Association Between Proinflammatory Cytokines IL-6 and TNF-Alpha, Psychological Stress and Chronic Spontaneous Urticaria Severity
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Methodology
4.2. Participants
4.3. Questionnaires
4.4. Serum Biomarker Analysis
4.5. Psychological Stress Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CU | chronic urticaria |
| QoL | quality of life |
| CSU | chronic spontaneous urticaria |
| TGF-β1 | tumor growth factor-β1 |
| MIP-1α | macrophage inflammatory protein 1α |
| RANTES | regulated upon activation, normal T-cell expressed and secreted |
| MCP-1 | monocyte chemoattractant protein 1 |
| DBC | differential blood count |
| ESR | erythrocyte sedimentation rate |
| CRP | C-reactive protein |
| IL | interleukin |
| TNF-α | tumor necrosis factor-alpha |
| SAM | sympatho-adrenal–medullary system |
| HPA | hypothalamic–pituitary–adrenal axis |
| CRH | corticotropin-releasing hormone |
| ACTH | adrenocorticotropic hormone (ACTH) |
| DLQI | Dermatology Life Quality Index |
| UAS | Urticaria Activity Score |
| ECLIA | electro-chemiluminescence immunoassay |
| PSS | perceived Stress Scale |
References
- Zuberbier, T.; Latiff, A.H.A.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; Bindslev-Jensen, C.; et al. The International EAACI/GA2LEN/EuroGuiDerm/APAAACI Guideline for The Definition, Classification, Diagnosis, and Management of Urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Stitt, J. Chronic Spontaneous Urticaria: Quality of Life and Economic Impacts. Immunol. Allergy Clin. N. Am. 2024, 44, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, L.; Zhang, H.; Song, Y.; Wang, W.; Hu, Z.; Wang, S.; Huang, L.; Wang, Y.; Wu, S.; et al. Beyond the Itch: The Complex Interplay of Immune, Neurological, and Psychological Factors in Chronic Urticaria. J. NeuroInflamm. 2025, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.R.; Pio-Abreu, J.L.; Figueiredo, A.; Misery, L. Pruritus, Allergy and Autoimmunity: Paving the Way for an Integrated Understanding of Psychodermatological Diseases? Front. Allery. 2021, 2, 688999. [Google Scholar] [CrossRef]
