Dendritic Polyglycerol Sulfate Reduces Inflammation Through Inhibition of the HMGB1/RAGE Axis in RAW 264.7 Macrophages
Abstract
1. Introduction
2. Results
2.1. Cloning, Recombinant Expression, and Purification of Human HMGB1 from E.coli
2.2. HMGB1/RAGE Interaction Is Reduced by dPGS
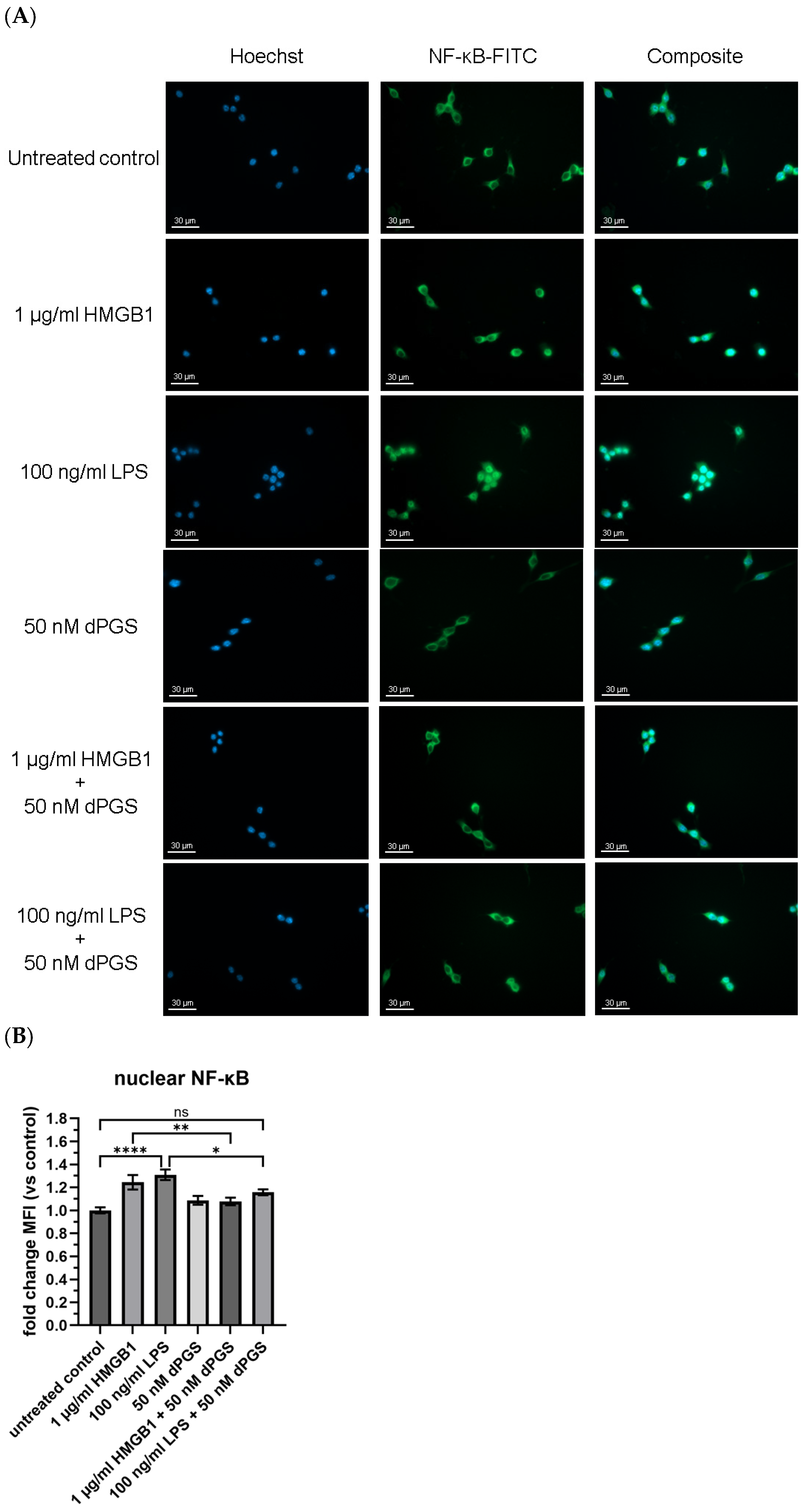
2.3. Shuttling of NF-κB in HMGB1 and LPS-Stimulated Macrophages Is Reduced by dPGS
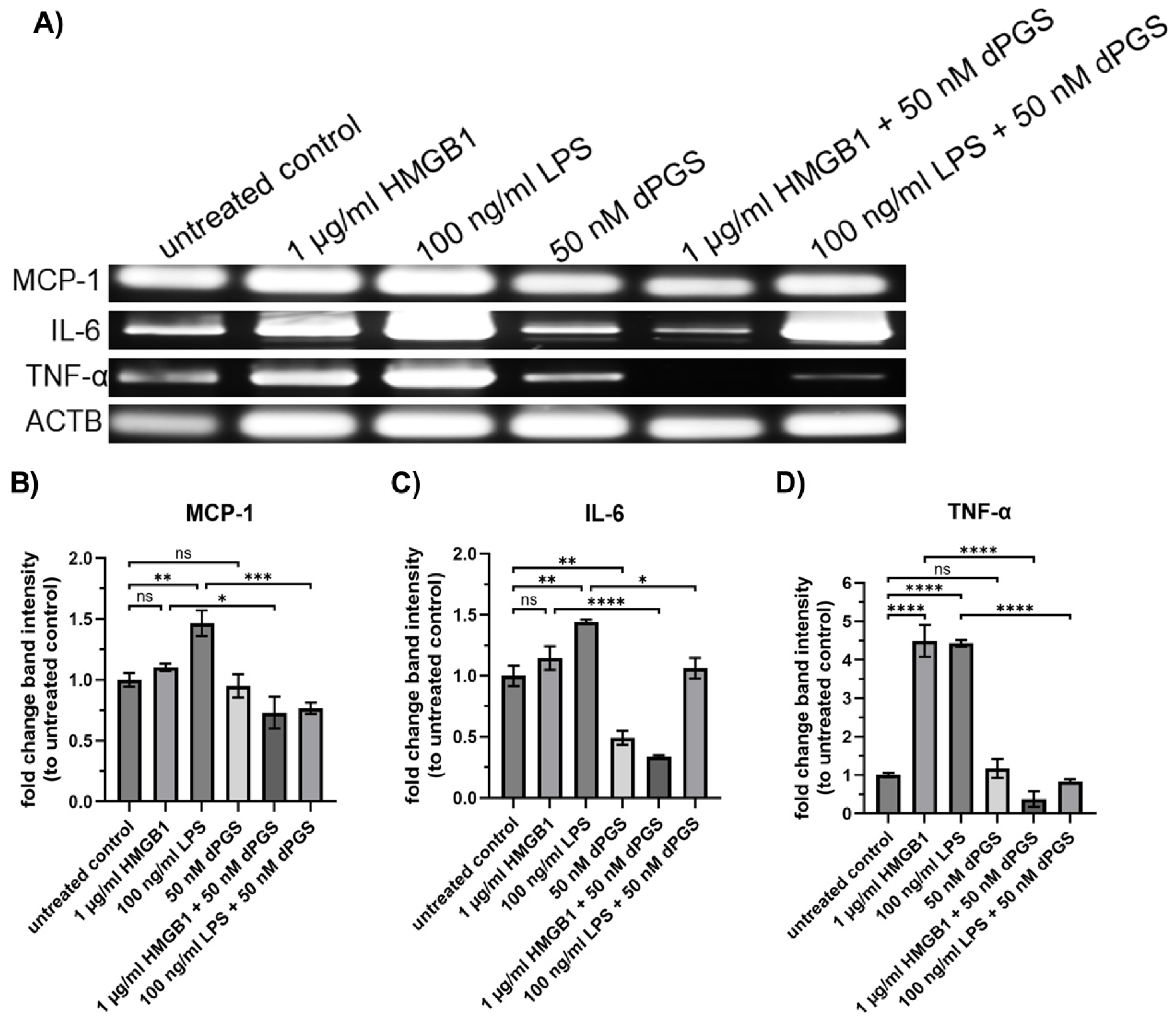
2.4. Dendritic Polyglycerol Sulfate Reduces mRNA Levels of Cytokines
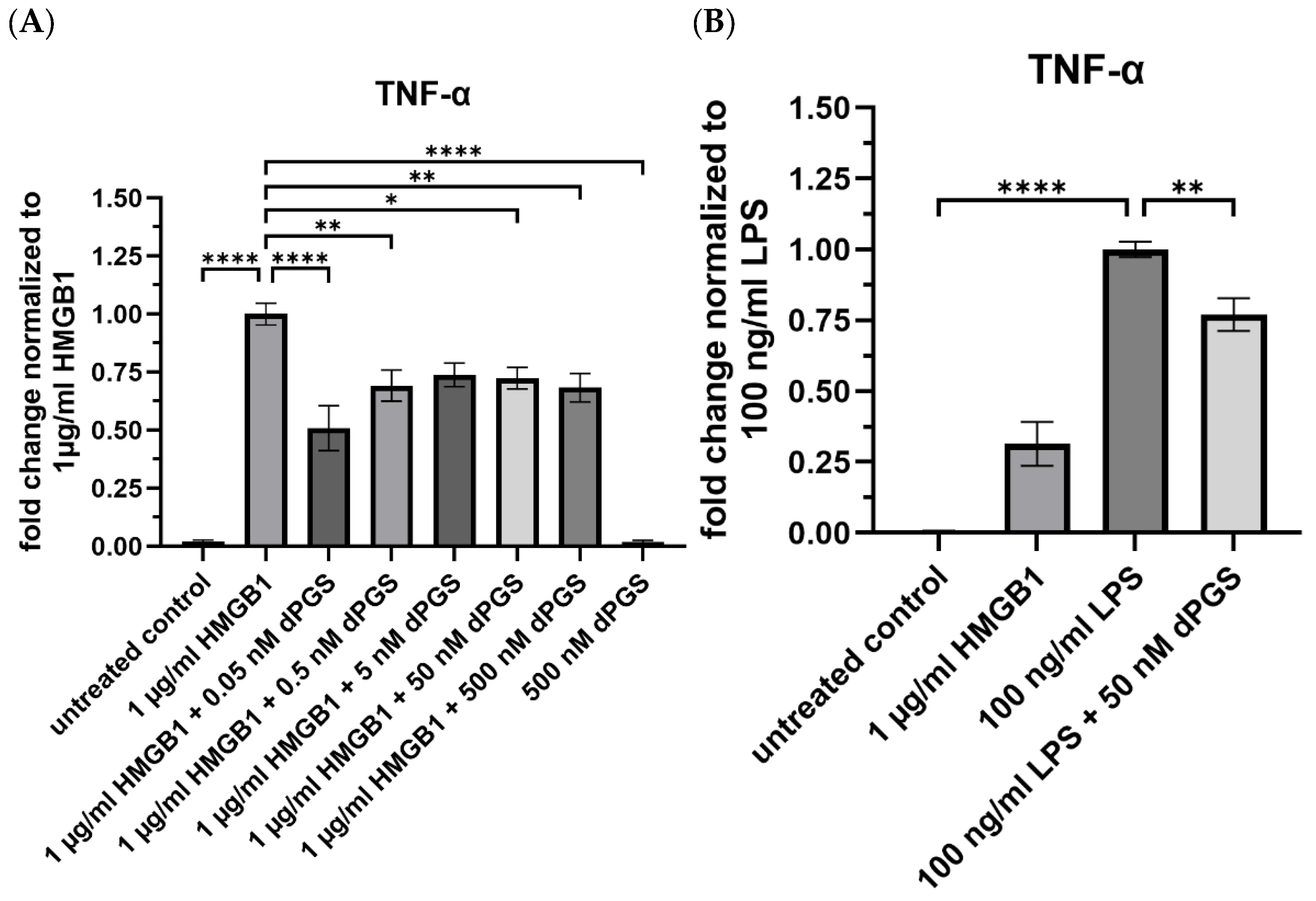
2.5. Dendritic Polyglycerol Sulfate Reduces the Release of the Protein TNF-α and NO in HMGB1 and LPS-Stimulated Mouse Macrophages
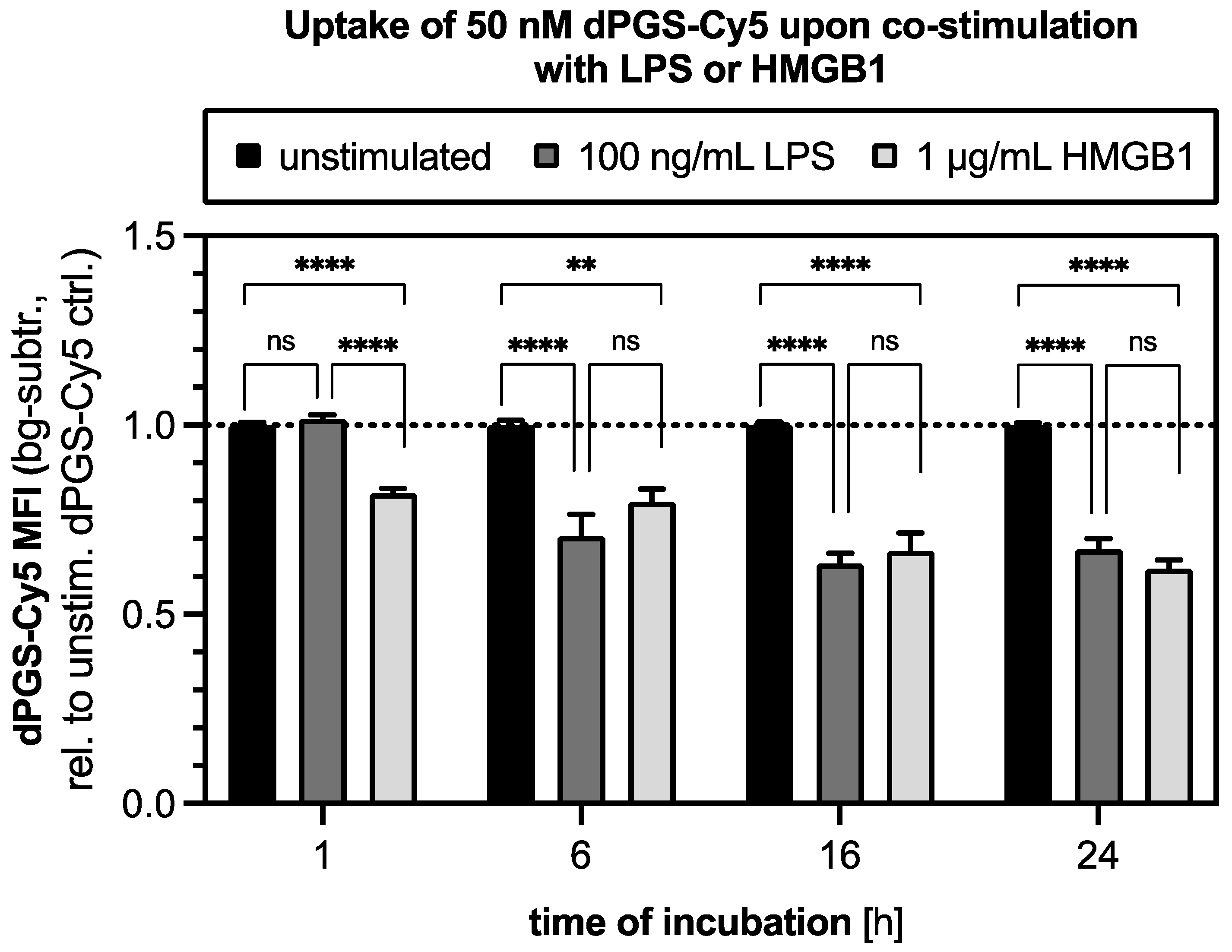
2.6. Uptake of dPGS in Mouse Macrophages Is Altered When Co-Stimulated with HMGB1 and LPS
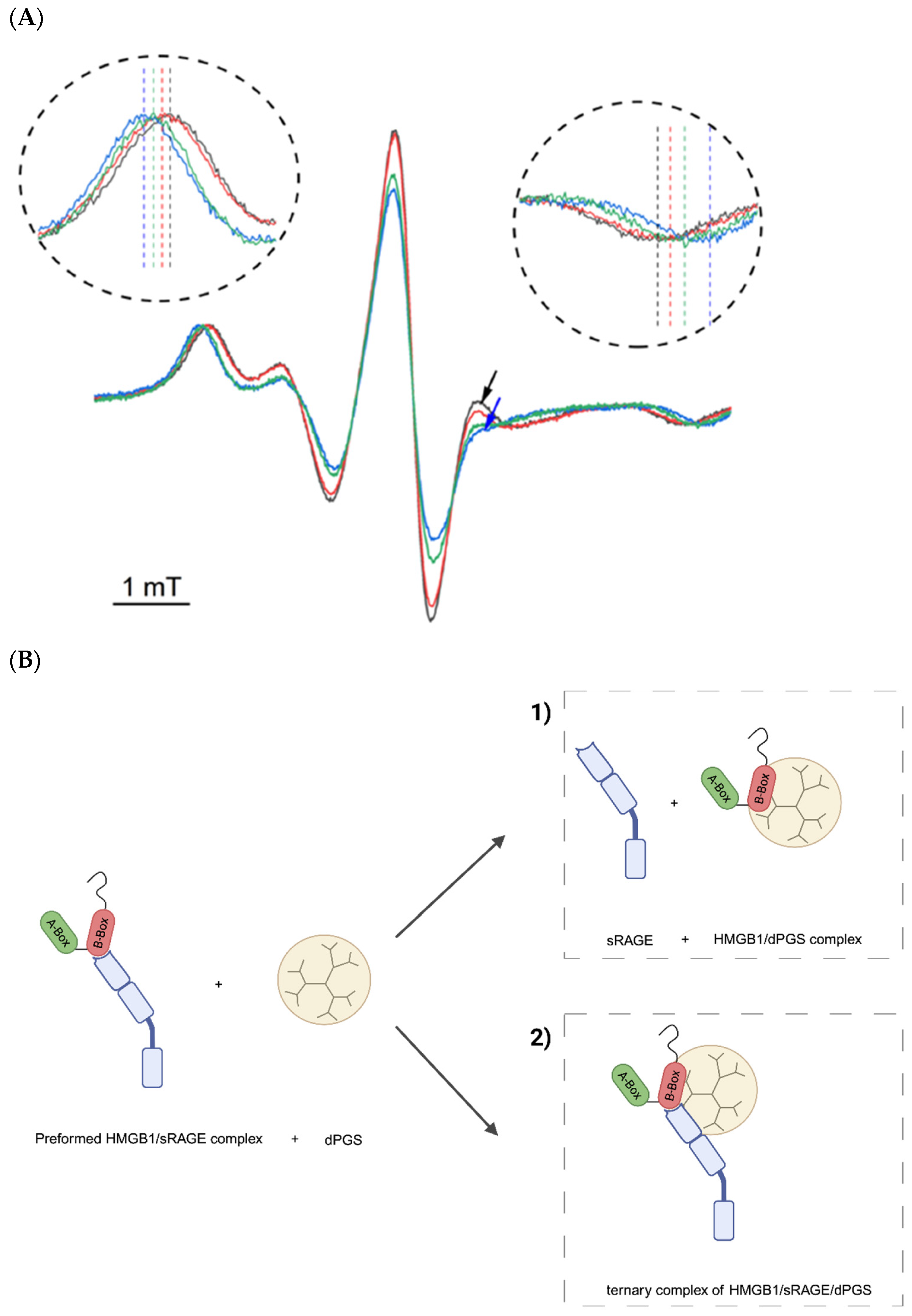
2.7. Spectroscopic Evidence for dPGS Interaction with HMGB1/sRAGE Complexes
3. Discussion
4. Materials and Methods
4.1. Cloning of Human HMGB1 into a Modified pET-22b(+) Vector
4.2. Recombinant Expression of hHMGB1
4.3. Affinity Chromatography
4.3.1. Ni-NTA Chromatography
4.3.2. Heparin-Sepharose Chromatography
4.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.5. Synthesis and Characterization of Dendritic Polyglycerol Sulfate (dPGS) and Cy5 Labeled Derivatives
4.6. RAW 264.7 Macrophage Culture
4.7. HMC3 Human Microglia Culture
4.8. PLA
4.9. NF-κB Shuttling
4.10. Reverse Transcription (RT)-PCR
4.11. Quantitative RT-PCR
4.12. NO Assay
4.13. ELISA
4.14. MTT-Assay
4.15. Time-Dependent dPGS-Cy5 Uptake Studies
4.15.1. Uptake of dPGS-Cy5 into RAW 264.7 Macrophages
4.15.2. Uptake of dPGS-Cy5 into Macrophages upon Co-Stimulation with LPS or HMGB1
4.16. Continuous Wave Electron Paramagnetic Resonance (Cw-EPR) Spectroscopy
4.17. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMGB1 | High-Mobility Group Box 1 |
| RAGE | Receptor for Advanced Glycation Endproducts |
| TLR | Toll-Like Receptor |
| dPGS | Dendritic Polyglycerol Sulfate |
| dPGA | Dendritic Polyglycerol Amine |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| TNF-α | Tumor Necrosis Factor alpha |
| IL-6 | Interleukin-6 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible Nitric Oxide Synthase |
