Diagnosis of Secondary Bacterial Meningitis via Aromatic Metabolites and Biomarkers in Cerebrospinal Fluid
Abstract
1. Introduction
2. Results and Discussion
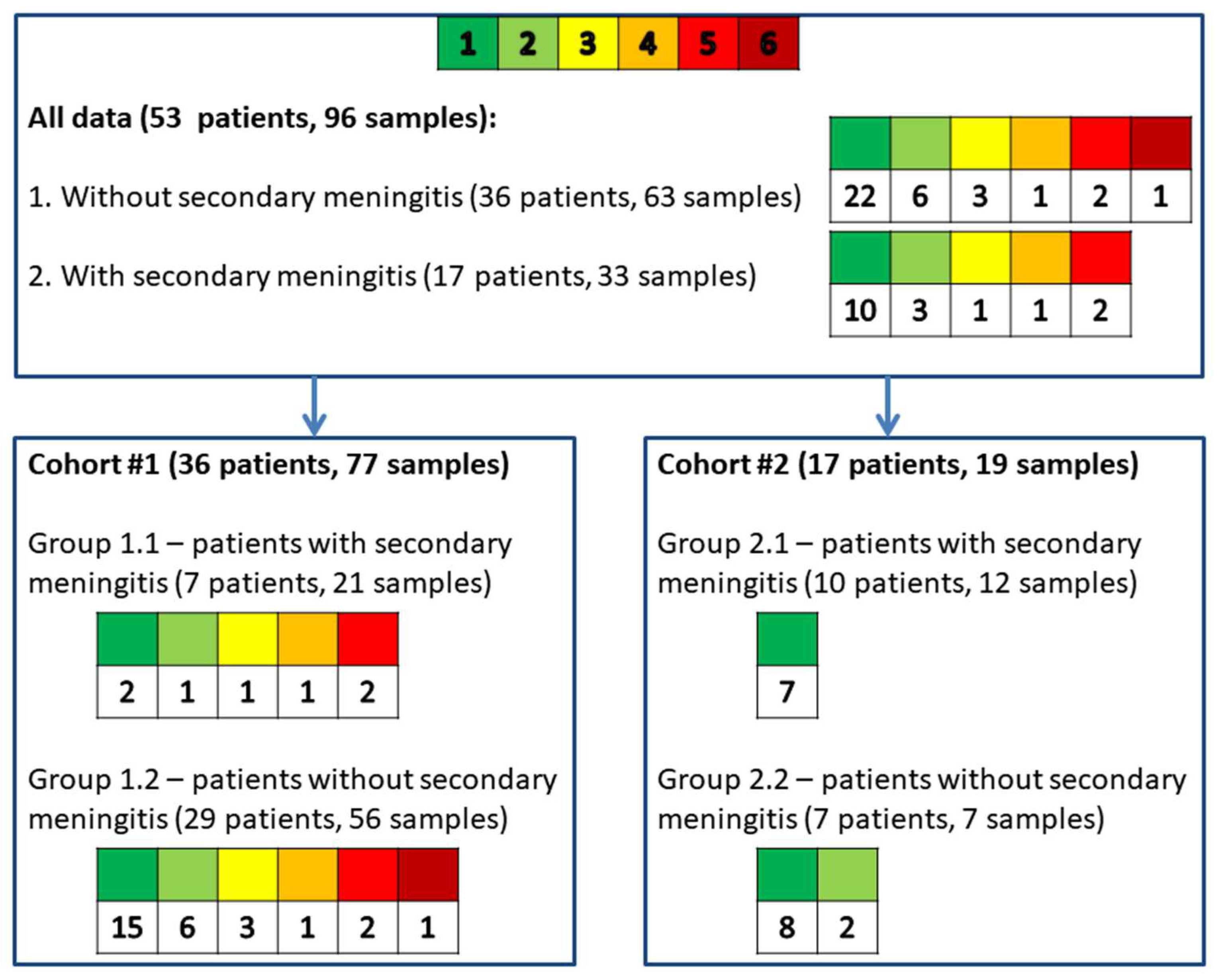
2.1. Description of Patients from Cohorts #1 and #2
2.2. Description of Cerebrospinal Fluid Samples from Cohorts #1 and #2
2.3. Cerebrospinal Fluid Composition from the Combined Sample Groups of Patients with or Without Secondary Meningitis
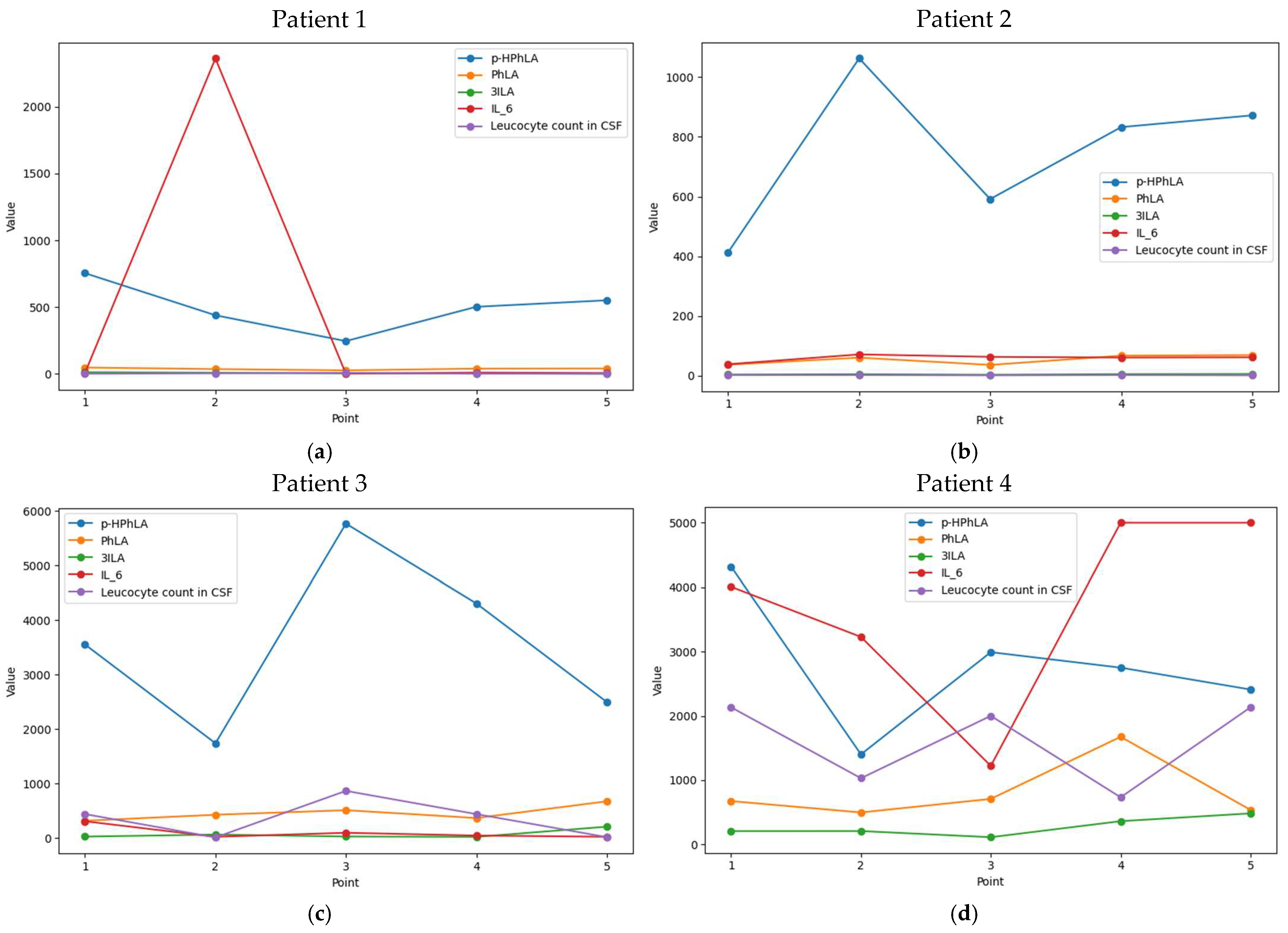
2.4. Dynamic Changes in CSF Parameters in Patients from Cohort #1
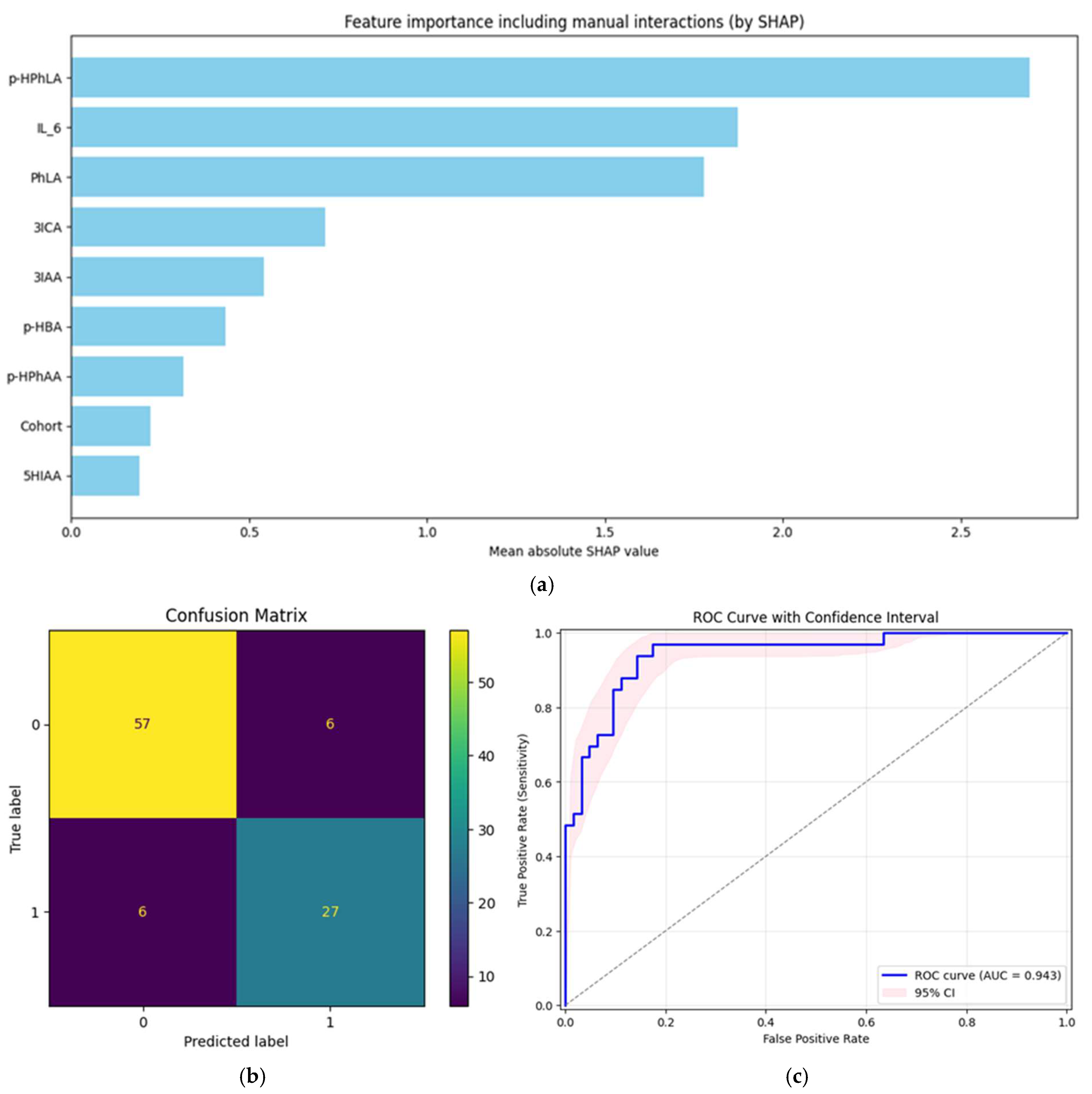
2.5. Prognostic Models for Secondary Bacterial Meningitis
3. Materials and Methods
3.1. Study Design
3.2. Collection of Clinical Data and Cerebrospinal Fluid Sample Analysis
- The patient’s medical records documented the growth of microorganisms in the CSF microbiological examination.
- There was a positive result from PCR testing.
- The patient’s medical records included a diagnosis of bacterial meningitis before the sample collection date. They met the CDC criteria [4], with threshold values defined as follows: leukocyte count in CSF greater than 300 cells/mm3, protein concentration above 1 g/L, and glucose concentration below 2.8 mmol/L.
3.3. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC-ROC | Area under the receiver operator characteristic curve |
| CDC | Centers for Disease Control |
| CI | Confidence interval |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| GC-MS | Gas chromatography–mass spectrometry |
| p-HBA | 4-hydroxybenzoic acid |
| 5HIAA | 5-hydroxyindole-3-acetic acid |
| p-HPhAA | 4-hydroxyphenylacetic acid |
| p-HPhLA | 4-hydroxyphenyllactic acid |
| p-HPhPA | 4-hydroxyphenylpropionic acid |
| 3IAA | Indole-3-acetic acid |
| 3ICA | Indole-3-carboxylic acid |
| IL-6 | Interleukin-6 |
| 3IPA | Indole-3-propionic acid |
| 3ILA | Indole-3-lactic acid |
| LLOQ | Lower limit of quantitation |
| NSE | Neuron-specific enolase |
| PCR | Polymerase chain reaction |
| PhPA | 3-phenylpropionic acid |
| PhLA | 3-phenyllactic acid |
| SHAP | SHapley Additive exPlanations |
| UPLC-MS/MS | Ultra-high-pressure liquid chromatography–tandem mass spectrometry |
References
- World Health Organization. WHO Guidelines on Meningitis Diagnosis, Treatment and Care; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.L.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef]