- Esen, M.; Demirbaş, A.; Diremsizoglu, E. Quality Of Life, Sleep, and Psychological Well-Being in Chronic Spontaneous Urticaria Patients Receiving Omalizumab: A Case-Control Study. Arch. Dermatol. Res. 2025, 317, 715. [Google Scholar] [CrossRef]
- Cetinkaya, P.O.; Kurt, B.O.; Aktaran, A.; Aksu, A.; Altunay, I.K. Sleep Disturbance and Psychological Stress: Two Interconnected Conditions in Chronic Spontaneous Urticaria. Sisli Etfal Hastan. Tıp Bul. 2025, 59, 35–43. [Google Scholar] [CrossRef]
- Bešlić, I.; Lugović-Mihić, L.; Vrtarić, A.; Bešlić, A.; Škrinjar, I.; Hanžek, M.; Crnković, D.; Artuković, M. Melatonin in Dermatologic Allergic Diseases and Other Skin Conditions: Current Trends and Reports. Int. J. Mol. Sci. 2023, 24, 4039. [Google Scholar] [CrossRef]
- Ben-Shoshan, M.; Blinderman, I.; Raz, A. Psychosocial Factors and Chronic Spontaneous Urticaria: A Systematic Review. Allergy 2013, 68, 131–141. [Google Scholar] [CrossRef]
- Donnelly, J.; Ridge, K.; O’Donovan, R.; Conlon, N.; Dunne, P.J. Psychosocial Factors and Chronic Spontaneous Urticaria: A Systematic Review. BMC Psychol. 2023, 11, 239. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.; Liu, R.; Zhu, L.; Peng, C. The Role of Crosstalk of Immune Cells in Pathogenesis of Chronic Spontaneous Urticaria. Front. Immunol. 2022, 31, 879754. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, P.K.; Chitkara, A.; Rani, S. To Evaluate the Role and Relevance of Cytokines IL-17, IL-18, IL-23 and TNF-α and Their Correlation With Disease Severity in Chronic Urticaria. Indian Dermatol. Online J. 2020, 11, 594–597. [Google Scholar] [CrossRef]
- Miyake, K.; Shibata, S.; Yoshikawa, S.; Karasuyama, H. Basophils and Their Effector Molecules in Allergic Disorders. Allergy 2021, 76, 1693–1706. [Google Scholar] [CrossRef]
- He, L.; Yi, W.; Huang, X.; Long, H.; Lu, Q. Chronic Urticaria: Advances in Understanding of the Disease and Clinical Management. Clin. Rev. Allergy Immunol. 2021, 61, 424–448. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Arnau, A.M.; de Montojoye, L.; Asero, R.; Cugno, M.; Kulthanan, K.; Yanase, Y.; Hide, M.; Kaplan, A.P. The Pathogenesis of Chronic Spontaneous Urticaria: The Role of Infiltrating Cells. J. Allergy Clin. Immunol. Pract. 2021, 9, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.; Kotrba, J.; Gaffal, E.; Katsoulis-Dimitriou, K.; Dudeck, A. Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation? Int. J. Mol. Sci. 2021, 22, 4589. [Google Scholar] [CrossRef] [PubMed]
