Analysis of the Circulating miRNome Expression Profile in Saliva Samples After Neoadjuvant Chemoradiotherapy in a Rectal Cancer Study Population Using Next-Generation Sequencing
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Cohort
2.2. General Description of Sequencing Results
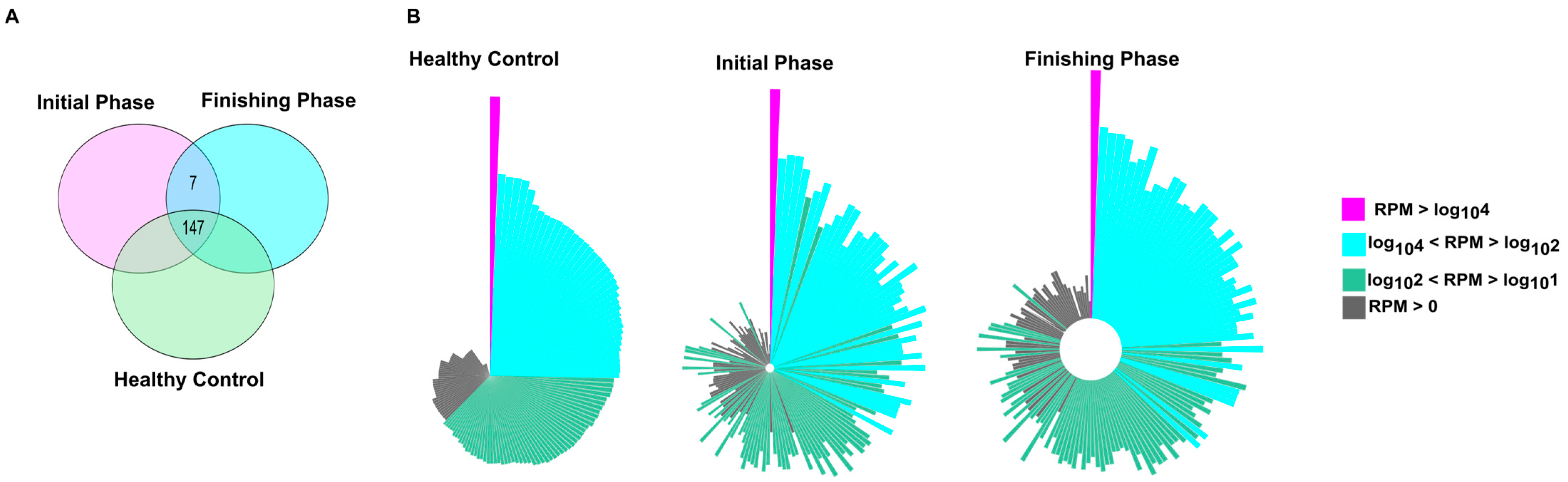
2.3. The Quantitative Evaluation of Small Non-Coding RNA Transcriptome Analyses Highlighted Both Overlapping and Cohort-Specific miRNAs
2.4. miRNA Expression Profiling in Rectal Cancer: Differential Signatures Associated with Disease Status and Radiotherapy Response
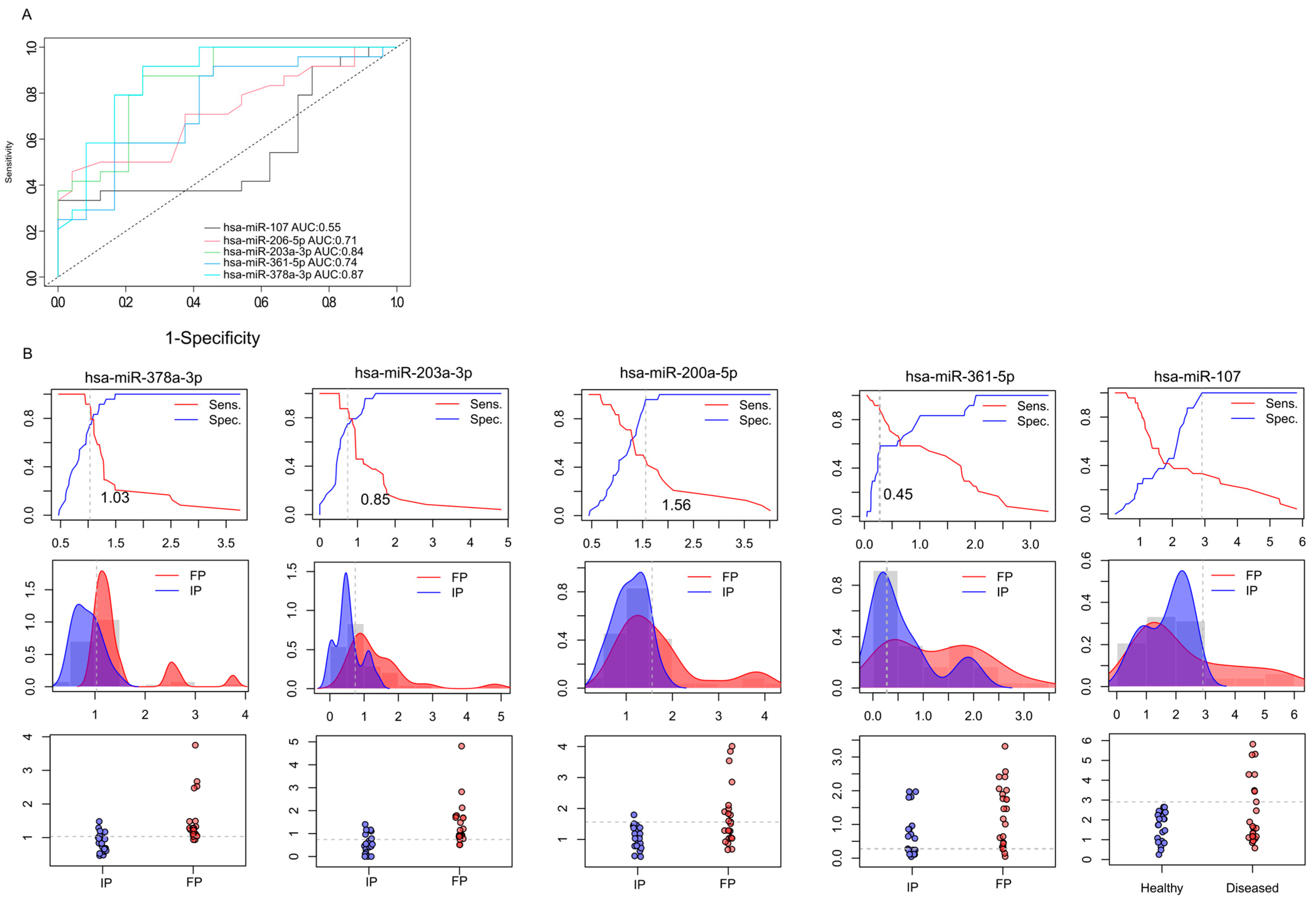
2.5. Validation of Differentially Expressed miRNAs by RT-qPCR (Reverse Transcription Quantitative Polymerase Chain Reaction)
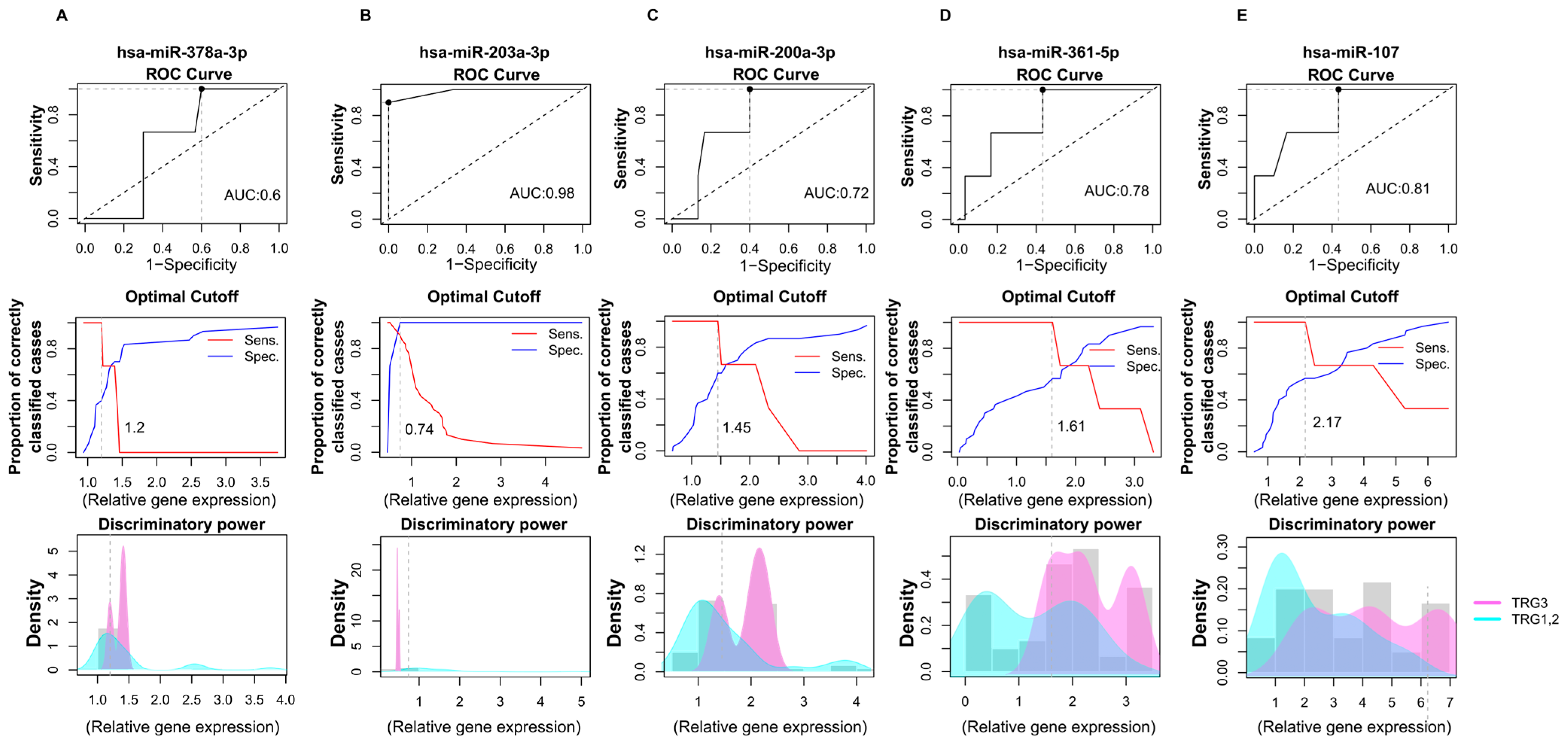
2.6. Prognostic Performance of miRNA Biomarkers from Saliva Samples Derived from Colon Cancer Patients
2.7. Biological Functions Affected by Dysregulated miRNAs in CR Patients
3. Discussion
4. Materials and Methods
4.1. Clinical Methods and Study Cohort
4.2. Sampling and RNA Extraction
4.3. Library Preparation and Sequencing
4.4. Bioinformatic Analyses
4.5. Validation of miRNA-Seq Data by RT-qPCR
4.6. Interaction Network Determination from KEGG and miRNA Database
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zavoral, M.; Suchanek, S.; Zavada, F.; Dusek, L.; Muzik, J.; Seifert, B.; Fric, P. Colorectal cancer screening in Europe. World J. Gastroenterol. 2009, 15, 5907–5915. [Google Scholar] [CrossRef]
- Döbrôssy, L.; Kovács, A.; Budai, A.; Cornides, A.; Ottó, S.; Tulassay, Z. The state of the colorectal screening in Hungary: Lessons of the pilot programs. Orvosi Hetil. 2007, 148, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, S. Colorectal cancer: Local results and significance in Hungary. J. Gastrointest. Oncol. 2024, 15, 2552–2577. [Google Scholar] [CrossRef]
- Wiegering, A.; Isbert, C.; Dietz, U.A.; Kunzmann, V.; Ackermann, S.; Kerscher, A.; Maeder, U.; Flentje, M.; Schlegel, N.; Reibetanz, J.; et al. Multimodal therapy in treatment of rectal cancer is associated with improved survival and reduced local recurrence—A retrospective analysis over two decades. BMC Cancer 2014, 14, 816. [Google Scholar] [CrossRef] [PubMed]
