Abstract
Osteoarthritis (OA) is the most common joint disorder globally, affecting approximately 595 million individuals and representing the first cause of chronic pain and disability. Recently, the infrapatellar fat pad (IFP), an intracapsular adipose tissue in the human knee joint, was recognized as an active and metabolically significant contributor to the pathophysiology of OA through the release of pro-inflammatory cytokines, adipokines, and growth factors that sustain inflammatory response, fibrotic remodeling, and neurogenic pain. The present review provides an overview of the pathophysiological significance of the IFP in OA and current and promising therapeutic strategies targeting this adipose structure. We summarize the available preclinical and translational evidence on conservative therapies, minimally invasive interventions, and surgical options as well as IFP-derived mesenchymal stromal cells as a potential cell source for cartilage repair. Overall, preclinical research indicates that the modulation of IFP inflammation and fibrosis could alleviate pain and delay the progression of the disease. The superficial location and its central role in the pathogenesis of OA make the IFP a promising therapeutic target in knee OA (KOA).
1. Introduction
Osteoarthritis (OA) is the most common joint disorder worldwide, and is recognized as a primary cause of chronic pain and disability in adults [1]. According to the data available from the Global Burden of Disease Study 2021, it has an impact on approximately 7.6% of the world’s population in 2020 (95% UI: 6.8–8.4%), affecting 595 million people (with a 95% uncertainty interval of 535–656 million) [2]. It is a degenerative joint disease involving the progressive disruption of articular cartilage, subchondral bone remodeling, synovial joint inflammation, and changes in periarticular structures [3].
Previously considered as a purely mechanical condition resulting from joint “wear and tear,” OA is currently recognized as a complex, multifactorial disease that affects all components of the joint, including articular cartilage, subchondral bone, ligaments, and surrounding muscles [4].
As defined by the Osteoarthritis Research Society International (OARSI), OA is characterized by joint impairment driven by cellular stress responses and the breakdown of the extracellular matrix [5]. The onset of the disease is determined by acute injuries or repetitive microtraumas which break the homeostatic balance of joint tissues leading to metabolic dysregulation, and may interact with genetic susceptibility and other environmental factors [6]. A modern approach to understanding OA reconsiders the Infrapatellar Fat Pad (IFP) and the adjacent synovial membrane (SM) as a single anatomo-functional unit (AFU) [7]. Several lines of evidence, ranging from macroscopic anatomy, imaging, histopathology, and molecular biology highlight the strict interaction between these structures [8,9,10]. The anatomical proximity and molecular interactions suggest a “mutual conditioning,” where pathological changes in one tissue influence the others [11]. This AFU is actively involved also in the pathogenesis and pain of OA, with histopathological analyses confirming that both the IFP and SM show increased inflammatory cells and increased vascular density in OA [7].
Studies show that low-grade chronic inflammation emerges as a key driver in the pathophysiology of OA, affecting not only the cartilage but also the joint structures and surrounding tissues [12]. Elevated levels of pro-inflammatory cytokines such as Interleukin (IL)-1β, Tumor Necrosis Factor (TNF)-α, and IL-6 in joint tissues promote the expression of catabolic enzymes, including matrix metalloproteinases (MMP) and aggrecanases, as well as molecules like nitric oxide (NO) and Prostaglandin E2 (PGE2), which collectively degrade the ECM and inhibit cartilage repair [13,14]. Synovitis is a frequent finding in OA and results from the activation of synovial cells by damage-associated molecular patterns (DAMPs), stimulating cytokine production and immune cell infiltration [15]. Oxidative stress exacerbates these inflammatory processes enhancing pro-inflammatory signaling and inducing chondrocyte apoptosis and senescence [16]. Furthermore, emerging evidence suggests that alterations in gut microbiota composition may disrupt host–microbe homeostasis, provoking immune activation and stimulating the gut–joint axis, thereby playing a role in the development of OA [17].
Taken together these events promote progressive morphological alterations such as degradation of cartilage, remodeling of subchondral bone, formation of osteophytes, and inflammation of the SM, ultimately leading to the loss of normal joint function and structural integrity.
Although any synovial (diarthrodial) joint could be affected, hips, knees, and interphalangeal and metacarpophalangeal joints of hands are most commonly affected sites [18,19]. The pathogenesis of the disease is multifactorial, influenced by systemic and local factors. Aging is the strongest non-modifiable risk factor, associated with cellular senescence and impaired cartilage matrix repair [3,20]. Genetic predisposition also plays a crucial role, suggesting a substantial genetic component to OA susceptibility, and the disease shows a higher prevalence in women, particularly after menopause [21]. Other significant factors include a history of major joint trauma (post-traumatic OA), overuse, and pre-existing anatomical abnormalities such as joint malalignment or dysplasia [22].
Among the modifiable and local risk factors, obesity is a major contributing factor through complex biomechanical and metabolic mechanisms acting at both systemic and local tissue levels [23,24]. Fatty tissue functions as a metabolically active secretory organ rather than an inert lipid reservoir, promoting the secretion of pro-inflammatory mediators, regulatory hormones, and additional bioactive mediators, collectively referred to as adipokines [25,26]. These secreted substances modulate critical biological functions including metabolic homeostasis, immune responses and they are critically involved in the regulation of bone remodeling, exerting their effects through endocrine, paracrine, and autocrine pathways [27,28]. They mediate the activity of osteoblasts and osteoclasts, influencing the dynamic equilibrium between bone formation and resorption and affecting cartilage metabolism [29]. Leptin, for instance, has been shown to exert both anabolic and catabolic effects on bone, depending on whether it acts centrally via the hypothalamus or peripherally on bone cells [30]. It induces chondrocytes to produce pro-inflammatory cytokines, adhesion molecules and matrix-degrading enzymes, thereby accelerating ECM breakdown [31]. Adiponectin shows a pro-osteogenic effect during bone remodeling [32,33] and inhibits osteoclastogenesis [34]. Although sometimes linked to bone-protective effects, it also induces the release of interleukins, MMPs and nitric oxide (NO) in chondrocytes, which can promote cartilage damage [35,36]. Other adipokines, such as resistin and visfatin, have been implicated in promoting a pro-inflammatory environment that may enhance osteoclast activity and bone resorption [37,38]. Visfatin, highly expressed in the IFP near osteophyte formation, reinforces local inflammation and enhances chondrocyte production of cytokines and chemokines, contributing further to cartilage catabolism [39,40].
IFP, synovium and osteophyte cells are active producers of inflammatory cytokines and adipokines [10]. These molecules, in particular leptin and adiponectin, can upregulate inflammatory mediators including PGE2, IL-6 and IL-8, TNF-α, and vascular cell adhesion molecule 1 (VCAM-1) within the synovial fluid of the knee [41,42] modulating the infiltration of inflammatory cells into cartilage, finally inducing a degenerative cascade that exacerbates the progression of OA. Correspondingly, studies have demonstrated that adipokine concentrations are markedly elevated in individuals with OA, and these increased levels remain significantly correlated with disease severity even after adjusting for confounding factors such as age, sex, and Body Mass Index (BMI) [43,44].
The dysregulation of adipokine signaling promotes chronic low-grade inflammation, which collectively accelerate cartilage degradation through upregulation of MMP and aggrecanases [45]. Furthermore, obesity-associated metabolic syndrome factors, including insulin resistance and dyslipidemia, contribute to synovial inflammation and chondrocyte dysfunction through advanced glycation end-product formation and oxidative stress pathways [46,47].
While this review primarily focuses on human KOA and findings from murine preclinical models, we need to consider the significant contribution of the canine model to our understanding of OA. A key study of Schmidli et al. investigated the inflammatory activity of the IFP in dogs with cranial cruciate ligament rupture (CCLD), a condition that leads to OA [48]. The results demonstrated that the IFP of dogs with CCLD presented a significant increase in immune cells, particularly T lymphocytes (CD3) and macrophages (CD14), compared to healthy dogs [48]. Additionally, increased gene and inflammatory markers levels, such as IL-1β, IL-6, MMP-1, and MMP-13, were found [48]. Adipokines analysis revealed increased adiponectin and decreased leptin secretion in the IFP of dogs with CCLD compared to controls [48]. As one of the earliest and most consistently observed abnormalities following a CCLD tear in dogs, the fat pad sign, characterized by the presence of edema on MRI (visualized as hyperintensity on T2-weighted sequences), volumetric increase, and fibrosis is able can be detected in inflamed or irritated Hoffa’s fat pad [49]. These data indicate that IFP is a potential contributing factor to the pathogenesis of chronic joint disease, due to its inflammatory phenotype and its proximity within the knee joint.
This review provides a comprehensive analysis of emerging therapeutic options targeting the IFP in Knee Osteoarthritis (KOA), examining their effects on inflammatory factors, fibrotic pathways and nervous sensitization. We combine preclinical and translational data to provide evidence of the central role of the IFP in OA pathophysiology, discussing future directions for clinical application.
Pathophysiological Roles of the Infrapatellar Fat Pad in OA
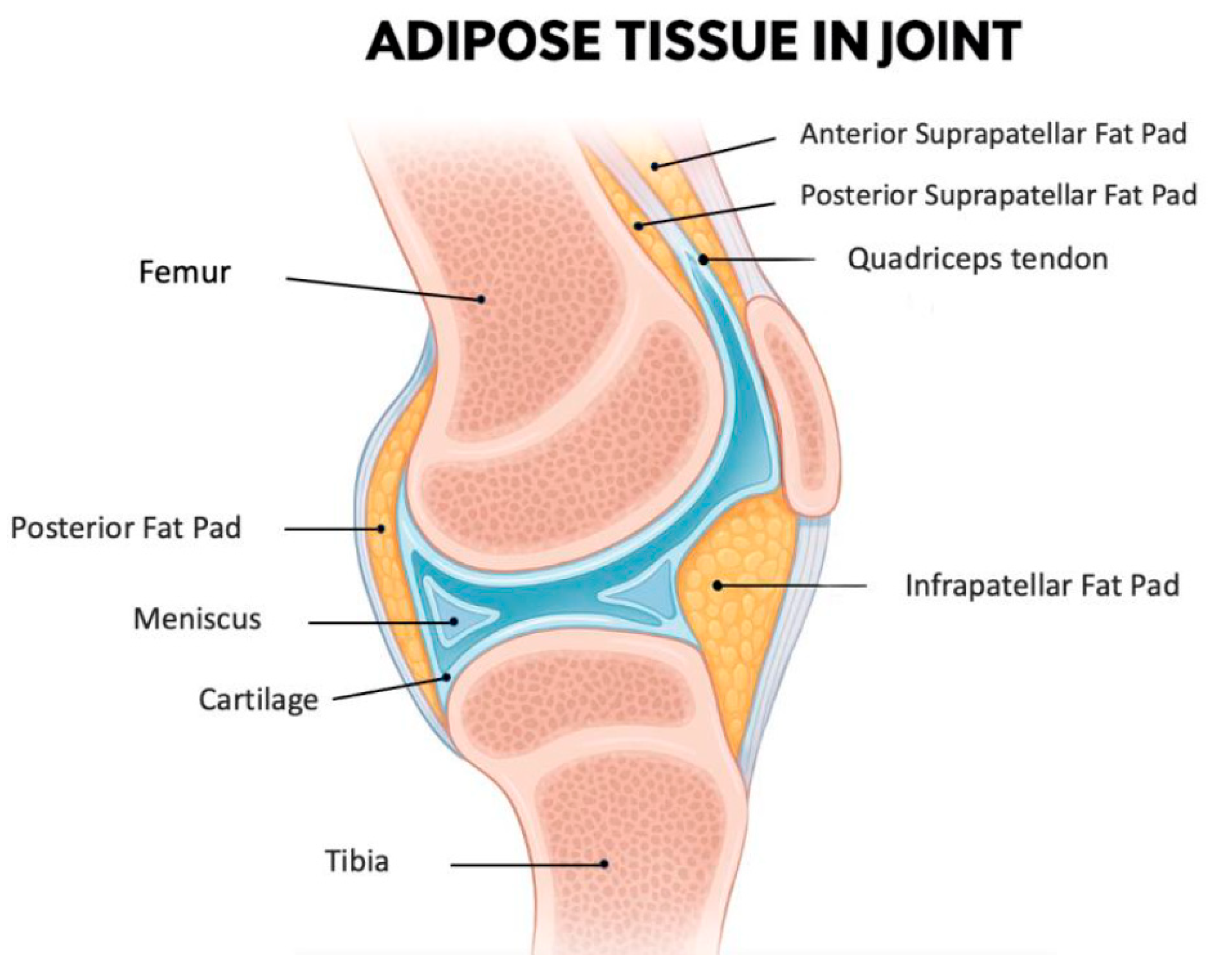
The IFP, also known as Hoffa’s fat pad, is an intra-articular but extra-synovial adipose tissue situated in the anterior region of the knee joint (Figure 1). This specialized fibrofatty tissue is strategically located between the patellar ligament and SM extending from the inferior patellar pole to the tibial tuberosity [50]. Functionally, it is a complex biomechanical structure that serves as a sophisticated cushion that optimizes joint movement by dynamically accommodating synovial fluid redistribution throughout the flexion-extension cycle [51]. Being an intra-articular fat depot, it is in direct anatomical continuity with synovial and cartilaginous structures making it particularly relevant to joint homeostasis.
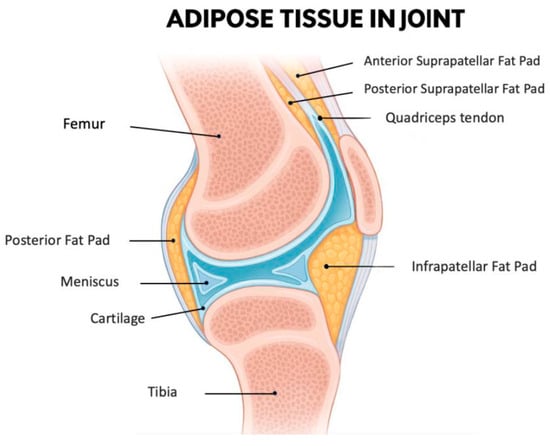
Figure 1.
