Adapting Crops to Rising Temperatures: Understanding Heat Stress and Plant Resilience Mechanisms
Abstract
1. Introduction
2. Plant Mechanisms and Responses During Heat Stress
2.1. Plant Morphological Responses to Stress
2.2. Physiological Adaptation of Plants to Heat Stress
2.2.1. Stomatal Conductance Activity During Heat Stress
2.2.2. Cell Membrane Thermostability During Heat Stress
2.3. Plant Hormonal Responses to Heat Stress
2.4. Plant Reproductive Responses to Heat Stress
2.4.1. Heat Stress Responses During Microsporogenesis/Megasporogenesis
2.4.2. Heat Stress Responses During Microgametogenesis/Megagametogenesis
2.5. Molecular Mechanism of Heats Stress Response
2.5.1. Role of Heat Shock Proteins in Heat Stress Response
2.5.2. Role of Dehydrins in Heat Stress Response
2.5.3. Transcriptomics and Proteomics Analyses
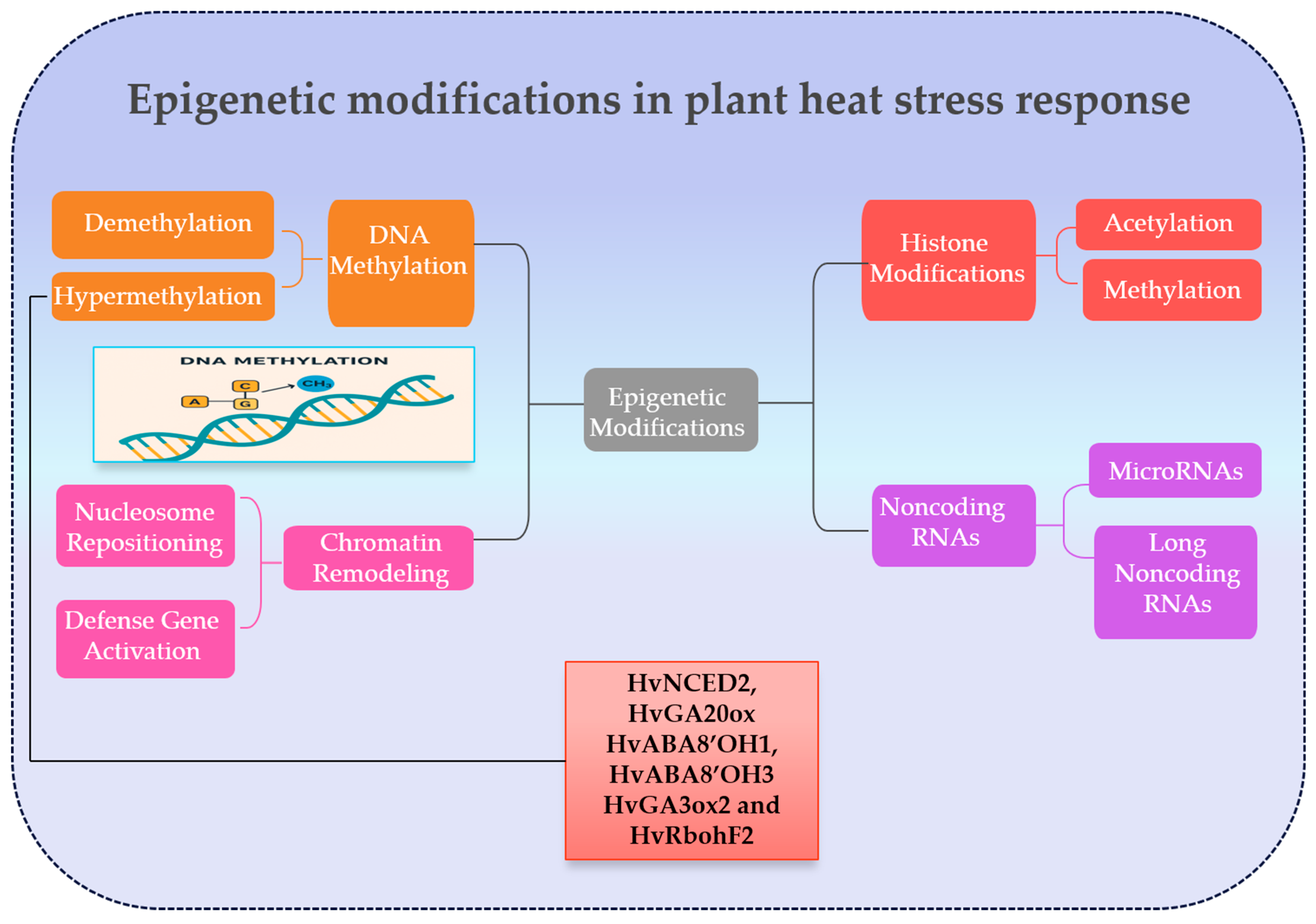
2.5.4. Epigenetic Modifications Regulate Plant Responses and Adaptation During Heat Stress
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Plant Heat Stress: Concepts Directing Future Research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef]
- Ugah, E.P.T.A.; Ndubuisi, O.G.; FNisafetyE, F.I.S.P.O.N. The Effects of Greenhouse Gas Emissions on Global Climate Patterns. Int. J. Innov. Biochem. Microbiol. Res. 2025, 13, 75–85. [Google Scholar]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Zhao, W.; Chou, J.; Li, J.; Xu, Y.; Li, Y.; Hao, Y. Impacts of Extreme Climate Events on Future Rice Yields in Global Major Rice-Producing Regions. Int. J. Environ. Res. Public Health 2022, 19, 4437. [Google Scholar] [CrossRef]
- Chhabra, S.; Maheshwari, C.; Umar, S.; Khan, M.I.R. Mitigating High-Temperature Stress in Plants: Strategies for Sustaining Crop Growth and Yield. In Optimizing Plant Health Under Abiotic-Stress Environments; CRC Press: Boca Raton, FL, USA, 2025. [Google Scholar]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Li, J.; Wang, M.; Shah, L.; Lu, S.; Wang, X.; Ma, C. Unraveling Field Crops Sensitivity to Heat Stress: Mechanisms, Approaches, and Future Prospects. Agronomy 2018, 8, 128. [Google Scholar] [CrossRef]
- Charney, D.S. Psychobiological Mechanisms of Resilience and Vulnerability: Implications for Successful Adaptation to Extreme Stress. AJP 2004, 161, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Kawakatsu, T.; Yamaguchi, N. Beyond Heat Waves: Unlocking Epigenetic Heat Stress Memory in Arabidopsis. Plant Physiol. 2024, 194, 1934–1951. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The Effect of Drought and Heat Stress on Reproductive Processes in Cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Lenaz, G.; Castelli, G.P. Membrane Fluidity: Molecular Basis and Physiological Significance. In Structure and Properties of Cell Membrane Structure and Properties of Cell Membranes; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of Reactive Oxygen Species and Hormones in Plant Responses to Temperature Changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants. Int. J. Mol. Sci. 2022, 23, 1995. [Google Scholar] [CrossRef]
- Bokszczanin, K.L.; Solanaceae Pollen Thermotolerance Initial Training Network (SPOT-ITN) Consortium; Fragkostefanakis, S. Perspectives on Deciphering Mechanisms Underlying Plant Heat Stress Response and Thermotolerance. Front. Plant Sci. 2013, 4, 315. [Google Scholar] [CrossRef]
- Kang, X.; Zhao, L.; Liu, X. Calcium Signaling and the Response to Heat Shock in Crop Plants. Int. J. Mol. Sci. 2024, 25, 324. [Google Scholar] [CrossRef]
- Szaker, H.M.; Gyula, P.; Szittya, G.; Csorba, T. Regulation of High-Temperature Stress Response by Small RNAs. In Plant microRNAs: Shaping Development and Environmental Responses; Springer International Publishing: Cham, Switzerland, 2020; pp. 171–197. [Google Scholar]
- Bhardwaj, R.; Lone, J.K.; Pandey, R.; Mondal, N.; Dhandapani, R.; Meena, S.K.; Khan, S.; Gayacharan. Insights into Morphological and Physio-Biochemical Adaptive Responses in Mungbean (Vigna radiata L.) under Heat Stress. Front. Genet. 2023, 14, 1206451. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ulhassan, Z.; Brestic, M.; Zivcak, M.; Zhou, W.; Allakhverdiev, S.I.; Yang, X.; Safdar, M.E.; Yang, W.; Liu, W. Photosynthesis Research under Climate Change. Photosynth. Res. 2021, 150, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Kathpalia, R.; Bhatla, S.C. Plant Water Relations. In Plant Physiology, Development and Metabolism; Bhatla, S.C., A. Lal, M., Eds.; Springer Nature: Singapore, 2018; pp. 3–36. ISBN 9789811320231. [Google Scholar]
- Buckley, T.N. How Do Stomata Respond to Water Status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Chachar, S.; Ahmed, N.; Hu, X. Drought-Induced Aesthetic Decline and Ecological Impacts on Ornamentals: Mechanisms of Damage and Innovative Strategies for Mitigation. Plant Biol. 2025, 27, e70074. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Djanaguiraman, M.; Stewart, Z.P.; Ciampitti, I.A. Agroclimatology of Maize, Sorghum, and Pearl Millet. In Agroclimatology: Linking Agriculture to Climate; Springer: Cham, Switzerland, 2020; Volume 60, pp. 201–241. [Google Scholar]
- Sandhu, N.; Aggarwal, H.; Kumar, A.; Augustine, G.; Vishnoi, R.; Pandey, A.K.; Chauhan, H.; Chhuneja, P. Regulating Plant Architecture to Enhance the Future of Cereal Crop Production. Physiol. Plant. 2025, 177, e70367. [Google Scholar] [CrossRef]
- Gruda, N.S.; Samuolienė, G.; Dong, J.; Li, X. Environmental Conditions and Nutritional Quality of Vegetables in Protected Cultivation. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70139. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Robinson, N. Stress Physiology: Abiotic Stresses. In Sugarcane: Physiology, Biochemistry, and Functional Biology; Springer: New York, NY, USA, 2013; pp. 411–434. [Google Scholar]
- Fan, C.; Hou, M.; Si, P.; Sun, H.; Zhang, K.; Bai, Z.; Wang, G.; Li, C.; Liu, L.; Zhang, Y. Response of Root and Root Hair Phenotypes of Cotton Seedlings under High Temperature Revealed with RhizoPot. Front. Plant Sci. 2022, 13, 1007145. [Google Scholar] [CrossRef]
- Vaughn, L.M.; Baldwin, K.L.; Jia, G.; Verdonk, J.C.; Strohm, A.K.; Masson, P.H. The Cytoskeleton and Root Growth Behavior. In The Plant Cytoskeleton; Liu, B., Ed.; Springer: New York, NY, USA, 2011; pp. 307–326. ISBN 978-1-4419-0987-9. [Google Scholar]
- Blume, Y.B.; Krasylenko, Y.A.; Yemets, A.I. The Role of the Plant Cytoskeleton in Phytohormone Signaling under Abiotic and Biotic Stresses. In Mechanism of Plant Hormone Signaling Under Stress; Springer: Cham, Switzerland, 2017; Volume 2, pp. 127–185. [Google Scholar]
- Calleja-Cabrera, J.; Boter, M.; Oñate-Sánchez, L.; Pernas, M. Root Growth Adaptation to Climate Change in Crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef]
- Waqas, M.A.; Wang, X.; Zafar, S.A.; Noor, M.A.; Hussain, H.A.; Nawaz, M.A.; Farooq, M. Thermal Stresses in Maize: Effects and Management Strategies. Plants 2021, 10, 293. [Google Scholar] [CrossRef]
- Arachchige, S.M.; Razzaq, A.; Dai, H.Y.; Wang, J. Confronting Heat Stress in Crops Amid Global Warming: Impacts, Defense Mechanisms, and Strategies for Enhancing Thermotolerance. Crop Breed. Genet. Genom. 2024, 6, e240011. [Google Scholar] [CrossRef]
- Khanzada, A.; Yan, K.; Hu, W.; Malko, M.; Khan, K.A.; Bao, Y.; Elboughdiri, N.; Li, Y. Heat Stress Response Mechanisms and Resilience Strategies in Wheat. J. Agron. Crop Sci. 2025, 211, e70023. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Narayanan, S.; Erdayani, E.; Prasad, P.V.V. Effects of High Temperature Stress during Anthesis and Grain Filling Periods on Photosynthesis, Lipids and Grain Yield in Wheat. BMC Plant Biol. 2020, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Beddington, J.R.; Asaduzzaman, M.; Fernández, A.; Clark, M.E.; Guillou, M.; Jahn, M.M.; Erda, L.; Mamo, T.; Bo, N.V.; Nobre, C.A.; et al. Achieving Food Security in the Face of Climate Change: Final Report from the Commission on Sustainable Agriculture and Climate Change; The CGIAR Research Program on Climate Change, Agriculture and Food Security (CCAFS): Wageningen, The Netherlands, 2012; pp. 1–64. [Google Scholar]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant Responses to Rising Vapor Pressure Deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed]
- Marquez, D.; Gardner, A.; Busch, F. Navigating Challenges in Interpreting Plant Physiology Responses through Gas Exchange Results in Stressed Plants. Plant Ecophysiol. 2025, 1, 2. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Zhang, H.; Fu, P.; Fan, Z. Stronger Cooling Effects of Transpiration and Leaf Physical Traits of Plants from a Hot Dry Habitat than from a Hot Wet Habitat. Funct. Ecol. 2017, 31, 2202–2211. [Google Scholar] [CrossRef]
- Zhao, C.; Haigh, A.M.; Holford, P.; Chen, Z.H. Roles of Chloroplast Retrograde Signals and Ion Transport in Plant Drought Tolerance. Int. J. Mol. Sci. 2018, 19, 963. [Google Scholar] [CrossRef]
- Batool, I.; Ayyaz, A.; Qin, T.; Wu, X.; Chen, W.; Hannan, F.; Zafar, Z.U.; Naeem, M.S.; Farooq, M.A.; Zhou, W. Morphological, Physiological, and Molecular Responses to Heat Stress in Brassicaceae. Plants 2025, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. In Climate Change and Plant Abiotic Stress Tolerance; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 209–250. ISBN 978-3-527-67526-5. [Google Scholar]
- Khan, S.; Saify, S.; Sofo, A.; Khan, N.A. The Mechanisms of Melatonin Action in Shielding Photosynthesis during Heat Stress. CABI Rev. 2024, 19, 1–16. [Google Scholar] [CrossRef]