- Falduto, G.H.; Pfeiffer, A.; Luker, A.; Metcalfe, D.D.; Olivera, A. Emerging Mechanisms Contributing to Mast Cell-Mediated Pathophysiology With Therapeutic Implications. Pharmacol. Ther. 2021, 220, 107718. [Google Scholar] [CrossRef]
- Saini, S.S.; Asero, R.; Cugno, M.; Park, H.S.; Oliver, E.T. Pathogenesis of Chronic Spontaneous Urticaria with or Without Angioedema. J. Allergy Clin. Immunol. Pract. 2025, 9, 2221–2228. [Google Scholar] [CrossRef]
- Lebedkina, M.; Kovalkova, E.; Andrenova, G.; Dushkin, A.; Chernov, A.; Nikitina, E.; Karaulov, A.; Lysenko, M.; Maurer, M.; Kocatürk, E.; et al. Chronic inducible urticaria—Having more than one is common and clinically relevant. Front. Immunol. 2025, 16, 1584771. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Du, S.; Yan, S.; Zeng, J. Advanced Biomarkers: Therapeutic and Diagnostic Targets in Urticaria. Int. Arch. Allergy Immunol. 2021, 182, 917–931. [Google Scholar] [CrossRef]
- Guo, H.; Guo, L.; Li, L.; Li, N.; Lin, X.; Wang, Y. Identification of Key Genes And Molecular Mechanisms of Chronic Urticaria Based on Bioinformatics. Ski. Res. Technol. 2024, 30, e13624. [Google Scholar] [CrossRef]
- Su, W.; Tian, Y.; Wei, Y.; Hao, F.; Ji, J. Key genes and immune infiltration in chronic spontaneous urticaria: A study of bioinformatics and systems biology. Front. Immunol. 2023, 14, 1279139. [Google Scholar] [CrossRef]
- Kuna, M.; Štefanović, M.; Ladika Davidović, B.; Mandušić, N.; Birkić Belanović, I.; Lugović-Mihić, L. Chronic Urticaria Biomarkers IL-6, ESR and CRP in Correlation with Disease Severity and Patient Quality of Life—A Pilot Study. Biomedicines 2023, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Deza, G.; Ricketti, P.A.; Giménez-Arnau, A.M.; Casale, T.B. Emerging Biomarkers and Therapeutic Pipelines for Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. Pract. 2018, 6, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Weller, K.; Zuberbier, T.; Maurer, M. Clinically Relevant Outcome Measures for Assessing Disease Activity, Disease Control, and Quality of Life Impairment in Patients with Chronic Spontaneous Urticaria and Recurrent Angioedema. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; André, F.; Church, M.K.; Maurer, M.; Metz, M. Potential Blood Biomarkers in Chronic Spontaneous Urticaria. Clin. Exp. Allergy 2017, 47, 19–36. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Grzanka, A.; Damasiewicz-Bodzek, A. IL-6 Trans-signaling in Patients with Chronic Spontaneous Urticaria. PLoS ONE 2015, 10, e0145751. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Zhao, X.; Wang, Y.; Chen, X.; Su, J. Role of Stress in Skin Diseases: A Neuroendocrine-Immune Interaction View. Brain Behav. Immun. 2024, 116, 286–302. [Google Scholar] [CrossRef]
- Keller, J.J. Cutaneous Neuropeptides: The Missing Link between Psychological Stress and Chronic Inflammatory Skin Disease? Arch. Dermatol. Res. 2023, 315, 1875–1881. [Google Scholar] [CrossRef]
- Konstantinou, G.N.; Konstantinou, G.N. Psychological Stress and Chronic Urticaria: A Neuro-immuno-cutaneous Crosstalk. A Systematic Review of the Existing Evidence. Clin. Ther. 2020, 42, 771–782. [Google Scholar] [CrossRef]
- Konstantinou, G.N.; Konstantinou, G.N. Psychiatric Comorbidity in Chronic Urticaria Patients: A Systematic Review and Meta-analysis. Clin. Transl. Allergy 2019, 9, 42. [Google Scholar] [CrossRef]
- Pondeljak, N.; Lugović-Mihić, L. Stress-Induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef]
- Tomaszewska, K.; Słodka, A.; Tarkowski, B.; Zalewska-Janowska, A. Neuro-Immuno-Psychological Aspects of Chronic Urticaria. J. Clin. Med. 2023, 12, 3134. [Google Scholar] [CrossRef] [PubMed]
- Reeshma, J.; Mariyath, O.K.R.; Narayan, K.D.; Devi, K.; Ajithkumar, K. Quality of Life and Perceived Stress in Chronic Spontaneous Urticaria: Counting the Burden. Indian Dermatol. Online J. 2024, 15, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Bansal, C.J.; Bansal, A.S. Stress, Pseudoallergens, Autoimmunity, Infection and Inflammation In Chronic Spontaneous Urticaria. Allergy Asthma Clin. Immunol. 2019, 15, 56. [Google Scholar] [CrossRef]
- Elçin, A.; Ayla, G.; Tuba Saadet Deveci, B.; Özlem, G.; Murat, Ö.; Ahmet Selim, B.; Behçet, C. Stressful Life Events, Psychiatric Comorbidities and Serum Neuromediator Levels in Patients with Chronic Spontaneous Urticaria Treated with Omalizumab. Allergol. Immunopathol. 2024, 52, 1–7. [Google Scholar]
