Key Nutrient Drivers for Biomass and C-Phycocyanin Production in Spirulina sp. Revealed by Media Optimization
Abstract
1. Introduction
2. Results
2.1. The Effect of Nutrient Modification on the Growth and Biomass Production of Spirulina
2.1.1. Growth Dynamics of Spirulina sp. Under Varying Nutrient Modifications
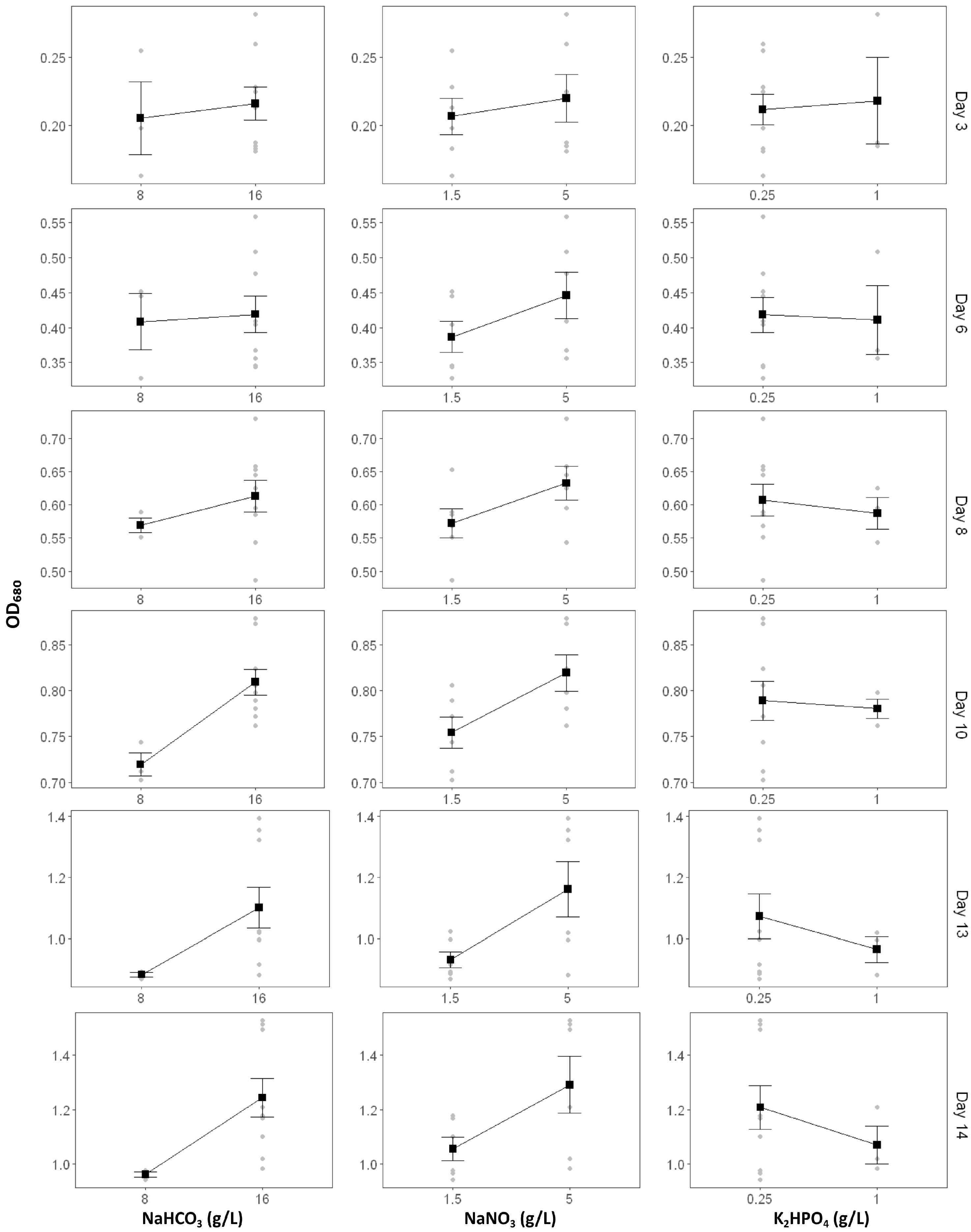
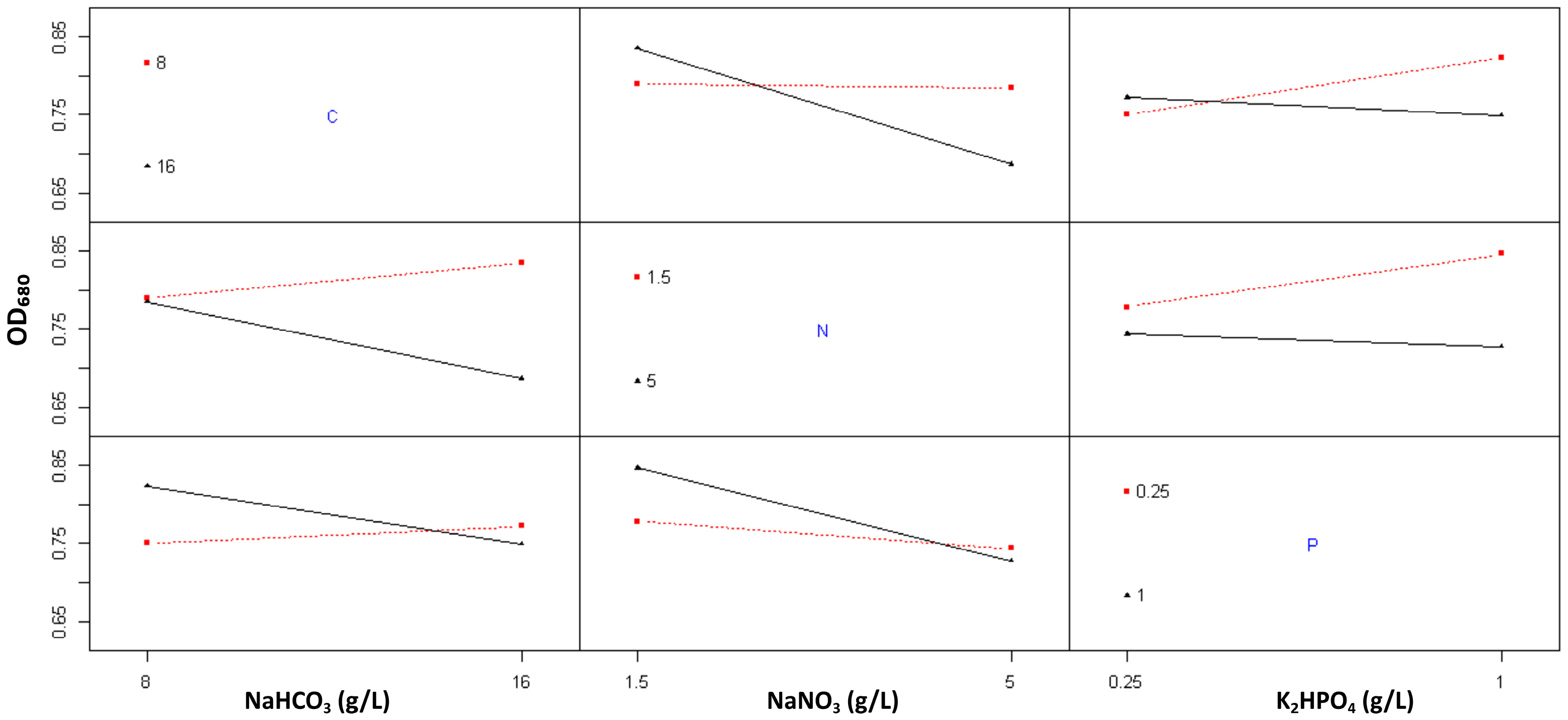
2.1.2. The Effect of Nutrient Modification on Spirulina Growth
2.1.3. The Effect of Nutrient Modification on Biomass Production
2.1.4. The Effect of Nutrient Modification on C-PC Production
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Culture and Cultivation Conditions
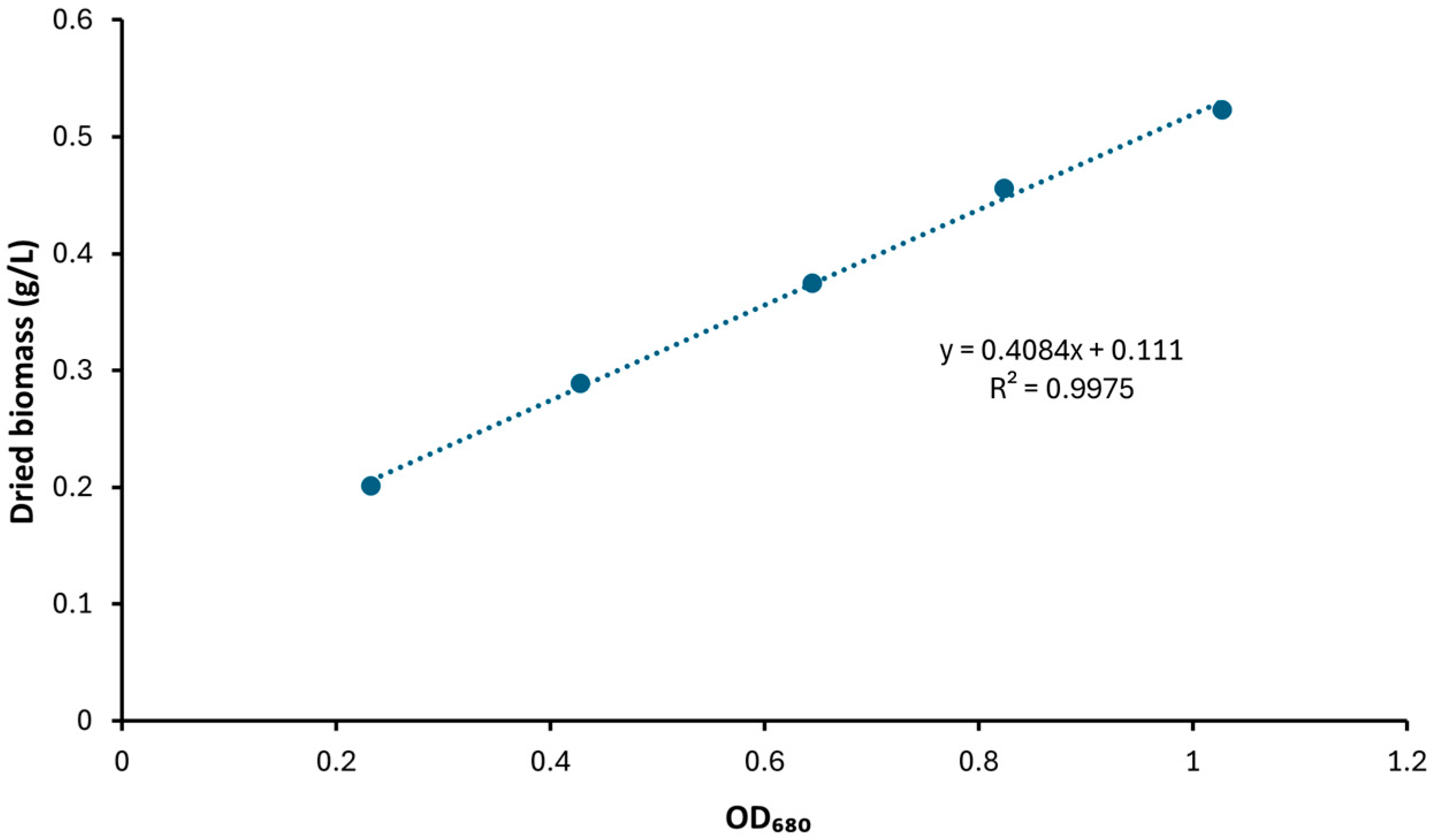
4.3. Dried Biomass and Productivity Analysis
4.4. C-Phycocyanin (C-PC) Content Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-PGA | 3-phosphoglicerate |
| ALA | aminolevulinic acid |
| ANOVA | Analysis of Variance |
| BBM | Bold Basal Medium |
| BG-11 | Blue Green-11 |
| C | Control |
| CA | Carbonic anhydrase |
| C-PC | C-Phycocyanin |
| F1–F8 | Formulations 1–8 |
| GAP | glyceraldehide-3-phosphate |
| OD | Optical density |
| PCB | Phycocyanobilin |
| PCD | Phycocyanin content in dried biomass |
| PCL | Phycocyanin content in liquid |
| PI | Purity index |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
Appendix A
| OD | Dried Biomass (g/L) | |
|---|---|---|
| Replicate 1 | 1.000 | 0.572 |
| 0.817 | 0.492 | |
| 0.621 | 0.390 | |
| 0.418 | 0.336 | |
| 0.223 | 0.234 | |
| Replicate 2 | 1.055 | 0.474 |
| 0.831 | 0.420 | |
| 0.668 | 0.360 | |
| 0.439 | 0.242 | |
| 0.242 | 0.168 | |
| Average | 1.027 | 0.523 |
| 0.824 | 0.456 | |
| 0.644 | 0.375 | |
| 0.428 | 0.289 | |
| 0.232 | 0.201 |
References
