Redox Homeostasis in Metabolic Syndrome and Type II Diabetes: Role of Skeletal Muscle and Impact of Gold-Standard Treatments
Abstract
1. Introduction
2. Redox Signaling in Healthy Skeletal Muscle
2.1. Physiolgical ROS/RNS and Its Roles in Skeletal Muscle
2.1.1. Introduction to ‘Redox’ Signaling
| ROS/RNS Molecule | Generated by | Reactivity * (L mol−1 s−1) [32] | Diffusion Capacity/Estimated Distance | Signaling Specificity | Half-Lives (Approximate) | References |
|---|---|---|---|---|---|---|
| Hydrogen Peroxide (H2O2) | SOD and oxidase enzymes | 2 × 10−2 | Membrane-permeable and use aquaporins; 100 µm | High | 40 ms–2 s | [33,34,35] |
| Hydroxyl Radical (OH•) | H2O2 reaction with Fe2+/Cu+ or ONOO− breakdown | 7 × 109 | Essentially no diffusion, attacks nearest targets; 12 nm | No signaling role | 3.5 ns | [36,37] |
| Nitric Oxide (NO•) | NOS enzymes | Very slow | Membrane permeable; 2–190 µm | High | 2 ms–2 s | [36,38] |
| Peroxynitrite (ONOO−) | Reaction of O2•− with NO | 2 × 10−1 | Limited diffusion; 96 µm | Pathologically high; limited physiological signaling | 0.77 s | [36,39] |
| Superoxide (O2•−) | NOX enzymes and mitochondrial respiration leak; one electron reduction of O2 | <0.3 | Poor membrane permeability; 0.8 µm | Low direct signaling, mainly a precursor to H2O2/ONOO− | 35 µs | [35,36,40] |
2.1.2. Sources of ROS/RNS in Skeletal Muscle
Mitochondrial ROS
NOX Enzymes
Additional Enzymatic Sources of ROS/RNS in Skeletal Muscle
Redox-Sensitive Systems in Skeletal Muscle
2.2. Antioxidant Defense Systems
2.3. Insulin Signaling Dependence on Redox-Sensitive Pathways
2.3.1. Overview of Insulin Signaling in Skeletal Muscle
2.3.2. Redox Actions in the Insulin Signaling Cascade
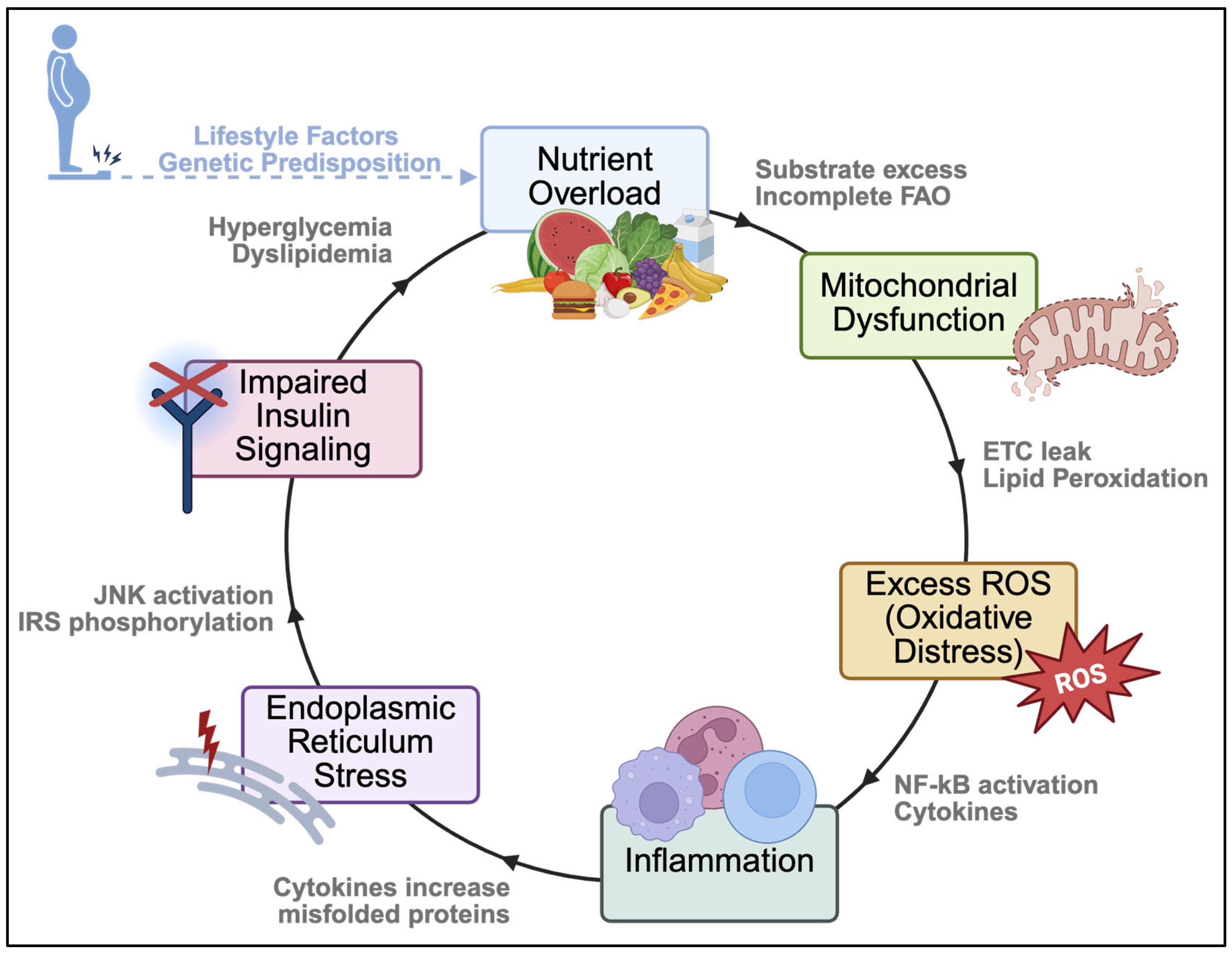
3. Redox Signaling During Metabolic Disease Progression
3.1. Redox Damage and Disruptions to Insulin Signaling
3.2. Redox-Induced PTMs
3.3. Mitochondrial Dysfunction
3.4. Lipid Accumulation
3.5. Inflammation
3.6. Antioxidant Defenses
3.7. Endoplasmic Reticulum Stress
4. Treatment-Based Changes to Redox Signaling in Skeletal Muscle
4.1. Biguanides (Metformin)
4.2. Insulin
4.3. Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors
4.4. Insulin Secretagogues
4.4.1. Sulfonylureas
4.4.2. Meglitinide Analogs
4.5. GLP-1 Receptor Agonists and Dual GLP-1/GIP Incretin Therapies
4.6. DPP-4 Inhibitors
4.7. Summary of Therapuetic Effects on Skeletal Muscle Redox Signaling
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HHE | 4-hydroxy-2-hexenal |
| 4-HNE | 4-hydroxy-2-nonenal |
| 4-ONE | 4-oxo-2-nonenal |
| AGE(s) | Advanced glycation end-product(s) |
| Akt | Protein kinase B |
| AMPK | AMP-activated kinase(s) |
| AP-1 | Activator protein 1 |
| CPT1 | Carnitine palmitoyltransferase 1 |
| CVD | Cardiovascular disease |
| COX(s) | Cyclooxygenase(s) |
| DAG(s) | Diacylglycerol(s) |
| DPP-4 | Dipeptidyl peptidase-4 |
| DRP1 | Dynamin-related protein 1 |
| ER | Endoplasmic reticulum |
| ERO1α | Oxidoreductin-1α |
| ETC | Electron transport chain |
| FOXO | Forkhead box class O |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-1RAs | Glucagon-like peptide-1 receptor agonist(s) |
| GLUT4 | Glucose transporter 4 |
| GPX(s) | Glutathione peroxidase(s) |
| GSH/GSSG | Glutathione (reduced/oxidized) |
| GSK3 | Glycogen synthase kinase 3 |
| H2O2 | Hydrogen peroxide |
| HFD | High-fat diet |
| IL | Interleukin |
| IR | Insulin receptor |
| IRS | Insulin receptor substrate(s) |
| JNK | c-Jun N-terminal kinase |
| LOX(s) | Lipoxygenase(s) |
| MAPK | Mitogen-activated kinase |
| MFN1/2 | Mitofusin 1/2 |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| NADH/NAD+ | Nicotinamide adenine dinucleotide (reduced/oxidized) |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| (e)(i)(n)NOS | (endothelial) (inducible) (neuronal) nitric oxide synthase |
| NOX | NAD(P)H oxidase |
| Nrf2 | Nuclear factor erythroid 2-related factor |
| O2•− | Superoxide |
| ONOO− | Peroxynitrite |
| OPA1 | Optic atrophy 1 (protein) |
| OXPHOS | Oxidative phosphorylation |
| PDI | Protein disulfide isomerase |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator-1α |
| PI3K | Phosphoinositide 3-kinase |
| PIP2 | Phosphatidylinositol bisphosphate |
| PIP3 | Phosphatidinositol-3,4,5-triphosphate |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PLA2 | Phospholipase A |
| PRX(s) | Peroxiredoxin(s) |
| PUFA(s) | Polyunsaturated fatty acid(s) |
| Rac1 | RAS-related C3 botulinum toxin substrate 1 |
| Redox | Oxidation-reduction |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SERCA | Sarco/endoplasmic reticulum calcium ATPase |
| SGLT2 | Sodium-glucose transport protein 2 |
| SOD | Superoxide dismutase |
| T2DM | Type 2 diabetes mellitus |