- Lee, J.H.; Bae, Y.J.; Lee, S.H.; Kim, S.C.; Lee, H.Y.; Ban, G.Y.; Shin, Y.S.; Park, H.S.; Kratzsch, J.; Ye, Y.M. Adaptation and Validation of the Korean Version of the Urticaria Control Test and Its Correlation With Salivary Cortisone. Allergy Asthma Immunol. Res. 2019, 11, 55–67. [Google Scholar] [CrossRef]
- Varghese, R.; Rajappa, M.; Chandrashekar, L.; Kattimani, S.; Archana, M.; Munisamy, M.; Revathy, G.; Thappa, D.M. Association Among Stress, Hypocortisolism, Systemic Inflammation, and Disease Severity in Chronic Urticaria. Ann. Allergy Asthma Immunol. 2016, 116, 344–348.e1. [Google Scholar] [CrossRef]
- Jia, Q.; Zang, D.; Yi, J.; Dong, H.; Niu, Y.; Zhai, Q.; Teng, Y.; Bin, P.; Zhou, W.; Huang, X.; et al. Cytokine Expression in Trichloroethylene-Induced Hypersensitivity Dermatitis: An In Vivo And In Vitro Study. Toxicol. Lett. 2012, 215, 31–39. [Google Scholar] [CrossRef]
- Tian, R.; Hou, G.; Li, D.; Yuan, T.F. A Possible Change Process of Inflammatory Cytokines in the Prolonged Chronic Stress and its Ultimate Implications for Health. Sci. World J. 2014, 2014, 780616. [Google Scholar] [CrossRef]
- Grzanka, R.; Damasiewicz-Bodzek, A.; Kasperska-Zajac, A. Tumor Necrosis Factor-Alpha and Fas/Fas Ligand Signaling Pathways in Chronic Spontaneous Urticaria. Allergy Asthma Clin. Immunol. 2019, 15, 15. [Google Scholar] [CrossRef]
- Tavakol, M.; Amirzargar, A.A.; Movahedi, M.; Aryan, Z.; Bidoki, A.Z.; Gharagozlou, M.; Aghamohammadi, A.; Nabavi, M.; Ahmadvand, A.; Behniafard, N.; et al. Interleukin-6 and Tumor Necrosis Factor-Alpha Gene Polymorphisms in Chronic Idiopathic Urticaria. Allergol. Immunopathol. 2014, 42, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Habal, F.; Huang, V. Angioedema Associated with Crohn’s Disease: Response to Biologics. World J. Gastroenterol. 2012, 18, 4787–4790. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.M.; Fernandez, A.P.; Lang, D.M. Biologic Therapy in the Treatment of Chronic Skin Disorders. Immunol. Allergy Clin. N. Am. 2017, 37, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Babaie, D.; Nabavi, M.; Arshi, S.; Gorjipour, H.; Darougar, S. The Relationship Between Serum Interleukin-6 Level and Chronic Urticaria. Immunoregulation 2019, 2, 41–46. [Google Scholar] [CrossRef]
