Abstract
Monoclonal antibodies (mAbs) represent one of the most successful classes of biopharmaceuticals, with more than 100 approved for treating oncological, immunological, and infectious diseases. Antibody discovery and development have been driven by diverse methodologies. Classical strategies such as mouse hybridoma technology, phage display, transgenic mouse models, and single B cell isolation have enabled the generation of high-affinity therapeutic antibodies. Beyond binding affinity, recent innovations in combinatorial antibody libraries have facilitated the selection of functional antibodies within cellular environments, revealing their ability to act as agonists or antagonists and influence signal transduction pathways. These insights expand therapeutic applications by enabling modulation of complex cellular responses. Recent breakthroughs in artificial intelligence, involving antibody generation supported by rapidly growing antibody sequence and structure databases, are transforming computational protein design. This review highlights five major approaches (hybridoma technology, phage display, transgenic mouse models, and single B cell isolation, de novo antibody design) for antibody discovery and development. These approaches offer innovative strategies designed to accelerate the discovery process and enhance therapeutic outcomes for human diseases.
1. Introduction
Antibodies are essential components of the adaptive immune system produced by B cells to specifically recognize and neutralize diverse antigens such as viruses, pathogens, and malignant cells. Since the pioneering work of Köhler and Milstein in developing hybridoma technology [1], monoclonal antibodies (mAbs) have become indispensable tools in both basic research and clinical medicine. To date, more than 100 therapeutic antibodies have been approved for clinical use [2], providing effective treatment options for autoimmune disorders, metabolic diseases, infectious diseases, and a wide range of cancers [2,3,4,5]. The development of therapeutic antibodies has traditionally relied on several strategies, including animal immunization, combinatorial antibody libraries, B cell cloning, and the use of transgenic mice [6,7,8]. These methods have enabled the discovery of high-affinity antibodies with desirable therapeutic properties. More recently, advances in computational biology and artificial intelligence (AI) have introduced powerful new approaches to antibody discovery [9,10,11,12]. These tools such as AlphaFold3 for structural prediction, molecular docking, and de novo design are increasingly combined with generative diffusion models to create antibodies with improved specificity, affinity, and efficacy [13]. In this review, we summarize the five major methodologies for antibody generation, these approaches illustrated in Figure 1 are expanding the landscape of therapeutic antibody discovery, offering innovative strategies to accelerate development and improve the treatment of human diseases.
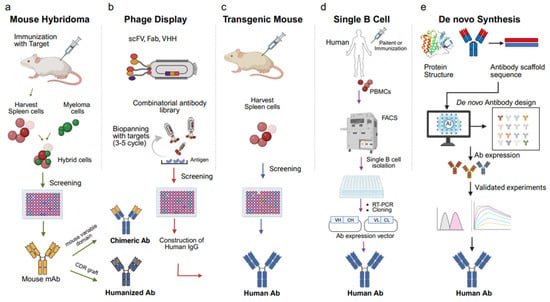
Figure 1.
Overview of five key approaches for therapeutic antibody development: (a) mouse hybridoma, (b) phage display, (c) transgenic mouse, (d) single B cell isolation, and (e) de novo design. Each method enables monoclonal antibody generation through distinct immunization, screening, or computational strategies. (a) Mouse hybridoma technology: Mice are immunized with the target antigen, and spleen B cells are fused with immortal myeloma cells to generate hybridomas. Hybridoma clones are screened for specific antibody production, followed by antibody humanization through complementarity-determining region (CDR) grafting. (b) Phage display: A combinatorial antibody library was generated in single-chain variable fragment (scFv), fragment antigen-binding (Fab), or single-domain antibody (VHH) formats. Repeated rounds of biopanning against immobilized antigens enrich high-affinity binders for screening and IgG reconstruction. (c) Transgenic mouse platforms: Genetically engineered mice expressing human immunoglobulin genes are immunized with target antigens. Human antibody-producing B cells are isolated from the spleen and screened to obtain fully human antibodies. (d) Single B cell isolation: Peripheral blood mononuclear cells (PBMCs) are collected from immunized or patient human donors. Antigen-specific B cells are isolated by fluorescence-activated cell sorting (FACS), and antibody genes are amplified via reverse transcription polymerase chain reaction (RT-PCR) and cloned into expression vectors to obtain fully human antibodies. (e) De novo antibody design: Using artificial intelligence (AI) and structure-based modeling, antibody sequences are generated computationally from scaffold structures. Predicted antibodies are expressed and experimentally validated for affinity, stability, and function.
2. Hybridoma Technology
For many years, antibodies have been an important tool in biomedical research, with potential applications in a variety of fields. The high specificity and selective binding of antibodies have expanded their application to flow cytometry, magnetic cell sorting, immunoassays, therapeutic methods, and more. In 1975, hybridoma-based technology was used to produce monoclonal antibodies with minimal inter-batch variability, allowing for continuous and unlimited production [6]. Hybridoma technology, first invented by Kohler and Milstein in 1975 as a method for obtaining monoclonal antibodies, involves fusing B cells produced in the spleen of immunized animals (such as mice) with immortalized myeloma cells [14,15]. Hybridoma technology is the original, most basic, and most successful method for isolating monoclonal antibodies. This technology is compelling and has been used to discover thousands of antibodies for various applications. The fundamental practical advantage of hybridoma technology is that once a hybridoma clone is established, the production of monoclonal antibodies becomes straightforward and efficient [16]. This technique remains extensively employed in the development of antibody therapeutics and diagnostic reagents, its core value lying in preserving the sequence integrity and biological activity of antibodies derived from the natural recombination process within B cells. The first therapeutic mAb, muromonab-CD3 (Orthoclone OKT3), was approved by the US FDA in 1986 and comprises a murine mAb against T cell-expressed CD3 that functions as an immunosuppressant for the treatment of acute transplant rejection [17]. To circumvent problems of diminished immunogenic potential and efficacy, while making possible the therapeutic use of antibodies for an extended duration, researchers developed techniques to transform rodent antibodies into structures more similar to human antibodies, without loss of binding properties. Then, the first mAb with an oncologic indication, rituximab, a chimeric anti-CD20 IgG1, was approved for the treatment of non-Hodgkin’s lymphoma in 1997 by the US FDA [18]. Recent innovations, such as electrofusion of antibody-secreting cells (ASCs) enriched via fluorescence-activated cell sorting (FACS), have significantly improved fusion efficiency and functional hybridoma yield. By selecting ASC subsets with high transmembrane activator and CAML interactor (TACI) and CD138 expression, researchers achieved more than 60% antigen-specific mAb production with nanomolar affinity. These refinements overcome prior limitations of random pairing and low PEG-mediated fusion, positioning hybridoma as a revitalized, high-yield platform for therapeutic and diagnostic antibody development [19].
