Potentiometric Studies of the Complexation Properties of Selected Lanthanide Ions with Schiff Base Ligand
Abstract
1. Introduction
2. Results and Discussion
2.1. FT-IR Spectroscopy
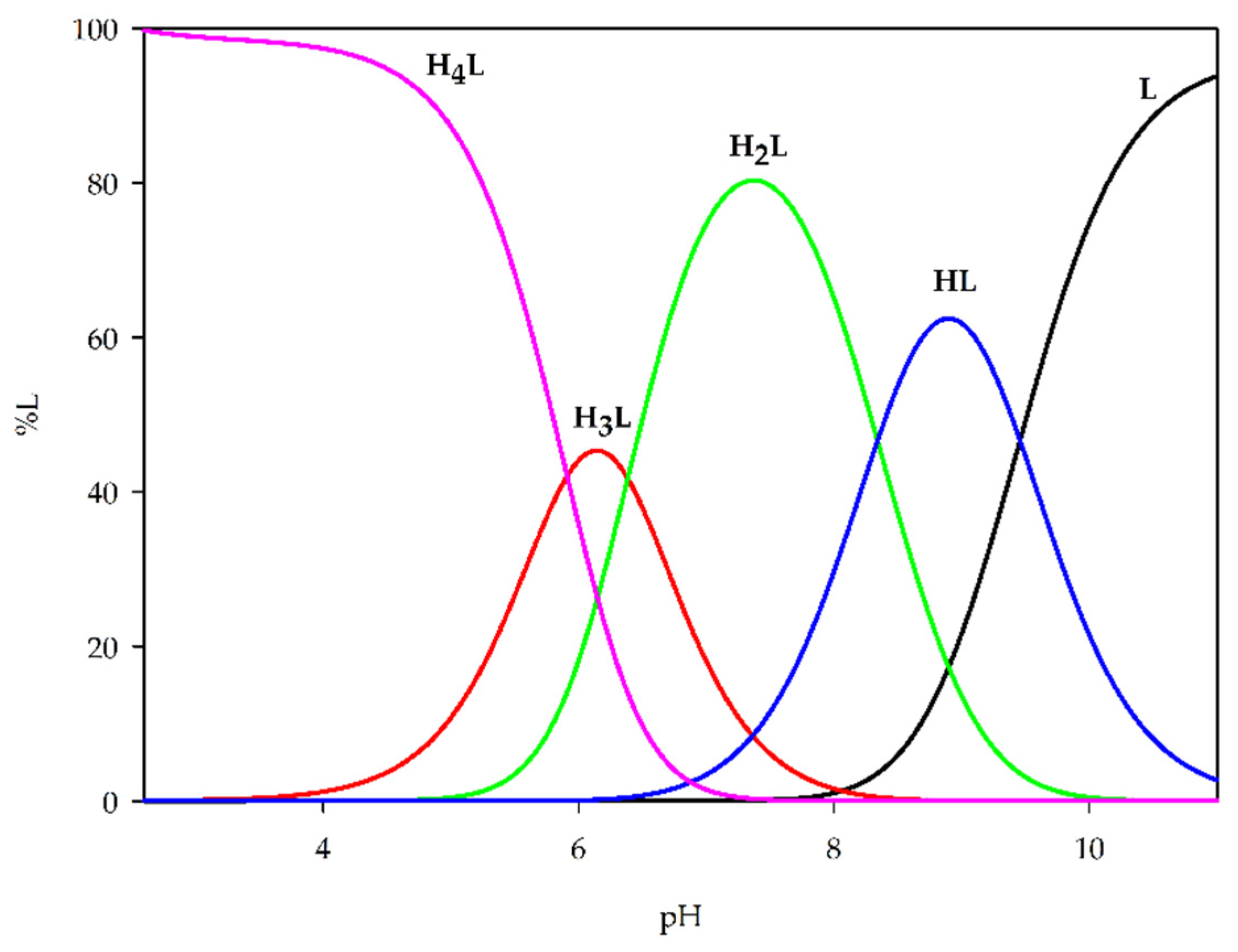
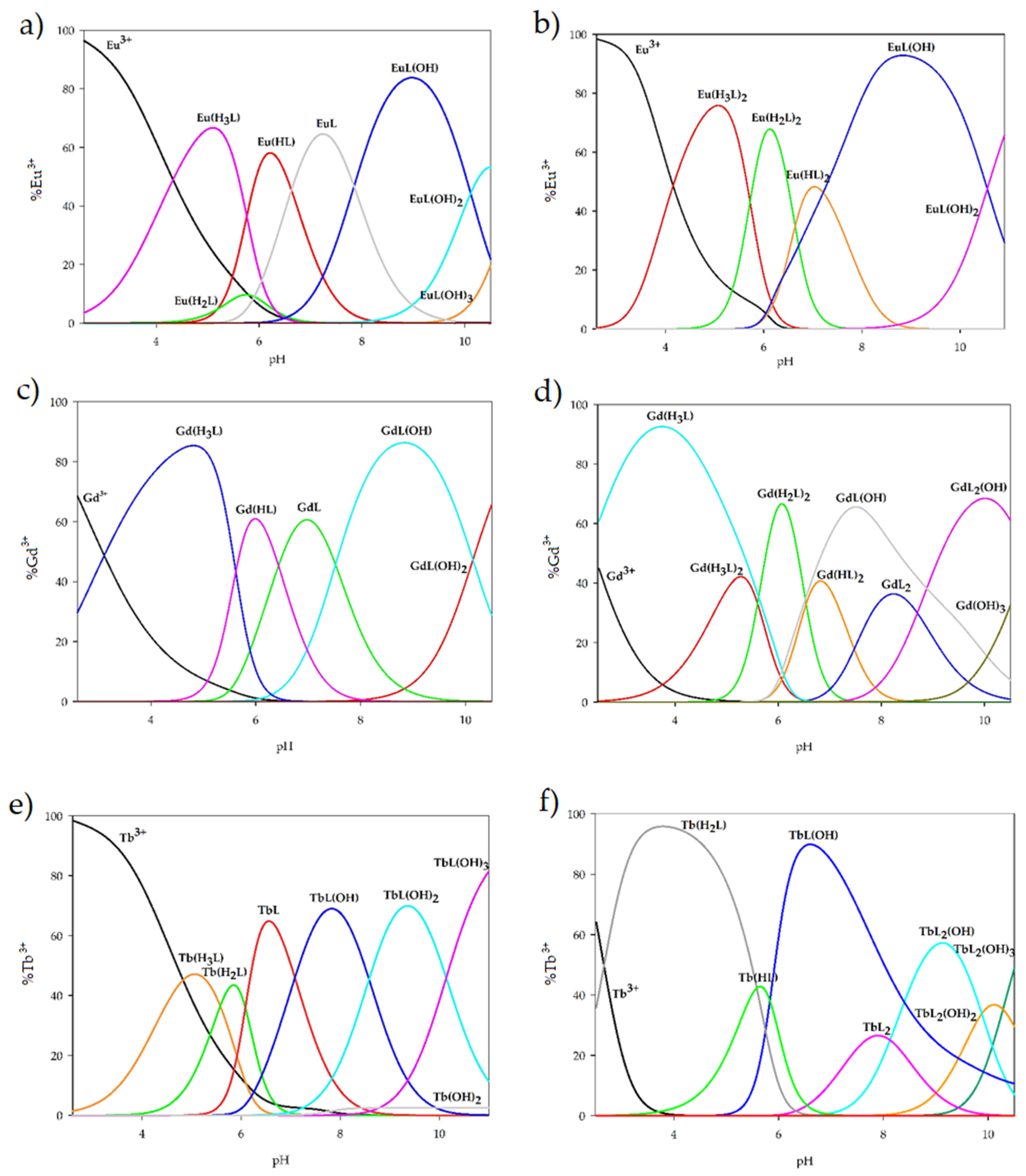
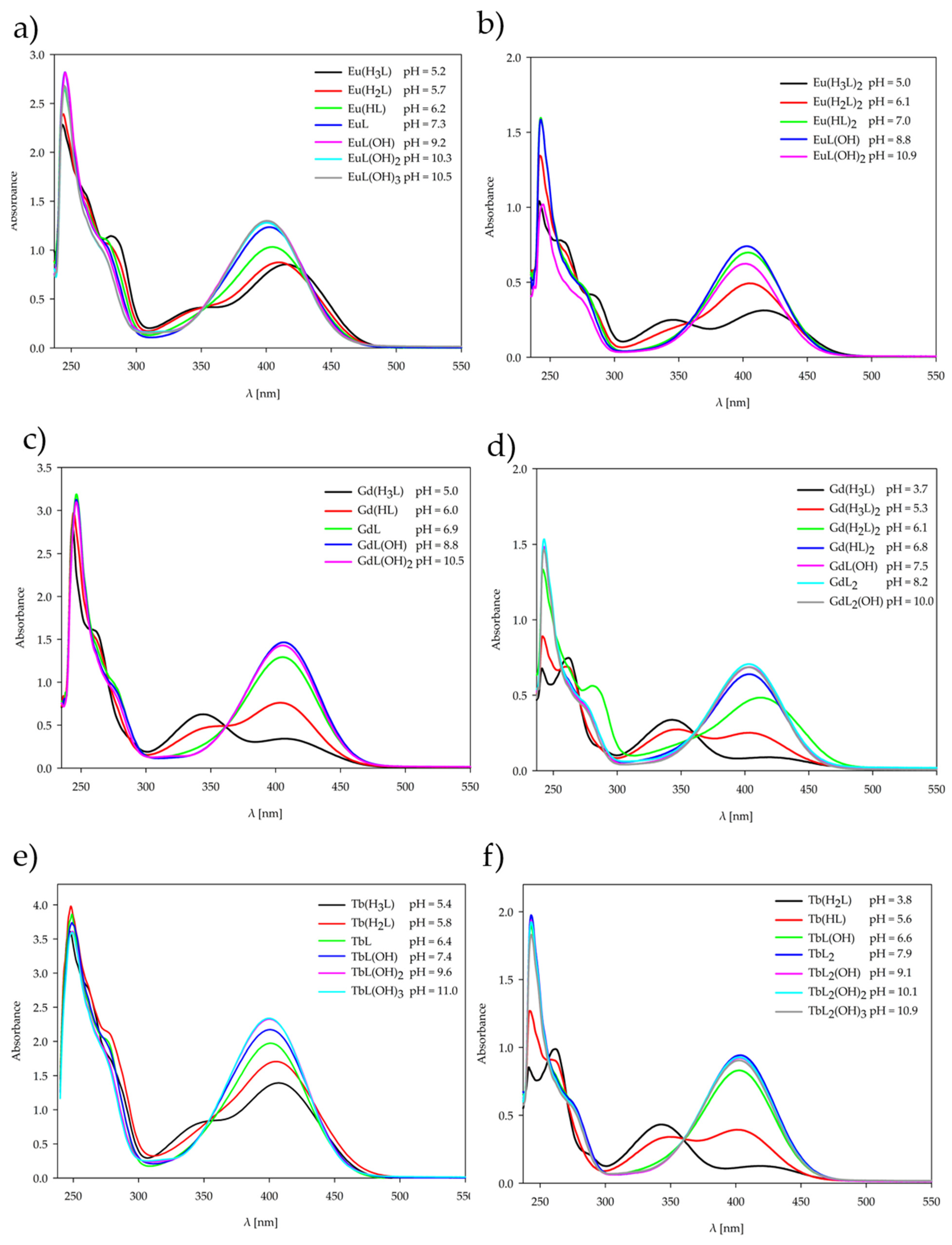
2.2. Equilibrium Study
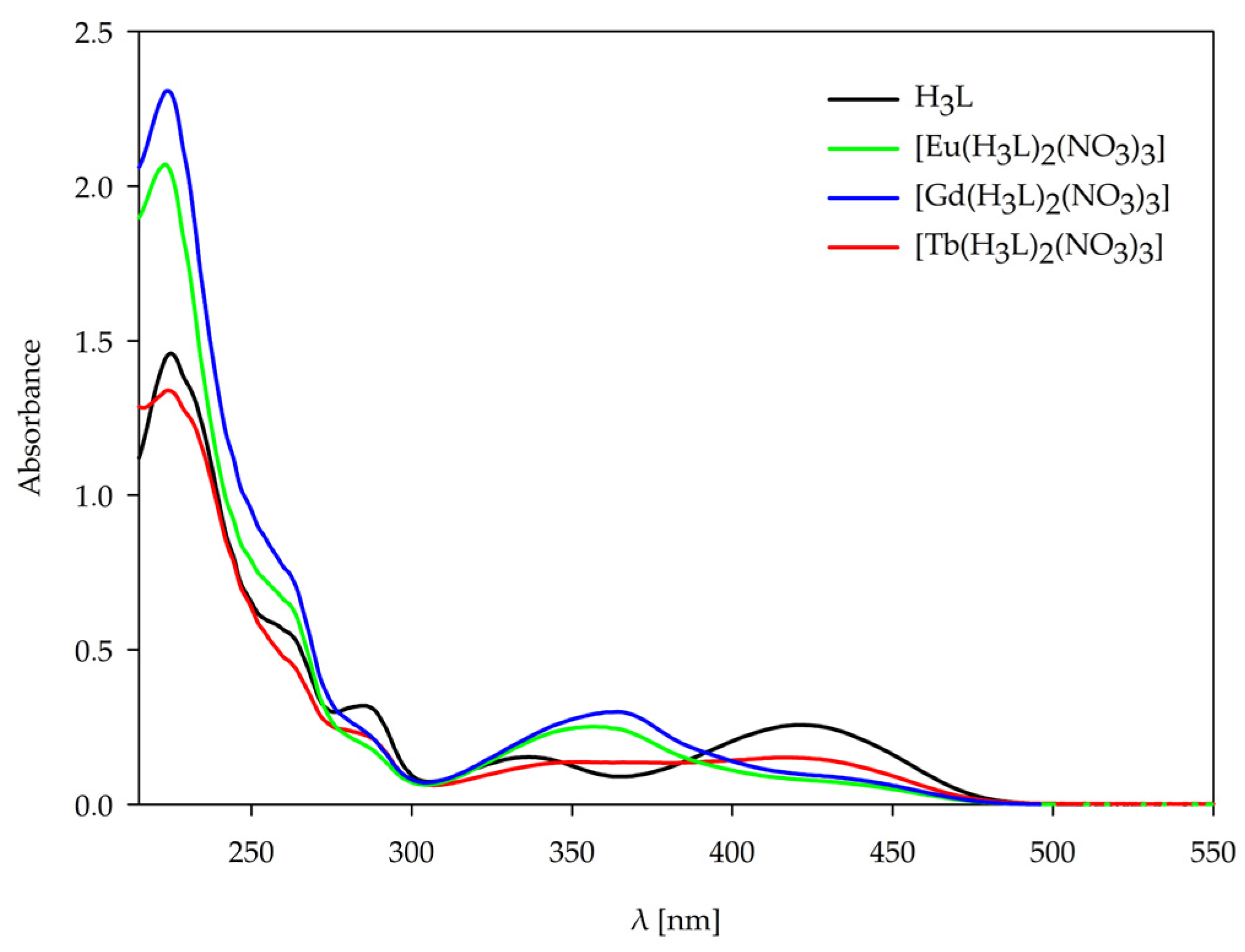
2.3. UV-Vis Spectroscopy
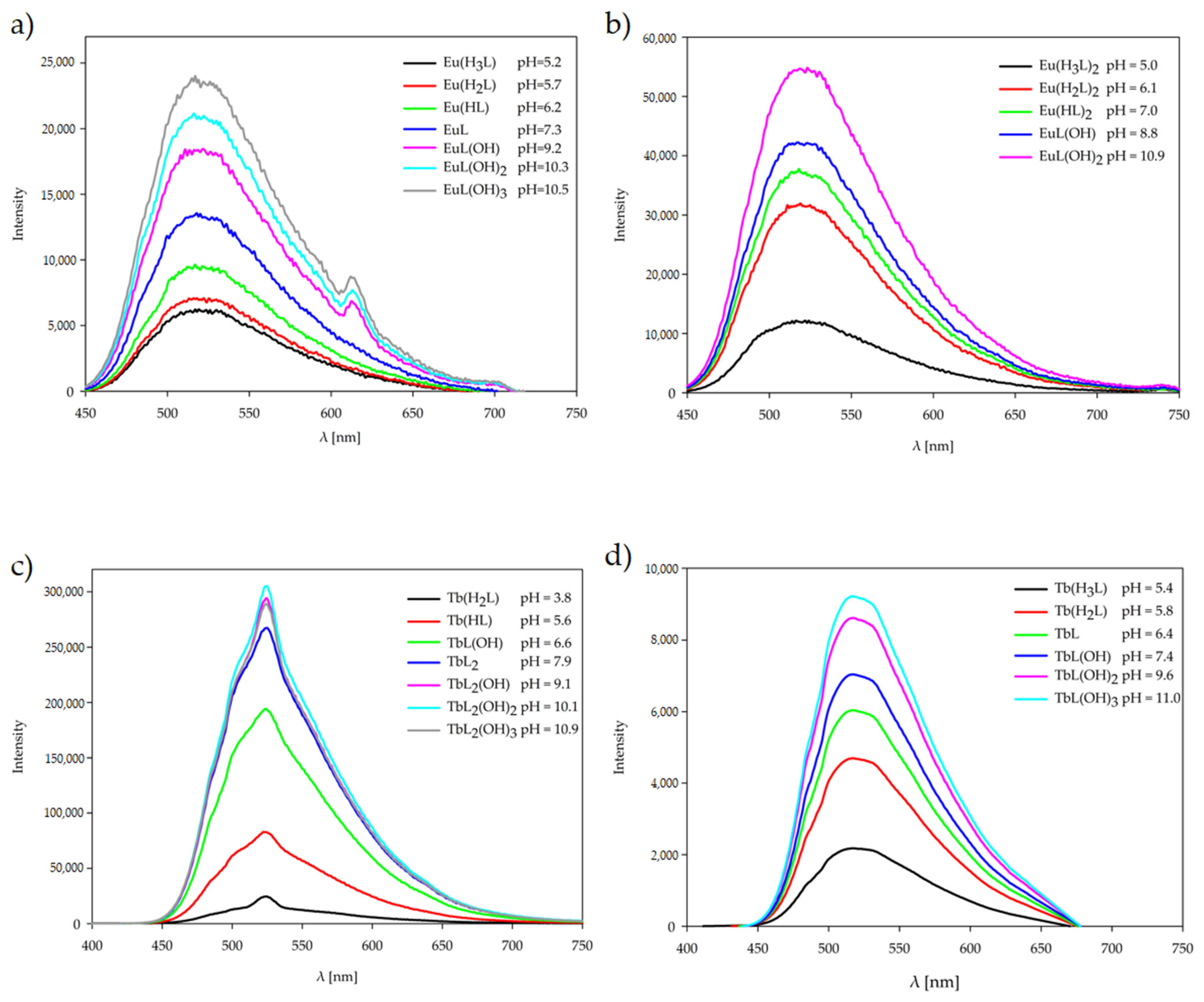
2.4. Luminescence Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Synthesis
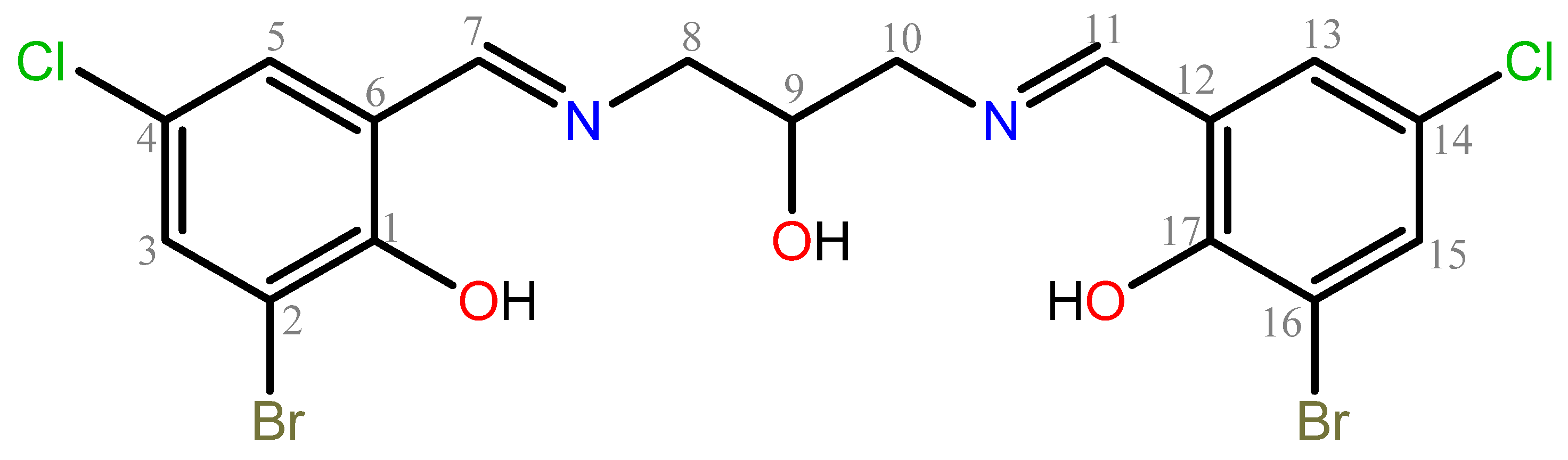
3.2.1. Synthesis of 1,3-Bis(3-bromo-5-chlorosalicylideneamino)-2-propanol, H3L
3.2.2. Synthesis of Europium(III) Complex [Eu(H3L)2(NO3)3]
3.2.3. Synthesis of Gadolinium(III) Complex [Gd(H3L)2(NO3)3]
3.2.4. Synthesis of Terbium(III) Complex [Tb(H3L)2(NO3)3]
3.3. Physical Measurements
3.4. Equilibrium Studies
3.5. Crystal Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaczmarek, M.T.; Pospieszna-Markiewicz, I.; Kubicki, M.; Radecka-Paryzek, W. Novel lanthanide salicylaldimine complexes with unusual coordination mode. Inorg. Chem. Commun. 2004, 7, 1247–1249. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Kubicki, M.; Mondry, A.; Janicki, R.; Radecka-Paryzek, W. Self-assembled lanthanide salicylaldimines with a unique coordination mode. Eur. J. Inorg. Chem. 2010, 2010, 2193–2200. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Al-Ghamdi, K.; Hamed, A.M.; Ibrahem, M.A.; Alharbi, S.K.; Abu Al-Ola, K.A.; Amin, M.S.; Eskander, T.N.A.; Abdel-Latif, S.A.; Abu-Dief, A.M. A robust synthesis, physicochemical characterization, stability determination and potential biomedical applications of novel Salen complexes supported by theoretical approaches. J. Mol. Struct. 2025, 1336, 142060–142082. [Google Scholar] [CrossRef]
