Chitosan Nanoformulations of Mycosporine-like Amino Acid (MAA)-Rich Extracts from Mazzaella laminarioides Effectively Protect Human Keratinocytes Against UVA Radiation Damage
Abstract
1. Introduction
2. Results
2.1. Extraction and Analysis of the MAA Content
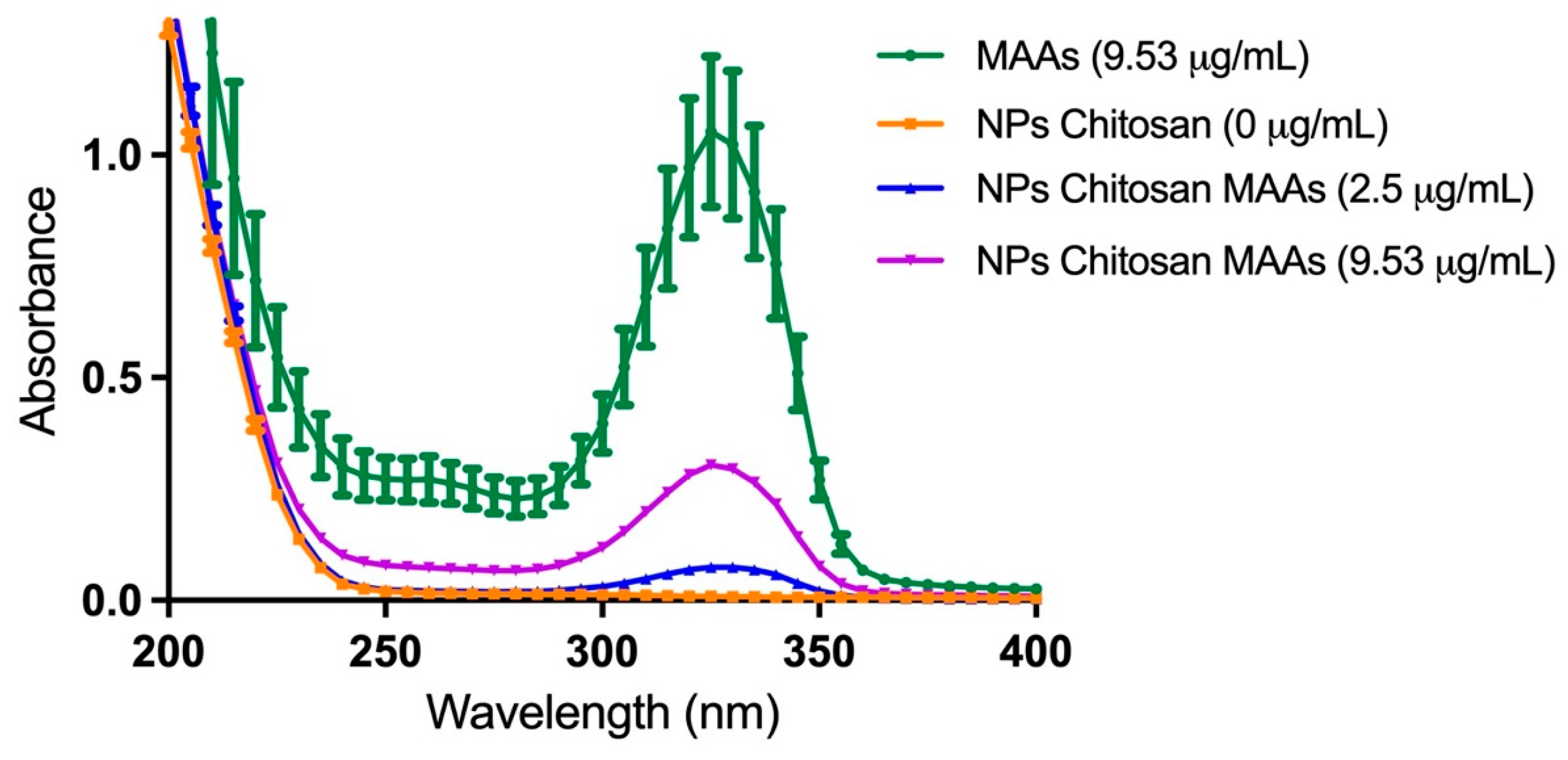
2.2. Characterization of the Aqueous MAA-Rich Extracts
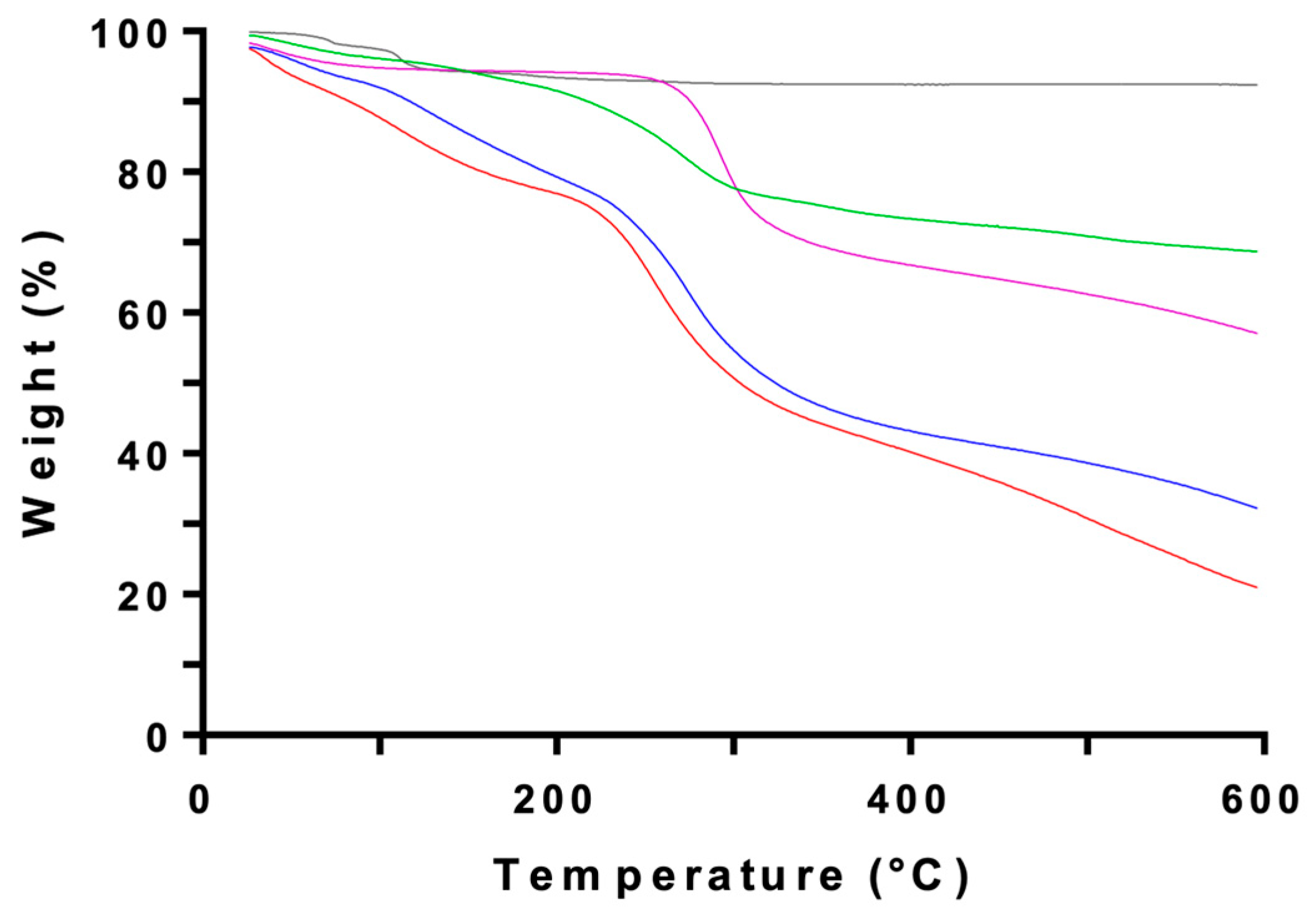
2.3. Nanoencapsulation of the MAA-Rich Extracts in Chitosan
2.4. In Vitro Photoprotection Experiments
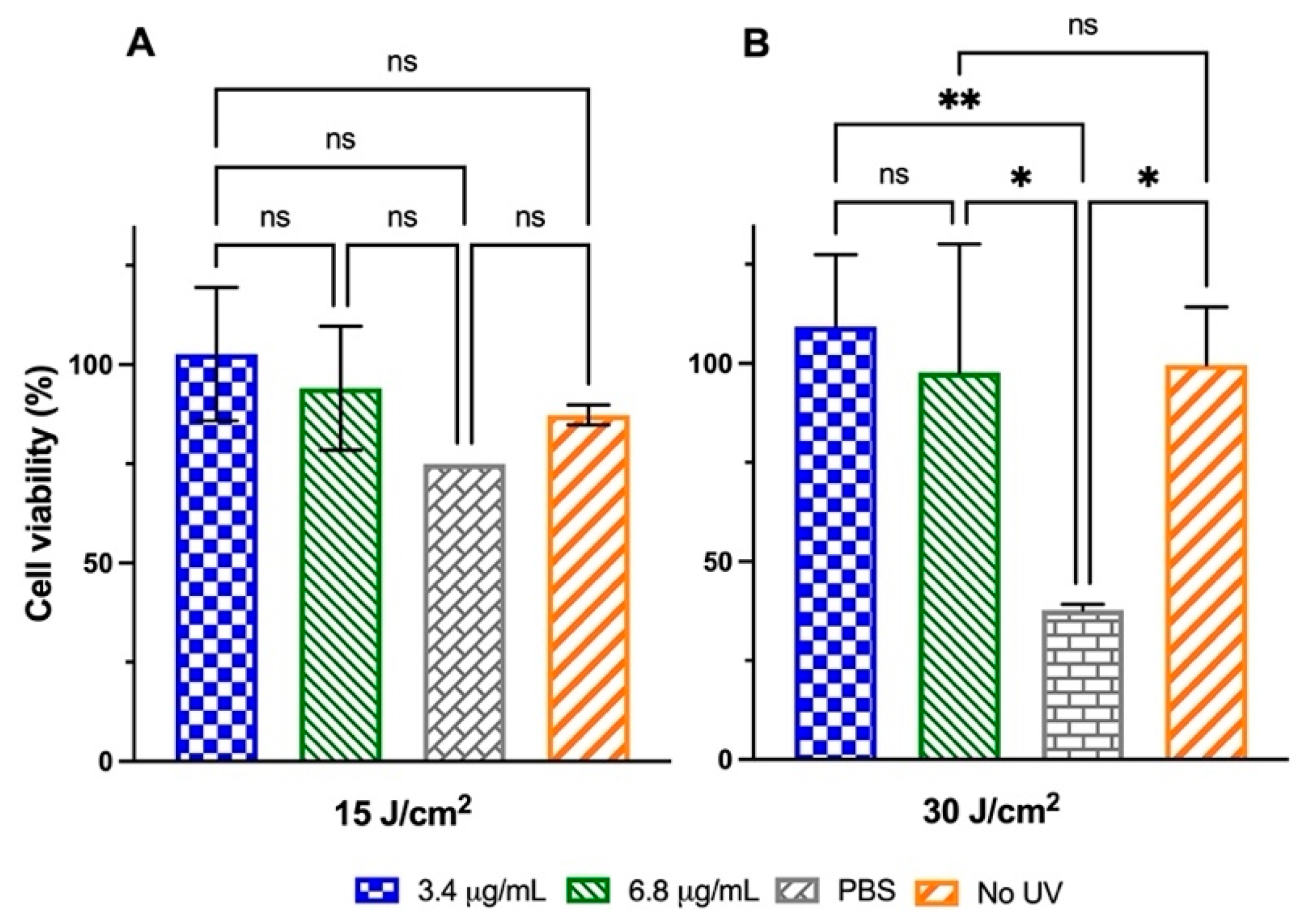
2.4.1. HaCaT Cells Exposure to Low-to-Medium UVA Radiation Doses
2.4.2. HaCaT Cells Exposure to High UVA Radiation Doses
2.5. Expression Analysis of Genes Associated with Photo-Oxidative Damage
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Reagents and Chemicals
4.3. Preparation and Analysis of Algal Extracts
4.4. Determination of Total Phenolic Content
4.5. HPLC Determination of the MAAs in the Algal Extract
4.6. Preparation of Chitosan-TPP Nanoformulations
4.7. Instrumental Characterization of CSNFs
4.8. ORAC Antioxidant Assay
4.9. Cell Culture
4.10. In Vitro UVA Irradiation
4.11. Cell Viability Assay
4.12. RNA Extraction and PCR Assays
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAAs | Mycosporine-like amino acids |
| UVR | ultraviolet radiation |
| CS | Chitosan |
| TPP | Sodium tripolyphosphate |
| CSNPs | Chitosan nanoparticles |
| ROS | Reactive oxygen species |
References
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef]
- Autier, P.; Doré, J.-F.; Eggermont, A.M.M.; Coebergh, J.W. Epidemiological Evidence That UVA Radiation Is Involved in the Genesis of Cutaneous Melanoma. Curr. Opin. Oncol. 2011, 23, 189–196. [Google Scholar] [CrossRef]
- Fadadu, R.P.; Wei, M.L. Ultraviolet A Radiation Exposure and Melanoma: A Review. Melanoma Res. 2022, 32, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kamenisch, Y.; Ivanova, I.; Drexler, K.; Berneburg, M. UVA, Metabolism and Melanoma: UVA Makes Melanoma Hungry for Metastasis. Exp. Dermatol. 2018, 27, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and Costs of Skin Cancer Treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, C.; Mention, M.M.; Brunissen, F.; Balaguer, P.; Allais, F. Innovative Bio-Based Organic UV-A and Blue Light Filters from Meldrum’s Acid. Molecules 2020, 25, 2178. [Google Scholar] [CrossRef]
- Andrady, A.; Aucamp, P.; Austin, A.; Bais, A.; Ballaré, C.; Barnes, P.; Bernhard, G.; Björn, L.; Bornman, J.; Congdon, N.; et al. Environmental Effects of Ozone Depletion and Its Interactions with Climate Change: Progress Report, 2016. Photochem. Photobiol. Sci. 2017, 16, 107–145. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Alonso, M.B.; Bertozzi, C.P.; Marigo, J.; Barbosa, L.; Cremer, M.; Secchi, E.R.; Domit, C.; Azevedo, A.; Lailson-Brito, J.; et al. First Determination of UV Filters in Marine Mammals. Octocrylene Levels in Franciscana Dolphins. Environ. Sci. Technol. 2013, 47, 5619–5625. [Google Scholar] [CrossRef]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The Mycosporine-like Amino Acids Porphyra-334 and Shinorine Are Antioxidants and Direct Antagonists of Keap1-Nrf2 Binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens Cause Coral Bleaching by Promoting Viral Infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Gries, T.; Fent, K. The Ultraviolet Filter 3-Benzylidene Camphor Adversely Affects Reproduction in Fathead Minnow (Pimephales Promelas). Toxicol. Sci. Off. J. Soc. Toxicol. 2006, 93, 311–321. [Google Scholar] [CrossRef]
