Au Nanoparticle Synthesis in the Presence of Thiolated Hyaluronic Acid
Abstract
1. Introduction
2. Results and Discussion
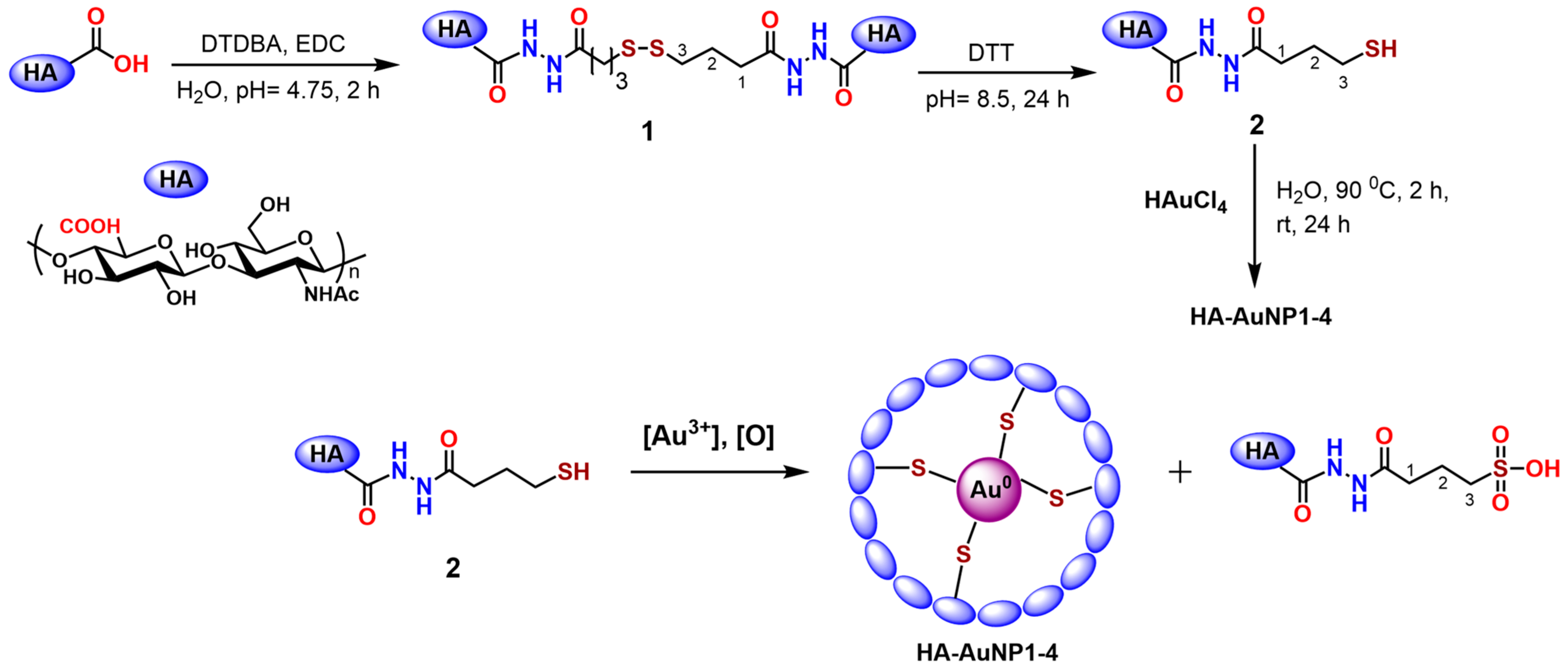
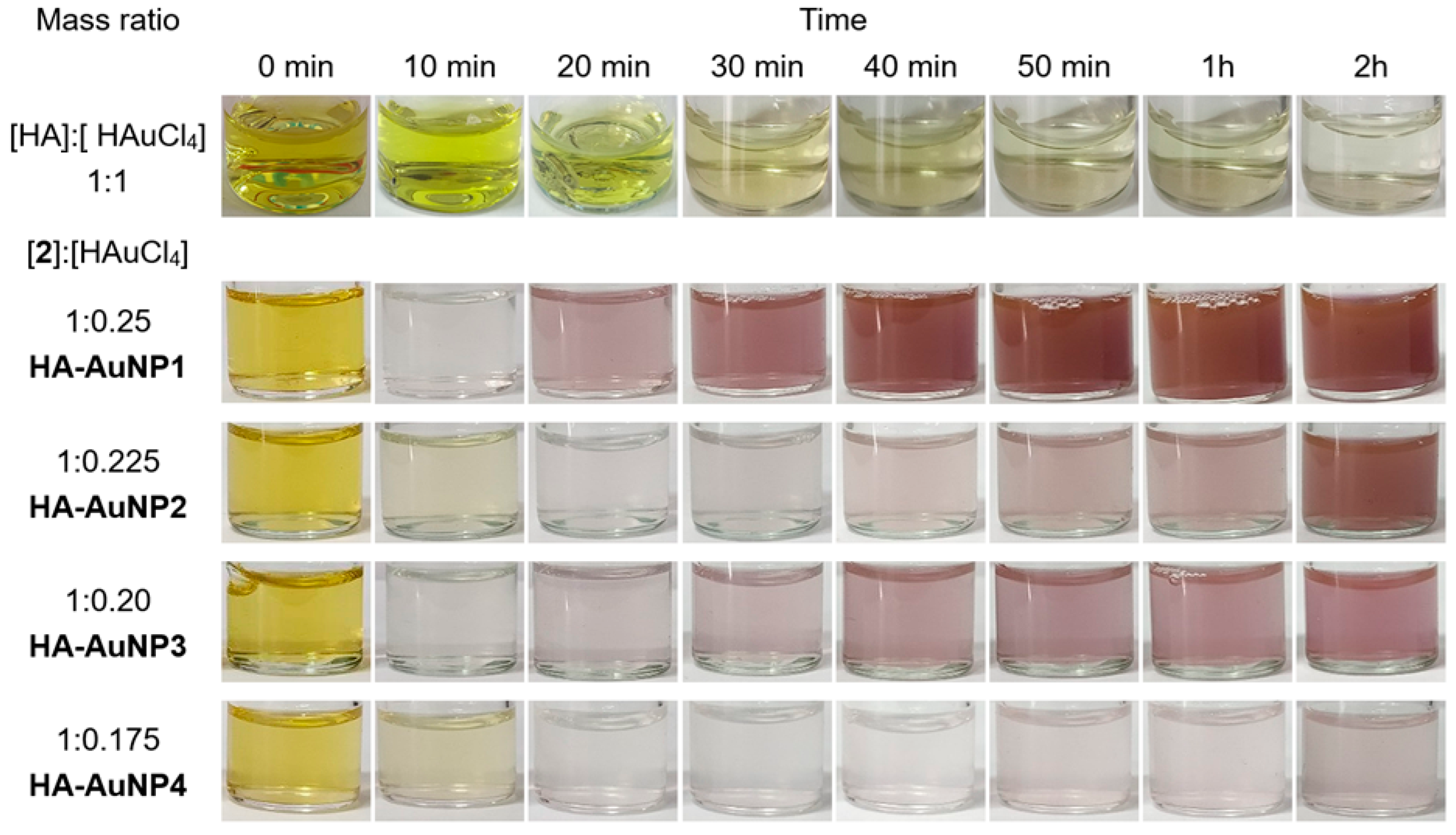
2.1. HA-AuNP Synthesis
2.2. HA-AuNP Characterization
2.2.1. UV–Visible Spectroscopy
2.2.2. Electron Microscopy and Photon Cross-Correlation Spectroscopy
2.2.3. X-Ray Photoelectron Spectroscopy
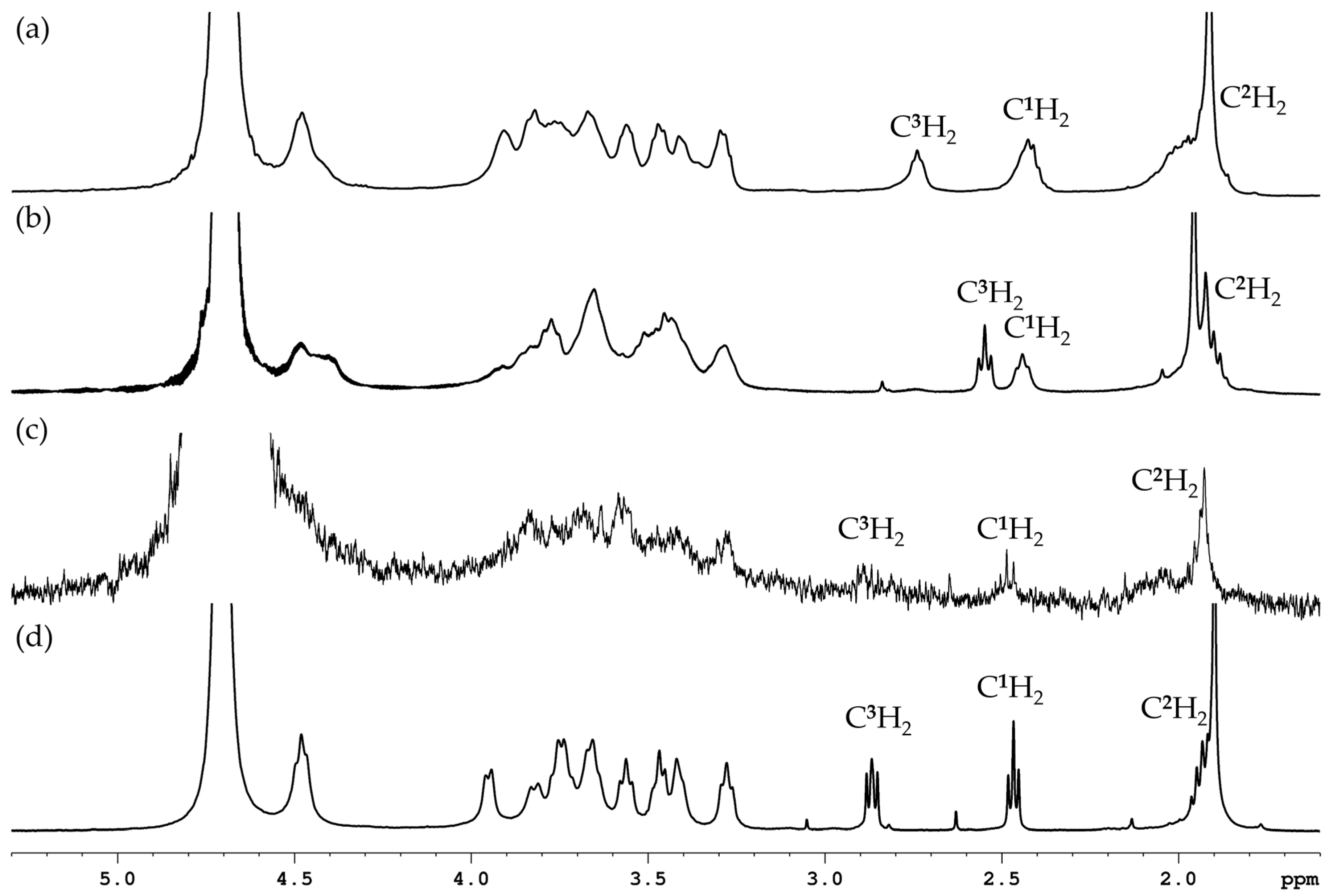
2.2.4. NMR Spectroscopy
2.2.5. ζ-Potential Measurements
3. Materials and Methods
3.1. General Information
3.2. Preparation of HA-AuNPs
3.3. Characterization of AuNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, A.; Rayhan, M.T.; Mobarak, M.H.; Rimon, M.I.H.; Hossain, N.; Islam, S.; Kafi, S.M.A.A. Advances and significances of gold nanoparticles in cancer treatment: A comprehensive review. Results Chem. 2024, 8, 101559. [Google Scholar] [CrossRef]
- Shevtsov, M.; Zhou, Y.; Khachatryan, W.; Multhoff, G.; Gao, H. Recent Advances in Gold Nanoformulations for Cancer Therapy. Curr. Drug Metab. 2018, 19, 768–780. [Google Scholar] [CrossRef]
- Abu-Dief, A.; Salaheldeen, M.; El-Dabea, T. Recent Advances in Development of Gold Nanoparticles for Drug Delivery Systems. J. Mod. Nanotechnol. 2021, 1, 1–14. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Ashour, M.; Faris, H.G.; Ahmed, H.; Mamdouh, S.; Thambiratnam, K.; Mohamed, T. Using Femtosecond Laser Pulses to Explore the Nonlinear Optical Properties of Au NP Colloids That Were Synthesized by Laser Ablation. Nanomaterials 2022, 12, 2980. [Google Scholar] [CrossRef]
- Sergievskaya, A.; Chauvin, A.; Konstantinidis, S. Sputtering onto liquids: A critical review. Beilstein J. Nanotechnol. 2022, 13, 10–53. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Bikmeeva, A.K.; Kovyazin, P.V.; Palatov, E.R.; Khalilov, L.M.; Ivanova, N.M.; Sergeev, S.N. Synthesis of Au nanoparticles by the reaction of HAuCl4·nH2O with organoaluminum compounds. J. Nanoparticle Res. 2025, 27, 129. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Tang, J.H.; Aziz, A.A.; Nowfal, S.H.; Jameel, M.S.; Alrosan, M.; Oladzadabbasabadi, N.; Ghasemlou, M. Green synthesis of gold nanoparticles and their emerging applications in cancer imaging and therapy: A review. Rev. Inorg. Chem. 2024. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, G.-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef]
- Yang, X.; Shi, X.; D’Arcy, R.; Tirelli, N.; Zhai, G. Amphiphilic polysaccharides as building blocks for self-assembled nanosystems: Molecular design and application in cancer and inflammatory diseases. J. Control. Release 2018, 272, 114–144. [Google Scholar] [CrossRef] [PubMed]
- Facchi, D.P.; da Cruz, J.A.; Bonafé, E.G.; Pereira, A.G.B.; Fajardo, A.R.; Venter, S.A.S.; Monteiro, J.P.; Muniz, E.C.; Martins, A.F. Polysaccharide-Based Materials Associated with or Coordinated to Gold Nanoparticles: Synthesis and Medical Application. Curr. Med. Chem. 2017, 24, 2701–2735. [Google Scholar] [CrossRef]
- Thodikayil, A.T.; Sharma, S.; Saha, S. Engineering Carbohydrate-Based Particles for Biomedical Applications: Strategies to Construct and Modify. ACS Appl. Bio Mater. 2021, 4, 2907–2940. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, M.; Meng, F.; Su, C.; Li, J. Polysaccharide-based gold nanomaterials: Synthesis mechanism, polysaccharide structure-effect, and anticancer activity. Carbohydr. Polym. 2023, 321, 121284. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H.; Wei, Y.; Cong, F. Hyaluronan-Inorganic Nanohybrid Materials for Biomedical Applications. Biomacromolecules 2017, 18, 1677–1696. [Google Scholar] [CrossRef]
- Cao, F.; Yan, M.; Liu, Y.; Liu, L.; Ma, G. Photothermally Controlled MHC Class I Restricted CD8+ T-Cell Responses Elicited by Hyaluronic Acid Decorated Gold Nanoparticles as a Vaccine for Cancer Immunotherapy. Adv. Healthc. Mater. 2018, 7, 1701439. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; McCoard, L.; Oottamasathien, S.; Prestwich, G.D. Dynamically Crosslinked Gold Nanoparticle—Hyaluronan Hydrogels. Adv. Mater. 2010, 22, 4736–4740. [Google Scholar] [CrossRef]