- Zou, X.; Du, C.; Dong, Y.; Li, G. Slow relaxation of two-dimensional salen type lanthanide coordination polymer. Inorg. Chim. Acta 2020, 507, 119455–119461. [Google Scholar] [CrossRef]
- Mediavilla, P.; Ribeiro, A.; Gutierrez, A.; Herrero, S.; Torralba, M.C. Novel 3-ethoxysalicylaldehyde lanthanide complexes obtained by decomposition of salen-type ligands. Inorganics 2025, 13, 93. [Google Scholar] [CrossRef]
- Zou, X.; Fei, B.; Li, G. Structural effect on NIR luminescence of three salen type heteropolynuclear 3d–4f erbium complexes. Polyhedron 2020, 192, 114811–114817. [Google Scholar] [CrossRef]
- Chakraborty, A.; Middya, P.; Chattapadhyay, S. An overview of the synthesis and properties of heterodinuclear europium(III)–transition metal complexes with salen type compartmental ligands having inner N2O2 and outer O4 cavities. Inorg. Chim. Acta 2025, 581, 122625–122654. [Google Scholar] [CrossRef]
- Shaw, S.; White, J.D. Asymmetric catalysis using chiral salen–metal complexes: Recent advances. Chem. Rev. 2019, 119, 9381–9426. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Correia, I. Salan vs. salen metal complexes in catalysis and medicinal applications: Virtues and pitfalls. Coord. Chem. Rev. 2019, 388, 227–247. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef]
- Mandal, S.S. Metallo-salen complexes show promise towards treatment of leukemia. Leuk. Res. 2011, 35, 571–572. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, H.; Li, S. Lanthanide complexes with salen-type ligands: Synthesis, luminescence and catalytic applications. Coord. Chem. Rev. 2017, 352, 30–52. [Google Scholar]