- Sahoo, R.; Samanta, D.; Sow, S.; Ranjan, S.; Nath, D.; Sadhu, S.; Kumar, N.; Rana, L.; Kumar, A.; Roy, D.K.; et al. Nexus between Photosynthesis and Radiation Use Efficiency towards Achieving Sustainability in the Era of Climate Change: An Overview. Discov. Environ. 2025, 3, 67. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Chen, J.H.; Tang, M.; Jin, X.Q.; Li, H.; Chen, L.S.; Wang, Q.L.; Sun, A.Z.; Yi, Y.; Guo, F.Q. Regulation of Calvin–Benson Cycle Enzymes under High Temperature Stress. Abiotech 2022, 3, 65–77. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Ranawana, S.R.W.M.C.J.K.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Role of Transpiration in Regulating Leaf Temperature and Its Application in Physiological Breeding. In Translating Physiological Tools to Augment Crop Breeding; Harohalli Masthigowda, M., Gopalareddy, K., Khobra, R., Singh, G., Pratap Singh, G., Eds.; Springer Nature: Singapore, 2023; pp. 91–119. ISBN 978-981-19749-8-4. [Google Scholar]
- Sullivan, C.Y.; Norcio, N.V.; Eastin, J.D. Plant Responses to High Temperatures. In Genetic Diversity in Plants; Muhammed, A., Aksel, R., von Borstel, R.C., Eds.; Springer: Boston, MA, USA, 1977; pp. 301–317. ISBN 978-1-4684-2886-5. [Google Scholar]
- Rogowska, A.; Szakiel, A. The Role of Sterols in Plant Response to Abiotic Stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.; Wang, H.; Zhou, X.; Wang, M.; Li, L.; Liu, F.; Sun, J.; Xiao, G. Peptide Hormone-Mediated Regulation of Plant Development and Environmental Adaptability. Adv. Sci. 2025, 12, e06590. [Google Scholar] [CrossRef]
- Lee, Z.; Lim, J.A.; Harikrishna, J.A.; Islam, T.; Abd Rahim, M.H.; Yaacob, J.S. Regulation of Plant Responses to Temperature Stress: A Key Factor in Food Security and for Mitigating Effects of Climate Change. Int. J. Plant Prod. 2024, 18, 141–159. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Skalicky, M.; Hussain, S.; Zulfiqar, U.; Anjum, M.Z.; Habib ur Rahman, M.; Brestic, M.; Ratnasekera, D.; Lamilla-Tamayo, L.; et al. Adaptation Strategies to Improve the Resistance of Oilseed Crops to Heat Stress Under a Changing Climate: An Overview. Front. Plant Sci. 2021, 12, 767150. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, Z.; Wang, Z.; Maruza, T.M.; Zhang, G. The Genetics and Breeding of Heat Stress Tolerance in Wheat: Advances and Prospects. Plants 2025, 14, 148. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Abiotic Stress, Generation of Reactive Oxygen Species, and Their Consequences. In Reactive Oxygen Species in Plants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 23–50. ISBN 978-1-119-32492-8. [Google Scholar]
- Dar, M.I.; Naikoo, M.I.; Khan, F.A.; Rehman, F.; Green, I.D.; Naushin, F.; Ansari, A.A. An Introduction to Reactive Oxygen Species Metabolism Under Changing Climate in Plants. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 25–52. ISBN 978-981-10-5254-5. [Google Scholar]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane Dynamics during Individual and Combined Abiotic Stresses in Plants and Tools to Study the Same. Physiol. Plant. 2021, 171, 653–676. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Kumar, A.K. An Updated Review On-Heat Stress Imbalance and Enhancement of Wheat. J. Xidian Univ. 2024, 18, 833–848. [Google Scholar] [CrossRef]
- Sahoo, R.; Samanta, D.; Sow, S.; Ranjan, S.; Nath, D.; Sadhu, S.; Kumar, N.; Rana, L.; Kumar, A.; Roy, D.K.; et al. Lipid Metabolism in Plants under Low-Temperature Stress: A Review. In Physiological Processes in Plants Under Low Temperature Stress; Springer: Cham, Switzerland, 2022; pp. 409–516. [Google Scholar]
- Dhaliwal, L.K.; Angeles-Shim, R.B. Cell Membrane Features as Potential Breeding Targets to Improve Cold Germination Ability of Seeds. Plants 2022, 11, 3400. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Lone, R. Heat shock proteins with an emphasis on HSP 60. Mol. Biol. Rep. 2021, 48, 6959–6969. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Pattnaik, D.; Dash, D.; Mishra, A.; Padhiary, A.K.; Dey, P.; Dash, G.K. Emerging Roles of Osmoprotectants in Alleviating Abiotic Stress Response Under Changing Climatic Conditions. In Climate Impacts on Sustainable Natural Resource Management; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 303–324. ISBN 978-1-119-79340-3. [Google Scholar]
- Kumar, H.; Chugh, V.; Kumar, M.; Gupta, V.; Prasad, S.; Kumar, S.; Singh, C.M.; Kumar, R.; Singh, B.K.; Panwar, G.; et al. Investigating the Impact of Terminal Heat Stress on Contrasting Wheat Cultivars: A Comprehensive Analysis of Phenological, Physiological, and Biochemical Traits. Front. Plant Sci. 2023, 14, 1189005. [Google Scholar] [CrossRef]
- Chaudhary, S.; Devi, P.; Bhardwaj, A.; Jha, U.C.; Sharma, K.D.; Prasad, P.V.; Siddique, K.H.; Bindumadhava, H.; Kumar, S.; Nayyar, H. Identification and Characterization of Contrasting Genotypes/Cultivars for Developing Heat Tolerance in Agricultural Crops: Current Status and Prospects. Front. Plant Sci. 2020, 11, 587264. [Google Scholar] [CrossRef]
- Senguttuvel, P.; Jaldhani, V.; Raju, N.S.; Balakrishnan, D.; Beulah, P.; Bhadana, V.P.; Mangrauthia, S.K.; Neeraja, C.N.; Subrahmanyam, D.; Rao, P.R.; et al. Breeding Rice for Heat Tolerance and Climate Change Scenario; Possibilities and Way Forward. A Review. Arch. Agron. Soil Sci. 2022, 68, 115–132. [Google Scholar] [CrossRef]
- Ul Hassan, M.; Rasool, T.; Iqbal, C.; Arshad, A.; Abrar, M.; Abrar, M.M.; Habib-ur-Rahman, M.; Noor, M.A.; Sher, A.; Fahad, S. Linking Plants Functioning to Adaptive Responses Under Heat Stress Conditions: A Mechanistic Review. J. Plant Growth Regul. 2022, 41, 2596–2613. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration Increases under High-Temperature Stress: Potential Mechanisms, Trade-Offs and Prospects for Crop Resilience in a Warming World. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Hafeez, U.; Ali, M.; Hassan, S.M.; Akram, M.A.; Zafar, A. Advances in Breeding and Engineering Climate-Resilient Crops: A Comprehensive Review. Int. J. Res. Adv. Agric. Sci. 2023, 2, 85–99. [Google Scholar]
- Jianing, G.; Yuhong, G.; Yijun, G.; Rasheed, A.; Qian, Z.; Zhiming, X.; Mahmood, A.; Shuheng, Z.; Zhuo, Z.; Zhuo, Z.; et al. Improvement of Heat Stress Tolerance in Soybean (Glycine max L.), by Using Conventional and Molecular Tools. Front. Plant Sci. 2022, 13, 993189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ye, H.; Kong, L.; Li, X.; Chen, Y.; Wang, S.; Liu, B. Multivariate Analysis Compares and Evaluates Heat Tolerance of Potato Germplasm. Plants 2024, 13, 142. [Google Scholar] [CrossRef]
- Prasad, V.B.R.; Govindaraj, M.; Djanaguiraman, M.; Djalovic, I.; Shailani, A.; Rawat, N.; Singla-Pareek, S.L.; Pareek, A.; Prasad, P.V.V. Drought and High Temperature Stress in Sorghum: Physiological, Genetic, and Molecular Insights and Breeding Approaches. Int. J. Mol. Sci. 2021, 22, 9826. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Moursi, Y.S.; Amro, A.; Baenziger, P.S.; Sallam, A. Investigation of Heat-Induced Changes in the Grain Yield and Grains Metabolites, with Molecular Insights on the Candidate Genes in Barley. Agronomy 2020, 10, 1730. [Google Scholar] [CrossRef]
- Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Ampomah-Dwamena, C.; Sodedji, F.A.K.; Asante, I.K.; Danquah, E.Y. Accelerating Breeding for Heat Tolerance in Tomato (Solanum lycopersicum L.): An Integrated Approach. Agronomy 2019, 9, 720. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, J.; Qian, Q.; Shang, L. Enhancement of Heat and Drought Stress Tolerance in Rice by Genetic Manipulation: A Systematic Review. Rice 2022, 15, 67. [Google Scholar] [CrossRef]
- Dev, W.; Sultana, F.; He, S.; Hu, D.; Geng, X.; Du, X.; Iqbal, B. Effects of High Temperatures on Pollen Germination and Physio-Morphological Traits in Upland Cotton (Gossypium hirsutum L.). J. Agron. Crop Sci. 2025, 211, e70080. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature Response of Photosynthesis in C3, C4, and CAM Plants: Temperature Acclimation and Temperature Adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Singh, J.; Thakur, J.K. Photosynthesis and Abiotic Stress in Plants. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; pp. 27–46. ISBN 978-981-10-9029-5. [Google Scholar]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Effects of Heat Stress on Photosynthetic Characteristics and Chloroplast Ultrastructure of a Heat-Sensitive and Heat-Tolerant Cultivar of Wucai (Brassica campestris L.). Acta Physiol. Plant 2016, 39, 30. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Rath, J.R.; Pandey, J.; Yadav, R.M.; Zamal, M.Y.; Ramachandran, P.; Mekala, N.R.; Allakhverdiev, S.I.; Subramanyam, R. Temperature-Induced Reversible Changes in Photosynthesis Efficiency and Organization of Thylakoid Membranes from Pea (Pisum sativum). Plant Physiol. Biochem. 2022, 185, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Wani, K.I.; Choudhary, S.; Naeem, M.; Khan, M.M.A.; Aftab, T. Involvement of Abscisic Acid in Silicon-Mediated Enhancement of Copper Stress Tolerance in Artemisia annua. Plant Physiol. Biochem. 2023, 195, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zahra, S.A.; Iqbal, J.; Abbasi, B.A.; Kanwal, S.; Alwahibi, M.S.; Elshikh, M.S.; Rizwan, M.; Iqbal, R.; Mahmood, T. Phylogenetic Analysis of Selected Species of Asteraceae on the Basis of RPS 11 Gene. Sci. Rep. 2024, 14, 24808. [Google Scholar] [CrossRef]
- Yamori, W. Photosynthetic Response to Fluctuating Environments and Photoprotective Strategies under Abiotic Stress. J. Plant Res. 2016, 129, 379–395. [Google Scholar] [CrossRef]