- El-Shall, N.A.; Jiang, S.; Farag, M.R.; Azzam, M.; Al-Abdullatif, A.A.; Alhotan, R.; Dhama, K.; Hassan, F.-U.; Alagawany, M. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth performance and immune modulation. Front. Immunol. 2023, 14, 1072787. [Google Scholar] [CrossRef]
- Fais, G.; Manca, A.; Bolognesi, F.; Borselli, M.; Concas, A.; Busutti, M.; Broggi, G.; Sanna, P.; Castillo-Aleman, Y.M.; Rivero-Jiménez, R.A.; et al. Wide Range Applications of Spirulina: From Earth to Space Missions. Mar. Drugs 2022, 20, 299. [Google Scholar] [CrossRef]
- Calella, P.; Cerullo, G.; Di Dio, M.; Liguori, F.; Di Onofrio, V.; Gallè, F.; Liguori, G. Antioxidant, anti-inflammatory and immunomodulatory effects of spirulina in exercise and sport: A systematic review. Front. Nutr. 2022, 9, 1048258. [Google Scholar] [CrossRef]
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A.I.R.N.A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592. [Google Scholar] [CrossRef]
- AsAshaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a super functional ingredient from algae; properties, purification characterization, and applications. Int. J. Biol. Macromol. 2021, 193, 2320–2331. [Google Scholar] [CrossRef]
- Pinto, L.F.R.; Ferreira, G.F.; Beatriz, F.P.; Cabral, F.A.; Filho, R.M. Lipid and phycocyanin extractions from Spirulina and economic assessment. J. Supercrit. Fluids 2022, 184, 105567. [Google Scholar] [CrossRef]
- Nikolova, K.; Petkova, N.; Mihaylova, D.; Gentscheva, G.; Gavrailov, G.; Pehlivanov, I.; Andonova, V. Extraction of Phycocyanin and Chlorophyll from Spirulina by “Green Methods”. Separations 2024, 11, 57. [Google Scholar] [CrossRef]
- Gorgich, M.; Passos, M.L.; Mata, T.M.; Martins, A.A.; Saraiva, M.L.M.; Caetano, N.S. Enhancing extraction and purification of phycocyanin from Arthrospira sp. with lower energy consumption. Energy Rep. 2020, 6, 312–318. [Google Scholar] [CrossRef]
