Investigation of Novel Predictive Biomarkers for Preeclampsia in Second-Trimester Amniotic Fluid
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Study Population
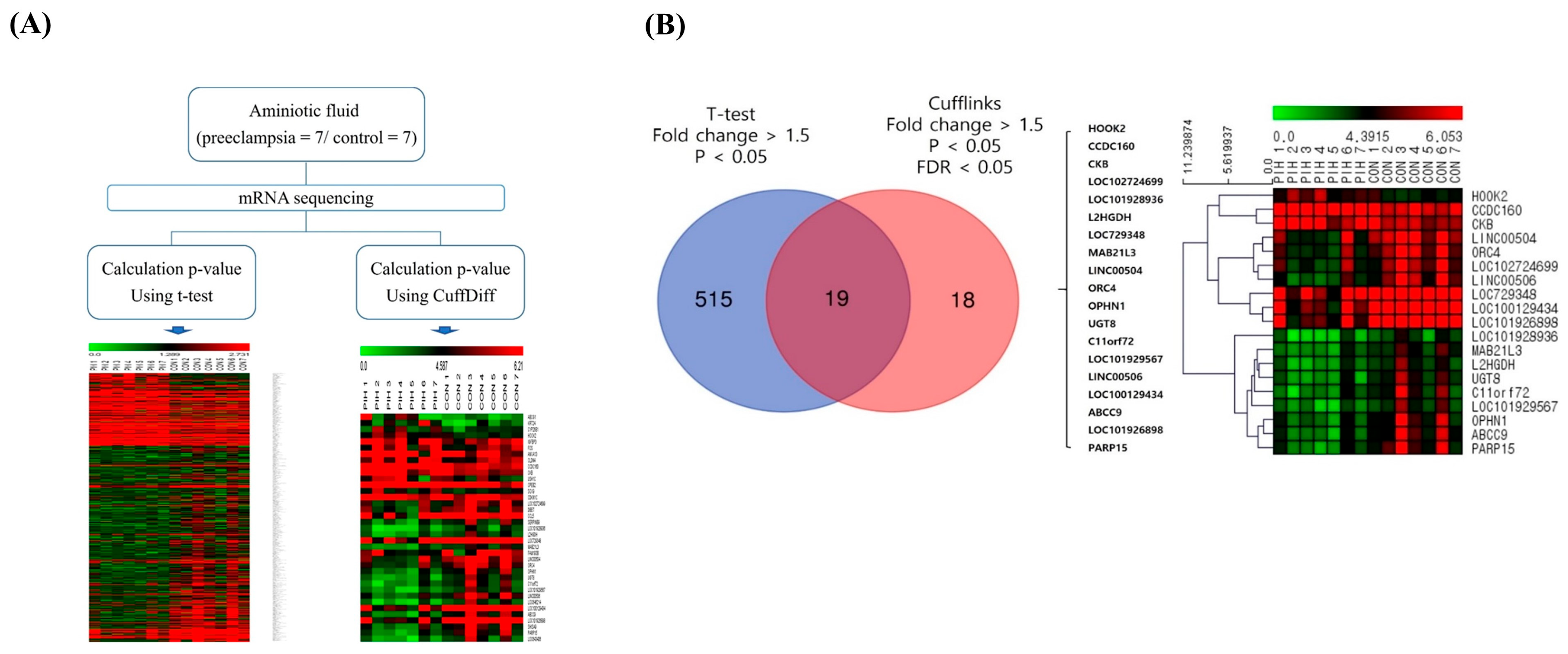
2.2. HOOK2 as the Most Overexpressed Gene in Preeclampsia
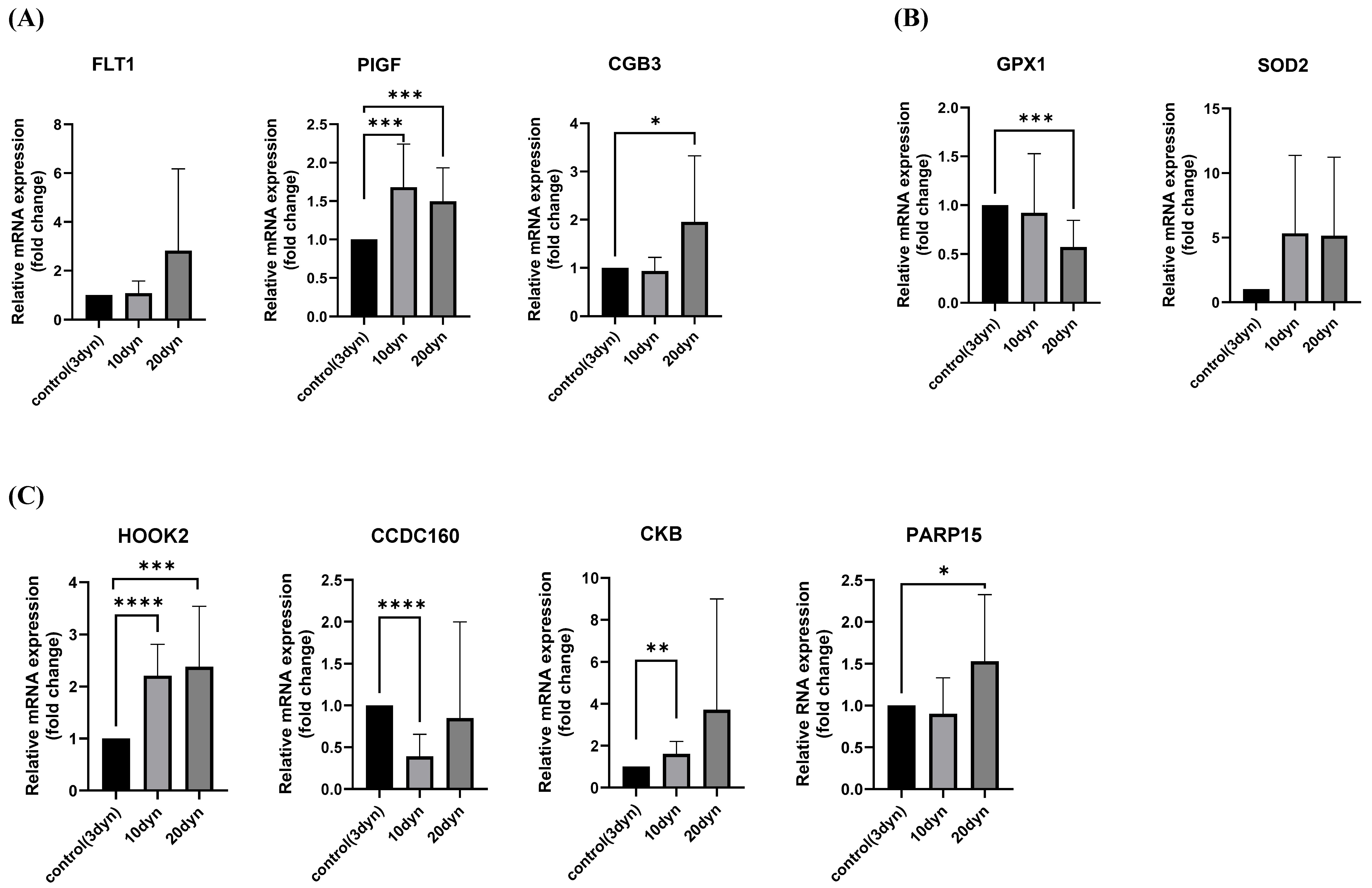
2.3. HOOK2 Expression Levels Were Increased in HTR-8/SVneo Cells by Fluid Shear Stress
2.4. Reducing HOOK2 Level Increased Invasiveness of HTR-8/SVneo Cells
3. Discussion
4. Materials and Methods
4.1. Patients and Amniotic Fluid Collection
4.2. RNA Sequencing and Analysis of Differentially Expressed Genes
4.3. Cell Culture
4.4. Fluid Shear Stress Experiments
4.5. RNA Extraction and Real-Time PCR
4.6. siRNA Transfection
4.7. Transwell Migration, Invasion Assay
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Saccone, G.; Gragnano, E.; Ilardi, B.; Marrone, V.; Strina, I.; Venturella, R.; Berghella, V.; Zullo, F. Maternal and perinatal complications according to maternal age: A systematic review and meta-analysis. Int. J. Gynecol. Obstet. 2022, 159, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M. Diagnosis and management of gestational hypertension and preeclampsia. Obstet. Gynecol. 2003, 102, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.C.; Brocklehurst, P.; E Green, M.; Hunter, R.; Hardy, P.; Juszczak, E.; Linsell, L.; Chiocchia, V.; Greenland, M.; Placzek, A.; et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): A randomised controlled trial. Lancet 2019, 394, 1181–1190. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Poon, L.C.; Nicolaides, K.H. First-trimester maternal factors and biomarker screening for preeclampsia. Prenat. Diagn. 2014, 34, 618–627. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Brace, R.A. Physiology of Amniotic Fluid Volume Regulation. Clin. Obstet. Gynecol. 1997, 40, 280–289. [Google Scholar] [CrossRef]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic Fluid: Not Just Fetal Urine Anymore. J. Perinatol. 2005, 25, 341–348. [Google Scholar] [CrossRef]