- Kono, M.; Oguchi, R.; Terashima, I. Photoinhibition of PSI and PSII in Nature and in the Laboratory: Ecological Approaches. In Progress in Botany Vol. 84; Lüttge, U., Cánovas, F.M., Risueño, M.-C., Leuschner, C., Pretzsch, H., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 241–292. ISBN 978-3-031-45754-8. [Google Scholar]
- Croce, R.; Carmo-Silva, E.; Cho, Y.B.; Ermakova, M.; Harbinson, J.; Lawson, T.; McCormick, A.J.; Niyogi, K.K.; Ort, D.R.; Patel-Tupper, D.; et al. Perspectives on Improving Photosynthesis to Increase Crop Yield. Plant Cell 2024, 36, 3944–3973. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.-D.; Zhang, Z.-S.; Sun, X.-B.; Zhao, M.; Sun, G.-Y.; Chow, W.S. Photoinhibition and Photoinhibition-like Damage to the Photosynthetic Apparatus in Tobacco Leaves Induced by Pseudomonas Syringae Pv. Tabaci under Light and Dark Conditions. BMC Plant Biol. 2016, 16, 29. [Google Scholar] [CrossRef]
- Yan, K.; Chen, P.; Shao, H.; Shao, C.; Zhao, S.; Brestic, M. Dissection of Photosynthetic Electron Transport Process in Sweet Sorghum under Heat Stress. PLoS ONE 2013, 8, e62100. [Google Scholar] [CrossRef]
- Krause, G.H. The Role of Oxygen in Photoinhibition of Photosynthesis. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Fahad, S.; Khan, F.A.; Pandupuspitasari, N.; Hussain, S.; Khan, I.A.; Saeed, M.; Saud, S.; Hassan, S.; Adnan, M.; Amanullah; et al. Suppressing Photorespiration for the Improvement in Photosynthesis and Crop Yields: A Review on the Role of S-Allantoin as a Nitrogen Source. J. Environ. Manag. 2019, 237, 644–651. [Google Scholar] [CrossRef]
- Lima-Melo, Y.; Alencar, V.T.C.B.; Lobo, A.K.M.; Sousa, R.H.V.; Tikkanen, M.; Aro, E.-M.; Silveira, J.A.G.; Gollan, P.J. Photoinhibition of Photosystem I Provides Oxidative Protection During Imbalanced Photosynthetic Electron Transport in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 916. [Google Scholar] [CrossRef]
- Mehdi, F.; Liu, X.; Riaz, Z.; Javed, U.; Aman, A.; Galani, S. Expression of Sucrose Metabolizing Enzymes in Different Sugarcane Varieties under Progressive Heat Stress. Front. Plant Sci. 2023, 14, 1269521. [Google Scholar] [CrossRef]
- Sharma, N.; Thakur, M.; Suryakumar, P.; Mukherjee, P.; Raza, A.; Prakash, C.S.; Anand, A. ‘Breathing Out’ under Heat Stress—Respiratory Control of Crop Yield under High Temperature. Agronomy 2022, 12, 806. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of Photosynthesis by Heat Stress: The Activation State of Rubisco as a Limiting Factor in Photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Z.; Jespersen, D.; Huang, B. Photosynthesis and Protein Metabolism Associated with Elevated CO2-Mitigation of Heat Stress Damages in Tall Fescue. Environ. Exp. Bot. 2014, 99, 75–85. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Zhou, J.; Zhou, Y.-H.; Yu, J.-Q. Role of Hormones in Plant Adaptation to Heat Stress. In Plant Hormones Under Challenging Environmental Factors; Ahammed, G.J., Yu, J.-Q., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–21. ISBN 978-94-017-7758-2. [Google Scholar]
- Sharma, L.; Priya, M.; Kaushal, N.; Bhandhari, K.; Chaudhary, S.; Dhankher, O.P.; Prasad, P.V.V.; Siddique, K.H.M.; Nayyar, H. Plant Growth-Regulating Molecules as Thermoprotectants: Functional Relevance and Prospects for Improving Heat Tolerance in Food Crops. J. Exp. Bot. 2020, 71, 569–594. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yin, B.; Gu, L.; Zhang, S.; Ren, J.; Wang, Y.; Duan, W.; Zhen, W. Heat Stress Affects Tassel Development and Reduces the Kernel Number of Summer Maize. Front. Plant Sci. 2023, 14, 1186921. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Ren, H.; Zhao, B.; Zhang, J.; Ren, B.; Liu, P. Maize (Zea mays L.) Responses to Heat Stress: Mechanisms That Disrupt the Development and Hormone Balance of Tassels and Pollen. J. Agron. Crop Sci. 2023, 209, 502–516. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of Orthodox Seeds during Ageing: Influencing Factors, Physiological Alterations and the Role of Reactive Oxygen Species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef]
- Żur, I.; Dubas, E.; Krzewska, M.; Janowiak, F.; Hura, K.; Pociecha, E.; Bączek-Kwinta, R.; Płażek, A. Antioxidant Activity and ROS Tolerance in Triticale (×Triticosecale Wittm.) Anthers Affect the Efficiency of Microspore Embryogenesis. Plant Cell Tissue Organ Cult. 2014, 119, 79–94. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Ren, H.; Zhao, B.; Ren, B.; Zhang, J.; Zhang, Z.; Li, Y.; Chen, Y.; Kuzyakov, Y.; et al. Inhibiting Reactive Oxygen Species Production Mitigates Endoplasmic Reticulum Damage in Florets of Developing Maize Ears under Heat Stress. Plant J. 2025, 122, e70243. [Google Scholar] [CrossRef]
- Chaudhry, A.; Chen, Z.; Gallavotti, A. Hormonal Influence on Maize Inflorescence Development and Reproduction. Plant Reprod. 2024, 37, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.-L.; Zheng, X.-L.; Zhou, C.-Y.; Kanwar, M.K.; Zhou, J. Functions of Redox Signaling in Pollen Development and Stress Response. Antioxidants 2022, 11, 287. [Google Scholar] [CrossRef]
- Duan, L.; Mo, Z.; Fan, Y.; Li, K.; Yang, M.; Li, D.; Ke, Y.; Zhang, Q.; Wang, F.; Fan, Y.; et al. Genome-Wide Identification and Expression Analysis of the bZIP Transcription Factor Family Genes in Response to Abiotic Stress in Nicotiana tabacum L. BMC Genom. 2022, 23, 318. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Jiang, Y.; Wang, Z.; Li, X.; Li, H.; Tang, S.; Zhang, J.; Xia, M.; Zhang, M.; Deng, X.; et al. Genome-Wide Identification and Expression Analysis of the HSP90 Gene Family in Relation to Developmental and Abiotic Stress in Ginger (Zingiber officinale Roscoe). Plants 2025, 14, 1660. [Google Scholar] [CrossRef]
- Arenas-M, A.; Castillo, F.M.; Godoy, D.; Canales, J.; Calderini, D.F. Transcriptomic and Physiological Response of Durum Wheat Grain to Short-Term Heat Stress during Early Grain Filling. Plants 2021, 11, 59. [Google Scholar] [CrossRef]
- Kumar, D.; Chattopadhyay, S. Glutathione Modulates the Expression of Heat Shock Proteins via the Transcription Factors BZIP10 and MYB21 in Arabidopsis. J. Exp. Bot. 2018, 69, 3729–3743. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef] [PubMed]
- Zwack, P.J.; Rashotte, A.M. Interactions between Cytokinin Signalling and Abiotic Stress Responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Interactions of Gibberellins with Phytohormones and Their Role in Stress Responses. Horticulturae 2022, 8, 241. [Google Scholar] [CrossRef]
- Barbosa, N.C.S.; Dornelas, M.C. The Roles of Gibberellins and Cytokinins in Plant Phase Transitions. Trop. Plant Biol. 2021, 14, 11–21. [Google Scholar] [CrossRef]
- Hussain, S.; Chang, J.; Li, J.; Chen, L.; Ahmad, S.; Song, Z.; Zhang, B.; Chen, X. Multifunctional Role of Cytokinin in Horticultural Crops. Int. J. Mol. Sci. 2025, 26, 1037. [Google Scholar] [CrossRef]
- Samanta, S.; Roychoudhury, A. Molecular Crosstalk of Jasmonate with Major Phytohormones and Plant Growth Regulators during Diverse Stress Responses. J. Plant Growth Regul. 2025, 44, 62–88. [Google Scholar] [CrossRef]
- Niharika; Singh, N.B.; Singh, A.; Khare, S.; Yadav, V.; Bano, C.; Yadav, R.K. Mitigating Strategies of Gibberellins in Various Environmental Cues and Their Crosstalk with Other Hormonal Pathways in Plants: A Review. Plant Mol. Biol. Rep. 2021, 39, 34–49. [Google Scholar] [CrossRef]
- Sarwar, R.; Zhu, K.M.; Jiang, T.; Ding, P.; Gao, Y.; Tan, X.L. DELLAs Directed Gibberellins Responses Orchestrate Crop Development: A Brief Review. Crop Sci. 2023, 63, 1–28. [Google Scholar] [CrossRef]
- Tavallali, V.; Darvishzadeh, M.D. Synergistic Effects of Fe Nanocomplex and Nitrophenolate-Based Biostimulant on Growth and Physiological Performance of Tomato Seedlings. BMC Plant Biol. 2025, 25, 905. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, W.; Peng, J.; Ju, J.; Ling, P.; Guo, X.; Yang, J.; Ma, Q.; Lin, H.; Li, J.; et al. GhGASA14 regulates the flowering time of upland cotton in response to GA3. Plant Cell Rep. 2024, 43, 170. [Google Scholar] [CrossRef]
- Singh, D.; Dhiman, V.K.; Pandey, H.; Dhiman, V.K.; Pandey, D. Crosstalk between Salicylic Acid and Auxins, Cytokinins, and Gibberellins under Biotic Stress. In Auxins, Cytokinins and Gibberellins Signaling in Plants; Springer International Publishing: Cham, Switzerland, 2022; pp. 249–262. [Google Scholar]
- Kumar, R.R.; Goswami, S.; Rai, G.K.; Jain, N.; Singh, P.K.; Mishra, D.; Chaturvedi, K.K.; Kumar, S.; Singh, B.; Singh, G.P.; et al. Protection from Terminal Heat Stress: A Trade-Off between Heat-Responsive Transcription Factors (HSFs) and Stress-Associated Genes (SAGs) under Changing Environment. Cereal Res. Commun. 2021, 49, 227–234. [Google Scholar] [CrossRef]
- Bouteraa, M.T.; Ben Romdhane, W.; Baazaoui, N.; Alfaifi, M.Y.; Chouaibi, Y.; Ben Akacha, B.; Ben Hsouna, A.; Kačániová, M.; Ćavar Zeljković, S.; Garzoli, S.; et al. GASA Proteins: Review of Their Functions in Plant Environmental Stress Tolerance. Plants 2023, 12, 2045. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic Acid-Induced Abiotic Stress Tolerance and Underlying Mechanisms in Plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Shah, S.A.; Arshad, M.; Aslam, S. Comprehensive Review on the Role of Exogenous Phytohormones in Enhancing Temperature Stress Tolerance in Plants. J. Crop Health 2025, 77, 136. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Ashraf, M.; Bajguz, A.; Ahmad, P. Brassinosteroids Regulate Growth in Plants Under Stressful Environments and Crosstalk with Other Potential Phytohormones. J. Plant Growth Regul. 2018, 37, 1007–1024. [Google Scholar] [CrossRef]