A midsagittal section of the knee joint illustrates the anatomical position of the IFP, along with the other smaller adipose structures: the posterior fat pad, the anterior suprapatellar fat pad, and the posterior suprapatellar fat pad. The IFP is located inferior to the patella and posterior to the patellar tendon. Anteriorly, it is enclosed by the joint capsule, while its articular-facing surface is lined by synovial membrane. As such, the IFP is included within the joint capsule (intracapsular) but outside the synovial cavity (extrasynovial). Additionally, it lies in close proximity to the articular cartilage surfaces, highlighting its potential functional significance in joint biomechanics. Figure created with the assistance of artificial intelligence and scientifically validated by the authors.
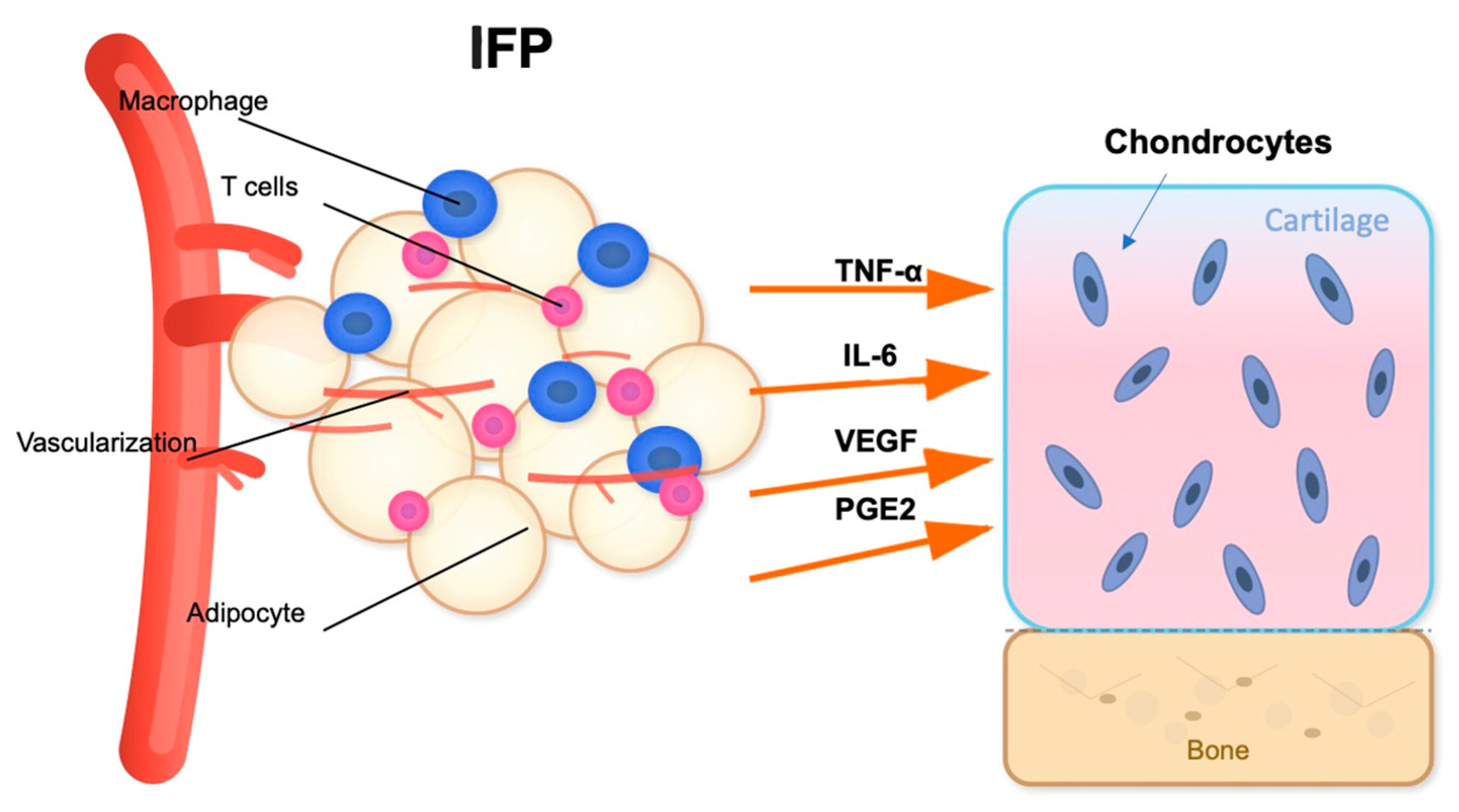
In addition to its mechanical function, the IFP acts as a metabolically active organ and a major player in obesity-related OA pathogenesis [52]. The IFP is a significant source of cytokines (IL-6, IL-1β, TNF-α), adipokines (leptin, resistin, adiponectin), and growth factors (like vascular endothelial growth factor, VEGF) [53] (Figure 2). Aging and obesity promote adipocyte hypertrophy and immune cell infiltration, especially pro-inflammatory M1 polarized macrophages and T cells, which contribute to local inflammation [54]. Simultaneously, the collection of senescent cells in the IFP drives the senescence-associated secretory phenotype (SASP) [55], releasing matrix-degrading enzymes and enhancing cytokine networks. A comparative study of IFP tissue from healthy young and elderly individuals found that aging is associated with distinct changes in adipocyte morphology and the ECM [56]. Specifically, adipocytes in the elderly group showed a significant increase in area and volume than younger one indicating age-related hypertrophy. Concurrently, the ECM in the older group was characterized by lower total collagen and a reduction in elastic fibers [56]. These physiological aging processes alter the cellular and structural composition of the IFP, which may compromise its biomechanical properties and contribute to the low-grade inflammatory state known as “inflammaging.” However, the structural changes in IFP, particularly regarding volume, remain a subject of debate with conflicting findings. Some studies suggest an increase in IFP volume in OA patients, potentially driven by local inflammation, adipocyte hypertrophy, and neovascularization, often correlating with higher BMI [57,58]. In contrast, other research reports a decrease in IFP volume or no significant change in OA, a phenomenon that could be attributed to the progressive development of fibrosis, leading to tissue contraction and atrophy in more advanced stages of the disease [59]. This discrepancy highlights the complex nature of IFP, suggesting that its volume may change dynamically depending on the stage of OA and the underlying pathological processes.
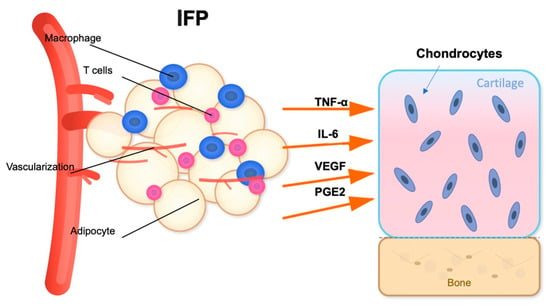
Figure 2.
Inflammatory signaling pathway between the IFP and articular cartilage. The IFP contains adipocytes, macrophages, and T cells within a highly vascularized environment. Upon activation, these cellular components release pro-inflammatory cytokines including TNF-α, IL-6, VEGF, and PGE2. These inflammatory mediators target the articular cartilage, potentially affecting chondrocyte metabolism and contributing to cartilage degradation [53]. The proximity of the IFP to the cartilage-subchondral bone interface facilitates this paracrine signaling mechanism in knee joint pathophysiology. Figure created with the assistance of artificial intelligence and scientifically validated by the authors.
Biological and biomechanical changes in IFP may also negatively impact adjacent synovium and cartilage via paracrine effects, eliciting synovitis and promoting cartilage degradation [60,61].
IFP can contribute to fibrotic remodeling, one of the main changes in OA, enhancing collagen deposition [10,62].
Remodeling is the final result of interconnected mechanisms, with Transforming growth factor-β (TGF-β) signaling pathways being the primary secretary, and pro-fibrotic M2 macrophages and constant low-grade inflammation offering a continuous stimulus for matrix production [63]. The fibrotic remodeling changes the essential biomechanical features of the IFP, alters its compliance, compromises its capacity to absorb joint stress effectively, and accelerates the deteriorative changes in the whole compartment [64]. Experimental results verify this mechanism demonstrating that post-inflammatory irreversible structural changes in the IFP plays a key role in sustaining chronic pain in rat OA models [65].
The neurogenic inflammation within the IFP also plays a central role in mediating OA pain [66]. The IFP is densely innervated by sensory and autonomic nerve fibers, especially by branches of the tibial and peroneal nerves [50]. In OA, the IFP shows increased expression of substance P and calcitonin gene-related peptide (CGRP), which play an important role in pain transmission and neurogenic inflammation [67,68]. Histological analysis showed an increase in the number of CGRP-positive nerve fiber endings in fibrous IFP, especially in the areas of neovascularization [69], with further increase as the OA progresses [70]. Additionally, IFP contains substance-P sensory nerve fibers that promote macrophage activation, which subsequently release of pro-inflammatory cytokines, such as IL-6 [66].
Recent research highlighted the role of mechanosensors, particularly the Piezo1 and Piezo2 ion channels, in mediating both mechanoceptive and inflammatory stimuli able to influence knee pain in OA [71]. Indeed, Piezo channels have been found to be involved in the inflammatory pathways of KOA, mediating the inflammatory response in chondrocytes and synovial cells [72]. Excessive mechanical stress, a risk factor for OA, upregulates Piezo1 expression, leading to a calcium influx that activates inflammatory responses and can further sensitize the channels, creating a pathogenic feedback loop [7]. Piezo2, in particular, is the most implicated in mechanical sensitization and pain responses, with higher immunoreactive vessel density in the IFP and SM compartments of the AFUs in OA compared to healthy subjects [7].
The presence of synovitis is associated with diminished pressure pain thresholds (PPTs) of the patella on Magnetic Resonance Imaging (MRI), which indicates an increased pain sensitivity and diminished pain functionality [73]. Synovitis has been shown to be significantly associated with reduced PPTs at the patella, showing an increase in local pain sensitivity [73]. This finding suggests that synovial inflammation contributes to peripheral sensitization, in which nociceptive neurons in the joint become hyper-responsive to mechanical stimuli. The lowering of PPTs indicates a lower tolerance to pressure-induced pain, which hesitates into impaired pain inhibitory mechanisms and reduced functional capacity in patients with joint pathology [74]. These changes in nociceptive processing are consistent with findings from previous studies linking synovial inflammation to clinical pain severity and structural progression in OA [75,76]. Furthermore, the role of synovitis in amplifying nociceptive signaling aligns with evidence showing that inflammatory mediators released within the SM, such as cytokines and prostaglandins, can directly sensitize joint afferents, thereby enhancing the subjective experience of pain [77,78]. The coexistence of inflammation, fibrosis and neo-innervation create the condition for a maladaptive pain amplification circuit potentially leading to a critical transition from acute-to-chronic OA pain while increasing overall nociceptive burden typical of knee KOA [79].
All the above-mentioned mechanisms are combined with its subcutaneous localization, making IFP an easily accessible site for minimal invasive interventions, a newer therapeutic target to treat KOA.
2. Therapeutic Strategies Targeting the Infrapatellar Fat Pad in Osteoarthritis
In the following sections, we will delineate the currently available therapeutic options targeting the IFP in KOA, ranging from conservative (Table 1) to minimally invasive (Table 2) and surgical treatments (Table 3).

Table 1.
Conservative Therapies. Summary of conservative therapeutic strategies for KOA, focusing on early and preventive interventions targeting inflammation, fibrosis, and biomechanical dysfunction. Treatments include pharmacological agents, lifestyle modifications and gene-based approaches, all aiming to modulate key pathological mechanisms in the IFP and surrounding joint environment.

Table 2.
Minimally Invasive Treatments. Overview of minimally invasive treatment strategies for KOA, highlighting therapeutic mechanisms, target tissues, and applicable disease stages. Approaches such as intra-articular injections, neuro-modulatory techniques, and vascular interventions address key pathological processes including inflammation, fibrosis, neo-innervation, and nociceptive signaling.

Table 3.
Surgical therapy. Surgical resection of the IFP as a therapeutic strategy for moderate to severe KOA. This intervention targets fibrotic and inflamed IFP tissue, aiming to reduce inflammation and alleviate pain by modifying IFP structure and its pathological contribution to OA progression [103,104].
2.1. Conservative Therapies
2.1.1. Anti-Inflammatory Agents
When comparing the inflammatory profile of the IFP from patients with KOA to subjects with traumatic knee injuries, such as an anterior cruciate ligament (ACL) rupture, distinct patterns related to inflammation are exhibited [107]. Studies showed that the IFP from OA patients is significantly more inflamed and vascularized than the IFP from patients with ACL injuries [107].
Furthermore, a study of Timur et al. demonstrated that the IFP from OA patients secretes significantly higher levels of prostanoids (PGE2, PGF2α, and PGD2) compared to IFP from subjects with cartilage defects [80], suggesting a distinctive inflammatory profile associated with OA pathogenesis. The secretion pattern of prostanoids in OA IFP samples can be stratified into two distinct subgroups: one characterized by markedly elevated prostanoid levels indicative of heightened inflammatory activity, and the other showing comparatively moderate reductions in prostanoids production [80]. This heterogeneity in prostanoids secretion reflects varying degrees of inflammatory microenviroment within the joint and may influence disease severity and symptoms [80]. Notably, the selective COX-2 inhibitor celecoxib has been shown to exert anti-inflammatory effects in both subgroups, primarily through the suppression of prostanoids synthesis by the IFP [80]. The efficacy of celecoxib appears particularly pronounced in individuals with higher baseline prostanoids secretion, underscoring its role in modulating inflammation mediated by lipid-derived mediators in OA [80]. These findings not only highlight the IFP as a dynamic source of pro-inflammatory prostanoids but also support targeted therapeutic strategies aiming to modulate local inflammatory pathways to alleviate joint inflammation and pain.
2.1.2. Low-Intensity Pulsed Ultrasound on the Fibrosis of the IFP
Low-Intensity Pulsed Ultrasound therapy (LIPUS) has demonstrated efficacy in alleviating KOA discomfort [108].
According to thermogenic mechanisms and high-frequency oscillatory stimulation, LIPUS exposure results in reduced edema and nociception and increased function [81,109].
Research conducted by Kitagawa and colleagues [110] demonstrated that LIPUS attenuated IFP fibrosis via multiple pathways. It inhibits Hypoxia Inducible Factor-1 (HIF-1) function, which correlates with macrophage phenotypic transformation [111], promoting M2 macrophage differentiation while reducing M1 phenotype (pro-inflammatory) prevalence. Furthermore, LIPUS has been documented to increase macrophage populations [112], decreases macrophage migration into the SM and reduce pro-inflammatory mediator release [113], showing a regulatory mechanism that promote modulation of macrophage activity through these combined effects [114].