| TCA | Tricarboxylic acid (cycle) |
| TFAM | Transcription factor α mitochondrial |
| TNF | Tumor necrosis factor |
| UPR | Unfolded protein response |
| XOR | Xanthine oxidoreductase |
References
- International Diabetes Federation. IDF Diabetes Atlas. Available online: http://www.diabetesatlas.org (accessed on 22 October 2025).
- Wysham, C.; Shubrook, J. Beta-cell failure in type 2 diabetes: Mechanisms, markers, and clinical implications. Postgrad. Med. 2020, 132, 676–686. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Gast, K.B.; Tjeerdema, N.; Stijnen, T.; Smit, J.W.; Dekkers, O.M. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS ONE 2012, 7, e52036. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, P.M.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, H.R.; Grundy, M.S.; Zimmet, Z.P.; Cleeman, I.J.; Donato, A.K.; Fruchart, J.-C.; James, T.P.W.; Loria, M.C.; Smith, C.S. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Cheeseman, H.K.; Slater, F.T. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Krishnamurthy, K.H.; Pereira, M.; Rajavelu, I.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Oxidative stress: Fundamentals and advances in quantification techniques. Front. Chem. 2024, 12, 1470458. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The Effect of Insulin on the Disposal of Intravenous Glucose: Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Sylow, L.; Tokarz, V.L.; Richter, E.A.; Klip, A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021, 33, 758–780. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef]
- Affourtit, C. Mitochondrial involvement in skeletal muscle insulin resistance: A case of imbalanced bioenergetics. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1678–1693. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48 (Suppl. S1), S27–S49. [Google Scholar] [CrossRef]
- Sena, C.M.; Bento, C.F.; Pereira, P.; Seiça, R. Diabetes mellitus: New challenges and innovative therapies. EPMA J. 2010, 1, 138–163. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Syeda, U.S.A.; Battillo, D.; Visaria, A.; Malin, S.K. The importance of exercise for glycemic control in type 2 diabetes. Am. J. Med. Open 2023, 9, 100031. [Google Scholar] [CrossRef]
- Kinaan, M.; Ding, H.; Triggle, C.R. Metformin: An Old Drug for the Treatment of Diabetes but a New Drug for the Protection of the Endothelium. Med. Princ. Pract. 2015, 24, 401–415. [Google Scholar] [CrossRef]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020, 34, 101517. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.J.; Duncan, T.G.; Goodman, A.M.; Mills, D.J.; Rohlf, J.L. Efficacy of metformin in type II diabetes: Results of a double-blind, placebo-controlled, dose-response trial. Am. J. Med. 1997, 103, 491–497. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Rizzo, M. The Emerging Role of Dual GLP-1 and GIP Receptor Agonists in Glycemic Management and Cardiovascular Risk Reduction. Diabetes Metab. Syndr. Obes. 2022, 15, 1023–1030. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rudich, A. Positive and Negative Regulation of Insulin Signaling by Reactive Oxygen and Nitrogen Species. Physiol. Rev. 2009, 89, 27–71. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef]
- Ledo, A.; Fernandes, E.; Salvador, A.; Laranjinha, J.; Barbosa, R.M. In vivo hydrogen peroxide diffusivity in brain tissue supports volume signaling activity. Redox Biol. 2022, 50, 102250. [Google Scholar] [CrossRef]
- Sousa, T.; Gouveia, M.; Travasso, D.M.R.; Salvador, A. How abundant are superoxide and hydrogen peroxide in the vasculature lumen, how far can they reach? Redox Biol. 2022, 58, 102527. [Google Scholar] [CrossRef]
- Imlay, A.J. The Barrier Properties of Biological Membranes Dictate How Cells Experience Oxidative Stress. Mol. Microbiol. 2025, 123, 454–463. [Google Scholar] [CrossRef]
- Möller, N.M.; Denicola, A. Diffusion of peroxynitrite, its precursors, and derived reactive species, and the effect of cell membranes. Redox Biochem. Chem. 2024, 9, 100033. [Google Scholar] [CrossRef]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, M.S.; Sharma, S.N.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef] [PubMed]
- Prolo, C.; Piacenza, L.; Radi, R. Peroxynitrite: A multifaceted oxidizing and nitrating metabolite. Curr. Opin. Chem. Biol. 2024, 80, 102459. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-M.; Li, Y.; Deng, S.-L.; Zhao, Y.; Lian, Z.-X.; Yu, K. Mitochondrial Function and Reactive Oxygen/Nitrogen Species in Skeletal Muscle. Front. Cell Dev. Biol. 2022, 10, 826981. [Google Scholar] [CrossRef]
- Anthony, T.G. Mechanisms of protein balance in skeletal muscle. Domest. Anim. Endocrinol. 2016, 56, S23–S32. [Google Scholar] [CrossRef]