- Kaewdam, S.; Jaturonglumlert, S.; Varith, J.; Nitatwichit, C.; Narkprasom, K. Kinetic Models for Phycocyanin Production by Fed-Batch Cultivation of the Spirulina plantesis. Int. J. GEOMATE 2019, 17, 187–194. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Teixeira, I.R.; Marczak, L.D.F.; Mercali, G.D. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Balseca, D.A.F.; Reyes, K.S.C.; Rodríguez, M.E.M. Optimization of an Alternative Culture Medium for Phycocyanin Production from Arthrospira platensis under Laboratory Conditions. Microorganisms 2024, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hou, X.; Yu, Q.; Huo, Y.; Wang, K.; Wen, X.; Ding, Y.; Li, Y.; Wang, Z. A novel two-stage culture strategy to enhance the C-phycocyanin productivity and purity of Arthrospira platensis. LWT 2023, 184, 115010. [Google Scholar] [CrossRef]
- Manirafasha, E.; Murwanashyaka, T.; Ndikubwimana, T.; Ahmed, N.R.; Liu, J.; Lu, Y.; Zeng, X.; Ling, X.; Jing, K. Enhancement of cell growth and phycocyanin production in Arthrospira (Spirulina) platensis by metabolic stress and nitrate fed-batch. Bioresour. Technol. 2018, 255, 293–301. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Garcia, G.M.; Boelen, P.; Buma, A.G.J. Enhancement of C-phycocyanin productivity by Arthrospira platensis when growing on palm oil mill effluent in a two-stage semi-continuous cultivation mode. J. Appl. Phycol. 2019, 31, 2855–2867. [Google Scholar] [CrossRef]
- Chen, G. Metabolites of microalgae. In Algal Biotechnology; CABI: Wallingford, UK, 2023; pp. 102–114. [Google Scholar]
- Magwell, P.F.R.; Djoudjeu, K.T.; Minyaka, E.; Tavea, M.-F.; Fotsop, O.W.; Tagnikeu, R.F.; Fofou, A.M.; Darelle, C.K.V.; Dzoyem, C.U.D.; Lehman, L.G. Sodium Bicarbonate (NaHCO3) Increases Growth, Protein and Photosynthetic Pigments Production and Alters Carbohydrate Production of Spirulina platensis. Curr. Microbiol. 2023, 80, 63. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.; Morsi, H.; Hassan, L. Growth enhancement of Spirulina platensis through optimization of media and nitrogen sources. Egypt. J. Bot. 2021, 61, 61–69. [Google Scholar] [CrossRef]