- Hui, L.; Bianchi, D. Cell-free fetal nucleic acids in amniotic fluid. Hum. Reprod. Update 2010, 17, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.W.; Shin, Y.J.; Shim, S.H.; Cha, D.H. An overview of current knowledge about cell-free RNA in amniotic fluid. J. Genet. Med. 2016, 13, 65–71. [Google Scholar] [CrossRef]
- Soltani, M.; Nemati, M.; Maralani, M.; A Estiar, M.; Andalib, S.; Fardiazar, Z.; Sakhinia, E. Cell-free fetal DNA in amniotic fluid supernatant for prenatal diagnosis. Cell. Mol. Biol. 2016, 62, 14–17. [Google Scholar] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Resnick, N.; Yahav, H.; Shay-Salit, A.; Shushy, M.; Schubert, S.; Zilberman, L.C.M.; Wofovitz, E. Fluid shear stress and the vascular endothelium: For better and for worse. Prog. Biophys. Mol. Biol. 2003, 81, 177–199. [Google Scholar] [CrossRef]
- James, J.L.; Cartwright, J.E.; Whitley, G.S.; Greenhill, D.R.; Hoppe, A. The regulation of trophoblast migration across endothelial cells by low shear stress: Consequences for vascular remodelling in pregnancy. Cardiovasc. Res. 2011, 93, 152–161. [Google Scholar] [CrossRef]
- Lecarpentier, E.; Atallah, A.; Guibourdenche, J.; Hebert-Schuster, M.; Vieillefosse, S.; Chissey, A.; Haddad, B.; Pidoux, G.; Evain-Brion, D.; Barakat, A.; et al. Fluid Shear Stress Promotes Placental Growth Factor Upregulation in Human Syncytiotrophoblast Through the cAMP–PKA Signaling Pathway. Hypertension 2016, 68, 1438–1446. [Google Scholar] [CrossRef]