- Lin, C.-M.; Kao, W.-C.; Yeh, C.-A.; Chen, H.-J.; Lin, S.-Z.; Hsieh, H.-H.; Sun, W.-S.; Chang, C.-H.; Hung, H.-S. Hyaluronic acid-fabricated nanogold delivery of the inhibitor of apoptosis protein-2 siRNAs inhibits benzo[a]pyrene-induced oncogenic properties of lung cancer A549 cells. Nanotechnology 2015, 26, 105101. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, V.; Caruso, V.C.; Cucci, L.M.; Inturri, R.; Vaccaro, S.; Satriano, C. Hyaluronan-Metal Gold Nanoparticle Hybrids for Targeted Tumor Cell Therapy. Int. J. Mol. Sci. 2020, 21, 3085. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Raja, M.D.; Sundar, D.S.; Gover Antoniraj, M.; Ruckmani, K. Hyaluronic acid co-functionalized gold nanoparticle complex for the targeted delivery of metformin in the treatment of liver cancer (HepG2 cells). Carbohydr. Polym. 2015, 128, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, N.; Su, Q.; Lv, Y.; Yang, C.; Zhan, H. Green Synthesis of Gold Nanoparticles and Study of Their Inhibitory Effect on Bulk Cancer Cells and Cancer Stem Cells in Breast Carcinoma. Nanomaterials 2022, 12, 3324. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Zhang, Y.; Zhang, W.; Ma, P.; Wang, X.; Song, D.; Sun, Y. One-pot synthesis of hyaluronic acid–coated gold nanoparticles as SERS substrate for the determination of hyaluronidase activity. Microchim. Acta 2020, 187, 604. [Google Scholar] [CrossRef]
- Vávrová, A.; Čapková, T.; Kuřitka, I.; Vícha, J.; Münster, L. One-step synthesis of gold nanoparticles for catalysis and SERS applications using selectively dicarboxylated cellulose and hyaluronate. Int. J. Biol. Macromol. 2022, 206, 927–938. [Google Scholar] [CrossRef]
- Lee, M.Y.; Yang, J.A.; Jung, H.S.; Beack, S.; Choi, J.E.; Hur, W.; Koo, H.; Kim, K.; Yoon, S.K.; Hahn, S.K. Hyaluronic acid-gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano 2012, 6, 9522–9531. [Google Scholar] [CrossRef]
- Kang, S.H.; Nafiujjaman, M.; Nurunnabi, M.; Li, L.; Khan, H.A.; Cho, K.J.; Huh, K.M.; Lee, Y.-k. Hybrid photoactive nanomaterial composed of gold nanoparticles, pheophorbide-A and hyaluronic acid as a targeted bimodal phototherapy. Macromol. Res. 2015, 23, 474–484. [Google Scholar] [CrossRef]