In addition to mouse hybridomas, rabbit monoclonal antibodies can also be generated from hybridoma technology. Rabbit antibody shows superior affinity, broader epitope recognition including cryptic or conformational sites and greater sensitivity for therapeutic and diagnostic use [20]. Rabbit monoclonal antibodies surpass their murine counterparts in several key immunological dimensions: (1) Superior Affinity—Enhanced binding kinetics enable more precise antigen detection and targeting. (2) Expanded Epitope Recognition—Capable of identifying epitopes that are structurally complex or poorly immunogenic in mice. (3) Sensitivity to Post-Translational Modifications—Ideal for detecting subtle biochemical variations in disease states. Rabbit monoclonal antibodies (RabMAbs) offer high specificity and affinity, often recognizing epitopes inaccessible to murine antibodies, making them valuable for antibody-drug conjugates (ADCs) and checkpoint inhibitors. The FDA approval of brolucizumab, a rabbit-derived ScFv targeting VEGF-A, marked the first therapeutic RabMAb. Brolucizumab is a single-chain variable fragment (ScFv) mAb that potently inhibits VEGF. Due to their small size, ScFvs can be delivered at higher concentrations compared to other therapeutic mAb formats and can penetrate tissue more effectively to exert their therapeutic effects. A key difference that sets brolucizumab apart from other mAbs, however, is that it is a humanized ScFv derived from rabbits, making this mAb the first of its kind in the market. In contrast, most other therapeutic mAbs are derived from mice [21,22,23]. Other examples include Zilovertamab vedotin, an ADC against receptor tyrosine kinase-like orphan receptor 1 (ROR1) [24,25], and OR502, targeting leukocyte immunoglobulin-like receptor B2 (LILRB2) to enhance antitumor immunity [26,27].
3. Phage Display
Phage display is the first technique for in vitro antibody screening and is still the most widely used method. The technology was first described in 1985 by George P. Smith, who demonstrated that filamentous bacteriophages could present foreign peptides on their surface via DNA insertion into coat protein genes, this technology has been extensively developed. Major advances came from Winter and McCafferty at the Laboratory of Molecular Biology (Cambridge, UK), Lerner and Barbas at The Scripps Research Institute (La Jolla, USA), and Breitling and Dübel at the German Cancer Research Center (Heidelberg, Germany), who pioneered the construction of combinatorial antibody libraries in filamentous phages [28,29,30,31,32]. These milestones firmly established phage display as a robust platform for therapeutic antibody engineering, leading to the development of fully human antibodies [33,34,35,36,37,38,39,40,41,42]. Combinatorial antibody libraries are constructed by amplifying VH and VL regions from B cells, immunized donors, or patients using PCR, followed by cloning into phagemid vectors fused to phage coat proteins such as pIII. This creates a direct genotype–phenotype linkage, enabling the display of antibody fragments on the phage surface. Libraries can exceed 1011 variants, providing extensive diversity. Iterative rounds of binding, washing, and elution against immobilized antigens enrich antigen-specific phages, after which binders are screened by ELISA, sequenced, and reformatted as scFv, Fab, or IgG molecules for testing. Importantly, phage display bypasses immune tolerance, allowing the generation of antibodies against self-antigens such as TNF-α. The approval of Adalimumab (Humira), the first phage display-derived fully human monoclonal antibody, illustrates its clinical significance [43]. There are phage display-derived human antibodies approved by the US FDA for the treatment of human disease, demonstrating the reliability of this technique as a platform for antibody discovery [33,44,45].
Beyond conventional binder selection, phage display has progressed to enable direct functional antibody discovery as shown as Figure 2 [46,47,48,49,50,51,52,53,54,55,56,57,58]. The identification of functional antibodies has become a central focus of research. In vivo and phenotypic screening approaches have further broadened phage display applications. Migration-based selection within whole organisms has revealed antibodies that regulate receptor pleiotropy and cell differentiation. These strategies enabled the identification of antibodies that drive stem cells into macrophage, microglia, brown adipocyte, or beta-like cell fates [52]. Morphology-based screening has even yielded antibodies that protect against virus-induced cell death [59]. In oncology, functional phage display has identified agonist antibodies activating non-canonical pathways and an anti-apoptotic intrabody against PKM2, revealing mechanisms of tumor survival [60,61,62]. These advances illustrate how phage display has evolved into a versatile discovery engine for antibodies with therapeutic functions beyond binding alone. In addition, antibodies obtained from hybridoma or phage display often undergo further engineering to improve binding. By introducing mutations into VH/VL regions and reselecting improved clones, phage display mimics natural affinity maturation that occurs in germinal centers after V(D)J recombination. This process enriches variants with higher antigen affinity, sometimes reaching low-picomolar binding strengths, and is critical for optimizing antibodies for therapeutic use [63,64].
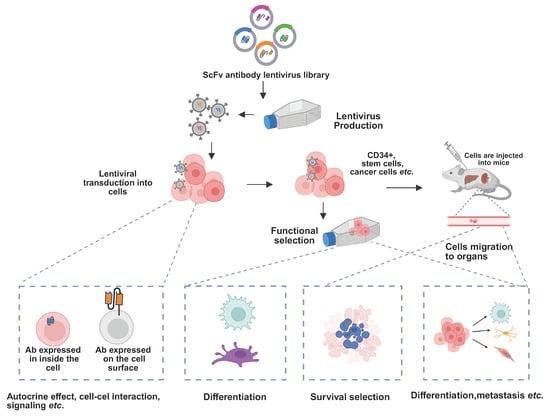
Figure 2.
Schematic of functional antibody discovery modulating cell fate. Lentiviral delivery of antibody libraries enables intracellular and surface expression of antibodies. Functional selection in cells and in vivo identifies antibodies that modulate signaling, differentiation, and cell fate. Functional antibodies can induce changes in cell fate, with the ability to be secreted, expressed in the cytoplasm, anchored on the plasma membrane. Through the use of antibody libraries, functional antibodies can be isolated in the cellular environment and modify the function of cell surface signaling components. These antibodies can also act in an autocrine or paracrine manner to influence cell survival, proliferation, and lineage differentiation, providing a powerful platform for discovering antibodies that regulate cellular homeostasis and fate determination.
4. Transgenic Mouse Technology
Antibodies derived from wild-type mice often require extensive downstream engineering to eliminate immunogenic murine sequences and to ensure proper interaction with human Fc receptors. While wild-type mice are widely available and straightforward to use, they are suboptimal for therapeutic antibody discovery. To address the challenge of immunogenicity, transgenic mouse models engineered to generate fully human antibodies. However, early generations of these models lacked murine constant regions, leading to defects in B-cell development and impaired antibody maturation within the mouse. The principle of transgenic mouse technology is to introduce human immunoglobulin (Ig) genes into the murine genome through genetic engineering, enabling mice to produce fully human antibodies. This is typically achieved by homologous recombination in embryonic stem (ES) cells or by microinjecting recombinant human antibody gene fragments into fertilized eggs, followed by embryo transfer and breeding to establish stable transgenic lines. The integrated human Ig genes cooperate with the murine immune system, ensuring normal B-cell development, somatic hypermutation, and affinity maturation. Compared with wild-type mice, these transgenic mice generate human antibodies with higher clinical relevance, reduced immunogenicity, and preserved immune surveillance. The first milestone was reported in 1989, when Brüggemann et al. [65] constructed a human heavy chain gene cassette containing two VH segments, diversity (D) elements, the JH cluster, and the μ constant region. This 25 kb construct was randomly integrated into the mouse genome by microinjection into fertilized eggs. Approximately 4% of B cells expressed human μ chains, and hybridomas producing human IgM could be generated from these mice. Later, Taylor et al. introduced a human κ light chain construct containing a single Vκ, Jκ cluster, and Cκ [66]. Mice co-expressing the human VH-D-JH-Cμ-Cγ1 and κ constructs were able to produce human antibodies, though at levels below 10% of total Ig, reflecting limited compatibility with endogenous murine Ig expression [66]. In parallel, knockout mouse models were developed to eliminate endogenous murine Ig production. In 1993, Chen et al. disrupted the murine JH and Jκ loci by targeted deletion, abolishing native Ig expression [67,68]. When crossed with human IgH and IgL transgenic lines, these knockout mice displayed broader human antibody repertoires. A major advance came in 1994 when Lonberg et al. generated HuMabMouse, the first mouse line carrying human IgH and Igκ genes in a murine Ig-deficient background. Although the full human IgH and Igκ loci span 1.29 Mb and 1.39 Mb, the introduced constructs were less than 80 kb, which constrained antibody diversity. Subsequently, yeast artificial chromosome (YAC) technology was applied. In 1993, researchers successfully assembled large genomic fragments of human Igκ (~300 kb) and IgH (~85 kb) using YACs [69,70]. Subsequently, Green et al. introduced YAC-based human Igκ (~170 kb) and IgH (~220 kb) into mouse ES cells via yeast spheroplast–ES fusion. Building on this, Mendez et al. generated even larger YAC constructs, including human Igκ (~700 kb) and IgH (~1 Mb), and introduced them into Ig-deficient mice, creating XenoMouse [71]. These mice expressed only fully human antibodies, free from murine Ig interference. Together, the development of transgenic animals provided groundbreaking platforms that enabled the efficient generation of fully human therapeutic antibodies. Beyond XenoMouse, an increasing number of advanced transgenic mouse platforms have been developed, such as the Atlas™ Mouse, the HuMab Mouse, and the VelocImmune® Mouse (Regeneron, Tarrytown, NY, USA). These models employ precise gene knock-in strategies to replace murine heavy- and light-chain variable regions with near-comprehensive human sequences, thereby enhancing antibody diversity while preserving natural structural characteristics. Newer generations also incorporate innovations such as fixed light-chain or binary light-chain strategies, which streamline the efficient generation of bispecific antibodies and improve developability and pharmacokinetic properties. Collectively, these innovations expand the functional versatility of therapeutic antibodies and provide greater flexibility in drug development pipelines. Although the technology remains costly and technically demanding, such transgenic mouse models are rapidly becoming an industry standard, demonstrating substantial value in addressing complex diseases and meeting high clinical demands [71,72,73,74].