- Powers, S.K.; Schrager, M. Redox signaling regulates skeletal muscle remodeling in response to exercise and prolonged inactivity. Redox Biol. 2022, 54, 102374. [Google Scholar] [CrossRef] [PubMed]
- Rathor, R.; Suryakumar, G. Myokines: A central point in managing redox homeostasis and quality of life. BioFactors 2024, 50, 885–909. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.A.; Packer, L.; Brooks, G.A. Exercise bioenergetics following sprint training. Arch. Biochem. Biophys. 1982, 215, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Vasilaki, A.; McArdle, A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic. Biol. Med. 2016, 98, 13–17. [Google Scholar] [CrossRef]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef]
- Díaz-Vegas, A.; Campos, C.A.; Contreras-Ferrat, A.; Casas, M.; Buvinic, S.; Jaimovich, E.; Espinosa, A. ROS Production via P2Y1-PKC-NOX2 Is Triggered by Extracellular ATP after Electrical Stimulation of Skeletal Muscle Cells. PLoS ONE 2015, 10, e0129882. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef]
- Xirouchaki, C.E.; Jia, Y.; McGrath, M.J.; Greatorex, S.; Tran, M.; Merry, T.L.; Hong, D.; Eramo, M.J.; Broome, S.C.; Woodhead, J.S.T.; et al. Skeletal muscle NOX4 is required for adaptive responses that prevent insulin resistance. Sci. Adv. 2021, 7, eabl4988. [Google Scholar] [CrossRef]
- Gong, M.C.; Arbogast, S.; Guo, Z.; Mathenia, J.; Su, W.; Reid, M.B. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J. Appl. Physiol. 2006, 100, 399–405. [Google Scholar] [CrossRef]
- Tengan, C.H.; Rodrigues, G.S.; Godinho, R.O. Nitric oxide in skeletal muscle: Role on mitochondrial biogenesis and function. Int. J. Mol. Sci. 2012, 13, 17160–17184. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Lei, H.; Qin, H.; Xia, Y. Molecular mechanisms of endothelial NO synthase uncoupling. Curr. Pharm. Des. 2014, 20, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Lee, M.; Rey, K.; Besler, K.; Wang, C.; Choy, J. Immunobiology of Nitric Oxide and Regulation of Inducible Nitric Oxide Synthase. In Macrophages: Origin, Functions and Biointervention; Kloc, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 181–207. [Google Scholar]
- Mueller, B.J.; Roberts, M.D.; Mobley, C.B.; Judd, R.L.; Kavazis, A.N. Nitric oxide in exercise physiology: Past and present perspectives. Front. Physiol. 2024, 15, 1504978. [Google Scholar] [CrossRef]
- Ortiz de Zevallos, J.; Woessner, M.N.; Kelley, E.E. Skeletal muscle as a reservoir for nitrate and nitrite: The role of xanthine oxidase reductase (XOR). Nitric Oxide 2022, 129, 102–109. [Google Scholar] [CrossRef]
- Upanan, S.; Lee, J.; Tunau-Spencer, K.J.; Rajvanshi, P.K.; Wright, E.C.; Noguchi, C.T.; Schechter, A.N. High nitrate levels in skeletal muscle contribute to nitric oxide generation via a nitrate/nitrite reductive pathway in mice that lack the nNOS enzyme. Front. Physiol. 2024, 15, 1352242. [Google Scholar] [CrossRef]
- Chen, T.H.; Koh, K.Y.; Lin, K.M.; Chou, C.K. Mitochondrial Dysfunction as an Underlying Cause of Skeletal Muscle Disorders. Int. J. Mol. Sci. 2022, 23, 12926. [Google Scholar] [CrossRef]
- Wong, H.-S.; Dighe, P.A.; Mezera, V.; Monternier, P.-A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017, 292, 16804–16809. [Google Scholar] [CrossRef]
- Koren, S.A.; Ahmed Selim, N.; De la Rosa, L.; Horn, J.; Farooqi, M.A.; Wei, A.Y.; Müller-Eigner, A.; Emerson, J.; Johnson, G.V.W.; Wojtovich, A.P. All-optical spatiotemporal mapping of ROS dynamics across mitochondrial microdomains in situ. Nat. Commun. 2023, 14, 6036, Erratum in Nat. Commun. 2023, 14, 8230. [Google Scholar] [CrossRef]
- Loschen, G.; Azzi, A.; Richter, C.; Flohé, L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974, 42, 68–72. [Google Scholar] [CrossRef]
- Boveris, A.; Chance, B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Javeshghani, D.; Magder, S.A.; Barreiro, E.; Quinn, M.T.; Hussain, S.N. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am. J. Respir. Crit. Care Med. 2013, 165, 412–418, Erratum in Am. J. Respir. Crit. Care Med. 2013, 188, 1271. [Google Scholar]
- Henríquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Baker, J.S.; Davison, G.W.; Yan, X. Redox signaling and skeletal muscle adaptation during aerobic exercise. iScience 2024, 27, 109643. [Google Scholar] [CrossRef]
- Truong, T.H.; Carroll, K.S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 332–356. [Google Scholar] [CrossRef]
- Bogeski, I.; Niemeyer, B.A. Redox regulation of ion channels. Antioxid. Redox Signal. 2014, 21, 859–862. [Google Scholar] [CrossRef]
- Priya Dharshini, L.C.; Vishnupriya, S.; Sakthivel, K.M.; Rasmi, R.R. Oxidative stress responsive transcription factors in cellular signalling transduction mechanisms. Cell. Signal. 2020, 72, 109670. [Google Scholar] [CrossRef]