- Weisbrod, C.J.; Kunz, P.Y.; Zenker, A.K.; Fent, K. Effects of the UV Filter Benzophenone-2 on Reproduction in Fish. Toxicol. Appl. Pharmacol. 2007, 225, 255–266. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-Based Active Photoprotectants for Sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Antioxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.-K. Photoprotective Substances Derived from Marine Algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N. Recent Advances in the Discovery of Novel Marine Natural Products and Mycosporine-like Amino Acid UV-Absorbing Compounds. Appl. Microbiol. Biotechnol. 2021, 105, 7053–7067. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- de la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant Activity of Mycosporine-like Amino Acids Isolated from Three Red Macroalgae and One Marine Lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Whittock, A.L.; Woolley, J.M.; Auckloo, N.; Corre, C.; Stavros, V.G. Investigating the Ultrafast Dynamics and Long-Term Photostability of an Isomer Pair, Usujirene and Palythene, from the Mycosporine-like Amino Acid Family. Molecules 2022, 27, 2272. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.-S.; Seo, H.H.; Song, M.Y.; Kulkarni, A.; Choi, Y.-H.; Kim, K.W.; Moh, S.H. Antiaging Effects of Algae-Derived Mycosporine-Like Amino Acids (MAAs) on Skin. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–8. ISBN 978-3-642-27814-3. [Google Scholar]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.H.; Nam, T.-J. Protective Effect of Porphyra-334 on UVA-Induced Photoaging in Human Skin Fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Suh, S.-S.; Oh, S.K.; Lee, S.G.; Kim, I.-C.; Kim, S. Porphyra-334, a Mycosporine-like Amino Acid, Attenuates UV-Induced Apoptosis in HaCaT Cells. Acta Pharm. 2017, 67, 257–264. [Google Scholar] [CrossRef]
- Rui, Y.; Zhaohui, Z.; Wenshan, S.; Bafang, L.; Hu, H. Protective Effect of MAAs Extracted from Porphyra Tenera against UV Irradiation-Induced Photoaging in Mouse Skin. J. Photochem. Photobiol. B 2019, 192, 26–33. [Google Scholar] [CrossRef]
- Wang, K.; Deng, Y.; He, Y.; Cao, J.; Zhang, L.; Qin, L.; Qu, C.; Li, H.; Miao, J. Protective Effect of Mycosporine-like Amino Acids Isolated from an Antarctic Diatom on UVB-Induced Skin Damage. Int. J. Mol. Sci. 2023, 24, 15055. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. New Insights on Unique Features and Role of Nanostructured Materials in Cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Knez, Ž.; Leitgeb, M. Sustainable Biodegradable Biopolymer-Based Nanoparticles for Healthcare Applications. Int. J. Mol. Sci. 2023, 24, 3188. [Google Scholar] [CrossRef]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan Nanoparticles for Enhancing Drugs and Cosmetic Components Penetration through the Skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.L.L.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.; Labidi, J.; Bulone, V. Exploiting Mycosporines as Natural Molecular Sunscreens for the Fabrication of UV-Absorbing Green Materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; Farag, M.A.; Kontominas, M.G.; Shakour, Z.T.; Ramadan, A.R. Nanoencapsulated Extract of a Red Seaweed (Rhodophyta) Species as a Promising Source of Natural Antioxidants. ACS Omega 2022, 7, 6539–6548. [Google Scholar] [CrossRef]
- Abdel-Aal, A.; El-khayat, Z.; Mohamed, N.; Rostom, M.; Tolba, E.; Galal El-Din Shams El-Din, N.; Mettwally, W.S.A.; Hamdy, A.A. Chitosan Nanoencapsulation of Turbinaria Triquetra Metabolites in the Management of Podocyturia in Nephrotoxic Rats. Sci. Rep. 2025, 15, 631. [Google Scholar] [CrossRef]
