Potassium-Hydroxide-Based Extraction of Nicotinamide Adenine Dinucleotides from Biological Samples Offers Accurate Assessment of Intracellular Redox Status
Abstract
1. Introduction
2. Results
2.1. KOH Extraction Is Superior to MeOH Extraction for Detection of Reduced Pyridine Nucleotides
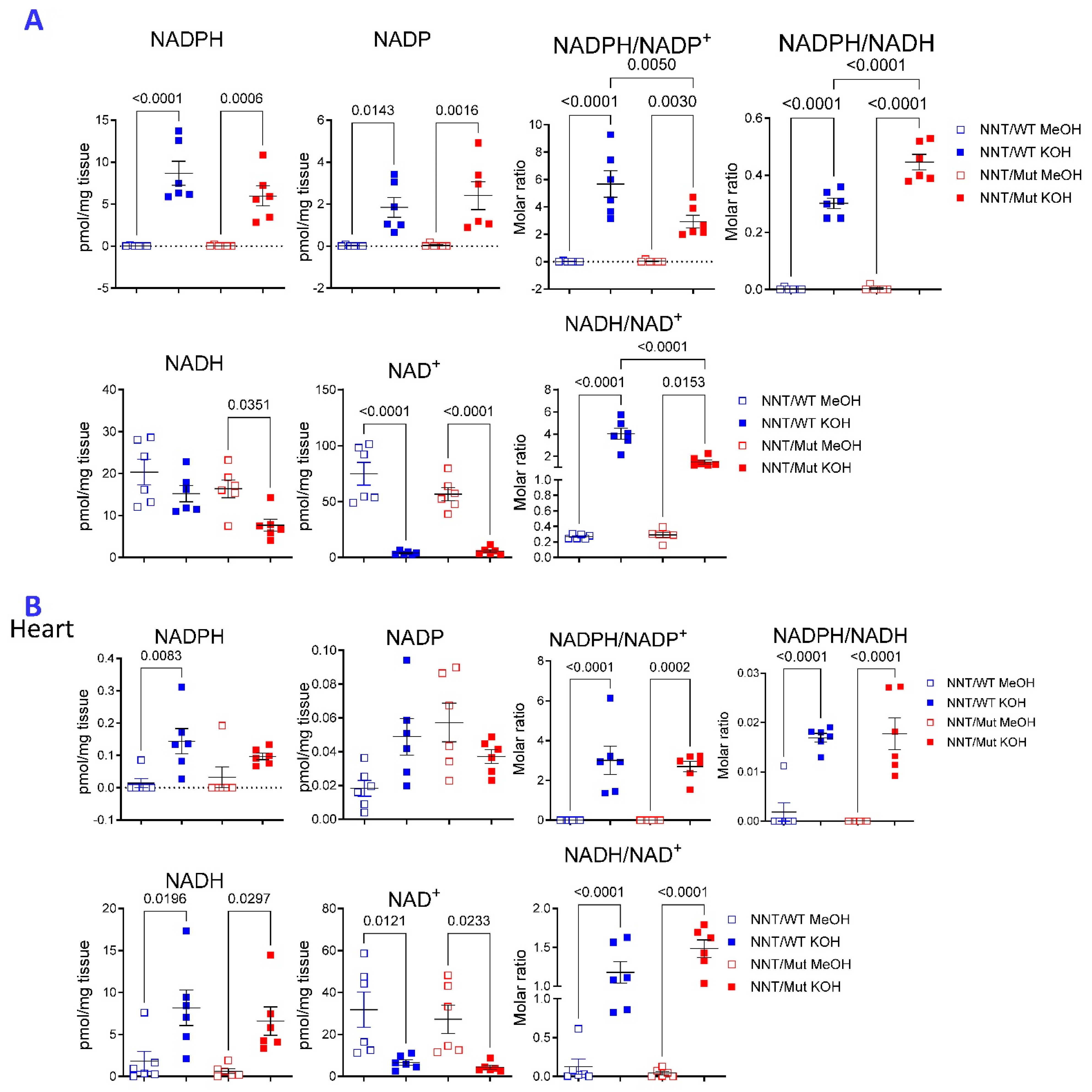
2.2. Compartmentalized Effect of NNT Deficiency on the Metabolome of the Liver
2.3. The Choice of Extraction Method Impacts the Metabolome Coverage, Which in Turn Affects the Discrimination of Genetically Different NNT/WT and NNT/Mut Mouse Strains
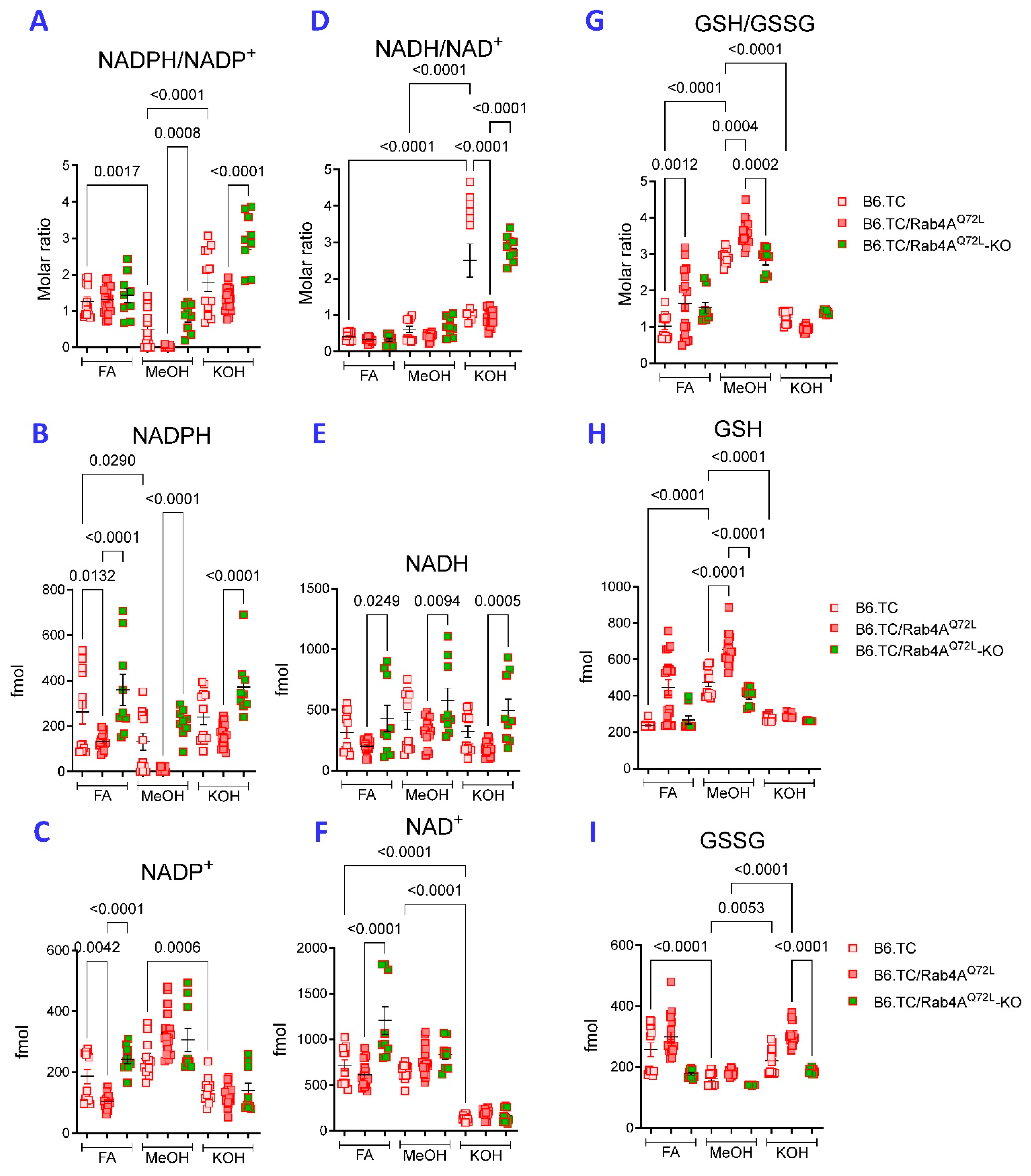
2.4. KOH Extraction Is Superior over FA Extraction for Detection of Reduced Pyridine Nucleotides from Hepatocytes
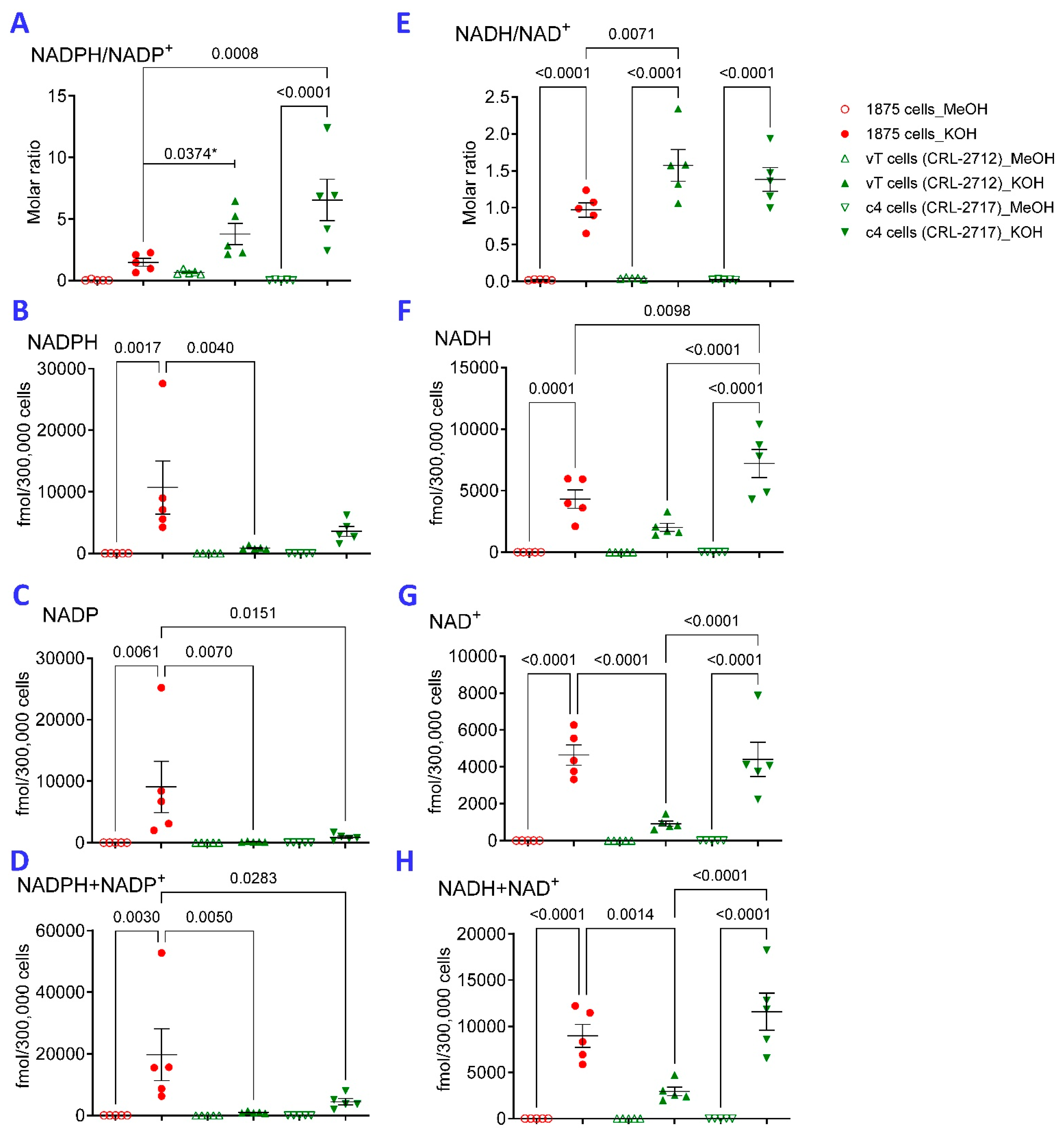
2.5. NADPH Predominates over NADP in A549 Cells
3. Discussion
4. Materials and Methods
4.1. Metabolite Extraction from Plate Bound Hepatocytes
4.1.1. Methanol Extraction
4.1.2. FA Extraction
4.1.3. KOH Extraction
4.2. Metabolite Extraction from Suspension Cells
4.2.1. Methanol Extraction
4.2.2. KOH Extraction
4.3. Metabolite Extraction from Mitochondria
4.3.1. Isolation of Mitochondria
4.3.2. Methanol Extraction
4.3.3. KOH Extraction
4.4. Metabolite Extraction from Organ Tissue
4.4.1. Methanol Extraction
4.4.2. KOH Extraction
4.5. High-Performance Liquid Chromatography with Ultraviolet Detection (HPLC-UV)
4.6. High-Performance Liquid Chromatography—Mass Spectroscopy (LC-MS)
4.7. Metabolite Steady-State, Pathway, and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FA | formic acid |
| GSH | reduced glutathione |
| GSSG | oxidized glutathione |
| HCC | hepatocellular carcinoma |
| HPLC-UV | high-performance liquid chromatography with ultraviolet detection |
| KOH | potassium hydroxide |
| LC-MS | liquid chromatography combined with mass spectrometry |
| MeOH | methanol |
| NADPH | reduced form of nicotinamide adenine dinucleotide phosphate |
| NADP | oxidized form of nicotinamide adenine dinucleotide phosphate |
| NADH | reduced form of nicotinamide adenine dinucleotide |
| NAD | oxidized form of nicotinamide adenine dinucleotide |
| SLE | systemic lupus erythematosus |
References
- Pollak, N.; Dolle, C.; Ziegler, M. The power to reduce: Pyridine nucleotides—Small molecules with a multitude of functions. Biochem. J. 2007, 402, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, H.N.; Rolfo, M.; Ferraris, A.M.; Gaetani, G.F. Mechanisms of Protection of Catalase by NADPH: Kinetics and stochiometry. J. Biol. Chem. 1999, 274, 13908–13914. [Google Scholar] [CrossRef]
- Perl, A.; Hanczko, R.; Telarico, T.; Oaks, Z.; Landas, S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol. Med. 2011, 7, 395–403. [Google Scholar] [CrossRef]