- Rahman, M.; Mofarrahi, M.; Kristof, A.S.; Nkengfac, B.; Harel, S.; Hussain, S.N. Reactive oxygen species regulation of autophagy in skeletal muscles. Antioxid. Redox Signal. 2014, 20, 443–459. [Google Scholar] [CrossRef]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sircar, E.; Bhattacharyya, C.; Choudhuri, A.; Mishra, A.; Dutta, S.; Bhatta, S.; Sachin, K.; Sengupta, R. S-Denitrosylation: A Crosstalk between Glutathione and Redoxin Systems. Antioxidants 2022, 11, 1921. [Google Scholar] [CrossRef]
- Yesupatham, A.; Saraswathy, R. Role of oxidative stress in prediabetes development. Biochem. Biophys. Rep. 2025, 43, 102069. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle–A focus on the molecular mechanisms of insulin resistance. Mol. Cell. Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef]
- Ishikura, S.; Koshkina, A.; Klip, A. Small G proteins in insulin action: Rab and Rho families at the crossroads of signal transduction and GLUT4 vesicle traffic. Acta Physiol. 2008, 192, 61–74. [Google Scholar] [CrossRef]
- Ishikura, S.; Bilan, P.J.; Klip, A. Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem. Biophys. Res. Commun. 2007, 353, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Hale, A.J.; Ter Steege, E.; den Hertog, J. Recent advances in understanding the role of protein-tyrosine phosphatases in development and disease. Dev. Biol. 2017, 428, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Di Cristofano, A.; Woo, M. Metabolic Role of PTEN in Insulin Signaling and Resistance. Cold Spring Harb. Perspect. Med. 2020, 10, a036137. [Google Scholar] [CrossRef]
- Czech, M.P.; Lawrence, J.C.; Lynn, W.S. Evidence for electron transfer reactions involved in the Cu2+-dependent thiol activation of fat cell glucose utilization. J. Biol. Chem. 1974, 249, 1001–1006. [Google Scholar] [CrossRef]
- May, J.M.; de Haën, C. The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J. Biol. Chem. 1979, 254, 9017–9021. [Google Scholar] [CrossRef]
- Hayes, G.R.; Lockwood, D.H. Role of insulin receptor phosphorylation in the insulinomimetic effects of hydrogen peroxide. Proc. Natl. Acad. Sci. USA 1987, 84, 8115–8119. [Google Scholar] [CrossRef]
- Higaki, Y.; Mikami, T.; Fujii, N.; Hirshman, M.F.; Koyama, K.; Seino, T.; Tanaka, K.; Goodyear, L.J. Oxidative stress stimulates skeletal muscle glucose uptake through a phosphatidylinositol 3-kinase-dependent pathway. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E889–E897. [Google Scholar] [CrossRef]
- Martínez Báez, A.; Ayala, G.; Pedroza-Saavedra, A.; González-Sánchez, H.M.; Chihu Amparan, L. Phosphorylation Codes in IRS-1 and IRS-2 Are Associated with the Activation/Inhibition of Insulin Canonical Signaling Pathways. Curr. Issues Mol. Biol. 2024, 46, 634–649. [Google Scholar] [CrossRef]
- Henriksen, E.J. Effects of H2O2 on insulin signaling the glucose transport system in mammalian skeletal muscle. Methods Enzymol. 2013, 528, 269–278. [Google Scholar]
- Henríquez-Olguín, C.; Gallero, S.; Reddy, A.; Persson, K.W.; Schlabs, F.L.; Voldstedlund, C.T.; Valentinaviciute, G.; Meneses-Valdés, R.; Sigvardsen, C.M.; Kiens, B.; et al. Revisiting insulin-stimulated hydrogen peroxide dynamics reveals a cytosolic reductive shift in skeletal muscle. Redox Biol. 2025, 82, 103607. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox regulation of the insulin signalling pathway. Redox Biol. 2021, 42, 101964. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.L.; Hotz-Wagenblatt, A.; Klein, H.; Dröge, W. Interdependent regulation of insulin receptor kinase activity by ADP and hydrogen peroxide. J. Biol. Chem. 2005, 280, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Ahn, Y.; Lee, S.-R.; Yeo, C.Y.; Hur, K.C. The Major Target of the Endogenously Generated Reactive Oxygen Species in Response to Insulin Stimulation Is Phosphatase and Tensin Homolog and Not Phosphoinositide-3 Kinase (PI-3 Kinase) in the PI-3 Kinase/Akt Pathway. Mol. Biol. Cell 2005, 16, 348–357. [Google Scholar] [CrossRef]
- Mahadev, K.; Zilbering, A.; Zhu, L.; Goldstein, B.J. Insulin-stimulated Hydrogen Peroxide Reversibly Inhibits Protein-tyrosine Phosphatase 1B in Vivo and Enhances the Early Insulin Action Cascade. J. Biol. Chem. 2001, 276, 21938–21942. [Google Scholar] [CrossRef]
- Su, Z.; Burchfield, J.G.; Yang, P.; Humphrey, S.J.; Yang, G.; Francis, D.; Yasmin, S.; Shin, S.-Y.; Norris, D.M.; Kearney, A.L. Global redox proteome and phosphoproteome analysis reveals redox switch in Akt. Nat. Commun. 2019, 10, 5486. [Google Scholar] [CrossRef]