- Bossa, R.; Di Colandrea, M.; Salbitani, G.; Carfagna, S. Phosphorous Utilization in Microalgae: Physiological Aspects and Applied Implications. Plants 2024, 13, 2127. [Google Scholar] [CrossRef] [PubMed]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Yang, Y.; Chou, S.; Ge, S.; Li, P.; Wang, X.; Zhuang, L.-L.; Zhang, J. Effect of N/P ratio on attached microalgae growth and the differentiated metabolism along the depth of biofilm. Environ. Res. 2023, 240, 117428. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Bogorad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1978, 58, 419–485. [Google Scholar] [CrossRef]
- Li, P.; Hu, Z.; Yin, Q.; Song, C. Improving the growth of Spirulina in CO2 absorption and microalgae conversion (CAMC) system through mixotrophic cultivation: Reveal of metabolomics. Sci. Total Environ. 2023, 858, 159920. [Google Scholar] [CrossRef]
- Rockwell, N.C.; Martin, S.S.; Lagarias, J.C. Elucidating the origins of phycocyanobilin biosynthesis and phycobiliproteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2300770120. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, W.; Huang, X.; Gu, P.; Li, Q.; Luo, X.; Zheng, Z. Phosphorus conditions change the cellular responses of Microcystis aeruginosa to perfluorooctanoic acid. Sci. Total Environ. 2023, 903, 166707. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Zhang, Z.; Zhou, J.; Li, J.; Chen, J.; Du, G.; Zhao, X. Biosynthesis, acquisition, regulation, and upcycling of heme: Recent advances. Crit. Rev. Biotechnol. 2024, 44, 1422–1438. [Google Scholar] [CrossRef]