- Krebs, H.A.; Veech, R.L. Equilibrium relations between pyridine nucleotides and adenine nucleotides and their roles in the regulation of metabolic processes. Adv. Enzym. Regul. 1969, 7, 397–413. [Google Scholar] [CrossRef]
- Marcuello, C.; de Miguel, R.; Martinez-Julvez, M.; Gomez-Moreno, C.; Lostao, A. Mechanostability of the Single-Electron-Transfer Complexes of Anabaena FerredoxinGÇôNADP+ Reductase. ChemPhysChem 2015, 16, 3161–3169. [Google Scholar] [CrossRef]
- Kra-ìun, D.; Lopes, L.R.; Cifuentes-Pagano, E.; Pagano, P.J. NADPH oxidases: Redox regulation of cell homeostasis and disease. Physiol. Rev. 2025, 105, 1291–1428. [Google Scholar] [CrossRef]
- Bouchard, M.; McAllister, A.; Bourlett, N.S.; Hoyt, C.; Calcul, L.; Walstrom, K.M.C. elegans Cytoplasmic Isocitrate Dehydrogenase Neomorphic G98N and R133H Mutants Produce the Oncometabolite 2-Hydroxyglutarate. Int. J. Mol. Sci. 2025, 26, 8238. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Miwa, M.; Miwa, K.; Hanayama, R.; Nagase, H.; Nagata, S.; Tanaka, M. Masking of Phosphatidylserine Inhibits Apoptotic Cell Engulfment and Induces Autoantibody Production in Mice. J. Exp. Med. 2004, 200, 459–467. [Google Scholar] [CrossRef]
- Nagy, G.; Koncz, A.; Fernandez, D.; Perl, A. Nitric oxide, mitochondrial hyperpolarization, and T cell activation. Free Radic. Biol. Med. 2007, 42, 1625–1631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banki, K.; Perl, A. Cell type-specific regulation of the pentose phosphate pathway during development and metabolic stress-driven autoimmune diseases: Relevance for inflammatory liver, renal, endocrine, cardiovascular and neurobehavioral comorbidities, carcinogenesis, and aging. Autoimmun. Rev. 2025; accepted. [Google Scholar][Green Version]
- Balsa, E.; Perry, E.A.; Bennett, C.F.; Jedrychowski, M.; Gygi, S.P.; Doench, J.G.; Puigserver, P. Defective NADPH production in mitochondrial disease complex I causes inflammation and cell death. Nat. Commun. 2020, 11, 2714. [Google Scholar] [CrossRef]
- Wang, H.; Cui, W.; Yue, S.; Zhu, X.; Li, X.; He, L.; Zhang, M.; Yang, Y.; Wei, M.; Wu, H.; et al. Malic enzymes in cancer: Regulatory mechanisms, functions, and therapeutic implications. Redox Biol. 2024, 75, 103273. [Google Scholar] [CrossRef]
- Ronchi, J.A.; Figueira, T.R.; Ravagnani, F.G.; Oliveira, H.C.F.; Vercesi, A.E.; Castilho, R.F. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic. Biol. Med. 2013, 63, 446–456. [Google Scholar] [CrossRef]
- Oaks, Z.; Patel, A.; Huang, N.; Choudhary, G.; Winans, T.; Faludi, T.; Krakko, D.; Duarte, M.; Lewis, J.; Beckford, M.; et al. Cytosolic aldose metabolism contributes to progression from cirrhosis to hepatocarcinogenesis. Nat. Metab. 2023, 5, 41–60, Erratum in Nat. Metab. 2023, 5, 349. [Google Scholar] [CrossRef]
