Multi-Omics Analysis Reveals the Regulation of Amino Acid Biosynthesis in Cyclocarya paliurus Leaves Under Salt Stress
Abstract
1. Introduction
2. Results
2.1. Amino Acids Accumulation in C. paliurus Leaves
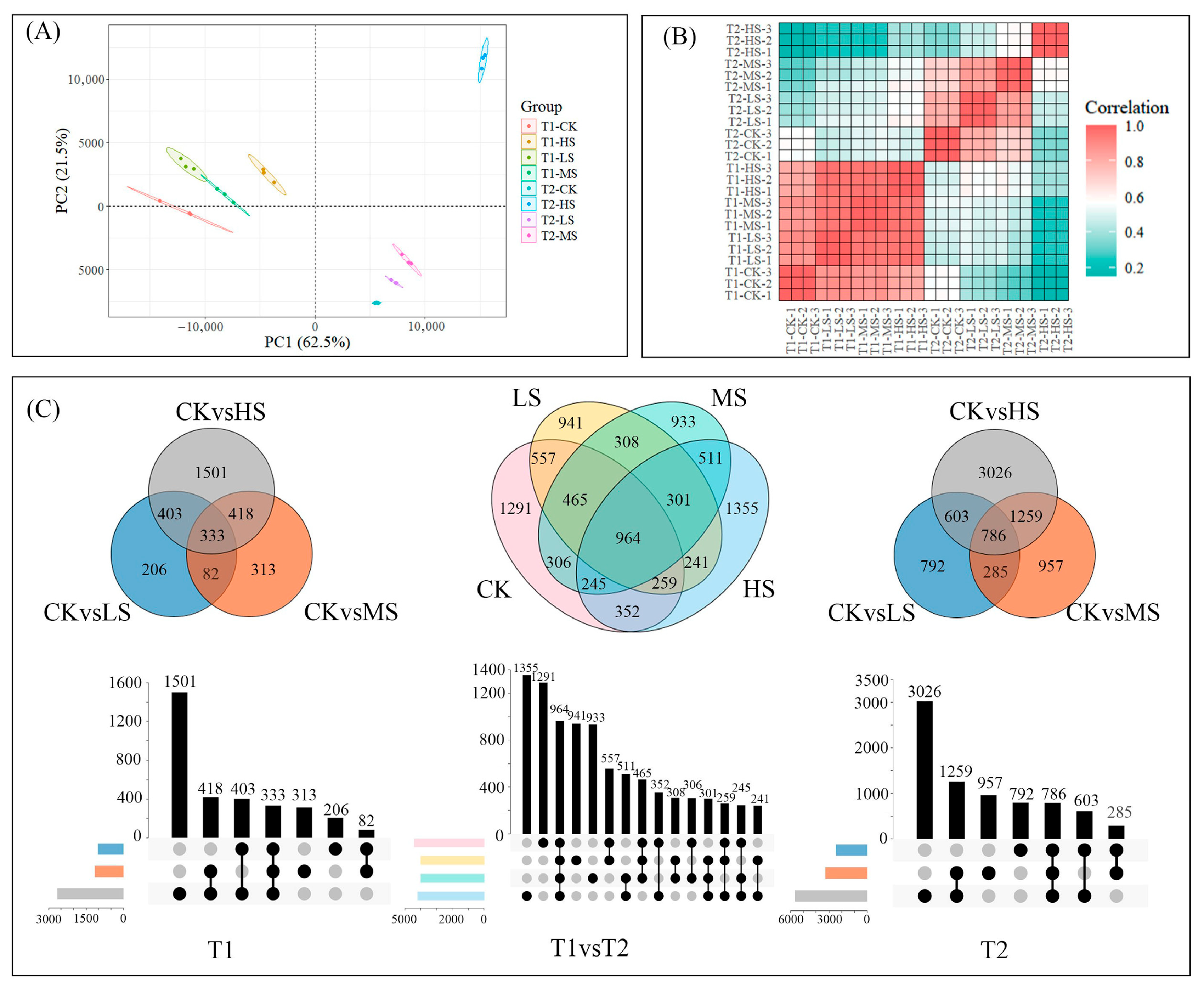
2.2. Transcriptomic Response in C. paliurus Leaves
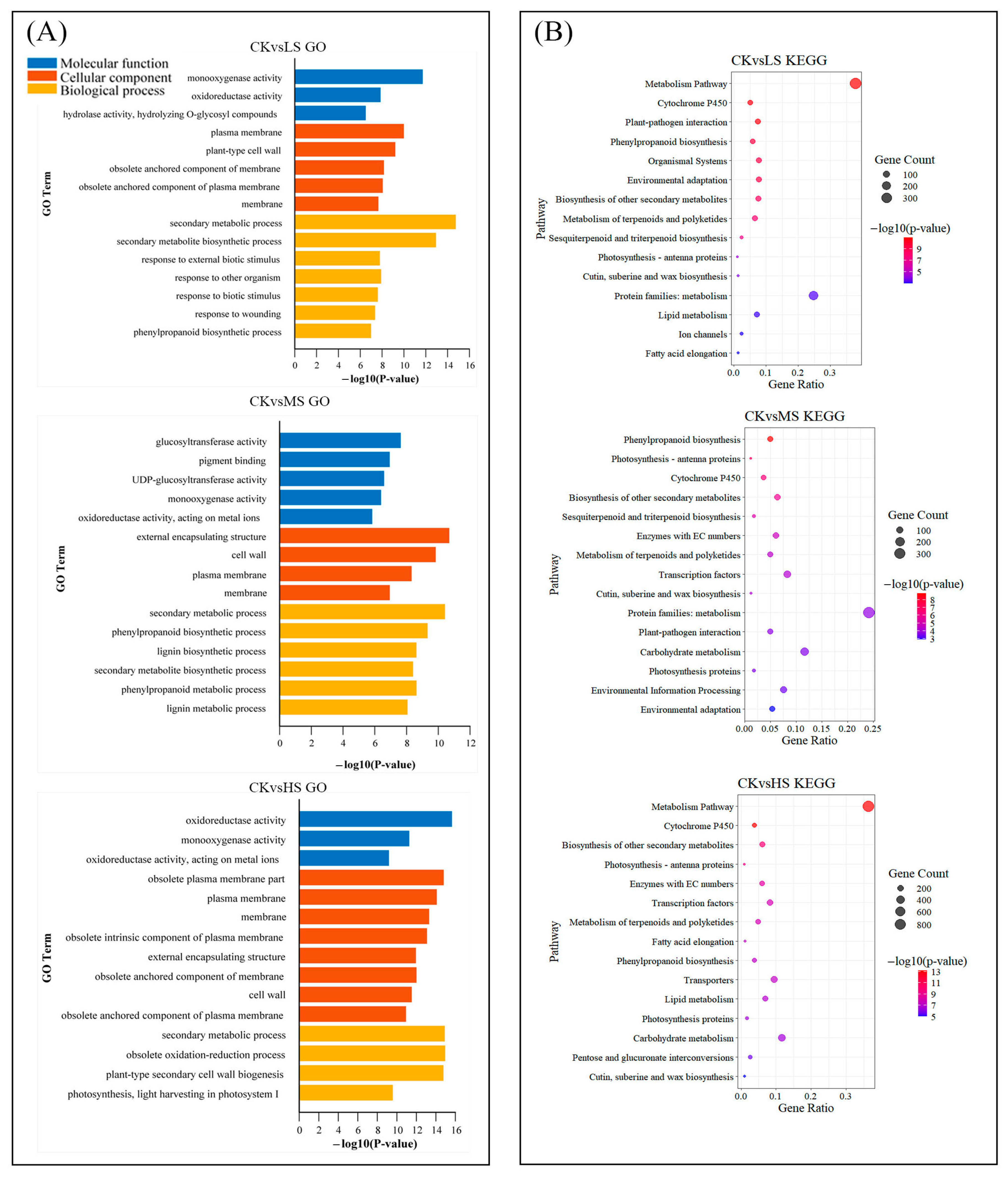
2.3. GO and KEGG Enrichment Analyses
2.4. Identification of WGCNA Modules Associated with Amino Acid Accumulation
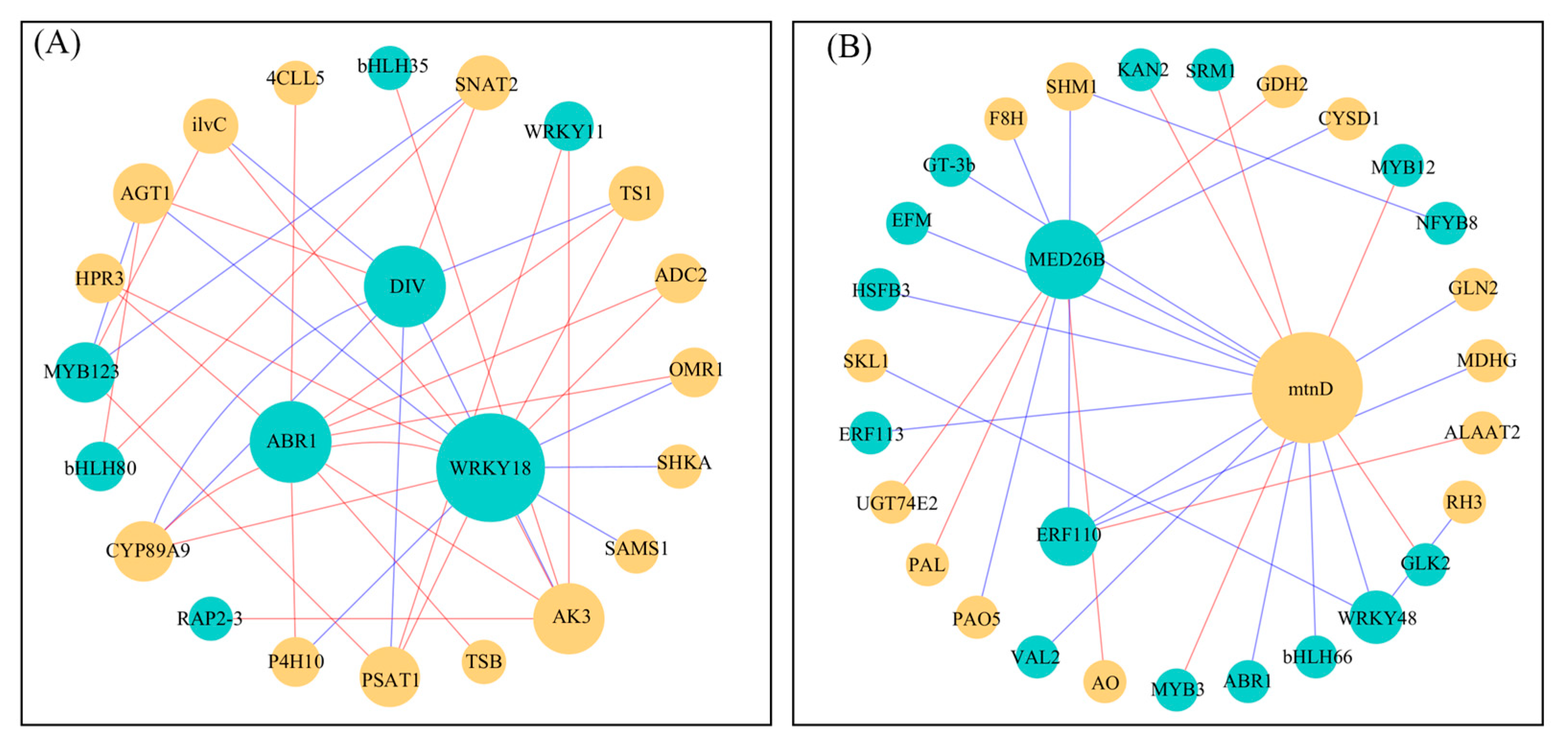
2.5. Construction of Gene Co-Expression Networks
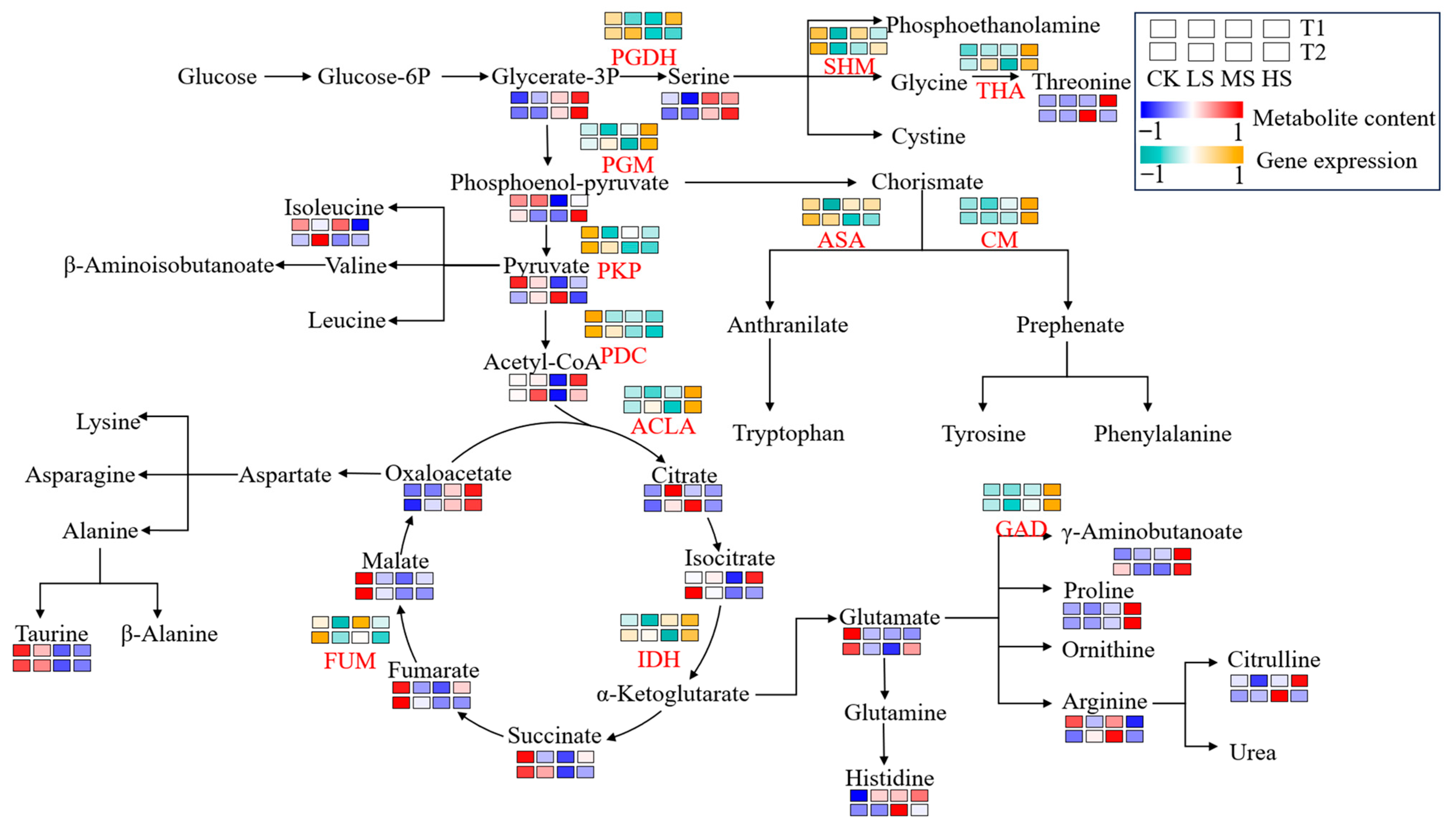
2.6. Gene Expression and Metabolite Accumulation in Carbohydrate and Amino Acid Metabolism Process
3. Discussion
3.1. Salinity Effects on Amino Acids Accumulation
3.2. Salinity Effects on Gene Expression
3.3. Regulated Gene Network of Amino Acids Biosynthesis
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Sample Collection, Amino Acids Extraction and Determination
4.3. Metabolomics Analysis
4.4. Transcriptomics Analysis
4.5. Weighted Gene Co-Expressed Network Analysis (WGCNA)
4.6. Statistics Analysis and Visualisations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Jiang, J.; Shan, Q.; Chen, G.; Wang, Y.; Shen, L.; Pan, C.; Wu, H.; Aljoy, A. Soil salinization and ecological remediation by planting trees in China. In Proceedings of the 2010 International Conference on Mechanic Automation and Control Engineering, Wuhan, China, 26–28 June 2010. [Google Scholar]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Hannachi, S.; Labeke, M.-C.V. Salt stress affects germination, seedling growth and physiological responses differentially in eggplant cultivars (Solanum melongena L.). Sci. Hortic. 2018, 228, 56–65. [Google Scholar] [CrossRef]
- Dhiman, P.; Rajora, N.; Bhardwaj, S.; Sudhakaran, S.S.; Kumar, A.; Raturi, G.; Chakraborty, K.; Gupta, O.P.; Devanna, B.N.; Tripathi, D.K.; et al. Fascinating role of silicon to combat salinity stress in plants: An updated overview. Plant Physiol. Biochem. 2021, 162, 110–123. [Google Scholar] [CrossRef]