- Gupta, A.; Mohan, D.; Saxena, R.K.; Singh, S. Phototrophic cultivation of NaCl-tolerant mutant of Spirulina platensis for enhanced C-phycocyanin production under optimized culture conditions and its dynamic modeling. J. Phycol. 2018, 54, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Gorelova, O.; Karpova, O.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Baulina, O.; Vinogradova, E.; Pugacheva, T.; Scherbakov, P.; et al. Phosphorus Feast and Famine in Cyanobacteria: Is Luxury Uptake of the Nutrient Just a Consequence of Acclimation to Its Shortage? Cells 2020, 9, 1933. [Google Scholar] [CrossRef]
- Zuorro, A.; Leal-Jerez, A.G.; Morales-Rivas, L.K.; Mogollón-Londoño, S.O.; Sanchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Solano, A.F. Enhancement of Phycobiliprotein Accumulation in Thermotolerant Oscillatoria sp. through Media Optimization. ACS Omega 2021, 6, 10527–10536. [Google Scholar] [CrossRef]
- El-Shouny, W.; Sharaf, M.; Abomohra, A.E.-F.; Abo-Eleneen, M.A.-E. Production Enhancement of Some Valuable Compounds of Arthrospira platensis. J. Basic Environ. Sci. 2015, 2, 74–83. [Google Scholar] [CrossRef]
- Chen, H.; Yang, B.; Li, T.; Wu, H.; Wu, H.; Xiang, W. Effects of Phosphorus Concentrations on Growth and Metabolism of Seawater Spirulina platensis. Biotechnol. Bull. 2019, 35, 103–110. [Google Scholar]
- Nurrusyda, F.S.; Subroto, T.; Hardianto, A.; Sumeru, H.A.; Ishmayana, S.; Pratomo, U.; Oktavia, D.N.; Latifah, R.G.; Dewi, D.A.S.L.A.; Rachmadona, N. Analyzing the Impact of Physicochemical Factors on Chlorella vulgaris Growth Through Design of Experiment (DoE) for Carbon Capture System. Mol. Biotechnol. 2024, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barton, E.E.; Lloyd, B.P.; Spriggs, A.D.; Gast, D.L. Visual Analysis of Graphic Data. In Single Case Research Methodology; Routledge: New York, NY, USA, 2018; pp. 179–214. [Google Scholar]