- Zhang, Y.; Liu, Z. Lanthanide Schiff base complexes: Synthesis, structure, luminescence and applications. Dalton Trans. 2014, 43, 8036–8050. [Google Scholar]
- Zou, X.; Du, C.; Dong, Y.; Li, G. Luminescence and structure of a family of salen type dinuclear lanthanide complexes. Inorg. Chim. Acta 2020, 512, 119860–119867. [Google Scholar] [CrossRef]
- Li, S.; Jansone-Popova, S.; Jiang, D. Insights into coordination and ligand trends of lanthanide complexes from the Cambridge Structural Database. Sci. Rep. 2024, 14, 11301. [Google Scholar] [CrossRef]
- Mortensen, S.S.; Nielsen, V.R.M.; Sørensen, T.J. Contrasting impact of coordination polyhedra and site symmetry on the electronic energy levels in nine-coordinated Eu(III) and Sm(III) crystal structures determined from single crystal luminescence spectra. Dalton Trans. 2024, 53, 10079–10089. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-R.; Tang, H.-H.; Wang, Y.-G.; Wang, X.; Fang, X.-M.; Ma, L.-H. Functionalized lanthanide(III) complexes constructed from azobenzene derivative and β-diketone ligands: Luminescent, magnetic, and reversible trans-to-cis photoisomerization properties. Inorg. Chem. 2017, 56, 3889–3900. [Google Scholar] [CrossRef]
- Patra, K.; Pal, H. Lanthanide-based metal–organic frameworks (Ln-MOFs): Synthesis, properties and applications. RSC Sustain. 2025, 3, 629–660. [Google Scholar] [CrossRef]
- McHugh, D.; Tong, W.; Bezrukov, A.; Farras, P.; Zaworotko, M.J.; Mayans, J.; Skelton, J.M.; Barnett, S.; Pallipurath, A.R.; Papatriantafyllopoulou, C. Lanthanide(III) MOFs (Ln = Gd, Tb, Dy) based on a C3 symmetrical tricarboxylate linker. Eur. J. Inorg. Chem. 2025, 28, e202400541. [Google Scholar] [CrossRef]