- Albrecht, E.D.; Pepe, G.J. Regulation of Uterine Spiral Artery Remodeling: A Review. Reprod. Sci. 2020, 27, 1932–1942. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The Uterine Spiral Arteries in Human Pregnancy: Facts and Controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Varberg, K.M.; Soares, M.J. Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling. Placenta 2021, 113, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.; Woods, A.; Jauniaux, E.; Kingdom, J. Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Pallesi-Pocachard, E.; Bazellieres, E.; Viallat-Lieutaud, A.; Delgrossi, M.-H.; Barthelemy-Requin, M.; Le Bivic, A.; Massey-Harroche, D. Hook2, a microtubule-binding protein, interacts with Par6α and controls centrosome orientation during polarized cell migration. Sci. Rep. 2016, 6, 33259. [Google Scholar] [CrossRef] [PubMed]
- Szebenyi, G.; Wigley, W.C.; Hall, B.; Didier, A.; Yu, M.; Thomas, P.; Krämer, H. Hook2 contributes to aggresome formation. BMC Cell Biol. 2007, 8, 19. [Google Scholar] [CrossRef]
- Szebenyi, G.; Hall, B.; Yu, R.; Hashim, A.I.; Krämer, H. Hook2 Localizes to the Centrosome, Binds Directly to Centriolin/CEP110 and Contributes to Centrosomal Function. Traffic 2006, 8, 32–46. [Google Scholar] [CrossRef]
- Johnston, J.A.; Ward, C.L.; Kopito, R.R. Aggresomes: A Cellular Response to Misfolded Proteins. J. Cell Biol. 1998, 143, 1883–1898. [Google Scholar] [CrossRef]
- Ritter, A.; Roth, S.; Kreis, N.-N.; Friemel, A.; Hoock, S.C.; Souto, A.S.; Eichbaum, C.; Neuhoff, A.; Chen, Q.; Solbach, C.; et al. Primary Cilia in Trophoblastic Cells. Hypertension 2020, 76, 1491–1505. [Google Scholar] [CrossRef]
- Iqbal, A.; Baldrighi, M.; Murdoch, J.N.; Fleming, A.; Wilkinson, C.J. Alpha-synuclein aggresomes inhibit ciliogenesis and multiple functions of the centrosome. Biol. Open 2020, 9, bio054338. [Google Scholar] [CrossRef]