- Hou, J.; Wan, H.; Zhang, J.; Liang, K.; Cui, B.; Ma, Y.; Chen, Y.; Wang, Y.; Liu, J.; Liu, X.; et al. Biochar amendment combined with partial root-zone drying irrigation alleviates salinity stress and improves root morphology and water use efficiency in cotton plant. Sci. Total Environ. 2023, 904, 166978. [Google Scholar] [CrossRef]
- Duan, H.; Tiika, J.; Cui, G.; Tian, F.; Yu, H.; Zhu, X.; Lu, Y.; Zhang, Q.; Wang, C.; Li, Y.; et al. SeXTH23 enhances salt tolerance by regulating succulence and osmotic adjustment in aspen hybrid. Plant Sci. 2025, 362, 112783. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Waqas, M.; Park, J.; Asif, S.; Kim, N.; Lee, I.; Kim, K. Drought and UV radiation stress tolerance in rice is improved by overaccumulation of non-enzymatic antioxidant flavonoids. Antioxidants 2022, 11, 917. [Google Scholar] [CrossRef]
- Wang, F.; Yang, X.; Li, W.; Ning, P.; Huang, X. Metabolomics and transcriptomics reveal the accumulation of key metabolites and flavor formation in the fresh leaves of wild and cultivated tea plants. Ind. Crops Prod. 2025, 235, 121780. [Google Scholar] [CrossRef]
- Turfan, N.; Khubalıyev, İ.; Tekşen, K.; Altuner, E. Investigating Exogenous Tyrosine Supplements on the Responses of the Kale Plant to Salinity Stress. Food Sci. Nutr. 2025, 13, e70660, Correction in Food Sci. Nutr. 2025, 13, e70873. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Dong, C.; Nie, S.; Li, F.; Wang, Z.; Shen, M.; Xie, M. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015, 186, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, X.; Shen, M.; Nie, S.; Zhang, H.; Li, C.; Gong, D.; Xie, M. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef]