- Yu, X.; Guo, W.; Hu, Z.; Li, P.; Zhang, Z.; Cheng, J.; Song, C.; Ye, Q. Flue gas CO2 supply methods for microalgae utilization: A review. Clean Energy Sci. Technol. 2023, 1, 78. [Google Scholar] [CrossRef]
- Cunningham, B.R.; John, S.G. The effect of iron limitation on cyanobacteria major nutrient and trace element stoichiometry. Limnol. Oceanogr. 2017, 62, 846–858. [Google Scholar] [CrossRef]
- Abd El-Monem, A.M.; Gharieb, M.M.; Doman, K.M. Chemical Constituents of Zarrouk’s Medium Affect Growth, Pigments and Metabolites Productions of Spirulina platensis. Egypt. J. Bot. 2021, 61, 681–691. [Google Scholar]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Devi, A.C.; Tavanandi, H.A.; Govindaraju, K.; Raghavarao, K.S.M.S. An effective method for extraction of high purity phycocyanins (C-PC and A-PC) from dry biomass of Arthrospira maxima. J. Appl. Phycol. 2020, 32, 1141–1151. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, P.C.; Tsai, C.J.; Lee, D.J.; Chang, J.S. Engineering strategies for simultaneous enhancement of C-phycocyanin production and CO2 fixation with Spirulina platensis. Bioresour. Technol. 2013, 145, 307–312. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef]
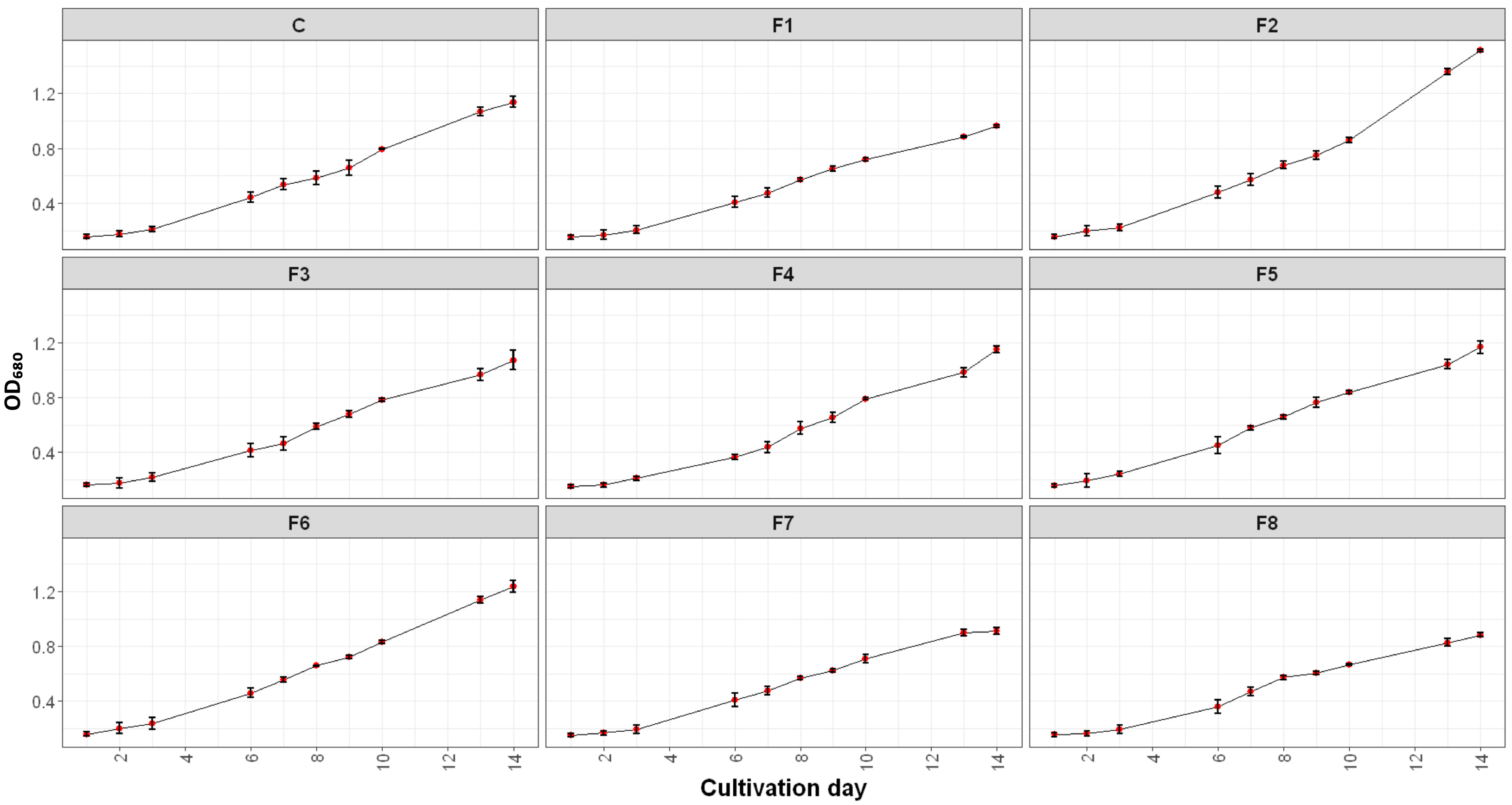
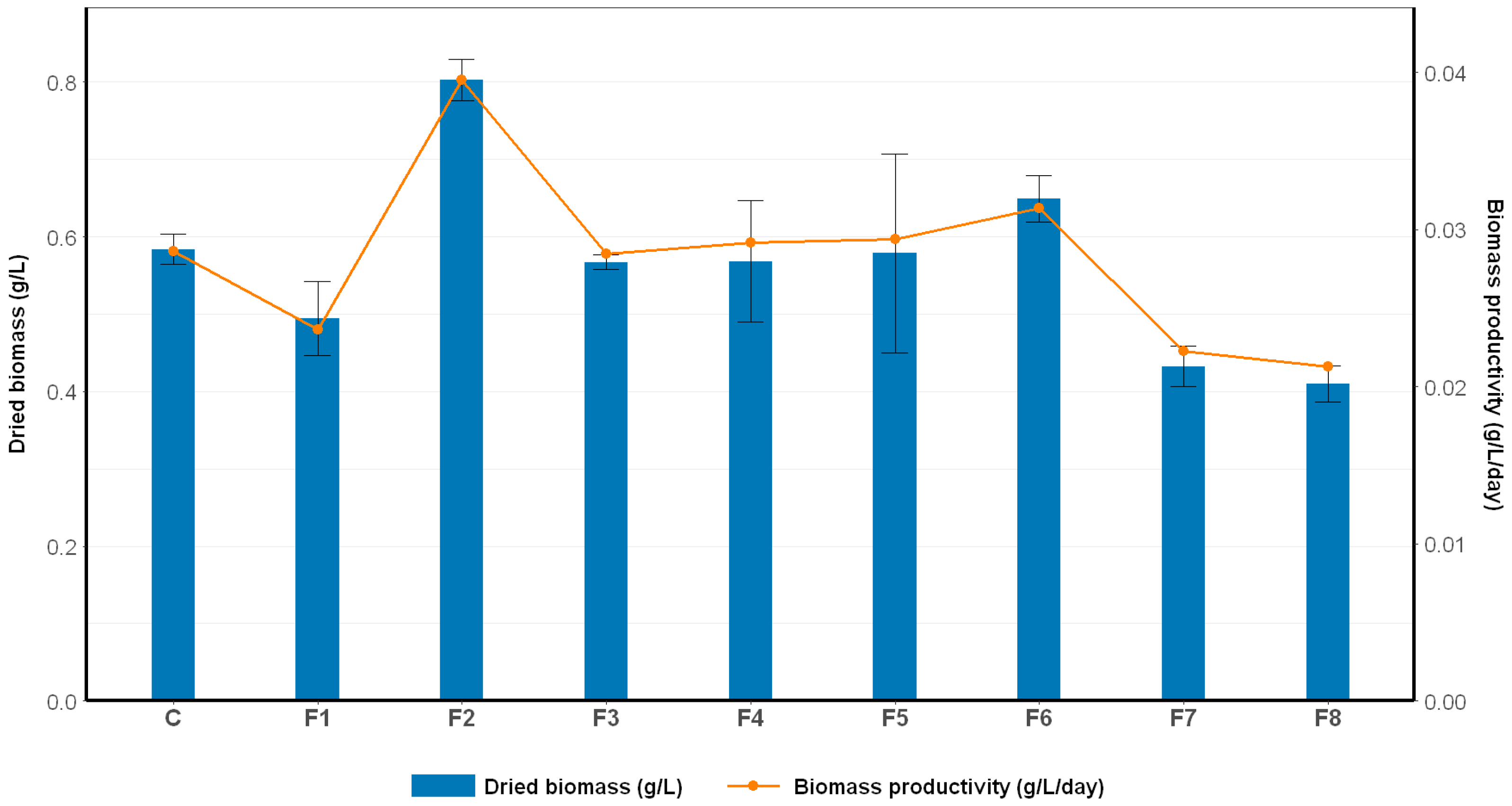
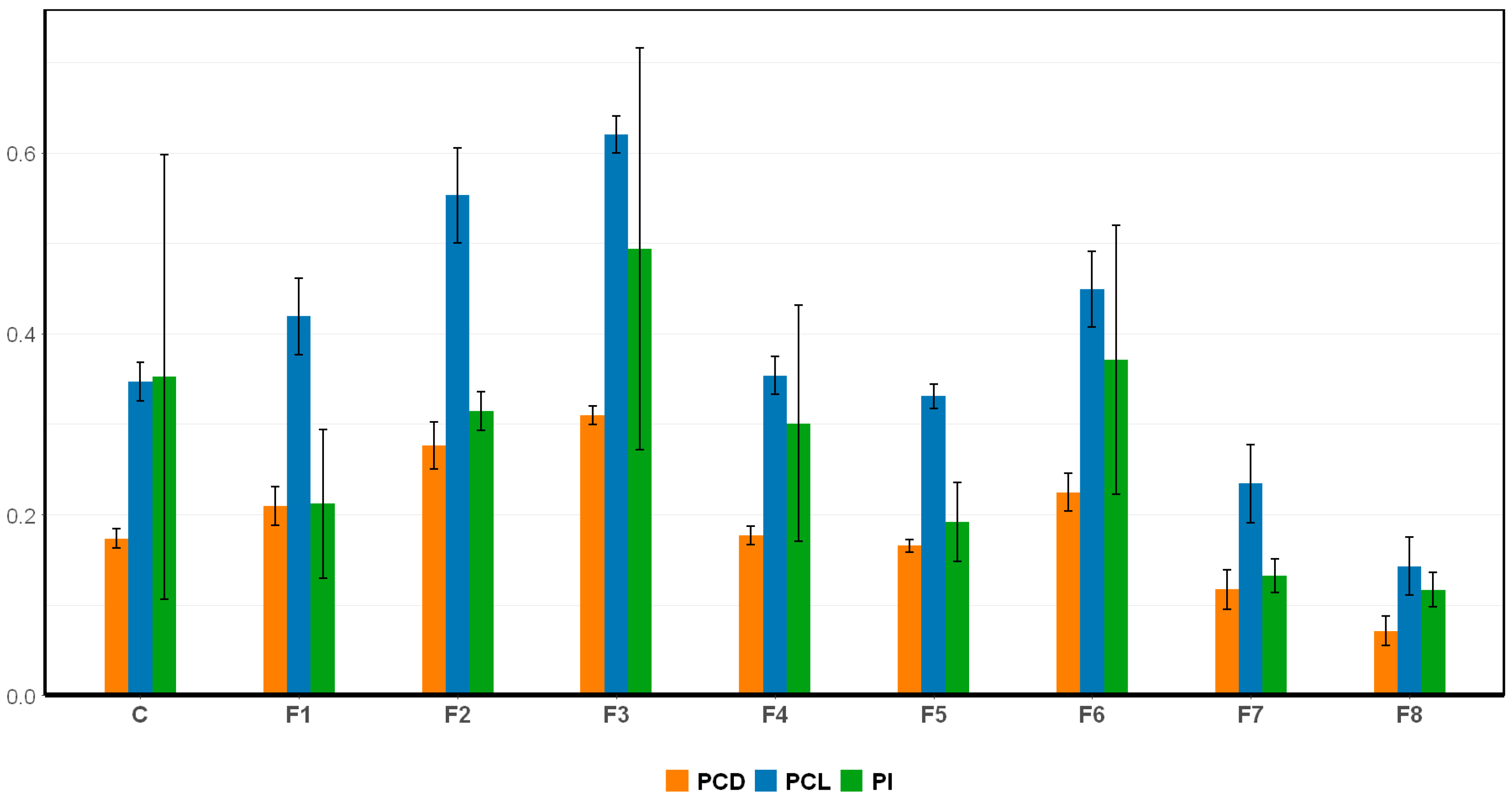
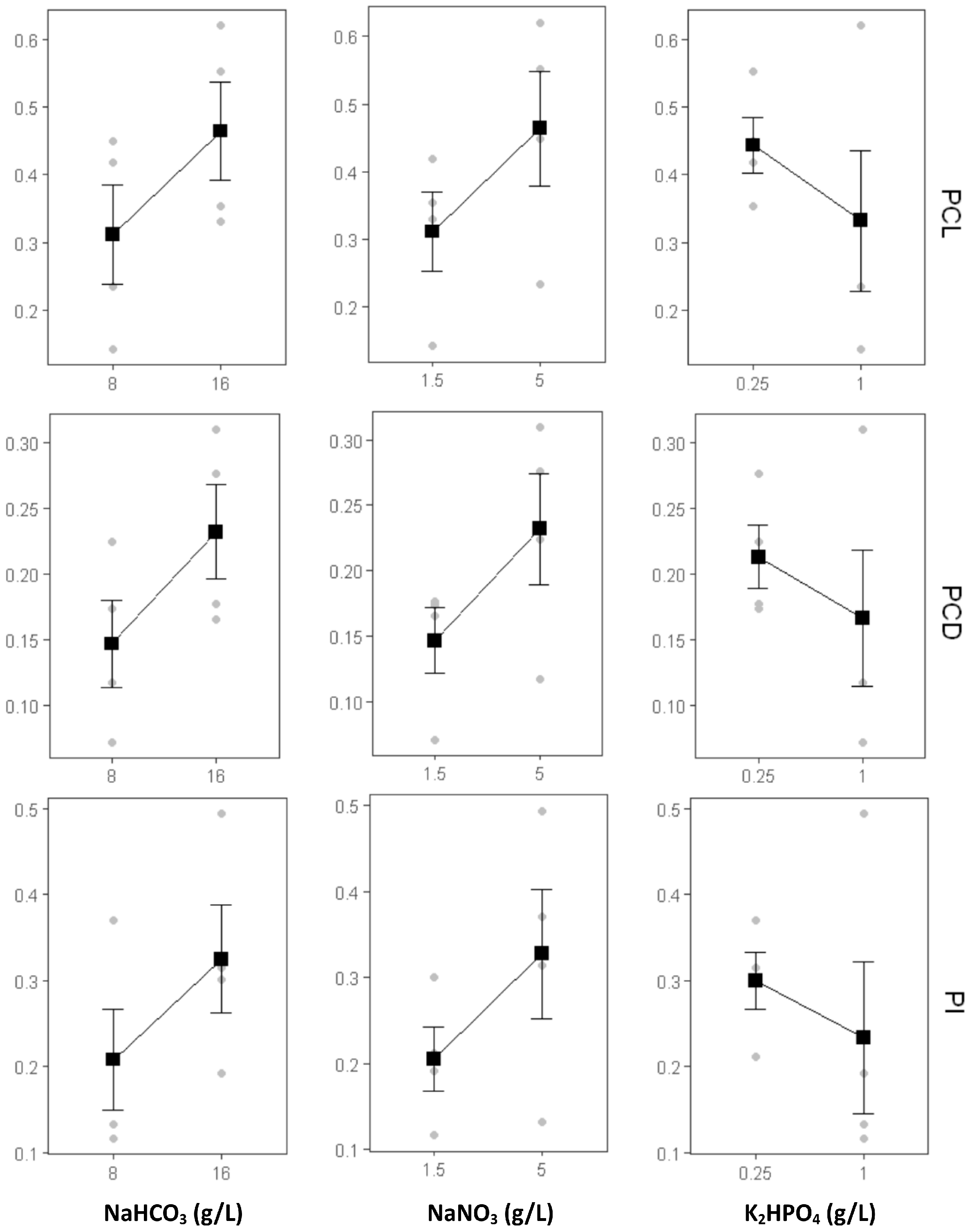
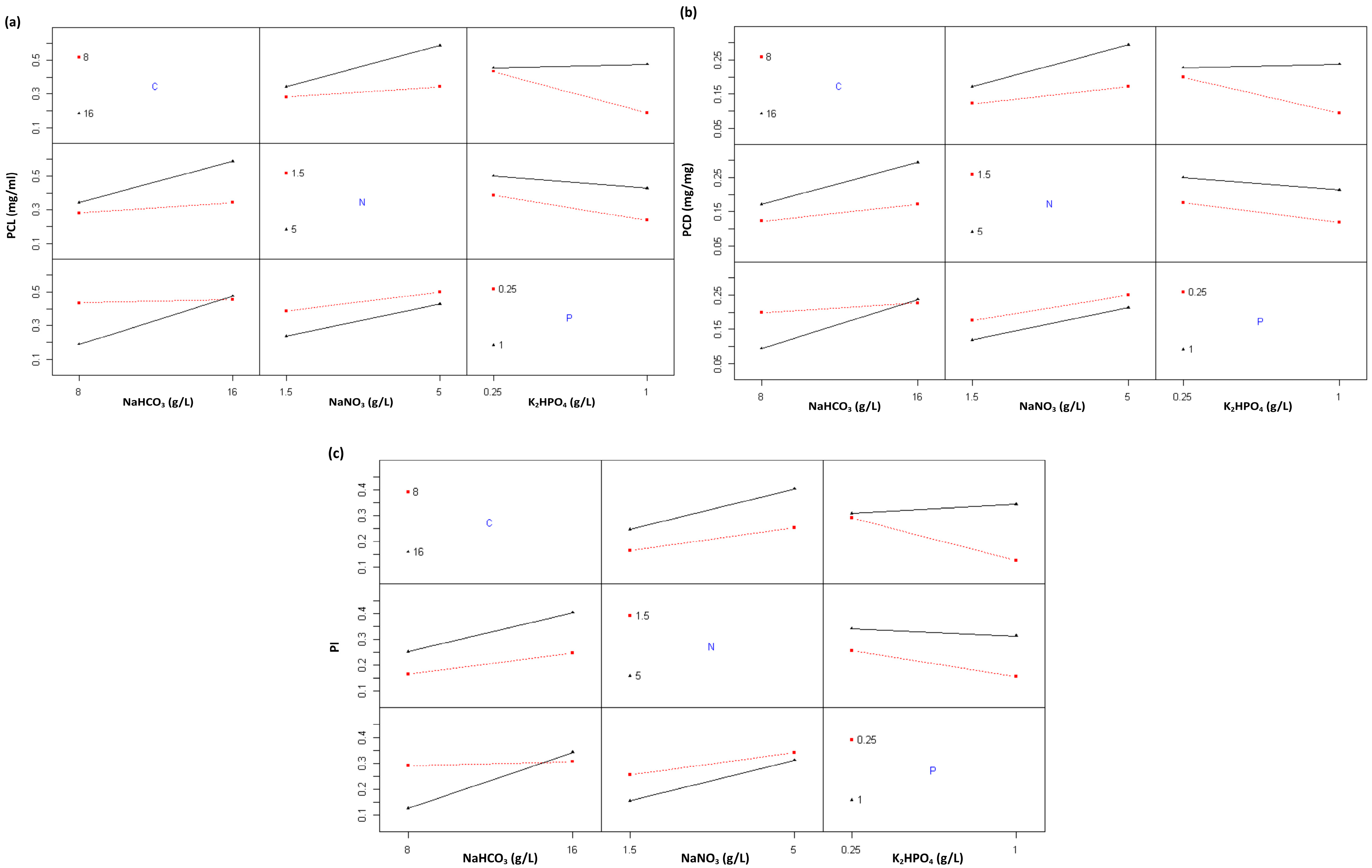
| Formulation | Growth Rate (ΔOD/Day) | OD680 Max |
|---|---|---|
| Control | 0.0820 ± 0.0035 | 1.136 ± 0.070 |
| F1 | 0.0654 ± 0.0030 | 0.963 ± 0.017 |
| F2 | 0.1030 ± 0.0028 | 1.512 ± 0.013 |
| F3 | 0.0727 ± 0.0032 | 1.070 ± 0.099 |
| F4 | 0.0775 ± 0.0008 | 1.149 ± 0.033 |
| F5 | 0.0792 ± 0.0060 | 1.164 ± 0.062 |
| F6 | 0.0901 ± 0.0040 | 1.233 ± 0.073 |