- Healy, M.D.; McNally, K.E.; Butkovič, R.; Chilton, M.; Kato, K.; Sacharz, J.; McConville, C.; Moody, E.R.; Shaw, S.; Planelles-Herrero, V.J.; et al. Structure of the endosomal Commander complex linked to Ritscher-Schinzel syndrome. Cell 2023, 186, 2219–2237.e29. [Google Scholar] [CrossRef]
- Di Giorgio, E.; Xodo, S.; Orsaria, M.; Mariuzzi, L.; Picco, R.; Tolotto, V.; Cortolezzis, Y.; D’ESte, F.; Grandi, N.; Driul, L.; et al. The central role of creatine and polyamines in fetal growth restriction. FASEB J. 2024, 38, e70222. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, X.; Han, X.; Shi, M.; Sun, L.; Liu, M.; Zhang, P.; Huang, Z.; Yang, X.; Li, R. Ferroptosis-related gene expression in the pathogenesis of preeclampsia. Front. Genet. 2022, 13, 927869. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torres, J.; Espino-Y-Sosa, S.; Martinez-Portilla, R.; Borboa-Olivares, H.; Estrada-Gutierrez, G.; Acevedo-Gallegos, S.; Ruiz-Ramirez, E.; Velasco-Espin, M.; Cerda-Flores, P.; Ramirez-Gonzalez, A.; et al. A Narrative Review on the Pathophysiology of Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7569. [Google Scholar] [CrossRef] [PubMed]
- Bisson, C.; Dautel, S.; Patel, E.; Suresh, S.; Dauer, P.; Rana, S. Preeclampsia pathophysiology and adverse outcomes during pregnancy and postpartum. Front. Med. 2023, 10, 1144170. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; Da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8, Correction in Nat. Rev. Dis. Primers 2023, 9, 35. [Google Scholar] [CrossRef]
- Bilban, M.; Tauber, S.; Haslinger, P.; Pollheimer, J.; Saleh, L.; Pehamberger, H.; Wagner, O.; Knöfler, M. Trophoblast invasion: Assessment of cellular models using gene expression signatures. Placenta 2010, 31, 989–996. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 12 June 2025).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://cran.r-project.org/ (accessed on 18 September 2025).