- Kaushalya, K.G.D.; Gunathilake, K.D.P.P. Encapsulation of Phlorotannins from Edible Brown Seaweed in Chitosan: Effect of Fortification on Bioactivity and Stability in Functional Foods. Food Chem. 2022, 377, 132012. [Google Scholar] [CrossRef]
- Tong, T.; Liu, X.; Yu, C. Extraction and Nano-Sized Delivery Systems for Phlorotannins to Improve Its Bioavailability and Bioactivity. Mar. Drugs 2021, 19, 625. [Google Scholar] [CrossRef]
- Panwar, R.; Pemmaraju, S.C.; Sharma, A.K.; Pruthi, V. Efficacy of Ferulic Acid Encapsulated Chitosan Nanoparticles against Candida Albicans Biofilm. Microb. Pathog. 2016, 95, 21–31. [Google Scholar] [CrossRef]
- Pattnaik, S.; Barik, S.; Muralitharan, G.; Busi, S. Ferulic Acid Encapsulated Chitosan--tripolyphosphate Nanoparticles Attenuate Quorum Sensing Regulated Virulence and Biofilm Formation in Pseudomonas Aeruginosa PAO1. IET Nanobiotech. 2018, 12, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Spósito, L.; Fonseca, D.; Carvalho, S.G.; Sábio, R.M.; Marena, G.D.; Bauab, T.M.; Meneguin, A.B.; Parreira, P.; Martins, M.C.L.; Chorilli, M. Engineering Resveratrol-Loaded Chitosan Nanoparticles for Potential Use against Helicobacter Pylori Infection. Eur. J. Pharm. Biopharm. 2024, 199, 114280. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, P.; Gómez, I.; Figueroa, F.L.; Ulloa, N.; Morales, V.; Lovengreen, C. Ultraviolet-Absorbing Mycosporine-like Amino Acids in Red Macroalgae from Chile. Bot. Mar. 2004, 47, 21–29. [Google Scholar] [CrossRef]
- Zwerger, M.; Schwaiger, S.; Ganzera, M. Efficient Isolation of Mycosporine-Like Amino Acids from Marine Red Algae by Fast Centrifugal Partition Chromatography. Mar. Drugs 2022, 20, 106. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Mohamed, M.I.; Ahmed, H.Y.; Elaasser, M.M.; Kandile, N.G. Fabrication and Characterization of Unique Sustain Modified Chitosan Nanoparticles for Biomedical Applications. Sci. Rep. 2024, 14, 13869. [Google Scholar] [CrossRef]
- Ali, M.E.A.; Aboelfadl, M.M.S.; Selim, A.M.; Khalil, H.F.; Elkady, G.M. Chitosan Nanoparticles Extracted from Shrimp Shells, Application for Removal of Fe(II) and Mn(II) from Aqueous Phases. Sep. Sci. Technol. 2018, 53, 2870–2881. [Google Scholar] [CrossRef]
- Kaur, I.; Goyal, D.; Agnihotri, S. Formulation of Cartap Hydrochloride Crosslinked Chitosan Tripolyphosphate Nanospheres and Its Characterization. Colloid. Polym. Sci. 2021, 299, 1407–1418. [Google Scholar] [CrossRef]
- Alehosseini, E.; Shahiri Tabarestani, H.; Kharazmi, M.S.; Jafari, S.M. Physicochemical, Thermal, and Morphological Properties of Chitosan Nanoparticles Produced by Ionic Gelation. Foods 2022, 11, 3841. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Ferreira, S.; Castillo, O.S.; Madera-Santana, J.T.; Mendoza-García, D.A.; Núñez-Colín, C.A.; Grijalva-Verdugo, C.; Villa-Lerma, A.G.; Morales-Vargas, A.T.; Rodríguez-Núñez, J.R. Production and Physicochemical Characterization of Chitosan for the Harvesting of Wild Microalgae Consortia. Biotechnol. Rep. 2020, 28, e00554. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.I.; Young, A.R. Ultraviolet Radiation-Induced Erythema in Human Skin. Methods 2002, 28, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Chadwick, C.A.; Harrison, G.I.; Nikaido, O.; Ramsden, J.; Potten, C.S. The Similarity of Action Spectra for Thymine Dimers in Human Epidermis and Erythema Suggests That DNA Is the Chromophore for Erythema. J. Investig. Dermatol. 1998, 111, 982–988. [Google Scholar] [CrossRef]