- Siebenhüner, A.R.; Güller, U.; Warschkow, R. Population-based SEER analysis of survival in colorectal cancer patients with or without resection of lung and liver metastases. BMC Cancer 2020, 20, 246. [Google Scholar] [CrossRef]
- O’Brien, T.; Hospers, G.; Conroy, T.; Lenz, H.J.; Smith, J.J.; Andrews, E.; O’nEill, B.; Leonard, G. The role of total neoadjuvant therapy in locally advanced rectal cancer: A survey of specialists attending the All-Ireland Colorectal Cancer Conference 2022 including lead investigators of OPRA, PRODIGE-23 and RAPIDO. Ir. J. Med. Sci. 2024, 193, 1183–1190. [Google Scholar] [CrossRef]
- Ochiai, K.; Bhutiani, N.; Ikeda, A.; Uppal, A.; White, M.G.; Peacock, O.; Messick, C.A.; Bednarski, B.K.; You, Y.-Q.N.; Skibber, J.M.; et al. Total Neoadjuvant Therapy for Rectal Cancer: Which Regimens to Use? Cancers 2024, 16, 2093. [Google Scholar] [CrossRef]
- Wo, J.Y.; Ashman, J.B.; Bhadkamkar, N.A.; Bradfield, L.; Chang, D.T.; Hanna, N.; Hawkins, M.; Holtz, M.; Kim, E.; Kelly, P.; et al. Radiation Therapy for Rectal Cancer: An ASTRO Clinical Practice Guideline Focused Update. Pract. Radiat. Oncol. 2025, 15, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Anker, C.J.; Ashman, J.B.; Bhadkamkar, N.A.; Bradfield, L.; Chang, D.T.; Dorth, J.; Garcia-Aguilar, J.; Goff, D.; Jacqmin, D.; et al. Radiation Therapy for Rectal Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2021, 11, 13–25. [Google Scholar] [CrossRef]
- Hanna, C.R.; Slevin, F.; Appelt, A.; Beavon, M.; Adams, R.; Arthur, C.; Beasley, M.; Duffton, A.; Gilbert, A.; Gollins, S.; et al. Intensity-modulated Radiotherapy for Rectal Cancer in the UK in 2020. Clin. Oncol. 2021, 33, 214–223. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, R.; Ke, Z.; Xia, F.; Li, G.; Wan, J.; Zhang, H.; Deng, Y.; Zhang, Z.; Wang, Y.; et al. Total neoadjuvant therapy in high-risk rectal cancer: Organ preservation and survival outcomes in a single-center retrospective cohort. Ther. Adv. Med. Oncol. 2025, 17, 17588359251332466. [Google Scholar] [CrossRef]
- Ma, Z.; Tan, L.; Liu, Z.L.; Xiao, J.W. Total neoadjuvant therapy or standard chemoradiotherapy for locally advanced rectal cancer: A systematic review and meta-analysis. Front. Surg. 2022, 9, 911538. [Google Scholar] [CrossRef]
- Sobral, D.; Martins, M.; Kaplan, S.; Golkaram, M.; Salmans, M.; Khan, N.; Vijayaraghavan, R.; Casimiro, S.; Fernandes, A.; Borralho, P.; et al. Genetic and microenvironmental intra-tumor heterogeneity impacts colorectal cancer evolution and metastatic development. Commun. Biol. 2022, 5, 937. [Google Scholar] [CrossRef] [PubMed]