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578, Erratum in Nat. Protoc. 2014, 9, 2513. [Google Scholar] [CrossRef]
- Kaartokallio, T.; Cervera, A.; Kyllönen, A.; Laivuori, K.; Kere, J.; Laivuori, H.; The FINNPEC Core Investigator Group; Heinonen, S.; Kajantie, E.; Kivinen, K.; et al. Gene expression profiling of pre-eclamptic placentae by RNA sequencing. Sci. Rep. 2015, 5, srep14107, Erratum in Sci. Rep. 2016, 6, 17245. [Google Scholar] [CrossRef]

| Sample | Age (Years) | Gestational Age at Amniocentesis (Weeks) | Indication of Amniocentesis |
|---|---|---|---|
| P1 | 40 | 17 + 3 | AMA |
| P2 | 32 | 17 + 2 | Positive screening test for neural tube defect |
| P3 | 33 | 17 + 0 | Positive screening test for Down syndrome |
| P4 | 34 | 17 + 3 | Nuchal translucency dilatation |
| P5 | 39 | 16 + 3 | AMA |
| P6 | 32 | 21 + 3 | Abnormal ultrasonography finding |
| P7 | 36 | 17 + 2 | AMA |
| C1 | 39 | 16 + 4 | Positive screening test for Down syndrome |
| C2 | 38 | 19 + 6 | AMA |
| C3 | 35 | 17 + 1 | AMA |
| C4 | 40 | 16 + 1 | AMA |
| C5 | 36 | 18 + 2 | Positive screening test for Down syndrome |
| C6 | 33 | 18 + 0 | Positive screening test for Down syndrome |
| C7 | 42 | 17 + 6 | AMA |
| Gene Symbol | Fold Change | p-Value | False Discovery Rate |
|---|---|---|---|
| PE/CON | PE/CON | PE/CON | |
| HOOK2 | 3.899 | 0.006 | 0.031 |
| CCDC160 | 3.444 | 0.015 | 0.031 |
| CKB | 3.413 | 0.023 | 0.031 |
| PARP15 | −1.179 | 0.042 | 0.031 |
| Genes | Sense | Antisense |
|---|---|---|
| FLT1 | AGAGGTGAGCACTGCAACAA | TCTCCTCCGAGCCTGAAAGT |
| PlGF | ACCTGATGGTAGGAGGCAGT | ACCAGAACAGATGCACAACCA |
| CGB3 | CACCCCAGCATCCTATCACC | ATCTCCATCCTTGGTGCGTC |
| GPX-1 | TATCGAGAATGTGGCGTCCC | TCTTGGCGTTCTCCTGATGC |
| SOD2 | GCTGGAAGCCATCAAACGTG | GCCTGTTGTTCCTTGCAGTG |
| HOOK2 | CCAACTGGAAGCTGAAGGTC | GTCCTGCTTTTTCTCGCAAC |
| CCDC160 | GCAAGCCAAAGAAGTCATCC | GCGGATCTTTGCCATTTCTA |
| CKB | TTCTCAGAGGTGGAGCTGGT | TACCAAGGGTGACGGAAGTC |
| PARP15 | TGGGACAGATGCAGACTCAG | GCTGTCTGGCTTGGAGTAGG |
| β-actin | GGACTTCGAGCAAGAGATGG | AGCACTGTGTTGGCGTACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.E.; Jeong, Y.; Kim, J.Y.; Shin, H.-Y.; Kim, Y.-H.; Kim, M.-A. Investigation of Novel Predictive Biomarkers for Preeclampsia in Second-Trimester Amniotic Fluid. Int. J. Mol. Sci. 2025, 26, 10530. https://doi.org/10.3390/ijms262110530
Lee HE, Jeong Y, Kim JY, Shin H-Y, Kim Y-H, Kim M-A. Investigation of Novel Predictive Biomarkers for Preeclampsia in Second-Trimester Amniotic Fluid. International Journal of Molecular Sciences. 2025; 26(21):10530. https://doi.org/10.3390/ijms262110530
Chicago/Turabian StyleLee, Hyo Eun, Yeonseong Jeong, Jue Young Kim, Ha-Yeon Shin, Young-Han Kim, and Min-A Kim. 2025. "Investigation of Novel Predictive Biomarkers for Preeclampsia in Second-Trimester Amniotic Fluid" International Journal of Molecular Sciences 26, no. 21: 10530. https://doi.org/10.3390/ijms262110530
APA StyleLee, H. E., Jeong, Y., Kim, J. Y., Shin, H.-Y., Kim, Y.-H., & Kim, M.-A. (2025). Investigation of Novel Predictive Biomarkers for Preeclampsia in Second-Trimester Amniotic Fluid. International Journal of Molecular Sciences, 26(21), 10530. https://doi.org/10.3390/ijms262110530






