Investigation on Applying Cyclodextrins in a Fermentation Process for Enhanced Biosurfactant Production by Bacillus licheniformis
Abstract
1. Introduction
2. Results
2.1. Cell Growth and Specific Growth Rate in a Reverse Spinning Personal Bioreactor (10 mL)
2.1.1. Impact of Cyclodextrin Derivatives on Maximum Specific Growth Rate (Umax)
2.1.2. Effect of Cyclodextrin Treatments on Biomass Concentration at 50 h
2.2. Fermentation Analysis of Shaking Flasks (150 mL)
2.3. Results of Surface Tension Measurments
2.4. Product Concentration
3. Discussion
4. Materials and Methods
4.1. Bacterium Strain
4.2. Cyclodextrin
4.3. Fermentation Process for Biosurfactant Production
4.4. Biomass Analysis
4.5. Glucose Analysis
4.6. Surface Tension
4.7. Isolation of Biosurfactant
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green surfactants (biosurfactants): A petroleum-free substitute for sustainability—Comparison, applications, market, and future prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Mahmoud, Z.H.; Hussein, U.A.-R.; Abduvalieva, D.; Alsultany, F.H.; Kianfar, E. Biosurfactants: Properties, applications and emerging trends. S. Afr. J. Chem. Eng. 2025, 53, 21–39. [Google Scholar] [CrossRef]
- Inès, M.; Mouna, B.; Marwa, E.; Dhouha, G. Biosurfactants as Emerging Substitutes of Their Synthetic Counterpart in Detergent Formula: Efficiency and Environmental Friendly. J. Polym. Environ. 2023, 31, 2779–2791. [Google Scholar] [CrossRef]
- Ciurko, D.; Czyżnikowska, Ż.; Kancelista, A.; Łaba, W.; Janek, T. Sustainable production of Biosurfactant from agro-industrial oil wastes by Bacillus subtilis and its potential application as antioxidant and ACE inhibitor. Int. J. Mol. Sci. 2022, 23, 10824. [Google Scholar] [CrossRef]
- De Almeida, D.G.; Soares Da Silva, R.D.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, D.; Sukhbir-Singh, G.M.; Karamchandani, B.M.; Aseri, G.K.; Banat, I.M.; Satpute, S.K. Biosurfactants: Forthcomings and regulatory affairs in food-based industries. Molecules 2023, 28, 2823. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Saharan, B.S.; Kapil, S. Structural properties of biosurfactants of lab. In Biosurfactants of Lactic Acid Bacteria; Springer: Berlin/Heidelberg, Germany, 2016; pp. 47–60. [Google Scholar] [CrossRef]