- Ograczyk-Piotrowska, A.; Gerlicz-Kowalczuk, Z.; Pietrzak, A.; Zalewska-Janowska, A.M. Stress, Itch And Quality Of Life in Chronic Urticaria Females. Adv. Dermatol. Allergol. 2018, 35, 156–160. [Google Scholar] [CrossRef]
- Eskeland, S.; Halvorsen, J.A.; Tanum, L. Antidepressants have Anti-inflammatory Effects that may be Relevant to Dermatology: A Systematic Review. Acta Derm. Venereol. 2017, 97, 897–905. [Google Scholar] [CrossRef]
- Chu, X.; Wang, J.; Ologundudu, L.; Brignardello-Petersen, R.; Guyatt, G.H.; Oykhman, P.; Bernstein, J.A.; Saini, S.S.; Beck, L.A.; Waserman, S. Efficacy and Safety of Systemic Corticosteroids for Urticaria: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Allergy Clin. Immunol. Pract. 2024, 12, 1879–1889.e8. [Google Scholar] [CrossRef]
- Lugović-Mihić, L.; Bukal, D.; Dolački, L.; Zanze, L.; Barac, E.; Tomašević, R.; Vilibić, M. Mental Health, Psychological Features and Psychiatric Comorbidity of Adolescents with Atopic Dermatitis: A Review. Pediatr. Rep. 2025, 17, 50. [Google Scholar] [CrossRef]
- Tat, T.S. Higher Levels of Depression and Anxiety in Patients with Chronic Urticaria. Med. Sci. Monit. 2019, 25, 115–120. [Google Scholar] [CrossRef]
- Konstantinou, G.N.; Podder, I.; Konstantinou, G. Mental Health Interventions in Refractory Chronic Spontaneous Urticaria: A Call to Expand Treatment Guidelines. Cureus 2025, 17, e81443. [Google Scholar] [CrossRef]
- Grieco, T.; Porzia, A.; Paolino, G.; Chello, C.; Sernicola, A.; Faina, V.; Carnicelli, G.; Moliterni, E.; Mainiero, F. IFN-γ/IL-6 and Related Cytokines in Chronic Spontaneous Urticaria: Evaluation of Their Pathogenetic Role And Changes During Omalizumab Therapy. Int. J. Dermatol. 2020, 59, 590–594. [Google Scholar] [CrossRef]
- Weller, K.; Groffik, A.; Church, M.K.; Hawro, T.; Krause, K.; Metz, M.; Martus, P.; Casale, T.B.; Staubach, P.; Maurer, M. Development and Validation of the Urticaria Control Test: A Patient-Reported Outcome Instrument for Assessing Urticaria Control. J. Allergy Clin. Immunol. 2014, 133, 1365–1372.e6. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A Simple Practical Measure for Routine Clinical Use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Pondeljak, N.; Lugović-Mihić, L.; Davidović, B.L.; Karlović, D.; Hanžek, M.; Neuberg, M. Serum Levels of IL-6 and TNF-α, Salivary Morning Cortisol and Intensity of Psychological Stress in Patients with Allergic Contact Hand Dermatitis and Healthy Subjects. Life 2025, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.D. The 10-Item Perceived Stress Scale as a Valid Measure of Stress Perception. Asia-Pac. Psychiatry 2021, 13, e12420. [Google Scholar] [CrossRef]
- Fazlić, H.; Brborović, O.; Vukušić Rukavina, T.; Fišter, K.; Milošević, M.; Mustajbegović, J. Karakteristike osoba sa opaženim stresom u hrvatskoj: Crohort istraživanje. Coll. Antropol. 2012, 36 (Suppl. 1), 165–169. [Google Scholar] [CrossRef]
- Schut, C.; Magerl, M.; Hawro, T.; Kupfer, J.; Rose, M.; Gieler, U.; Maurer, M.; Peters, E.M.J. Disease Activity and Stress Are Linked in a Subpopulation of Chronic Spontaneous Urticaria Patients. Allergy 2020, 75, 224–226. [Google Scholar] [CrossRef]

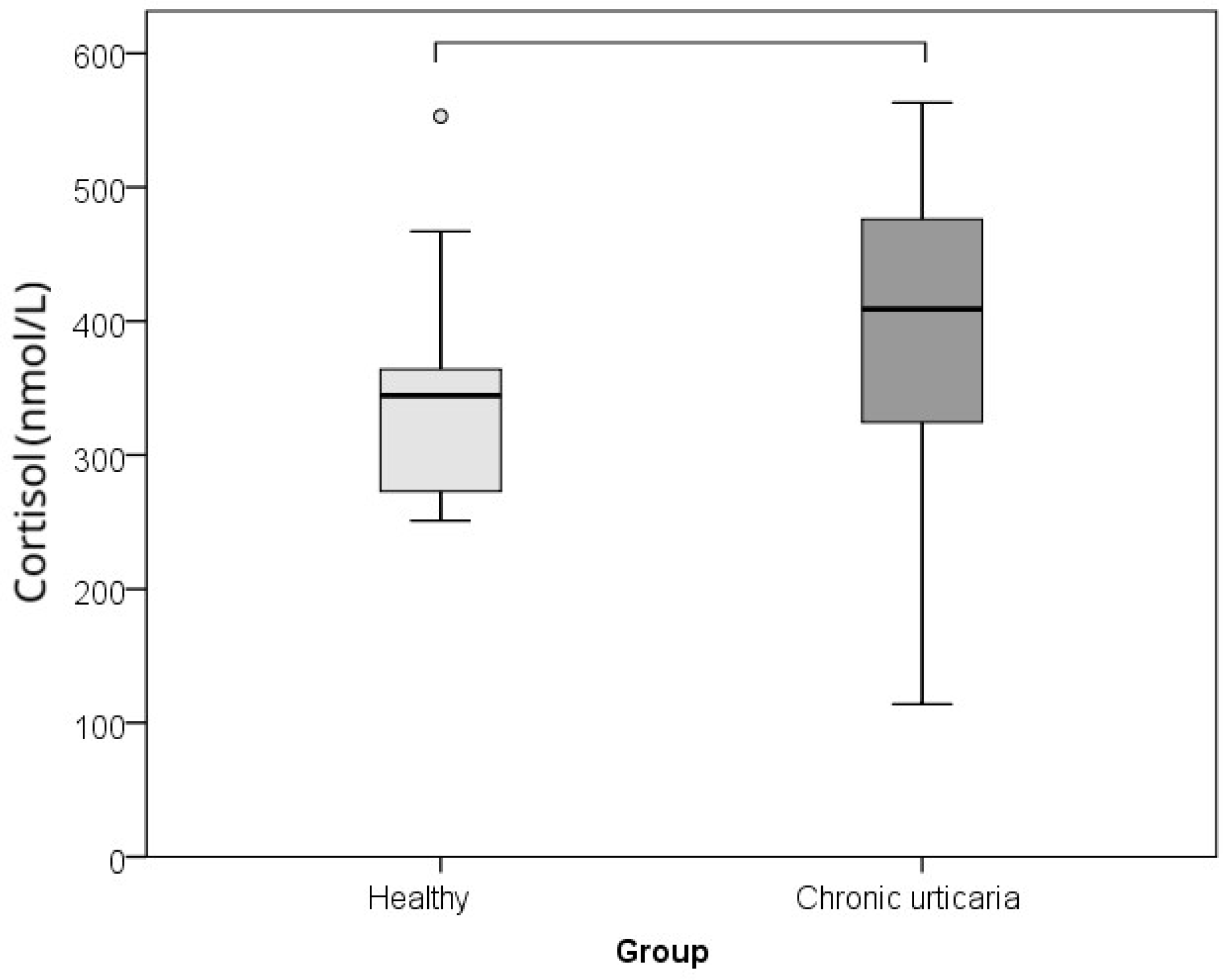
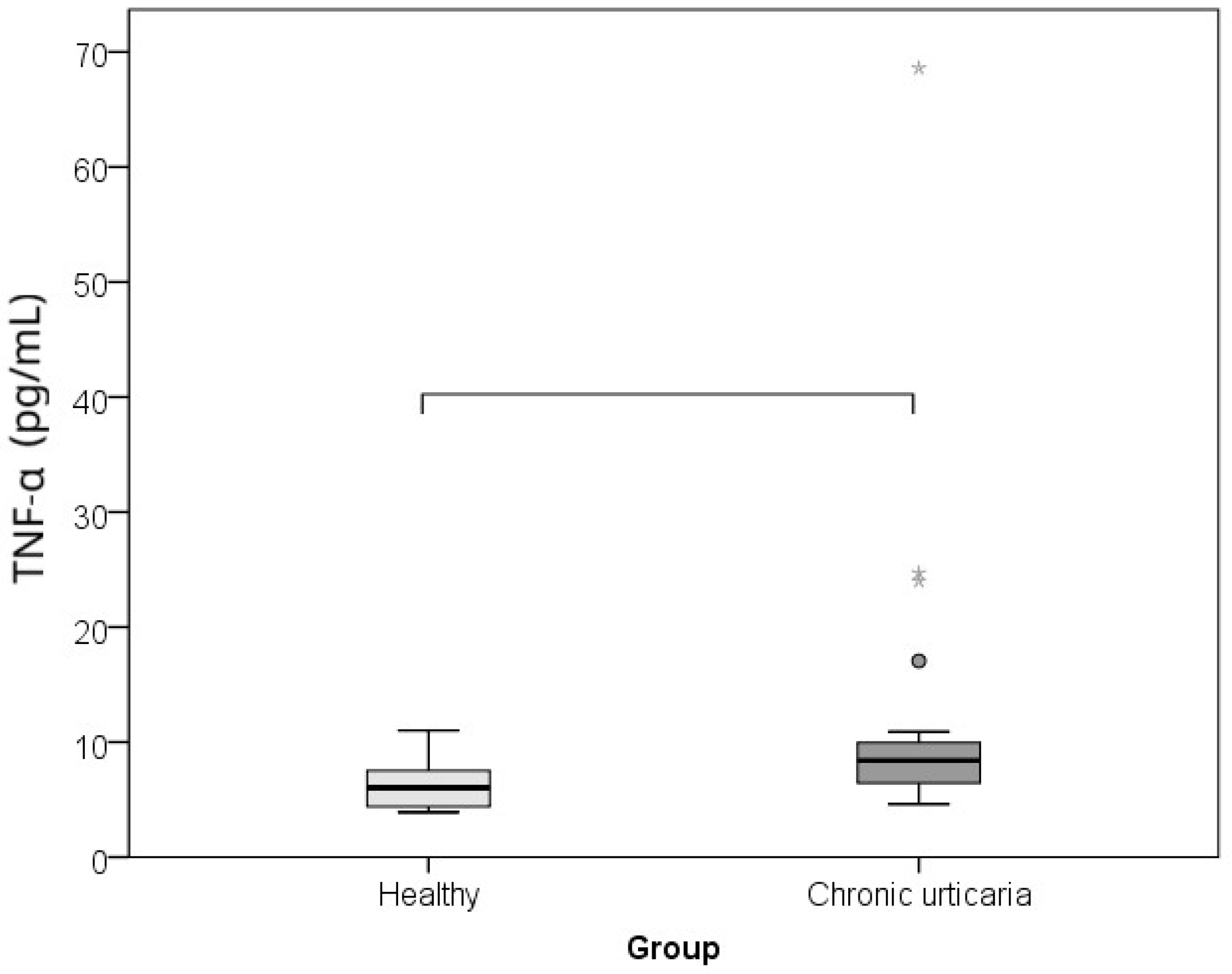
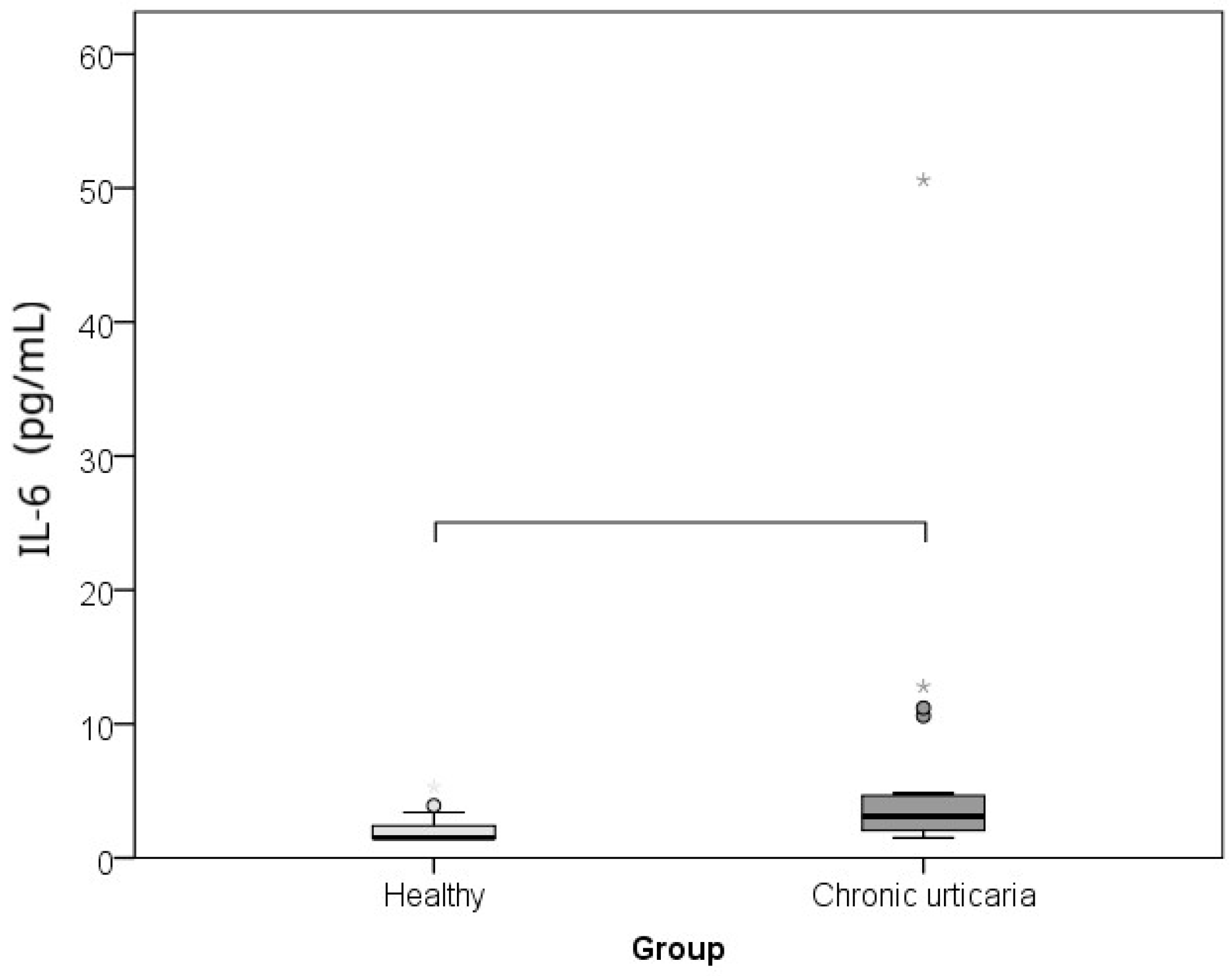
| Parameter | Healthy Controls | Chronic Spontaneous Urticaria Patients | p * | r ** |
|---|---|---|---|---|
| Age (years) | 34 (27–48) | 35 (29–48) | 0.486 | −0.115 |
| IL-6 (pg/mL) | 1.5 (1.5–2.7) | 3.1 (1.9–4.7) | 0.002 | −0.460 |
| TNF-α (pg/mL) | 6.0 (4.3–7.7) | 8.4 (6.4–10.3) | 0.001 | −0.485 |
| Cortisol (nmol/L) | 344.0 (273.0–364.0) | 409.0 (322.0–476.0) | 0.015 | −0.360 |
| Perceived stress (PSS, scalar points) | 25 (21–29) | 16 (12–22) | <0.001 | −0.608 |
| Disease Duration | Disease Severity (UAS7) | Impairment of Quality of Life (DLQI) | IL-6 | TNF-α | Stress (PSS) | Cortisol | Age | ||
|---|---|---|---|---|---|---|---|---|---|
| Disease duration (months) | r | 1 | 0.092 | −0.069 | 0.079 | −0.081 | −0.107 | −0.115 | 0.174 |
| p | 0.678 | 0.756 | 0.72 | 0.712 | 0.627 | 0.601 | 0.426 | ||
| Disease severity (UAS7, scalar points) | r | 0.092 | 1 | 0.715 | 0.214 | −0.141 | 0.367 | 0.463 | −0.323 |
| p | 0.678 | <0.001 | 0.328 | 0.520 | 0.085 | 0.026 | 0.132 | ||
| Impairment of quality of life (DLQI, scalar points) | r | −0.069 | 0.715 | 1 | 0.113 | 0.018 | 0.523 | 0.475 | −0.529 |