Additionally, LIPUS leads to the downregulation of the genetic transcription of TGF-β [115], suppressing TGF-β-mediated fibrotic processes in the cultured synovial fibroblasts [116], inhibiting the osteoclastogenesis through the interference of the TGF-β1/Smad3 pathway [117], and subsequently the subchondral bone resorption.
2.1.3. Gene Therapy
As previously mentioned, fibrosclerosis of Hoffa’s fat pad has a central role in the pathogenesis of degenerative knee arthropathy through histiocytic invasion, angiogenesis, and expansion of nociceptive innervation, resulting in persistent pain [69].
Monocyte chemoattractant protein-1 (CCL2/MCP-1), a strong chemotactic factor for histiocytes, shows increased expression in various fibrotic disorders like hepatic cirrhosis [118], nephrosclerosis [119], and progressive systemic sclerosis [120].
The CCL2/C-C Motif Chemokine Receptor 2 (CCR2) signaling pathway is the most frequently documented mechanism responsible for the recruitment of circulating monocytes in OA [121,122] as well as for pain development in murine OA models [123]. Elevated levels of CCL2 have been observed in the IFP, synovial tissue [10,62] and blood serum of individuals with OA [124]. N-terminally truncated or chemically altered CCL2 variants have been identified as functional receptor antagonists [82]. Research suggests that targeting CCL2 could be a promising strategy to inhibit osteoclast formation, as the 7ND variant has been shown to prevent human osteoclast differentiation [125]. In addition, administration of 7ND markedly decreased osteolysis induced by wear particles and reduced both inflammatory cell infiltration and osteoclast numbers [126].
In a study conducted by Yoshimura et al. [82], employing a rat inflammatory arthropathy model, gene delivery of 7ND significantly suppressed the histological fibrosis in the IFP, decreased macrophage infiltration in synovial tissues, and led to a notable reduction in local CCL2 levels, apparently produced by infiltrating macrophages, ultimately inhibiting CCL2-mediated macrophage migration, and lowering the production of inflammatory cells and additional chemokines in the IFP.
Targeting CCL2 appears to play a central role in mitigating IFP fibrosis and limiting the inflammatory activation process [82]. Nevertheless, the precise in vivo mechanisms through which 7ND exerts its antagonistic effects still remain insufficiently understood [127]. Additional investigations are required to fully clarify biological pathways influenced by 7ND.
2.1.4. Diet
Several studies, using high fat-diet-induced murine OA models, reported that IFP volume or area was increased after administration of lipid-enriched diet [128,129,130].
In humans, however, the association between systemic anthropometric indices, such as BMI, and the size of the IFP is more complex, with conflicting results across studies. Some research, for instance, did not find a significant correlation between measures of adiposity and IFP volume in either OA patients or control subjects [131,132], suggesting that local joint factors may have a greater influence than systemic body composition in determining IFP size. In contrast, other studies have identified a positive relationship [133], although this can be influenced by factors such as sex [134]. For example, a significant correlation between IFP volume and body mass has been observed in females but not in males, highlighting potential sex-specific differences in adipose tissue metabolism within the knee [134]. The conflicting aspects of these findings may be attributed to differences in study methodologies, patient populations (including OA severity and demographics), and imaging techniques.
Radakovich et al. [83] indicated that the size of IFP was not dependent on body weight in the guinea pigs exposed to different dietary conditions. At the same time, the gene expression profiles within the IFP significantly varied. Specifically, this study showed higher transcription of pro-inflammatory genes (like Nuclear factor kappa light chain enhancer of activated B cells, NF-κB) in the IFP of hyperlipidic diet-fed guinea pigs than in the obese guinea and hypocaloric standard diet-fed pigs, enhancing expression of inflammatory signaling molecules including IL-1, IL-6, and TNF-a that may directly act on pathologic processes related with inflammation [83,84]. In that context, the COX-2 cascade and MCP-1 which is known to stimulate MMP activity, were overexpressed in hyperlipidic diet cohort inducing breakdown of type II and IV collagen within articular cartilage [83].
Taken together, this evidence demonstrated that immune stimulation is greater in the knee joint of obese and hyperlipidic diet groups versus lean. In addition, these data support the concept that low-caloric diets with a low-fat content result in reduced knee joint inflammation.
2.1.5. Exercise
It has been established that the IFP exhibits dynamic movement during knee joint motion [135]. Several studies suggest that the IFP plays a functional role promoting efficient joint movement, maintaining joint space, and acting as a cushion to absorb mechanical loads, thereby protecting the joint structures [135,136].
Histological analyses in knee immobilization in rat models [137,138] and in OA rat models induced by monoiodoacetate [85], demonstrated pathological changes in the IFP, including adipose tissue atrophy, fibrosis, and vascular congestion, particularly in the anterior region [85].
Although there is no direct evidence on the combined effect of joint mobilization and range-of-motion (ROM) exercises on adipose tissue atrophy and the structural integrity of the IFP, studies suggest that such interventions may improve joint function and positively influence the surrounding tissues.
In particular, a study of Griffin et al. showed, using murine models, that while starting of daily exercise temporarily triggered inflammation in the synovium and IFP along with changes in tissue architecture, continued daily exercise supported the recovery and maintenance of IFP homeostasis. Takeda et al. [86], also using murine models, demonstrated that joint movement not only reduced adipose cell degeneration in the IFP compared to non-treated controls, but may also exert a prophylactic effect. Nonetheless, the precise mechanisms underlying these therapeutic effects remain unclear and warrant further investigation to optimize treatment strategies.
2.2. Minimally Invasive Treatments
2.2.1. Intra-Articular Injective Therapies
Hyaluronic acid (HA), a macromolecular glycosaminoglycan, is a key substance produced by the SM and present in hyaline cartilage [139]. Intra-articular administration of HA provides mechanical viscosupplementation to articular surfaces, diminishes cartilaginous degradation, promotes trophic support to chondrocytes, and enhances synthesis of native hyaluronan, consequently slowing the progression of OA [87,88].
Studies of Chen and Qu [140,141] collectively highlight that targeting the IFP with HA can effectively modulate local inflammation and promote both structural preservation and symptomatic relief in OA. In particular in the study of Chen et al. [141] the researchers found, employing an in vitro model, that treatment with a combination of HA and platelet-rich plasma (PRP) significantly reduced the secretion of pro-inflammatory cytokines and adipokines from the IFP adipocytes. This anti-inflammatory effect helped to restore chondrocytes’ ability to produce a cartilage-like ECM, suggesting that HA can positively influence the local joint environment and protect cartilage by modifying the behavior of inflamed IFP cells [141]. Study of Qu [140] extended this concept into an in vivo setting using a rat model of OA. Here, HA was applied in the form of a biodegradable sheet placed directly onto the IFP. The treatment led to a marked reduction in fibrotic tissue remodeling and nerve fiber ingrowth within the fat pad, both known contributors to pain in OA. Together, these studies suggest that HA does more than lubricate the joint; whether delivered as an injectable compound (with PRP) or as a physical implant (in sheet form), HA appears capable of altering the IFP’s pathological signaling in OA, offering a potential disease-modifying and analgesic effect.
Platelet-rich plasma (PRP): Recent advances in tissue biology elucidated the crucial role of growth factors (GFs) in maintaining tissue homeostasis and orchestrating reparative responses to pathological insults, with extensive in vitro and in vivo studies examining their impact on chondral regeneration [142]. PRP intra-articular injection causes an initial burst, then a sustained release of biologically active substances, including key growth factors such as platelet-derived growth factor (PDGF), TGF-β, insulin-like growth factor I (IGF-I), and VEGF [89]. These signaling proteins play essential roles in tissue repair processes, including the prevention of chondrocyte apoptosis, promotion of angiogenesis and osteogenesis, regulation of the inflammatory response, and stimulation of collagen production [143,144]. Moreover, additional components released by platelets, such as fibrin, contribute to tissue regeneration by serving as both a structural matrix and a chemoattractant, facilitating the recruitment of stem cells and other reparative cell populations to the site of injury [145,146].
Research conducted by Araya and colleagues [90] revealed that intra-articular administration of pure PRP can reduce pain and suppress the advancement of synovial inflammation, infrapatellar adipose tissue architectural alterations, and articular cartilage deterioration in the short-term period. Furthermore, it was detected a reduction in the expression of CGRP nerve fibers in IFP of KOA patients treated with pure PRP [90]. The diminished presence of CGRP-positive nerve fibers after PRP treatment suggests that PRP has the ability to modulate the local neural environment mitigating nociceptive sensitization. Notably, this effect appears to be independent of the degree of cartilage damage within the knee joint [147]. This observation is consistent with findings from prior studies, which have similarly indicated that PRP can exert analgesic effects through mechanisms beyond cartilage repair [148,149].
Corticosteroids (CCS): Steroids modulate immune cells, as they regulate the polarization of macrophages, promoting immune cells switching from the pro-inflammatory M1 phenotype into an anti-inflammatory M2 phenotype [91] and inhibiting T-cell activity and proliferation [92]. Moreover, CCS also have powerful anti-catabolic effects as they inhibit the production of MMPs and other proteolytic enzymes responsible for cartilage degradation [93].
The analgesic efficacy of CCS in IFP-related pathology acts through multiple mechanisms, including the suppression of inflammatory mediator production, attenuation of peripheral nerve sensitization, and modulation of nociceptive signal transduction [150,151]. Additionally, these therapeutic agents exhibit anti-fibrotic properties by interfering with TGF-β signaling pathways, suggesting their potential role in reducing excessive collagen deposition and promoting favorable tissue remodeling within the fat pad [152,153]. This multifaceted mechanism of action positions CCS as valuable therapeutic interventions for addressing both the inflammatory and structural alterations characteristic of IFP pathology in OA conditions.
Research conducted by Heard and colleagues [103] revealed that in a surgically induced IFP impingement, a single intra-articular injection of CCS administered during the operative procedure effectively attenuated the acute inflammatory response within the articular space, primarily by suppressing cellular proliferation. However, this intervention proved inadequate for sustaining joint protection through the 9-week postoperative period.
It was also demonstrated that MPA (Methylprednisolone acetate), in ovine model, attenuated the IL1β-mediated transcriptional upregulation of MMP genes within the hyaline cartilage of various joint surfaces (including the kneecap and trochlear groove, distal femoral articular surfaces, and proximal tibial articular surface), synovial tissue, and IFP, demonstrating a dose-dependent suppressive effect [104].
The GLITTERS randomized controlled trial, the first trial that investigated corticosteroid administration directly into the IFP rather than the intra-articular space, failed to demonstrate favorable outcomes [154]. The study revealed that targeted corticosteroid infiltration of the infrapatellar adipose tissue showed no significant efficacy in mitigating articular pain or diminishing the volume of effusion-synovitis in patients with inflammatory KOA [154]. Nevertheless, the emergence of encouraging trends warrants further investigation through large-scale, multicentric clinical trials to comprehensively evaluate the therapeutic potential and clinical relevance of this intervention.
C-type natriuretic peptides (CNP): C-type natriuretic peptide belongs to the natriuretic peptide superfamily and has been documented to suppress the transcriptional activity of type I collagen in both pulmonary alveolar epithelial cells and cardiac myocytes through downregulation of the TGF-β signaling cascade [155,156].
Research conducted by An and colleagues [94] demonstrated that the administration of CNP via intra-articular injection effectively inhibited fibroproliferative alterations within the IFP, resulting in significant mitigation of chronic knee pain. Furthermore, local articular administration of CNP was shown to attenuate the progressive deterioration of hyaline cartilage [94]. These findings indicate that CNP represents a promising disease-modifying OA drug.
2.2.2. Electroacupuncture
Electroacupuncture (EA), extensively used in clinical settings, involves the application of pulsed electrical stimulation to acupuncture points following needle insertion [157]. This non-invasive therapeutic modality is broadly employed in the management of KOA, with various acupuncture techniques having demonstrated clinical efficacy [158,159]. As a conventional intervention for pain management, its therapeutic properties including analgesia, sedative effects, enhancement of circulatory function, and modulation of muscular tone have been validated through extensive clinical evidence, leading to improvement of joint pain, edema and functional mobility associated with KOA [95,96,97].
Research conducted by Zhang and colleagues [98] revealed that EA not only mitigated cartilaginous deterioration but also attenuated synovial inflammatory processes and IFP fibrotic changes, suppressing NLR family pyrin domain containing 3 (NLRP3) inflammasome activation, thereby reducing the inflammatory microenvironment characteristic of KOA.
2.2.3. Genicular Artery Embolization (GAE)
The primary blood supply to the IFP originates from branches of the genicular arterial system, which penetrate the tissue and form an extensive network of anastomotic connections throughout the structure [160]. This rich periarticular vascular network surrounding the knee joint is clinically significant, as it influences the fat pad’s response to injury, its healing potential, and its involvement in various pathological conditions affecting the knee [161]. Within this perspective, GAE has been introduced during the last ten years as an innovative percutaneous intervention for managing pain associated with degenerative knee joint disease [99]. This procedure specifically targets the abnormal neovascularization in arthritic knee joint, a vascular network believed to contribute to inflammatory processes, promoting nociception and synovial hyperplasia [162]. Through the selective obstruction of pathological branches of the genicular vessels, GAE diminishes blood flow to the synovium and reduce inflammatory activity, consequently providing pain relief while preserving the mechanical integrity of the joint [99,100].
A study of Sun et al. [163] evaluated the safety and efficacy of GAE in 33 patients with mild to severe KOA. At 12-month follow-up, the procedure demonstrated a significant reduction in pain and improvement in function in both patients with mild-to-moderate and severe OA, showing that GAE is a well-tolerated and effective treatment for improving symptoms and function in this kind of patients.
GAE shows also a favorable safety profile over a two-year follow-up period [164]. The most common adverse events are minor and self-limiting, primarily consisting of temporary skin discoloration and one groin hematoma, making it a promising therapeutic option for patients where surgery is contraindicated [164].
However, therapeutic efficacy is not uniformly achieved [163]. Therefore, careful candidate identification and appropriate prognostic counseling remains crucial.