- Saoudi González, N.; Salvà, F.; Ros, J.; Baraibar, I.; Rodríguez-Castells, M.; García, A.; Alcaráz, A.; Vega, S.; Bueno, S.; Tabernero, J.; et al. Unravelling the Complexity of Colorectal Cancer: Heterogeneity, Clonal Evolution, and Clinical Implications. Cancers 2023, 15, 4020. [Google Scholar] [CrossRef]
- Valdeolivas, A.; Amberg, B.; Giroud, N.; Richardson, M.; Gálvez, E.J.C.; Badillo, S.; Julien-Laferrière, A.; Túrós, D.; von Voithenberg, L.V.; Wells, I.; et al. Profiling the heterogeneity of colorectal cancer consensus molecular subtypes using spatial transcriptomics. npj Precis. Oncol. 2024, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.T.D.; Tagliaferri, P.; Tassone, P. MicroRNA in cancer therapy: Breakthroughs and challenges in early clinical applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef]
- Lindholm, E.M.; Aure, M.R.; Haugen, M.H.; Sahlberg, K.K.; Kristensen, V.N.; Nebdal, D.; Børresen-Dale, A.-L.; Lingjaerde, O.C.; Engebraaten, O. miRNA expression changes during the course of neoadjuvant bevacizumab and chemotherapy treatment in breast cancer. Mol. Oncol. 2019, 13, 2278–2296. [Google Scholar] [CrossRef] [PubMed]
- Hummel, R.; Sie, C.; Watson, D.I.; Wang, T.; Ansar, A.; Michael, M.Z.; Van der Hoek, M.; Haier, J.; Hussey, D.J. MicroRNA signatures in chemotherapy resistant esophageal cancer cell lines. World J. Gastroenterol. 2014, 20, 14904–14912. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Yu, J.; Chan, M.T.V.; Wu, W.K.K. MicroRNAs predict and modulate responses to chemotherapy in colorectal cancer. Cell Prolif. 2015, 48, 503–510. [Google Scholar] [CrossRef]
- Valenzuela, G.; Contreras, H.R.; Marcelain, K.; Burotto, M.; González-Montero, J. Understanding microRNA-Mediated Chemoresistance in Colorectal Cancer Treatment. Int. J. Mol. Sci. 2025, 26, 1168. [Google Scholar] [CrossRef]
- Badr, D.; Fouad, M.A.; Hussein, M.; Salem, S.; Zekri, A.; Shouman, S. Rebound increase in microRNA levels at the end of 5-FU-based therapy in colorectal cancer patients. Sci. Rep. 2023, 13, 14237. [Google Scholar] [CrossRef]
- Huang, C.-M.; Huang, M.-Y.; Chen, Y.-C.; Chen, P.-J.; Su, W.-C.; Chang, T.-K.; Li, C.-C.; Huang, C.-W.; Tsai, H.-L.; Wang, J.-Y. miRNA-148a Enhances the Treatment Response of Patients with Rectal Cancer to Chemoradiation and Promotes Apoptosis by Directly Targeting c-Met. Biomedicines 2021, 9, 1371. [Google Scholar] [CrossRef]