- Moorthy, R.K.; Sarkar, H.; Rajshekhar, V. Conservative Antibiotic Policy in Patients Undergoing Non-Trauma Cranial Surgery Does Not Result in Higher Rates of Postoperative Meningitis: An Audit of Nine Years of Narrow-Spectrum Prophylaxis. Br. J. Neurosurg. 2013, 27, 497–502. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN Surveillance Definition of Health Care–Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Hussein, K.; Bitterman, R.; Shofty, B.; Paul, M.; Neuberger, A. Management of Post-Neurosurgical Meningitis: Narrative Review. Clin. Microbiol. Infect. 2017, 23, 621–628. [Google Scholar] [CrossRef]
- Borowiak, A.; Safranow, K.; Sarna, A.; Łoniewska, B. Diagnostic Utility of Procalcitonin and Lactate Determination in Cerebrospinal Fluid for the Diagnosis of Neonatal Meningitis. J. Clin. Med. 2025, 14, 414. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Yang, Y.; Kong, Y.; Peng, Y. The Value of Elevated Cerebrospinal Fluid Lactate Concentrations in Post-Neurosurgical Bacterial Meningitis. BMC Neurol. 2023, 23, 377. [Google Scholar] [CrossRef]
- Suryaningtyas, W.; Meizikri, R.; Parenrengi, M.; Utomo, B.; Al Fauzi, A.; Bajamal, A. Risk Factors for Mortality in Patients with Bacterial Meningitis Following a Neurosurgical Procedure: A Meta-analysis. World Acad. Sci. J. 2024, 6, 59. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.M. The Quantitative Determination of Indolic Microbial Tryptophan Metabolites in Human and Rodent Samples: A Systematic Review. J. Chromatogr. B 2021, 1186, 123008. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, N.V. Serum Aromatic Microbial Metabolites as Biological Markers in Intensive Care; Springer: Cham, Switzerland, 2023; pp. 245–268. [Google Scholar]
- Pautova, A.K.; Meglei, A.Y.; Chernevskaya, E.A.; Alexandrova, I.A.; Beloborodova, N.V. 4-Hydroxyphenyllactic Acid in Cerebrospinal Fluid as a Possible Marker of Post-Neurosurgical Meningitis: Retrospective Study. J. Pers. Med. 2022, 12, 399. [Google Scholar] [CrossRef]
- Pautova, A.K.; Meinarovich, P.A.; Zakharchenko, V.E.; Sobolev, P.D.; Burnakova, N.A.; Beloborodova, N.V. Differences in the Profile of Aromatic Metabolites in the Corresponding Blood Serum and Cerebrospinal Fluid Samples of Patients with Secondary Bacterial Meningitis. Metabolites 2025, 15, 527. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, P.D.; Burnakova, N.A.; Revelsky, A.I.; Zakharchenko, V.E.; Beloborodova, N.V.; Pautova, A.K. A Sensitive Method for the Profiling of Phenyl- and Indole-Containing Metabolites in Blood Serum and Cerebrospinal Fluid Samples of Patients with Severe Brain Damage Using Ultra-High-Pressure Liquid Chromatography-Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2025, 260, 116803. [Google Scholar] [CrossRef]
- Goktas, S.Y.; Oral, A.Y.; Yılmaz, E.; Akalın, E.H.; Guvenc, F.; Ozkaya, G.; Kocaeli, H.; Dogan, S.; Yılmazlar, S.; Oral, H.B. Diagnostic Value of Cerebrospinal Fluid Levels of D-Lactate, Tumour Necrosis Factor-Alpha and Interleukin-6, -8, and -17 in Suspected Nosocomial Meningitis. Singap. Med. J. 2024, 65, 430–437. [Google Scholar] [CrossRef]