2.2.4. Genicular Nerve-Targeted Cooled and Pulsed Radiofrequency Ablation
The IFP is richly innervated, creating a complex neural network that contributes significantly to knee pain experience in KOA patients. The fat pad is primarily innervated by branches of the femoral, common peroneal, and saphenous nerves, with particular contribution from the infrapatellar branch of the saphenous nerve and articular branches that form part of the genicular nerve complex. [50,165]
The dense neural supply of the IFP includes both sensory and sympathetic nerve fibers, containing numerous nociceptors and mechanoreceptors that make it particularly sensitive to mechanical stress and inflammatory mediators [68]. In KOA, the fat pad often becomes fibrotic, inflamed, and hyper innervated, leading to enhanced pain transmission and contributing to the overall pain experience [69]. Radiofrequency ablation has emerged as promising interventional approach for managing chronic knee pain by targeting the neural structures innervating the joint, including those supplying the IFP [166,167].
The technique involves using thermal or pulsed energy to disrupt nerve conduction, thereby interrupting pain signal transmission [101,102].
Conventional radiofrequency ablation creates thermal lesions at temperatures exceeding 80 °C [168], while cooled radiofrequency maintains tissue temperatures around 60–70 °C through internal probe cooling, allowing for larger lesion volumes [169].
A systematic review and meta-analysis by Soetjahjo et al. found that both cooled and pulsed Radiofrequency Ablation (RFA) techniques targeting genicular nerves provided significant pain reduction in KOA patients at all follow-up intervals (1, 3, 6, and 12 months post-treatment), with no significant difference in analgesic effectiveness between the two methods [170]. Pain scores, measured using Visual Analog Scale (VAS) or Numeric Rating Scale (NRS), showed substantial improvement particularly at the 6-month mark [170]. The meta-analysis revealed also that both techniques were generally safe with minimal adverse events reported, including only minor complications such as injection site pain, numbness, and stiffness that resolved quickly [170]. Both techniques offer effective pain relief for KOA patients for at least 6 months, though the long-term benefits beyond 12 months remain uncertain, possibly due to nerve regeneration [170].
This suggests that these non-conventional RFA techniques provide a valuable minimally invasive treatment option for managing chronic KOA pain when conservative therapies have failed.
2.3. Surgery
Evidence demonstrates that specific anatomical regions within the IFP, specifically the upper and lower sections, exhibit heightened vulnerability to biomechanical stress and loading forces during articular motion [171]. Such focal loading patterns induce hypoxic tissue damage, inflammatory responses, and promote releasing of inflammatory mediators and adipose-derived factors, thereby intensifying the manifestations of KOA [172].
The significant nociceptive innervation throughout the IFP also makes these compression zones primary generators of articular pain, perpetuating the pathological cascade [171].
Studies demonstrated that selective removal of the IFP, whether through partial or total excision, successfully diminished articular inflammation while preserving chondral structure, thereby slowing the progression of KOA [105,106].
Notably, targeted excision of adipose tissue enhanced biomechanical stability of the joint, maintained the structural integrity of the subchondral osseous tissue, and diminished degradation of the articular surface. Furthermore, immunohistochemistry evaluation revealed significant suppression of pro-inflammatory mediators, including IL-6, TNF-a, and MMP-3, alongside optimized retention of type II collagen [173].
The best approach to the IFP during total knee arthroplasty in patients with KOA remains controversial. A systematic review by Yao et al. [174] found inconsistent evidence regarding whether IFP resection leads to significantly inferior outcomes compared to preservation. Subsequently, Rajbhandari et al. [175] reported that IFP resection yielded marginally superior patient-reported functional outcomes as measured by Oxford Knee Scores; however, no significant difference in patient satisfaction was observed between the resection and preservation groups based on SF-12 scores.
3. The Use of Infrapatellar Mesenchymal Stromal Cells in Joint Cartilage Repair
Multipotent stromal cells (MSCs) exhibit immunomodulatory and nutritive properties involving anti-inflammatory, vasculogenic, and fibrosis-inhibiting mechanisms [176].
Research demonstrated that progenitor cells exhibiting stem cell properties are found within IFP, sharing characteristics with, though not being identical to, mesenchymal stromal cells of bone marrow origin [177].
These Infrapatellar Fat Pad-derived Stem Cells (IFPSCs) demonstrate reparative capacity and immunomodulatory properties, modulating macrophage phenotypic polarization, while exhibiting superior cartilage-forming potential compared to other mesenchymal stromal cells [177,178,179] due to their proximity to the knee joint and similarity to subcutaneous adipose tissue cells.
However, the therapeutic use of autologous stem cells shows significant challenges as the pathological state of the donor tissue is a critical factor. There is compelling evidence that IFP-SCs isolated from patients with advanced OA can exhibit a primed pro-inflammatory phenotype [180]. These cells may present significant expression of HLA-DR anti Fas/FasL, and a null expression of the CD38/NADase gene, indicating that the stem cells are from IFP of OA subjects in a chronic state of immune activation, without a substantial counteracting [180]. This characteristic could potentially undermine their regenerative function or even exacerbate the inflammatory environment within the OA joint if used without pre-treatment or appropriate cell selection. Despite this challenge, the promising therapeutic properties of IFP-SCs have driven their assessment within clinical contexts. Several early-phase clinical trials have been conducted to establish the safety and efficacy of implanting autologous IFP-derived MSCs for treating KOA and chondral defects [179,181]. These initial studies have generally reported positive outcomes regarding safety, pain reduction, and functional improvement, laying the groundwork for further research [182].
The mesenchymal-derived extracellular vesicles from the infrapatellar adipose tissue (MSCIPFP-Exos) mitigated OA progression in vivo through suppression of apoptosis, induction of ECM production, and downregulation of degradative mediators in vitro [183]. Furthermore, MSCIPFP-Exos substantially elevated autophagic activity within cartilage cells, mediated in part through microRNA 100-5p (miR100-5p)-dependent suppression of mTOR autophagy signaling cascade [183].
For these reasons, the stromal cells of IFP tissue have been focused on as potential therapeutic cell source for localized chondral defect in knee joint due to their anatomical location and surgical accessibility [184,185]. Nonetheless, although promising, its adoption into mainstream clinical practice is still in the preliminary stages. Key questions regarding optimal cell dosage, long-term efficacy, and strategies to mitigate the pro-inflammatory potential of cells from OA donors must be addressed through larger, well-controlled randomized clinical trials.
While MSCs often show poor adhesion, migration, and survival post-injection, a study of Yang et al. [186] demonstrates that using tropoelastin (TE) as an injection medium significantly improves IPFP-MSC adhesion, migration, and chondrogenic differentiation compared to standard carriers (normal saline, hyaluronic acid, platelet-rich plasma). TE also boosts ECM production in co-cultured osteoarthritic chondrocytes [186].
Moreover, when cultured within a HA-enriched environment, IFPSCs exhibit significantly enhanced chondrogenic differentiation capacity, characterized by increased expression of cartilage-specific genes and improved ECM synthesis [187].
Consequently, combined use of an HA-enriched microenvironment and IFPSC-based therapeutic strategies could improve the quality and long-term efficacy of joint cartilage repair, especially in degenerative or post-traumatic conditions [188].
Research conducted by Wang and colleagues [189] demonstrated that viral vector-mediated gene transfer altered the cartilage-forming, and fat-forming capabilities of IPFSCs, affecting mitotically exhausted IPFSCs proliferation rates and lineage commitment preferences. These observations suggest that both acellular matrix cultivation and life-extension methodologies may be employed for proliferation-exhausted adult stem cells’ proliferation and cell-type specialization, representing an opportunity for future cellular-mediated tissue repair therapies [189].
Preconditioning represents a promising strategy for enhancing the therapeutic potential of extracellular vesicles derived from IFPSCs in KOA management [190,191].
Specifically, research conducted by Wu and colleagues [192] demonstrated that TNF-a priming enhanced extracellular vesicle production from IFPSCs compared to non-primed cells (via activation of the Phosphoinositide 3-kinase/Protein Kinase B, PI3K/AKT, signaling cascade), improving therapeutic efficacy in relieving disease-associated joint alterations in murine OA models.
4. Discussion
This review presents an in-depth evaluation of novel therapeutic approaches targeting the IFP in KOA, with particular focus on their impact on inflammatory mediators, fibrotic remodeling processes, and nociceptive sensitization. By integrating findings from both preclinical models and translational research, we highlight the pivotal role of the IFP in the pathophysiological mechanisms underlying OA and examine translational relevance for patient care.
It has been studied how, in patients with OA, the IFP not merely represents a fat storage and a biomechanical shock absorber, but also an active inflammatory organ, displaying hypertrophic adipocytes, infiltration of pro-inflammatory M1 macrophages, and a senescence-associated secretory phenotype (SASP) [38,54,55].
This structure also stimulates the secretion of prostaglandins such as PGE2, PGF2a, and PGD2, which contribute, through paracrine stimulation, to the development of a chronic inflammatory trigger in the synovium and cartilage of adjacent tissues [193].
Among the pathological changes in the IFP in KOA, fibrotic remodeling is the most important, predominantly driven by the TGF-β signaling pathway and profibrotic M1 macrophages [194]. These structural changes alter the biomechanical properties of the tissue, promoting a change in loading distribution and thus supporting degeneration of joint structures [64].
The IFP is also a densely innervated structure, influencing pain stimulation [69]. In KOA, increased expression of CGRP and substance P within IFP gives rise to a neurogenic inflammatory loop that contributes to the transition from acute to chronic OA pain [69,70].
Several treatments have been developed specifically to target the IFP, ranging from pharmacological therapies to interventional procedures. The use of nonsteroidal anti-inflammatory drugs, particularly celecoxib, has demonstrated efficacy in inhibiting prostanoid production, particularly in patients with significant inflammatory profiles [80]. However, inflammatory patterns are highly heterogeneous, so phenotyping of the IFP may be useful to establish personalized therapy and achieve improved therapeutic outcomes [195,196].
LIPUS, a minimally invasive technique, shows therapeutic potential in reducing IFP fibrosis through several mechanisms, such as HIF-1 inhibition and macrophage phenotypic modulation [110,197]. Specifically, this minimally invasive approach inhibits TGF-β signaling and stimulates macrophage differentiation into M2, reducing the population of pro-inflammatory M1 macrophages [112].
Gene therapy has also shown promising results, demonstrating how CCL2 receptor antagonists can suppress OA-related histopathological changes in the IFP, reducing macrophage infiltration into synovial tissues and suppressing additional intrinsic chemokine production [82]. Intra-articular injective therapies have also demonstrated efficacy, particularly when used in combination (e.g., PRP with HA), thanks to a synergistic mechanism that simultaneously modulates inflammation and biomechanical function [198]. However, the limited duration of the therapeutic effect, approximately 9 months, should prompt clinicians to consider treatment cycles rather than single administrations [198].
Genicular artery embolization has been associated with functional improvement in 70–80% of patients, suggesting a potential role for treating the vascular component that contributes to chronic pain through the ablation of neovascularized areas [163].
Regarding the surgical approach, studies indicate that selective excision of the IFP may help reduce the progression of KOA; however, there is no conclusive evidence supporting the removal of this structure during knee replacement surgery in patients with KOA, and high-quality studies highlighting the best surgical strategy are lacking [105,106].
The most innovative therapeutic approach involves the use of IFP-derived mesenchymal stromal cells, which have a natural tissue regenerative ability. These cells exhibit enhanced chondrogenic potential than other mesenchymal populations, suppress apoptosis, and stimulate matrix production, contributing to reduce the rate of OA progression [182,199,200].
Despite these advances, significant gaps persist in our knowledge and capacity to therapeutically target the IFP.
The pathological involvement of the IFP varies significantly among patients [10], and it would be important to identify biomarkers to stratify patients, working toward patient-specific treatment. Novel imaging tool able to detect more accurately the level of tissue inflammation, not just structural characteristics, are also needed. Moreover, the time course of IFP pathology during disease development has not been fully defined, limiting optimization of intervention timing [65].
Additionally, the predominance of rodent models in translation of preclinical findings may not fully capture the complexity of human IFP pathology, particularly regarding chronic OA development and the impact of comorbidities.
Future research must give priority to the development of non-invasive IFP phenotyping methods, advanced imaging techniques, and artificial intelligence-enhanced analysis to promote a personalized therapeutic approach.
5. Conclusions
In conclusion, the IFP emerges as a promising therapeutic target in OA management, offering multiple intervention opportunities across the spectrum of conservative, minimally invasive, and surgical approaches. The convergence of mechanistic understanding, preclinical efficacy data, and early clinical observations supports the continued investigation of IFP-targeted therapies. However, the translation of these insights into standardized clinical practice requires more standardized studies like randomized controlled trials specifically designed to evaluate IFP-targeted interventions, the development of reliable biomarkers for patient selection and the creation of algorithms that consider IFP assessment in routine OA management protocols.