- Levine, A.J.; Puzio-Kuter, A.M. The Control of the Metabolic Switch in Cancers by Oncogenes and Tumor Suppressor Genes. Science 2010, 330, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; van der Stelt, I.; Li, W.; Hu, L.; Song, J.; Grefte, S.; van de Westerlo, E.; Zhang, D.; van Schothorst, E.M.; der Grinten, H.C.-V.; et al. Mitochondrial Nicotinamide Nucleotide Transhydrogenase: Role in Energy Metabolism, Redox Homeostasis, and Cancer. Antioxid. Redox Signal. 2024, 41, 927–956. [Google Scholar] [CrossRef]
- Lin, S.J.; Guarente, L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr. Opin. Cell Biol. 2003, 15, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L.; Eggleston, L.V.; Krebs, H.A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem. J. 1969, 115, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Albeck, J.G.; Tantama, M.; Yellen, G. Imaging Cytosolic NADH-NAD+ Redox State with a Genetically Encoded Fluorescent Biosensor. Cell Metab. 2011, 14, 545–554. [Google Scholar] [CrossRef]
- Cracan, V.; Titov, D.V.; Shen, H.; Grabarek, Z.; Mootha, V.K. A genetically encoded tool for manipulation of NADP+/NADPH in living cells. Nat. Chem. Biol. 2017, 13, 1088–1095. [Google Scholar] [CrossRef]
- Lowry, O.H.; Passonneau, J.V.; Rock, M.K. The Stability of Pyridine Nucleotides. J. Biol. Chem. 1961, 236, 2756–2759. [Google Scholar] [CrossRef]
- Zhang, Y.; Krahnert, I.; Bolze, A.; Gibon, Y.; Fernie, A.R. Adenine Nucleotide and Nicotinamide Adenine Dinucleotide Measurements in Plants. Curr. Protoc. Plant Biol. 2020, 5, e20115. [Google Scholar] [CrossRef]
- Zerez, C.R.; Lee, S.J.; Tanaka, K.R. Spectrophotometric determination of oxidized and reduced pyridine nucleotides in erythrocytes using a single extraction procedure. Anal. Biochem. 1987, 164, 367–373. [Google Scholar] [CrossRef]
- Banki, K.; Hutter, E.; Colombo, E.; Gonchoroff, N.J.; Perl, A. Glutathione Levels and Sensitivity to Apoptosis Are Regulated by changes in Transaldolase expression. J. Biol. Chem. 1996, 271, 32994–33001. [Google Scholar] [CrossRef]
- Qian, Y.; Banerjee, S.; Grossman, C.E.; Amidon, W.; Nagy, G.; Barcza, M.; Niland, B.; Karp, D.R.; Middleton, F.A.; Banki, K.; et al. Transaldolase deficiency influences the pentose phosphate pathway, mitochondrial homoeostasis and apoptosis signal processing. Biochem. J. 2008, 415, 123–134. [Google Scholar] [CrossRef]