| F7 | 0.0612 ± 0.0050 | 0.913 ± 0.042 |
| F8 | 0.0599 ± 0.0045 | 0.983 ± 0.164 |
| p-Value | ||||||
|---|---|---|---|---|---|---|
| Day 3 | Day 6 | Day 8 | Day 10 | Day 13 | Day 14 | |
| C | 9.09 × 10−1 | 9.50 × 10−1 | 4.93 × 10−1 | 2.93 × 10−2 * | 3.25 × 10−3 ** | 1.69 × 10−5 *** |
| N | 2.77 × 10−1 | 5.81 × 10−2 | 1.46 × 10−3 ** | 2.86 × 10−6 *** | 1.79 × 10−8 *** | 2.82 × 10−7 *** |
| P | 9.28 × 10−1 | 9.13 × 10−1 | 1.57 × 10−1 | 3.21 × 10−2 * | 3.52 × 10−6 *** | 8.73 × 10−6 *** |
| C:N | 2.91 × 10−1 | 8.23 × 10−1 | 2.11 × 10−1 | 5.97 × 10−6 *** | 1.28 × 10−2 * | 4.47 × 10−4 *** |
| C:P | 9.41 × 10−1 | 5.94 × 10−1 | 1.83 × 10−1 | 4.23 × 10−4 *** | 1.44 × 10−5 *** | 4.26 × 10−5 *** |
| N:P | 8.01 × 10−1 | 1.76 × 10−1 | 9.03 × 10−2 | 1.38 × 10−3 ** | 5.63 × 10−8 *** | 4.36 × 10−6 *** |
| C:N:P | 6.90 × 10−1 | 6.41 × 10−1 | 8.55 × 10−2 | 5.02 × 10−2 * | 2.83 × 10−4 *** | 2.56 × 10−4 *** |
| Df | Sum Sq | Mean Sq | F Value | p-Value | ||
|---|---|---|---|---|---|---|
| C | 1 | 363.0 | 363.0 | 122.1 | 9.60 × 10−9 | *** |
| N | 1 | 348.5 | 348.5 | 117.2 | 2.60 × 10−9 | *** |
| P | 1 | 186.9 | 186.9 | 62.8 | 2.79 × 10−7 | *** |
| C:N | 1 | 126.2 | 126.2 | 42.4 | 4.00 × 10−6 | *** |
| C:P | 1 | 268.6 | 268.6 | 90.3 | 1.95 × 10−8 | *** |
| N:P | 1 | 21.6 | 21.6 | 7.28 | 1.47 × 10−2 | * |
| C:N:P | 1 | 0.8 | 0.8 | 0.26 | 6.15 × 10−1 | |
| Residuals | 18 | 53.3 | 2.97 |
| Run | NaHCO3 (g/L) | NaNO3 (g/L) | K2HPO4 (g/L) |
|---|---|---|---|
| F1 | 8 | 1.5 | 0.25 |
| F2 | 16 | 5 | 0.25 |
| F3 | 16 | 5 | 1 |
| F4 | 16 | 1.5 | 0.25 |
| F5 | 16 | 1.5 | 1 |
| F6 | 8 | 5 | 0.25 |
| F7 | 8 | 5 | 1 |
| F8 | 8 | 1.5 | 1 |
| Component | Zarrouk (g/L) | F1 (g/L) | F2 (g/L) | F3 (g/L) | F4 (g/L) | F5 (g/L) | F6 (g/L) | F7 (g/L) | F8 (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| NaHCO3 | 16.8 | 8 | 16 | 16 | 16 | 16 | 8 | 8 | 8 |