| NO | Nitric Oxide |
| PLA | Proximity Ligation Assay |
| cw-EPR | continuous wave Electron Paramagnetic Resonance |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| RT-qPCR | Quantitative Real-time-PCR |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| Ni-NTA | Nickel–Nitrilotriacetic Acid |
| sRAGE | Soluble Receptor for Advanced Glycation Endproducts |
| PBS | Phosphate-Buffered Saline |
| FBS | Fetal Bovine Serum |
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Yumoto, Y.; Shirakawa, H.; Yoshida, M.; Suwa, A.; Watanabe, F.; Teraoka, H. High mobility group proteins 1 and 2 can function as DNA-binding regulatory components for DNA-dependent protein kinase in vitro. J. Biochem. 1998, 124, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Bustin, M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell Biol. 1999, 19, 5237–5246. [Google Scholar] [CrossRef]
- Bustin, M.; Reeves, R. High-mobility-group chromosomal proteins: Architectural components that facilitate chromatin function. Prog. Nucleic Acid. Res. Mol. Biol. 1996, 54, 35–100. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Neeper, M.; Schmidt, A.M.; Brett, J.; Yan, S.D.; Wang, F.; Pan, Y.C.; Elliston, K.; Stern, D.; Shaw, A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 1992, 267, 14998–15004. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef]
- Yan, S.D.; Bierhaus, A.; Nawroth, P.P.; Stern, D.M. RAGE and Alzheimer’s disease: A progression factor for amyloid-beta-induced cellular perturbation? J. Alzheimer’s Dis. 2009, 16, 833–843. [Google Scholar] [CrossRef]
- Xue, J.; Manigrasso, M.; Scalabrin, M.; Rai, V.; Reverdatto, S.; Burz, D.S.; Fabris, D.; Schmidt, A.M.; Shekhtman, A. Change in the Molecular Dimension of a RAGE-Ligand Complex Triggers RAGE Signaling. Structure 2016, 24, 1509–1522. [Google Scholar] [CrossRef]
- Rauvala, H.; Rouhiainen, A. Physiological and pathophysiological outcomes of the interactions of HMGB1 with cell surface receptors. Biochim. Biophys. Acta 2010, 1799, 164–170. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef]
- Bai, W.; Zhou, J.; Zhou, N.; Liu, Q.; Cui, J.; Zou, W.; Zhang, W. Hypoxia-increased RAGE expression regulates chemotaxis and pro-inflammatory cytokines release through nuclear translocation of NF-κ B and HIF1α in THP-1 cells. Biochem. Biophys. Res. Commun. 2018, 495, 2282–2288. [Google Scholar] [CrossRef]