- Roesky, P.W.; Bhunia, A.; Lan, Y.; Powell, A.K.; Kureti, S. Salen-based metal–organic frameworks of nickel and the lanthanides. Chem. Commun. 2011, 47, 2035–2037. [Google Scholar] [CrossRef]
- Yao, Y.; Yin, H.-Y.; Ning, Y.; Wang, J.; Meng, Y.-S.; Huang, X.; Zhang, W.; Kang, L.; Zhang, J.-L. Strong fluorescent lanthanide salen complexes: Photophysical properties, excited-state dynamics, and bioimaging. Inorg. Chem. 2019, 58, 1806–1814. [Google Scholar] [CrossRef]
- Summers, T.J.; Taylor, M.G.; Augustine, L.J.; Janssen, J.; Perez, D.; Batista, E.R.; Yang, P. On the importance of configuration search to the predictivity of lanthanide selectivity. JACS Au 2024, 5, 631–641. [Google Scholar] [CrossRef]
- Yarullin, D.N.; Slavova, S.O.; Abramova, E.O.; Zavalishin, M.N.; Tolstoy, P.M.; Gamov, G.A.; Grachova, E.V. Conformer-specific differences in solid-phase emission of pyridoxal 5′-phosphate hydrazones containing heteroaromatic cycles. Opt. Mater. 2025, 159, 116593–116602. [Google Scholar] [CrossRef]
- Khodov, I.A.; Belov, K.V.; Pogonin, A.E.; Savenkova, M.A.; Gamov, G.A. Spatial structure and conformations of hydrazones derived from pyridoxal 5′-phosphate and 2-, 3-pyridinecarbohydrazide in the light of NMR study and quantum chemical calculations. J. Mol. Liq. 2021, 342, 117372–117383. [Google Scholar] [CrossRef]
- Mikuriya, M.; Tsuchimoto, N.; Koyama, Y.; Mitsuhashi, R.; Tsuboi, M. Crystal structure of 1,3-bis(3,5-dibromosalicylideneamino)-2-propanol. X-ray Struct. Anal. Online 2022, 38, 3–5. [Google Scholar] [CrossRef]