- Liu, J.-F.; Mbadinga, S.M.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Chemical structure, property and potential applications of biosurfactants produced by Bacillus subtilis in petroleum re-covery and spill mitigation. Int. J. Mol. Sci. 2015, 16, 4814–4837. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Teixeira, J.A. Bacillus licheniformis: The unexplored alternative for the anaerobic production of lipopeptide biosurfactants? Biotechnol. Adv. 2022, 60, 108013. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Nerurkar, A.S. Structural and molecular characteristics of lichenysin and its relationship with surface activity. In Biosurfactants; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 304–315. [Google Scholar] [CrossRef]
- Grangemard, I.; Wallach, J.; Maget-Dana, R.; Peypoux, F. Lichenysin: A more efficient cation chelator than surfactin. Appl. Biochem. Biotechnol. 2001, 90, 199–210. [Google Scholar] [CrossRef]
- Sousa, M.; Melo, V.M.M.; Rodrigues, S.; Sant’ana, H.B.; Gonçalves, L.R.B. Screening of biosurfactant-producing Bacillus strains using glycerol from the biodiesel synthesis as main carbon source. Bioprocess Biosyst. Eng. 2012, 35, 897–906. [Google Scholar] [CrossRef]
- Wu, B.; Xiu, J.; Yu, L.; Huang, L.; Yi, L.; Ma, Y. Biosurfactant production by Bacillus subtilis SL and its potential for enhanced oil recovery in low permeability reservoirs. Sci. Rep. 2022, 12, 7785. [Google Scholar] [CrossRef] [PubMed]
- Moshtagh, B.; Hawboldt, K.; Zhang, B. Optimization of biosurfactant production by Bacillus subtilis N3-1P using the brewery waste as the carbon source. Environ. Technol. 2018, 40, 3371–3380. [Google Scholar] [CrossRef]
- Amodu, O.S.; Ntwampe, S.K.O.; Ojumu, T.V. Optimization of biosurfactant production by Bacillus licheniformis STK 01 grown exclusively on Beta vulgaris waste using response surface methodology. BioResources 2014, 9, 5045–5065. [Google Scholar] [CrossRef]