- Hanczko, R.; Fernandez, D.; Doherty, E.; Qian, Y.; Vas, G.; Niland, B.; Telarico, T.; Garba, A.; Banerjee, S.; Middleton, F.A.; et al. Prevention of hepatocarcinogenesis and acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine. J. Clin. Investig. 2009, 119, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Qian, Y.; Chohan, K.R.; Shirley, C.R.; Amidon, W.; Banerjee, S.; Middleton, F.A.; Conkrite, K.L.; Barcza, M.; Gonchoroff, N.; et al. Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. Proc. Natl. Acad. Sci. USA 2006, 103, 14813–14818. [Google Scholar] [CrossRef]
- Lu, W.; Wang, L.; Chen, L.; Hui, S.; Rabinowitz, J.D. Extraction and Quantitation of Nicotinamide Adenine Dinucleotide Redox Cofactors. Antioxid. Redox Signal. 2017, 28, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Prot. 2012, 7, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Banki, K.; Halladay, D.; Perl, A. Cloning and expression of the human gene for transaldolase: A novel highly repetitive element constitutes an integral part of the coding sequence. J. Biol. Chem. 1994, 269, 2847–2851. [Google Scholar] [CrossRef]
- Lee, B.L.; Moon, J.E.; Shu, J.H.; Yuan, L.; Newman, Z.R.; Schekman, R.; Barton, G.M. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife Sci. 2013, 2, e00291. [Google Scholar] [CrossRef]
- Hoffman, E.C.; Reyes, H.; Chu, F.F.; Sander, F.; Conley, L.H.; Brooks, B.A.; Hankinson, O. Cloning of a Factor Required for Activity of the Ah (Dioxin) Receptor. Science 1991, 252, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Toye, A.A.; Lippiat, J.D.; Proks, P.; Shimomura, K.; Bentley, L.; Hugill, A.; Mijat, V.; Goldsworthy, M.; Moir, L.; Haynes, A.; et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia 2005, 48, 675–686. [Google Scholar] [CrossRef]
- Freeman, H.C.; Hugill, A.; Dear, N.T.; Ashcroft, F.M.; Cox, R.D. Deletion of Nicotinamide Nucleotide Transhydrogenase: A New Quantitive Trait Locus Accounting for Glucose Intolerance in C57BL/6J Mice. Diabetes 2006, 55, 2153, Erratum in Diabetes 2014, 73, 815. [Google Scholar] [CrossRef]
- Williams, J.L.; Hall, C.L.; Meimaridou, E.; Metherell, L.A. Loss of nnt increases expression of oxidative phosphorylation complexes in c57bl/6j hearts. Int. J. Mol. Sci. 2021, 22, 6101. [Google Scholar] [CrossRef]
- Vozenilek, A.E.; Vetkoetter, M.; Green, J.M.; Shen, X.; Traylor, J.G.; Klein, R.L.; Orr, A.W.; Woolard, M.D.; Krzywanski, D.M. Absence of Nicotinamide Nucleotide Transhydrogenase in C57BL/6J Mice Exacerbates Experimental Atherosclerosis. J. Vasc. Res. 2018, 55, 98–110. [Google Scholar] [CrossRef]