- Wani, R.; Qian, J.; Yin, L.; Bechtold, E.; King, S.B.; Poole, L.B.; Paek, E.; Tsang, A.W.; Furdui, C.M. Isoform-specific regulation of Akt by PDGF-induced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2011, 108, 10550–10555. [Google Scholar] [CrossRef]
- Alcala, M.; Calderon-Dominguez, M.; Serra, D.; Herrero, L.; Ramos, M.P.; Viana, M. Short-term vitamin E treatment impairs reactive oxygen species signaling required for adipose tissue expansion, resulting in fatty liver and insulin resistance in obese mice. PLoS ONE 2017, 12, e0186579. [Google Scholar] [CrossRef]
- Fernández-Puente, E.; Martín-Prieto, E.; Márquez, C.M.; Palomero, J. Effect of RONS-Induced Intracellular Redox Homeostasis in 6-NBDG/Glucose Uptake in C2C12 Myotubes and Single Isolated Skeletal Muscle Fibres. Int. J. Mol. Sci. 2023, 24, 8082. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Lai, Y.C. Redox Regulation of Metabolic Syndrome: Recent Developments in Skeletal Muscle Insulin Resistance and Non-alcoholic Fatty Liver Disease (NAFLD). Curr. Opin. Physiol. 2019, 9, 79–86. [Google Scholar] [CrossRef]
- Wang, X.; Gu, C.; He, W.; Ye, X.; Chen, H.; Zhang, X.; Hai, C. Glucose oxidase induces insulin resistance via influencing multiple targets in vitro and in vivo: The central role of oxidative stress. Biochimie 2012, 94, 1705–1717. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Liemburg-Apers, D.C.; Willems, P.H.; Koopman, W.J.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, M.H. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Homko, C.; Barrero, A.C.; Stein, P.T.; Chen, X.; Cheung, P.; Fecchio, C.; Koller, S.; Merali, S. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci. Transl. Med. 2015, 7, 304re7. [Google Scholar] [CrossRef] [PubMed]
- Ingram, H.K.; Hill, H.; Moellering, R.D.; Hill, G.B.; Lara-Castro, C.; Newcomer, B.; Brandon, J.L.; Ingalls, P.C.; Penumetcha, M.; Rupp, C.J.; et al. Skeletal Muscle Lipid Peroxidation and Insulin Resistance in Humans. J. Clin. Endocrinol. Metab. 2012, 97, E1182–E1186. [Google Scholar] [CrossRef]
- Frohnert, I.B.; Bernlohr, A.D. Protein Carbonylation, Mitochondrial Dysfunction, and Insulin Resistance. Adv. Nutr. 2013, 4, 157–163. [Google Scholar] [CrossRef]
- Shearn, C.T.; Fritz, K.S.; Reigan, P.; Petersen, D.R. Modification of Akt2 by 4-hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry 2011, 50, 3984–3996. [Google Scholar] [CrossRef]
- Soulage, O.C.; Puig, S.L.; Soulère, L.; Zarrouki, B.; Guichardant, M.; Lagarde, M.; Pillon, J.N. Skeletal muscle insulin resistance is induced by 4-hydroxy-2-hexenal, a by-product of n-3 fatty acid peroxidation. Diabetologia 2018, 61, 688–699. [Google Scholar] [CrossRef]
- Eshima, H.; Ishihara, T.; Tabuchi, A.; Kano, Y.; Kurokawa, K.; Shchepinov, M.S. Deuterium-reinforced polyunsaturated fatty acids protect against muscle atrophy by STZ-induced diabetic mice. Free Radic. Biol. Med. 2025, 230, 273–282. [Google Scholar] [CrossRef]
- Mckeegan, K.; Mason, A.S.; Trewin, J.A.; Keske, A.M.; Wadley, D.G.; Gatta, D.A.P.; Nikolaidis, G.M.; Parker, L. Reactive oxygen species in exercise and insulin resistance: Working towards personalized antioxidant treatment. Redox Biol. 2021, 44, 102005. [Google Scholar] [CrossRef]
- Parker, L.; Shaw, C.S.; Stepto, N.K.; Levinger, I. Exercise and Glycemic Control: Focus on Redox Homeostasis and Redox-Sensitive Protein Signaling. Front. Endocrinol. 2017, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Archuleta, L.T.; Lemieux, M.A.; Saengsirisuwan, V.; Teachey, K.M.; Lindborg, A.K.; Kim, S.J.; Henriksen, J.E. Oxidant stress-induced loss of IRS-1 and IRS-2 proteins in rat skeletal muscle: Role of p38 MAPK. Free Radic. Biol. Med. 2009, 47, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, A.; Guarente, L.; Bose, A. Acute Oxidative Stress Can Reverse Insulin Resistance by Inactivation of Cytoplasmic JNK. J. Biol. Chem. 2010, 285, 21581–21589. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Soos, J.T.; Li, X.; Wu, J.; Degennaro, M.; Sun, X.; Littman, R.D.; Birnbaum, J.M.; Polakiewicz, D.R. Protein Kinase C θ Inhibits Insulin Signaling by Phosphorylating IRS1 at Ser1101. J. Biol. Chem. 2004, 279, 45304–45307. [Google Scholar] [CrossRef]
- Mothe, I.; Obberghen, V.E. Phosphorylation of Insulin Receptor Substrate-1 on Multiple Serine Residues, 612, 632, 662, and 731, Modulates Insulin Action. J. Biol. Chem. 1996, 271, 11222–11227. [Google Scholar] [CrossRef]
- Werner, D.E.; Lee, J.; Hansen, L.; Yuan, M.; Shoelson, E.S. Insulin Resistance Due to Phosphorylation of Insulin Receptor Substrate-1 at Serine 302. J. Biol. Chem. 2004, 279, 35298–35305. [Google Scholar] [CrossRef]
- Zhou, H.-L.; Grimmett, W.Z.; Venetos, M.N.; Stomberski, T.C.; Qian, Z.; Mclaughlin, J.P.; Bansal, K.P.; Zhang, R.; Reynolds, D.J.; Premont, T.R.; et al. An enzyme that selectively S-nitrosylates proteins to regulate insulin signaling. Cell 2023, 186, 5812–5825.e21. [Google Scholar] [CrossRef]