- Ramirez-Olea, H.; Reyes-Ballesteros, B.; Chavez-Santoscoy, R.A. Potential application of the probiotic Bacillus licheniformis as an adjuvant in the treatment of diseases in humans and animals: A systematic review. Front. Microbiol. 2022, 13, 993451. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Wang, F.; Xu, B.; Safdar, B.; Ullah, A.; Naveed, M.; Wang, C.; Rashid, M.T. Production and Application of Biosurfactant Produced by Bacillus licheniformis Ali5 in Enhanced Oil Recovery and Motor Oil Removal from Contaminated Sand. Molecules 2019, 24, 4448. [Google Scholar] [CrossRef]
- Théatre, A.; Cano-Prieto, C.; Bartolini, M.; Laurin, Y.; Deleu, M.; Niehren, J.; Fida, T.; Gerbinet, S.; Alanjary, M.; Medema, M.H.; et al. The surfactin-like lipopeptides from Bacillus spp.: Natural Biodiversity and synthetic biology for a broader Application Range. Front. Bioeng. Biotechnol. 2021, 9, 623701. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Hong, S.-J.; Park, B.-R.; Lee, H.-N.; Jang, D.E.; Kang, H.-J.; Ameer, K.; Kim, S.-J.; Kim, Y.-M. Carbohydrate-binding module of cycloisomaltooligosaccharide glucanotransferase from Thermoanaerobacter thermocopriae improves its cyclodextran production. Enzym. Microb. Technol. 2022, 157, 110023. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of Cyclodextrin Functional Nanofibers for Drug Delivery Applications. Pharmaceutics 2019, 11, 6. [Google Scholar] [CrossRef]
- Dalal, D.S.; Patil, D.R.; Tayade, Y.A. β-Cyclodextrin: A green and efficient supramolecular catalyst for organic transformations. Chem. Rec. 2018, 18, 1560–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xiao, Z.; Zhou, R.; Zhu, Y. Study of production and pyrolysis characteristics of sweet orange flavor-β-cyclodextrin inclusion complex. Carbohydr. Polym. 2014, 105, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Yamamura, H.; Sugiyama, Y.; Murata, K.; Yokoi, T.; Kurata, R.; Miyagawa, A.; Sakamoto, K.; Komagoe, K.; Inoue, T.; Katsu, T. Synthesis of antimicrobial cyclodextrins bearing polyarylamino and polyalkylamino groups via click chemistry for bacterial membrane disruption. Chem. Commun. 2014, 50, 5444–5446. [Google Scholar] [CrossRef]