| p | 0.756 | <0.001 | 0.608 | 0.935 | 0.010 | 0.022 | 0.009 | ||
| Serum IL-6 (pg/mL) | r | 0.079 | 0.214 | 0.113 | 1 | −0.235 | −0.285 | 0.108 | 0.038 |
| p | 0.72 | 0.328 | 0.608 | 0.28 | 0.187 | 0.623 | 0.863 | ||
| Serum TNF-α (pg/mL) | r | −0.081 | −0.141 | 0.018 | −0.235 | 1 | −0.030 | −0.145 | −0.229 |
| p | 0.712 | 0.52 | 0.935 | 0.28 | 0.891 | 0.51 | 0.294 | ||
| Perceived stress (PSS, scalar points) | r | −0.107 | 0.367 | 0.523 | −0.285 | −0.030 | 1 | 0.196 | −0.133 |
| p | 0.627 | 0.085 | 0.01 | 0.187 | 0.891 | 0.370 | 0.546 | ||
| Serum cortisol (nmol/L) | r | −0.115 | 0.463 | 0.475 | 0.108 | −0.145 | 0.196 | 1 | −0.303 |
| p | 0.601 | 0.026 | 0.022 | 0.623 | 0.510 | 0.370 | 0.159 | ||
| Age (years) | r | 0.174 | −0.323 | −0.529 | 0.038 | −0.229 | −0.133 | −0.303 | 1 |
| p | 0.426 | 0.132 | 0.009 | 0.863 | 0.294 | 0.546 | 0.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugović-Mihić, L.; Štrajtenberger, M.; Kuna, M.; Ladika-Davidović, B.; Barac, E.; Vilibić, M. Association Between Proinflammatory Cytokines IL-6 and TNF-Alpha, Psychological Stress and Chronic Spontaneous Urticaria Severity. Int. J. Mol. Sci. 2025, 26, 10384. https://doi.org/10.3390/ijms262110384
Lugović-Mihić L, Štrajtenberger M, Kuna M, Ladika-Davidović B, Barac E, Vilibić M. Association Between Proinflammatory Cytokines IL-6 and TNF-Alpha, Psychological Stress and Chronic Spontaneous Urticaria Severity. International Journal of Molecular Sciences. 2025; 26(21):10384. https://doi.org/10.3390/ijms262110384
Chicago/Turabian StyleLugović-Mihić, Liborija, Maja Štrajtenberger, Matea Kuna, Blaženka Ladika-Davidović, Ema Barac, and Maja Vilibić. 2025. "Association Between Proinflammatory Cytokines IL-6 and TNF-Alpha, Psychological Stress and Chronic Spontaneous Urticaria Severity" International Journal of Molecular Sciences 26, no. 21: 10384. https://doi.org/10.3390/ijms262110384
APA StyleLugović-Mihić, L., Štrajtenberger, M., Kuna, M., Ladika-Davidović, B., Barac, E., & Vilibić, M. (2025). Association Between Proinflammatory Cytokines IL-6 and TNF-Alpha, Psychological Stress and Chronic Spontaneous Urticaria Severity. International Journal of Molecular Sciences, 26(21), 10384. https://doi.org/10.3390/ijms262110384