- Pouyani, T.; Prestwich, G.D. Functionalized Derivatives of Hyaluronic Acid Oligosaccharides: Drug Carriers and Novel Biomaterials. Bioconjugate Chem. 1994, 5, 339–347. [Google Scholar] [CrossRef]
- Varghese, O.P.; Sun, W.; Hilborn, J.; Ossipov, D.A. In Situ Cross-Linkable High Molecular Weight Hyaluronan−Bisphosphonate Conjugate for Localized Delivery and Cell-Specific Targeting: A Hydrogel Linked Prodrug Approach. J. Am. Chem. Soc. 2009, 131, 8781–8783. [Google Scholar] [CrossRef]
- Henglein, A. Radiolytic Preparation of Ultrafine Colloidal Gold Particles in Aqueous Solution: Optical Spectrum, Controlled Growth, and Some Chemical Reactions. Langmuir 1999, 15, 6738–6744. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Hien, N.Q.; Van Phu, D.; Duy, N.N.; Quoc, L.A. Radiation synthesis and characterization of hyaluronan capped gold nanoparticles. Carbohydr. Polym. 2012, 89, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Meen, T.-H.; Tsai, J.-K.; Chao, S.-M.; Lin, Y.-C.; Wu, T.-C.; Chang, T.-Y.; Ji, L.-W.; Water, W.; Chen, W.-R.; Tang, I.T.; et al. Surface plasma resonant effect of gold nanoparticles on the photoelectrodes of dye-sensitized solar cells. Nanoscale Res. Lett. 2013, 8, 450. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of Binding Energy Positions with C1s for XPS Results. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Anderson, D.P.; Alvino, J.F.; Gentleman, A.; Qahtani, H.A.; Thomsen, L.; Polson, M.I.J.; Metha, G.F.; Golovko, V.B.; Andersson, G.G. Chemically-synthesised, atomically-precise gold clusters deposited and activated on titania. Phys. Chem. Chem. Phys. 2013, 15, 3917–3929. [Google Scholar] [CrossRef] [PubMed]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Shin, D.; Kim, H.R.; Hong, B.H. Gold nanoparticle-mediated non-covalent functionalization of graphene for field-effect transistors. Nanoscale Adv. 2021, 3, 1404–1412. [Google Scholar] [CrossRef]
