Long Noncoding RNA Lnc-MTPAP-1 Overexpressed by Particulate Matter Suppresses Apoptosis in Non-Small Cell Lung Cancer (NSCLC) Cells
Abstract
1. Introduction
2. Results
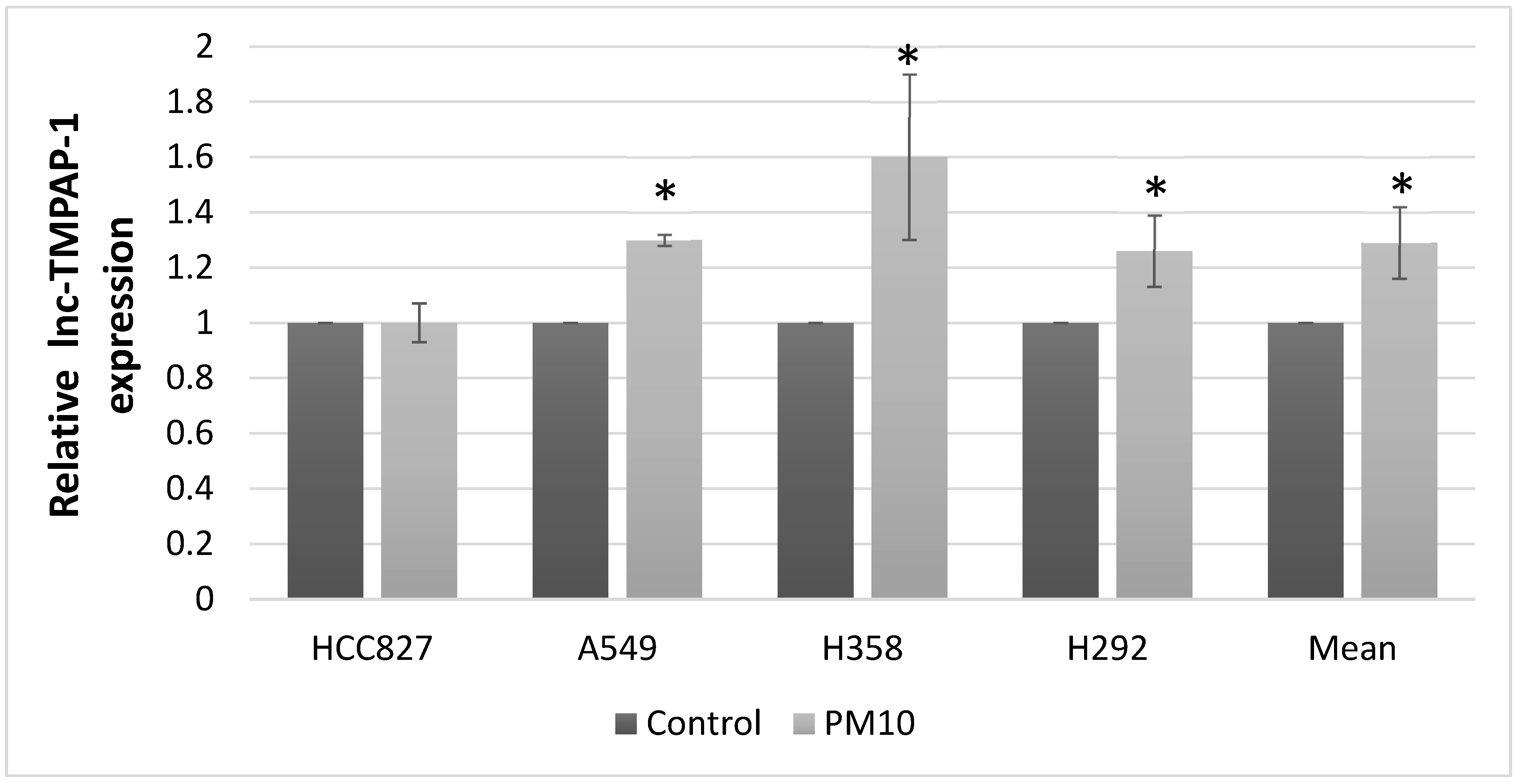
2.1. Lnc-MTPAP-1 Is Overexpressed in Lung Cancer Cell Lines After PM10 Exposure
2.2. Screening for Function of Lnc-MTPAP-1 Using Mini-FACS
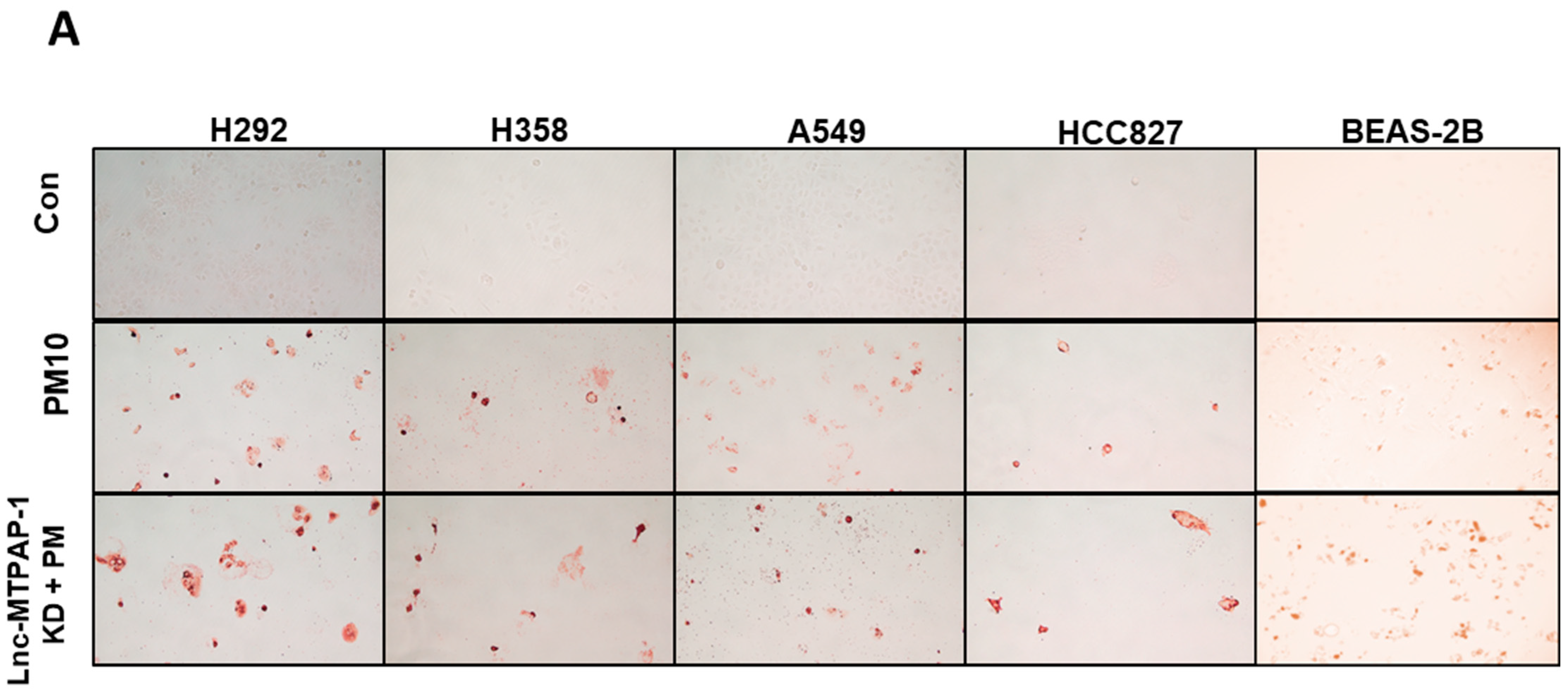
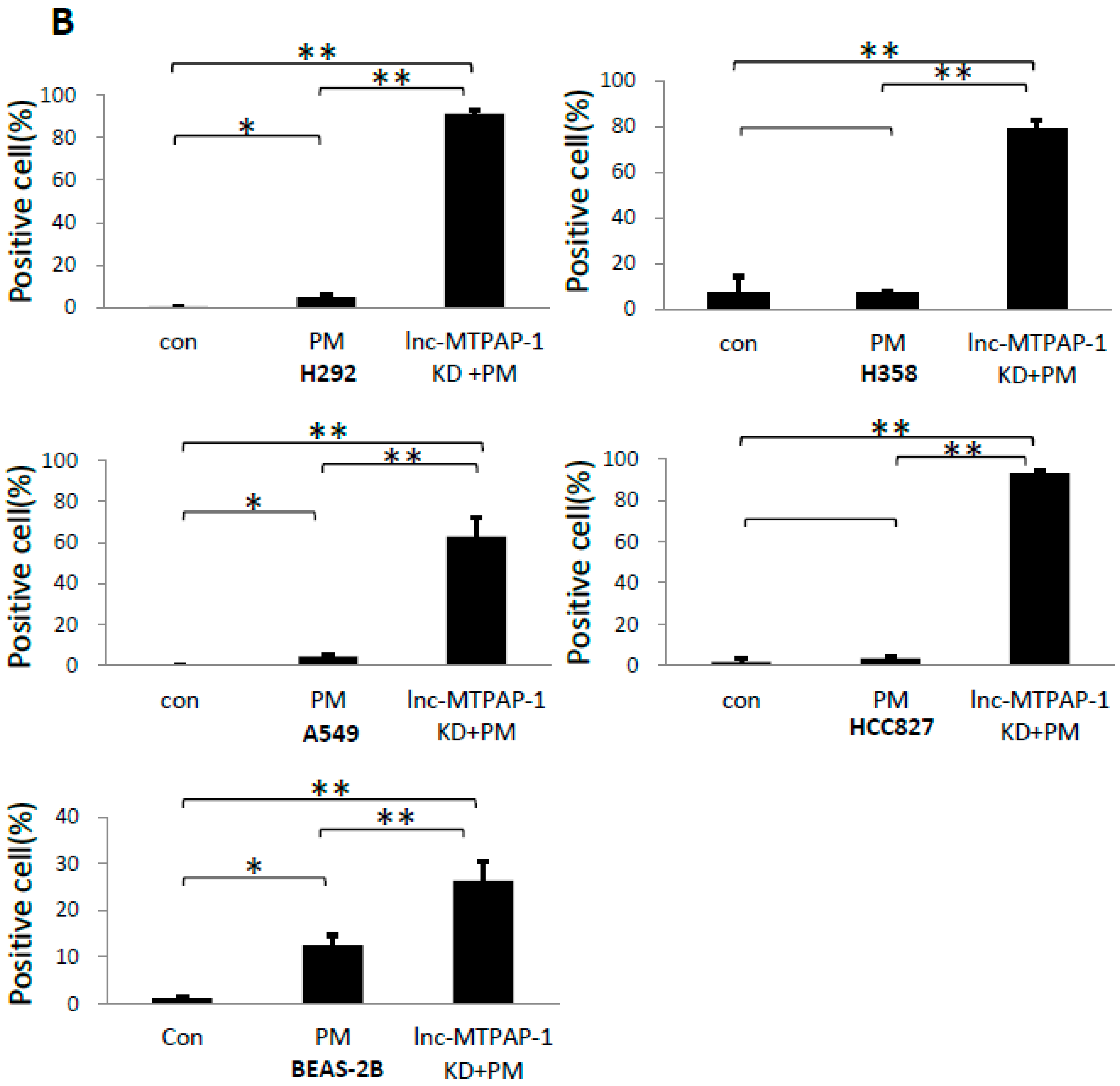
2.3. Lnc-MTPAP-1 Knockdown Induces Apoptosis in Lung Cancer Cell Lines
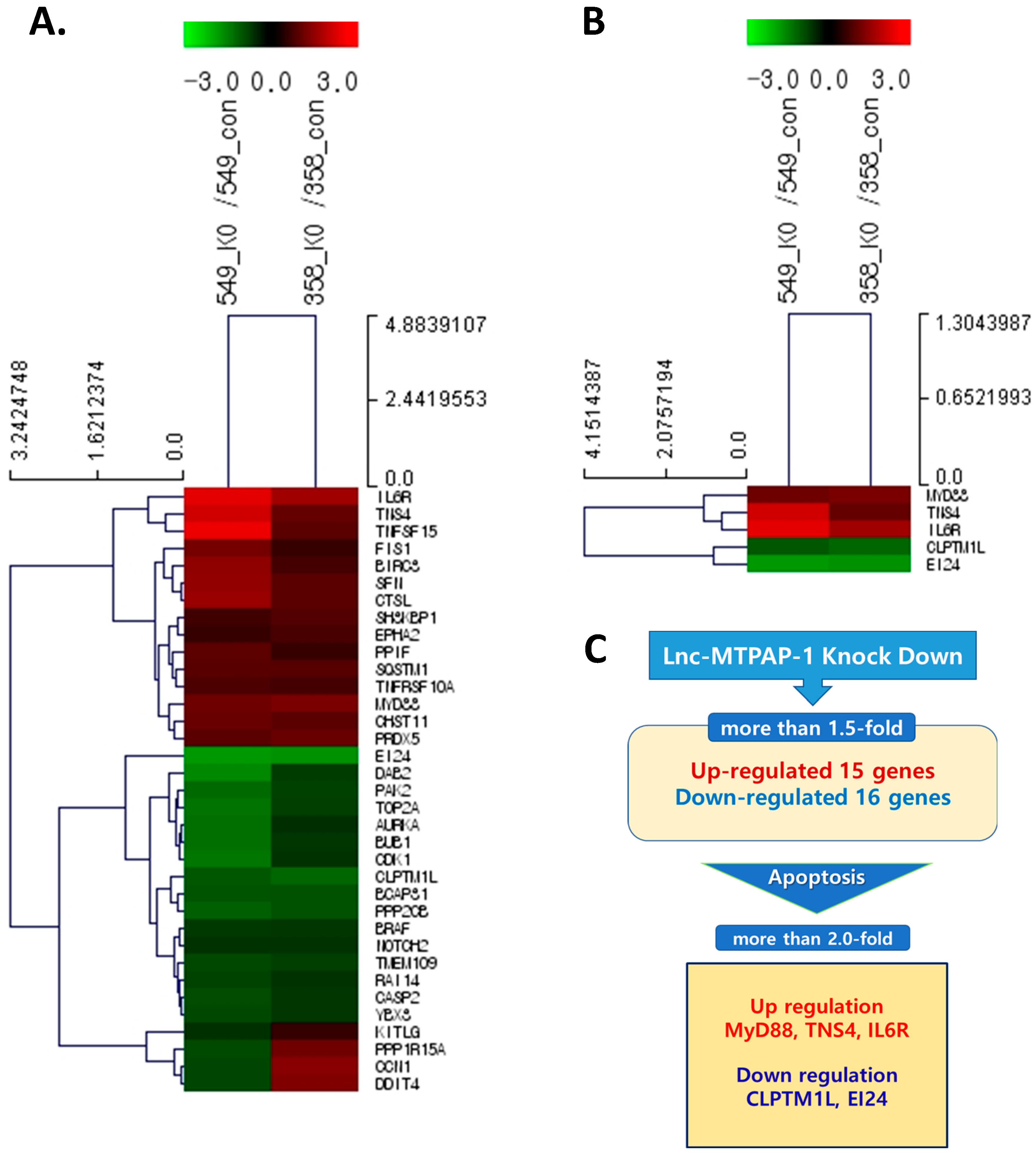
2.4. Search for Target Gene of Lnc-MTPAP-1 Using Next Generation Sequencing (NGS)