- Yang, H.; Yin, Z.; Zhao, M.; Jiang, C.; Zhang, J.; Pan, K. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry 2018, 151, 119–127. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Chen, P.; Chen, R.; Cheng, T. Sequential determination of tannin and total amino acid contents in tea for taste assessment by a fluorescent flow-injection analytical system. Food Chem. 2010, 118, 876–881. [Google Scholar] [CrossRef]
- Nahar, M.N.-E.-N.; Islam, M.M.; Hoque, M.A.; Yonezawa, A.; Prodhan, M.Y.; Nakamura, T.; Nakamura, Y.; Munemasa, S.; Murata, Y. Exogenous proline enhances the sensitivity of Tobacco BY-2 cells to arsenate. Biosci. Biotechnol. Biochem. 2017, 81, 1726–1731. [Google Scholar] [CrossRef][Green Version]
- Kaspal, M.; Kanapaddalagamage, M.H.; Ramesh, S.A. Emerging roles of γ aminobutyric acid (GABA) gated channels in plant stress tolerance. Plants 2021, 10, 2178. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Gu, Z.; Tao, Y.; Shen, C.; Zhou, Y.; Han, Y.; Yang, R. Ca2+ involved in GABA signal transduction for phenolics accumulation in germinated hulless barley under NaCl stress. Food Chem. X 2019, 2, 100023, Erratum in Food Chemistry. 2021, 12, 100111. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Su, N.; Huang, X.; Cui, J.; Shabala, L.; Zhou, M.; Yu, M.; Shabala, S. Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun. 2021, 2, 100188. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Shams, M.; Turan, M.; Ucar, S.; Yaprak, E.; Yuksel, E.A.; Aydin, M.; Ilhan, E.; Agar, G.; Ercisli, S.; et al. Chitosan mitigated the adverse effect of Cd by regulating antioxidant activities, hormones, and organic acids contents in pepper (Capsicum annum L.). Heliyon 2024, 10, e36867. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; McFarland, J.; Blazewicz, S.J.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B.; et al. Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature 2015, 521, 208–212. [Google Scholar] [CrossRef]
- Li, C.; Wan, Y.; Shang, X.; Fang, S. Integration of transcriptomic and metabolomic analysis unveils the response mechanism of sugar metabolism in Cyclocarya paliurus seedlings subjected to PEG-induced drought stress. Plant Physiol. Biochem. 2023, 201, 107856. [Google Scholar] [CrossRef]