Author Contributions
Conceptualization, I.M.; methodology, I.M.; data curation, I.M.; writing—original draft preparation, I.M.; writing—review and editing, A.F. and S.G.; visualization, S.G. and A.F.; supervision, S.G. and A.F.; project administration, S.G. and A.F.; funding acquisition, S.G. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Institutional Review Board Statement, Informed Consent Statement and Data Availability Statement. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| ACL | Anterior Cruciate Ligament |
| AFU | Anatomo-Functional Unit |
| BMI | Body Mass Index |
| BMP-7 | Bone Morphogenetic Protein-7 |
| BMP-14 | Bone Morphogenetic Protein-14 |
| CCL2 | Monocyte chemoattractant protein-1 |
| CCLD | Cranial Cruciatus Ligament Disease |
| CCR2 | C-C Motif Chemokine Receptor 2 |
| CCS | Corticosteroids |
| CNP | C-type natriuretic peptide |
| DAMPS | Damage-Associated Molecular Patterns |
| EA | Electroacupuncture |
| FABP4 | Fatty Acid-Binding Protein-4 |
| GAE | Genicular Artery Embolization |
| GFs | Growth Factors |
| HA | Hyaluronic Acid |
| HIF-1 | Activation of hypoxia-inducible factor 1 |
| IFP | Infrapatellar Fat Pad |
| IFPSCs | Infrapatellar Fat Pad-derived Stem Cells |
| IL-6 | Interleukin-6 |
| IL-1 | Interleukin-1 |
| iNOS | Inducible Nitric Oxide Synthase |
| KOA | Knee Osteoarthritis |
| LIPUS | Low-intensity pulsed ultrasound |
| MCP-1 | Monocyte chemoattractant protein-1 |
| miR100-5p | MicroRNA 100-5-p |
| MSCIPFP-Exos | Mesenchymal-derived extracellular vesicles from the infrapatellar adipose tissue |
| MMP-1 | Matrix Metalloproteinase 1 |
| MMP-3 | Matrix Metalloproteinase 3 |
| MPA | Methylprednisolone acetate |
| MRI | Magnetic Resonance |
| m-TOR | Mechanistic Target of Rapamycin |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| NO | Nitric Oxide |
| NRS | Numeric Rating Scale |
| OA | Osteoarthritis |
| PGE2 | Prostaglandin E2 |
| PGF2α | Prostaglandin F2α |
| PGD2 | Prostaglandin D2 |
| PI3K/AKT | Phosphoinositide 3-kinase/Protein Kinase B. |
| PPAR-γ2 | Peroxisome Proliferator-Activated Receptor Gamma 2 |
| PPTs | Pressure Pain Thresholds |
| PRP | Platelet-Rich Plasma |
| RFA | Radiofrequency Ablation |
| RUNX2 | Runt-related transcription factor 2 |
| SASP | Secretory phenotype |
| SM | Synovial Membrane |
| SMAD3 | Mothers against decapentaplegic homolog 3 |
| SOX-9 | SRY-related HMG-box gene 9 |
| TE | Tropoelastin |
| TGF-β | Transforming growth factor-β |
| TIMP | Tissue Inhibitor of Metalloproteinases |
| TNF-α | Tumor necrosis factor |
| VAS | Visual Analog Scale |
| VCAM1 | Vascular Cell Adhesion Molecule 1 |
| VEGF | Vascular endothelial growth factor |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
References
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Z.; Liang, X.-Z.; Sun, Y.-Q.; Jia, H.-F.; Li, J.-C.; Li, G. Global, regional, and national burdens of osteoarthritis from 1990 to 2021: Findings from the 2021 global burden of disease study. Front. Med. 2024, 11, 1476853. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Kraus, V.; Blanco, F.; Englund, M.; Karsdal, M.; Lohmander, L. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.-M.; Ding, C.; et al. Osteoarthritis. Nat. Rev. Dis. Primers 2025, 11, 10. [Google Scholar] [CrossRef]
- Emmi, A.; Stocco, E.; Boscolo-Berto, R.; Contran, M.; Belluzzi, E.; Favero, M.; Ramonda, R.; Porzionato, A.; Ruggieri, P.; De Caro, R.; et al. Infrapatellar Fat Pad-Synovial Membrane Anatomo-Fuctional Unit: Microscopic Basis for Piezo1/2 Mechanosensors Involvement in Osteoarthritis Pain. Front. Cell Dev. Biol. 2022, 10, 886604. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Klein-Wieringa, I.R.; de Lange-Brokaar, B.J.; Yusuf, E.; Andersen, S.N.; Kwekkeboom, J.C.; Kroon, H.M.; van Osch, G.J.; Zuurmond, A.-M.; Stojanovic-Susulic, V.; Nelissen, R.G.; et al. Inflammatory Cells in Patients with Endstage Knee Osteoarthritis: A Comparison between the Synovium and the Infrapatellar Fat Pad. J. Rheumatol. 2016, 43, 771–778. [Google Scholar] [CrossRef]
- Favero, M.; El-Hadi, H.; Belluzzi, E.; Granzotto, M.; Porzionato, A.; Sarasin, G.; Rambaldo, A.; Iacobellis, C.; Cigolotti, A.; Fontanella, C.G.; et al. Infrapatellar fat pad features in osteoarthritis: A histopathological and molecular study. Rheumatology 2017, 56, 1784–1793. [Google Scholar] [CrossRef]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Knights, A.J.; Redding, S.J.; Maerz, T. Inflammation in osteoarthritis: The latest progress and ongoing challenges. Curr. Opin. Rheumatol. 2023, 35, 128–134. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Namane, M.; Cicuttini, F. Osteoarthritis. Lancet 2025, 405, 71–85, Correction in Lancet 2025, 405, 2278. [Google Scholar] [CrossRef]
- Wei, Y.; Bai, L. Recent advances in the understanding of molecular mechanisms of cartilage degeneration, synovitis and subchondral bone changes in osteoarthritis. Connect. Tissue Res. 2016, 57, 245–261. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; MacGregor, A.J. Risk factors for osteoarthritis: Genetics11supported by Procter & Gamble Pharmaceuticals, Mason, OH. Osteoarthr. Cartil. 2004, 12, 39–44. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Binvignat, M.; Sellam, J.; Berenbaum, F.; Felson, D.T. The role of obesity and adipose tissue dysfunction in osteoarthritis pain. Nat. Rev. Rheumatol. 2024, 20, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Linares, N.; Eymard, F.; Berenbaum, F.; Houard, X. Role of adipose tissues in osteoarthritis. Curr. Opin. Rheumatol. 2021, 33, 84–93. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the art paper Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 2, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Drevon, C.A.; Eckel, J. Secreted proteins from adipose tissue and skeletal muscle–adipokines, myokines and adipose/muscle cross-talk. Arch. Physiol. Biochem. 2011, 117, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Karastergiou, K.; Mohamed-Ali, V. The autocrine and paracrine roles of adipokines. Mol. Cell Endocrinol. 2010, 318, 69–78. [Google Scholar] [CrossRef]
- Neumann, E.; Junker, S.; Schett, G.; Frommer, K.; Müller-Ladner, U. Adipokines in bone disease. Nat. Rev. Rheumatol. 2016, 12, 296–302. [Google Scholar] [CrossRef]
- Reid, I.R.; Baldock, P.A.; Cornish, J. Effects of Leptin on the Skeleton. Endocr. Rev. 2018, 39, 938–959. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef]
- Kanazawa, I.; Yamaguchi, T.; Yano, S.; Yamauchi, M.; Yamamoto, M.; Sugimoto, T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 2007, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Chen, C.Y.; Chuang, T.Y.; Lin, Y.; Liu, H.Y.; Mersmann, H.J.; Wu, S.C.; Ding, S.T. Adiponectin receptor 1 regulates bone formation and osteoblast differentiation by GSK-3β/β-Catenin signaling in mice. Bone 2014, 64, 147–154. [Google Scholar] [CrossRef]
- Chen, G.; Huang, L.; Wu, X.; Liu, X.; Xu, Q.; Li, F.; Dai, M.; Zhang, B. Adiponectin inhibits osteoclastogenesis by suppressing NF-κB and p38 signaling pathways. Biochem. Biophys. Res. Commun. 2018, 503, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, A.; Juslin, S.; Nieminen, R.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res. Ther. 2011, 13, R184. [Google Scholar] [CrossRef]
- Lago, R.; Gomez, R.; Otero, M.; Lago, F.; Gallego, R.; Dieguez, C.; Gomez-Reino, J.; Gualillo, O. A new player in cartilage homeostasis: Adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthr. Cartil. 2008, 16, 1101–1109. [Google Scholar] [CrossRef]
- Cheleschi, S.; Gallo, I.; Barbarino, M.; Giannotti, S.; Mondanelli, N.; Giordano, A.; Tenti, S.; Fioravanti, A. MicroRNA Mediate Visfatin and Resistin Induction of Oxidative Stress in Human Osteoarthritic Synovial Fibroblasts Via NF-κB Pathway. Int. J. Mol. Sci. 2019, 20, 5200. [Google Scholar] [CrossRef] [PubMed]
- Economou, A.; Mallia, I.; Fioravanti, A.; Gentileschi, S.; Nacci, F.; Randone, S.B.; Lepri, G.; Guiducci, S. The Role of Adipokines between Genders in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 10865. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Barbarino, M.; Giannotti, S.; Bellisai, F.; Frati, E.; Fioravanti, A. Exploring the Crosstalk between Hydrostatic Pressure and Adipokines: An In Vitro Study on Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2021, 22, 2745. [Google Scholar] [CrossRef]
- Laiguillon, M.-C.; Houard, X.; Bougault, C.; Gosset, M.; Nourissat, G.; Sautet, A.; Jacques, C.; Berenbaum, F.; Sellam, J. Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis. Arthritis Res. Ther. 2014, 16, R38. [Google Scholar] [CrossRef]
- Klein-Wieringa, I.R.; Kloppenburg, M.; Bastiaansen-Jenniskens, Y.M.; Yusuf, E.; Kwekkeboom, J.C.; El-Bannoudi, H.; Nelissen, R.G.H.H.; Zuurmond, A.; Stojanovic-Susulic, V.; Van Osch, G.J.V.M.; et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann. Rheum. Dis. 2011, 70, 851–857. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, Y.; Yan, C.H.; Zhang, W. Adipokine Signaling Pathways in Osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 865370. [Google Scholar] [CrossRef]
- de Boer, T.; van Spil, W.; Huisman, A.; Polak, A.; Bijlsma, J.; Lafeber, F.; Mastbergen, S. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthr. Cartil. 2012, 20, 846–853. [Google Scholar] [CrossRef]
- Bas, S.; Finckh, A.; Puskas, G.J.; Suva, D.; Hoffmeyer, P.; Gabay, C.; Lübbeke, A. Adipokines correlate with pain in lower limb osteoarthritis: Different associations in hip and knee. Int. Orthop. 2014, 38, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.H.; Lee, Y.J.; Kim, T.K.; Chang, C.B.; Chung, J.-H.; Shin, K.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Adiponectin is a potential catabolic mediator in osteoarthritis cartilage. Arthritis Res. Ther. 2010, 12, R231. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Sellam, J.; Berenbaum, F. Metabolic syndrome-associated osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 214–222. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Zhang, H.; Wu, W.; Xue, S.; Zhu, Z.; Ding, C. Inflammatory Mechanisms Underlying Metabolic Syndrome-associated Osteoarthritis and Potential Treatments. Osteoarthr. Cartil. Open 2025, 7, 100614. [Google Scholar] [CrossRef] [PubMed]
- Schmidli, M.R.; Fuhrer, B.; Kurt, N.; Senn, D.; Drögemüller, M.; Rytz, U.; Spreng, D.E.; Forterre, S. Inflammatory pattern of the infrapatellar fat pad in dogs with canine cruciate ligament disease. BMC Vet. Res. 2018, 14, 161. [Google Scholar] [CrossRef]
- Widmer, W.R.; Buckwalter, K.A.; Braunstein, E.M.; Hill, M.A.; O’Connor, B.L.; Visco, D.M. Radiographic and Magnetic Resonance imaging of the stifle joint in experimental osteoarthritis of dogs. Vet. Radiol. Ultrasound 1994, 35, 371–384. [Google Scholar] [CrossRef]
- Leese, J.; Davies, D.C. An investigation of the anatomy of the infrapatellar fat pad and its possible involvement in anterior pain syndrome: A cadaveric study. J. Anat. 2020, 237, 20–28. [Google Scholar] [CrossRef]
- Fontanella, C.G.; Carniel, E.L.; Frigo, A.; Macchi, V.; Porzionato, A.; Sarasin, G.; Rossato, M.; De Caro, R.; Natali, A.N. Investigation of biomechanical response of Hoffa’s fat pad and comparative characterization. J. Mech. Behav. Biomed. Mater. 2017, 67, 1–9. [Google Scholar] [CrossRef]
- Chou, L.S.; Zhang, J.; Jildeh, T.R. Metabolic Functions of the Infrapatellar Fat Pad: Implications for Knee Health and Pathology. JBJS Rev. 2024, 12, e24.00110. [Google Scholar] [CrossRef]
- Yue, S.; Zhai, G.; Zhao, S.; Liang, X.; Liu, Y.; Zheng, J.; Chen, X.; Dong, Y. The biphasic role of the infrapatellar fat pad in osteoarthritis. Biomed. Pharmacother. 2024, 179, 117364. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Adipose tissue, immune aging, and cellular senescence. Semin. Immunopathol. 2020, 42, 573–587. [Google Scholar] [CrossRef]
- Jeon, O.H.; David, N.; Campisi, J.; Elisseeff, J.H. Senescent cells and osteoarthritis: A painful connection. J. Clin. Investig. 2018, 128, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Belluzzi, E.; Contran, M.; Boscolo-Berto, R.; Picardi, E.; Guidolin, D.; Fontanella, C.G.; Olivotto, E.; Filardo, G.; Borile, G.; et al. Age-Dependent Remodeling in Infrapatellar Fat Pad Adipocytes and Extracellular Matrix: A Comparative Study. Front. Med. 2021, 8, 661403. [Google Scholar] [CrossRef] [PubMed]
- Davulcu, C.; Celayir, A. Exploring the Correlation Between Body Mass Index and Knee Hoffa Fat Pad Size in MRI Sagittal Plane. Ann. Med. Res. 2024, 1, 226. [Google Scholar] [CrossRef]