2.5. Validation of the Relative Expression of Apoptosis-Related Target Genes of Lnc-MTPAP-1
3. Discussion
4. Materials and Methods
4.1. Cell Culture and PM10 Treatment
4.2. Microarray Analysis
4.2.1. Amplification and Hybridization
4.2.2. Signal Analysis
4.3. siRNA-Mediated Knockdown of Lnc-MTPAP-1 in Lung Cancer Cells
4.3.1. Cell Plating and LncMTPAP−1 Gene Knockdown
4.3.2. RNA Isolation
4.3.3. qPCR Analysis of LncRNA
4.4. Transferase-Mediated dUTP Nick End-Labeling (TUNEL) Staining
4.5. Next-Generation Sequencing After Knockdown of Lnc-MTPAP-1
4.5.1. Library Preparation and Sequencing
4.5.2. Data Analysis
4.6. qPCR of mRNA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Liu, D.; Lu, X.; Huang, W.; Zhuang, W. Long non-coding RNAs in non-small cell lung cancer: Implications for EGFR-TKI resistance. Front. Genet. 2023, 14, 1222059. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Liu, T.; Liu, Y.-Y.; He, Y.; Liu, T.; Xu, Z.; Wang, Z.-W.; Luo, F. Long Non-Coding RNAs in Lung Cancer: The Role in Tumor Microenvironment. Front. Cell Dev. Biol. 2022, 9, 795874. [Google Scholar] [CrossRef] [PubMed]
- Gencel-Augusto, J.; Wu, W.; Bivona, T.G. Long Non-Coding RNAs as Emerging Targets in Lung Cancer. Cancers 2023, 15, 3135. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R. Non-coding RNA: It’s Not Junk. Dig. Dis. Sci. 2017, 62, 1107–1109, Erratum in Dig. Dis. Sci. 2017, 62, 3260. https://doi.org/10.1007/s10620-017-4746-0. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278, Erratum in Dig. Dis. Sci. 2017, 62, 3260. https://doi.org/10.1007/s10620-017-4746-0. [Google Scholar] [CrossRef]
- Cao, Z.; Oyang, L.; Luo, X.; Xia, L.; Hu, J.; Lin, J.; Tan, S.; Tang, Y.; Zhou, Y.; Cao, D.; et al. The roles of long non-coding RNAs in lung cancer. J. Cancer 2022, 13, 174–183. [Google Scholar] [CrossRef]
- Feng, F.; Jiao, P.; Wang, J.; Li, Y.; Bao, B.; Luoreng, Z.; Wang, X. Role of Long Noncoding RNAs in the Regulation of Cellular Immune Response and Inflammatory Diseases. Cells 2022, 11, 3642. [Google Scholar] [CrossRef]
- Gutti, R.K.; Sangeeth, A.; Malleswarapu, M.; Mishra, A. Long Non-coding RNA Therapeutics: Recent Advances and Challenges. Curr. Drug Targets 2022, 23, 1457–1464. [Google Scholar] [CrossRef]
- World Health Organization (WHO). List of Classifications Agents Classified by the IARC Monographs. 2024. Available online: https://monographs.iarc.who.int/list-of classifications (accessed on 1 January 2024).