- Williams, J.L.; Paudyal, A.; Awad, S.; Nicholson, J.; Grzesik, D.; Botta, J.; Meimaridou, E.; Maharaj, A.V.; Stewart, M.; Tinker, A.; et al. Mylk3 null c57bl/6n mice develop cardiomyopathy, whereas nnt null c57bl/6j mice do not. Life Sci. Alliance 2020, 3, e201900593. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.; Engel, D.F.; Figueira, T.R.; Rogerio, F.; de Bem, A.F.; Castilho, R.F. Mitochondrial NAD(P)+ Transhydrogenase is Unevenly Distributed in Different Brain Regions, and its Loss Causes Depressive-like Behavior and Motor Dysfunction in Mice. Neuroscience 2020, 440, 210–229. [Google Scholar] [CrossRef]
- Rydstrom, J. Mitochondrial NADPH, transhydrogenase and disease. Biochim. Biophys. Acta 2006, 1757, 721–726. [Google Scholar] [CrossRef]
- Simon, M.M.; Greenaway, S.; White, J.K.; Fuchs, H.; Gailus-Durner, V.R.; Wells, S.; Sorg, T.; Wong, K.; Bedu, E.; Cartwright, E.J.; et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013, 14, R82. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Chen, C.H.; Ursell, P.; Huang, T.T. Genetic modifier of mitochondrial superoxide dismutase-deficient mice delays heart failure and prolongs survival. Mamm. Genome 2010, 21, 534–542. [Google Scholar] [CrossRef]
- Nickel, A.G.; von Hardenberg, A.; Hohl, M.; Loffler, J.R.; Kohlhaas, M.; Becker, J.; Reil, J.C.; Kazakov, A.; Bonnekoh, J.; Stadelmaier, M.; et al. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metab. 2015, 22, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Naeemuddin, M.; Elchuri, S.; Yamaguchi, M.; Kozy, H.M.; Carlson, E.J.; Epstein, C.J. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum. Mol. Genet. 2006, 15, 1187–1194. [Google Scholar] [CrossRef]
- Iyama, T.; Abolhassani, N.; Tsuchimoto, D.; Nonaka, M.; Nakabeppu, Y. NUDT16 is a (deoxy)inosine diphosphatase, and its deficiency induces accumulation of single-strand breaks in nuclear DNA and growth arrest. Nucl. Acids Res. 2010, 38, 4834–4843. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Yang, X.; Zhang, X.; Liu, J. The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes. Brit. J. Pharmacol. 2013, 168, 163–171. [Google Scholar] [CrossRef]
- Xiang, F.; Zhang, Z.; Xie, J.; Xiong, S.; Yang, C.; Liao, D.; Xia, B.; Lin, L. Comprehensive review of the expanding roles of the carnitine pool in metabolic physiology: Beyond fatty acid oxidation. J. Transl. Med. 2025, 23, 324. [Google Scholar] [CrossRef] [PubMed]
- Rockenfeller, P.; Koska, M.; Pietrocola, F.; Minois, N.; Knittelfelder, O.; Sica, V.; Franz, J.; Carmona-Gutierrez, D.; Kroemer, G.; Madeo, F. Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ. 2015, 22, 499–508. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Nagy, G.M. Dopamine-Derived Salsolinol Derivatives as Endogenous Monoamine Oxidase Inhibitors: Occurrence, Metabolism and Function in Human Brains. NeuroToxicology 2004, 25, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Sanayama, H.; Ito, K.; Ookawara, S.; Uemura, T.; Sakiyama, Y.; Sugawara, H.; Tabei, K.; Igarashi, K.; Soda, K. Whole Blood Spermine/Spermidine Ratio as a New Indicator of Sarcopenia Status in Older Adults. Biomedicines 2023, 11, 1403. [Google Scholar] [CrossRef]