- Kálmán, A.; Párkányi, L.; Argay, G. Classification of the isostructurality of organic molecules in the crystalline state. Acta Cryst. B 1993, 49, 1039–1049. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Potentiometric determination of novel complexes of selected lanthanide ions with N,N′-bis(5-methylsalicylidene)-4-methyl-1,3-phenylenediamine. J. Iran. Chem. Soc. 2018, 15, 407–414. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Jastrząb, R.; Radecka-Paryzek, W. Potentiometric study of lanthanide salicylaldimine Schiff base complexes. J. Solut. Chem. 2013, 42, 18–26. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Skrobanska, M.; Zabiszak, M.; Walesa-Chorab, M.; Kubicki, M.; Jastrzab, R. Coordination properties of N,N′-bis(5-methylsalicylidene)-2-hydroxy-1,3-propanediamine with d- and f-electron ions: Crystal structure, stability in the solution, spectroscopic and spectroelectrochemical studies. RSC Adv. 2018, 8, 30994–31007. [Google Scholar] [CrossRef]
- Sarwar, A.; Shamsuddin, M.; Kassim, K.; Kakar, E.; Iqbal, S. New luminescent Eu(III) and Er(III) Schiff base complexes: Synthesis, characterization and luminescence properties. J. Iran. Chem. Soc. 2024, 21, 2933–2942. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Kubicki, M.; Hnatejko, Z. Two types of lanthanide Schiff base complexes: Synthesis, structure and spectroscopic studies. Polyhedron 2015, 102, 224–232. [Google Scholar] [CrossRef]
