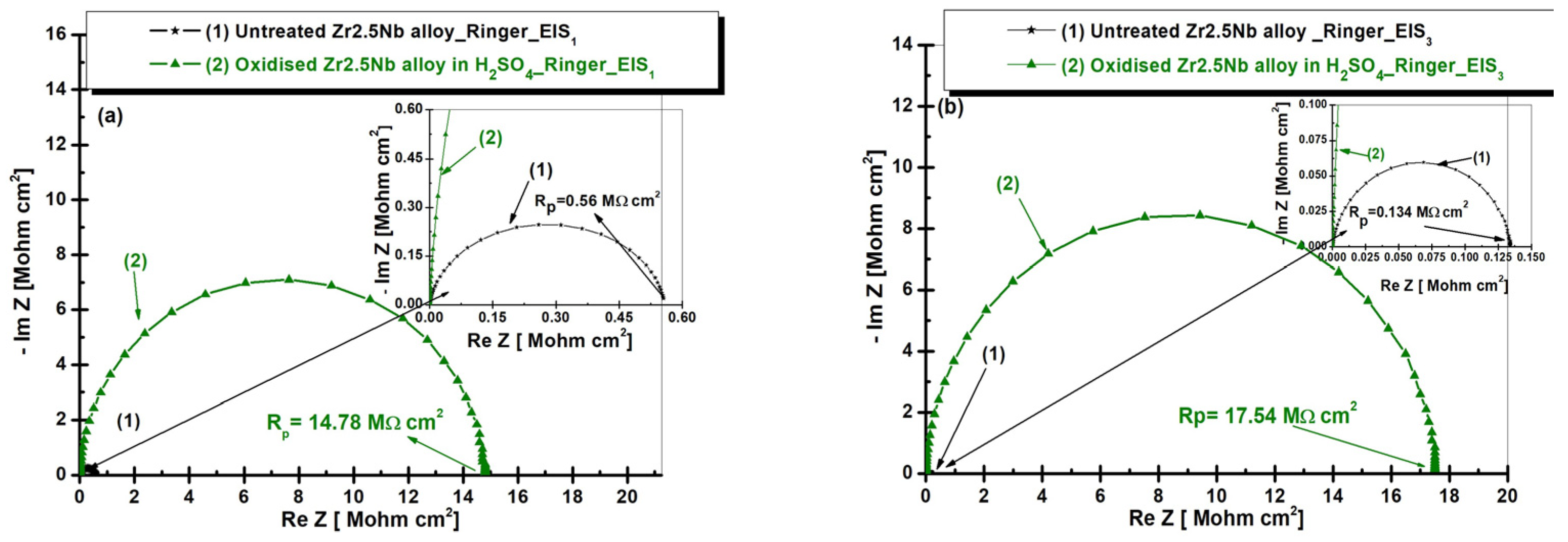
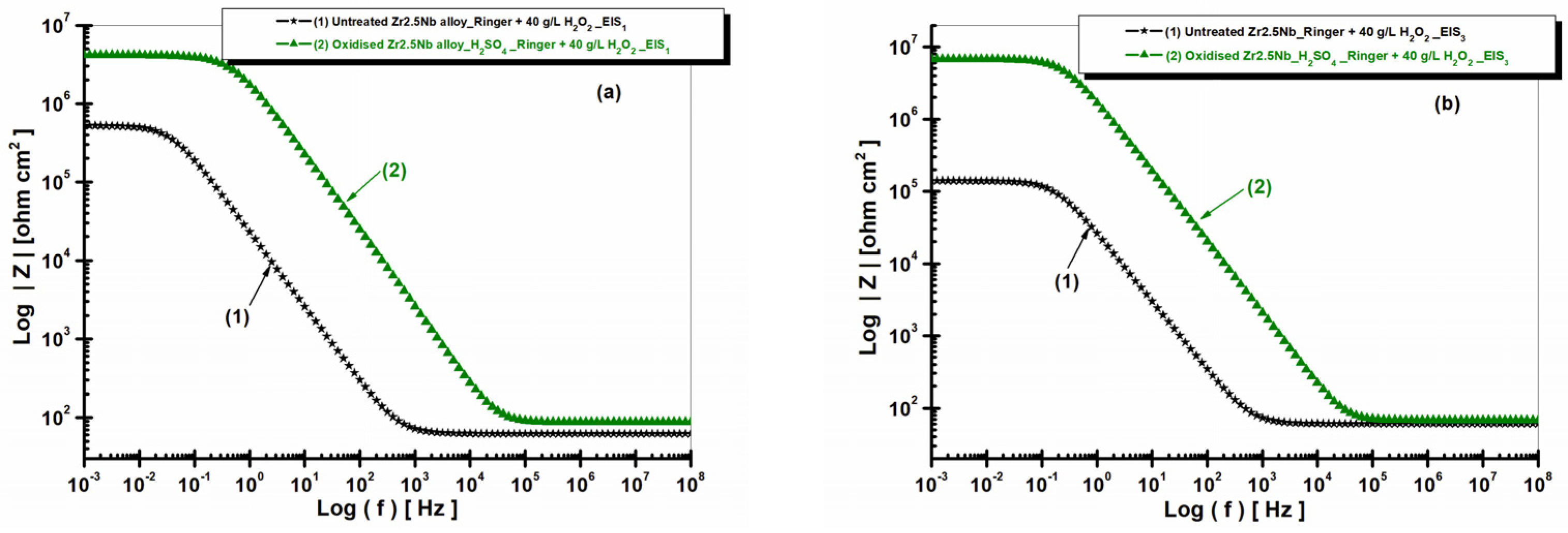
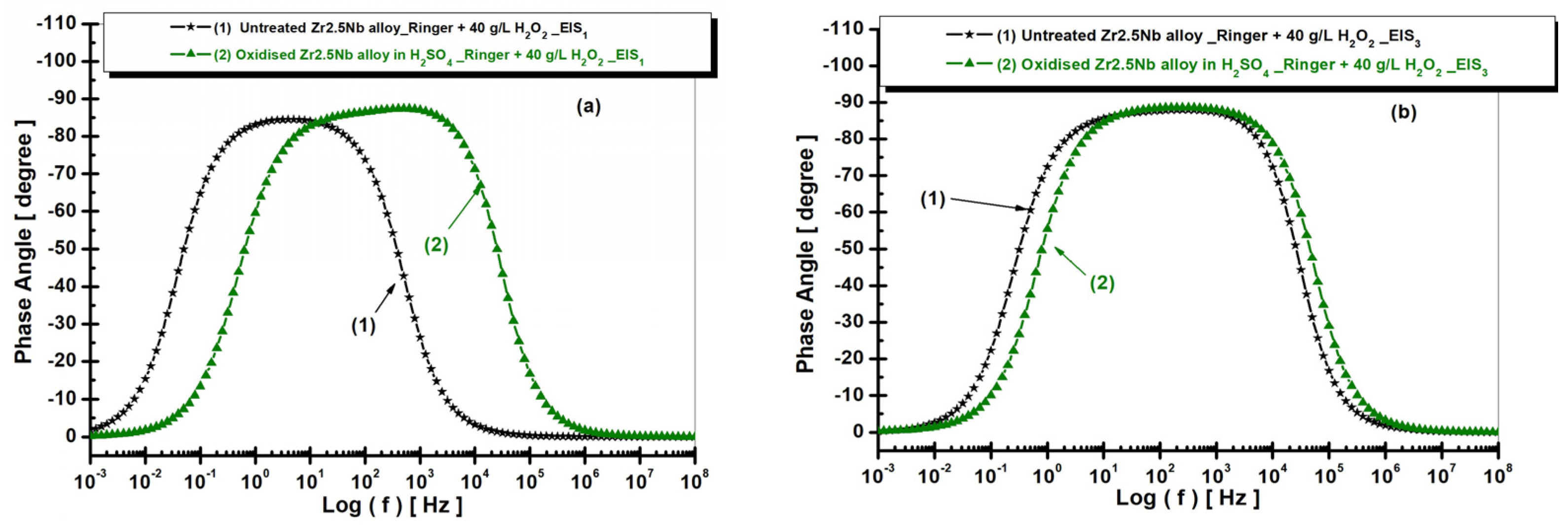

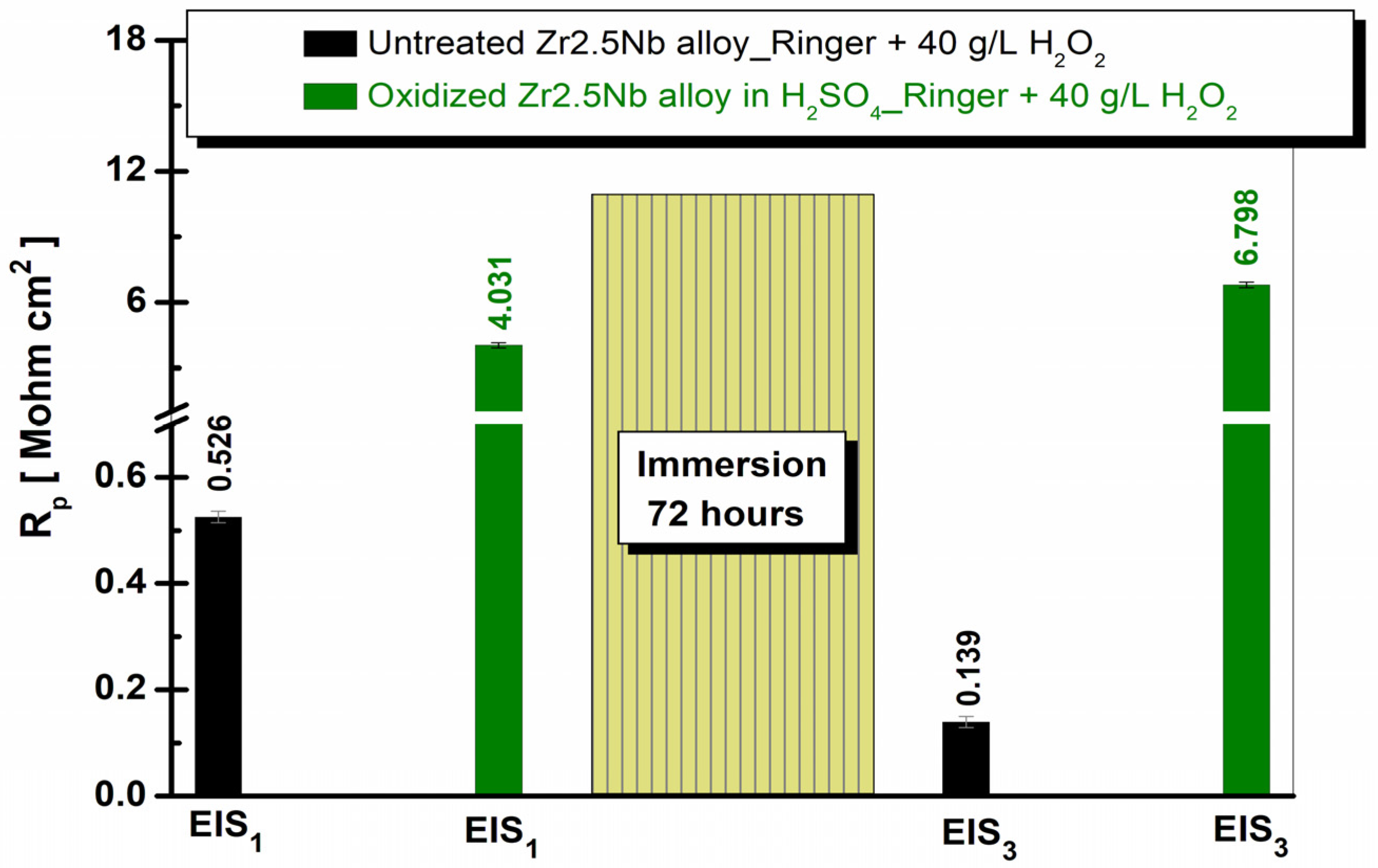
| Equivalent Electrical Circuit Elements/Measuring Unit | Untreated Zr2.5Nb EIS1/EIS3 | Oxidized Zr2.5Nb Eox 200 V–1 min 1 M H2SO4 EIS1/EIS3 |
|---|---|---|
| Rs [Ω cm2] | 46.92 / 48.59 | 66.53 / 59.37 |
| CPE-P [F/cm2] | 6.999 × 10−6 / 5.887 × 10−6 | 6.422 × 10−8 / 2.296 × 10−9 |
| α | 0.920 / 0.924 | 0.977 / 0.971 |
| Rp [MΩ cm2] | 0.5581 / 0.1342 | 16.82 × 10−6 / 3.375 × 10−6 |
| CPE-Tox [F/cm2] | - | 1.447 × 10−9 / 6.411 × 10−8 |
| α | - | 0.831 / 0.976 |
| Rp [MΩ cm2] | - | 14.7810 / 17.5420 |
| Equivalent Electrical Circuit Elements/Measuring Unit | Untreated Zr2.5Nb EIS1/EIS3 | Oxidized Zr2.5Nb Eox 200 V–1 min 1 M H2SO4 EIS1/EIS3 |