- Galvão, V.C.; Collani, S.; Horrer, D.; Schmid, M. Gibberellic Acid Signaling Is Required for Ambient Temperature-Mediated Induction of Flowering in Arabidopsis thaliana. Plant J. 2015, 84, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I.; Voytenko, L.; Vasyuk, V.; Shcherbatiuk, M. ABA-Induced Alterations in Cytokinin Homeostasis of Triticum aestivum and Triticum spelta under Heat Stress. Plant Stress 2024, 11, 100353. [Google Scholar] [CrossRef]
- Voytenko, L.; Kosakivska, I. AUXINS as regulators of growth and development of cereal crops under abiotic stresses: A review. Adv. Biol. Earth Sci. 2025, 10, 5–31. [Google Scholar] [CrossRef]
- Boussora, F.; Allam, M.; Guasmi, F.; Ferchichi, A.; Rutten, T.; Hansson, M.; Youssef, H.M.; Börner, A. Spike developmental stages and ABA role in spikelet primordia abortion contribute to the final yield in barley (Hordeum vulgare L.). Bot. Stud. 2019, 60, 13. [Google Scholar] [CrossRef]
- Iqbal, H.; Yaning, C.; Raza, S.T.; Karim, S.; Shareef, M.; Waqas, M. From Lab to Field: Harnessing H2O2-Mediated Upregulation of Plant Capacities under Abiotic Stresses. Physiol. Plant. 2025, 177, e70488. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Hasan, M.M.; Hazzazi, Y.; Alharbi, B.M.; Ghani, M.U.; Ahmad, P.; Carriquí, M. Potential Mechanisms for the Rapid Post-Drought Reversal of ABA-Induced Stomatal Closure by Melatonin, 5-Aminolevulinic Acid, and Brassinosteroids. Photosynthetica 2025, 63, 104–115. [Google Scholar] [CrossRef]
- Tao, Z.; Yan, P.; Zhang, X.; Wang, D.; Wang, Y.; Ma, X.; Yang, Y.; Liu, X.; Chang, X.; Sui, P.; et al. Physiological Mechanism of Abscisic Acid-Induced Heat-Tolerance Responses to Cultivation Techniques in Wheat and Maize. Agronomy 2022, 12, 1579. [Google Scholar] [CrossRef]
- Kosakivska, I.; Voytenko, L.; Vasyuk, V.; Shcherbatiuk, M. Aba-Induced Changes in Cytokinin Homeostasis of Cereals Under Heat Stress; Kholodny Institute of Botany, National Academy of Sciences of Ukraine: Kyiv, Ukraine, 2023. [Google Scholar]
- Kosakivska, I.V.; Vasyuk, V.A.; Voytenko, L.V.; Shcherbatiuk, M.M. Changes in Hormonal Status of Winter Wheat (Triticum aestivum L.) and Spelt Wheat (Triticum spelta L.) after Heat Stress and in Recovery Period. Cereal Res. Commun. 2022, 50, 821–830. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Liu, J.; Zhang, P.; Kudoyarova, G.; Liu, C.J.; Zhang, K. Spatially distributed cytokinins: Metabolism, signaling, and transport. Plant Commun. 2024, 5, 100987. [Google Scholar] [CrossRef]
- Yao, L.; Li, S.; Zhou, N.; Guo, Y. The Mechanism of Seed Priming with Abscisic Acid for Enhancing Cuticle Deposition Under Drought Stress: Phenotypic and Transcriptomic Insights. Agriculture 2025, 15, 1124. [Google Scholar] [CrossRef]
- Onyemaobi, I.O. Genetic Analysis of Meiotic-Stage Water Stress Tolerance in Wheat (Triticum aestivum L.). Ph.D. Thesis, The University of Western Australia, Perth, Australia, 2018; pp. 1–89. [Google Scholar]
- Kosakivska, I.V.; Voytenko, L.V.; Vasyuk, V.A.; Shcherbatiuk, M.M. Abscisic Acid-Induced Response of Triticum aestivum and T. spelta Phytohormonal System to Moderate Soil Drought. Zemdirb.-Agric. 2023, 110, 111–120. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Vasyuk, V.A.; Voytenko, L.V.; Shcherbatiuk, M.M. The Effects of Moderate Soil Drought on Phytohormonal Balance of Triticum aestivum L. and Triticum spelta L. Cereal Res. Commun. 2023, 51, 627–638. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Voytenko, L.V.; Vasyuk, V.A.; Shcherbatiuk, M.M. Effect of Pre-Sowing Priming of Seeds with Exogenous Abscisic Acid on Endogenous Hormonal Balance of Spelt Wheat under Heat Stress. Zemdirb.-Agric. 2022, 109, 21–26. [Google Scholar] [CrossRef]
- Han, H.; Tian, Z.; Fan, Y.; Cui, Y.; Cai, J.; Jiang, D.; Cao, W.; Dai, T. Water-deficit treatment followed by re-watering stimulates seminal root growth associated with hormone balance and photosynthesis in wheat (Triticum aestivum L.) seedlings. Plant Growth Regul. 2015, 77, 201–210. [Google Scholar] [CrossRef]
- Hudeček, M.; Nožková, V.; Plíhalová, L.; Plíhal, O. Plant hormone cytokinin at the crossroads of stress priming and control of photosynthesis. Front. Plant Sci. 2023, 13, 1103088. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Poór, P.; Nawaz, K.; Gupta, R.; Ashfaque, F.; Khan, M.I.R. Ethylene Involvement in the Regulation of Heat Stress Tolerance in Plants. Plant Cell Rep. 2022, 41, 675–698. [Google Scholar] [CrossRef]
- Janaagal, M.; Sharma, P.; Kumari, G.; Gulia, H.; Suresh, G.; Tallapragada, S.; Devi, S.; Lakra, N.; Arya, S.S.; Pooja, P. Revolutionizing High Temperature Stress Relief: Exploring the Latest Advances in Salicylic Acid Application. J. Crop Health 2024, 76, 1293–1305. [Google Scholar] [CrossRef]
- Rehman, A.; Khan, I.; Farooq, M. Secondary Metabolites Mediated Reproductive Tolerance Under Heat Stress in Plants. J. Plant Growth Regul. 2024, 43, 2993–3011. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential Role of Phytohormones and Plant Growth-Promoting Rhizobacteria in Abiotic Stresses: Consequences for Changing Environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.; Tomovicova, L.; Panzarova, K.; Fajkus, J.; Hejatko, J.; Skalak, J. Epigenetics and plant hormone dynamics: A functional and methodological perspective. J. Exp. Bot. 2024, 75, 5267–5294. [Google Scholar] [CrossRef] [PubMed]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. High Temperature Susceptibility of Sexual Reproduction in Crop Plants. J. Exp. Bot. 2020, 71, 555–568. [Google Scholar] [CrossRef]
- Burgess, A.J.; Masclaux-Daubresse, C.; Strittmatter, G.; Weber, A.P.; Taylor, S.H.; Harbinson, J.; Yin, X.; Long, S.; Paul, M.J.; Westhoff, P.; et al. Improving crop yield potential: Underlying biological processes and future prospects. Food Energy Secur. 2023, 12, e435. [Google Scholar] [CrossRef]
- Resentini, F.; Orozco-Arroyo, G.; Cucinotta, M.; Mendes, M.A. The Impact of Heat Stress in Plant Reproduction. Front. Plant Sci. 2023, 14, 1271644. [Google Scholar] [CrossRef]
- Mehmood, M.; Tanveer, N.A.; Joyia, F.A.; Ullah, I.; Mohamed, H.I. Effect of High Temperature on Pollen Grains and Yield in Economically Important Crops: A Review. Planta 2025, 261, 141. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Tsuchiya, T.; Hamada, K.; Kawamura, S.; Yano, K.; Ohshima, M.; Higashitani, A.; Watanabe, M.; Kawagishi-Kobayashi, M. High Temperatures Cause Male Sterility in Rice Plants with Transcriptional Alterations during Pollen Development. Plant Cell Physiol. 2009, 50, 1911–1922. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Pal, M. Response of Rice (Oryza sativa L.) to Increasing Temperature and Atmospheric CO2. In Climate Change and Crops; Singh, S.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 63–80. ISBN 978-3-540-88246-6. [Google Scholar]
- Usman, B.; Nawaz, G.; Zhao, N.; Liu, Y.; Li, R. Generation of High Yielding and Fragrant Rice (Oryza sativa L.) Lines by CRISPR/Cas9 Targeted Mutagenesis of Three Homoeologs of Cytochrome P450 Gene Family and OsBADH2 and Transcriptome and Proteome Profiling Revealed Changes Triggered by Mutations. Plants 2020, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.T. Short Day Induction of Inflorescence Initiation in Some Winter Wheat Varieties. Funct. Plant Biol. 1987, 14, 277–286. [Google Scholar] [CrossRef]
- Shitsukawa, N.; Kinjo, H.; Takumi, S.; Murai, K. Heterochronic Development of the Floret Meristem Determines Grain Number per Spikelet in Diploid, Tetraploid and Hexaploid Wheats. Ann. Bot. 2009, 104, 243–251. [Google Scholar] [CrossRef]
- Wang, H.M.; Enns, J.L.; Nelson, K.L.; Brost, J.M.; Orr, T.D.; Ferrie, A.M.R. Improving the Efficiency of Wheat Microspore Culture Methodology: Evaluation of Pretreatments, Gradients, and Epigenetic Chemicals. Plant Cell Tissue Organ Cult. 2019, 139, 589–599. [Google Scholar] [CrossRef]
- Ugarte, C.; Calderini, D.F.; Slafer, G.A. Grain Weight and Grain Number Responsiveness to Pre-Anthesis Temperature in Wheat, Barley and Triticale. Field Crops Res. 2007, 100, 240–248. [Google Scholar] [CrossRef]
- Calderini, D.F.; Reynolds, M.P. Changes in Grain Weight as a Consequence of De-Graining Treatments at Pre- and Post-Anthesis in Synthetic Hexaploid Lines of Wheat (Triticum durum × T. tauschii). Funct. Plant Biol. 2000, 27, 183–191. [Google Scholar] [CrossRef]
- Kaur, V.; Behl, R. Grain Yield in Wheat as Affected by Short Periods of High Temperature, Drought and Their Interaction during Pre- and Post-Anthesis Stages. Communications 2010, 38, 514–520. [Google Scholar] [CrossRef]
- Brenciiley, W.E. The Development of the Flower and Grain of Barley. J. Inst. Brew. 1920, 26, 615–632. [Google Scholar] [CrossRef]
- Jacquard, C.; Mazeyrat-Gourbeyre, F.; Devaux, P.; Boutilier, K.; Baillieul, F.; Clément, C. Microspore Embryogenesis in Barley: Anther Pre-Treatment Stimulates Plant Defence Gene Expression. Planta 2009, 229, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Abdalla Eltantawy, A. Key Factors Involved in Stress-Induced Microspore Embryogenesis in Barley and Rapeseed: DNA Methylation, Arabinogalactan Proteins and Auxin; Universidad Complutense de Madrid: Madrid, Spain, 2016; pp. 1–279. [Google Scholar]