- Bianchi, M.; Falciola, L.; Ferrari, S.; Lilley, D. The DNA binding site of HMG1 protein is composed of two similar segments (HMG boxes), both of which have counterparts in other eukaryotic regulatory proteins. EMBO J. 1992, 11, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kokkola, R.; Tabibzadeh, S.; Yang, R.; Ochani, M.; Qiang, X.; Harris, H.E.; Czura, C.J.; Wang, H.; Ulloa, L.; et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 2003, 9, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hori, O.; Brett, J.; Slattery, T.; Cao, R.; Zhang, J.; Chen, J.X.; Nagashima, M.; Lundh, E.R.; Vijay, S.; Nitecki, D.; et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 1995, 270, 25752–25761. [Google Scholar] [CrossRef] [PubMed]
- Venereau, E.; Casalgrandi, M.; Schiraldi, M.; Antoine, D.J.; Cattaneo, A.; De Marchis, F.; Liu, J.; Antonelli, A.; Preti, A.; Raeli, L.; et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012, 209, 1519–1528. [Google Scholar] [CrossRef]
- Venereau, E.; Schiraldi, M.; Uguccioni, M.; Bianchi, M.E. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol. Immunol. 2013, 55, 76–82. [Google Scholar] [CrossRef]
- Yang, H.; Antoine, D.J.; Andersson, U.; Tracey, K.J. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 2013, 93, 865–873. [Google Scholar] [CrossRef]
- Zandarashvili, L.; Sahu, D.; Lee, K.; Lee, Y.S.; Singh, P.; Rajarathnam, K.; Iwahara, J. Real-time kinetics of high-mobility group box 1 (HMGB1) oxidation in extracellular fluids studied by in situ protein NMR spectroscopy. J. Biol. Chem. 2013, 288, 11621–11627. [Google Scholar] [CrossRef]
- Sirois, C.M.; Jin, T.; Miller, A.L.; Bertheloot, D.; Nakamura, H.; Horvath, G.L.; Mian, A.; Jiang, J.; Schrum, J.; Bossaller, L.; et al. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. J. Exp. Med. 2013, 210, 2447–2463. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Li, F.; Carey, L.B.; Sun, C.; Ling, K.; Tachikawa, H.; Fujita, M.; Gao, X.-D.; Nakanishi, H. Receptor for advanced glycation end-products (RAGE) mediates phagocytosis in nonprofessional phagocytes. Commun. Biol. 2022, 5, 824. [Google Scholar] [CrossRef]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Zenerino, C.; Nuzzo, A.M.; Giuffrida, D.; Biolcati, M.; Zicari, A.; Todros, T.; Rolfo, A. The HMGB1/RAGE Pro-Inflammatory Axis in the Human Placenta: Modulating Effect of Low Molecular Weight Heparin. Molecules 2017, 22, 1997. [Google Scholar] [CrossRef]