- Yamamura, H.; Isshiki, K.; Fujita, Y.; Kato, H.; Katsu, T.; Masuda, K.; Osawa, K.; Miyagawa, A. Gramicidin S-inspired antimicrobial cyclodextrin to disrupt gram-negative and gram-positive bacterial membranes. MedChemComm 2019, 10, 1432–1437. [Google Scholar] [CrossRef]
- Wong, C.E.; Dolzhenko, A.V.; Lee, S.M.; Young, D.J. Cyclodextrins: A weapon in the fight against antimicrobial resistance. J. Mol. Eng. Mater. 2017, 5, 1740006, Erratum in J. Mol. Eng. Mater. 2017, 5, 1792001. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Shan, M.; Zhao, H.; Lu, Z.; Lu, Y. A mini-review: Mechanism of antimicrobial action and application of surfactin. World J. Microbiol. Biotechnol. 2022, 38, 143. [Google Scholar] [CrossRef]
- Adhikary, R.; Maiti, P.K.; Ghosh, N.; Rajbanshi, B.; Roy, M.N.; Mandal, S.; Mandal, V. Lipopeptide iturin C3 from endophytic Bacillus sp. effectively inhibits biofilm formation and prevents the adhesion of topical and food-borne pathogens in vitro and on biomedical devices. Arch. Microbiol. 2025, 207, 62. [Google Scholar] [CrossRef]
- Yeak, K.Y.C.; Perko, M.; Staring, G.; Fernandez-Ciruelos, B.M.; Wells, J.M.; Abee, T.; Wells-Bennik, M.H.J. Lichenysin Production by Bacillus licheniformis Food Isolates and Toxicity to Human Cells. Front. Microbiol. 2022, 13, 831033. [Google Scholar] [CrossRef]
- Márton, R.; Margl, M.; Tóth, L.K.; Fenyvesi, É.; Szente, L.; Molnár, M. The Impact of Cyclodextrins on the Physiology of Candida boidinii: Exploring New Opportunities in the Cyclodextrin Application. Molecules 2024, 29, 3698. [Google Scholar] [CrossRef]
- Yáñez, C.; Cañete-Rosales, P.; Castillo, J.P.; Catalán, N.; Undabeytia, T.; Morillo, E. Cyclodextrin inclusion complex to improve physicochemical properties of herbicide bentazon: Exploring better formula-tions. PLoS ONE 2012, 7, e41072. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Kamigauchi, M.; Kawanishi, K.; Sugiura, M.; Ohishi, H.; Ishida, T. γ-Cyclodextrin as Inhibitor of the Precipitation Reaction between Berberine and Glycyrrhizin in Decoctions of Natural Medicines: Interaction Studies of Cyclodextrins with Glycyrrhizin and Glycyrrhetic Acid by 1H-NMR Spectroscopy and Molecular-Dynamics Calculation. Helv. Chim. Acta 2008, 91, 1614–1624. [Google Scholar] [CrossRef]
- Coronel-León, J.; Marqués, A.; Bastida, J.; Manresa, A. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. J. Appl. Microbiol. 2016, 120, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Li, Y.; Shang, Y.; Ali, N.; Wang, F.; Zhang, D.; Liao, Y. Optimization of Medium Components for Fed-Batch Fermentation Using Central Composite Design to Enhance Lichenysin Production by Bacillus licheniformis Ali5. Fermentation 2022, 8, 712. [Google Scholar] [CrossRef]
- Ahmadi-Ashtiani, H.-R.; Baldisserotto, A.; Cesa, E.; Manfredini, S.; Zadeh, H.S.; Gorab, M.G.; Khanahmadi, M.; Zakizadeh, S.; Buso, P.; Vertuani, S. Microbial Biosurfactants as Key Multifunctional Ingredients for Sustainable Cosmetics. Cosmetics 2020, 7, 46. [Google Scholar] [CrossRef]
- Spiridon, I.; Anghel, N. Cyclodextrins as Multifunctional Platforms in Drug Delivery and Beyond: Structural Features, Functional Applications, and Future Trends. Molecules 2025, 30, 3044. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef]
- Łagiewka, J.; Girek, T.; Ciesielski, W. Cyclodextrins-Peptides/Proteins Conjugates: Synthesis, Properties and Applications. Polymers 2021, 13, 1759. [Google Scholar] [CrossRef] [PubMed]
- Rusznyák, Á.; Malanga, M.; Fenyvesi, É.; Szente, L.; Váradi, J.; Bácskay, I.; Vecsernyés, M.; Vasvári, G.; Haimhoffer, Á.; Fehér, P.; et al. Investigation of the Cellular Effects of Beta- Cyclodextrin Derivatives on Caco-2 Intestinal Epithelial Cells. Pharmaceutics 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Li, Z.; Uematsu, K.; Kobayashi, T.; Horikoshi, K. Antibacterial activity of cyclodextrins against Bacillus strains. Arch. Microbiol. 