- Ahmad, P.; Sana, J.; Slavik, M.; Slampa, P.; Smilek, P.; Slaby, O. MicroRNAs Involvement in Radioresistance of Head and Neck Cancer. Dis. Markers 2017, 2017, 8245345. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, H.T.; Nguyen, X.H.; Do, X.H.; Mai, B.T.; Ngoc Nguyen, H.T.; Than, U.T.T.; Nguyen, T.-H. Regulation and therapeutic potentials of microRNAs to non-small cell lung cancer. Heliyon 2023, 9, e22080. [Google Scholar] [CrossRef] [PubMed]
- Imedio, L.; Cristóbal, I.; Rubio, J.; Santos, A.; Rojo, F.; García-Foncillas, J. MicroRNAs in Rectal Cancer: Functional Significance and Promising Therapeutic Value. Cancers 2020, 12, 2040. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, X.; Li, Z.; Chen, X.; Zhao, J. miR-152-3p facilitates cell adhesion and hepatic metastases in colorectal cancer via targeting AQP11. Pathol. Res. Pract. 2023, 244, 154389. [Google Scholar] [CrossRef]
- Quillet, A.; Saad, C.; Ferry, G.; Anouar, Y.; Vergne, N.; Lecroq, T.; Dubessy, C. Improving Bioinformatics Prediction of microRNA Targets by Ranks Aggregation. Front. Genet. 2019, 10, 1330. [Google Scholar] [CrossRef]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [PubMed]
- Nonaka, T.; Wong, D.T.W. Saliva-Exosomics in Cancer: Molecular Characterization of Cancer-Derived Exosomes in Saliva. Enzymes 2017, 42, 125–151. [Google Scholar]
- Nonaka, T.; Wong, D.T.W. Saliva diagnostics: Salivaomics, saliva exosomics, and saliva liquid biopsy. J. Am. Dent. Assoc. 2023, 154, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Apweiler, R.; Balgley, B.M.; Boontheung, P.; Bundy, J.L.; Cargile, B.J.; Cole, S.; Fang, X.; Gonzalez-Begne, M.; Griffin, T.J.; et al. Systematic comparison of the human saliva and plasma proteomes. Proteom. Clin. Appl. 2009, 3, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Schwab, S.; Nonaka, T. Circulating miRNAs as liquid biopsy biomarkers for diagnosis in patients with colorectal cancer: A systematic review and meta-analysis. Front. Genet. 2025, 16, 1574586. [Google Scholar] [CrossRef]