- Maysinger, D.; Zhang, I.; Wu, P.Y.; Kagelmacher, M.; Luo, H.D.; Kizhakkedathu, J.N.; Dernedde, J.; Ballauff, M.; Haag, R.; Shobo, A.; et al. Sulfated Hyperbranched and Linear Polyglycerols Modulate HMGB1 and Morphological Plasticity in Neural Cells. ACS Chem. Neurosci. 2023, 14, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Dernedde, J.; Rausch, A.; Weinhart, M.; Enders, S.; Tauber, R.; Licha, K.; Schirner, M.; Zügel, U.; von Bonin, A.; Haag, R. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 19679–19684. [Google Scholar] [CrossRef]
- Weinhart, M.; Gröger, D.; Enders, S.; Dernedde, J.; Haag, R. Synthesis of Dendritic Polyglycerol Anions and Their Efficiency Toward L-Selectin Inhibition. Biomacromolecules 2011, 12, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Magna, M.; Pisetsky, D.S. The Role of HMGB1 in the Pathogenesis of Inflammatory and Autoimmune Diseases. Mol. Med. 2014, 20, 138–146. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. Production of functional rat HMG1 protein in Escherichia coli. Gene 1991, 104, 271–275. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Mason, J.M.; Levine, J.; Yu, M.; Ulloa, L.; Czura, C.J.; Tracey, K.J.; Yang, H. Recombinant HMGB1 with cytokine-stimulating activity. J. Immunol. Methods 2004, 289, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Chavan, S.S.; Andersson, U. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol. Med. 2015, 21, S6–S12. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Yang, H.; Harris, H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin. Immunol. 2018, 38, 40–48. [Google Scholar] [CrossRef]
- Xu, D.; Young, J.; Song, D.; Esko, J.D. Heparan sulfate is essential for high mobility group protein 1 (HMGB1) signaling by the receptor for advanced glycation end products (RAGE). J. Biol. Chem. 2011, 286, 41736–41744. [Google Scholar] [CrossRef]