- Oshino, T.; Abiko, M.; Saito, R.; Ichiishi, E.; Endo, M.; Kawagishi-Kobayashi, M.; Higashitani, A. Premature Progression of Anther Early Developmental Programs Accompanied by Comprehensive Alterations in Transcription during High-Temperature Injury in Barley Plants. Mol. Genet. Genom. 2007, 278, 31–42. [Google Scholar] [CrossRef]
- García, G.A.; Serrago, R.A.; Dreccer, M.F.; Miralles, D.J. Post-Anthesis Warm Nights Reduce Grain Weight in Field-Grown Wheat and Barley. Field Crops Res. 2016, 195, 50–59. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Park, J.-H.; Cho, S.-H.; Yang, M.-S.; Park, C.-M. Phenological Growth Stages of Brachypodium Distachyon: Codification and Description. Weed Res. 2011, 51, 612–620. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, M.B.; Bhalla, P.L. Ultrastructure of Microsporogenesis and Microgametogenesis in Brachypodium Distachyon. Protoplasma 2015, 252, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-J.; Dickinson, D.B. Ability of Pollen to Germinate Prior to Anthesis and Effect of Desiccation on Germination 1. Plant Physiol. 1984, 74, 746–748. [Google Scholar] [CrossRef]
- Peng, Y.; Zeng, X.; Houx III, J.H.; Boardman, D.L.; Li, C.; Fritschi, F.B. Pre- and Post-Silking Carbohydrate Concentrations in Maize Ear-Leaves and Developing Ears in Response to Nitrogen Availability. Crop Sci. 2016, 56, 3218–3227. [Google Scholar] [CrossRef]
- Amelework, A.; Laing, M.; Shimelis, H. Evaluation of Effective Gametocides for Selective Induction of Male Sterility in Sorghum. Czech J. Genet. Plant Breed. 2016, 52, 163–170. [Google Scholar] [CrossRef]
- Bartek, M.S.; Hodnett, G.L.; Burson, B.L.; Stelly, D.M.; Rooney, W.L. Pollen Tube Growth after Intergeneric Pollinations of Iap-Homozygous Sorghum. Crop Sci. 2012, 52, 1553–1560. [Google Scholar] [CrossRef]
- Laza, H.E.; Kaur-Kapoor, H.; Xin, Z.; Payton, P.R.; Chen, J. Morphological Analysis and Stage Determination of Anther Development in Sorghum [Sorghum bicolor (L.) Moench]. Planta 2022, 255, 86. [Google Scholar] [CrossRef]
- Worland, B.; Robinson, N.; Jordan, D.; Schmidt, S.; Godwin, I. Post-Anthesis Nitrate Uptake Is Critical to Yield and Grain Protein Content in Sorghum bicolor. J. Plant Physiol. 2017, 216, 118–124. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rai, K.N.; Singh, P.; Ameta, V.L.; Gupta, S.K.; Jayalekha, A.K.; Mahala, R.S.; Pareek, S.; Swami, M.L.; Verma, Y.S. Seed Set Variability under High Temperatures during Flowering Period in Pearl Millet (Pennisetum glaucum L. (R.) Br.). Field Crops Res. 2015, 171, 41–53. [Google Scholar] [CrossRef]
- Miao, Y.; Cao, J.; Huang, L.; Yu, Y.; Lin, S. FLA14 Is Required for Pollen Development and Preventing Premature Pollen Germination under High Humidity in Arabidopsis. BMC Plant Biol. 2021, 21, 254. [Google Scholar] [CrossRef]
- Osorio Zaldumbide, E.E. Effect of High Temperature on Ovule Development in Field Pea (Pisum sativum L.). Ph.D. Dissertation, University of Saskatchewan, Saskatoon, SK, Canada, 2020; pp. 1–191. [Google Scholar]
- Jerca, I.O.; Cîmpeanu, S.M.; Teodorescu, R.I.; Drăghici, E.M.; Nițu, O.A.; Sannan, S.; Arshad, A. A Comprehensive Assessment of the Morphological Development of Inflorescence, Yield Potential, and Growth Attributes of Summer-Grown, Greenhouse Cherry Tomatoes. Agronomy 2024, 14, 556. [Google Scholar] [CrossRef]
- Mattioli, R.; Biancucci, M.; El Shall, A.; Mosca, L.; Costantino, P.; Funck, D.; Trovato, M. Proline Synthesis in Developing Microspores Is Required for Pollen Development and Fertility. BMC Plant Biol. 2018, 18, 356. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Z.; Huang, X.; Xu, C. Molecular Regulation of Tomato Male Reproductive Development. aBIOTECH 2023, 4, 72–82. [Google Scholar] [CrossRef]
- Tran, L.T.; Sugimoto, K.; Kasozi, M.; Mitalo, O.W.; Ezura, H. Pollination, Pollen Tube Growth, and Fertilization Independently Contribute to Fruit Set and Development in Tomato. Front. Plant Sci. 2023, 14, 1205816. [Google Scholar] [CrossRef]
- Patterson, B.D.; Mutton, L.; Paull, R.E.; Nguyen, V.Q. Tomato Pollen Development: Stages Sensitive to Chilling and a Natural Environment for the Selection of Resistant Genotypes. Plant Cell Environ. 1987, 10, 363–368. [Google Scholar] [CrossRef]
- Farooq, M.; Rehman, A.; Wahid, A.; Siddique, K.H.M. Physiology of Grain Development in Cereals. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Wang, C.; Yang, X.; Li, G. Molecular Insights into Inflorescence Meristem Specification for Yield Potential in Cereal Crops. Int. J. Mol. Sci. 2021, 22, 3508. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Sahu, B.B. Botany of Rice Plant. In Panicle Architecture of Rice and Its Relationship with Grain Filling; Mohapatra, P.K., Sahu, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 27–48. ISBN 978-3-030-67897-5. [Google Scholar]
- Xie, W.; He, P.; Ma, H.; Huang, X.; Fan, G.; Yang, H. Straw Mulching Combined with Phosphorus Fertilizer Increases Fertile Florets of Wheat by Enhancing Leaf Photosynthesis and Assimilate Utilization. Agronomy 2023, 13, 2342. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Vedenicheva, N.P.; Babenko, L.M.; Voytenko, L.V.; Romanenko, K.O.; Vasyuk, V.A. Exogenous Phytohormones in the Regulation of Growth and Development of Cereals under Abiotic Stresses. Mol. Biol. Rep. 2022, 49, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.K.; Sahu, B.B. Ontogeny of Organ Development in Rice Plant. In Panicle Architecture of Rice and its Relationship with Grain Filling; Mohapatra, P.K., Sahu, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 49–61. ISBN 978-3-030-67897-5. [Google Scholar]
- Liu, Z. Mutant Analysis in a Small Weed Reveals the Blueprint of Floral Patterning. Dev. Biol. 2025, 521, 138–141. [Google Scholar] [CrossRef]
- Bowman, J.L.; Moyroud, E. Reflections on the ABC Model of Flower Development. Plant Cell 2024, 36, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Pfannebecker, K.C.; Lange, M.; Rupp, O.; Becker, A. An Evolutionary Framework for Carpel Developmental Control Genes. Mol. Biol. Evol. 2016, 34, 330–348. [Google Scholar] [CrossRef]
- Cong, D.; Zhao, X.; Ni, C.; Li, M.; Han, L.; Cheng, J.; Liu, H.; Liu, H.; Yao, D.; Liu, S.; et al. The SEPALLATA-like Gene HrSEP1 in Hippophae Rhamnoides Regulates Flower Development by Interacting with Other MADS-Box Subfamily Genes. Front. Plant Sci. 2025, 15, 1503346. [Google Scholar] [CrossRef]
- Adhikari, P.B.; Kasahara, R.D. An Overview on MADS Box Members in Plants: A Meta-Review. Int. J. Mol. Sci. 2024, 25, 8233. [Google Scholar] [CrossRef]
- Zhou, L.; Iqbal, A.; Yang, M.; Yang, Y. Research Progress on Gene Regulation of Plant Floral Organogenesis. Genes 2025, 16, 79. [Google Scholar] [CrossRef]
- Magon, G. The Regulation of Gene Expression in Grapevine Flowering Process. Ph.D. Thesis, University of Padova, Padova, Italy, 2023; pp. 1–304. [Google Scholar]
- Zhang, Z.; Hu, M.; Xu, W.; Wang, Y.; Huang, K.; Zhang, C.; Wen, J. Understanding the molecular mechanism of anther development under abiotic stresses. Plant Mol. Biol. 2021, 105, 1–10. [Google Scholar] [CrossRef]
- Zenda, T.; Wang, N.; Dong, A.; Zhou, Y.; Duan, H. Reproductive-stage heat stress in cereals: Impact, plant responses and strategies for tolerance improvement. Int. J. Mol. Sci. 2022, 23, 6929. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, I.G.; Massiah, A.J.; Thomas, B. Florigenic and Antiflorigenic Signaling in Plants. Plant Cell Physiol. 2012, 53, 1827–1842. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, P.; Msanne, J.; Rabara, R. Phytochrome and Phytohormones: Working in Tandem for Plant Growth and Development. Front. Plant Sci. 2018, 9, 1037. [Google Scholar] [CrossRef]
- Loka, D.A.; Oosterhuis, D.M. Increased Night Temperatures during Cotton’s Early Reproductive Stage Affect Leaf Physiology and Flower Bud Carbohydrate Content Decreasing Flower Bud Retention. J. Agron. Crop Sci. 2016, 202, 518–529. [Google Scholar] [CrossRef]
- Chiluwal, A.; Bheemanahalli, R.; Kanaganahalli, V.; Boyle, D.; Perumal, R.; Pokharel, M.; Oumarou, H.; Jagadish, S.V.K. Deterioration of Ovary Plays a Key Role in Heat Stress-Induced Spikelet Sterility in Sorghum. Plant Cell Environ. 2020, 43, 448–462. [Google Scholar] [CrossRef]
- Kaur, I.; Kathpalia, R.; Koul, M. Understanding Megasporogenesis through Model Plants: Contemporary Evidence and Future Insights. Int. J. Dev. Biol. 2024, 68, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Diwan, G.; Rawte, S.; Jha, Z. Haploids: Then and Now. In Doubled Haploids: Technological Advances and Role in Crop Improvement; Jha, Z., Verulkar, S.B., Penna, S., Eds.; Springer Nature: Singapore, 2025; pp. 1–56. ISBN 978-981-9623-39-6. [Google Scholar]
- Wang, X.T.; Yuan, C.; Yuan, T.T.; Cui, S.J. The Arabidopsis LFR Gene Is Required for the Formation of Anther Cell Layers and Normal Expression of Key Regulatory Genes. Mol. Plant 2012, 5, 993–1000. [Google Scholar] [CrossRef][Green Version]
- Ma, H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu. Rev. Plant Biol. 2005, 56, 393–434. [Google Scholar] [CrossRef]
- Qian, Z.; Shi, D.; Zhang, H.; Li, Z.; Huang, L.; Yan, X.; Lin, S. Transcription Factors and Their Regulatory Roles in the Male Gametophyte Development of Flowering Plants. Int. J. Mol. Sci. 2024, 25, 566. [Google Scholar] [CrossRef]
- De Jaeger-Braet, J.; Schnittger, A. Heating up Meiosis—Chromosome Recombination and Segregation under High Temperatures. Curr. Opin. Plant Biol. 2024, 80, 102548. [Google Scholar] [CrossRef]