- Chaves-Peña, P.; de la Coba, F.; Figueroa, F.L.; Korbee, N. Quantitative and Qualitative HPLC Analysis of Mycosporine-Like Amino Acids Extracted in Distilled Water for Cosmetical Uses in Four Rhodophyta. Mar. Drugs 2019, 18, 27. [Google Scholar] [CrossRef]
- Sun, Y.; Han, X.; Hu, Z.; Cheng, T.; Tang, Q.; Wang, H.; Deng, X.; Han, X. Extraction, Isolation and Characterization of Mycosporine-like Amino Acids from Four Species of Red Macroalgae. Mar. Drugs 2021, 19, 615. [Google Scholar] [CrossRef]
- Kim, S.; You, D.H.; Han, T.; Choi, E.-M. Modulation of Viability and Apoptosis of UVB-Exposed Human Keratinocyte HaCaT Cells by Aqueous Methanol Extract of Laver (Porphyra Yezoensis). J. Photochem. Photobiol. B 2014, 141, 301–307. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Syafitri, S.M.; Geraldine, B.A.F.D.; Hamdin, C.D.; Frediansyah, A.; Miyake, M.; Kobayashi, D.; Hazama, A.; Sunarpi, H. UVA Photoprotective Activity of Brown Macroalgae Sargassum Cristafolium. Biomedicines 2019, 7, 77. [Google Scholar] [CrossRef]
- Carpena, M.; Garcia-Perez, P.; Garcia-Oliveira, P.; Chamorro, F.; Otero, P.; Lourenço-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Biological Properties and Potential of Compounds Extracted from Red Seaweeds. Phytochem. Rev. 2022, 1, 1–32. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative Study on Skin Protection Activity of Polyphenol-Rich Extract and Polysaccharide-Rich Extract from Sargassum Vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.; Climstein, M.; Boyle, G.M.; Thanh Nguyen, D.; Feng, Y. Exploring Mycosporine-like Amino Acid UV-Absorbing Natural Products for a New Generation of Environmentally Friendly Sunscreens. Mar. Drugs 2023, 21, 253. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Shchelkanov, M.Y. Antiviral Effects of Polyphenols from Marine Algae. Biomedicines 2021, 9, 200. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent Advances in Extracting Phenolic Compounds from Food and Their Use in Disease Prevention and as Cosmetics. Crit. Rev. Food Sci. Nutr. 2021, 61, 1130–1151. [Google Scholar] [CrossRef]
- Jayawardhana, H.H.a.C.K.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Liyanage, N.M.; Nagahawatta, D.P.; Lee, H.-G.; Kim, J.-I.; Jeon, Y.-J. Marine Algal Polyphenols as Skin Protective Agents: Current Status and Future Prospectives. Mar. Drugs 2023, 21, 285. [Google Scholar] [CrossRef] [PubMed]
- Kasanah, N.; Ulfah, M.; Imania, O.; Hanifah, A.N.; Marjan, M.I.D. Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application. Molecules 2022, 27, 7788. [Google Scholar] [CrossRef] [PubMed]
- Kazłowska, K.; Hsu, T.; Hou, C.-C.; Yang, W.-C.; Tsai, G.-J. Anti-Inflammatory Properties of Phenolic Compounds and Crude Extract from Porphyra Dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of Phlorotannins Isolated from Ecklonia Cava on Melanogenesis and Their Protective Effect against Photo-Oxidative Stress Induced by UV-B Radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Piao, M.J.; Zhang, R.; Lee, N.H.; Hyun, J.W. Protective Effect of Triphlorethol-A against Ultraviolet B-Mediated Damage of Human Keratinocytes. J. Photochem. Photobiol. B 2012, 106, 74–80. [Google Scholar] [CrossRef]
- Wang, L.; Je, J.-G.; Yang, H.-W.; Jeon, Y.-J.; Lee, S. Dieckol, an Algae-Derived Phenolic Compound, Suppresses UVB-Induced Skin Damage in Human Dermal Fibroblasts and Its Underlying Mechanisms. Antioxidants 2021, 10, 352. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Stalikas, C.D. Phenolic Acids and Flavonoids: Occurrence and Analytical Methods. In Free Radicals and Antioxidant Protocols; Methods in Molecular Biology; Uppu, R.M., Murthy, S.N., Pryor, W.A., Parinandi, N.L., Eds.; Humana Press: Totowa, NJ, USA, 2010; Volume 610, pp. 65–90. ISBN 978-1-58829-710-5. [Google Scholar]