- Momayezi, M.R.; Zaharah, A.R.; Hanafi, M.M.; Razi, M. Amino acids status in Iranian rice (Oryza sativa L.) as affected by different salt compositions. Int. J. Ecol. Dev. 2010, 17, 77–88. [Google Scholar]
- Celi, G.E.A.; Tenesaca, L.F.L.; Calzada, K.P.; Alves, R.d.C.; Cruz, M.C.P.d.; Junior, J.S.P.; Carrega, W.C.; Gratão, P.L. Exogenous ascorbic acid mitigates salt-induced damage in soybean by modulating photosynthesis, antioxidant defense, and ionic homeostasis. Acta Physiol. Plant. 2025, 47, 26. [Google Scholar] [CrossRef]
- Targino, V.A.; Dias, T.J.; Sousa, V.F.d.O.; Silva, M.d.M.; Silva, A.J.d.; Ribeiro, J.E.d.S.; Silva, R.F.d.; Batista, D.S.; Henschel, J.M.; Rêgo, M.M.d. Growth, gas exchange, and phytochemical quality of nasturtium (Tropaeolum majus L.) subjected to proline concentrations and salinity. Plants 2025, 14, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Preet, K.; Dhansu, P.; Sehrawat, N.; Kumar, R.; Appunu, C.; Vengavasi, K.; Arunkumar, R.; Rana, R.; Kumar, S.; Joon, V. Morpho-physiological analysis of salinity tolerance in sugarcane genotypes. Plant Physiol. Rep. 2024, 29, 356–366. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, X.; Liu, B.; Wang, J.; Shan, J.; Wang, J.; Zhang, Y.; Li, G.; Jia, Y.; Wang, R. Transcriptome and metabolome reveal the primary and secondary metabolism changes in Larix gmelinii seedlings under abiotic stress. BMC Plant Biol. 2024, 24, 1128. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.; Long, Q.; Xing, J.; Guo, P.; Liu, Y.; Zhang, C.; Zhang, Y.; Fernie, A.R.; Shi, Y.; et al. OsBCAT2, a gene responsible for the degradation of branched-chain amino acids, positively regulates salt tolerance by promoting the synthesis of vitamin B5. New Phytol. 2024, 241, 2558–2574. [Google Scholar] [CrossRef]