- Pi, J.; Li, T.; Liu, J.; Su, X.; Wang, R.; Yang, F.; Bai, H.; Jin, H.; Cai, J. Detection of lipopolysaccharide induced inflammatory responses in RAW264.7 macrophages using atomic force microscope. Micron 2014, 65, 1–9. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Tracey, K.J. HMGB1 in sepsis. Scand. J. Infect. Dis. 2003, 35, 577–584. [Google Scholar] [CrossRef]
- Dumitriu, I.E.; Baruah, P.; Bianchi, M.E.; Manfredi, A.A.; Rovere-Querini, P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur. J. Immunol. 2005, 35, 2184–2190. [Google Scholar] [CrossRef]
- Hreggvidsdóttir, H.S.; Lundberg, A.M.; Aveberger, A.-C.; Klevenvall, L.; Andersson, U.; Harris, H.E. High Mobility Group Box Protein 1 (HMGB1)-Partner Molecule Complexes Enhance Cytokine Production by Signaling Through the Partner Molecule Receptor. Mol. Med. 2012, 18, 224–230. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.R.M.T.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef] [PubMed]
- Isas, J.M.; Langen, R.; Haigler, H.T.; Hubbell, W.L. Structure and dynamics of a helical hairpin and loop region in annexin 12: A site-directed spin labeling study. Biochemistry 2002, 41, 1464–1473. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, C.W.; Jeong, M.S.; Kwon, T.J.; Choi, J.Y.; Jang, S.B. Cryo-EM structure of HMGB1-RAGE complex and its inhibitory effect on lung cancer. Biomed. Pharmacother. 2025, 187, 118088. [Google Scholar] [CrossRef]
- Liang, G.; He, Z. High Mobility Group Proteins in Sepsis. Front. Immunol. 2022, 13, 911152. [Google Scholar] [CrossRef]
- DeWulf, B.; Minsart, L.; Verdonk, F.; Kruys, V.; Piagnerelli, M.; Maze, M.; Saxena, S. High Mobility Group Box 1 (HMGB1): Potential Target in Sepsis-Associated Encephalopathy. Cells 2023, 12, 1088. [Google Scholar] [CrossRef]
- Park, J.S.; Gamboni-Robertson, F.; He, Q.; Svetkauskaite, D.; Kim, J.Y.; Strassheim, D.; Sohn, J.W.; Yamada, S.; Maruyama, I.; Banerjee, A.; et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 2006, 290, C917–C924. [Google Scholar] [CrossRef]