- Guo, Y.; Ye, Q.; Deng, P.; Cao, Y.; He, D.; Zhou, Z.; Wang, C.; Zaytseva, Y.Y.; Schwartz, C.E.; Lee, E.Y.; et al. Spermine synthase and MYC cooperate to maintain colorectal cancer cell survival by repressing Bim expression. Nat. Commun. 2020, 11, 3243. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Womack, T.R.; Vollert, C.T.; Ohia-Nwoko, O.; Schmitt, M.; Montazari, S.; Beckett, T.L.; Mayerich, D.; Murphy, M.P.; Eriksen, J.L. Prostacyclin Promotes Degenerative Pathology in a Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2022, 16, 769347. [Google Scholar] [CrossRef]
- Huang, N.; Winans, T.; Wyman, B.; Oaks, Z.; Faludi, T.; Choudhary, G.; Lai, Z.-W.; Lewis, J.; Beckford, M.; Duarte, D.; et al. Rab4A-directed endosome traffic shapes pro-inflammatory mitochondrial metabolism in T cells via mitophagy, CD98 expression, and kynurenine-sensitive mTOR activation. Nat. Commun. 2024, 15, 2598. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Jeon, S.M.; Chandel, N.S.; Hay, N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012, 485, 661–665. [Google Scholar] [CrossRef]
- Singh, A.; Happel, C.; Manna, S.K.; Acquaah-Mensah, G.; Carrerero, J.; Kumar, S.; Nasipuri, P.; Krausz, K.W.; Wakabayashi, N.; Dewi, R.; et al. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Investig. 2013, 123, 2921–2934. [Google Scholar] [CrossRef]
- Moreno-Sanchez, R.; Gallardo-Perez, J.C.; Rodriguez-Enriquez, S.; Saavedra, E.; Marin-Hernandez, A. Control of the NADPH supply for oxidative stress handling in cancer cells. Free Radic. Biol. Med. 2017, 112, 149–161. [Google Scholar] [CrossRef]
- Krebs, H.A.; Eggleston, L.V. The regulation of the pentose phosphate cycle in rat liver. Adv. Enzym. Regul. 1974, 12, 421–434. [Google Scholar] [CrossRef]
- Zhang, J.; Pierick, A.T.; van Rossum, H.M.; Seifar, R.M.; Ras, C.; Daran, J.M.; Heijnen, J.J.; Wahl, S.J. Determination of the Cytosolic NADPH/NADP Ratio in Saccharomyces cerevisiae using Shikimate Dehydrogenase as Sensor Reaction. Sci. Rep. 2015, 5, 12846. [Google Scholar] [CrossRef]
- Jones, D.P. Determination of pyridine dinucleotides in cell extracts by high-performance liquid chromatography. J. Chromatog. B Biomed. Sci. Appl. 1981, 225, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Ser, Z.; Liu, X.; Tang, N.N.; Locasale, J.W. Extraction parameters for metabolomics from cultured cells. Anal. Biochem. 2015, 475, 22–28. [Google Scholar] [CrossRef]