2008, 190, 605–609. [Google Scholar] [CrossRef]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Cyclodextrin-Elicited Bryophyllum Suspension Cultured Cells: Enhancement of the Production of Bioactive Compounds. Int. J. Mol. Sci. 2019, 20, 5180. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Esmaeilishirazifard, E.; De Vizio, D.; Moschos, S.A.; Keshavarz, T. Genomic and molecular characterization of a novel quorum sensing molecule in Bacillus licheniformis. AMB Express 2017, 7, 78. [Google Scholar] [CrossRef]
- Shumilin, I.; Tanbuz, A.; Harries, D. Self-association of cyclodextrin inclusion complexes in a deep eutectic solvent enhances guest solubility. Carbohydr. Polym. 2025, 351, 123067. [Google Scholar] [CrossRef]
- Jarak, I.; Ramos, S.; Caldeira, B.; Domingues, C.; Veiga, F.; Figueiras, A. The Many Faces of Cyclodextrins within Self-Assembling Polymer Nanovehicles: From Inclusion Complexes to Valuable Structural and Functional Elements. Int. J. Mol. Sci. 2024, 25, 9516. [Google Scholar] [CrossRef] [PubMed]
- Cova, T.F.; Cruz, S.M.; Valente, A.J.; Abreu, P.E.; Marques, J.M.; Pais, A.A. Aggregation of Cyclodextrins: Fundamental Issues and Applications; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Tsianou, M.; Fajalia, A.I. Cyclodextrins and surfactants in aqueous solution above the critical micelle concentration: Where are the cyclodextrins located? Langmuir 2014, 30, 13754–13764. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, S.; Mu, B. Structural Characterization of Lipopeptide Methyl Esters Produced by Bacillus licheniformis HSN 221. Chem. Biodivers. 2010, 7, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Fenyvesi, É. Cyclodextrin-Lipid Complexes: Cavity Size Matters. Struct. Chem. 2017, 28, 479–492. [Google Scholar] [CrossRef]
- Joshi, S.J.; Geetha, S.; Yadav, S.; Desai, A.J. Optimization of bench-scale production of biosurfactant by Bacillus licheniformis R2. APCBEE Procedia 2013, 5, 232–236. [Google Scholar] [CrossRef]
- Sakiyo, J.J.; Németh, Á. Optimization of Bacillus licheniformis DSM13 for Biosurfactant production using response surface methodology. Hung. J. Ind. Chem. 2022, 50, 51–55. [Google Scholar] [CrossRef]