- Golubev, A.M. Enolases: Limitations for Implementation in Clinical Practice (Critical Review). Gen. Reanimatol. 2024, 20, 53–64. [Google Scholar]
- Singh, P.; Ali, S.A. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef]
- Meinarovich, P.; Pautova, A.; Zuev, E.; Sorokina, E.; Chernevskaya, E.; Beloborodova, N. An Integrated Approach Based on Clinical Data Combined with Metabolites and Biomarkers for the Assessment of Post-Operative Complications after Cardiac Surgery. J. Clin. Med. 2024, 13, 5054. [Google Scholar] [CrossRef]
- Grechko, A.V.; Gurkova, M.M.; Zhdanova, M.A.; Zurabov, A.Y.; Zurabov, F.M.; Kuzovlev, A.N.; Petrova, M.V.; Polyakov, P.A.; Cheboksarov, D.V.; Chernevskaya, E.A.; et al. Prevention of Nosocomial Pneumonia Recurrence Using a Bacteriophage Cocktail in Intensive Care Unit. Russ. J. Anesthesiol. Reanimatol. 2024, 2024, 39–48. [Google Scholar] [CrossRef]
- Likhvantsev, V.V.; Berikashvili, L.B.; Yadgarov, M.Y.; Yakovlev, A.A.; Kuzovlev, A.N. The Tri-Steps Model of Critical Conditions in Intensive Care: Introducing a New Paradigm for Chronic Critical Illness. J. Clin. Med. 2024, 13, 3683. [Google Scholar] [CrossRef]
- Ellis, J.; Cresswell, F.V.; Rhein, J.; Ssebambulidde, K.; Boulware, D.R. Cryptococcal Meningitis and Tuberculous Meningitis Co-Infection in HIV-Infected Ugandan Adults. Open Forum Infect. Dis. 2018, 5, ofy193, Erratum in Open Forum Infect. Dis. 2018, 6, ofy252. [Google Scholar] [CrossRef] [PubMed]
- Widerström, M. Significance of Staphylococcus Epidermidis in Health Care-Associated Infections, from Contaminant to Clinically Relevant Pathogen: This Is a Wake-Up Call! J. Clin. Microbiol. 2016, 54, 1679. [Google Scholar] [CrossRef]
- Schwieler, L.; Larsson, M.K.; Skogh, E.; Kegel, M.E.; Orhan, F.; Abdelmoaty, S.; Finn, A.; Bhat, M.; Samuelsson, M.; Lundberg, K.; et al. Increased Levels of IL-6 in the Cerebrospinal Fluid of Patients with Chronic Schizophrenia—Significance for Activation of the Kynurenine Pathway. J. Psychiatry Neurosci. 2015, 40, 126–133. [Google Scholar] [CrossRef]
- Casmiro, M.; Maitan, S.; De Pasquale, F.; Cova, V.; Scarpa, E.; Vignatelli, L. Cerebrospinal Fluid and Serum Neuron-specific Enolase Concentrations in a Normal Population. Eur. J. Neurol. 