- Stańczak, P.; Łuczkowski, M.; Juszczyk, P.; Grzonka, Z.; Kozłowski, H. Interactions of Cu2+ ions with chicken prion tandem repeats. Dalton Trans. 2004, 14, 2102–2107. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Jastrząb, R. Phosphoserine and specific types of its coordination in copper(II) and adenosine nucleotides systems—Potentiometric and spectroscopic studies. J. Inorg. Biochem. 2009, 103, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Bregier-Jarzebowska, R.; Gasowska, A.; Jastrząb, R.; Łomozik, L. Noncovalent interactions and coordination reactions in the systems consisting of copper(II) ions, aspartic acid and diamines. J. Inorg. Biochem. 2009, 103, 1228–1235. [Google Scholar] [CrossRef]
- Bruker. SAINT, V8.41; Bruker AXS Inc.: Madison, WI, USA, 2025.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
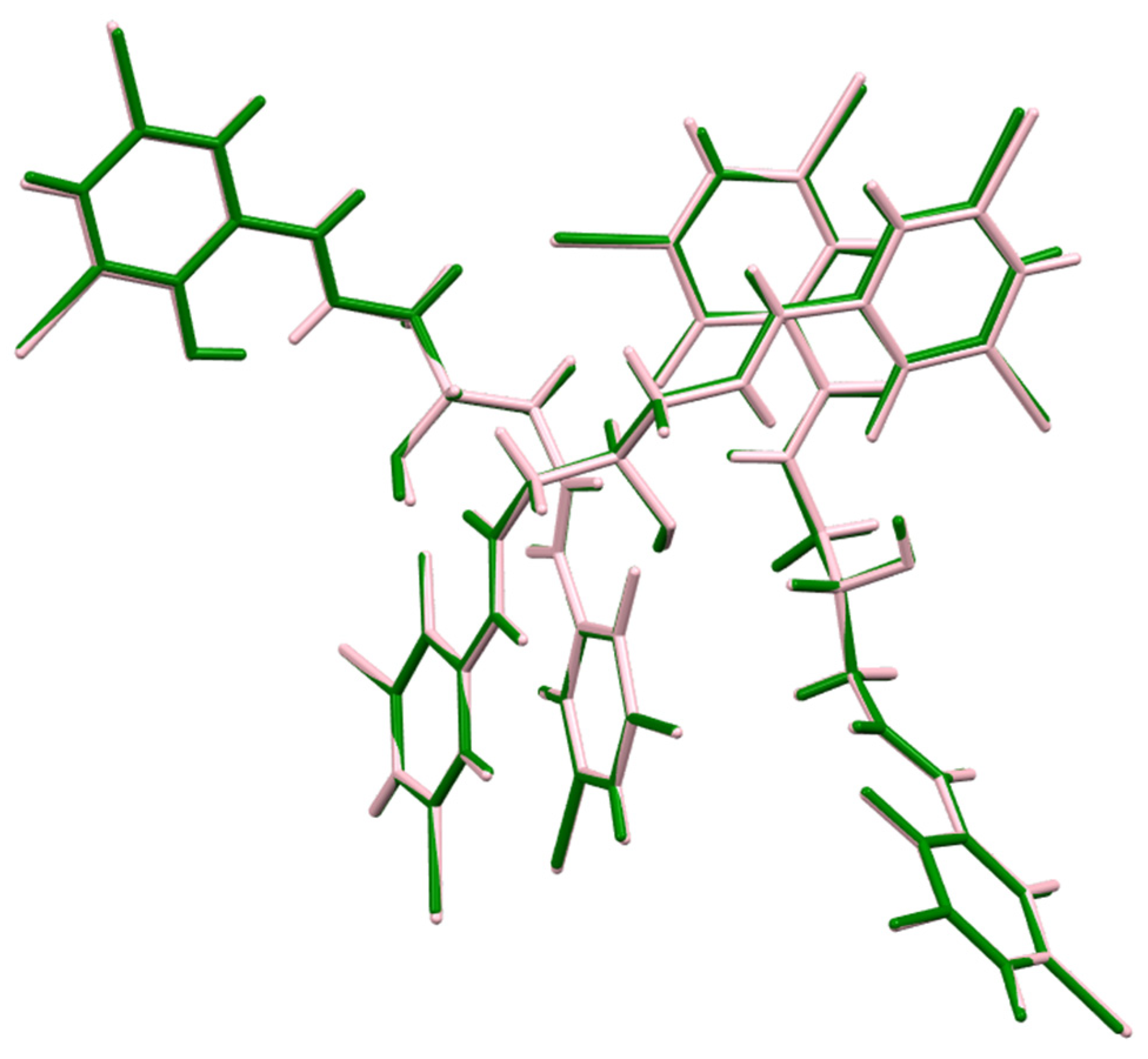

| D—H···A | D—H (Å) | H···A (Å) | D···A (Å) | D—H···A (°) |
|---|---|---|---|---|
| O10—H10···O21 | 0.84 | 2.01 | 2.836 (5) | 166.7 |
| N12—H12···O15 | 0.88 | 1.88 | 2.599 (5) | 137.1 |
| N48—H48···O41 | 0.88 | 1.94 | 2.619 (6) | 133.1 |
| N28—H28···O21 | 0.88 | 1.96 | 2.630 (5) | 132.4 |
| N28—H28···O54 | 0.88 | 2.27 | 2.917 (5) | 130.7 |
| N8—H8···O1 | 0.88 | 1.87 | 2.569 (5) | 134.9 |
| O30—H30···O1 | 0.84 | 1.96 | 2.774 (5) | 162.0 |
| N32—H32···O35 | 0.88 | 1.82 | 2.532 (5) | 137.1 |
| N52—H52···O21 | 0.88 | 2.59 | 3.222 (6) | 129.2 |
| N52—H52···O54 | 0.88 | 1.83 | 2.557 (5) | 138.1 |
| O50—H50···O15 | 0.84 | 1.91 | 2.754 (5) | 178.3 |
| Species | logβ | logKe |
|---|---|---|
| H4L | 30.09 (3) | 5.91 |
| H3L | 24.19 (3) | 6.39 |
| H2L | 17.79 (2) | 8.34 |
| HL | 9.46 (2) | 9.46 |
| Species | Eu(III) | Gd(III) | Tb(III) | ||||
|---|---|---|---|---|---|---|---|
| logβ | logKe | logβ | logKe | logβ | logKe | ||
| 1:1 | M(H3L) | 28.65 (4) | 4.86 | 29.88 (5) | 6.09 | 28.26 (7) | 4.47 |