- Sahoo, S.R.; Ke, S.-C. Spin-Orbit Coupling Effects in Au 4f Core-Level Electronic Structures in Supported Low-Dimensional Gold Nanoparticles. Nanomaterials 2021, 11, 554. [Google Scholar] [CrossRef]
- Juodkazis, K.; Juodkazyt, J.; Jasulaitien, V.; Lukinskas, A.; Šebeka, B. XPS studies on the gold oxide surface layer formation. Electrochem. Commun. 2000, 2, 503–507. [Google Scholar] [CrossRef]
- Wei, H.; Li, B.; Du, Y.; Dong, S.; Wang, E. Nucleobase−Metal Hybrid Materials: Preparation of Submicrometer-Scale, Spherical Colloidal Particles of Adenine−Gold(III) via a Supramolecular Hierarchical Self-Assembly Approach. Chem. Mater. 2007, 19, 2987–2993, Correction in: Chem. Mater. 2009, 21, 432. [Google Scholar] [CrossRef]
- Krozer, A.; Rodahl, M. X-ray photoemission spectroscopy study of UV/ozone oxidation of Au under ultrahigh vacuum conditions. J. Vac. Sci. Technol. A Vac. Surf. Film. 1997, 15, 1704–1709. [Google Scholar] [CrossRef]
- Kepenienė, V.; Stagniūnaitė, R.; Tamašauskaitė-Tamašiūnaitė, L.; Pakštas, V.; Jasulaitienė, V.; Léger, B.; Rousseau, J.; Ponchel, A.; Monflier, E.; Norkus, E. Co3O4/C and Au supported Co3O4/C nanocomposites—Peculiarities of fabrication and application towards oxygen reduction reaction. Mater. Chem. Phys. 2020, 241, 122332. [Google Scholar] [CrossRef]
- Nyholm, R.; Berndtsson, A.; Martensson, N. Core level binding energies for the elements Hf to Bi (Z=72-83). J. Phys. C Solid State Phys. 1980, 13, L1091. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J.; King, R.C. Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Chanhassen, MN, USA, 1995. [Google Scholar]
- Eatoo, M.A.; Wehbe, N.; Kharbatia, N.; Guo, X.; Mishra, H. Why do some metal ions spontaneously form nanoparticles in water microdroplets? Disentangling the contributions of the air–water interface and bulk redox chemistry. Chem. Sci. 2025, 16, 1115–1125. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q. Adsorption of phosphorylated chitosan on mineral surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 656–663. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Genet, M.J. XPS analysis of bio-organic systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black Hydroxylated Titanium Dioxide Prepared via Ultrasonication with Enhanced Photocatalytic Activity. Sci. Rep. 2015, 5, 11712. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-ray Photoelectron Spectroscopy Sulfur 2p Study of Organic Thiol and Disulfide Binding Interactions with Gold Surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Lindberg, B.J.; Hamrin, K.; Johansson, G.; Gelius, U.; Fahlman, A.; Nordling, C.; Siegbahn, K. Molecular Spectroscopy by Means of ESCA II. Sulfur compounds. Correlation of electron binding energy with structure. Phys. Scr. 1970, 1, 286. [Google Scholar] [CrossRef]
- Shanthi, P.M.; Hanumantha, P.J.; Ramalinga, K.; Gattu, B.; Datta, M.K.; Kumta, P.N. Sulfonic Acid Based Complex Framework Materials (CFM): Nanostructured Polysulfide Immobilization Systems for Rechargeable Lithium–Sulfur Battery. J. Electrochem. Soc. 2019, 166, A1827. [Google Scholar] [CrossRef]
- Zhang, Q.; Tao, Q.; He, H.; Liu, H.; Komarneni, S. An efficient SO2-adsorbent from calcination of natural magnesite. Ceram. Int. 2017, 43, 12557–12562. [Google Scholar] [CrossRef]
- Tudino, T.C.; Nunes, R.S.; Mandelli, D.; Carvalho, W.A. Influence of Dimethylsulfoxide and Dioxygen in the Fructose Conversion to 5-Hydroxymethylfurfural Mediated by Glycerol’s Acidic Carbon. Front. Chem. 2020, 8, 2020. [Google Scholar] [CrossRef] [PubMed]
- Cano-Serrano, E.; Campos-Martin, J.M.; Fierro, J.L.G. Sulfonic acid-functionalized silica through quantitative oxidation of thiol groups. Chem. Commun. 2003, 9, 246–247. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Luo, Y.; Roberts, M.C.; Prestwich, G.D. Disulfide Cross-Linked Hyaluronan Hydrogels. Biomacromolecules 2002, 3, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.J. Organic Chemistry Data Collection. Available online: https://organicchemistrydata.org/hansreich/resources/nmr/?page=05-hmr-15-aabb%2F (accessed on 9 January 2020).