5. Single B Cell Technology
In the human immune system, antibody responses are robust, highly specific, and often potently neutralizing. Traditional strategies to generate therapeutic monoclonal antibodies, such as murine hybridoma technology or the use of transgenic mice, require lengthy immunization protocols and extensive screening. Moreover, murine-derived antibodies carry a significant risk of immunogenicity in humans, frequently leading to the development of human anti-mouse antibody (HAMA) responses. To overcome these limitations, an early alternative was the immortalization of human B cells using Epstein–Barr virus (EBV) transformation [75]. While this approach enabled the production of human antibodies under certain conditions, it suffered from key drawbacks, including inefficiency in some donors and instability of EBV-transformed clones. A transformative advance came with single B cell technologies, which allow direct recovery of human antibodies without animal immunization. Using reverse transcription polymerase chain reaction (RT-PCR), the variable heavy (VH) and light (VL) chains from individual B cells can be amplified and expressed recombinantly [76,77]. B cells are typically isolated from peripheral blood mononuclear cells (PBMCs), bone marrow, or lymphoid tissues, often after density gradient centrifugation. Antigen-specific B cells are identified and sorted using FACS based on stage-specific markers. Earlier methods employed antigen-coated beads to enrich for rare B cells, leading to the first human monoclonal antibodies against viral pathogens [78]. Today, multiparameter flow cytometry and high-throughput single-cell cloning enable rapid identification and expression of paired VH/VL genes. These methods have revolutionized antibody discovery by offering an efficient, animal-free strategy particularly suited to urgent public health crises like emerging viral outbreaks such as pandemic influenza [7,8,79]. Applications of this strategy have been widely demonstrated. For example, broadly neutralizing antibodies (bnAbs) against HIV-1 were isolated from infected or vaccinated individuals by using HIV envelope proteins as baits [80,81,82,83]. These antibodies often target conserved epitopes on viral membrane glycoproteins, enabling potent and cross-reactive neutralization. More recently, single B cell methods have enabled the rapid identification of potent neutralizing antibodies against SARS-CoV-2. Panels of antibodies targeting the spike protein were isolated and characterized shortly after the COVID-19 pandemic began, providing critical insights into both therapeutic and vaccine design [84,85,86]. Collectively, single B cell antibody discovery platforms represent a transformative advance compared to traditional hybridoma methods, enabling faster, more direct, and clinically relevant recovery of fully human monoclonal antibodies.
6. De Novo Synthesis
Traditional antibody discovery and optimization techniques, such as hybridoma technology, phage display, transgenic mice, B-cell cloning, etc., are widely used for therapeutic antibody screening. However, some of these are labor-intensive, time-consuming, and costly, it usually takes more than six months to produce feasible antibodies [87,88]. Subsequently, through homology modeling, molecular docking, and structure-based design strategies, optimization steps were performed to enhance binding affinity, biophysical stability and developability [89,90,91,92]. Recent advances in protein data acquisition, GPU computers, and machine learning (ML) are expected to revolutionize the process of antibody discovery and optimized screening. The availability of extensive protein structure, interaction, and function data provides a large dataset for training sophisticated machine learning models, while enhanced computational power enables efficient execution of complex models and simulations. Machine learning models have been widely used in protein research, covering technologies such as neural networks, transducers, and protein language modeling [93,94]. De novo antibody design refers to the generation of new antibody sequences without relying on natural templates. Recent studies have used this approach to computationally predict sequences with high binding affinity based on accurate modeling of intermolecular interactions by simulating the antigen-antibody interaction interface [95]. The AlphaFold3 [13,96] and RoseTTAFold [97,98] models have achieved high accuracy in predicting protein structures directly from amino acid sequences. Generative models such as ProteinBERT [99], ProteinMPNN, [100,101] and RFdiffusion [102] have advanced computational protein designs by predicting protein backbones, designing sequences for specific structures, and filtering low-quality protein candidates. Researchers can use these models to design and optimize proteins with desired properties, such as enzymatic activity and binding. Specialized models focus on antibody design, particularly targeting the complementarity-determining regions (CDRs). IgFold can rapidly predict antibody structures using pre-trained language models and graph neural networks [103], and models like DiffAb can be used for the joint generation of antibody sequences and structures targeting CDR optimization [104].
Machine learning for functional protein design spans three core modalities: sequence-based, structure-based, and function-based models (Figure 3).
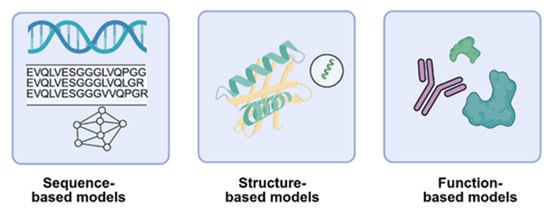
Figure 3.
Schematic of machine learning modalities for antibody design. Sequence-based models leverage amino acid sequences to learn representations and generate novel variants and enabling de novo design. Structure-based models incorporate structural constraints to guide sequence generation and optimization. Function-based models utilize experimental, published data or predicted functional protein such as catalytic activity or binding for supervised learning.