- Vijay, J.; Je-Gun, J.; Zhangjun, F.; Georg, J. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef]
- Nefissi Ouertani, R.; Arasappan, D.; Abid, G.; Ben Chikha, M.; Jardak, R.; Mahmoudi, H.; Mejri, S.; Ghorbel, A.; Ruhlman, T.A.; Jansen, R.K. Transcriptomic analysis of salt-stress-responsive genes in barley roots and leaves. Int. J. Mol. Sci. 2021, 22, 8155. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Qiu, Y.; Tao, Y.; Zhang, L.; Okita, T.W.; Yan, Y.; Tian, L. RNA-seq analysis reveals transcriptome reprogramming and alternative splicing during early response to salt stress in tomato root. Front. Plant Sci. 2024, 15, 1394223. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Gao, C.; Fu, F.; Wang, T.; El-Kassaby, Y.A.; Wang, G. Amino acid metabolism reprogramming in response to changing growth environment in Ginkgo biloba leaves. LWT 2021, 144, 111276. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.; Wang, Y.; Xu, Y.; Li, J.; Zhao, S.; Wang, D.; Ma, Z.; Yan, F.; Liu, Y. Combined transcriptomic and metabolomic analysis reveals the role of phenylpropanoid biosynthesis pathway in the salt tolerance process of Sophora alopecuroides. Int. J. Mol. Sci. 2021, 22, 2399. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Guo, B.; Wang, B.; Li, X.; Yang, T.; Li, N.; Wang, J.; Yu, Q. Integrated metabolomic and transcriptomic analysis reveals the role of phenylpropanoid biosynthesis pathway in tomato roots during salt stress. Front. Plant Sci. 2022, 13, 1023696. [Google Scholar] [CrossRef]
- Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB transcription factors as regulators of secondary metabolism in plants. Biology 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, L.; Zhang, P.; Liu, J.; Li, L.; Li, H.; Wang, X.; Bai, Y.; Jiang, G.; Qin, P. Comparative transcriptomes and WGCNA reveal hub genes for spike germination in different quinoa lines. BMC Genom. 2024, 25, 1231. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Fang, S.; Liu, Y.; Shang, X. Integrative analysis of metabolome and transcriptome reveals molecular regulatory mechanism of flavonoid biosynthesis in Cyclocarya paliurus under salt stress. Ind. Crops Prod. 2021, 170, 113823. [Google Scholar] [CrossRef]