- Jofre, J.; Celis-Plá, P.S.M.; Figueroa, F.L.; Navarro, N.P. Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds. Mar. Drugs 2020, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-Protective Compounds in Red Macroalgae from Brittany: Considerable Diversity in Mycosporine-like Amino Acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Navarro, N.P.; Figueroa, F.L.; Korbee, N. Mycosporine-like Amino Acids vs Carrageenan Yield in Mazzaella Laminarioides (Gigartinales; Rhodophyta) under High and Low UV Solar Irradiance. Phycologia 2017, 56, 570–578. [Google Scholar] [CrossRef]
- Navarro, N.; Korbee, N.; Jofre, J.; Figueroa, F. Short-Term Variations of Mycosporine-like Amino Acids in the Carrageenan-Producing Red Macroalga Mazzaella Laminarioides (Gigartinales, Rhodophyta) Are Related to Nitrate Availability. J. Appl. Phycol. 2021, 33, 2537–2546. [Google Scholar] [CrossRef]
- Navarro, N.P.; Figueroa, F.L.; Korbee, N.; Mansilla, A.; Matsuhiro, B.; Barahona, T.; Plastino, E.M. The Effects of NO3(-) Supply on Mazzaella Laminarioides (Rhodophyta, Gigartinales) from Southern Chile. Photochem. Photobiol. 2014, 90, 1299–1307. [Google Scholar] [CrossRef]
- Navarro, N.P.; Figueroa, F.L.; Korbee, N.; Mansilla, A.; Plastino, E.M. Differential Responses of Tetrasporophytes and Gametophytes of Mazzaella Laminarioides (Gigartinales, Rhodophyta) under Solar UV Radiation. J. Phycol. 2016, 52, 451–462. [Google Scholar] [CrossRef]
- Richa; Sinha, R.P. Biochemical Characterization of Sunscreening Mycosporine-like Amino Acids from Two Nostoc Species Inhabiting Diverse Habitats. Protoplasma 2015, 252, 199–208. [Google Scholar] [CrossRef]
- Singh, D.K.; Pathak, J.; Pandey, A.; Rajneesh; Singh, V.; Sinha, R.P. Purification, Characterization and Assessment of Stability, Reactive Oxygen Species Scavenging and Antioxidative Potentials of Mycosporine-like Amino Acids (MAAs) Isolated from Cyanobacteria. J. Appl. Phycol. 2022, 34, 3157–3175. [Google Scholar] [CrossRef]
- Singh, V.K.; Das, B.; Jha, S.; Rana, P.; Kumar, R.; Sinha, R.P. Characterization, DFT Study and Evaluation of Antioxidant Potentials of Mycosporine-like Amino Acids (MAAs) in the Cyanobacterium Anabaenopsis Circularis HKAR-22. J. Photochem. Photobiol. B Biol. 2024, 257, 112975. [Google Scholar] [CrossRef] [PubMed]
- Enujiugha, V.N.; Talabi, J.Y.; Malomo, S.A.; Olagunju, A.I. DPPH Radical Scavenging Capacity of Phenolic Extracts from African Yam Bean (Sphenostylis Stenocarpa). Food Nutr. Sci. 2012, 3, 7–13. [Google Scholar] [CrossRef]
- Larsson, P.; Andersson, E.; Johansson, U.; Ollinger, K.; Rosdahl, I. Ultraviolet A and B Affect Human Melanocytes and Keratinocytes Differently. A Study of Oxidative Alterations and Apoptosis. Exp. Dermatol. 2005, 14, 117–123. [Google Scholar] [CrossRef]
- Jiang, Y.; Rabbi, M.; Kim, M.; Ke, C.; Lee, W.; Clark, R.L.; Mieczkowski, P.A.; Marszalek, P.E. UVA Generates Pyrimidine Dimers in DNA Directly. Biophys. J. 2009, 96, 1151–1158. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-Photosensitizers: Mediators of Skin Photodamage and Novel Targets for Skin Photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively Generated Damage to Cellular DNA by UVB and UVA Radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef]