- Tóth, P.; Németh, Á. Exploring the Impact of Alginite Mineral on Lactic Acid Bacteria. Fermentation 2025, 11, 482. [Google Scholar] [CrossRef]
- Czinkóczky, R.; Németh, Á. Techno-economic assessment of Bacillus fermentation to produce surfactin and lichenysin. Biochem. Eng. J. 2020, 163, 107719. [Google Scholar] [CrossRef]
- Czinkóczky, R.; Németh, Á. The Effect of pH on Biosurfactant Production by Bacillus subtilis DSM10. Hung. J. Ind. Chem. 2020, 48, 37–43. [Google Scholar] [CrossRef]
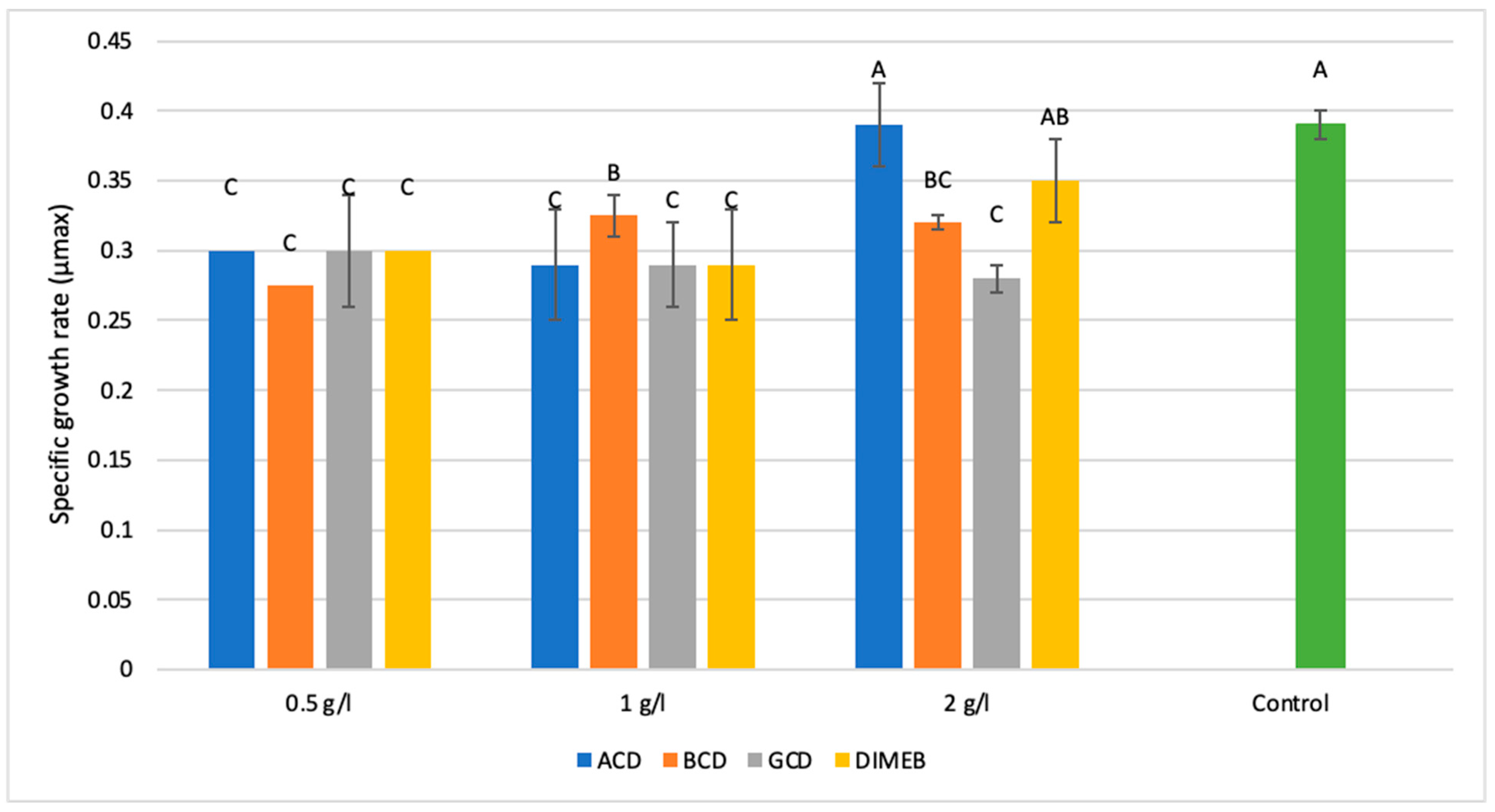
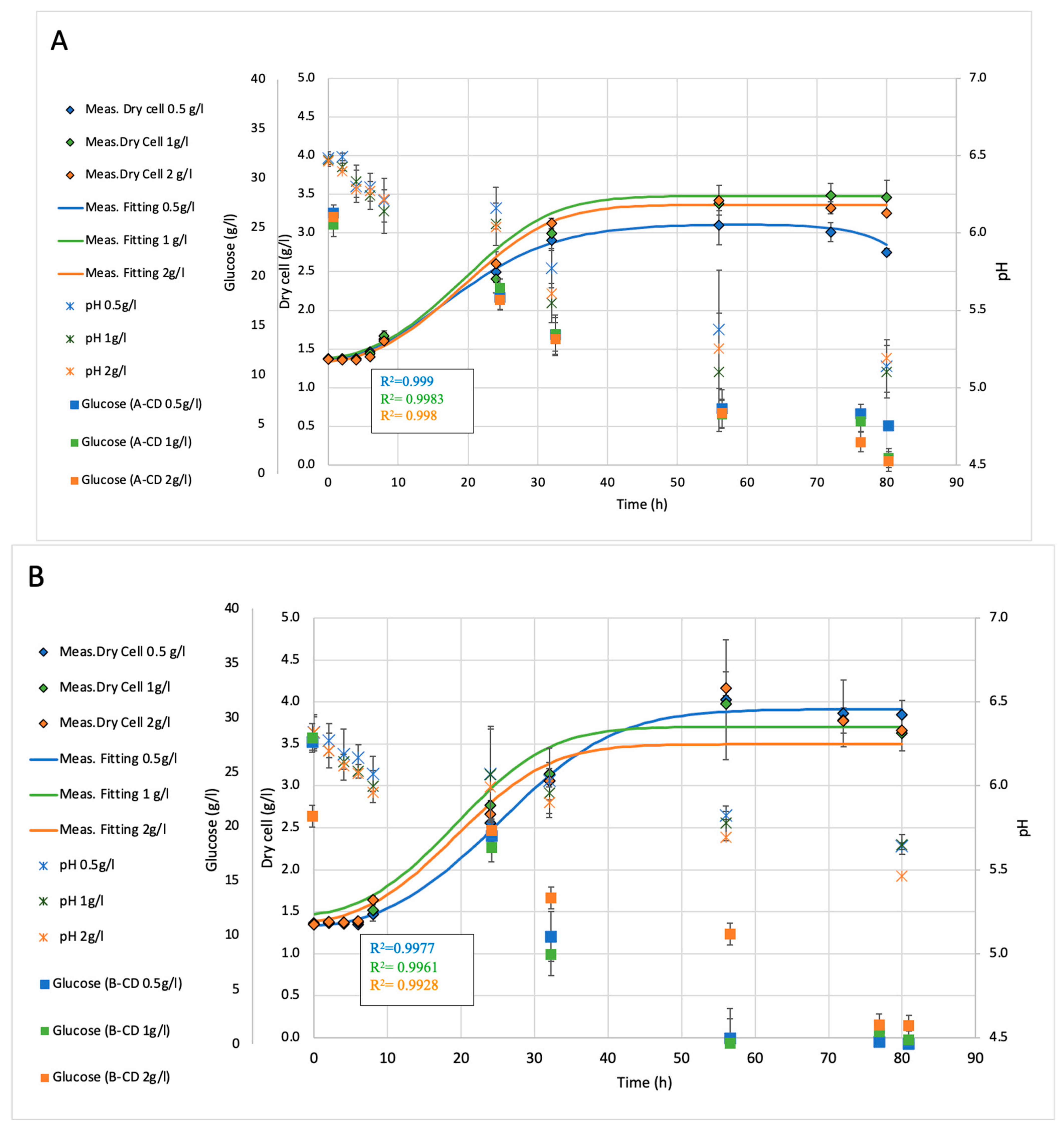
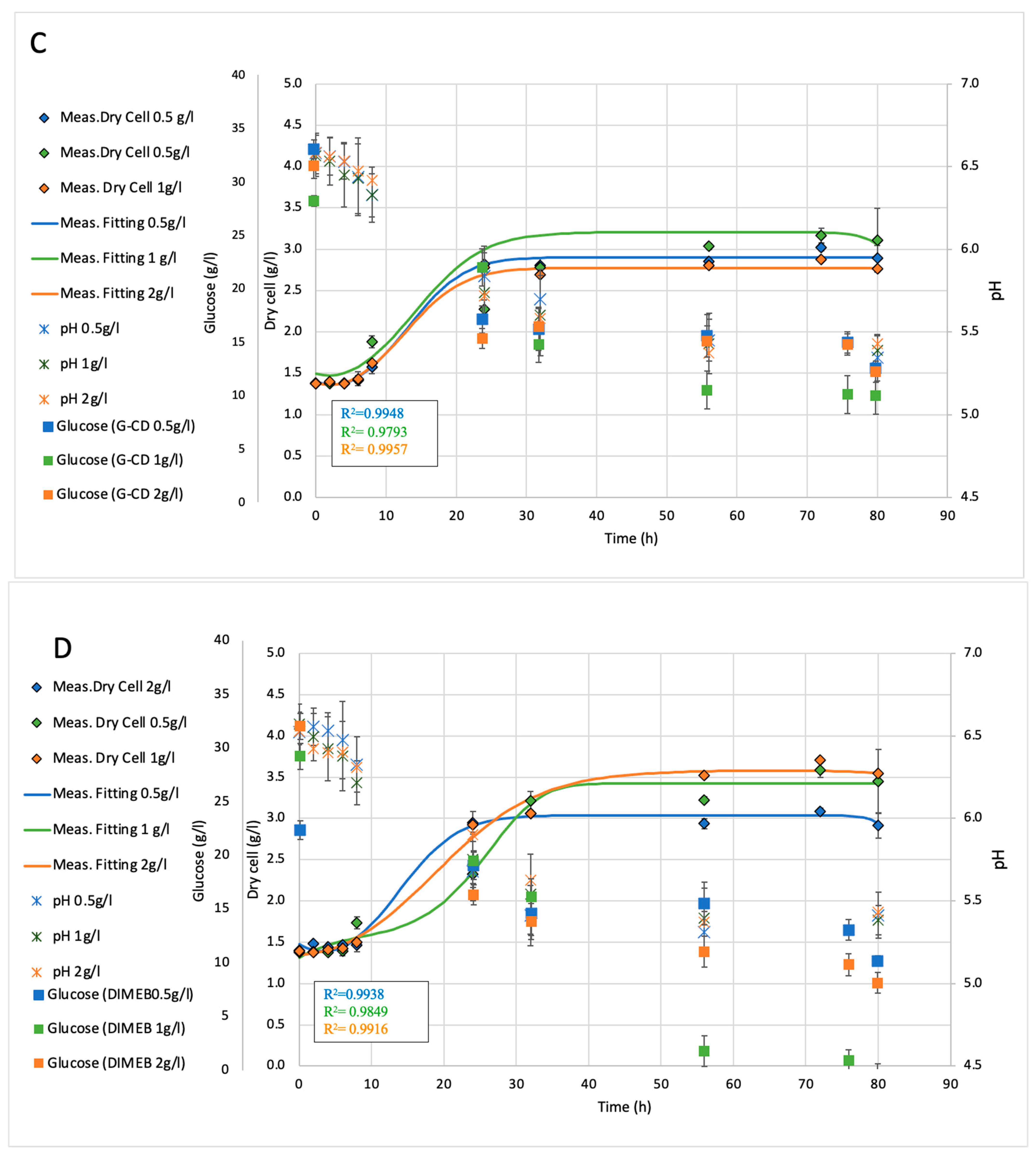
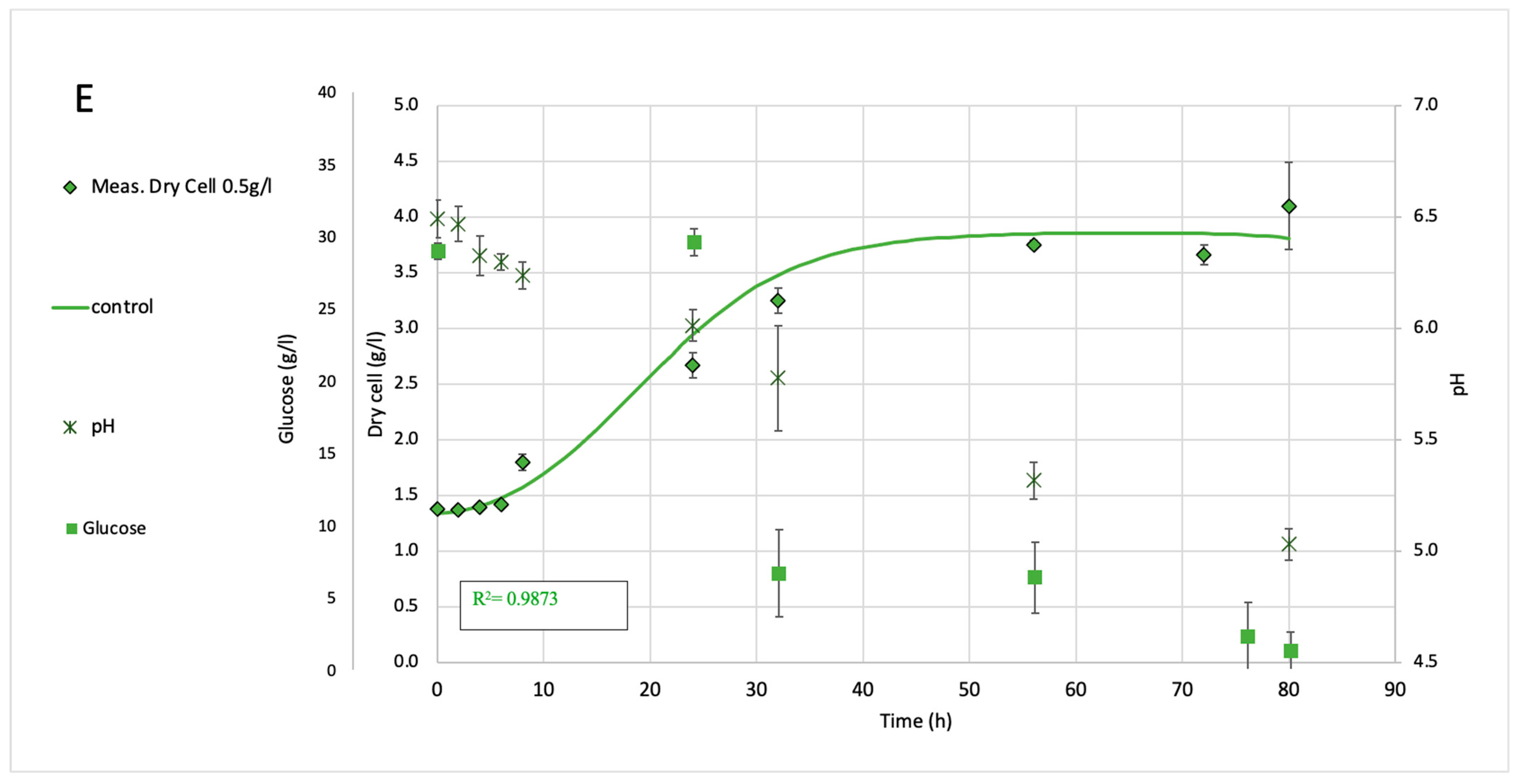


| Treatment | Concentration (g/L) | Mean OD850 | Std Dev | p-Value vs. Control | Significance |
|---|---|---|---|---|---|
| Control | 0 | 2.980 | 0.149 | – | – |
| ACD | 0.5 | 2.983 | 0.202 | 0.983 | n.s. |
| ACD | 1.0 | 2.940 | 0.142 | 0.753 | n.s. |
| ACD | 2.0 | 3.787 | 0.463 | 0.045 | * |
| BCD | 0.5 | 2.867 | 0.059 | 0.320 | n.s. |
| BCD | 1.0 | 3.243 | 0.881 | 0.658 | n.s. |
| BCD | 2.0 | 2.637 | 0.278 | 0.154 | n.s. |
| GCD | 0.5 | 2.857 | 0.827 | 0.822 | n.s. |
| GCD | 1.0 | 2.517 | 0.582 | 0.300 | n.s. |
| GCD | 2.0 | 2.823 | 1.104 | 0.830 | n.s. |
| DIMEB | 0.5 | 2.300 | 0.830 | 0.290 | n.s. |
| DIMEB | 1.0 | 2.177 | 0.460 | 0.045 | * |
| DIMEB | 2.0 | 3.497 | 0.638 | 0.294 | n.s. |
| Trophophase | Idiophase | ||||||
|---|---|---|---|---|---|---|---|
| Umax (1/h) | Biomass Increment RTS (g/L) | Biomass Increment in Flasks (g/L) | Product Conc. (g/L) | Surface Tension Decrement | Specific Product Formation Yield (g/g Cell) | ||
| α-CD | 0.5 g/L | 0.30 ± 0.05 b | 2.7 ± 0.16 a | 1.39 ± 0.04 b | 3.61 ± 0.14 b | 3.44 ± 1.16 b | 2.60 ± 0.17 b |
| 1.0 g/L | 0.29 ± 0.03 b | 2.69 ± 0.09 a | 2.09 ± 0.21 b | 4.34 ± 0.22 ab | 5.91 ± 2.14 a | 2.08 ± 0.13 ab | |
| 2.0 g/L | 0.39 ± 0.03 a | 3.5 ± 0.32 c | 1.89 ± 0.03 b | 5.07 ± 0.1 ab | 3.9 ± 1.52 b | 2.68 ± 0.23 b | |
| b-CD | 0.5 g/L | 0.28 ± 0.02 b | 2.56 ± 0.02 a | 2.48 ± 0.16 a | 5.29 ± 0.22 a | 2.46 ± 0.66 b | 2.13 ± 0.07 ab |