- Gad, G.; Rachel, A. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar]
- Habib, A.; Maachia, S.B.; Namsi, A.; Slimane, M.H.B.; Jeandet, P.; Aziz, A. Evaluation of salt stress-induced changes in polyamine, amino acid, and phytoalexin profiles in mature fruits of grapevine cultivars grown in Tunisian Oases. Plants 2023, 12, 4031. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, B.; Chen, Z.; Huo, Y. Metabolic engineering of Corynebacterium glutamicum for producing branched chain amino acids. Microb. Cell Factories 2021, 20, 230. [Google Scholar] [CrossRef]
- Karamat, U.; Guo, J.; Jiang, S.; Khan, I.; Lu, M.; Fu, M.; Li, G. Comprehensive, genome-wide identification and expression analyses of phenylalanine ammonia-lyase family under abiotic stresses in Brassica oleracea. Int. J. Mol. Sci. 2024, 25, 10276. [Google Scholar] [CrossRef]
- Tian, J.; Xu, R.; Chang, K.; Yuan, S.; Huang, C.; Wang, J.; Li, S.; Liu, F.; Zhong, F. Identification of PAL gene in purple cabbage and functional analysis related to anthocyanin synthesis. Horticulturae 2023, 9, 469. [Google Scholar] [CrossRef]
- Mu, D.; Chen, L.; Wang, H.; Hu, Z.; Chen, S.; Chen, S.; Cai, N.; Xu, Y.; Tang, J. The identification of phenylalanine ammonia-lyase (PAL) genes from Pinus yunnanensis and an analysis of enzyme activity in vitro. Phyton-Int. J. Exp. Bot. 2024, 93, 503–516. [Google Scholar] [CrossRef]
- Yuan, D.; Wu, X.; Gong, B.; Huo, R.; Zhao, L.; Li, J.; Lü, G.; Gao, H. GABA metabolism, transport and their roles and mechanisms in the regulation of abiotic stress (hypoxia, salt, drought) resistance in plants. Metabolites 2023, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Siarhei, D.A.; Stanislav, I.V. The role of the γ-aminobutyric acid (GABA) in plant salt stress tolerance. Horticulturae 2023, 9, 230. [Google Scholar] [CrossRef]