2005, 12, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Missler, U.; Wiesmann, M. Measurement of S-100 Protein in Human Blood and Cerebrospinal Fluid: Analytical Method and Preliminary Clinical Results. Clin. Chem. Lab. Med. 1995, 33, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Reavis, Z.W.; Mirjankar, N.; Sarangi, S.; Boyle, S.H.; Kuhn, C.M.; Matson, W.R.; Babyak, M.A.; Matson, S.A.; Siegler, I.C.; Kaddurah-Daouk, R.; et al. Sex and Race Differences of Cerebrospinal Fluid Metabolites in Healthy Individuals. Metabolomics 2021, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice Guidelines for the Management of Bacterial Meningitis. Clin. Infect. Dis. 2004, 39, 1267–1284. [Google Scholar] [CrossRef]
- Seehusen, D.A.; Reeves, M.M.; Fomin, D.A. Cerebrospinal Fluid Analysis. Am. Fam. Physician 2003, 68, 1103–1109. [Google Scholar] [PubMed]
- Gruol, D.L.; Nelson, T.E. Physiological and Pathological Roles of Interleukin-6 in the Central Nervous System. Mol. Neurobiol. 1997, 15, 307–339. [Google Scholar] [CrossRef]
- Kazakov, A.S.; Sokolov, A.S.; Permyakova, M.E.; Litus, E.A.; Uversky, V.N.; Permyakov, E.A.; Permyakov, S.E. Specific Cytokines of Interleukin-6 Family Interact with S100 Proteins. Cell Calcium 2022, 101, 102520. [Google Scholar] [CrossRef]
- Pautova, A.K.; Sergeev, A.A.; Beloborodova, N.V. Prospects for Monitoring Aromatic and Mitochondrial Metabolites Using Gas Chromatography–Mass Spectrometry during Extracorporeal Blood Purification in Patients with Sepsis. J. Anal. Chem. 2024, 79, 1951–1955. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Alnomasy, S.F.; Alotaibi, B.S.; Mujamammi, A.H.; Hassan, E.A.; Ali, M.E. Microbial Aspects and Potential Markers for Differentiation between Bacterial and Viral Meningitis among Adult Patients. PLoS ONE 2021, 16, e0251518. [Google Scholar] [CrossRef]
- Delannoy, Q.; Pean-de-Ponfilly, G.; Mesnil, C.; Severin, C.; Robert, J.; Plaisance, P.; Freund, Y.; Hausfater, P.; Cambau, E.; Jacquier, H.; et al. Validation of the Bacterial Meningitis Score in Adults Consulting at an Emergency Department: A Retrospective Multicentric Study. Eur. J. Emerg. Med. 2020, 27, 447–453. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, P.; Prieto, B.; Martínez-Morillo, E.; Rodríguez, V.; Álvarez, F.V. Interleukin-6 in Cerebrospinal Fluid as a Biomarker of Acute Meningitis. Ann. Clin. Biochem. Int. J. Lab. Med. 2016, 53, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Cao, Y.; Chen, Y.; Zeng, Z. Diagnostic Performance of Interleukin-6 and Interleukin-8 for Bacterial Meningitis: A Meta-Analysis. Int. J. Clin. Exp. Med. 2015, 8, 7059. [Google Scholar]
- Nugrahanto, A.P.; Triono, A.; Damroni, R.A.; Herini, E.S. Diagnostic Value of Serum Procalcitonin, CSF Neutrophil-to-Lymphocyte Ratio, and CSF Lactate in Pediatric Bacterial Meningoencephalitis. Ann. Indian. Acad. Neurol. 2024, 27, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.; Medeiros Santos, D.A.; Nery, E.K.; Mori, D.P.; de Carvalho Lucas, P.C.; Cammarota, D.; Florez Montero, G.L.; Barcellos Filho, F.N.; Frugis Yu, A.L.; Carvalhanas, T.R.M.P. Improving Meningitis Surveillance and Diagnosis with Machine Learning: Insights from São Paulo. PLoS Digit. Health 2025, 4, e0000925. [Google Scholar] [CrossRef]