Each leveraging distinct data type to optimize protein properties. Sequence-based models include classical alignment-based approaches using multiple sequence alignments to capture evolutionary constraints, and conditional generative models like variational autoencoders and transformers that generate novel sequences based on family-specific or sequence homology contexts [105,106]. A Generative Adversarial Network (GAN) was designed [107] and trained on over 400,000 human antibody light and heavy chain sequences effectively learns the underlying principles of antibody formation [108]. Structure-based models encompass structure prediction tools such as AlphaFold3 and RoseTTAFold [97], RF-diffusion models [109] for 3D fold creation, and design models that optimize sequences within structural frameworks. David Baker’s team recently developed RFdiffusion, a generative model for de novo design of VHHs and scFvs targeting influenza hemagglutinin and Clostridium difficile toxin B (TcdB). It achieves atomic-level precision in both structure and epitope targeting. [110]. These approaches integrate sequence and structural data are increasingly used to iteratively refine antibody designs, combining the strengths of both modalities for enhanced functional outcomes [111]. Function-based models focus on engineering antibodies with specific biological activities, often starting from known scaffolds and introducing targeted mutations to enhance binding, specificity. These approaches frequently reference receptor pockets, natural ligands, or agonist, antagonist interactions to guide the design of functional antibodies [112]. These models enable rational design of agonists, antagonists, and therapeutic proteins. Together, these modalities offer complementary strategies for accelerating protein engineering, with growing applications in antibody design, optimization, and synthetic biology. Collectively, recent strategies converge within generative AI frameworks that can be broadly classified into three categories: Language Models, Diffusion Models, and Hybrid Models (Figure 4).
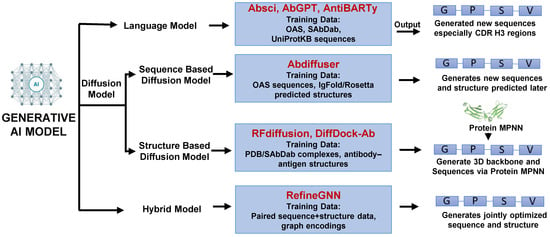
Figure 4.
Schematic diagram of antibody generation using generative AI models. Generative AI approaches include Language models (sequence-based design), Diffusion models (sequence- or structure-guided generation), and Hybrid models (integrating sequence and structure for simultaneous optimization). An integrative framework transforms antibody discovery from published empirical screening to data-driven design.
Language models (e.g., Absci, AbGPT, AntiBARTy) [113,114] are trained on large antibody sequence repositories to generate novel sequences, particularly within highly diverse regions such as CDRH3. Diffusion models are subdivided into structure-based (e.g., RFdiffusion, DiffDock-A) [110,115] which use experimentally solved antibody–antigen complexes from PDB or SAbDab to design new 3D backbones followed by sequence optimization via Protein MPNN, and sequence-based (e.g., Abdiffuser) [116] which leverage antibody sequence datasets with predicted structures to generate novel sequences. Hybrid models (e.g., Refine GNN) [117] integrate paired sequence and structure data, enabling the simultaneous optimization of both sequences and structures. Together, these generative frameworks provide complementary strategies that expand the landscape of antibody discovery and accelerate the design of functional therapeutic candidates.
Now, AI-assisted antibody design has demonstrated remarkable versatility across diverse targets, including virus proteins, membrane receptor oncogenic proteins [117,118,119,120]. As computational modeling and high-throughput experimentation converge, de novo antibody generation is poised to become an automated, iterative process driven by machine learning and structural bioinformatics. This integration promises faster, more precise development of therapeutic antibodies, accelerating breakthroughs in cancer, infectious diseases, and immunotherapy. AI-assisted antibody design represents a new trend that is reshaping antibody discovery [110,121].
7. Summary and Outlook
This review highlights diverse strategies for antibody discovery, including hybridoma technology, phage display libraries, transgenic mice, single B cell isolation, and de novo synthetic approaches. Despite these advances, each antibody discovery approach still presents unique challenges and trade-offs. Hybridoma methods are limited by species-specific immune responses such immune tolerance, while phage display may introduce library bias and lose native antibody pairing. Transgenic mouse platforms are costly, and single B-cell technologies face throughput and data interpretation constraints. Moreover, current AI-driven and de novo computational design approaches still struggle with accurately predicting antibody folding, dynamics, and functional efficacy, underscoring the need for integrated, multi-platform strategies in next-generation antibody development. In addition, beyond these five antibody generation strategies, post-production engineering such as Fc modification, half-life extension, and glycoengineering also continues to enhance antibody efficacy, stability, and therapeutic index. These innovations complement discovery platforms, bridging antibody generation with clinical optimization for next-generation biologics. Taken together, in this review, we summarize five major antibody generation methodologies that are expanding the frontiers of therapeutic antibody discovery, providing innovative strategies to accelerate the development process and enhance the therapeutic efficacy of human diseases. Collectively, these platforms have enabled the rapid generation of not only conventional neutralizing antibodies but also functional agonists that modulate signaling pathways and drive cellular differentiation. Emerging methods such as autocrine-based and migration-based selection, in vivo functional screening, and morphology-guided assays further expand the potential of antibody discovery, particularly in cancer and immunology. These advances provide powerful means to target viruses, tumors, and immune receptors, while also offering new insights into receptor pleiotropy and cell fate regulation. De novo design, increasingly coupled with AI-driven modeling and high-throughput screening, represents a new trend that is reshaping antibody discovery. Looking forward, the convergence of these approaches with next-generation computational tools will accelerate the development of innovative therapeutic antibodies across infectious diseases, oncology, and beyond.
Funding