- Jin, S.-G.; Padron, F.; Pfeifer, G.P. UVA Radiation, DNA Damage, and Melanoma. ACS Omega 2022, 7, 32936–32948. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular Photoprotection of Human Keratinocytes in Vitro by the Naturally Occurring Mycosporine-like Amino Acid Palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of Titanium Dioxide Nanoparticles in Cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 7), 34–46. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Wen, Z.-S.; Liu, L.-J.; Qu, Y.-L.; OuYang, X.-K.; Yang, L.-Y.; Xu, Z.-R. Chitosan Nanoparticles Attenuate Hydrogen Peroxide-Induced Stress Injury in Mouse Macrophage RAW264.7 Cells. Mar. Drugs 2013, 11, 3582–3600. [Google Scholar] [CrossRef] [PubMed]
- Wardani, G.; Nugraha, J.; Mustafa, M.R.; Kurnijasanti, R.; Sudjarwo, S.A. Antioxidative Stress and Antiapoptosis Effect of Chitosan Nanoparticles to Protect Cardiac Cell Damage on Streptozotocin-Induced Diabetic Rat. Oxid. Med. Cell Longev. 2022, 2022, 3081397. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Zacaron, T.M.; Silva, M.L.S.e.; Costa, M.P.; Silva, D.M.e.; Silva, A.C.; Apolônio, A.C.M.; Fabri, R.L.; Pittella, F.; Rocha, H.V.A.; Tavares, G.D. Advancements in Chitosan-Based Nanoparticles for Pulmonary Drug Delivery. Polymers 2023, 15, 3849. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.; Palei, N.N.; Pathak, P.; Verma, A.; Yadav, J.P. Chitosan-Based Nanoformulations: Preclinical Investigations, Theranostic Advancements, and Clinical Trial Prospects for Targeting Diverse Pathologies. AAPS PharmSciTech 2024, 25, 263. [Google Scholar] [CrossRef]
- Megna, M.; Lembo, S.; Balato, N.; Monfrecola, G. “Active” Photoprotection: Sunscreens with DNA Repair Enzymes. G. Ital. Dermatol. Venereol. 2017, 152, 302–307. [Google Scholar] [CrossRef]
- Ikehata, H.; Yamamoto, M. Roles of the KEAP1-NRF2 System in Mammalian Skin Exposed to UV Radiation. Toxicol. Appl. Pharmacol. 2018, 360, 69–77. [Google Scholar] [CrossRef]
- Álvarez-Gomez, F.; Korbee, N.; Figueroa, F.L. Analysis of Antioxidant Capacity and Bioactive Compounds in Marine Macroalgal and Lichenic Extracts Using Different Solvents and Evaluation Methods. Cienc. Mar. 2016, 42, 271–288. [Google Scholar] [CrossRef]
- Yamamoto, R.; Toriumi, S.; Kawagoe, C.; Saburi, W.; Kishimura, H.; Kumagai, Y. Extraction and Antioxidant Capacity of Mycosporine-like Amino Acids from Red Algae in Japan. Biosci. Biotechnol. Biochem. 2024, 88, 830–838. [Google Scholar] [CrossRef]
- Ryu, J.; Kwon, M.-J.; Nam, T.-J. Nrf2 and NF-κB Signaling Pathways Contribute to Porphyra-334-Mediated Inhibition of UVA-Induced Inflammation in Skin Fibroblasts. Mar. Drugs 2015, 13, 4721–4732. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Arancibia, R.; Tapia, C.; Acuña-Rougier, C.; Diaz-Dosque, M.; Cáceres, M.; Martínez, J.; Smith, P.C. Chitosan and Platelet-Derived Growth Factor Synergistically Stimulate Cell Proliferation in Gingival Fibroblasts. J. Periodontal Res. 2013, 48, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the Degree of Acetylation on Some Biological Properties of Chitosan Films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A Review on Nanoparticle Based Treatment for Wound Healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Amor, I.B.; Emran, T.B.; Hemmami, H.; Zeghoud, S.; Laouini, S.E. Nanomaterials Based on Chitosan for Skin Regeneration: An Update. Int. J. Surg. 2023, 109, 594–596. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Loo, H.L.; Goh, B.H.; Lee, L.-H.; Chuah, L.H. Application of Chitosan-Based Nanoparticles in Skin Wound Healing. Asian J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Calvo, N.L.; Sreekumar, S.; Svetaz, L.A.; Lamas, M.C.; Moerschbacher, B.M.; Leonardi, D. Design and Characterization of Chitosan Nanoformulations for the Delivery of Antifungal Agents. Int. J. Mol. Sci. 2019, 20, 3686. [Google Scholar] [CrossRef]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the War Against Inflammation With Natural Products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef]