- Boateng, K.A. Studies on Arabidopsis Proteins Required for the Establishment and Release of Sister Chromatid Cohesion. Ph.D. Thesis, Miami University, Oxford, OH, USA, 2007; pp. 1–226. [Google Scholar]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. Heat-Triggered Transcriptional Reprogramming in Microspores Disrupts Progression of Pollen Development in Brassica napus L. BioRxiv 2024, 11, 15.62384. [Google Scholar]
- Maroto, M.; Torvisco, S.N.; García-Merino, C.; Fernández-González, R.; Pericuesta, E. Mechanisms of Hormonal, Genetic, and Temperature Regulation of Germ Cell Proliferation, Differentiation, and Death During Spermatogenesis. Biomolecules 2025, 15, 500. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pant, J.; Dell, B.; Bell, R.W. Effects of Boron Deficiency on Anther Development and Floret Fertility in Wheat (Triticum aestivum L. ‘Wilgoyne’). Ann. Bot. 2000, 85, 493–500. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Wu, Y.; Zheng, X.; Guo, X.; Sun, W.; Sun, Z.; Wang, Z.; Zhang, Y. A Dynamic Regulation of Nitrogen on Floret Primordia Development in Wheat. Crop J. 2024, 12, 271–280. [Google Scholar] [CrossRef]
- Rutley, N.; Twell, D. A Decade of Pollen Transcriptomics. Plant Reprod. 2015, 28, 73–89. [Google Scholar] [CrossRef]
- Basiri, E.; Jafari Marandi, S.; Arbabian, S.; Majd, A.; Malboobi, M.A. Development of Male and Female Gametophytes and Embryogenesis in the Arabidopsis thaliana. Biologia 2021, 76, 853–863. [Google Scholar] [CrossRef]
- Grossniklaus, U.; Schneitz, K. The Molecular and Genetic Basis of Ovule and Megagametophyte Development. Semin. Cell Dev. Biol. 1998, 9, 227–238. [Google Scholar] [CrossRef]
- Sprunck, S.; Groß-Hardt, R. Nuclear Behavior, Cell Polarity, and Cell Specification in the Female Gametophyte. Sex. Plant Reprod. 2011, 24, 123–136. [Google Scholar] [CrossRef]
- Shi, W.; Yang, J.; Kumar, R.; Zhang, X.; Impa, S.M.; Xiao, G.; Jagadish, S.V.K. Heat Stress During Gametogenesis Irreversibly Damages Female Reproductive Organ in Rice. Rice 2022, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Erfatpour, M.; MacLean, D.; Lahlali, R.; Jiang, Y. Ovule and seed development of crop plants in response to climate change. Front. Sustain. Food Syst. 2024, 8, 1495610. [Google Scholar] [CrossRef]
- Hedhly, A. Sensitivity of Flowering Plant Gametophytes to Temperature Fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of soluble sugars in metabolism and sensing under abiotic stress. In Plant Growth Regulators: Signalling Under Stress Conditions; Springer International Publishing: Cham, Switzerland, 2021; pp. 305–334. [Google Scholar]
- Kumar, S.; Thakur, M.; Mitra, R.; Basu, S.; Anand, A. Sugar Metabolism during Pre- and Post-Fertilization Events in Plants under High Temperature Stress. Plant Cell Rep. 2022, 41, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.M.; Vijayalakshmi, G.; Naik, M.L.; Basha, P.O.; Sergeant, K.; Hausman, J.F.; Khan, P.S.S.V. Pollen Development and Function under Heat Stress: From Effects to Responses. Acta Physiol. Plant 2019, 41, 47. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Kong, X.; Khan, A.; Ullah, N.; Zhang, X. Plant Coping with Cold Stress: Molecular and Physiological Adaptive Mechanisms with Future Perspectives. Cells 2025, 14, 110. [Google Scholar] [CrossRef]
- Dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.-R.; Gao, J.; Lin, H.-X.; Lin, Y. The Molecular Basis of Heat Stress Responses in Plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef]
- Somero, G.N. The Cellular Stress Response and Temperature: Function, Regulation, and Evolution. J. Exp. Zool. A Ecol. Integr. Physiol. 2020, 333, 379–397. [Google Scholar] [CrossRef]
- Wen, J.; Qin, Z.; Sun, L.; Zhang, Y.; Wang, D.; Peng, H.; Yao, Y.; Hu, Z.; Ni, Z.; Sun, Q.; et al. Alternative Splicing of TaHSFA6e Modulates Heat Shock Protein–Mediated Translational Regulation in Response to Heat Stress in Wheat. New Phytol. 2023, 239, 2235–2247. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Gao, H.; Yu, S.; Liu, C.; Wang, Y.; Sun, Y.; Zhang, D. Alternative Splicing of Functional Genes in Plant Growth, Development, and Stress Responses. Int. J. Mol. Sci. 2025, 26, 5864. [Google Scholar] [CrossRef]
- Merret, R.; Carpentier, M.C.; Favory, J.J.; Picart, C.; Descombin, J.; Bousquet-Antonelli, C.; Tillard, P.; Lejay, L.; Deragon, J.M.; Charng, Y.Y. Heat Shock Protein HSP101 Affects the Release of Ribosomal Protein mRNAs for Recovery after Heat Shock. Plant Physiol. 2017, 174, 1216–1225. [Google Scholar] [CrossRef]
- Tian, X.; Qin, Z.; Zhao, Y.; Wen, J.; Lan, T.; Zhang, L.; Wang, F.; Qin, D.; Yu, K.; Zhao, A.; et al. Stress Granule-Associated TaMBF1c Confers Thermotolerance through Regulating Specific mRNA Translation in Wheat (Triticum aestivum). New Phytol. 2022, 233, 1719–1731. [Google Scholar] [CrossRef]
- Blombach, F.; Launay, H.; Snijders, A.P.; Zorraquino, V.; Wu, H.; de Koning, B.; Brouns, S.J.; Ettema, T.J.; Camilloni, C.; Cavalli, A.; et al. Archaeal MBF1 binds to 30S and 70S ribosomes via its helix–turn–helix domain. Biochem. J. 2014, 462, 373–384. [Google Scholar] [CrossRef]
- Mahmood, T.; Safdar, W.; Abbasi, B.H.; Naqvi, S.M.S. An Overview on the Small Heat Shock Proteins. Afr. J. Biotechnol. 2010, 9, 927–939. [Google Scholar] [CrossRef]
- Pirkkala, L.; Nykänen, P.; Sistonen, L.E.A. Roles of the Heat Shock Transcription Factors in Regulation of the Heat Shock Response and Beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of Heat Shock Transcription Factors and Their Roles in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Kumar, P.; Paul, D.; Jhajhriya, S.; Kumar, R.; Dutta, S.; Siwach, P.; Das, S. Understanding Heat-Shock Proteins’ Abundance and Pivotal Function under Multiple Abiotic Stresses. J. Plant Biochem. Biotechnol. 2024, 33, 492–513. [Google Scholar] [CrossRef]
- Lu, F.; Feng, B.; Chen, L.; Qiu, J.; Wei, X. How Does Rice Cope with High-Temperature Stress During Its Growth and Development, Especially at the Grain-Filling Stage? Agronomy 2025, 15, 623. [Google Scholar] [CrossRef]
- Swindell, W.R.; Huebner, M.; Weber, A.P. Transcriptional Profiling of Arabidopsis Heat Shock Proteins and Transcription Factors Reveals Extensive Overlap between Heat and Non-Heat Stress Response Pathways. BMC Genom. 2007, 8, 125. [Google Scholar] [CrossRef]
- Yurina, N.P. Heat shock proteins in plant protection from oxidative stress. Mol. Biol. 2023, 57, 951–964. [Google Scholar] [CrossRef]
- Pérez-Salamó, I.; Papdi, C.; Rigó, G.; Zsigmond, L.; Vilela, B.; Lumbreras, V.; Nagy, I.; Horváth, B.; Domoki, M.; Darula, Z.; et al. The heat shock factor A4A confers salt tolerance and is regulated by oxidative stress and the mitogen-activated protein kinases MPK3 and MPK6. Plant Physiol. 2014, 165, 319–334. [Google Scholar] [CrossRef]
- Waters, E.R.; Lee, G.J.; Vierling, E. Evolution, Structure and Function of the Small Heat Shock Proteins in Plants. J. Exp. Bot. 1996, 47, 325–338. [Google Scholar] [CrossRef]
- Heinemann, B.; Künzler, P.; Eubel, H.; Braun, H.-P.; Hildebrandt, T.M. Estimating the Number of Protein Molecules in a Plant Cell: Protein and Amino Acid Homeostasis during Drought. Plant Physiol. 2021, 185, 385–404. [Google Scholar] [CrossRef]
- Kolesnichenko, A.V.; Zykova, V.V.; Grabelnych, O.I.; Sumina, O.N.; Pobezhimova, T.P.; Voinikov, V.K. Screening of Mitochondrial Proteins in Winter Rye, Winter Wheat, Elymus and Maize with an Immunochemical Affinity to the Stress Protein 310 kD and Their Intramitochondrial Localization in Winter Wheat. J. Therm. Biol. 2000, 25, 245–249. [Google Scholar] [CrossRef]
- Suzuki, N. Fine tuning of ROS, redox and energy regulatory systems associated with the functions of chloroplasts and mitochondria in plants under heat stress. Int. J. Mol. Sci. 2023, 24, 1356. [Google Scholar] [CrossRef]
- Yadav, M.R.; Choudhary, M.; Singh, J.; Lal, M.K.; Jha, P.K.; Udawat, P.; Gupta, N.K.; Rajput, V.D.; Garg, N.K.; Maheshwari, C.; et al. Impacts, tolerance, adaptation, and mitigation of heat stress on wheat under changing climates. Int. J. Mol. Sci. 2022, 23, 2838. [Google Scholar] [CrossRef]
- Nasr, M.A.; Dovbeshko, G.I.; Bearne, S.L.; El-Badri, N.; Matta, C.F. Heat Shock Proteins in the “Hot” Mitochondrion: Identity and Putative Roles. BioEssays 2019, 41, 1900055. [Google Scholar] [CrossRef]
- Schmitt, M.; Neupert, W.; Langer, T. The Molecular Chaperone Hsp78 Confers Compartment-Specific Thermotolerance to Mitochondria. J. Cell Biol. 1996, 134, 1375–1386. [Google Scholar] [CrossRef]
- Webster, J.M.; Darling, A.L.; Uversky, V.N.; Blair, L.J. Small Heat Shock Proteins, Big Impact on Protein Aggregation in Neurodegenerative Disease. Front. Pharmacol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Young, T.E.; Ling, J.; Geisler-Lee, C.J.; Tanguay, R.L.; Caldwell, C.; Gallie, D.R. Developmental and Thermal Regulation of the Maize Heat Shock Protein, HSP101. Plant Physiol. 2001, 127, 777–791. [Google Scholar] [CrossRef][Green Version]
- Tiwari, L.D.; Kumar, R.; Sharma, V.; Sahu, A.K.; Sahu, B.; Naithani, S.C.; Grover, A. Stress and Development Phenotyping of Hsp101 and Diverse Other Hsp Mutants of Arabidopsis thaliana. J. Plant Biochem. Biotechnol. 2021, 30, 889–905. [Google Scholar] [CrossRef]
- Haq, N.U.; Ammar, M.; Bano, A.; Luthe, D.S.; Heckathorn, S.A.; Shakeel, S.N. Molecular Characterization of Chenopodium Album Chloroplast Small Heat Shock Protein and Its Expression in Response to Different Abiotic Stresses. Plant Mol. Biol. Rep. 2013, 31, 1230–1241. [Google Scholar] [CrossRef]
- Khan, Z.; Shahwar, D. Role of Heat Shock Proteins (HSPs) and Heat Stress Tolerance in Crop Plants. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 211–234. ISBN 978-3-030-45669-6. [Google Scholar]