- Fan, H.; Tang, H.-B.; Chen, Z.; Wang, H.-Q.; Zhang, L.; Jiang, Y.; Li, T.; Yang, C.-F.; Wang, X.-Y.; Li, X.; et al. Inhibiting HMGB1-RAGE axis prevents pro-inflammatory macrophages/microglia polarization and affords neuroprotection after spinal cord injury. J. Neuroinflammation 2020, 17, 295. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, L.; Chen, J.; Chen, C.; Jiang, P.; Mei, Z.; Jiang, Q.; Hua, X. HMGB1 Inhibition Alleviates Chronic Nonbacterial Prostatitis by Suppressing M1 Polarization of Macrophages. J. Inflamm. Res. 2025, 18, 6735–6748. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Fang, Y.; Deng, S.; Li, X.; Zhou, Y.; Gong, Y.; Zhu, H.; Wang, W. Glycyrrhizin Protects Rats from Sepsis by Blocking HMGB1 Signaling. Biomed. Res. Int. 2017, 2017, 9719647. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F.; Yao, Y.M.; Sheng, Z.Y. Novel insights for high mobility group box 1 protein-mediated cellular immune response in sepsis: A systemic review. World J. Emerg. Med. 2012, 3, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, M.; Wang, D.; Ousaka, D.; Wake, H. High Mobility Group Box-1 and Blood-Brain Barrier Disruption. Cells 2020, 9, 2650. [Google Scholar] [CrossRef] [PubMed]
- Otazu, G.K.; Dayyani, M.; Badie, B. Role of RAGE and Its Ligands on Inflammatory Responses to Brain Tumors. Front. Cell Neurosci. 2021, 15, 770472. [Google Scholar] [CrossRef] [PubMed]
- Joma, N.; Kagelmacher, M.; Zhang, I.; Herrmann, A.; Dernedde, J.; Haag, R.; Maysinger, D. Charged dendrimers reduce glioblastoma viability by modulating lysosomal activity and HMG1-RAGE interaction. Biochem. Pharmacol. 2025, 238, 116969. [Google Scholar] [CrossRef]
- Wu, H.; Reimann, S.; Siddiqui, S.; Haag, R.; Siegmund, B.; Dernedde, J.; Glauben, R. dPGS Regulates the Phenotype of Macrophages via Metabolic Switching. Macromol. Biosci. 2019, 19, 1900184. [Google Scholar] [CrossRef]
- Pierce, J.W.; Schoenleber, R.; Jesmok, G.; Best, J.; Moore, S.A.; Collins, T.; Gerritsen, M.E. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 1997, 272, 21096–21103. [Google Scholar] [CrossRef]
- Juliana, C.; Fernandes-Alnemri, T.; Wu, J.; Datta, P.; Solorzano, L.; Yu, J.W.; Meng, R.; Quong, A.A.; Latz, E.; Scott, C.P.; et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010, 285, 9792–9802. [Google Scholar] [CrossRef]