This work was financially supported by China Medical University Yingcai Scholar Fund CMU110-YTY-03. “Cancer Biology and Precision Therapeutics Center, China Medical University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. MOE Taiwan scholarship program. 2025 Hannam University Research Fund.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This work was financially supported by the “Cancer Biology and Precision Therapeutics Center, China Medical University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. MOE (Ministry of Education Taiwan scholarship program), China Medical University Yingcai Scholar Fund CMU110-YTY-03. This work also was supported by the 2025 Hannam University Research Fund.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves 100th monoclonal antibody product. Nat. Rev. Drug Discov. 2021, 20, 491–495. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Tsuchikama, K.; Anami, Y.; Ha, S.Y.Y.; Yamazaki, C.M. Exploring the next generation of antibody-drug conjugates. Nat. Rev. Clin. Oncol. 2024, 21, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Jeong, M.; In, H.; Kim, J.H.; Lin, C.-W.; Han, K.H. Trends in the development of antibody-drug conjugates for cancer therapy. Antibodies 2023, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Martyn, G.D.; Carter, P.J. Fifty years of monoclonals: The past, present and future of antibody therapeutics. Nat. Rev. Immunol. 2025, 25, 745–765. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.; Ramos, A.S.F.; Vu, M.; Maurer, D.P.; Rosado, V.C.; Lingwood, D.; Bajic, G.; Schmidt, A.G. Human naïve B cells recognize prepandemic influenza virus hemagglutinins. Sci. Immunol. 2025, 10, eado9572. [Google Scholar] [CrossRef]
- Abu-Shmais, A.A.; Freeman, G.; Creanga, A.; Vukovich, M.J.; Malla, T.; Mantus, G.E.; Shimberg, G.D.; Gillespie, R.A.; Guerra Canedo, V.; Dadonaite, B. Cross-neutralizing and potent human monoclonal antibodies against historical and emerging H5Nx influenza viruses. Nat. Microbiol. 2025, 1–16. [Google Scholar] [CrossRef]
- Chakravarty, D.; Lee, M.; Porter, L.L. Proteins with alternative folds reveal blind spots in AlphaFold-based protein structure prediction. Curr. Opin. Struct. Biol. 2025, 90, 102973. [Google Scholar] [CrossRef]
- Harmalkar, A.; Lyskov, S.; Gray, J.J. Reliable protein–protein docking with AlphaFold, Rosetta, and replica exchange. eLife 2025, 13, RP94029. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, K.; Zhu, K.; Zhang, H.; Shen, C.; Hou, T. From Traditional Methods to Deep Learning Approaches: Advances in Protein–Protein Docking. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2025, 15, e70016. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, J.; Zhou, J.; Cong, Q. Recent progress and future challenges in structure-based protein-protein interaction prediction. Mol. Ther. 2025, 33, 2252–2268. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Crowe, J.E., Jr. Use of Human Hybridoma Technology to Isolate Human Monoclonal Antibodies. Microbiol. Spectr. 2015, 3, AID-0027-2014. [Google Scholar] [CrossRef]
- Mitra, S.; Tomar, P.C. Hybridoma technology; advancements, clinical significance, and future aspects. J. Genet. Eng. Biotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Parray, H.A.; Shukla, S.; Samal, S.; Shrivastava, T.; Ahmed, S.; Sharma, C.; Kumar, R. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int. Immunopharmacol. 2020, 85, 106639. [Google Scholar] [CrossRef]
- Smith, S.L. Ten years of Orthoclone OKT3 (muromonab-CD3): A review. J. Transpl. Coord. 1996, 6, 109–119. [Google Scholar] [CrossRef]
- Grillo-Lopez, A.J.; White, C.A.; Dallaire, B.K.; Varns, C.L.; Shen, C.D.; Wei, A.; Leonard, J.E.; McClure, A.; Weaver, R.; Cairelli, S.; et al. Rituximab: The first monoclonal antibody approved for the treatment of lymphoma. Curr. Pharm. Biotechnol. 2000, 1, 1–9. [Google Scholar] [CrossRef]
- Rousseau, F.; Menier, C.; Brochard, P.; Simon, S.; Perez-Toralla, K.; Wijkhuisen, A. Targeted fusion of antibody-secreting cells: Unlocking monoclonal antibody production with hybridoma technology. mAbs 2025, 17, 2510336. [Google Scholar] [CrossRef]
- Weber, J.; Peng, H.; Rader, C. From rabbit antibody repertoires to rabbit monoclonal antibodies. Exp. Mol. Med. 2017, 49, e305. [Google Scholar] [CrossRef]
- Karasavvidou, E.M.; Tranos, P.; Panos, G.D. Brolucizumab for the Treatment of Degenerative Macular Conditions: A Review of Clinical Studies. Drug Des. Devel Ther. 2022, 16, 2659–2680. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef]
- Honda, S.; Maruyama-Inoue, M.; Otsuji, T.; Kyo, A.; Kobayashi, Y.; Yamamoto, Y.; Gomi, F. Efficacy and safety of brolucizumab every 6 weeks induction therapy for neovascular age-related macular degeneration. Sci. Rep. 2025, 15, 5705. [Google Scholar] [CrossRef]
- Wang, M.L.; Barrientos, J.C.; Furman, R.R.; Mei, M.; Barr, P.M.; Choi, M.Y.; de Vos, S.; Kallam, A.; Patel, K.; Kipps, T.J. Zilovertamab vedotin targeting of ROR1 as therapy for lymphoid cancers. NEJM Evid. 2022, 1, EVIDoa2100001. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Gutierrez, M.; Sanz-Garcia, E.; Villa, D.; Zhang, J.; Friedmann, J.; Yan, F.; Socinski, M.A.; Sarantopoulos, J.; Raez, L.E. Phase 2 Study of Zilovertamab Vedotin in Participants with Metastatic Solid Tumors. Cancer Res. Commun. 2025, 5, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Safety and efficacy of OR502, an antibody targeting leukocyte immunoglobulin-like receptor B2 (LILRB2), ± cemiplimab in patients with advanced solid tumors from a phase 1 study. J. Clin. Oncol. 2025, 43, 2524. [CrossRef]
- Phase 2 expansions of OR502, an antibody targeting leukocyte immunoglobulin-like receptor B2 (LILRB2) ± cemiplimab in patients with advanced solid tumors. J. Clin. Oncol. 2025, 43, TPS2669. [CrossRef]
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef]
- Barbas, C.F., 3rd; Kang, A.S.; Lerner, R.A.; Benkovic, S.J. Assembly of combinatorial antibody libraries on phage surfaces: The gene III site. Proc. Natl. Acad. Sci. USA 1991, 88, 7978–7982. [Google Scholar] [CrossRef]
- Clackson, T.; Hoogenboom, H.R.; Griffiths, A.D.; Winter, G. Making antibody fragments using phage display libraries. Nature 1991, 352, 624–628. [Google Scholar] [CrossRef]
- Hoogenboom, H.R.; Griffiths, A.D.; Johnson, K.S.; Chiswell, D.J.; Hudson, P.; Winter, G. Multi-subunit proteins on the surface of filamentous phage: Methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991, 19, 4133–4137. [Google Scholar] [CrossRef] [PubMed]
- Breitling, F.; Dubel, S.; Seehaus, T.; Klewinghaus, I.; Little, M. A surface expression vector for antibody screening. Gene 1991, 104, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, M.A.; Alsaab, H.O.; Mahmoud, A.B.; Alkayyal, A.A.; Jones, M.L.; Mahler, S.M.; Hashem, A.M. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front. Immunol. 2020, 11, 1986. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, A.; Schirrmann, T.; Hust, M. Phage display-derived human antibodies in clinical development and therapy. mAbs 2016, 8, 1177–1194. [Google Scholar] [CrossRef]