- Paganelli, A.; Pisciotta, A.; Bertani, G.; Di Tinco, R.; Tagliaferri, N.; Orlandi, G.; Azzoni, P.; Bertoni, L. Food Supplements for Skin Health: In Vitro Efficacy of a Combination of Rhodiola Rosea, Tribulus Terrestris, Moringa Oleifera and Undaria Pinnatifida on UV-Induced Damage. Cosmetics 2023, 10, 83. [Google Scholar] [CrossRef]
- Kämpfer, H.; Bräutigam, L.; Geisslinger, G.; Pfeilschifter, J.; Frank, S. Cyclooxygenase-1-Coupled Prostaglandin Biosynthesis Constitutes an Essential Prerequisite for Skin Repair. J. Investig. Dermatol. 2003, 120, 880–890. [Google Scholar] [CrossRef]
- Ling, X.; Zhu, L.; Yan, Y.; Qian, H.; Kang, Z.; Ye, W.; Xie, Z.; Xue, C. Ferulic Acid Protects Human Lens Epithelial Cells Against UVA-Induced Oxidative Damage by Downregulating the DNA Demethylation of the Keap1 Promoter. J. Biochem. Mol. Toxicol. 2024, 38, e70031. [Google Scholar] [CrossRef]
- Palsamy, P.; Bidasee, K.R.; Shinohara, T. Valproic Acid Suppresses Nrf2/Keap1 Dependent Antioxidant Protection through Induction of Endoplasmic Reticulum Stress and Keap1 Promoter DNA Demethylation in Human Lens Epithelial Cells. Exp. Eye Res. 2014, 121, 26–34. [Google Scholar] [CrossRef]
- Wang, M.-L.; Zhong, Q.-Y.; Lin, B.-Q.; Liu, Y.-H.; Huang, Y.-F.; Chen, Y.; Yuan, J.; Su, Z.-R.; Zhan, J.Y.-X. Andrographolide Sodium Bisulfate Attenuates UV-Induced Photo-Damage by Activating the Keap1/Nrf2 Pathway and Downregulating the NF-κB Pathway in HaCaT Keratinocytes. Int. J. Mol. Med. 2020, 45, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chan, F.; Sun, H.; Yan, J.; Fan, D.; Zhao, D.; An, J.; Zhou, D. Resveratrol Protects Human Keratinocytes HaCaT Cells from UVA-Induced Oxidative Stress Damage by Downregulating Keap1 Expression. Eur. J. Pharmacol. 2011, 650, 130–137. [Google Scholar] [CrossRef]
- Ngoennet, S.; Nishikawa, Y.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. A Method for the Isolation and Characterization of Mycosporine-Like Amino Acids from Cyanobacteria. Methods Protoc. 2018, 1, 46. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Sawall, T.; Hanelt, D.; Bischof, K.; Figueroa, F.L.; Flores-Moya, A.; Wiencke, C. An Inventory of UV-Absorbing Mycosporine-Like Amino Acids in Macroalgae from Polar to Warm-Temperate Regions. Bot. Mar. 1998, 41, 443–454. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Quantification of Tannins in Tree Foliage: A Laboratory Manual for the FAO/IAEA Coordinated Research Project on “Use of Nuclear and Related Techniques to Develop Simple Tannin Assays for Predicting and Improving the Safety and Efficiency of Feeding Ruminants on Tanniniferous Tree Foliage”; International Atomic Energy Agency (IAEA): Vienna, Austria, 2000. [Google Scholar]
- Pant, A.; Negi, J.S. Novel Controlled Ionic Gelation Strategy for Chitosan Nanoparticles Preparation Using TPP-β-CD Inclusion Complex. Eur. J. Pharm. Sci. 2018, 112, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC−Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.; Rajasekaran, A.; Brochu-Gaudreau, K.; Dubois, C.; Farmilo, A.J.; Gris, P.; Khatiz, A.; Matthews, A.; Piltonen, M.; Amrani, A.; et al. A Convenient Analytic Method for Gel Quantification Using ImageJ Paired with Python or R. PLoS ONE 2024, 19, e0308297. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Yamaguchi, M.; Kasai, K. Substance P Stimulates Release of RANKL via COX-2 Expression in Human Dental Pulp Cells. Inflamm. Res. 2006, 55, 78–84. [Google Scholar] [CrossRef]