- Falzone, L.; Scola, L.; Zanghì, A.; Biondi, A.; Di Cataldo, A.; Libra, M.; Candido, S. Integrated analysis of colorectal cancer microRNA datasets: Identification of microRNAs associated with tumor development. Aging 2018, 10, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, S.; Zhen, T.; Shi, H.; Zhang, F.; Yang, Y.; Kang, L.; Liang, Y.; Han, A. Clinical and biological significance of miR-378a-3p and miR-378a-5p in colorectal cancer. Eur. J. Cancer 2014, 50, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.; Brzozowska, A.; Małecka-Massalska, T.; Powrózek, T. Plasma Circulating lncRNAs: MALAT1 and NEAT1 as Biomarkers of Radiation-Induced Adverse Effects in Laryngeal Cancer Patients. Diagnostics 2025, 15, 676. [Google Scholar] [CrossRef]
- Castellani, G.; Buccarelli, M.; Lulli, V.; Ilari, R.; De Luca, G.; Pedini, F.; Boe, A.; Felli, N.; Biffoni, M.; Pilozzi, E.; et al. MiR-378a-3p Acts as a Tumor Suppressor in Colorectal Cancer Stem-Like Cells and Affects the Expression of MALAT1 and NEAT1 lncRNAs. Front. Oncol. 2022, 12, 867886. [Google Scholar] [CrossRef]
- Qian, Z.; Gong, L.; Mou, Y.; Han, Y.; Zheng, S. MicroRNA-203a-3p is a candidate tumor suppressor that targets thrombospondin 2 in colorectal carcinoma. Oncol. Rep. 2019, 42, 1825–1832. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Yang, Y.; Luo, M.; Zhang, M.; Wang, X.; Liu, L.; Hou, N.; Guo, Q.; Song, T.; et al. MiR-99b-5p and miR-203a-3p Function as Tumor Suppressors by Targeting IGF-1R in Gastric Cancer. Sci. Rep. 2018, 8, 10119, Correction to Sci. Rep. 2023, 13, 906. [Google Scholar] [CrossRef]
- Chen, L.; Gao, H.; Liang, J.; Qiao, J.; Duan, J.; Shi, H.; Zhen, T.; Li, H.; Zhang, F.; Zhu, Z.; et al. miR-203a-3p promotes colorectal cancer proliferation and migration by targeting PDE4D. Am. J. Cancer Res. 2018, 8, 2387–2401. [Google Scholar]
- Pichler, M.; Ress, A.L.; Winter, E.; Stiegelbauer, V.; Karbiener, M.; Schwarzenbacher, D.; Scheideler, M.; Ivan, C.; Jahn, S.W.; Kiesslich, T.; et al. MiR-200a regulates epithelial to mesenchymal transition-related gene expression and determines prognosis in colorectal cancer patients. Br. J. Cancer 2014, 110, 1614–1621. [Google Scholar] [CrossRef]
- Ono, H.; Imoto, I.; Kozaki, K.; Tsuda, H.; Matsui, T.; Kurasawa, Y.; Muramatsu, T.; Sugihara, K.; Inazawa, J. SIX1 promotes epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation. Oncogene 2012, 31, 4923–4934. [Google Scholar] [CrossRef]