- Kaneki, M.; Shimizu, N.; Yamada, D.; Chang, K. Nitrosative Stress and Pathogenesis of Insulin Resistance. Antioxid. Redox Signal. 2007, 9, 319–329. [Google Scholar] [CrossRef]
- Zhong, Q.; Zheng, K.; Li, W.; An, K.; Liu, Y.; Xiao, X.; Hai, S.; Dong, B.; Li, S.; An, Z.; et al. Post-translational regulation of muscle growth, muscle aging and sarcopenia. J. Cachexia Sarcopenia Muscle 2023, 14, 1212–1227. [Google Scholar] [CrossRef]
- Xu, H.; Remmen, V.H. The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: A potential target for intervention in aging and skeletal muscle pathologies. Skelet. Muscle 2021, 11, 25. [Google Scholar] [CrossRef]
- Koves, R.T.; Ussher, R.J.; Noland, C.R.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, R.B.J.; Newgard, B.C.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.M.; Shulman, I.G. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Consitt, A.L.; Koves, R.T.; Muoio, M.D.; Nakazawa, M.; Newton, A.C.; Houmard, A.J. Plasma acylcarnitines during insulin stimulation in humans are reflective of age-related metabolic dysfunction. Biochem. Biophys. Res. Commun. 2016, 479, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Mera, P.; Malandrino, I.M.; Mir, F.J.; Herrero, L. Mitochondrial Fatty Acid Oxidation in Obesity. Antioxid. Redox Signal. 2013, 19, 269–284. [Google Scholar] [CrossRef]
- Fiorenza, M.; Onslev, J.; Henríquez-Olguín, C.; Persson, K.W.; Hesselager, S.A.; Jensen, T.E.; Wojtaszewski, J.F.P.; Hostrup, M.; Bangsbo, J. Reducing the mitochondrial oxidative burden alleviates lipid-induced muscle insulin resistance in humans. Sci. Adv. 2024, 10, eadq4461. [Google Scholar] [CrossRef]
- Fealy, E.C.; Mulya, A.; Axelrod, L.C.; Kirwan, P.J. Mitochondrial dynamics in skeletal muscle insulin resistance and type 2 diabetes. Transl. Res. 2018, 202, 69–82. [Google Scholar] [CrossRef]
- Eshima, H. Influence of Obesity and Type 2 Diabetes on Calcium Handling by Skeletal Muscle: Spotlight on the Sarcoplasmic Reticulum and Mitochondria. Front. Physiol. 2021, 12, 758316. [Google Scholar] [CrossRef]
- Wolf, C.; Amo, D.L.V.; Arndt, S.; Bueno, D.; Tenzer, S.; Hanschmann, E.-M.; Berndt, C.; Methner, A. Redox Modifications of Proteins of the Mitochondrial Fusion and Fission Machinery. Cells 2020, 9, 815. [Google Scholar] [CrossRef]
- del Campo, A.; Parra, V.; Vásquez-Trincado, C.; Gutiérrez, T.; Morales, E.P.; López-Crisosto, C.; Bravo-Sagua, R.; Navarro-Marquez, F.M.; Verdejo, E.H.; Contreras-Ferrat, A.; et al. Mitochondrial fragmentation impairs insulin-dependent glucose uptake by modulating Akt activity through mitochondrial Ca2+ uptake. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E1–E13. [Google Scholar] [CrossRef]
- Mezincescu, M.A.; Rudd, A.; Cheyne, L.; Horgan, G.; Philip, S.; Cameron, D.; Loon, V.L.; Whitfield, P.; Gribbin, R.; Hu, K.M.; et al. Comparison of intramyocellular lipid metabolism in patients with diabetes and male athletes. Nat. Commun. 2024, 15, 3690. [Google Scholar] [CrossRef]
- Kiefer, S.L.; Fabian, J.; Rospleszcz, S.; Lorbeer, R.; Machann, J.; Kraus, S.M.; Roemer, F.; Rathmann, W.; Meisinger, C.; Heier, M.; et al. Distribution patterns of intramyocellular and extramyocellular fat by magnetic resonance imaging in subjects with diabetes, prediabetes and normoglycaemic controls. Diabetes Obes. Metab. 2021, 23, 1868–1878. [Google Scholar] [CrossRef]
- Blackwood, S.J.; Horwath, O.; Moberg, M.; Pontén, M.; Apró, W.; Ekblom, M.M.; Larsen, F.J.; Katz, A. Insulin resistance after a 3-day fast is associated with an increased capacity of skeletal muscle to oxidize lipids. Am. J. Physiol.-Endocrinol. Metab. 2023, 324, E390–E401. [Google Scholar] [CrossRef] [PubMed]
- Kitessa, S.; Abeywardena, M. Lipid-Induced Insulin Resistance in Skeletal Muscle: The Chase for the Culprit Goes from Total Intramuscular Fat to Lipid Intermediates, and Finally to Species of Lipid Intermediates. Nutrients 2016, 8, 466. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, E.; Blachnio-Zabielska, A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Jani, S.; Eira, D.D.; Hadday, I.; Bikopoulos, G.; Mohasses, A.; Pinho, D.A.R.; Ceddia, B.R. Distinct mechanisms involving diacylglycerol, ceramides, and inflammation underlie insulin resistance in oxidative and glycolytic muscles from high fat-fed rats. Sci. Rep. 2021, 11, 19160. [Google Scholar] [CrossRef]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: A review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef]
- Whytock, L.K.; Goodpaster, H.B. Unraveling Skeletal Muscle Insulin Resistance: Molecular Mechanisms and the Restorative Role of Exercise. Circ. Res. 2025, 137, 184–204. [Google Scholar] [CrossRef]