- Kim, J.W.; Cho, A.H.; Shin, H.G.; Jang, S.H.; Cho, S.Y.; Lee, Y.R.; Lee, S. Development and Characterization of Phage Display-Derived Monoclonal Antibodies to the S2 Domain of Spike Proteins of Wild-Type SARS-CoV-2 and Multiple Variants. Viruses 2023, 15, 174. [Google Scholar] [CrossRef]
- Daly, J.M.; Lim, T.S.; Gough, K.C. Therapeutic Phage Display-Derived Single-Domain Antibodies for Pandemic Preparedness. Antibodies 2023, 12, 7. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamamoto, N.; Kurosawa, G.; Tajima, K.; Kondo, M.; Hiramatsu, N.; Kato, Y.; Tanaka, M.; Yamaguchi, H.; Kurosawa, Y.; et al. A Novel High-Throughput Screening Method for a Human Multicentric Osteosarcoma-Specific Antibody and Biomarker Using a Phage Display-Derived Monoclonal Antibody. Cancers 2022, 14, 5829. [Google Scholar] [CrossRef]
- Ghaderi, S.S.; Riazi-Rad, F.; Qamsari, E.S.; Bagheri, S.; Rahimi-Jamnani, F.; Sharifzadeh, Z. Development of a human phage display-derived anti-PD-1 scFv antibody: An attractive tool for immune checkpoint therapy. BMC Biotechnol. 2022, 22, 22. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Gao, H.; Qing, G. Phage display derived peptides for Alzheimer’s disease therapy and diagnosis. Theranostics 2022, 12, 2041–2062. [Google Scholar] [CrossRef]
- Moreira, G.; Gronow, S.; Dubel, S.; Mendonca, M.; Moreira, A.N.; Conceicao, F.R.; Hust, M. Phage Display-Derived Monoclonal Antibodies Against Internalins A and B Allow Specific Detection of Listeria monocytogenes. Front. Public Health 2022, 10, 712657. [Google Scholar] [CrossRef]
- Hietanen, E.; Tripathi, L.; Brockmann, E.C.; Merilahti, P.; Lamminmaki, U.; Susi, P. Isolation and characterization of phage display-derived scFv antibodies against human parechovirus 1 VP0 protein. Sci. Rep. 2022, 12, 13453. [Google Scholar] [CrossRef]
- Berge-Seidl, S.; Nielsen, N.V.; Rodriguez Alfonso, A.A.; Etscheid, M.; Kandanur, S.P.S.; Haug, B.E.; Stensland, M.; Thiede, B.; Karacan, M.; Preising, N.; et al. Identification of a Phage Display-Derived Peptide Interacting with the N-Terminal Region of Factor VII Activating Protease (FSAP) Enables Characterization of Zymogen Activation. ACS Chem. Biol. 2022, 17, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Weinblatt, M.E.; Keystone, E.C.; Furst, D.E.; Moreland, L.W.; Weisman, M.H.; Birbara, C.A.; Teoh, L.A.; Fischkoff, S.A.; Chartash, E.K. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis Rheum. 2003, 48, 35–45, Erratum in Arthritis Rheum. 2003, 48, 855. [Google Scholar] [CrossRef] [PubMed]
- Kaleli, N.E.; Karadag, M.; Kalyoncu, S. Phage display derived therapeutic antibodies have enriched aliphatic content: Insights for developability issues. Proteins 2019, 87, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Lerner, R.A. Antibody libraries as tools to discover functional antibodies and receptor pleiotropism. Int. J. Mol. Sci. 2021, 22, 4123. [Google Scholar] [CrossRef]
- Lerner, R.A. Combinatorial antibody libraries: New advances, new immunological insights. Nat. Rev. Immunol. 2016, 16, 498–508. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Sato, A.K. Phage display technology and its impact in the discovery of novel protein-based drugs. Expert Opin. Drug Discov. 2024, 19, 887–915. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Yea, K.; Lerner, R.A. Autocrine signaling based selection of combinatorial antibodies that transdifferentiate human stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 8099–8104. [Google Scholar] [CrossRef]
- Lerner, R.A.; Grover, R.K.; Zhang, H.; Xie, J.; Han, K.H.; Peng, Y.; Yea, K. Antibodies from combinatorial libraries use functional receptor pleiotropism to regulate cell fates. Q. Rev. Biophys. 2015, 48, 389–394. [Google Scholar] [CrossRef]
- Yea, K.; Zhang, H.; Xie, J.; Jones, T.M.; Lin, C.W.; Francesconi, W.; Berton, F.; Fallahi, M.; Sauer, K.; Lerner, R.A. Agonist antibody that induces human malignant cells to kill one another. Proc. Natl. Acad. Sci. USA 2015, 112, E6158–E6165. [Google Scholar] [CrossRef]
- Lin, C.W.; Ho Han, K. Antibody Libraries as Platforms to Exploring Target and Receptor Pleiotropy. Isr. J. Chem. 2023, 63, e202300050. [Google Scholar] [CrossRef]
- Han, K.H.; Arlian, B.M.; Macauley, M.S.; Paulson, J.C.; Lerner, R.A. Migration-based selections of antibodies that convert bone marrow into trafficking microglia-like cells that reduce brain amyloid beta. Proc. Natl. Acad. Sci. USA 2018, 115, E372–E381. [Google Scholar] [CrossRef]
- Andre, A.S.; Moutinho, I.; Dias, J.N.R.; Aires-da-Silva, F. In vivo Phage Display: A promising selection strategy for the improvement of antibody targeting and drug delivery properties. Front. Microbiol. 2022, 13, 962124. [Google Scholar] [CrossRef]
- Tao, P.; Kuang, Y.; Li, Y.; Li, W.; Gao, Z.; Liu, L.; Qiang, M.; Zha, Z.; Fan, K.; Ma, P.; et al. Selection of a Full Agonist Combinatorial Antibody that Rescues Leptin Deficiency In Vivo. Adv. Sci. 2020, 7, 2000818. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Arlian, B.M.; Lin, C.W.; Jin, H.Y.; Kang, G.H.; Lee, S.; Lee, P.C.; Lerner, R.A. Agonist Antibody Converts Stem Cells into Migrating Brown Adipocyte-Like Cells in Heart. Cells 2020, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Gonzalez-Quintial, R.; Peng, Y.; Baccala, R.; Theofilopoulos, A.N.; Lerner, R.A. An agonist antibody that blocks autoimmunity by inducing anti-inflammatory macrophages. FASEB J. 2016, 30, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Park, H.W.; Han, K.H. Antibodies Regulate Dual-Function Enzyme IYD to Induce Functional Synergy between Metabolism and Thermogenesis. Int. J. Mol. Sci. 2022, 23, 7834. [Google Scholar] [CrossRef]
- Lee, E.J.; Baek, S.H.; Song, C.H.; Choi, Y.H.; Han, K.H. Agonist (P1) Antibody Converts Stem Cells into Migrating Beta-Like Cells in Pancreatic Islets. J. Microbiol. Biotechnol. 2022, 32, 1615–1621. [Google Scholar] [CrossRef]
- Xie, J.; Yea, K.; Zhang, H.; Moldt, B.; He, L.; Zhu, J.; Lerner, R.A. Prevention of cell death by antibodies selected from intracellular combinatorial libraries. Chem. Biol. 2014, 21, 274–283. [Google Scholar] [CrossRef]
- Lin, C.W.; Xie, J.; Zhang, D.; Han, K.H.; Grande, G.; Wu, N.C.; Yang, Z.; Yea, K.; Lerner, R.A. Immunity against cancer cells may promote their proliferation and metastasis. Proc. Natl. Acad. Sci. USA 2020, 117, 426–431. [Google Scholar] [CrossRef]
- Song, C.H.; Lin, C.W.; Han, K.H. Cell cycle-based antibody selection for suppressing cancer cell growth. FASEB J. 2025, 39, e70402. [Google Scholar] [CrossRef]
- Liu, T.; Kuwana, T.; Zhang, H.; Vander Heiden, M.G.; Lerner, R.A.; Newmeyer, D.D. Phenotypic selection with an intrabody library reveals an anti-apoptotic function of PKM2 requiring Mitofusin-1. PLoS Biol. 2019, 17, e2004413. [Google Scholar] [CrossRef]