| M(H2L) | 22.31 (1) | 4.78 | - | - | 22.68 (9) | 5.15 | |
| M(HL) | 17.13 (4) | 7.77 | 18.64 (5) | 9.28 | - | - | |
| ML | 10.50 (6) | 10.50 | 12.20 (5) | 12.20 | 10.51 (1) | 10.51 | |
| ML(OH) | 2.65 (4) | 6.66 | 4.68 (5) | 6.98 | 3.42 (4) | 7.41 | |
| ML(OH)2 | −7.46 (5) | 4.39 | −5.46 (6) | 4.36 | −5.16 (8) | 5.92 | |
| ML(OH)3 | −18.39 (7) | 3.57 | - | - | −15.30 (9) | 4.36 | |
| 1:2 | M(H3L)2 | 57.71 (7) | 10.13 | 58.47 (1) | 10.90 | - | - |
| M(H2L)2 | 46.27 (7) | 11.20 | 47.28 (8) | 12.22 | - | - | |
| M(HL)2 | 32.99 (8) | 14.27 | 34.19 (1) | 15.48 | - | - | |
| ML2 | - | - | 19.25 (1) | 19.25 | 23.36 (8) | 23.36 | |
| M(H3L) | - | - | - | - | 30.43 (8) | 6.65 | |
| M(H2L) | - | - | - | - | 27.47 (4) | 9.94 | |
| M(HL) | - | - | - | - | 21.89 (5) | 12.53 | |
| ML2(OH) | - | - | 10.55 (8) | 5.80 | 15.23 (6) | 6.37 | |
| ML2(OH)2 | - | - | - | - | 5.35 (7) | 4.62 | |
| ML2(OH)3 | - | - | - | - | −4.93 (7) | 4.22 | |
| ML(OH) | 4.46 (8) | 18.96 | 6.13 (1) | 20.63 | 10.23 (5) | 24.73 | |
| ML(OH)2 | −6.09 (1) | 3.95 | - | - | - | - | |
| 1,3-Bis(3-bromo-5-chlorosalicylideneamino)-2-propanol | |
|---|---|
| Crystal data | |
| Chemical formula | 3(C17H14Br2Cl2N2O3) |
| Mr | 1575.06 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 100 |
| a, b, c (Å) | 16.445 (3), 18.111 (3), 18.956 (4) |
| â (°) | 92.978 (9) |
| V (Å3) | 5638.2 (17) |
| Z | 4 |
| Radiation type | Cu K |
| m (mm−1) | 8.27 |
| Crystal size (mm) | 0.2 × 0.05 × 0.05 |
| Data collection | |
| Diffractometer | Bruker D8 QUEST |
| Absorption correction | Multi-scan |
| Tmin, Tmax | 0.250, 0.383 |
| No. of measured, independent and observed [I > 2ó(I)] reflections | 42,240, 10,291, 8425 |