- Zhang, I.; Lépine, P.; Han, C.; Lacalle-Aurioles, M.; Chen, C.X.-Q.; Haag, R.; Durcan, T.M.; Maysinger, D. Nanotherapeutic Modulation of Human Neural Cells and Glioblastoma in Organoids and Monocultures. Cells 2020, 9, 2434. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Y.; Wang, J.; Shi, X.; Liu, Q.; Liu, Z.; Li, Y.; Scott, M.J.; Xiao, G.; Li, S.; et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 2014, 21, 1229–1239. [Google Scholar] [CrossRef]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]
- Muhammad, W.; Zhu, J.; Zhai, Z.; Xie, J.; Zhou, J.; Feng, X.; Feng, B.; Pan, Q.; Li, S.; Venkatesan, R.; et al. ROS-responsive polymer nanoparticles with enhanced loading of dexamethasone effectively modulate the lung injury microenvironment. Acta Biomater. 2022, 148, 258–270. [Google Scholar] [CrossRef]
- Koskela, E.V.; Frey, A.D. Homologous recombinatorial cloning without the creation of single-stranded ends: Exonuclease and ligation-independent cloning (ELIC). Mol. Biotechnol. 2015, 57, 233–240. [Google Scholar] [CrossRef]
- Bergeron, S.; Anderson, D.K.; Swanson, P.C. RAG and HMGB1 proteins: Purification and biochemical analysis of recombination signal complexes. Methods Enzymol. 2006, 408, 511–528. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- De St. Groth, S.F.; Webster, R.G.; Datyner, A. Two new staining procedures for quantitative estimation of proteins on electrophoretic strips. Biochim. Biophys. Acta 1963, 71, 377–391. [Google Scholar] [CrossRef]
- Wallert, M.; Plaschke, J.; Dimde, M.; Ahmadi, V.; Block, S.; Haag, R. Automated Solvent-Free Polymerization of Hyperbranched Polyglycerol with Tailored Molecular Weight by Online Torque Detection. Macromol. Mater. Eng. 2021, 306, 2000688. [Google Scholar] [CrossRef]
- Alban, S.; Kraus, J.; Franz, G. Synthesis of laminarin sulfates with anticoagulant activity. Arzneimittelforschung 1992, 42, 1005–1008. [Google Scholar] [PubMed]
- Türk, H.; Haag, R.; Alban, S. Dendritic Polyglycerol Sulfates as New Heparin Analogues and Potent Inhibitors of the Complement System. Bioconjugate Chem. 2004, 15, 162–167. [Google Scholar] [CrossRef]
- Arenhoevel, J.; Kuppe, A.; Addante, A.; Wei, L.F.; Boback, N.; Butnarasu, C.; Zhong, Y.; Wong, C.; Graeber, S.Y.; Duerr, J.; et al. Thiolated polyglycerol sulfate as potential mucolytic for muco-obstructive lung diseases. Biomater. Sci. 2024, 12, 4376–4385. [Google Scholar] [CrossRef]