- Pi, J.; Qu, W.; Reece, J.M.; Kumagai, Y.; Waalkes, M.P. Transcription Factor Nrf2 Activation by Inorganic Arsenic in Cultured Keratinocytes: Involvement of Hydrogen Peroxide. Exp. Cell Res. 2003, 290, 234–245. [Google Scholar] [CrossRef]







| Cell Line | MAAs | Formulation | MAAs Dose | UV Dose | Viability Without MAAs | Viability with MAAs | Photoprotective Efficacy (Fold Change in Viability) | Refs. |
|---|---|---|---|---|---|---|---|---|
| HaCat | Porphyra-334 | HPLC-purified porphyra-334 in methanol 50% (v/v) | 0.1 mg/mL | 20 J/cm2, 300–400 nm (UVB/UVA) | 39% | 83% | 2.2× | [22,24] |
| HaCaT (ATCC 12191) | MAA-rich algal extract of laver (Porphyra yezoensis) | 80% (v/v) methanol extract of laver | 0.5–3.0 mg/mL DW alga | 70 mJ/cm2, (UVB) | 68% | 78% (with 3.0 mg/mL) | 1.1× (post-treatment) | [51] |
| Human skin fibroblasts CCD-986sk | Porphyra-334 | HPLC-purified porphyra-334 in 0.1% acetic acid (v/v) | 0–40 µM (0–18.9 μg/mL) | 10 J/cm2 (UVA) | 68% | 90% (with 18.9 μg/mL) | 1.4× | [23] |
| HaCaT | Palythine | HPLC-purified palythine in PBS | 0.3–10% w/v (3–100 mg/mL) | 20 J/cm2 (UVA) | 47% | 100% | 2.2× | [82] |
| HaCat | MAA-rich algal extract of luga cuchara (Mazaella laminarioides) | Nanoformulation | 3.4–17 μg/mL | Low to medium UVA dose (5–30 J/cm2) | 38% | 92% (with CSNFs-10.2) | 2.3–2.6× (pre-treatment) | This study |
| High UVA dosis (60 J/cm2) | 15% | 62.5% (with CSNFs-17) | 4.2× (pre-treatment) 8.5× (post-treatment) |
| Primer | Forward | Reverse | Fragment Size | Ref. |
|---|---|---|---|---|
| COX 1 | 5′-GGGCTTGGGCCATGGGGTAG-3′ | 5′-AGCTGCTCATCGCCCCAGGT-3′ | 318 pb | [117] |
| Keap 1 | 5′-CAGAGGTGGTGGTGTTGCTTAT-3′ | 5′-AGCTCGTTCATGATGCCAAAG-3′ | 244 pb | [118] |
| β-actin | 5′-AGAGATGGCCACGGCTGCTT-3′ | 5′-ATTTGCGGTGGACGATGGAG-3′ | 406 pb | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez, O.; Contreras-Trigo, B.; Castillo, E.; Contreras, N.; Lemus, J.; Zuniga, F.A.; Oyarce, K.; Núñez, D.; Díaz-García, V.; Oyarzún, P. Chitosan Nanoformulations of Mycosporine-like Amino Acid (MAA)-Rich Extracts from Mazzaella laminarioides Effectively Protect Human Keratinocytes Against UVA Radiation Damage. Int. J. Mol. Sci. 2025, 26, 10394. https://doi.org/10.3390/ijms262110394
Vásquez O, Contreras-Trigo B, Castillo E, Contreras N, Lemus J, Zuniga FA, Oyarce K, Núñez D, Díaz-García V, Oyarzún P. Chitosan Nanoformulations of Mycosporine-like Amino Acid (MAA)-Rich Extracts from Mazzaella laminarioides Effectively Protect Human Keratinocytes Against UVA Radiation Damage. International Journal of Molecular Sciences. 2025; 26(21):10394. https://doi.org/10.3390/ijms262110394
Chicago/Turabian StyleVásquez, Osmán, Braulio Contreras-Trigo, Eileen Castillo, Neriel Contreras, Jessica Lemus, Felipe A. Zuniga, Karina Oyarce, Dariela Núñez, Víctor Díaz-García, and Patricio Oyarzún. 2025. "Chitosan Nanoformulations of Mycosporine-like Amino Acid (MAA)-Rich Extracts from Mazzaella laminarioides Effectively Protect Human Keratinocytes Against UVA Radiation Damage" International Journal of Molecular Sciences 26, no. 21: 10394. https://doi.org/10.3390/ijms262110394
APA StyleVásquez, O., Contreras-Trigo, B., Castillo, E., Contreras, N., Lemus, J., Zuniga, F. A., Oyarce, K., Núñez, D., Díaz-García, V., & Oyarzún, P. (2025). Chitosan Nanoformulations of Mycosporine-like Amino Acid (MAA)-Rich Extracts from Mazzaella laminarioides Effectively Protect Human Keratinocytes Against UVA Radiation Damage. International Journal of Molecular Sciences, 26(21), 10394. https://doi.org/10.3390/ijms262110394