| 1.0 g/L | 0.33 ± 0.01 b | 2.56 ± 0.02 a | 2.29 ± 0.41 a | 5.52 ± 0.11 a | 4.33 ± 0.63 b | 2.41 ± 0.16 b | |
| 2.0 g/L | 0.32 ± 0.01 b | 2.56 ± 0.02 a | 2.31 ± 0.15 a | 5.42 ± 0.39 a | 4.92 ± 1.75 b | 2.35 ± 0.43 b | |
| g-CD | 0.5 g/L | 0.30 ± 0.04 b | 2.99 ± 0.10 c | 1.51 ± 0.14 b | 3.48 ± 0.15 b | 1.91 ± 0.89 b | 2.30 ± 0.23 b |
| 1.0 g/L | 0.29 ± 0.01 b | 2.54 ± 0.24 a | 1.73 ± 0.37 b | 3.54 ± 0.19 b | 1.83 ± 0.75 b | 2.05 ± 0.24 ab | |
| 2.0 g/L | 0.28 ± 0.01 b | 2.48 ± 0.82 a | 1.39 ± 0.01 b | 3.26 ± 0.2 b | 5.95 ± 0.5 ab | 2.35 ± 0.45 b | |
| DIMEB | 0.5 g/L | 0.30 ± 0.05 b | 1.92 ± 0.58 b | 1.51 ± 0.23 b | 5.31 ± 0.2 c | 2.5 ± 1.15 b | 3.52 ± 0.14 c |
| 1.0 g/L | 0.29 ± 0.03 b | 1.92 ± 0.58 b | 2.07 ± 0.57 ab | 6.24 ± 0.64 c | 9.64 ± 0.31 c | 3.01 ± 0.55 c | |
| 2.0 g/L | 0.35 ± 0.01 ab | 3.1 ± 0.36 c | 2.14 ± 0.56 ab | 7 ± 0.17 c | 14.59 ± 0.73 c | 3.27 ± 0.89 c | |
| control | 0.39 ± 0.04 a | 2.55 ± 0.05 a | 2.72 ± 0.22 a | 4.96 ± 0.22 a | 5.9 ± 0.99 a | 1.82 ± 0.86 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakiyo, J.J.; Németh, Á. Investigation on Applying Cyclodextrins in a Fermentation Process for Enhanced Biosurfactant Production by Bacillus licheniformis. Int. J. Mol. Sci. 2025, 26, 10518. https://doi.org/10.3390/ijms262110518
Sakiyo JJ, Németh Á. Investigation on Applying Cyclodextrins in a Fermentation Process for Enhanced Biosurfactant Production by Bacillus licheniformis. International Journal of Molecular Sciences. 2025; 26(21):10518. https://doi.org/10.3390/ijms262110518
Chicago/Turabian StyleSakiyo, Jesse John, and Áron Németh. 2025. "Investigation on Applying Cyclodextrins in a Fermentation Process for Enhanced Biosurfactant Production by Bacillus licheniformis" International Journal of Molecular Sciences 26, no. 21: 10518. https://doi.org/10.3390/ijms262110518
APA StyleSakiyo, J. J., & Németh, Á. (2025). Investigation on Applying Cyclodextrins in a Fermentation Process for Enhanced Biosurfactant Production by Bacillus licheniformis. International Journal of Molecular Sciences, 26(21), 10518. https://doi.org/10.3390/ijms262110518