- Eisen, H.N. Affinity enhancement of antibodies: How low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol. Res. 2014, 2, 381–392. [Google Scholar] [CrossRef]
- Hoogenboom, H.R. Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 2005, 23, 1105–1116. [Google Scholar] [CrossRef]
- Bruggemann, M.; Caskey, H.M.; Teale, C.; Waldmann, H.; Williams, G.T.; Surani, M.A.; Neuberger, M.S. A repertoire of monoclonal antibodies with human heavy chains from transgenic mice. Proc. Natl. Acad. Sci. USA 1989, 86, 6709–6713. [Google Scholar] [CrossRef]
- Taylor, L.D.; Carmack, C.E.; Schramm, S.R.; Mashayekh, R.; Higgins, K.M.; Kuo, C.C.; Woodhouse, C.; Kay, R.M.; Lonberg, N. A transgenic mouse that expresses a diversity of human sequence heavy and light chain immunoglobulins. Nucleic Acids Res. 1992, 20, 6287–6295. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Trounstine, M.; Alt, F.W.; Young, F.; Kurahara, C.; Loring, J.F.; Huszar, D. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int. Immunol. 1993, 5, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Trounstine, M.; Kurahara, C.; Young, F.; Kuo, C.C.; Xu, Y.; Loring, J.F.; Alt, F.W.; Huszar, D. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J. 1993, 12, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.P.; Rosewell, I.R.; Richardson, J.C.; Cook, G.P.; Neuberger, M.S.; Brownstein, B.H.; Norris, M.L.; Brüggemann, M. Creation of mice expressing human antibody light chains by introduction of a yeast artificial chromosome containing the core region of the human immunoglobulin kappa locus. Biotechnology 1993, 11, 911–914. [Google Scholar] [CrossRef]
- Choi, T.K.; Hollenbach, P.W.; Pearson, B.E.; Ueda, R.M.; Weddell, G.N.; Kurahara, C.G.; Woodhouse, C.S.; Kay, R.M.; Loring, J.F. Transgenic mice containing a human heavy chain immunoglobulin gene fragment cloned in a yeast artificial chromosome. Nat. Genet. 1993, 4, 117–123, Erratum in Nat. Genet. 1993, 4, 320. [Google Scholar] [CrossRef]
- Jakobovits, A.; Amado, R.G.; Yang, X.; Roskos, L.; Schwab, G. From XenoMouse technology to panitumumab, the first fully human antibody product from transgenic mice. Nat. Biotechnol. 2007, 25, 1134–1143. [Google Scholar] [CrossRef]
- Foltz, I.N.; Gunasekaran, K.; King, C.T. Discovery and bio-optimization of human antibody therapeutics using the XenoMouse® transgenic mouse platform. Immunol. Rev. 2016, 270, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Garambois, V.; Glaussel, F.; Foulquier, E.; Ychou, M.; Pugnière, M.; Luo, R.X.; Bezabeh, B.; Pèlegrin, A. Fully human IgG and IgM antibodies directed against the carcinoembryonic antigen (CEA) Gold 4 epitope and designed for radioimmunotherapy (RIT) of colorectal cancers. BMC Cancer 2004, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Green, L.L. Antibody engineering via genetic engineering of the mouse: XenoMouse strains are a vehicle for the facile generation of therapeutic human monoclonal antibodies. J. Immunol. Methods 1999, 231, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Traggiai, E.; Becker, S.; Subbarao, K.; Kolesnikova, L.; Uematsu, Y.; Gismondo, M.R.; Murphy, B.R.; Rappuoli, R.; Lanzavecchia, A. An efficient method to make human monoclonal antibodies from memory B cells: Potent neutralization of SARS coronavirus. Nat. Med. 2004, 10, 871–875. [Google Scholar] [CrossRef]
- Tiller, T.; Meffre, E.; Yurasov, S.; Tsuiji, M.; Nussenzweig, M.C.; Wardemann, H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods 2008, 329, 112–124, Erratum in J. Immunol. Methods 2008, 334, 142. [Google Scholar] [CrossRef]
- Coronella, J.; Telleman, P.; Truong, T.; Ylera, F.; Junghans, R. Amplification of IgG VH and VL (Fab) from single human plasma cells and B cells. Nucleic Acids Res. 2000, 28, e85. [Google Scholar] [CrossRef][Green Version]
- Pedrioli, A.; Oxenius, A. Single B cell technologies for monoclonal antibody discovery. Trends Immunol. 2021, 42, 1143–1158. [Google Scholar] [CrossRef]
- Spangler, A.; Shimberg, G.D.; Mantus, G.E.; Malek, R.; Cominsky, L.Y.; Tsybovsky, Y.; Li, N.; Gillespie, R.A.; Ravichandran, M.; Creanga, A. Early influenza virus exposure shapes the B cell response to influenza vaccination in individuals 50 years later. Immunity 2025, 58, 728–744.e729. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.-Y.; Li, Y.; Hogerkorp, C.-M.; Schief, W.R.; Seaman, M.S.; Zhou, T.; Schmidt, S.D.; Wu, L.; Xu, L. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010, 329, 856–861. [Google Scholar] [CrossRef]
- Scheid, J.F.; Mouquet, H.; Feldhahn, N.; Seaman, M.S.; Velinzon, K.; Pietzsch, J.; Ott, R.G.; Anthony, R.M.; Zebroski, H.; Hurley, A. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 2009, 458, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; de Alwis, A.R.; Kose, N.; Jadi, R.S.; de Silva, A.M.; Crowe, J.E., Jr. Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones. J. Virol. 2014, 88, 12233–12241. [Google Scholar] [CrossRef]
- Leggat, D.J.; Cohen, K.W.; Willis, J.R.; Fulp, W.J.; Decamp, A.C.; Kalyuzhniy, O.; Cottrell, C.A.; Menis, S.; Finak, G.; Ballweber-Fleming, L. Vaccination induces HIV broadly neutralizing antibody precursors in humans. Science 2022, 378, eadd6502. [Google Scholar] [CrossRef] [PubMed]
- Qiang, M.; Ma, P.; Li, Y.; Liu, H.; Harding, A.; Min, C.; Liu, L.; Yuan, M.; Ji, Q.; Tao, P.; et al. Potent SARS-CoV-2 neutralizing antibodies selected from a human antibody library constructed decades ago. bioRxiv, 2020; preprint. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Makdasi, E.; Alcalay, R.; Mechaly, A.; Levy, Y.; Bercovich-Kinori, A.; Zauberman, A.; Tamir, H.; Yahalom-Ronen, Y.; Israeli, M.; et al. A panel of human neutralizing mAbs targeting SARS-CoV-2 spike at multiple epitopes. Nat. Commun. 2020, 11, 4303. [Google Scholar] [CrossRef]
- Li, W.; Chen, C.; Drelich, A.; Martinez, D.R.; Gralinski, L.E.; Sun, Z.; Schafer, A.; Kulkarni, S.S.; Liu, X.; Leist, S.R.; et al. Rapid identification of a human antibody with high prophylactic and therapeutic efficacy in three animal models of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2020, 117, 29832–29838. [Google Scholar] [CrossRef]
- Shirai, H.; Ikeda, K.; Yamashita, K.; Tsuchiya, Y.; Sarmiento, J.; Liang, S.; Morokata, T.; Mizuguchi, K.; Higo, J.; Standley, D.M. High-resolution modeling of antibody structures by a combination of bioinformatics, expert knowledge, and molecular simulations. Proteins Struct. Funct. Bioinform. 2014, 82, 1624–1635. [Google Scholar] [CrossRef]
- Leem, J.; Dunbar, J.; Georges, G.; Shi, J.; Deane, C.M. Bodybuilder: Automated antibody structure prediction with data–driven accuracy estimation. mAbs 2016, 8, 1259–1268. [Google Scholar] [CrossRef]
- Sivasubramanian, A.; Sircar, A.; Chaudhury, S.; Gray, J.J. Toward high-resolution homology modeling of antibody Fv regions and application to antibody–antigen docking. Proteins Struct. Funct. Bioinform. 2009, 74, 497–514. [Google Scholar] [CrossRef]