- Diaz-Vegas, A.; Madsen, S.; Cooke, K.C.; Carroll, L.; Khor, J.X.Y.; Turner, N.; Lim, X.Y.; Astore, M.A.; Morris, J.C.; Don, A.S.; et al. Mitochondrial electron transport chain, ceramide, and coenzyme Q are linked in a pathway that drives insulin resistance in skeletal muscle. eLife 2023, 12, RP87340. [Google Scholar] [CrossRef]
- Zemski Berry, K.A.; Garfield, A.; Jambal, P.; Zarini, S.; Perreault, L.; Bergman, B.C. Oxidised phosphatidylcholine induces sarcolemmal ceramide accumulation and insulin resistance in skeletal muscle. Diabetologia 2024, 67, 2819–2832. [Google Scholar] [CrossRef]
- Shastry, A.; Wilkinson, M.S.; Miller, D.M.; Kuriakose, M.; Veeneman, J.; Smith, M.R.; Hindmarch, C.C.T.; Dunham-Snary, K.J. Multi-tissue metabolomics reveal mtDNA- and diet-specific metabolite profiles in a mouse model of cardiometabolic disease. Redox Biol. 2025, 81, 103541. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, K.; Whaley-Connell, T.A.; Stump, S.C.; Ibdah, A.J.; Sowers, R.J. Skeletal muscle insulin resistance: Role of inflammatory cytokines and reactive oxygen species. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R673–R680. [Google Scholar] [CrossRef] [PubMed]
- de Alvaro, C.; Teruel, T.; Hernandez, R.; Lorenzo, M. Tumor Necrosis Factor α Produces Insulin Resistance in Skeletal Muscle by Activation of Inhibitor κB Kinase in a p38 MAPK-dependent Manner. J. Biol. Chem. 2004, 279, 17070–17078. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.M.; Liu, Z.-G. Reactive Oxygen Species in TNFα-Induced Signaling and Cell Death. Mol. Cells 2010, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Werida, H.R.; El-Gharbawy, M.N.; Mostafa, M.T. Circulating IL-6, clusterin and irisin in obese subjects with different grades of obesity: Association with insulin resistance and sexual dimorphism. Arch. Endocrinol. Metab. 2021, 65, 126–136. [Google Scholar] [CrossRef]
- Mohamed, M.E.A.E.-R.; Ahmed, Y.K.; Ghamry, E.M.; Khedr, M.A.E.-H. Study of Serum Tumor Necrosis Factor Alpha Level in Prediabetics and Type 2 Diabetic Egyptian Patients. Al-Azhar Med. J. 2022, 51, 1193–1198. [Google Scholar] [CrossRef]
- Tan, C.H.; Hsu, W.J.; Tai, S.E.; Chacko, S.; Kovalik, J.-P.; Jahoor, F. The Impact of Bariatric Surgery on Glutathione Synthesis in Individuals with Severe Obesity. Antioxidants 2024, 13, 967. [Google Scholar] [CrossRef]
- Andrich, E.D.; Melbouci, L.; Ou, Y.; Auclair, N.; Mercier, J.; Grenier, J.-C.; Lira, S.F.; Barreiro, B.L.; Danialou, G.; Comtois, A.-S.; et al. A Short-Term High-Fat Diet Alters Glutathione Levels and IL-6 Gene Expression in Oxidative Skeletal Muscles of Young Rats. Front. Physiol. 2019, 10, 372. [Google Scholar] [CrossRef]
- Maddux, A.B.; See, W.; Lawrence, C.J.; Goldfine, L.A.; Goldfine, D.I.; Evans, L.J. Protection Against Oxidative Stress—Induced Insulin Resistance in Rat L6 Muscle Cells by Micromolar Concentrations of α-Lipoic Acid. Diabetes 2001, 50, 404–410. [Google Scholar] [CrossRef]
- Ostrom, L.E.; Stuppard, R.; Mattson-Hughes, A.; Marcinek, J.D. Inducible and reversible SOD2 knockdown in mouse skeletal muscle drives impaired pyruvate oxidation and reduced metabolic flexibility. Free Radic. Biol. Med. 2025, 226, 237–250. [Google Scholar] [CrossRef]
- Ji, F.; Lee, H.; Rheem, H.; Liu, J.; Kim, J.-H. Differential ferroptosis regulation in red and white gastrocnemius under obesity and its Attenuation by exercise and dietary restriction. Sci. Rep. 2025, 15, 23821. [Google Scholar] [CrossRef]
- Park, S.; Park, S.-Y. Can antioxidants be effective therapeutics for type 2 diabetes? Yeungnam Univ. J. Med. 2021, 38, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, A.; Yang, C.; Liu, Y.; Tan, Y.; Bond, T.S.; Walker, S.; Sikora, T.; Laskowski, A.; Sharma, A.; Haan, D.B.J.; et al. SOD2 in skeletal muscle: New insights from an inducible deletion model. Redox Biol. 2021, 47, 102135. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.S.; Goldstein, E.; Schrager, M.; Ji, L.L. Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update. Antioxidants 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, C.; Chung, N.; Schmidt, U.; Kreutz, T.; Lenzen, E.; Schiffer, T.; Geisler, S.; Graf, C.; Montiel-Garcia, G.; Renner, R.; et al. Training alters the skeletal muscle antioxidative capacity in non-insulin-dependent type 2 diabetic men. Scand. J. Med. Sci. Sports 2012, 22, 462–470. [Google Scholar] [CrossRef]
- Samjoo, A.I.; Safdar, A.; Hamadeh, J.M.; Raha, S.; Tarnopolsky, A.M. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr. Diabetes 2013, 3, e88. [Google Scholar] [CrossRef]