- Burda, B.; Steidle-Kloc, E.; Dannhauer, T.; Wirth, W.; Ruhdorfer, A.; Eckstein, F. Variance in infra-patellar fat pad volume: Does the body mass index matter?—Data from osteoarthritis initiative participants without symptoms or signs of knee disease. Ann. Anat.—Anat. Anz. 2017, 213, 19–24. [Google Scholar] [CrossRef]
- Fontanella, C.G.; Belluzzi, E.; Rossato, M.; Olivotto, E.; Trisolino, G.; Ruggieri, P.; Rubini, A.; Porzionato, A.; Natali, A.; De Caro, R.; et al. Quantitative MRI analysis of infrapatellar and suprapatellar fat pads in normal controls, moderate and end-stage osteoarthritis. Ann. Anat.—Anat. Anz. 2019, 221, 108–114. [Google Scholar] [CrossRef]
- Gross, J.-B.; Guillaume, C.; Gegout-Pottie, P.; Reboul, P.; Jouzeau, J.-Y.; Mainard, D.; Presle, N. The infrapatellar fat pad induces inflammatory and degradative effects in articular cells but not through leptin or adiponectin. Clin. Exp. Rheumatol. 2017, 35, 53–60. [Google Scholar]
- Terada, H.; Kojima, T.; Takasu, C.; Kawabata, S.; Shimada, N.; Nihei, K.; Takayanagi, K.; Kanemura, N.; Murata, K. Fibrosis of the infrapatellar fat pad induces gradual cartilage degeneration in a rat model. Tissue Cell 2025, 95, 102851. [Google Scholar] [CrossRef]
- Bastiaansen-Jenniskens, Y.M.; Wei, W.; Feijt, C.; Waarsing, J.H.; Verhaar, J.A.N.; Zuurmond, A.; Hanemaaijer, R.; Stoop, R.; van Osch, G.J.V.M. Stimulation of Fibrotic Processes by the Infrapatellar Fat Pad in Cultured Synoviocytes from Patients With Osteoarthritis: A Possible Role for Prostaglandin F2a. Arthritis Rheum. 2013, 65, 2070–2080. [Google Scholar] [CrossRef]
- Tung, N.T.C.; Nogami, M.; Iwasaki, M.; Yahara, Y.; Seki, S.; Makino, H.; Kamei, K.; He, Z.; Kawaguchi, Y. M2-like macrophages derived from THP-1 cells promote myofibroblast differentiation of synovial fibroblasts in association with the TGF-β1/SMAD2/3 signaling pathway. Sci. Rep. 2025, 15, 25505. [Google Scholar] [CrossRef]
- Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Favero, M.; Ruggieri, P.; Macchi, V.; Carniel, E.L. Mechanical behavior of infrapatellar fat pad of patients affected by osteoarthritis. J Biomech 2022, 131, 110931. [Google Scholar] [CrossRef]
- Inomata, K.; Tsuji, K.; Onuma, H.; Hoshino, T.; Udo, M.; Akiyama, M.; Nakagawa, Y.; Katagiri, H.; Miyatake, K.; Sekiya, I.; et al. Time course analyses of structural changes in the infrapatellar fat pad and synovial membrane during inflammation-induced persistent pain development in rat knee joint. BMC Musculoskelet. Disord. 2019, 20, 8. [Google Scholar] [CrossRef]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. Biomed. Res. Int. 2019, 2019, 6390182. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.A.; Mapp, P.I.; Kelly, S. Calcitonin gene-related peptide in the joint: Contributions to pain and inflammation. Br. J. Clin. Pharmacol. 2015, 80, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, M.; Meier, F.; Walter, G.F.; Hurschler, C.; Schmolke, S.; Wirth, C.J.; Rühmann, O. Distribution of substance-P nerves inside the infrapatellar fat pad and the adjacent synovial tissue: A neurohistological approach to anterior knee pain syndrome. Arch. Orthop. Trauma. Surg. 2005, 125, 592–597. [Google Scholar] [CrossRef]
- Onuma, H.; Tsuji, K.; Hoshino, T.; Inomata, K.; Udo, M.; Nakagawa, Y.; Katagiri, H.; Miyatake, K.; Watanabe, T.; Sekiya, I.; et al. Fibrotic changes in the infrapatellar fat pad induce new vessel formation and sensory nerve fiber endings that associate prolonged pain. J. Orthop. Res. 2020, 38, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, J.; Uchida, K.; Takano, S.; Inoue, G.; Minatani, A.; Miyagi, M.; Iwase, D.; Sekiguchi, H.; Mukai, M.; Takaso, M. Expression of calcitonin gene-related peptide in the infrapatellar fat pad in knee osteoarthritis patients. J. Orthop. Surg. Res. 2017, 12, 65. [Google Scholar] [CrossRef]
- Gao, W.; Hasan, H.; Anderson, D.E.; Lee, W. The Role of Mechanically-Activated Ion Channels Piezo1, Piezo2, and TRPV4 in Chondrocyte Mechanotransduction and Mechano-Therapeutics for Osteoarthritis. Front. Cell Dev. Biol. 2022, 10, 885224. [Google Scholar] [CrossRef]
- Lee, W.; Nims, R.J.; Savadipour, A.; Zhang, Q.; Leddy, H.A.; Liu, F.; McNulty, A.L.; Chen, Y.; Guilak, F.; Liedtke, W.B. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2001611118. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Guermazi, A.; Roemer, F.; Nevitt, M.C.; Scholz, J.; Arendt-Nielsen, L.; Woolf, C.; Niu, J.; Bradley, L.A.; Quinn, E.; et al. Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2016, 68, 654–661. [Google Scholar] [CrossRef]
- Kuni, B.; Wang, H.; Rickert, M.; Ewerbeck, V.; Schiltenwolf, M. Pain threshold correlates with functional scores in osteoarthritis patients. Acta Orthop. 2015, 86, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.C.; Zaki, S.; Ravi, V.; Schiavinato, A.; Smith, M.M.; Little, C.B. The relationship between synovial inflammation, structural pathology, and pain in post-traumatic osteoarthritis: Differential effect of stem cell and hyaluronan treatment. Arthritis Res. Ther. 2020, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Tsuji, K.; Onuma, H.; Udo, M.; Ueki, H.; Akiyama, M.; Abula, K.; Katagiri, H.; Miyatake, K.; Watanabe, T.; et al. Persistent synovial inflammation plays important roles in persistent pain development in the rat knee before cartilage degradation reaches the subchondral bone. BMC Musculoskelet. Disord. 2018, 19, 291. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Syx, D.; Tran, P.B.; Miller, R.E.; Malfait, A.-M. Peripheral Mechanisms Contributing to Osteoarthritis Pain. Curr. Rheumatol. Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, C.; Wang, Z.; Li, G.; Xiao, J. Neural and immune roles in osteoarthritis pain: Mechanisms and intervention strategies. J. Orthop. Translat. 2024, 48, 123–132. [Google Scholar] [CrossRef]
- Timur, U.; Caron, M.; Bastiaansen-Jenniskens, Y.; Welting, T.; van Rhijn, L.; van Osch, G.; Emans, P. Celecoxib-mediated reduction of prostanoid release in Hoffa’s fat pad from donors with cartilage pathology results in an attenuated inflammatory phenotype. Osteoarthr. Cartil. 2018, 26, 697–706. [Google Scholar] [CrossRef]
- Kitano, M.; Kawahata, H.; Okawa, Y.; Handa, T.; Nagamori, H.; Kitayama, Y.; Miyashita, T.; Sakamoto, K.; Fukumoto, Y.; Kudo, S. Effects of low-intensity pulsed ultrasound on the infrapatellar fat pad in knee osteoarthritis: A randomized, double blind, placebo-controlled trial. J. Phys. Ther. Sci. 2023, 35, 2022–2085. [Google Scholar] [CrossRef]
- Yoshimura, H.; Nakagawa, Y.; Muneta, T.; Koga, H. A CCL2/MCP-1 antagonist attenuates fibrosis of the infrapatellar fat pad in a rat model of arthritis. BMC Musculoskelet. Disord. 2024, 25, 674. [Google Scholar] [CrossRef]
- Radakovich, L.B.; Marolf, A.J.; Culver, L.A.; Santangelo, K.S. Calorie restriction with regular chow, but not a high-fat diet, delays onset of spontaneous osteoarthritis in the Hartley guinea pig model. Arthritis Res. Ther. 2019, 21, 145. [Google Scholar] [CrossRef]
- Cortez, M.; Carmo, L.S.; Rogero, M.M.; Borelli, P.; Fock, R.A. A High-Fat Diet Increases IL-1, IL-6, and TNF-α Production by Increasing NF-κB and Attenuating PPAR-γ Expression in Bone Marrow Mesenchymal Stem Cells. Inflammation 2013, 36, 379–386. [Google Scholar] [CrossRef]
- Clements, K.M.; Ball, A.D.; Jones, H.B.; Brinckmann, S.; Read, S.J.; Murray, F. Cellular and histopathological changes in the infrapatellar fat pad in the monoiodoacetate model of osteoarthritis pain. Osteoarthr. Cartil. 2009, 17, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Takeshima, E.; Kojima, S.; Watanabe, M.; Matsuzaki, T.; Hoso, M. Daily and short-term application of joint movement for the prevention of infrapatellar fat pad atrophy due to immobilization. J. Phys. Ther. Sci. 2019, 31, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Glinkowski, W.; Śladowski, D.; Tomaszewski, W. Molecular Mechanisms and Therapeutic Role of Intra-Articular Hyaluronic Acid in Osteoarthritis: A Precision Medicine Perspective. J. Clin. Med. 2025, 14, 2547. [Google Scholar] [CrossRef]
- Oliveira, M.Z.; Albano, M.B.; Stirma, G.A.; Namba, M.M.; Vidigal, L.; da Cunha, L.A.M. Intra-articular viscosupplementation of hyaluronic acids in an experimental osteoarthritis model. Rev. Bras. Ortop. 2018, 53, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Sundman, E.A.; Cole, B.J.; Karas, V.; Della Valle, C.; Tetreault, M.W.; Mohammed, H.O.; Fortier, L.A. The Anti-inflammatory and Matrix Restorative Mechanisms of Platelet-Rich Plasma in Osteoarthritis. Am. J. Sports Med. 2014, 42, 35–41. [Google Scholar] [CrossRef]
- Araya, N.; Miyatake, K.; Tsuji, K.; Katagiri, H.; Nakagawa, Y.; Hoshino, T.; Onuma, H.; An, S.; Nishio, H.; Saita, Y.; et al. Intra-articular Injection of Pure Platelet-Rich Plasma Is the Most Effective Treatment for Joint Pain by Modulating Synovial Inflammation and Calcitonin Gene-Related Peptide Expression in a Rat Arthritis Model. Am. J. Sports Med. 2020, 48, 2004–2012. [Google Scholar] [CrossRef]
- Du, Q.; Dickinson, A.; Nakuleswaran, P.; Maghami, S.; Alagoda, S.; Hook, A.L.; Ghaemmaghami, A.M. Targeting Macrophage Polarization for Reinstating Homeostasis following Tissue Damage. Int. J. Mol. Sci. 2024, 25, 7278. [Google Scholar] [CrossRef]
- Albarracin Melo, L.T.; Abdulkhakov, N.; Han, I.; El-Bizri, A.; Brunner-Weinzierl, M.; Schraven, B.; Simeoni, L. The immunosuppressive effect of glucocorticoids in human primary T cells is mainly mediated via a rapid inhibition of the IL-2/IL-2R signaling axis. Cell Commun. Signal. 2025, 23, 268. [Google Scholar] [CrossRef]
- Richardson, D.W.; Dodge, G.R. Dose-dependent effects of corticosteroids on the expression of matrix-related genes in normal and cytokine-treated articular chondrocytes. Inflamm. Res. 2003, 52, 39–49. [Google Scholar] [CrossRef]
- An, J.-S.; Tsuji, K.; Onuma, H.; Araya, N.; Isono, M.; Hoshino, T.; Inomata, K.; Hino, J.; Miyazato, M.; Hosoda, H.; et al. Inhibition of fibrotic changes in infrapatellar fat pad alleviates persistent pain and articular cartilage degeneration in monoiodoacetic acid-induced rat arthritis model. Osteoarthr. Cartil. 2021, 29, 380–388. [Google Scholar] [CrossRef]
- Ng, M.M.L.; Leung, M.C.P.; Poon, D.M.Y. The Effects of Electro-Acupuncture and Transcutaneous Electrical Nerve Stimulation on Patients with Painful Osteoarthritic Knees: A Randomized Controlled Trial with Follow-Up Evaluation. J. Altern. Complement. Med. 2003, 9, 641–649. [Google Scholar] [CrossRef]
- Yuan, X.-C.; Wang, Q.; Su, W.; Li, H.-P.; Wu, C.-H.; Gao, F.; Xiang, H.-C.; Zhu, H.; Lin, L.-X.; Hu, X.-F.; et al. Electroacupuncture potentiates peripheral CB2 receptor-inhibited chronic pain in a mouse model of knee osteoarthritis. J. Pain Res. 2018, 11, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, R.; Xu, B.; Yang, Y.; Wang, Y.; Xu, W.; Li, P.; Yu, A.; Ning, S.; Fu, Q.; et al. The efficacy and safety of electrical acupoint stimulation (EAS) for knee osteoarthritis (KOA): A GRADE-assessed systematic review, meta-analysis and trial sequential analysis. PLoS ONE 2025, 20, e0331568. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Yang, S.; Wen, B.; Chen, J.; Chang, J. Electroacupuncture ameliorates knee osteoarthritis in rats via inhibiting NLRP3 inflammasome and reducing pyroptosis. Mol. Pain 2023, 19, 1–12. [Google Scholar] [CrossRef]
- Taslakian, B.; Miller, L.E.; Mabud, T.S.; Macaulay, W.; Samuels, J.; Attur, M.; Alaia, E.F.; Kijowski, R.; Hickey, R.; Sista, A.K. Genicular artery embolization for treatment of knee osteoarthritis pain: Systematic review and meta-analysis. Osteoarthr. Cartil. Open 2023, 5, 100342. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.; Ahmed, S.S.; Koethe, Y.; Raskind, A.; Ahmed, O. Genicular Artery Embolization for Primary Knee Osteoarthritis. Semin. Interv. Radiol. 2022, 39, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-H.; Shen, P.-C.; Lu, C.-C.; Liu, Z.-M.; Tien, Y.-C.; Huang, P.-J.; Chou, C.-M.; Shih, C.-L. Comparison of Efficacy among Three Radiofrequency Ablation Techniques for Treating Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2021, 18, 7424. [Google Scholar] [CrossRef]
- Kidd, V.D.; Strum, S.R.; Strum, D.S.; Shah, J. Genicular Nerve Radiofrequency Ablation for Painful Knee Arthritis: The Why and the How. JBJS Essent. Surg. Tech. 2019, 9, e10. [Google Scholar] [CrossRef]