- Hu, Y.; Zhang, T.; Liu, Y.; Li, Y.; Wang, M.; Zhu, B.; Liao, D.; Yun, T.; Huang, W.; Zhang, W.; et al. Pumpkin (Cucurbita Moschata) HSP20 Gene Family Identification and Expression Under Heat Stress. Front. Genet. 2021, 12, 753953. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef]
- Momcilovic, I.; Ristic, Z. Localization and Abundance of Chloroplast Protein Synthesis Elongation Factor (EF-Tu) and Heat Stability of Chloroplast Stromal Proteins in Maize. Plant Sci. 2004, 166, 81–88. [Google Scholar] [CrossRef]
- Razzaq, M.K.; Rani, R.; Xing, G.; Xu, Y.; Raza, G.; Aleem, M.; Iqbal, S.; Arif, M.; Mukhtar, Z.; Nguyen, H.T.; et al. Genome-Wide Identification and Analysis of the Hsp40/J-Protein Family Reveals Its Role in Soybean (Glycine max) Growth and Development. Genes 2023, 14, 1254. [Google Scholar] [CrossRef]
- Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- DeRocher, A.E.; Vierling, E. Developmental Control of Small Heat Shock Protein Expression during Pea Seed Maturation. Plant J. 1994, 5, 93–102. [Google Scholar] [CrossRef]
- Charfeddine, S.; Saïdi, M.N.; Charfeddine, M.; Gargouri-Bouzid, R. Genome-Wide Identification and Expression Profiling of the Late Embryogenesis Abundant Genes in Potato with Emphasis on Dehydrins. Mol. Biol. Rep. 2015, 42, 1163–1174. [Google Scholar] [CrossRef]
- Bies-Ethève, N.; Gaubier-Comella, P.; Debures, A.; Lasserre, E.; Jobet, E.; Raynal, M.; Cooke, R.; Delseny, M. Inventory, Evolution and Expression Profiling Diversity of the LEA (Late Embryogenesis Abundant) Protein Gene Family in Arabidopsis thaliana. Plant Mol. Biol. 2008, 67, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bañuelos, M.L.; Gardea, A.A.; Winzerling, J.J.; Vazquez-Moreno, L. Characterization of a Midwinter-Expressed Dehydrin (DHN) Gene from Apple Trees (Malus Domestica). Plant Mol. Biol. Rep. 2009, 27, 476–487. [Google Scholar] [CrossRef]
- Ismail, A.M.; Hall, A.E.; Close, T.J. Allelic Variation of a Dehydrin Gene Cosegregates with Chilling Tolerance during Seedling Emergence. Proc. Natl. Acad. Sci. USA 1999, 96, 13566–13570. [Google Scholar] [CrossRef]
- Ganguly, M.; Datta, K.; Roychoudhury, A.; Gayen, D.; Sengupta, D.N.; Datta, S.K. Overexpression of Rab16A Gene in Indica Rice Variety for Generating Enhanced Salt Tolerance. Plant Signal. Behav. 2012, 7, 502–509. [Google Scholar] [CrossRef]
- Rorat, T. Plant Dehydrins—Tissue Location, Structure and Function. Cell Mol. Biol. Lett. 2006, 11, 536–556. [Google Scholar] [CrossRef]
- Campbell, S.A.; Close, T.J. Dehydrins: Genes, Proteins, and Associations with Phenotypic Traits. New Phytol. 1997, 137, 61–74. [Google Scholar] [CrossRef]
- Zaidi, I.; Hanin, M.; Saidi, M.N.; Soltani, N.; Brini, F. The Barley Dehydrin 4 and Stress Tolerance: From Gene to Function. Environ. Exp. Bot. 2024, 224, 105802. [Google Scholar] [CrossRef]
- Soltani, N.; Zaidi, I.; Saidi, M.N.; Brini, F. Group 1 LEA Proteins in Durum Wheat: Evolution, Expression, and Roles in Abiotic Stress Tolerance. Plants 2025, 14, 2817. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-G.; Ahsan, N.; Lee, S.-H.; Kang, K.Y.; Bahk, J.D.; Lee, I.-J.; Lee, B.-H. A Proteomic Approach in Analyzing Heat-Responsive Proteins in Rice Leaves. Proteomics 2007, 7, 3369–3383. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Close, T.J. Expression of Dehydrins under Heat Stress and Their Relationship with Water Relations of Sugarcane Leaves. Biol. Plant 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Marcon, C.; Schützenmeister, A.; Schütz, W.; Madlung, J.; Piepho, H.P.; Hochholdinger, F. Nonadditive Protein Accumulation Patterns in Maize (Zea mays L.) Hybrids during Embryo Development. J. Proteome Res. 2010, 9, 6511–6522. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, J.; Shi, W.; Cao, X.; Luo, J.; Polle, A.; Luo, Z.-B. Comparative Transcriptomic Analysis Reveals the Roles of Overlapping Heat-/Drought-Responsive Genes in Poplars Exposed to High Temperature and Drought. Sci. Rep. 2017, 7, 43215. [Google Scholar] [CrossRef]
- Singh, S.; Praveen, A.; Dudha, N.; Bhadrecha, P. Integrating Physiological and Multi-Omics Methods to Elucidate Heat Stress Tolerance for Sustainable Rice Production. Physiol. Mol. Biol. Plants 2024, 30, 1185–1208. [Google Scholar] [CrossRef]
- Liu, X.-H.; Lyu, Y.-S.; Yang, W.; Yang, Z.-T.; Lu, S.-J.; Liu, J.-X. A Membrane-Associated NAC Transcription Factor OsNTL3 Is Involved in Thermotolerance in Rice. Plant Biotechnol. J. 2020, 18, 1317–1329. [Google Scholar] [CrossRef]
- Shu, Y.; Zhou, Y.; Mu, K.; Hu, H.; Chen, M.; He, Q.; Huang, S.; Ma, H.; Yu, X. A Transcriptomic Analysis Reveals Soybean Seed Pre-Harvest Deterioration Resistance Pathways under High Temperature and Humidity Stress. Genome 2020, 63, 115–124. [Google Scholar] [CrossRef]
- Fang, S.; Cammarano, D.; Zhou, G.; Tan, K.; Ren, S. Effects of Increased Day and Night Temperature with Supplemental Infrared Heating on Winter Wheat Growth in North China. Eur. J. Agron. 2015, 64, 67–77. [Google Scholar] [CrossRef]
- Ali, I.; Ullah, S.; Iqbal, A.; Quan, Z.; Liang, H.; Ahmad, S.; Muhammad, I.; Amanullah; Imran; Guo, Z.; et al. Combined Application of Biochar and Nitrogen Fertilizer Promotes the Activity of Starch Metabolism Enzymes and the Expression of Related Genes in Rice in a Dual Cropping System. BMC Plant Biol. 2021, 21, 600. [Google Scholar] [CrossRef]
- Kino, R.I.; Pellny, T.K.; Mitchell, R.A.C.; Gonzalez-Uriarte, A.; Tosi, P. High Post-Anthesis Temperature Effects on Bread Wheat (Triticum aestivum L.) Grain Transcriptome during Early Grain-Filling. BMC Plant Biol. 2020, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Lv, H.; Yu, Z.; Zhang, D.; Zhu, W. Identification of changes in Triticum aestivum L. leaf proteome in response to drought stress by 2D-PAGE and MALDI-TOF/TOF mass spectrometry. Acta Physiol. Plant. 2014, 36, 1385–1398. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Hu, M.; Sun, L.; Liu, Q.; Zhang, Y.; Li, Q.; Wang, P.; Ma, W.; Li, H.; et al. Transcriptome and Proteome Analysis Revealed the Influence of High-Molecular-Weight Glutenin Subunits (HMW-GSs) Deficiency on Expression of Storage Substances and the Potential Regulatory Mechanism of HMW-GSs. Foods 2023, 12, 361. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and Night Heat Stress Trigger Different Transcriptomic Responses in Green and Ripening Grapevine (Vitis Vinifera) Fruit. BMC Plant Biol. 2014, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Balfagón, D.; Zandalinas, S.I.; Mittler, R.; Gómez-Cadenas, A. High temperatures modify plant responses to abiotic stress conditions. Physiol. Plant. 2020, 170, 335–344. [Google Scholar] [CrossRef]
- Riaz, A.; Thomas, J.; Ali, H.H.; Zaheer, M.S.; Ahmad, N.; Pereira, A. High night temperature stress on rice (Oryza sativa)—Insights from phenomics to physiology: A review. Funct. Plant Biol. 2024, 51, FP24057. [Google Scholar] [CrossRef]
- Teng, Z.; Chen, Y.; Meng, S.; Duan, M.; Zhang, J.; Ye, N. Environmental Stimuli: A Major Challenge during Grain Filling in Cereals. Int. J. Mol. Sci. 2023, 24, 2255. [Google Scholar] [CrossRef]
- Seni, S.; Kaur, S.; Malik, P.; Yadav, I.S.; Sirohi, P.; Chauhan, H.; Kaur, A.; Chhuneja, P. Transcriptome based identification and validation of heat stress transcription factors in wheat progenitor species Aegilops speltoides. Sci. Rep. 2021, 11, 22049. [Google Scholar] [CrossRef]
- Tomás, D.; Viegas, W.; Silva, M. Grain Transcriptome Dynamics Induced by Heat in Commercial and Traditional Bread Wheat Genotypes. Front. Plant Sci. 2022, 13, 842599. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, Z.; Liu, K.; Zhao, Y.; Nie, Z.; Zhang, L.; Muhammad Fahim, A.; Yang, X. Comparative Transcriptome Analysis Reveals Differential Gene Expression Pattern Associated with Heat Tolerance in Pepper (Capsicum annuum L.). Horticulturae 2023, 9, 801. [Google Scholar] [CrossRef]
- Ni, Z.; Li, H.; Zhao, Y.; Peng, H.; Hu, Z.; Xin, M.; Sun, Q. Genetic Improvement of Heat Tolerance in Wheat: Recent Progress in Understanding the Underlying Molecular Mechanisms. Crop J. 2018, 6, 32–41. [Google Scholar] [CrossRef]
- Sharma, E.; Borah, P.; Kaur, A.; Bhatnagar, A.; Mohapatra, T.; Kapoor, S.; Khurana, J.P. A Comprehensive Transcriptome Analysis of Contrasting Rice Cultivars Highlights the Role of Auxin and ABA Responsive Genes in Heat Stress Response. Genomics 2021, 113, 1247–1261. [Google Scholar] [CrossRef]
- Gul, R.M.S.; Rauf, S.; Ortiz, R.; Waqas Khalid, M.; Kaya, Y. Understanding Abscisic Acid-Mediated Stress Signaling to Affect Rice Development under Stress. Front. Sustain. Food Syst. 2024, 8, 1477994. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.H.; Ma, X.; Luo, D.X.; Gong, Z.H.; Lu, M.H. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, X.; Gu, C.; Lu, Z.; Ma, R.; Wang, X.; Lu, Y.; Cai, K.; Tang, Z.; Zhou, Z.; et al. Rice heat stress response: Physiological changes and molecular regulatory network research progress. Plants 2025, 14, 2573. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, L.; Onkarappa, D.; Yogendra, K.; Bose, J.; Sharma, R.A. Molecular Basis and Engineering Strategies for Transcription Factor-Mediated Reproductive-Stage Heat Tolerance in Crop Plants. Agronomy 2024, 14, 159. [Google Scholar] [CrossRef]
- Lamba, K.; Kumar, M.; Singh, V.; Chaudhary, L.; Gupta, V. Transcriptome Analysis for Heat Stress Related Genes in Wheat Genotype WH-730. Cereal Res. Commun. 2025, 53, 793–805. [Google Scholar] [CrossRef]