- Herke, R.; Rasheed, K. Addition of bisulfite to α-olefins: Synthesis of n-alkane sulfonates and characterization of intermediates. J. Am. Oil Chem. Soc. 1992, 69, 47–51. [Google Scholar] [CrossRef]
- He, R.; Wang, J.; Yu, Z.-H.; Moyers, J.S.; Michael, M.D.; Durham, T.B.; Cramer, J.W.; Qian, Y.; Lin, A.; Wu, L.; et al. Structure-Based Design of Active-Site-Directed, Highly Potent, Selective, and Orally Bioavailable Low-Molecular-Weight Protein Tyrosine Phosphatase Inhibitors. J. Med. Chem. 2022, 65, 13892–13909. [Google Scholar] [CrossRef]
- Witt, D. Recent Developments in Disulfide Bond Formation. Synthesis 2008, 2008, 2491–2509. [Google Scholar] [CrossRef]
- McNeil, N.M.R.; McDonnell, C.; Hambrook, M.; Back, T.G. Oxidation of Disulfides to Thiolsulfinates with Hydrogen Peroxide and a Cyclic Seleninate Ester Catalyst. Molecules 2015, 20, 10748–10762. [Google Scholar] [CrossRef]
- Spiliopoulou, N.; Kokotos, C.G. Photochemical metal-free aerobic oxidation of thiols to disulfides. Green Chem. 2021, 23, 546–551. [Google Scholar] [CrossRef]
- Koval, I.V. The chemistry of disulfides. Russ. Chem. Rev. 1994, 63, 735. [Google Scholar] [CrossRef]
- Bagiyan, G.A.; Koroleva, I.K.; Soroka, N.V.; Ufimtsev, A.V. Oxidation of thiol compounds by molecular oxygen in aqueous solutions. Russ. Chem. Bull. 2003, 52, 1135–1141. [Google Scholar] [CrossRef]
- van Bergen, L.A.H.; Roos, G.; De Proft, F. From Thiol to Sulfonic Acid: Modeling the Oxidation Pathway of Protein Thiols by Hydrogen Peroxide. J. Phys. Chem. A 2014, 118, 6078–6084. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639, Erratum in Can. J. Chem. Eng. 2023, 101, 6672. [Google Scholar] [CrossRef]
- Chauvin, J.-P.R.; Pratt, D.A. On the Reactions of Thiols, Sulfenic Acids, and Sulfinic Acids with Hydrogen Peroxide. Angew. Chem. Int. Ed. 2017, 56, 6255–6259. [Google Scholar] [CrossRef]
- Vercruysse, K.P.; Marecak, D.M.; Marecek, J.F.; Prestwich, G.D. Synthesis and In Vitro Degradation of New Polyvalent Hydrazide Cross-Linked Hydrogels of Hyaluronic Acid. Bioconjug. Chem. 1997, 8, 686–694. [Google Scholar] [CrossRef] [PubMed]