- Ma, F.; Song, H.; Guo, B.; Zhang, Y.; Zheng, Y.; Lin, C.; Wu, Y.; Guan, G.; Sha, R.; Zhou, Q.; et al. MiR-361-5p inhibits colorectal and gastric cancer growth and metastasis by targeting staphylococcal nuclease domain containing-1. Oncotarget 2015, 6, 17404–17416. [Google Scholar] [CrossRef]
- Cao, Z.G.; Huang, Y.N.; Yao, L.; Liu, Y.R.; Hu, X.; Hou, Y.F.; Shao, Z.-M. Positive expression of miR-361-5p indicates better prognosis for breast cancer patients. J. Thorac. Dis. 2016, 8, 1772–1779. [Google Scholar] [CrossRef]
- Othman, N.; Nagoor, N.H. Overexpression of miR-361-5p plays an oncogenic role in human lung adenocarcinoma through the regulation of SMAD2. Int. J. Oncol. 2019, 54, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; He, P. miR-361-5p regulates ovarian cancer cell proliferation and apoptosis by targeting TRAF3. Exp. Ther. Med. 2021, 21, 199. [Google Scholar] [CrossRef]
- Yin, L.C.; Xiao, G.; Zhou, R.; Huang, X.P.; Li, N.L.; Tan, C.L.; Xie, F.-J.; Weng, J.; Liu, L.-X. MicroRNA-361-5p Inhibits Tumorigenesis and the EMT of HCC by Targeting Twist1. BioMed Res. Int. 2020, 2020, 8891876. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tao, T.; Xu, B.; Chen, S.; Liu, C.; Zhang, L.; Lu, K.; Huang, Y.; Jiang, L.; Zhang, X.; et al. MiR-361-5p acts as a tumor suppressor in prostate cancer by targeting signal transducer and activator of transcription-6(STAT6). Biochem. Biophys. Res. Commun. 2014, 445, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.A.; Li, C.C.; Lin, Y.J.; Wang, T.F.; Chen, M.C.; Su, Y.H.; Yeh, Y.-L.; Padma, V.V.; Liao, P.-H.; Huang, C.-Y. Hsa-miR-107 regulates chemosensitivity and inhibits tumor growth in hepatocellular carcinoma cells. Aging 2021, 13, 12046–12057. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, D.; Hou, L.; Wang, Y.; Huang, X.; Zhou, C.; Zhu, L.; Wang, Y.; Li, L.; Gu, Y.; et al. MiR-107 Confers Chemoresistance to Colorectal Cancer by Targeting Calcium-Binding Protein 39. Br. J. Cancer 2020, 122, 705–714. [Google Scholar] [CrossRef]
- Lo, H.C.; Hsu, J.H.; Lai, L.C.; Tsai, M.H.; Chuang, E.Y. MicroRNA-107 enhances radiosensitivity by suppressing granulin in PC-3 prostate cancer cells. Sci. Rep. 2020, 10, 14584. [Google Scholar] [CrossRef]
- Feng, L.; Xie, Y.; Zhang, H.; Wu, Y. miR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cells. Med. Oncol. 2012, 29, 856–863. [Google Scholar] [CrossRef]