- Pedregosa, F.; Michel, V.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Vanderplas, J.; Cournapeau, D.; Pedregosa, F.; Varoquaux, G.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the SciPy 2010, Austin, TX, USA, 28 June–3 July 2010; pp. 92–96. [Google Scholar]
- Chen, Y.-M.; Weng, Y.-T.; Dong, X.; Tsong, Y. Wald Tests for Variance-Adjusted Equivalence Assessment with Normal Endpoints. J. Biopharm. Stat. 2017, 27, 308–316. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver Operating Characteristic Curve: Overview and Practical Use for Clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Raschka, S. Model Evaluation, Model Selection, and Algorithm Selection in Machine Learning. arXiv 2018, arXiv:1811.12808. [Google Scholar] [CrossRef]
- Cleophas, T.J.; Zwinderman, A.H. Random Effects Models in Clinical Research. Int. J. Clin. Pharmacol. Ther. 2008, 46, 421–427. [Google Scholar] [CrossRef]
- Silveira, L.T.Y.D.; Ferreira, J.C.; Patino, C.M. Mixed-Effects Model: A Useful Statistical Tool for Longitudinal and Cluster Studies. J. Bras. Pneumol. 2023, 49, e20230137. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Bobadilla, A.V.; Schmitt, V.; Maier, C.S.; Mensing, S.; Stodtmann, S. Practical Guide to SHAP Analysis: Explaining Supervised Machine Learning Model Predictions in Drug Development. Clin. Transl. Sci. 2024, 17, e70056. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Cohort #1 | Cohort #2 | ||||
|---|---|---|---|---|---|---|
| All Patients/Samples | Group 1.1 with Secondary Meningitis | Group 1.2 Without Secondary Meningitis | All Patients/Samples | Group 2.1 with Secondary Meningitis | Group 2.2 Without Secondary Meningitis | |
| Number of patients, n | 36 | 7 | 29 | 17 | 10 | 7 |
| Number of CSF samples, n | 77 | 21 | 56 | 19 | 12 | 7 |
| Sex, males | 21 | 3 | 19 | 13 | 7 | 6 |
| Age, years | 50 (40, 61) | 32 (50, 65) | 50 (39, 59) | 39 (26, 54) | 38 (27, 45) | 48 (31, 50) |
| Deaths | 9 | 6 | 3 | 0 | 0 | 0 |
| Traumatic brain injury | 15 | 1 | 14 | - | - | - |
| Hemorrhagic events | 8 | 3 | 5 | - | - | - |
| Ischemic stroke | 7 | 1 | 6 | - | - | - |
| CNS tumor | 5 | 1 | 4 | 17 | 10 | 7 |
| CNS infection | 1 | 1 | 0 | - | - | - |
| Pneumonia | 24 | 5 | 19 | 0 | 0 | 0 |
| No growth in CSF | 29 | 2 | 27 | 15 | 9 | 6 |
| Staphylococcus epidermidis | 2 | 0 | 2 | 1 | 0 | 1 |
| Acinetobacter baumanii | - | 1 | - | - | - | - |