|---|---|---|
| Rs [Ω cm2] | 62.22 / 60.9 | 87.79 / 67.70 |
| CPE-P [F/cm2] | 7.466 × 10−6 / 6.451 × 10−6 | 6.189 × 10−8 / 7.984 × 10−8 |
| α | 0.952 / 0.952 | 0.998 / 0.998 |
| Rp [MΩ cm2] | 0.5257 / 0.1397 | 0.14625 / 0.02361 |
| CPE-Tox [F/cm2] | - | 3.366 × 10−8 / 6.943 × 10−8 |
| α | - | 0.735 / 0.450 |
| Rp [MΩ cm2] | - | 4.0304 / 6.7985 |
| Be | Hf | Ni | Cr | Ti | Al | O | Pb | Nb | Zr |
|---|---|---|---|---|---|---|---|---|---|
| 0.003 | 0.01 | 0.02 | 0.02 | 0.007 | 0.008 | 0.06–0.1 | 0.005 | 2.4–2.7 | Rest |
| Alloy | Modulus of Elasticity (Young) [GPa] | Breaking Strength [MPa] | Elongation δ [%] | Hardness HB [kgf/mm2] |
|---|---|---|---|---|
| Zr2.5Nb | 95 | 569 | 28 | 64–67 |
| Nr. Crt. | Chemical Compound | Solution Name Simplified Ringer [g/L] | Simplified Ringer + 40 g/L H2O2 |
|---|---|---|---|
| 1 | NaCl | 8.402 | 8.402 |
| 2 | KCl | 0.302 | 0.302 |
| 3 | CaCl2 | 0.298 | 0.298 |
| 4 | H2O (distilled water) | Rest | Rest |
| 5 | pH | 6.67 | 5.85 |
| 6 | Conductivity [mS/cm] | 14.4 | 12.3 |
| 7 | Salinity [ppt] | 8.4 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benea, L.; Neaga, V.; Bogatu, N.; Axente, E.R. Monitoring and Evaluation of Corrosion at the Interface of Zirconium Alloy Biomaterials Under Simulated Oxidative Biological Environment. Int. J. Mol. Sci. 2025, 26, 10537. https://doi.org/10.3390/ijms262110537
Benea L, Neaga V, Bogatu N, Axente ER. Monitoring and Evaluation of Corrosion at the Interface of Zirconium Alloy Biomaterials Under Simulated Oxidative Biological Environment. International Journal of Molecular Sciences. 2025; 26(21):10537. https://doi.org/10.3390/ijms262110537
Chicago/Turabian StyleBenea, Lidia, Veaceslav Neaga, Nicoleta Bogatu, and Elena Roxana Axente. 2025. "Monitoring and Evaluation of Corrosion at the Interface of Zirconium Alloy Biomaterials Under Simulated Oxidative Biological Environment" International Journal of Molecular Sciences 26, no. 21: 10537. https://doi.org/10.3390/ijms262110537
APA StyleBenea, L., Neaga, V., Bogatu, N., & Axente, E. R. (2025). Monitoring and Evaluation of Corrosion at the Interface of Zirconium Alloy Biomaterials Under Simulated Oxidative Biological Environment. International Journal of Molecular Sciences, 26(21), 10537. https://doi.org/10.3390/ijms262110537