- Moya, L.; Meijer, J.; Schubert, S.; Matin, F.; Batra, J. Assessment of miR-98-5p, miR-152-3p, miR-326 and miR-4289 Expression as Biomarker for Prostate Cancer Diagnosis. Int. J. Mol. Sci. 2019, 20, 1154. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Qin, F.; Dai, S. miR-152 as a tumor suppressor microRNA: Target recognition and regulation in cancer. Oncol. Lett. 2016, 11, 3911–3916. [Google Scholar] [CrossRef] [PubMed]
- Khalighfard, S.; Kalhori, M.R.; Amiriani, T.; Poorkhani, A.; Khori, V.; Esmati, E.; Lashkari, M.; Najafi, A.; Alizadeh, A.M. A systematic approach introduced novel targets in rectal cancer by considering miRNA/mRNA interactions in response to radiotherapy. Cancer Biomark. 2022, 33, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Kiener, M.; Chen, L.; Krebs, M.; Grosjean, J.; Klima, I.; Kalogirou, C.; Riedmiller, H.; Kneitz, B.; Thalmann, G.N.; Snaar-Jagalska, E.; et al. miR-221-5p regulates proliferation and migration in human prostate cancer cells and reduces tumor growth in vivo. BMC Cancer 2019, 19, 627. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.M.; Gay, H.; Jackson, A.; Tucker, S.L.; Deasy, J.O. Radiation dose-volume effects in radiation-induced rectal injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. S3), S123–S129, Erratum in Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1185. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]



| Patient ID | Gender | Age | Initial Clinical Stage | After Neoadjuvant Therapy Clinical Stage | After Surgery Pathological Stage | Grade | Modified Ryan Scheme TRG | Localization |
|---|---|---|---|---|---|---|---|---|
| 58511 | Female | 66 | T3bN2 | T2N1 | ypT3ypN1a | G1 | TRG 1 | upper |
| 66921 | Male | 68 | T3cN2 | T3bN0 | ypT3ypN0 | G2 | TRG 1 | middle |
| 66935 | Female | 62 | T3cN1 | T3bN0 | ypT3ypN1a | G2 | TRG 3 | middle |
| 67028 | Male | 62 | T1N1 | T1N1 | ypT1ypN0 | G2 | TRG 2 | lower |
| 67035 | Male | 50 | T2N1 | T1N0 | ypT2ypN0 | G2 | TRG 2 | lower |
| 67558 | Male | 70 | T4N2 | T2N0 | ypT2ypN0 | G2 | TRG 2 | lower |
| 67594 | Female | 58 | T3bN1 | T3bN0 | ypT3ypN0 | G2 | TRG 2 | middle |
| 67673 | Female | 70 | T3bN2 | T3bN1 | ypT3ypN1a | G2 | TRG 2 | upper |
| 67858 | Male | 70 | T3cN2 | T2N0 | ypT2ypN0 | G2 | TRG 2 | lower |
| 67892 | Male | 71 | T3cN2 | T3bN0 | ypT3ypN0 | G2 | TRG 2 | lower |
| 67898 | Female | 72 | T3bN2 | T3bN0 | yPT3ypN0 | G2 | TRG 2 | middle |
| Clinical Parameters (Patients n = 11) | ||