| Klebsiella pneumonia | - | 2 | - | - | - | - |
| Cryptococcus neoformans | - | 1 | - | - | - | - |
| Staphylococcus aureus | - | 0 | - | - | 1 | - |
| Unknown (positive Gram strain but no growth) | - | 1 | - | - | - | - |
| Parameter | Reference Values | Cohort #1 | Cohort #2 | p Cohorts #1 and #2 | p 1.1 and 2.1 | p 1.2 and 2.2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Samples (n = 77) | Group 1.1 with Secondary Meningitis (n = 21) | Group 1.2 Without Secondary Meningitis (n = 56) | p 1.1 and 1.2 | All Samples (n = 19) | Group 2.1 with Secondary Meningitis (n = 12) | Group 2.2 Without Secondary Meningitis (n = 7) | p 2.1 and 2.2 | |||||
| Leucocyte count, cells/mm3 | 2–8 | 11 (3, 96) | 733 (139, 2000) | 6 (2, 18) | <0.001 | 163 (18, 710) | 258 (136, 866) | 14 (5, 88) | 0.21 | 0.27 | 0.62 | <0.001 |
| Lymphocytes, % | 90–95 | 61 (30, 94) | 11 (5, 36) | 75 (54, 100) | <0.001 | 4 (3, 6) | 4 (3, 8) | 4 (3, 5) | 0.55 | <0.001 | 0.08 | <0.001 |
| Neutrophils, % | 3–5 | 47 (25, 68) | 84 (69, 92) | 40 (22, 50) | <0.001 | 88 (44, 96) | 96 (87, 97) | 37 (11, 63) | <0.001 | <0.001 | 0.31 | 0.57 |
| Protein, g/L | 0.1–0.3 | 0.7 (0.5, 1.2) | 1.6 (0.6, 4.5) | 0.7 (0.5, 1.0) | <0.001 | 2.6 (1.2, 4.2) | 3.6 (2.4, 4.7) | 1.2 (0.5, 2.2) | 0.06 | <0.001 | 0.36 | 0.03 |
| Glucose, mmol/L | 2.8–3.9 | 3.1 (2.2, 3.8) | 0.6 (0.2, 3.5) | 3.2 (2.6, 3.8) | 0.12 | 2.6 (2.2, 3.5) | 2.2 (1.9, 2.7) | 3.7 (3.3, 4.2) | <0.001 | 0.42 | 0.99 | 0.78 |
| IL-6, pg/mL | 1.5 (1.0, 2.2) [23] | 71 (10, 897) | 3228 (104, 5000) | 42 (7, 234) | <0.001 | 2336 (420, 5000) | 4017 (1455, 5000) | 271 (144, 3371) | 0.19 | <0.001 | 0.46 | 0.03 |
| NSE, ng/mL | 17.3 ± 4.6 [24] | 1.6 (0.9, 3.2) | 2.0 (1.3, 9.3) | 1.3 (0.7, 2.9) | 0.06 | 12.1 (5.0, 37.2) | 20.5 (9.0, 35.7) | 5.0 (3.3, 64.7) | 0.7 | 0.14 | 0.4 | <0.001 |
| S100, μg/L | 1.4 ± 0.5 [25] | 0.8 (0.4, 3.3) | 3.7 (0.7, 18.0) | 0.6 (0.3, 1.2) | <0.001 | 6.5 (2.5, 23.1) | 5.5 (2.3, 26.0) | 9.2 (3.2, 19.6) | 0.98 | <0.001 | 0.7 | <0.001 |
| p-HPhLA, nmol/L | 237 (196, 319) [26] | 633 (370, 1246) | 2750 (1734, 4206) | 491 (281, 840) | <0.001 | 925 (533, 1485) | 1172 (861, 1726) | 522 (424, 725) | 0.46 | 0.7 | 0.03 | 0.1 |
| p-HBA, nmol/L | no data | 33 (23, 61) | 35 (23, 99) | 32 (22, 60) | 0.26 | 30 (17, 41) | 22 (14, 36) | 35 (33, 41) | 0.38 | 0.32 | 0.31 | 0.7 |
| p-HPhAA, nmol/L | no data | 223 (101, 469) | 906 (362, 3167) | 152 (77, 278) | 0.03 | 100 (60, 191) | 92 (66, 233) | 103 (60, 138) | 0.7 | 0.46 | 0.32 | 0.79 |
| PhLA, nmol/L | no data | 67 (43, 173) | 538 (361, 736) | 52 (36, 77) | <0.001 | 100 (59, 131) | 108 (94, 145) | 47 (35, 68) | 0.26 | 0.57 | 0.16 | 0.43 |