- Wang, T.; Huang, K.; Chen, C.; Chang, Y.; Chen, H.; Hsueh, C.; Liu, Y.; Yang, S.; Yang, P. PM2.5 promotes lung cancer progression through activation of the AhR-TMPRSS2-IL18 pathway. EMBO Mol. Med. 2023, 15, e17014. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-J.; Ho, T.-L.; Chao, C.-C.; He, X.-Y.; Chen, P.-C.; Cheng, F.-J.; Huang, W.-C.; Huang, C.-L.; Liu, P.-I.; Tang, C.-H. Particulate matter facilitates amphiregulin-dependent lung cancer proliferation through glutamine metabolism. Int. J. Biol. Sci. 2024, 20, 3126–3139. [Google Scholar] [CrossRef] [PubMed]
- Aghaei-Zarch, S.M.; Alipourfard, I.; Rasoulzadeh, H.; Najafi, S.; Aghaei-Zarch, F.; Partov, S.; Movafagh, A.; Jahanara, A.; Toolabi, A.; Sheikhmohammadi, A.; et al. Non-coding RNAs: An emerging player in particulate matter 2.5-mediated toxicity. Int. J. Biol. Macromol. 2023, 235, 123790. [Google Scholar] [CrossRef]
- Das, S.; Samaddar, S. Recent Advances in the Clinical Translation of Small-Cell Lung Cancer Therapeutics. Cancers 2025, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Bräuner, E.V.; Bønløkke, J.H.; Andersen, Z.J. Particulate matter air pollution as a cause of lung cancer: Epidemiological and experimental evidence. Br. J. Cancer 2025, 132, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Rinaldi, E.; Ferrari, L.; Caimi, M.; Pedersini, R.; Ferrari, V.D.; Dominoni, F.; Marini, M.; Ardighieri, L.; Berruti, A.; et al. 167P Five-years incidence of small-cell lung cancer and analysis of PM2.5 in Brescia, Italy. J. Thorac. Oncol. 2023, 18, S1204. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Ambient(outdoor) Air Pollution. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-%28outdoor%29-air-quality-and-health (accessed on 1 January 2024).
- Misiukiewicz-Stepien, P.; Paplinska-Goryca, M. Biological effect of PM10 on airway epithelium-focus on obstructive lung diseases. Clin. Immunol. 2021, 227, 108754. [Google Scholar] [CrossRef] [PubMed]
- Kyung, S.Y.; Jeong, S.H. Particulate-Matter Related Respiratory Diseases. Tuberc. Respir. Dis. 2020, 83, 116–121. [Google Scholar] [CrossRef]
- Fajersztajn, L.; Veras, M.; Barrozo, L.V.; Saldiva, P. Air pollution: A potentially modifiable risk factor for lung cancer. Nat. Rev. Cancer 2013, 13, 674–678. [Google Scholar] [CrossRef] [PubMed]
- García-Cuellar, C.M.; Santibáñez-Andrade, M.; Chirino, Y.I.; Quintana-Belmares, R.; Morales-Bárcenas, R.; Quezada-Maldonado, E.M.; Sánchez-Pérez, Y. Particulate Matter (PM10) Promotes Cell Invasion through Epithelial-Mesenchymal Transition (EMT) by TGF-β Activation in A549 Lung Cells. Int. J. Mol. Sci. 2021, 22, 12632. [Google Scholar] [CrossRef]
- Santibáñez-Andrade, M.; Sánchez-Pérez, Y.; Chirino, Y.I.; Morales-Bárcenas, R.; García-Cuellar, C.M. Long non-coding RNA NORAD upregulation induced by airborne particulate matter (PM10) exposure leads to aneuploidy in A549 lung cells. Chemosphere 2021, 266, 128994. [Google Scholar] [CrossRef]
- Santibáñez-Andrade, M.; Sánchez-Pérez, Y.; Chirino, Y.I.; Morales-Bárcenas, R.; Quintana-Belmares, R.; García-Cuellar, C.M. Particulate matter (PM10) destabilizes mitotic spindle through downregulation of SETD2 in A549 lung cancer cells. Chemosphere 2022, 295, 133900. [Google Scholar] [CrossRef]