|---|---|---|
| Gender, n (%) | ||
| Male | 6 (54.54%) | |
| Female | 5 (45.46%) | |
| Age, median | 68 | |
| G1, n (%) | 1 (9.09%) | |
| G2, n (%) | 10 (90.91%) | |
| Location, n (%) | ||
| upper | 2 (18.18%) | |
| middle | 4 (36.36%) | |
| lower | 5 (45.46%) | |
| cT, n (%) | ||
| 1 | 1 (9.09%) | |
| 2 | 1 (9.09%) | |
| 3 | 8 (72.73%) | |
| 4 | 1 (9.09%) | |
| cN, n (%) | ||
| 1 | 4 (36.36%) | |
| 2 | 7 (63.64%) | |
| TRG (modified Ryan scheme), n (%) | ||
| 1 | 2 (18.18%) | |
| 2 | 8 (72.73%) | |
| 3 | 1 (9.09%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gál, K.; Dávid, P.; Paholcsek, M.; Barabás, M.; Szilágyi, E.; Balogh, K.; Solymosi, D.; Miklós, S.; Mikáczó, J.; Trási, K.; et al. Analysis of the Circulating miRNome Expression Profile in Saliva Samples After Neoadjuvant Chemoradiotherapy in a Rectal Cancer Study Population Using Next-Generation Sequencing. Int. J. Mol. Sci. 2025, 26, 10506. https://doi.org/10.3390/ijms262110506
Gál K, Dávid P, Paholcsek M, Barabás M, Szilágyi E, Balogh K, Solymosi D, Miklós S, Mikáczó J, Trási K, et al. Analysis of the Circulating miRNome Expression Profile in Saliva Samples After Neoadjuvant Chemoradiotherapy in a Rectal Cancer Study Population Using Next-Generation Sequencing. International Journal of Molecular Sciences. 2025; 26(21):10506. https://doi.org/10.3390/ijms262110506
Chicago/Turabian StyleGál, Kristóf, Péter Dávid, Melinda Paholcsek, Márton Barabás, Endre Szilágyi, Krisztina Balogh, Dóra Solymosi, Szidónia Miklós, Johanna Mikáczó, Krisztina Trási, and et al. 2025. "Analysis of the Circulating miRNome Expression Profile in Saliva Samples After Neoadjuvant Chemoradiotherapy in a Rectal Cancer Study Population Using Next-Generation Sequencing" International Journal of Molecular Sciences 26, no. 21: 10506. https://doi.org/10.3390/ijms262110506
APA StyleGál, K., Dávid, P., Paholcsek, M., Barabás, M., Szilágyi, E., Balogh, K., Solymosi, D., Miklós, S., Mikáczó, J., Trási, K., Csiki, E., Simon, M., Fauszt, P., Póliska, S., Remenyik, J., Kovács, Á., & Szilágyi-Tolnai, E. (2025). Analysis of the Circulating miRNome Expression Profile in Saliva Samples After Neoadjuvant Chemoradiotherapy in a Rectal Cancer Study Population Using Next-Generation Sequencing. International Journal of Molecular Sciences, 26(21), 10506. https://doi.org/10.3390/ijms262110506