| 5HIAA, nmol/L | 71 (55, 102) [26] | 112 (22, 224) | 162 (85, 306) | 72 (<20, 212) | 0.35 | 122 (81, 204) | 143 (103, 215) | 72 (62, 146) | 0.91 | 0.87 | 0.77 | 0.93 |
| 3ILA, nmol/L | 9 (7–12) [26] | 11 (4, 27) | 202 (22, 472) | 5 (3, 15) | <0.001 | 25 (18, 85) | 60 (25, 93) | 10 (6, 18) | 0.39 | 0.95 | 0.26 | 0.03 |
| 3ICA, nmol/L | no data | 11 (6, 14) | 12 (11, 20) | 8 (6, 13) | 0.03 | 8 (6, 10) | 8 (7, 11) | 5 (4, 9) | 0.7 | 0.47 | 0.17 | 0.38 |
| 3IAA, nmol/L | 20 (14–32) [26] | 39 (22, 79) | 96 (47, 204) | 26 (18, 44) | 0.12 | 71 (43, 118) | 73 (65, 88) | 47 (35, 177) | 0.47 | 0.14 | 0.6 | 0.03 |
| 3IPA, nmol/L | 0.11 ± 0.02 [10] | <2 (<2, 4) | 2 (<2, 4) | <2 (<2, 4) | <0.001 | 9 (3, 15) | 9 (4, 16) | 2 (<2, 14) | 0.56 | 0.27 | 0.88 | <0.001 |
| Parameter | Group 1. CSF Samples from Patients with Secondary Bacterial Meningitis (n = 33) | Group 2. CSF Samples from Patients without Secondary Bacterial Meningitis (n = 63) | p |
|---|---|---|---|
| Leucocyte count, cells/mm3 | 434 (139, 1685) | 7 (2, 18) | <0.001 |
| Lymphocytes, % | 10 (5, 20) | 74 (43, 98) | <0.001 |
| Neutrophils, % | 89 (77, 96) | 37 (20, 50) | <0.001 |
| Protein, g/L | 2.6 (1.3, 4.7) | 0.7 (0.5, 1.1) | <0.001 |
| Glucose, mmol/L | 2.1 (0.4, 3.0) | 3.4 (2.6, 3.9) | <0.001 |
| IL-6, pg/mL | 3228 (323, 5000) | 61 (9, 370) | <0.001 |
| NSE, ng/mL | 6.3 (1.5, 25.4) | 1.7 (0.9, 3.2) | 0.1 |
| S100, μg/L | 4.7 (1.1, 18.0) | 0.7 (0.4, 2.5) | <0.001 |
| p-HPhLA, nmol/L | 1923 (1248, 2991) | 502 (306, 828) | <0.001 |
| p-HBA, nmol/L | 30 (19, 58) | 34 (23, 58) | 0.49 |
| p-HPhAA, nmol/L | 362 (158, 1938) | 149 (75, 271) | 0.05 |
| PhLA, nmol/L | 311 (108, 668) | 52 (36, 76) | <0.001 |
| 5HIAA, nmol/L | 153 (97, 234) | 72 (<20, 206) | 0.18 |
| 3ILA, nmol/L | 91 (24, 208) | 6 (3, 16) | <0.001 |
| 3ICA, nmol/L | 12 (9, 14) | 8 (6, 13) | 0.15 |
| 3IAA, nmol/L | 77 (50, 176) | 29 (20, 52) | 0.21 |
| 3IPA, nmol/L | 4 (<2, 10) | <2 (<2, 4) | 0.2 |
| Parameter | AUC-ROC, 95% CI | Sensitivity, 95% CI | Specificity, 95% CI | Threshold Value, 95% CI |
|---|---|---|---|---|
| Leucocyte count, cells/mm3 | 0.91 (0.84, 0.96) | 0.80 (0.67, 1.00) | 0.94 (0.71, 1.00) | 139 (18, 244) |
| Lymphocytes, % | 0.88 (0.82, 0.94) | 0.91 (0.79, 1.00) | 0.79 (0.63, 0.92) | 48 (16, 68) |
| Neutrophils, % | 0.93 (0.87, 0.98) | 0.88 (0.73, 1.00) | 0.90 (0.74, 1.00) | 58 (46, 81) |
| Protein, g/L | 0.83 (0.75, 0.91) | 0.77 (0.68, 0.87) | 0.84 (0.72, 0.95) | 1.3 (1.0, 2.6) |
| Glucose, mmol/L | 0.78 (0.66, 0.87) | 0.71 (0.42, 0.86) | 0.82 (0.62, 1.00) | 2.6 (1.3, 3.0) |
| IL-6, pg/mL | 0.82 (0.73, 0.92) | 0.75 (0.52, 1.00) | 0.84 (0.45, 0.96) | 869 (16, 5000) |
| NSE, ng/mL | 0.71 (0.59, 0.84) | 0.55 (0.38, 0.86) | 0.91 (0.53, 0.97) | 6.3 (4.6, 24.4) |