- Gröger, D.; Paulus, F.; Licha, K.; Welker, P.; Weinhart, M.; Holzhausen, C.; Mundhenk, L.; Gruber, A.D.; Abram, U.; Haag, R. Synthesis and biological evaluation of radio and dye labeled amino functionalized dendritic polyglycerol sulfates as multivalent anti-inflammatory compounds. Bioconjugate Chem. 2013, 24, 1507–1514. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| TNF-α | GTTCTGTCCCTTTCACT CACTG | GGTAGA GAATGGATGAACACC |
| iNOS | CTGCAGCACTTGGATCA GAACCTG | GGGA GTAGCCTGTGTGCACCTGGAA |
| IL-6 | CATGTTCTCTG GAAATCGTGG | AACGCA CTAGGTT TGCCGAGTA |
| COX-2 | TTTGCCCAGCACTTCACCCAT | AAGTGGTAAC CGCTCAGGTGT |
| MCP-1 | TGATCCCAATGAGTAGGCTG GAG | ATGTCTG GACCCATTCTTTCTTG |
| β-actin | GTGGGCCGCCCTAGGACCAG | GGAGGAAGAG GATGCGGCAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagelmacher, M.; Quella, C.S.; Kautz, E.; Klumpp, A.; Weichert, F.; Zhang, I.; Maysinger, D.; Wedamulla, P.G.; Straus, S.K.; Risse, T.; et al. Dendritic Polyglycerol Sulfate Reduces Inflammation Through Inhibition of the HMGB1/RAGE Axis in RAW 264.7 Macrophages. Int. J. Mol. Sci. 2025, 26, 10440. https://doi.org/10.3390/ijms262110440
Kagelmacher M, Quella CS, Kautz E, Klumpp A, Weichert F, Zhang I, Maysinger D, Wedamulla PG, Straus SK, Risse T, et al. Dendritic Polyglycerol Sulfate Reduces Inflammation Through Inhibition of the HMGB1/RAGE Axis in RAW 264.7 Macrophages. International Journal of Molecular Sciences. 2025; 26(21):10440. https://doi.org/10.3390/ijms262110440
Chicago/Turabian StyleKagelmacher, Marten, Cristina S. Quella, Emma Kautz, Anna Klumpp, Felix Weichert, Issan Zhang, Dusica Maysinger, Poornima G. Wedamulla, Suzana K. Straus, Thomas Risse, and et al. 2025. "Dendritic Polyglycerol Sulfate Reduces Inflammation Through Inhibition of the HMGB1/RAGE Axis in RAW 264.7 Macrophages" International Journal of Molecular Sciences 26, no. 21: 10440. https://doi.org/10.3390/ijms262110440
APA StyleKagelmacher, M., Quella, C. S., Kautz, E., Klumpp, A., Weichert, F., Zhang, I., Maysinger, D., Wedamulla, P. G., Straus, S. K., Risse, T., Haag, R., Pigaleva, M., & Dernedde, J. (2025). Dendritic Polyglycerol Sulfate Reduces Inflammation Through Inhibition of the HMGB1/RAGE Axis in RAW 264.7 Macrophages. International Journal of Molecular Sciences, 26(21), 10440. https://doi.org/10.3390/ijms262110440