- Chaudhary, C.; Sharma, N.; Khurana, P. Decoding the wheat awn transcriptome and overexpressing Ta Rca1β in rice for heat stress tolerance. Plant Mol. Biol. 2021, 105, 133–146. [Google Scholar] [CrossRef]
- Talarico, E.; Zambelli, A.; Araniti, F.; Greco, E.; Chiappetta, A.; Bruno, L. Unravelling the Epigenetic Code: DNA Methylation in Plants and Its Role in Stress Response. Epigenomes 2024, 8, 30. [Google Scholar] [CrossRef]
- Sharma, M.; Sidhu, A.K.; Samota, M.K.; Gupta, M.; Koli, P.; Choudhary, M. Post-Translational Modifications in Histones and Their Role in Abiotic Stress Tolerance in Plants. Proteomes 2023, 11, 38. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Zhang, Z.; Mullasseri, S.; Kalendar, R.; Ahmad, Z.; Sharma, A.; Liu, G.; Zhou, M.; Wei, Q. Epigenetic Stress Memory: A New Approach to Study Cold and Heat Stress Responses in Plants. Front. Plant Sci. 2022, 13, 1075279. [Google Scholar] [CrossRef]
- Workman, J.L.; Kingston, R.E. Alteration of Nucleosome Structure as a Mechanism of Transcriptional Regulation. Annu. Rev. Biochem. 1998, 67, 545–579. [Google Scholar] [CrossRef]
- Luo, M.; Liu, X.; Singh, P.; Cui, Y.; Zimmerli, L.; Wu, K. Chromatin Modifications and Remodeling in Plant Abiotic Stress Responses. Biochim. Et Biophys. Acta (BBA)—Gene Regul. Mech. 2012, 1819, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bure, I.V.; Nemtsova, M.V.; Kuznetsova, E.B. Histone Modifications and Non-Coding RNAs: Mutual Epigenetic Regulation and Role in Pathogenesis. Int. J. Mol. Sci. 2022, 23, 5801. [Google Scholar] [CrossRef]
- Saha, C.; Saha, S.; Bhattacharyya, N.P. LncRNAOmics: A Comprehensive Review of Long Non-Coding RNAs in Plants. Genes 2025, 16, 765. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Chachar, M.; Ali, A.; Chachar, Z.; Zhang, P.; Riaz, A.; Ahmed, N.; Chachar, S. Epigenetic Regulation for Heat Stress Adaptation in Plants: New Horizons for Crop Improvement under Climate Change. Agronomy 2024, 14, 2105. [Google Scholar] [CrossRef]
- Shanker, A.K.; Bhanu, D.; Maheswari, M. Epigenetics and Transgenerational Memory in Plants under Heat Stress. Plant Physiol. Rep. 2020, 25, 583–593. [Google Scholar] [CrossRef]
- Vaschetto, L.M. DNA Methylation, Histone Modifications, and Non-coding RNA Pathways. In Epigenetics in Crop Improvement: Safeguarding Food Security in an Ever-Changing Climate; Springer Nature: Cham, Switzerland, 2024; pp. 15–27. [Google Scholar]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA methylation. PLoS Genet. 2020, 16, e1009034. [Google Scholar] [CrossRef]
- Ali, S.; Tang, Y. Noncoding RNA-Mediated Regulation of DNA Methylation: Insights into Plant Epigenetic Mechanisms. J. Plant Growth Regul. 2025, 44, 373–388. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Zhang, Y.; Lin, C.; Gong, D.; Guan, Y.; Hu, J. Reactive Oxygen Species and Gibberellin Acid Mutual Induction to Regulate Tobacco Seed Germination. Front. Plant Sci. 2018, 9, 1279. [Google Scholar] [CrossRef]
- Zdunek-Zastocka, E.; Grabowska, A. The Interplay of PsABAUGT1 with Other Abscisic Acid Metabolic Genes in the Regulation of ABA Homeostasis during the Development of Pea Seeds and Germination in the Presence of H2O2. Plant Sci. 2019, 285, 79–90. [Google Scholar] [CrossRef]
- Sakai, Y.; Suriyasak, C.; Inoue, M.; Hamaoka, N.; Ishibashi, Y. Heat stress during grain filling regulates seed germination through alterations of DNA methylation in barley (Hordeum vulgare L.). Plant Mol. Biol. 2022, 110, 325–332. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Wang, J.; Wang, Y.; Ji, F.; Sun, L.; Zhang, G.; Hao, F. H3K4me2, H4K5ac and DNA Methylation Function in Short- and Long-Term Heat Stress Responses through Affecting the Expression of the Stress-Related Genes in G. Hirsutum. Environ. Exp. Bot. 2022, 194, 104699. [Google Scholar] [CrossRef]
- Benamar, K.; Dehbi, I.; Radi, M.; Ez-Zouggari, R.; Fikri Benbrahim, K.; Jiang, Y.; Lahlali, R. Epigenetics for Combating Heat Stress in Plants: Updated Methods and Current Achievements. In Epigenetics for Climate-Smart and Sustainable Agriculture; CABI: Wallingford, UK, 2025; pp. 178–195. [Google Scholar]
- Gan, L.; Wei, Z.; Yang, Z.; Li, F.; Wang, Z. Updated Mechanisms of GCN5—The Monkey King of the Plant Kingdom in Plant Development and Resistance to Abiotic Stresses. Cells 2021, 10, 979. [Google Scholar] [CrossRef]
- Tahir, M.S.; Tian, L. HD2-Type Histone Deacetylases: Unique Regulators of Plant Development and Stress Responses. Plant Cell Rep. 2021, 40, 1603–1615. Available online: https://link.springer.com/article/10.1007/s00299-021-02688-3 (accessed on 6 May 2021). [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Margalha, L.; Elias, A.; Belda-Palazón, B.; Peixoto, B.; Confraria, A.; Baena-González, E. HOS1 Promotes Plant Tolerance to Low-Energy Stress via the SnRK1 Protein Kinase. Plant J. 2023, 115, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, N.; Martinez-Garcia, M.; Varas, J.; Gil-Dones, F.; Santos, J.L.; Pradillo, M. The Scaffold Nucleoporins SAR1 and SAR3 Are Essential for Proper Meiotic Progression in Arabidopsis thaliana. Front. Cell Dev. Biol. 2023, 11, 1285695. [Google Scholar] [CrossRef] [PubMed]

| Crop | Growth Stage | Control Temperature | Extreme Temperature | Plant Response Studied | References |
|---|---|---|---|---|---|
| (Degree Celsius) | (Degree Celsius) | ||||
| Rice (Oryza Sativa) | Inflorescence development | 25 | 37 | Pollen sterility | [152] |
| Microsporogenesis | 28 | 33 | Reduced pollen production, Pollen inviability | [152] | |
| Pollen maturation | 28 | 39 | Down regulation of expression of tapetum genes | [153] | |
| Anthesis | 30 | >33.7 | Pollen sterility | [154] | |
| Pollination | 28 | 38 | Spikelet sterility | [155] | |
| Wheat (T. aestivum) | Inflorescence initiation | 25 | 30 | Early anthesis | [156] |
| Inflorescence development | 26 | 33 | Meiotic abnormalities | [157] | |
| Microsporogenesis | 20 | 30 | Male sterility | [158] | |
| Anthesis | 28 | 38 | Less grains per ear | [159] | |
| Post anthesis | 18 | 30 | Reduced kernel weight | [160] | |
| Post fertilization | 20 | 35 | Yield reduction | [161] | |
| Barley (Hordeum vulgare) | Inflorescence development | 20 | 30 | Pollen inviability | [162] |
| Microsporogenesis | 20 | 30 | Abnormal microspores | [163] | |
| Microgametogenesis | 20 | 30 | Pollen abortion | [164] | |
| Pollen maturation | 20 | 30 | Anther wall degradation | [165] | |
| Post anthesis | 20 | 40 | Reduced grain weight | [166] | |
| Brachypodium (Brachypodiumd istachyon) | Inflorescence initiation | 24 | 32 | Less tillering | [167] |
| Microgametogenesis | 24 | 36 | Pollen development ceases | [168] | |
| Anthesis | 24 | 36 | Anther indehiscence | [168] | |
| Pre-fertilization | 24 | 32 | Reduce pollen germination | [168] | |
| Pollination | 22 | 27 | Reduced grain weight | [168] | |
| MAIZE (Zea mays) | Inflorescence development | 33.9 | >35 | Male and female sterility | [104] |
| Anthesis | 27 | 38 | Reduced pollen germination | [169] | |
| Pollination | 27 | 38 | Poor kernel set | [169] | |
| Pre silking | 25 | 35 | Decrease in ear weight | [170] | |
| Sorghum (Sorghum bicolor) | Inflorescence initiation | 25 | 37 | Floret sterility | [171] |
| Inflorescence development | 30 | 38 | Reduced pollen germination | [172] | |
| Anthesis | 28 | 33 | Embryo abortion | [173] | |
| Post anthesis | 32 | 40 | Lesser grain yield | [174] | |
| Pearl millet (Pennisetumglacum) | Inflorescence development | 35 | >42 | Reduced seed set | [175] |
| Arabidopsis (A. thaliana) | Inflorescence development | 22 | 42 | Pollen release is impaired | [176] |
| Peas (Pisum sativum) | Inflorescence development | 20 | 33 | Abortion of floral buds | [177] |
| Inflorescence development | 20 | 30 | Less flowering nodes | [177] | |
| Anthesis | 20 | 28 | Lesser number of seeds per pod | [177] | |
| Post fertilization | 24 | 32 | Reduction in yield | [140] | |
| Tomato (Solanum lycopersicum) | Inflorescence initiation | 28 | >29 | Reduction in fruit yield | [178] |
| Inflorescence development | 18 | 28 | Reduce Stigma Surface Area | [178] | |
| Microsporogenesis | 28 | 32 | Reduced expression of Proline Transporter I | [179] | |
| Microgametogenesis | 28 | 35 | Stigma exertion without anthesis | [180] | |
| Pollen maturation | 25 | 29 | Decrease fruit set | [181] | |
| Anthesis | 28 | 32 | Inviable pollen | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Muthuramalingam, P.; Kumar, R.; Tiwari, S.; Verma, L.; Park, S.; Shin, H. Adapting Crops to Rising Temperatures: Understanding Heat Stress and Plant Resilience Mechanisms. Int. J. Mol. Sci. 2025, 26, 10426. https://doi.org/10.3390/ijms262110426
Kumar A, Muthuramalingam P, Kumar R, Tiwari S, Verma L, Park S, Shin H. Adapting Crops to Rising Temperatures: Understanding Heat Stress and Plant Resilience Mechanisms. International Journal of Molecular Sciences. 2025; 26(21):10426. https://doi.org/10.3390/ijms262110426
Chicago/Turabian StyleKumar, Anand, Pandiyan Muthuramalingam, Reetesh Kumar, Savitri Tiwari, Laxmidas Verma, Sujeong Park, and Hyunsuk Shin. 2025. "Adapting Crops to Rising Temperatures: Understanding Heat Stress and Plant Resilience Mechanisms" International Journal of Molecular Sciences 26, no. 21: 10426. https://doi.org/10.3390/ijms262110426
APA StyleKumar, A., Muthuramalingam, P., Kumar, R., Tiwari, S., Verma, L., Park, S., & Shin, H. (2025). Adapting Crops to Rising Temperatures: Understanding Heat Stress and Plant Resilience Mechanisms. International Journal of Molecular Sciences, 26(21), 10426. https://doi.org/10.3390/ijms262110426