- Hajihoseinlou, S.; Rezayian, M.; Niknam, V.; Esfandiari, E. Exogenous γ-aminobutyric acid and penconazole mitigated salt stress-induced impairments in Triticum aestivum plants. Cereal Res. Commun. 2025, 53, 1413–1425. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Y.; Wang, R.; He, Y.; Ma, X.; Shen, J.; He, Z.; Lai, H. Effects of exogenous abscisic acid on the physiological and biochemical responses of Camellia oleifera seedlings under drought stress. Plants 2024, 13, 225. [Google Scholar] [CrossRef]
- Song, J.; Wang, J.; Qin, R.; Ji, G.; Cui, C.; Sun, N.; Qi, S.; Ding, C.; Zhang, H. RNA-Seq-based WGCNA reveals the physiological and molecular responses of poplar leaves to NaHCO3 stress. Trees 2024, 39, 3. [Google Scholar]
- Sifau, A.A.; Bosede, O.; Agnes, A.O.; Abiodun, A.; Omotayo, A.D. Anatomical changes, osmolytes accumulation and distribution in the native plants growing on Pb-contaminated sites. Environ. Geochem. Health 2020, 43, 1537–1549. [Google Scholar]
- Yang, H.; Wu, Y.; Che, J.; Lyu, L.; Wu, W.; Cao, F.; Li, W. Effects of cadmium stress on the growth, physiology, mineral uptake, cadmium accumulation and fruit quality of ‘Sharpblue’ blueberry. Sci. Hortic. 2024, 337, 113593. [Google Scholar] [CrossRef]
- Geng, W.; Zhang, Y.; Li, C.; Song, G.; Shi, S. Effect of exogenous γ-aminobutyric acid (GABA) on the growth, photosynthetic pigment, antioxidant and GABA metabolism of Festuca arundinacea (tall fescues) under cadmium stress. Plants 2025, 14, 383. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Anokhina, G.B.; Shakhov, Z.N.; Moskvina, P.P.; Igamberdiev, A.U. The role of glutamate metabolism and the GABA shunt in bypassing the tricarboxylic acid cycle in the light. Int. J. Mol. Sci. 2024, 25, 12711. [Google Scholar] [CrossRef]
- Fang, S.; Wang, J.; Wei, Z.; Zhu, Z. Methods to break seed dormancy in Cyclocarya paliurus (Batal) Iljinskaja. Sci. Hortic. 2006, 110, 305–309. [Google Scholar] [CrossRef]