- Heard, B.J.; Solbak, N.M.; Chung, M.; Achari, Y.; Shrive, N.G.; Frank, C.B.; Hart, D.A. The infrapatellar fat pad is affected by injury induced inflammation in the rabbit knee: Use of dexamethasone to mitigate damage. Inflamm. Res. 2016, 65, 459–470. [Google Scholar] [CrossRef]
- Barton, K.I.; Chung, M.; Frank, C.B.; Shrive, N.G.; Hart, D.A. Methylprednisolone acetate mitigates IL1β induced changes in matrix metalloproteinase gene expression in skeletally immature ovine explant knee tissues. Inflamm. Res. 2021, 70, 99–107. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Q. Partial excision of infrapatellar fat pad for the treatment of knee osteoarthritis. J. Orthop. Surg. Res. 2024, 19, 631. [Google Scholar] [CrossRef]
- Afzali, M.F.; Radakovich, L.B.; Sykes, M.M.; Campbell, M.A.; Patton, K.M.; Sanford, J.L.; Vigon, N.; Ek, R.; Narez, G.E.; Marolf, A.J.; et al. Early removal of the infrapatellar fat pad/synovium complex beneficially alters the pathogenesis of moderate stage idiopathic knee osteoarthritis in male Dunkin Hartley guinea pigs. Arthritis Res. Ther. 2022, 24, 282. [Google Scholar] [CrossRef]
- Belluzzi, E.; Macchi, V.; Fontanella, C.G.; Carniel, E.L.; Olivotto, E.; Filardo, G.; Sarasin, G.; Porzionato, A.; Granzotto, M.; Pozzuoli, A.; et al. Infrapatellar Fat Pad Gene Expression and Protein Production in Patients with and without Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 6016. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Zhang, X.; Sun, M. Effects of low-intensity pulsed ultrasound on knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2022, 36, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Feltham, T.; Paudel, S.; Lobao, M.; Schon, L.; Zhang, Z. Low-Intensity Pulsed Ultrasound Suppresses Synovial Macrophage Infiltration and Inflammation in Injured Knees in Rats. Ultrasound Med. Biol. 2021, 47, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Kawahata, H.; Kudo, S. Effect of Low-Intensity Pulsed Ultrasound on Macrophage Properties and Fibrosis in the Infrapatellar Fat Pad in a Carrageenan-Induced Knee Osteoarthritis Rat Model. Cureus 2024, 16, e59246. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fan, C.; Ji, Y.; Su, Q.; Zhao, F.; Xie, C.; Chen, X.; Zhang, Y.; Chen, Y. SENP3 facilitates M1 macrophage polarization via the HIF-1α/PKM2 axis in lipopolysaccharide-induced acute lung injury. Innate Immun. 2023, 29, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.A.A.; Aboulhoda, B.E.; Abdelwahed, O.M.; Abdallah, H.; Rashed, L.; Hussein, R.E.; Sharawy, N. Low-intensity pulsed ultrasound (LIPUS) switched macrophage into M2 phenotype and mitigated necroptosis and increased HSP 70 in gentamicin-induced nephrotoxicity. Life Sci. 2023, 314, 121338. [Google Scholar] [CrossRef]
- Nakamura, T.; Fujihara, S.; Yamamoto-Nagata, K.; Katsura, T.; Inubushi, T.; Tanaka, E. Low-Intensity Pulsed Ultrasound Reduces the Inflammatory Activity of Synovitis. Ann. Biomed. Eng. 2011, 39, 2964–2971. [Google Scholar] [CrossRef]
- Qin, H.; Luo, Z.; Sun, Y.; He, Z.; Qi, B.; Chen, Y.; Wang, J.; Li, C.; Lin, W.; Han, Z.; et al. Low-intensity pulsed ultrasound promotes skeletal muscle regeneration via modulating the inflammatory immune microenvironment. Int. J. Biol. Sci. 2023, 19, 1123–1145. [Google Scholar] [CrossRef]
- Gurkan, I.; Ranganathan, A.; Yang, X.; Horton, W.E., Jr.; Todman, M.; Huckle, J.; Pleshko, N.; Spencer, R.G. Modification of osteoarthritis in the guinea pig with pulsed low-intensity ultrasound treatment. Osteoarthr. Cartil. 2010, 18, 724–733. [Google Scholar] [CrossRef]
- Liao, B.; Guan, M.; Tan, Q.; Wang, G.; Zhang, R.; Huang, J.; Liu, M.; Chen, H.; Li, K.; Bai, D.; et al. Low-intensity pulsed ultrasound inhibits fibroblast-like synoviocyte proliferation and reduces synovial fibrosis by regulating Wnt/β-catenin signaling. J. Orthop. Transl. 2021, 30, 41–50. [Google Scholar] [CrossRef]
- Yi, X.; Wu, L.; Liu, J.; Qin, Y.; Li, B.; Zhou, Q. Low-intensity pulsed ultrasound protects subchondral bone in rabbit temporomandibular joint osteoarthritis by suppressing TGF-β1/Smad3 pathway. J. Orthop. Res. 2020, 38, 2505–2512. [Google Scholar] [CrossRef]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.G.; Puthumana, J.; Coca, S.G.; Gentry, M.; Parikh, C.R. Biomarkers for the detection of renal fibrosis and prediction of renal outcomes: A systematic review. BMC Nephrol. 2017, 18, 72. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Akhmetshina, A.; Schett, G.; Distler, O. Monocyte chemoattractant proteins in the pathogenesis of systemic sclerosis. Rheumatology 2009, 48, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.H.; Masuko-Hongo, K.; Sakata, M.; Tsuruha, J.; Onuma, H.; Nakamura, H.; Aoki, H.; Kato, T.; Nishioka, K. The role of C-C chemokines and their receptors in osteoarthritis. Arthritis Rheum. 2001, 44, 1056–1070. [Google Scholar] [CrossRef]
- Miller, R.E.; Malfait, A.-M. Can we target CCR2 to treat osteoarthritis? The trick is in the timing! Osteoarthr. Cartil. 2017, 25, 799–801. [Google Scholar] [CrossRef]
- Zarebska, J.M.; Chanalaris, A.; Driscoll, C.; Burleigh, A.; Miller, R.; Malfait, A.; Stott, B.; Vincent, T. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthr. Cartil. 2017, 25, 406–412. [Google Scholar] [CrossRef]
- Longobardi, L.; Jordan, J.; Shi, X.; Renner, J.; Schwartz, T.; Nelson, A.; Barrow, D.; Kraus, V.; Spagnoli, A. Associations between the chemokine biomarker CCL2 and knee osteoarthritis outcomes: The Johnston County Osteoarthritis Project. Osteoarthr. Cartil. 2018, 26, 1257–1261. [Google Scholar] [CrossRef]
- Morrison, N.A.; Day, C.J.; Nicholson, G.C. Dominant Negative MCP-1 Blocks Human Osteoclast Differentiation. J. Cell Biochem. 2014, 115, 303–312. [Google Scholar] [CrossRef]
- Jiang, X.; Sato, T.; Yao, Z.; Keeney, M.; Pajarinen, J.; Lin, T.; Loi, F.; Egashira, K.; Goodman, S.; Yang, F. Local delivery of mutant CCL2 protein-reduced orthopaedic implant wear particle-induced osteolysis and inflammation in vivo. J. Orthop. Res. 2016, 34, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Quan, J.; Liu, Y.; Li, J.; Gong, Q.; Jiang, H. 7ND protein exerts inhibitory effects on both osteoclast differentiation in vitro and lipopolysaccharide-induced bone erosion in vivo. Mol. Med. Rep. 2020, 22, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Barboza, E.; Hudson, J.; Chang, W.; Kovats, S.; Towner, R.A.; Silasi-Mansat, R.; Lupu, F.; Kent, C.; Griffin, T.M. Profibrotic Infrapatellar Fat Pad Remodeling Without M1 Macrophage Polarization Precedes Knee Osteoarthritis in Mice With Diet-Induced Obesity. Arthritis Rheumatol. 2017, 69, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ochi, H.; Hara, Y.; Tagawa, M.; Koga, D.; Okawa, A.; Asou, Y. Initial Responses of Articular Tissues in a Murine High-Fat Diet-Induced Osteoarthritis Model: Pivotal Role of the IPFP as a Cytokine Fountain. PLoS ONE 2013, 8, e60706. [Google Scholar] [CrossRef]
- Chang, W.; DeMoe, J.; Kent, C.; Kovats, S.; Garteiser, P.; Doblas, S.; Towner, R.; Griffin, T. Infrapatellar Fat Pad hypertrophy without inflammation in a diet-induced mouse model of obeshy and osteoarthritis. Osteoarthr. Cartil. 2011, 19, S66. [Google Scholar] [CrossRef]
- Chuckpaiwong, B.; Charles, H.C.; Kraus, V.B.; Guilak, F.; Nunley, J.A. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J. Orthop. Res. 2010, 28, 1149–1154. [Google Scholar] [CrossRef]
- Han, W.; Cai, S.; Liu, Z.; Jin, X.; Wang, X.; Antony, B.; Cao, Y.; Aitken, D.; Cicuttini, F.; Jones, G.; et al. Infrapatellar fat pad in the knee: Is local fat good or bad for knee osteoarthritis? Arthritis Res. Ther. 2014, 16, R145. [Google Scholar] [CrossRef]
- Kurt, M.; Öner, A.Y.; Uçar, M.; Aladag Kurt, S. The relationship between patellofemoral arthritis and fat tissue volume, body mass index and popliteal artery intima-media thickness through 3T knee MRI. Turk. J. Med. Sci. 2019, 49, 844–853. [Google Scholar] [CrossRef]
- Diepold, J.; Ruhdorfer, A.; Dannhauer, T.; Wirth, W.; Steidle, E.; Eckstein, F. Sex-differences of the healthy infra-patellar (Hoffa) fat pad in relation to intermuscular and subcutaneous fat content—Data from the Osteoarthritis Initiative. Ann. Anat.—Anat. Anz. 2015, 200, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.M.; Sopher, R.; Tullie, S.; Amis, A.A.; Ball, S.; Williams, A. The infrapatellar fat pad is a dynamic and mobile structure, which deforms during knee motion, and has proximal extensions which wrap around the patella. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3515–3524. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Wulidasari, E.; Brady, S.R.E.; Wang, Y.; Wluka, A.E.; Ding, C.; Giles, G.G.; Cicuttini, F.M. A large infrapatellar fat pad protects against knee pain and lateral tibial cartilage volume loss. Arthritis Res. Ther. 2015, 17, 318. [Google Scholar] [CrossRef]
- Takahashi, I.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Disuse histological changes of an unloading environment on joint components in rat knee joints. Osteoarthr. Cartil. Open 2019, 1, 100008. [Google Scholar] [CrossRef]
- Watanabe, M.; Hoso, M.; Hibino, I.; Matsuzaki, T.; Kojima, S. Histopathological Changes of Joint Capsule after Joint Immobility Compared with Aging in Rats. J. Phys. Ther. Sci. 2010, 22, 369–374. [Google Scholar] [CrossRef]
- Bastow, E.R.; Byers, S.; Golub, S.B.; Clarkin, C.E.; Pitsillides, A.A.; Fosang, A.J. Hyaluronan synthesis and degradation in cartilage and bone. Cell. Mol. Life Sci. 2008, 65, 395–413. [Google Scholar] [CrossRef]
- Qu, Z.; Koga, H.; Tsuji, K.; Tang, G.; Yang, Y.; Yoshihara, A.; Katakura, M.; Katagiri, H.; Miyatake, K.; Nakamura, T.; et al. Hyaluronic acid sheet transplantation attenuates infrapatellar fat pad fibrosis and pain in a rat arthritis model. J. Orthop. Res. 2023, 41, 2442–2454. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Lin, C.-M.; Huang, C.-F.; Hsu, W.-C.; Lee, C.-H.; Ou, K.-L.; Dubey, N.K.; Deng, W.-P. Functional Recovery in Osteoarthritic Chondrocytes Through Hyaluronic Acid and Platelet-Rich Plasma–Inhibited Infrapatellar Fat Pad Adipocytes. Am. J. Sports Med. 2016, 44, 2696–2705. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Buda, R.; Timoncini, A.; Di Martino, A.; Cenacchi, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 528–535. [Google Scholar] [CrossRef]
- Cassuto, J.; Folestad, A.; Göthlin, J.; Malchau, H.; Kärrholm, J. VEGF-A,-C,-D, VEGFR1,-2,-3, PDGF-BB and FGF-2 join forces to induce vascular and lymphatic angiogenesis during bone healing of hip implants. Bone Rep. 2025, 26, 101856. [Google Scholar] [CrossRef]
- Wen, C.; Xu, L.; Xu, X.; Wang, D.; Liang, Y.; Duan, L. Insulin-like growth factor-1 in articular cartilage repair for osteoarthritis treatment. Arthritis Res. Ther. 2021, 23, 277. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Vogel, S. Platelets in tissue repair: Control of apoptosis and interactions with regenerative cells. Blood 2013, 122, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- Burchard, R.; Huflage, H.; Soost, C.; Richter, O.; Bouillon, B.; Graw, J.A. Efficiency of platelet-rich plasma therapy in knee osteoarthritis does not depend on level of cartilage damage. J. Orthop. Surg. Res. 2019, 14, 153. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Zhang, H.; Zhang, X.; Liu, R.; Li, X.; Wang, J.; Li, T. Platelet-rich plasma relieves inflammation and pain by regulating M1/M2 macrophage polarization in knee osteoarthritis rats. Sci. Rep. 2025, 15, 12805. [Google Scholar] [CrossRef]
- Yan, X.; Ye, Y.; Wang, L.; Xue, J.; Shen, N.; Li, T. Platelet-rich plasma alleviates neuropathic pain in osteoarthritis by downregulating microglial activation. BMC Musculoskelet. Disord. 2024, 25, 331. [Google Scholar] [CrossRef]
- Jørgensen, T.S.; Graven-Nielsen, T.; Ellegaard, K.; Danneskiold-Samsøe, B.; Bliddal, H.; Henriksen, M. Intra-Articular Analgesia and Steroid Reduce Pain Sensitivity in Knee OA Patients: An Interventional Cohort Study. Pain. Res. Treat. 2014, 2014, 710490. [Google Scholar] [CrossRef]
- Bensa, A.; Salerno, M.; Boffa, A.; de Girolamo, L.; Laver, L.; Magalon, J.; Sánchez, M.; Tischer, T.; Filardo, G. Corticosteroid injections for the treatment of osteoarthritis present a wide spectrum of effects ranging from detrimental to disease-modifying: A systematic review of preclinical evidence by the ESSKA Orthobiologic Initiative. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 2725–2745. [Google Scholar] [CrossRef]