- Ni, Z.; Tao, K.; Chen, G.; Chen, Q.; Tang, J.; Luo, X.; Yin, P.; Tang, J.; Wang, X.; Roemer, K. CLPTM1L Is Overexpressed in Lung Cancer and Associated with Apoptosis. PLoS ONE 2012, 7, e52598. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Chen, J.; Teng, J. EI24 promotes cell adaption to ER stress by coordinating IRE1 signaling and calcium homeostasis. Embo Rep. 2022, 23, e51679. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Ma, J.; Yang, W.; Cao, L.; Wang, X.; Niu, L.; Li, Y.; Zhou, W.; Zhang, Y.; Liu, J.; et al. EI24 Inhibits Cell Proliferation and Drug Resistance of Esophageal Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 1570. [Google Scholar] [CrossRef]
- Sun, G.; Wang, T.; Shi, M.; Zhou, H.; Wang, J.; Huang, Z.; Zhang, H.; Shi, J. Low expression of IL6R predicts poor prognosis for lung adenocarcinoma. Ann. Transl. Med. 2021, 9, 1057. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Q.; Yue, C.; Lan, L.; Jiang, J.; Shen, Y.; Lu, B. Prognostic value of IL-6R mRNA in lung adenocarcinoma and squamous cell carcinoma. Oncol. Lett. 2018, 16, 2935–2948. [Google Scholar] [CrossRef]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Cheng, Z.; Huang, Y.; Zheng, W.; Yang, S.; Lin, C.; Ye, J. MyD88 mediates colorectal cancer cell proliferation, migration and invasion via NF-κB/AP-1 signaling pathway. Int. J. Mol. Med. 2019, 45, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sawazaki, S.; Oshima, T.; Sakamaki, K.; Aoyama, T.; Sato, T.; Shiozawa, M.; Yoshikawa, T.; Rino, Y.; Imada, T.; Masuda, M. Clinical Significance of Tensin 4 Gene Expression in Patients with Gastric Cancer. Vivo 2017, 31, 1065–1071. [Google Scholar] [CrossRef][Green Version]
- Lo, S.H. C-terminal tensin-like (CTEN): A promising biomarker and target for cancer. Int. J. Biochem. Cell Biol. 2014, 51, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, X.; Gu, X.; Li, X.; Shang, L. Progress in understanding the role of lncRNA in programmed cell death. Cell Death Discov. 2021, 7, 30. [Google Scholar] [CrossRef]
- Frank, F.; Kavousi, N.; Bountali, A.; Dammer, E.B.; Mourtada-Maarabouni, M.; Ortlund, E.A. The lncRNA Growth Arrest Specific 5 Regulates Cell Survival via Distinct Structural Modules with Independent Functions. Cell Rep. 2020, 32, 107933. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z. Long non-coding RNA growth arrest-specific transcript 5 in tumor biology. Oncol. Lett. 2015, 10, 1953–1958. [Google Scholar] [CrossRef]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA Gas5 Is a Growth Arrest- and Starvation-Associated Repressor of the Glucocorticoid Receptor. Sci. Signal. 2010, 3, ra8. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, D.; Yang, F.; Tian, M.; Wang, Y.; Shen, D.; Wang, Q.; Chen, Q.; Zhang, L. Long Noncoding RNA Highly Upregulated in Liver Cancer Regulates the Tumor Necrosis Factor-α-Induced Apoptosis in Human Vascular Endothelial Cells. DNA Cell Biol. 2016, 35, 296–300. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118, Correction in Nat. Rev. Mol. Cell Biol. 2021, 22, 159. https://doi.org/10.1038/s41580-021-00330-4. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.; Hennig, J.; Jagtap, P.K.A.; Sonntag, M.; Valcárcel, J.; Sattler, M. Structure, dynamics and RNA binding of the multi-domain splicing factor TIA-1. Nucleic Acids Res. 2014, 42, 5949–5966. [Google Scholar] [CrossRef]