| Rint | 0.064 |
| (sinθ/λmax (Å−1) | 0.603 |
| Refinement | |
| R[F2 > 2ó(F2)], wR(F2), S | 0.046, 0.113, 1.02 |
| No. of reflections | 10,291 |
| No. of parameters | 802 |
| Δρmax, Δρmin (e Å−3) | 1.56, −1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barańska, J.; Koroniak-Szejn, K.; Zabiszak, M.; Grześkiewicz, A.; Skrobanska, M.; Nowak, M.; Jastrzab, R.; Kaczmarek, M.T. Potentiometric Studies of the Complexation Properties of Selected Lanthanide Ions with Schiff Base Ligand. Int. J. Mol. Sci. 2025, 26, 10379. https://doi.org/10.3390/ijms262110379
Barańska J, Koroniak-Szejn K, Zabiszak M, Grześkiewicz A, Skrobanska M, Nowak M, Jastrzab R, Kaczmarek MT. Potentiometric Studies of the Complexation Properties of Selected Lanthanide Ions with Schiff Base Ligand. International Journal of Molecular Sciences. 2025; 26(21):10379. https://doi.org/10.3390/ijms262110379
Chicago/Turabian StyleBarańska, Julia, Katarzyna Koroniak-Szejn, Michał Zabiszak, Anita Grześkiewicz, Monika Skrobanska, Martyna Nowak, Renata Jastrzab, and Małgorzata T. Kaczmarek. 2025. "Potentiometric Studies of the Complexation Properties of Selected Lanthanide Ions with Schiff Base Ligand" International Journal of Molecular Sciences 26, no. 21: 10379. https://doi.org/10.3390/ijms262110379
APA StyleBarańska, J., Koroniak-Szejn, K., Zabiszak, M., Grześkiewicz, A., Skrobanska, M., Nowak, M., Jastrzab, R., & Kaczmarek, M. T. (2025). Potentiometric Studies of the Complexation Properties of Selected Lanthanide Ions with Schiff Base Ligand. International Journal of Molecular Sciences, 26(21), 10379. https://doi.org/10.3390/ijms262110379