- Wen, F.-Q.; Kohyama, T.; Sköld, C.M.; Zhu, Y.K.; Liu, X.; Romberger, D.J.; Stoner, J.; Rennard, S.I. Glucocorticoids Modulate TGF-β Production by Human Fetal Lung Fibroblasts. Inflammation 2003, 27, 9–19. [Google Scholar] [CrossRef]
- Wen, F.-Q.; Sköld, C.M.; Liu, X.-D.; Ertl, R.F.; Zhu, Y.K.; Kohyama, T.; Wang, H.; Rennard, S.I. Glucocorticoids and TGF-β1 Synergize in Augmenting Fibroblast Mediated Contraction of Collagen Gels. Inflammation 2001, 25, 109–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, G.; Zheng, P.; Zhu, Z.; Chen, H.; Fan, T.; Jiang, L.; Ding, C. Efficacy and safety of GLucocorticoid injections into InfrapaTellar faT pad in patients with knee ostEoarthRitiS: A randomized clinical trial. Osteoarthr. Cartil. 2025, 33, S223. [Google Scholar] [CrossRef]
- Soeki, T.; Kishimoto, I.; Okumura, H.; Tokudome, T.; Horio, T.; Mori, K.; Kangawa, K. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nojiri, T.; Hino, J.; Hosoda, H.; Miura, K.; Shintani, Y.; Inoue, M.; Zenitani, M.; Takabatake, H.; Miyazato, M.; et al. C-type natriuretic peptide ameliorates pulmonary fibrosis by acting on lung fibroblasts in mice. Respir. Res. 2016, 17, 19. [Google Scholar] [CrossRef]
- Hahm, T.S. Electroacupuncture. Korean J. Anesthesiol. 2009, 57, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xing, C.; Wan, Q.; Guo, G.; Li, W. Electroacupuncture superiority in knee osteoarthritis: A meta-analysis of four acupuncture techniques. Front. Med. 2025, 12, 1563715. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liang, Y.; Luo, K.-T.; Shao, X.-M.; Tu, M.-Q.; Wu, X.-T.; Liu, F.; Li, X.-W.; Chen, Y.-D.; Zhang, Q.-F.; et al. Efficacy of Different Acupuncture Techniques for Pain and Dysfunction in Patients with Knee Osteoarthritis: A Randomized Controlled Trial. Pain. Ther. 2025, 14, 737–751. [Google Scholar] [CrossRef]
- Nemschak, G.; Pretterklieber, M.L. The Patellar Arterial Supply via the Infrapatellar Fat Pad (of Hoffa): A Combined Anatomical and Angiographical Analysis. Anat. Res. Int. 2012, 2012, 713838. [Google Scholar] [CrossRef]
- Gallagher, J.; Tierney, P.; Murray, P.; O’Brien, M. The infrapatellar fat pad: Anatomy and clinical correlations. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 268–272. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Blakeney, W.G.; Soares, J. Selective Genicular Artery Embolization in the Management of Osteoarthritic Knee Pain—A Narrative Review. J. Clin. Med. 2024, 13, 3256. [Google Scholar] [CrossRef]
- Sun, C.; Chen, Y.; Gao, Z.; Wu, L.; Lu, R.; Zhao, C.; Yang, H.; Chen, Y. Genicular Artery Embolization for the Treatment of Knee Pain Secondary to Mild to Severe Knee Osteoarthritis: One Year Clinical Outcomes. Eur. J. Radiol. 2024, 175, 111443. [Google Scholar] [CrossRef]
- Little, M.W.; O’grady, A.; Briggs, J.; Gibson, M.; Speirs, A.; Al-Rekabi, A.; Yoong, P.; Ariyanayagam, T.; Davies, N.; Tayton, E.; et al. Genicular Artery embolisation in Patients with Osteoarthritis of the Knee (GENESIS) Using Permanent Microspheres: Long-Term Results. Cardiovasc. Interv. Radiol. 2024, 47, 1750–1762. [Google Scholar] [CrossRef]
- Fonkoué, L.; Behets, C.; Kouassi, J.K.; Coyette, M.; Detrembleur, C.; Thienpont, E.; Cornu, O. Distribution of sensory nerves supplying the knee joint capsule and implications for genicular blockade and radiofrequency ablation: An anatomical study. Surg. Radiol. Anat. 2019, 41, 1461–1471. [Google Scholar] [CrossRef]
- Jamison, D.E.; Cohen, S.P. Radiofrequency Techniques to Treat Chronic Knee Pain: A Comprehensive Review of Anatomy, Effectiveness, Treatment Parameters, and Patient Selection. J. Pain Res. 2018, 11, 1879–1888. [Google Scholar] [CrossRef]
- Kulhari, S.; Eghrari, N.; Chen, M.; Grandhi, N.; Kim, C. Infrapatellar Branch of the Saphenous Nerve Radiofrequency Ablation for Refractory Knee Pain: Case Series. Pain Med. Case Rep. 2024, 8, 195–197. [Google Scholar]
- Karaman, H.; Tüfek, A.; Kavak, G.Ö.; Yildirim, Z.B.; Uysal, E.; Çelik, F.; Kaya, S. Intra-articularly applied pulsed radiofrequency can reduce chronic knee pain in patients with osteoarthritis. J. Chin. Med. Assoc. 2011, 74, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.D.; Hawkins, J.K. Analgesia and Improved Performance in a Patient Treated by Cooled Radiofrequency for Pain and Dysfunction Postbilateral Total Knee Replacement. Pain Pract. 2015, 15, 12292. [Google Scholar] [CrossRef] [PubMed]
- Soetjahjo, B.; Adriansyah, D.; Yudistira, M.B.; Rahman, A.N.; Herman, H.; Diwan, S. Systematic Review the Analgesic Effectiveness of Genicular Nerve-targeted Cooled and Pulsed Radiofrequency Ablation for Osteoarthritis Knee Pain: A Systematic Review and Meta-Analysis. [Online]. Available online: www.painphysicianjournal.com (accessed on 1 September 2025).
- Bohnsack, M.; Hurschler, C.; Demirtas, T.; Rühmann, O.; Stukenborg-Colsman, C.; Wirth, C. Infrapatellar fat pad pressure and volume changes of the anterior compartment during knee motion: Possible clinical consequences to the anterior knee pain syndrome. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 135–141. [Google Scholar] [CrossRef]
- Ali, M.M.; Phillips, S.A.; Mahmoud, A.M. HIF1α/TET1 Pathway Mediates Hypoxia-Induced Adipocytokine Promoter Hypomethylation in Human Adipocytes. Cells 2020, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, P.; Yao, H.; Yang, S.; Tu, B.; Kong, L.; Ning, R. Observation of the Effects of Infrapatellar Fat Pad Excision on the Inflammatory Progression of Knee Osteoarthritis in Mice. J. Inflamm. Res. 2025, 18, 6653–6672. [Google Scholar] [CrossRef]
- Yao, B.; Samuel, L.T.; Acuña, A.J.; Faour, M.; Roth, A.; Kamath, A.F.; Mont, M.A. Infrapatellar Fat Pad Resection or Preservation during Total Knee Arthroplasty: A Systematic Review. J. Knee Surg. 2021, 34, 415–421. [Google Scholar] [CrossRef]
- Rajbhandari, A.; Banskota, B.; Bhusal, R.; Banskota, A.K. Effect of Infrapatellar Fat Pad Preservation vs Resection on Clinical Outcomes After Total Knee Arthroplasty in Patient with End-Stage Osteoarthritis. Indian. J. Orthop. 2023, 57, 863–867. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Sun, X.; Xing, Y.; Wang, X.; Yang, Q. Immunomodulation of MSCs and MSC-Derived Extracellular Vesicles in Osteoarthritis. Front. Bioeng. Biotechnol. 2020, 8, 575057. [Google Scholar] [CrossRef]
- Kouroupis, D.; Kaplan, L.D.; Best, T.M. Human infrapatellar fat pad mesenchymal stem cells show immunomodulatory exosomal signatures. Sci. Rep. 2022, 12, 3609. [Google Scholar] [CrossRef]
- Hindle, P.; Khan, N.; Biant, L.; Péault, B. The Infrapatellar Fat Pad as a Source of Perivascular Stem Cells with Increased Chondrogenic Potential for Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 77–87. [Google Scholar] [CrossRef]
- Chen, H.-H.; Chen, Y.-C.; Yu, S.-N.; Lai, W.-L.; Shen, Y.-S.; Shen, P.-C.; Lin, S.-H.; Chang, C.-H.; Lee, S.-M. Infrapatellar fat pad-derived mesenchymal stromal cell product for treatment of knee osteoarthritis: A first-in-human study with evaluation of the potency marker. Cytotherapy 2022, 24, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Piccione, M.; Belluzzi, E.; Petrelli, L.; Pozzuoli, A.; Ramonda, R.; Rossato, M.; Favero, M.; Ruggieri, P.; et al. Infrapatellar Fat Pad Stem Cells Responsiveness to Microenvironment in Osteoarthritis: From Morphology to Function. Front. Cell Dev. Biol. 2019, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Choi, Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef]
- Vahedi, P.; Moghaddamshahabi, R.; Webster, T.J.; Koyuncu, A.C.C.; Ahmadian, E.; Khan, W.S.; Mohamed, A.J.; Eftekhari, A. The Use of Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells in Articular Cartilage Regeneration: A Review. Int. J. Mol. Sci. 2021, 22, 9215. [Google Scholar] [CrossRef]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.-N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Pei, M. Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential. Biomaterials 2017, 117, 10–23. [Google Scholar] [CrossRef]
- Meng, H.; Lu, V.; Khan, W. Adipose Tissue-Derived Mesenchymal Stem Cells as a Potential Restorative Treatment for Cartilage Defects: A PRISMA Review and Meta-Analysis. Pharmaceuticals 2021, 14, 1280. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Fan, Y.; Song, X.; Wu, J.; Fu, Z.; Li, T.; Huang, Y.; Tang, Z.; Meng, S.; et al. Tropoelastin improves adhesion and migration of intra-articular injected infrapatellar fat pad MSCs and reduces osteoarthritis progression. Bioact. Mater. 2022, 10, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Song, X.; Li, T.; Xiao, J.; Chen, Y.; Gong, X.; Zeng, W.; Yang, L.; Chen, C. Pellet coculture of osteoarthritic chondrocytes and infrapatellar fat pad-derived mesenchymal stem cells with chitosan/hyaluronic acid nanoparticles promotes chondrogenic differentiation. Stem Cell Res. Ther. 2017, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Wu, K.-C.; Chou, H.-L.; Hung, W.-T.; Liu, H.-W.; Chu, T.-Y. Human Infrapatellar Fat Pad-Derived Stromal Cells have more Potent Differentiation Capacity than other Mesenchymal Cells and can be Enhanced by Hyaluronan. Cell Transplant. 2015, 24, 1221–1232. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, Y.A.; Sun, Y.; Zhou, S.; Zhang, X.-B.; Pei, M. Stem cells immortalized by hTERT perform differently from those immortalized by SV40LT in proliferation, differentiation, and reconstruction of matrix microenvironment. Acta Biomater. 2021, 136, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, X.; Qu, Z.; Hao, J.; Zhang, W. Hypoxia-Preconditioned Extracellular Vesicles from Mesenchymal Stem Cells Improve Cartilage Repair in Osteoarthritis. Membranes 2022, 12, 225. [Google Scholar] [CrossRef]
- Duan, A.; Shen, K.; Li, B.; Li, C.; Zhou, H.; Kong, R.; Shao, Y.; Qin, J.; Yuan, T.; Ji, J.; et al. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res. Ther. 2021, 12, 427. [Google Scholar] [CrossRef]
- Wu, J.; Wu, J.; Xiang, W.; Gong, Y.; Feng, D.; Fang, S.; Wu, Y.; Liu, Z.; Li, Y.; Chen, R.; et al. Engineering exosomes derived from TNF-α preconditioned IPFP-MSCs enhance both yield and therapeutic efficacy for osteoarthritis. J. Nanobiotechnol. 2024, 22, 555. [Google Scholar] [CrossRef]
- Aikawa, J.; Uchida, K.; Takano, S.; Inoue, G.; Iwase, D.; Miyagi, M.; Mukai, M.; Shoji, S.; Sekiguchi, H.; Takaso, M. Regulation of calcitonin gene-related peptide expression through the COX-2/mPGES-1/PGE2 pathway in the infrapatellar fat pad in knee osteoarthritis. Lipids Health Dis. 2018, 17, 215. [Google Scholar] [CrossRef]
- Bolia, I.K.; Mertz, K.; Faye, E.; Sheppard, J.; Telang, S.; Bogdanov, J.; Hasan, L.K.; Haratian, A.; Evseenko, D.; E Weber, A.; et al. Cross-Communication Between Knee Osteoarthritis and Fibrosis: Molecular Pathways and Key Molecules. Open Access J. Sports Med. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Pu, H.; Gao, C.; Zou, Y.; Zhao, L.; Li, G.; Liu, C.; Zhao, L.; Zheng, M.; Sheng, G.; Sun, X.; et al. Single cell transcriptome profiling of infrapatellar fat pad highlights the role of interstitial inflammatory fibroblasts in osteoarthritis. Int. Immunopharmacol. 2024, 131, 111888. [Google Scholar] [CrossRef] [PubMed]
- Kouroupis, D.; Best, T.M.; Kaplan, L.D.; Correa, D.; Griswold, A.J. Single-Cell RNA-Sequencing Identifies Infrapatellar Fat Pad Macrophage Polarization in Acute Synovitis/Fat Pad Fibrosis and Cell Therapy. Bioengineering 2021, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Kawahata, H.; Aoki, M.; Kudo, S. Inhibitory effect of low-intensity pulsed ultrasound on the fibrosis of the infrapatellar fat pad through the regulation of HIF-1α in a carrageenan-induced knee osteoarthritis rat model. Biomed. Rep. 2022, 17, 79. [Google Scholar] [CrossRef]
- José, F.S.D. Lana Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J. Stem Cells Regen. Med. 2016, 12, 69–78. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Pei, M. Comparative advantages of infrapatellar fat pad: An emerging stem cell source for regenerative medicine. Rheumatology 2018, 57, 2072–2086. [Google Scholar] [CrossRef]
- Kouroupis, D.; Willman, M.A.; Best, T.M.; Kaplan, L.D.; Correa, D. Infrapatellar fat pad-derived mesenchymal stem cell-based spheroids enhance their therapeutic efficacy to reverse synovitis and fat pad fibrosis. Stem Cell Res. Ther. 2021, 12, 44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).