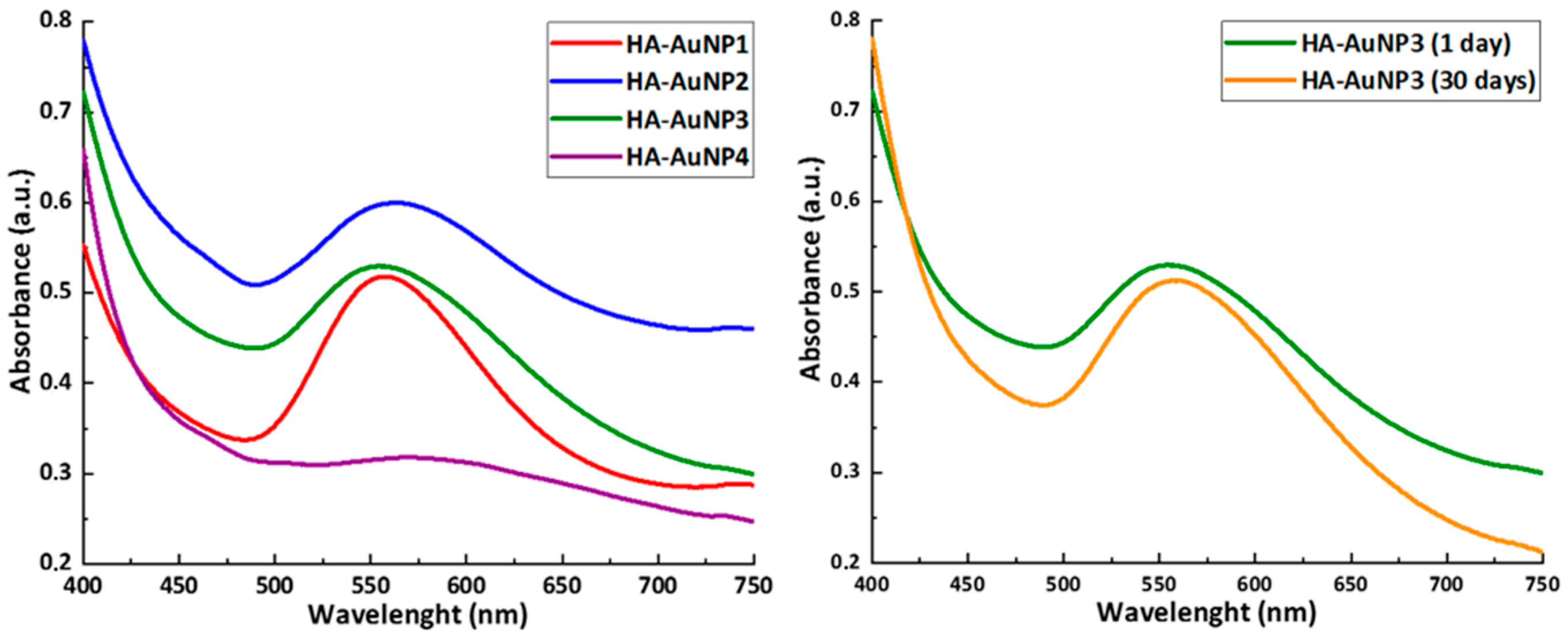


| Sample | λmax (1 Day), nm | λmax (30 Days), nm |
|---|---|---|
| HA-AuNP1 | 557 | - |
| HA-AuNP2 | 563 | - |
| HA-AuNP3 | 555 | 559 |
| HA-AuNP4 | 569 | - |
| Sample | Au | Binding Energy (eV) 4f7/2 | Binding Energy (eV) 4f5/2 | Rel. Area (%) |
|---|---|---|---|---|
| HAuCl4 | Au3+ | 86.9 | 90.6 | 47.88 ± 1.06 |
| Au1+ | 85.3 | 88.9 | 35.62 ± 0.79 | |
| Au0 | 84.6 | 88.3 | 16.48 ± 0.36 | |
| HA-AuNP3 | Au3+ | - | - | - |
| Au1+ | 84.6 | 88.3 | 40.79 ± 1.23 | |
| Au0 | 84.1 | 87.8 | 59.21 ± 1.31 |
| HA-AuNP1 | HA-AuNP2 | HA-AuNP3 | HA-AuNP4 | |
|---|---|---|---|---|
| Zeta-potential (mV) | −27.6 ± 3.4 | −28.6 ± 1.7 | −41.0 ± 4.0 | −41.5 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parfenova, L.V.; Alibaeva, E.I.; Gil’fanova, G.U.; Galimshina, Z.R.; Mescheryakova, E.S.; Khalilov, L.M.; Sergeev, S.N.; Penkov, N.V.; Subrahmanyam, C. Au Nanoparticle Synthesis in the Presence of Thiolated Hyaluronic Acid. Int. J. Mol. Sci. 2025, 26, 10532. https://doi.org/10.3390/ijms262110532
Parfenova LV, Alibaeva EI, Gil’fanova GU, Galimshina ZR, Mescheryakova ES, Khalilov LM, Sergeev SN, Penkov NV, Subrahmanyam C. Au Nanoparticle Synthesis in the Presence of Thiolated Hyaluronic Acid. International Journal of Molecular Sciences. 2025; 26(21):10532. https://doi.org/10.3390/ijms262110532
Chicago/Turabian StyleParfenova, Lyudmila V., Eliza I. Alibaeva, Guzel U. Gil’fanova, Zulfiya R. Galimshina, Ekaterina S. Mescheryakova, Leonard M. Khalilov, Semen N. Sergeev, Nikita V. Penkov, and Challapalli Subrahmanyam. 2025. "Au Nanoparticle Synthesis in the Presence of Thiolated Hyaluronic Acid" International Journal of Molecular Sciences 26, no. 21: 10532. https://doi.org/10.3390/ijms262110532
APA StyleParfenova, L. V., Alibaeva, E. I., Gil’fanova, G. U., Galimshina, Z. R., Mescheryakova, E. S., Khalilov, L. M., Sergeev, S. N., Penkov, N. V., & Subrahmanyam, C. (2025). Au Nanoparticle Synthesis in the Presence of Thiolated Hyaluronic Acid. International Journal of Molecular Sciences, 26(21), 10532. https://doi.org/10.3390/ijms262110532