- Lu, W.; Su, X.; Klein, M.S.; Lewis, I.A.; Fiehn, O.; Rabinowitz, J.D. Metabolite measurement: Pitfalls to avoid and practices to follow. Ann. Rev. Biochem. 2017, 86, 277–304. [Google Scholar] [CrossRef]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef]
- Lepoittevin, M.; Blancart-Remaury, Q.; Kerforne, T.; Pellerin, L.; Hauet, T.; Thuillier, R. Comparison between 5 extractions methods in either plasma or serum to determine the optimal extraction and matrix combination for human metabolomics. Cell. Mol. Biol. Lett. 2023, 28, 43. [Google Scholar] [CrossRef]
- Perl, A.; Hanczko, R.; Doherty, E. Assessment of mitochondrial dysfunction in lymphocytes of patients with systemic lupus erythematosus. In Autoimmunity: Methods and Protocols; Perl, A., Ed.; Springer: Clifton, NJ, USA, 2012; p. 61. [Google Scholar]
- Meimaridou, E.; Goldsworthy, M.; Chortis, V.; Fragouli, E.; Foster, P.A.; Arlt, W.; Cox, R.; Metherell, L.A. NNT is a key regulator of adrenal redox homeostasis and steroidogenesis in male mice. J. Endocrinol. 2018, 236, 13–28. [Google Scholar] [CrossRef]
- Hofmann, D.; Wirtz, A.; Santiago-Schubel, B.; Disko, U.; Pohl, M. Structure elucidation of the thermal degradation products of the nucleotide cofactors NADH and NADPH by nano-ESI-FTICR-MS and HPLC-MS. Anal. Bioanal. Chem. 2010, 398, 2803–2811. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimakawa, G.; Tabata, H.; Kusama, S.; Miyake, C.; Nakanishi, S. Quantification of NAD(P)H in cyanobacterial cells by a phenol extraction method. Photosynth. Res. 2021, 148, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, T.; Wang, Y.; Ji, F.; Zhang, L. The spatio-temporal trends and determinants of liver cancer attributable to specific etiologies: A systematic analysis from the Global Burden of Disease Study 2021. Glob. Health Res. Policy 2025, 10, 22. [Google Scholar] [CrossRef]
- Pons-Estel, G.J.; Ugarte-Gil, M.F.; Alarcon, G.S. Epidemiology of systemic lupus erythematosus. Exp. Rev. Clin. Immunol. 2017, 13, 799–814. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Yao, X.; Huang, Y.; Lu, Q. Global epidemiology of systemic lupus erythematosus: A comprehensive systematic analysis and modelling study. Ann. Rheum. Dis. 2023, 82, 351. [Google Scholar] [CrossRef]
- Luo, L.; Gáo, X.; Yang, J.; Zhang, X.; Liu, Z.; Li, C. Musculoskeletal disorders in women of childbearing age: Global trends, socio-demographic disparities, and future projections. Ann. Med. 2025, 57, 2532860. [Google Scholar] [CrossRef]
- Kono, H.; Kyogoku, C.; Suzuki, T.; Tsuchiya, N.; Honda, H.; Yamamoto, K.; Tokunaga, K.; Honda, Z.I. FcγRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum. Mol. Genet. 2005, 14, 2881–2892. [Google Scholar] [CrossRef]