| Top 5 Up-Regulated LncRNA Genes | |||||
| Gene | FC | Mean FC | |||
| HCC827 | A549 | H358 | H292 | ||
| lnc-MTPAP-1 | 1.1412245 | 1.0393772 | 6.814241 | 2.1546037 | 2.7873616 |
| lnc-PLXND1-1 | 1.11237 | 1.2055317 | 4.872442 | 2.4244833 | 2.4037068 |
| lnc-DHX40-1 | 1.2124051 | 1.2725444 | 4.540834 | 1.8083729 | 2.2085391 |
| THAP9−AS1 | 1.1720212 | 1.8104212 | 3.9262705 | 1.0608561 | 1.9923923 |
| lnc-USP28-1 | 1.3274853 | 2.2720706 | 2.4233804 | 1.3167702 | 1.8349266 |
| Top 5 Down-Regulated LncRNA Genes | |||||
| Gene | FC | Mean FC | |||
| HCC827 | A549 | H358 | H292 | ||
| lnc-TRA2A-1 | 0.5045274 | 0.8241876 | 0.21710351 | 0.25026 | 0.4490196 |
| HID1−AS1 | 0.746688 | 0.25143218 | 0.46924052 | 0.55981 | 0.5067927 |
| MIR200CHG | 0.4364092 | 0.4170083 | 0.4236734 | 0.7643422 | 0.5103583 |
| lnc-PBX1-2 | 0.7775569 | 0.5471765 | 0.4881028 | 0.3748579 | 0.5469235 |
| LOC101929217 | 0.4969948 | 0.398749 | 0.582493 | 0.7563759 | 0.5586532 |
| Gene | Sequence (5′ to 3′) |
|---|---|
| CLPTM1L | F: AGAGGAGAGCCAGGGATTGA/ R: CCACCTTCTTGTGAGCATCC |
| EI24 | F: GCTGGTGCAGGAAATGAAGA/ R: TGAGTCTGGTAGGCGATGAA |
| IL6R | F: AAGGTGCGGATGAGTTTGAG/ R: CTTCCACGATGCTGATGTTG |
| MyD88 | F: TGTGTGGTGAAGATGCGTTT/ R: TGGGAAAGGAGAGGTGGAAG |
| TNS4 | F: ATAGAGCGTGGTCCAGTTCG/ R: GGTCCACCTCCGAGTTCTTC |
| GAPDH | F: CTCCTGCACCACCAACTGCT/ R: GGGCATGGACTGTGGTCATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.W.; Kang, D.; Lee, M.H.; Lee, Y.; Lee, S.Y.; Woo, S.Y.; Yang, K.-J.; Jeong, I.B.; Park, H.S.; Son, J.W.; et al. Long Noncoding RNA Lnc-MTPAP-1 Overexpressed by Particulate Matter Suppresses Apoptosis in Non-Small Cell Lung Cancer (NSCLC) Cells. Int. J. Mol. Sci. 2025, 26, 10486. https://doi.org/10.3390/ijms262110486
Park JW, Kang D, Lee MH, Lee Y, Lee SY, Woo SY, Yang K-J, Jeong IB, Park HS, Son JW, et al. Long Noncoding RNA Lnc-MTPAP-1 Overexpressed by Particulate Matter Suppresses Apoptosis in Non-Small Cell Lung Cancer (NSCLC) Cells. International Journal of Molecular Sciences. 2025; 26(21):10486. https://doi.org/10.3390/ijms262110486
Chicago/Turabian StylePark, Ji Won, Daeun Kang, Min Hyeok Lee, Yeonwoo Lee, Su Yel Lee, Sin Yung Woo, Keum-Jin Yang, In Beom Jeong, Hee Sun Park, Ji Woong Son, and et al. 2025. "Long Noncoding RNA Lnc-MTPAP-1 Overexpressed by Particulate Matter Suppresses Apoptosis in Non-Small Cell Lung Cancer (NSCLC) Cells" International Journal of Molecular Sciences 26, no. 21: 10486. https://doi.org/10.3390/ijms262110486
APA StylePark, J. W., Kang, D., Lee, M. H., Lee, Y., Lee, S. Y., Woo, S. Y., Yang, K.-J., Jeong, I. B., Park, H. S., Son, J. W., & Kwon, S. J. (2025). Long Noncoding RNA Lnc-MTPAP-1 Overexpressed by Particulate Matter Suppresses Apoptosis in Non-Small Cell Lung Cancer (NSCLC) Cells. International Journal of Molecular Sciences, 26(21), 10486. https://doi.org/10.3390/ijms262110486