| S100,μg/L | 0.74 (0.61, 0.90) | 0.71 (0.38, 1.00) | 0.75 (0.53, 0.96) | 1.7 (0.7, 11.5) |
| p-HPhLA, nmol/L | 0.91 (0.84, 0.96) | 0.79 (0.65, 0.92) | 0.97 (0.84, 1.00) | 1248 (924, 1510) |
| p-HBA, nmol/L | 0.53 (0.43, 0.65) | 0.22 (0.12, 0.95) | 0.96 (0.26, 1.00) | 180 (14, 466) |
| p-HPhAA, nmol/L | 0.70 (0.56, 0.82) | 0.67 (0.44, 0.83) | 0.75 (0.62, 0.94) | 262 (156, 452) |
| PhLA, nmol/L | 0.92 (0.85, 0.97) | 0.84 (0.71, 0.95) | 0.88 (0.78, 0.98) | 99 (78, 173) |
| 5HIAA, nmol/L | 0.65 (0.44, 0.75) | 0.86 (0.73, 1.00) | 0.53 (0.34, 0.70) | 79 (69, 178) |
| 3ILA, nmol/L | 0.91 (0.85, 0.96) | 0.90 (0.74, 1.00) | 0.82 (0.70, 0.98) | 18 (15, 47) |
| 3ICA, nmol/L | 0.65 (0.42, 0.76) | 0.81 (0.68, 0.96) | 0.56 (0.26, 0.68) | 9 (5, 13) |
| 3IAA, nmol/L | 0.79 (0.26, 0.88) | 0.87 (0.79, 1.00) | 0.71 (0.07, 0.85) | 45 (37, 332) |
| 3IPA, nmol/L | 0.65 (0.36, 0.76) | 0.63 (0.33, 1.00) | 0.70 (0.02, 0.93) | 3 (2, 35) |
| Multivariate model | 0.94 (0.89; 0.98) | 0.94 (0.86; 1.00) | 0.86 (0.75; 0.96) | - |
| Part of Analysis | Formula for Each Model | ||
|---|---|---|---|
| Target Variable | Fixed Effects | Random Effects | |
| Statistical comparison of groups (the Wald test) | Concentration of a single metabolite, biomarker, or clinical variable | Group or Cohort | Patient’s ID |
| ROC analysis | Group | Concentration of a single metabolite, biomarker, or clinical variable | Patient’s ID |
| Final model | Group | Selected metabolites and biomarkers; Cohort | Patient’s ID |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meinarovich, P.A.; Sorokina, E.A.; Beloborodova, N.V.; Pautova, A.K. Diagnosis of Secondary Bacterial Meningitis via Aromatic Metabolites and Biomarkers in Cerebrospinal Fluid. Int. J. Mol. Sci. 2025, 26, 10522. https://doi.org/10.3390/ijms262110522
Meinarovich PA, Sorokina EA, Beloborodova NV, Pautova AK. Diagnosis of Secondary Bacterial Meningitis via Aromatic Metabolites and Biomarkers in Cerebrospinal Fluid. International Journal of Molecular Sciences. 2025; 26(21):10522. https://doi.org/10.3390/ijms262110522
Chicago/Turabian StyleMeinarovich, Petr A., Ekaterina A. Sorokina, Natalia V. Beloborodova, and Alisa K. Pautova. 2025. "Diagnosis of Secondary Bacterial Meningitis via Aromatic Metabolites and Biomarkers in Cerebrospinal Fluid" International Journal of Molecular Sciences 26, no. 21: 10522. https://doi.org/10.3390/ijms262110522
APA StyleMeinarovich, P. A., Sorokina, E. A., Beloborodova, N. V., & Pautova, A. K. (2025). Diagnosis of Secondary Bacterial Meningitis via Aromatic Metabolites and Biomarkers in Cerebrospinal Fluid. International Journal of Molecular Sciences, 26(21), 10522. https://doi.org/10.3390/ijms262110522