- Pollak, N.; Niere, M.; Ziegler, M. NAD Kinase Levels Control the NADPH Concentration in Human Cells. J. Biol. Chem. 2007, 282, 33562–33571. [Google Scholar] [CrossRef]
- Merker, M.P.; Bongard, R.D.; Kettenhofen, N.J.; Okamoto, Y.; Dawson, C.A. Intracellular redox status affects transplasma membrane electron transport in pulmonary arterial endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L36–L43. [Google Scholar] [CrossRef]
- Brookes, P.S.; Shiva, S.; Patel, R.P.; Darley-Usmar, V.M. Measurement of mitochondrial respiratory thresholds and the control of respiration by nitric oxide. In Methods in Enzymology Nitric Oxide, Part D: Oxide Detection, Mitochondria and Cell Functions, and Peroxynitrite Reactions; Academic Press: New York, NY, USA, 2002; p. 305. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef]
- Xia, J.; Mandal, R.; Sinelnikov, I.V.; Broadhurst, D.; Wishart, D.S. MetaboAnalyst 2.0-a comprehensive server for metabolomic data analysis. Nucl. Acids Res. 2012, 40, W127–W133. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faludi, T.; Krakko, D.; Nolan, J.; Hanczko, R.; Patel, A.; Oaks, Z.; Ruggiero, E.; Lewis, J.; Wang, X.; Huang, T.-T.; et al. Potassium-Hydroxide-Based Extraction of Nicotinamide Adenine Dinucleotides from Biological Samples Offers Accurate Assessment of Intracellular Redox Status. Int. J. Mol. Sci. 2025, 26, 10371. https://doi.org/10.3390/ijms262110371
Faludi T, Krakko D, Nolan J, Hanczko R, Patel A, Oaks Z, Ruggiero E, Lewis J, Wang X, Huang T-T, et al. Potassium-Hydroxide-Based Extraction of Nicotinamide Adenine Dinucleotides from Biological Samples Offers Accurate Assessment of Intracellular Redox Status. International Journal of Molecular Sciences. 2025; 26(21):10371. https://doi.org/10.3390/ijms262110371
Chicago/Turabian StyleFaludi, Tamas, Daniel Krakko, Jessica Nolan, Robert Hanczko, Akshay Patel, Zach Oaks, Evan Ruggiero, Joshua Lewis, Xiaojing Wang, Ting-Ting Huang, and et al. 2025. "Potassium-Hydroxide-Based Extraction of Nicotinamide Adenine Dinucleotides from Biological Samples Offers Accurate Assessment of Intracellular Redox Status" International Journal of Molecular Sciences 26, no. 21: 10371. https://doi.org/10.3390/ijms262110371
APA StyleFaludi, T., Krakko, D., Nolan, J., Hanczko, R., Patel, A., Oaks, Z., Ruggiero, E., Lewis, J., Wang, X., Huang, T.-T., Molnar-Perl, I., & Perl, A. (2025). Potassium-Hydroxide-Based Extraction of Nicotinamide Adenine Dinucleotides from Biological Samples Offers Accurate Assessment of Intracellular Redox Status. International Journal of Molecular Sciences, 26(21), 10371. https://doi.org/10.3390/ijms262110371