- Krieger, E.; Nabuurs, S.B.; Vriend, G. Homology modeling. Struct. Bioinform. 2003, 44, 509–523. [Google Scholar]
- Diller, D.J.; Li, R. Kinases, homology models, and high throughput docking. J. Med. Chem. 2003, 46, 4638–4647. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Jimenez, X.; Zhang, H.; Bohlen, P.; Witte, L.; Zhu, Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int. J. Cancer 2002, 97, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Khakzad, H.; Igashov, I.; Schneuing, A.; Goverde, C.; Bronstein, M.; Correia, B. A new age in protein design empowered by deep learning. Cell Syst. 2023, 14, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Notin, P.; Rollins, N.; Gal, Y.; Sander, C.; Marks, D. Machine learning for functional protein design. Nat. Biotechnol. 2024, 42, 216–228. [Google Scholar] [CrossRef]
- Erlach, L.; Friedensohn, S.; Neumeier, D.; Mason, D.M.; Reddy, S.T. Antibody affinity engineering using antibody repertoire data and machine learning. bioRxiv, 2025; preprint. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Krishna, R.; Wang, J.; Ahern, W.; Sturmfels, P.; Venkatesh, P.; Kalvet, I.; Lee, G.R.; Morey-Burrows, F.S.; Anishchenko, I.; Humphreys, I.R. Generalized biomolecular modeling and design with RoseTTAFold All-Atom. Science 2024, 384, eadl2528. [Google Scholar] [CrossRef]
- Baek, M.; McHugh, R.; Anishchenko, I.; Jiang, H.; Baker, D.; DiMaio, F. Accurate prediction of protein–nucleic acid complexes using RoseTTAFoldNA. Nat. Methods 2024, 21, 117–121. [Google Scholar] [CrossRef]
- Brandes, N.; Ofer, D.; Peleg, Y.; Rappoport, N.; Linial, M. ProteinBERT: A universal deep-learning model of protein sequence and function. Bioinformatics 2022, 38, 2102–2110. [Google Scholar] [CrossRef]
- Sumida, K.H.; Núñez-Franco, R.; Kalvet, I.; Pellock, S.J.; Wicky, B.I.; Milles, L.F.; Dauparas, J.; Wang, J.; Kipnis, Y.; Jameson, N. Improving protein expression, stability, and function with ProteinMPNN. J. Am. Chem. Soc. 2024, 146, 2054–2061. [Google Scholar] [CrossRef]
- Dauparas, J.; Anishchenko, I.; Bennett, N.; Bai, H.; Ragotte, R.J.; Milles, L.F.; Wicky, B.I.; Courbet, A.; de Haas, R.J.; Bethel, N. Robust deep learning–based protein sequence design using ProteinMPNN. Science 2022, 378, 49–56. [Google Scholar] [CrossRef]
- Watson, J.L.; Juergens, D.; Bennett, N.R.; Trippe, B.L.; Yim, J.; Eisenach, H.E.; Ahern, W.; Borst, A.J.; Ragotte, R.J.; Milles, L.F. De novo design of protein structure and function with RFdiffusion. Nature 2023, 620, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, J.A.; Chu, L.-S.; Mahajan, S.P.; Gray, J.J. Fast, accurate antibody structure prediction from deep learning on massive set of natural antibodies. Nat. Commun. 2023, 14, 2389. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Su, Y.; Peng, X.; Wang, S.; Peng, J.; Ma, J. Antigen-specific antibody design and optimization with diffusion-based generative models for protein structures. Adv. Neural Inf. Process. Syst. 2022, 35, 9754–9767. [Google Scholar]
- Liu, G.; Zeng, H.; Mueller, J.; Carter, B.; Wang, Z.; Schilz, J.; Horny, G.; Birnbaum, M.E.; Ewert, S.; Gifford, D.K. Antibody complementarity determining region design using high-capacity machine learning. Bioinformatics 2020, 36, 2126–2133. [Google Scholar] [CrossRef]
- Mason, D.M.; Friedensohn, S.; Weber, C.R.; Jordi, C.; Wagner, B.; Meng, S.M.; Ehling, R.A.; Bonati, L.; Dahinden, J.; Gainza, P. Optimization of therapeutic antibodies by predicting antigen specificity from antibody sequence via deep learning. Nat. Biomed. Eng. 2021, 5, 600–612. [Google Scholar] [CrossRef]
- Goodfellow, I.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative adversarial networks. Commun. ACM 2020, 63, 139–144. [Google Scholar] [CrossRef]
- Amimeur, T.; Shaver, J.M.; Ketchem, R.R.; Taylor, J.A.; Clark, R.H.; Smith, J.; Van Citters, D.; Siska, C.C.; Smidt, P.; Sprague, M. Designing feature-controlled humanoid antibody discovery libraries using generative adversarial networks. bioRxiv, 2020; preprint. [Google Scholar] [CrossRef]
- Wang, R.; Wu, F.; Gao, X.; Wu, J.; Zhao, P.; Yao, J. Iggm: A generative model for functional antibody and nanobody design. bioRxiv, 2024; preprint. [Google Scholar] [CrossRef]
- Bennett, N.R.; Watson, J.L.; Ragotte, R.J.; Borst, A.J.; See, D.L.; Weidle, C.; Biswas, R.; Shrock, E.L.; Leung, P.J.; Huang, B. Atomically accurate de novo design of single-domain antibodies. bioRxiv Prepr. Serv. Biol. 2024; preprint. [Google Scholar]
- Schneider, C.; Buchanan, A.; Taddese, B.; Deane, C.M. DLAB: Deep learning methods for structure-based virtual screening of antibodies. Bioinformatics 2022, 38, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Bio, N.; Biswas, S. De novo design of hundreds of functional GPCR-targeting antibodies enabled by scaling test-time compute. bioRxiv, 2025; preprint. [Google Scholar]
- Venderley, J. AntiBARTy Diffusion for Property Guided Antibody Design. arXiv 2023, arXiv:2309.13129. [Google Scholar] [CrossRef]
- Shanehsazzadeh, A.; Bachas, S.; McPartlon, M.; Kasun, G.; Sutton, J.M.; Steiger, A.K.; Shuai, R.; Kohnert, C.; Rakocevic, G.; Gutierrez, J.M. Unlocking de novo antibody design with generative artificial intelligence. bioRxiv, 2023; preprint. [Google Scholar]
- Corso, G.; Stärk, H.; Jing, B.; Barzilay, R.; Jaakkola, T. DiffDock: Diffusion Steps, Twists, and Turns for Molecular Docking. arXiv 2022, arXiv:2210.01776. [Google Scholar]
- Martinkus, K.; Ludwiczak, J.; Cho, K.; Lian, W.-C.; Lafrance-Vanasse, J.; Hotzel, I.; Rajpal, A.; Wu, Y.; Bonneau, R.; Gligorijevic, V.; et al. AbDiffuser: Full-Atom Generation of In-Vitro Functioning Antibodies. Adv. Neural Inf. Process. Syst. 2023, 36, 40729–40759. [Google Scholar]
- Dewaker, V.; Morya, V.K.; Kim, Y.H.; Park, S.T.; Kim, H.S.; Koh, Y.H. Revolutionizing oncology: The role of Artificial Intelligence (AI) as an antibody design, and optimization tools. Biomark. Res. 2025, 13, 52. [Google Scholar] [CrossRef]
- Kang, Y.; Jin, K.; Pan, L. AI designed, mutation resistant broad neutralizing antibodies against multiple SARS-CoV-2 strains. Sci. Rep. 2025, 15, 15533. [Google Scholar] [CrossRef]
- Venkataraman, S.; Li, Y.C.; Hung, Z.W.; Hsu, Y.C.; Yang, Z.; Tsai, T.I.; Hung, M.C.; Han, K.H.; Lin, C.W. Epitope-guided selection of CXCR4-targeting antibodies using AlphaFold3 for GPCR modulation and cancer therapy. Am. J. Cancer Res. 2025, 15, 2127–2139. [Google Scholar] [CrossRef]
- Kim, J.; McFee, M.; Fang, Q.; Abdin, O.; Kim, P.M. Computational and artificial intelligence-based methods for antibody development. Trends Pharmacol. Sci. 2023, 44, 175–189. [Google Scholar] [CrossRef]
- Clifford, J.N.; Richardson, E.; Peters, B.; Nielsen, M. AbEpiTope-1.0: Improved antibody target prediction by use of AlphaFold and inverse folding. Sci. Adv. 2025, 11, eadu1823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).