| NaNO3 | 1.5 | 1.5 | 5 | 5 | 1.5 | 1.5 | 5 | 5 | 1.5 |
| K2HPO4 | 0.5 | 0.25 | 0.25 | 1 | 0.25 | 1 | 0.25 | 1 | 1 |
| NaCl | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| CaCl2.2H2O | 0.04 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| FeSO4.7H2O | 0.01 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 |
| Na2EDTA | 0.08 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| K2SO4 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| MgSO4.7H2O | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Trace element | 1 mL | 0.5 mL | 0.5 mL | 0.5 mL | 0.5 mL | 0.5 mL | 0.5 mL | 0.5 mL | 0.5 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurjannah, I.; Subroto, T.; Hardianto, A.; Adinisa, L.; Mochida, K. Key Nutrient Drivers for Biomass and C-Phycocyanin Production in Spirulina sp. Revealed by Media Optimization. Int. J. Mol. Sci. 2025, 26, 10425. https://doi.org/10.3390/ijms262110425
Nurjannah I, Subroto T, Hardianto A, Adinisa L, Mochida K. Key Nutrient Drivers for Biomass and C-Phycocyanin Production in Spirulina sp. Revealed by Media Optimization. International Journal of Molecular Sciences. 2025; 26(21):10425. https://doi.org/10.3390/ijms262110425
Chicago/Turabian StyleNurjannah, Ivani, Toto Subroto, Ari Hardianto, Lucy Adinisa, and Keiichi Mochida. 2025. "Key Nutrient Drivers for Biomass and C-Phycocyanin Production in Spirulina sp. Revealed by Media Optimization" International Journal of Molecular Sciences 26, no. 21: 10425. https://doi.org/10.3390/ijms262110425
APA StyleNurjannah, I., Subroto, T., Hardianto, A., Adinisa, L., & Mochida, K. (2025). Key Nutrient Drivers for Biomass and C-Phycocyanin Production in Spirulina sp. Revealed by Media Optimization. International Journal of Molecular Sciences, 26(21), 10425. https://doi.org/10.3390/ijms262110425