- YAO, R. Calcium alleviation of sodium toxicity in salt-treated Cyclocarya paliurus seedlings. Afr. J. Agric. Res. 2011, 6, 5967–5971. [Google Scholar] [CrossRef]
- Zhou, M.; Hua, T.; Ma, X.; Sun, H.; Xu, L. Protein content and amino acids profile in 10 cultivars of ginkgo (Ginkgo biloba L.) nut from China. R. Soc. Open Sci. 2019, 6, 181571. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Guo, Y.; Gao, C.; Wang, M.; Fu, F.-F.; El-Kassaby, Y.A.; Wang, T.; Wang, G. Metabolome and transcriptome analyses reveal flavonoids biosynthesis differences in Ginkgo biloba associated with environmental conditions. Ind. Crops Prod. 2020, 158, 112963. [Google Scholar] [CrossRef]
- Michael, K.; Sebastian, W.; Bettina, W. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Hong, K.; Zhang, Z.; Shang, X.; Fang, S. Multi-Omics Analysis Reveals the Regulation of Amino Acid Biosynthesis in Cyclocarya paliurus Leaves Under Salt Stress. Int. J. Mol. Sci. 2025, 26, 10444. https://doi.org/10.3390/ijms262110444
Zhang L, Hong K, Zhang Z, Shang X, Fang S. Multi-Omics Analysis Reveals the Regulation of Amino Acid Biosynthesis in Cyclocarya paliurus Leaves Under Salt Stress. International Journal of Molecular Sciences. 2025; 26(21):10444. https://doi.org/10.3390/ijms262110444
Chicago/Turabian StyleZhang, Lei, Kun Hong, Zijie Zhang, Xulan Shang, and Shengzuo Fang. 2025. "Multi-Omics Analysis Reveals the Regulation of Amino Acid Biosynthesis in Cyclocarya paliurus Leaves Under Salt Stress" International Journal of Molecular Sciences 26, no. 21: 10444. https://doi.org/10.3390/ijms262110444
APA StyleZhang, L., Hong, K., Zhang, Z., Shang, X., & Fang, S. (2025). Multi-Omics Analysis Reveals the Regulation of Amino Acid Biosynthesis in Cyclocarya paliurus Leaves Under Salt Stress. International Journal of Molecular Sciences, 26(21), 10444. https://doi.org/10.3390/ijms262110444