- Henriquez-Olguin, C.; Meneses-Valdes, R.; Raun, S.H.; Gallero, S.; Knudsen, J.R.; Li, Z.; Li, J.; Sylow, L.; Jaimovich, E.; Jensen, T.E. NOX2 deficiency exacerbates diet-induced obesity and impairs molecular training adaptations in skeletal muscle. Redox Biol. 2023, 65, 102842. [Google Scholar] [CrossRef]
- Bond, J.M.; Dzubanova, M.; Addington, A.K.; Najt, C.P.; Gilbert, E.R.; Tencerova, M.; Craige, S.M. Sex-specific metabolic responses to high-fat diet in mice with NOX4 deficiency. Redox Biol. 2025, 85, 103698. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Cesarini, L.; Grignaffini, F.; Alisi, A.; Pastore, A. Alterations in Glutathione Redox Homeostasis in Metabolic Dysfunction-Associated Fatty Liver Disease: A Systematic Review. Antioxidants 2024, 13, 1461. [Google Scholar] [CrossRef]
- Masenga, K.S.; Kabwe, S.L.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Jackson, J.M.; Pollock, N.; Staunton, C.; Jones, S.; Mcardle, A. Redox Control of Signalling Responses to Contractile Activity and Ageing in Skeletal Muscle. Cells 2022, 11, 1698. [Google Scholar] [CrossRef]
- Mcginnis, A.; Klichko, I.V.; Orr, C.W.; Radyuk, N.S. Hyperoxidation of Peroxiredoxins and Effects on Physiology of Drosophila. Antioxidants 2021, 10, 606. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Kitada, M.; Xu, J.; Ogura, Y.; Monno, I.; Koya, D. Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease. Front. Physiol. 2020, 11, 755. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, N.; Wolf, A.M.; Yokota, T.; Nito, C.; Takahashi, H.; Ohta, S. Transgenic type2 diabetes mouse models for in vivo redox measurement of hepatic mitochondrial oxidative stress. Biochim. Biophys. Acta BBA Gen. Subj. 2023, 1867, 130302. [Google Scholar] [CrossRef] [PubMed]
- Back, H.S.; Kaufman, J.R. Endoplasmic Reticulum Stress and Type 2 Diabetes. Annu. Rev. Biochem. 2012, 81, 767–793. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, X.; Xu, Z.; Liu, Y. Endoplasmic reticulum stress mechanisms and exercise intervention in type 2 diabetes mellitus. Biomed. Pharmacother. 2024, 177, 117122. [Google Scholar] [CrossRef]
- Tu, P.B.; Weissman, S.J. Oxidative protein folding in eukaryotes. J. Cell Biol. 2004, 164, 341–346. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Liu, S.-H.; Su, C.-C.; Fang, K.-M.; Yang, T.-Y.; Liu, J.-M.; Chen, Y.-W.; Chang, K.-C.; Chuang, H.-L.; Wu, C.-T.; et al. Methylmercury Induces Mitochondria- and Endoplasmic Reticulum Stress-Dependent Pancreatic β-Cell Apoptosis via an Oxidative Stress-Mediated JNK Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 2858. [Google Scholar] [CrossRef]
- Li, G.; Mongillo, M.; Chin, K.-T.; Harding, H.; Ron, D.; Marks, R.A.; Tabas, I. Role of ERO1-α–mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress–induced apoptosis. J. Cell Biol. 2009, 186, 783–792. [Google Scholar] [CrossRef]
- Brookes, S.P.; Yoon, Y.; Robotham, L.J.; Anders, W.M.; Sheu, S.-S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol.-Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Deldicque, L.; Cani, D.P.; Philp, A.; Raymackers, J.-M.; Meakin, J.P.; Ashford, J.L.M.; Delzenne, M.N.; Francaux, M.; Baar, K. The unfolded protein response is activated in skeletal muscle by high-fat feeding: Potential role in the downregulation of protein synthesis. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E695–E705. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, S.; Zhang, Q.; Shao, W.; Han, X.; Wang, Y.; Du, Y. Endoplasmic reticulum stress mediates JNK-dependent IRS-1 serine phosphorylation and results in Tau hyperphosphorylation in amyloid β oligomer-treated PC12 cells and primary neurons. Gene 2016, 587, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.R.; Logie, L.; Patel, K.; Erhardt, S.; Bacon, S.; Middleton, P.; Harthill, J.; Forteath, C.; Coats, J.T.; Kerr, C.; et al. Metformin selectively targets redox control of complex I energy transduction. Redox Biol. 2018, 14, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Maurer, J.; Zhao, X.; Irmler, M.; Gudiksen, A.; Pilmark, N.S.; Li, Q.; Goj, T.; Beckers, J.; Angelis, M.H.d.; Birkenfeld, A.L.; et al. Redox state and altered pyruvate metabolism contribute to a dose-dependent metformin-induced lactate production of human myotubes. Am. J. Physiol.-Cell Physiol. 2023, 325, C1131–C1143. [Google Scholar] [CrossRef] [PubMed]
- Bubak, M.P.; Davidyan, A.; O’Reilly, C.L.; Mondal, S.A.; Keast, J.; Doidge, S.M.; Borowik, A.K.; Taylor, M.E.; Volovičeva, E.; Kinter, M.T.; et al. Metformin treatment results in distinctive skeletal muscle mitochondrial remodeling in rats with different intrinsic aerobic capacities. Aging Cell 2024, 23, e14235. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Chen, Y.; Zhao, Q. PGC-1 mediates the regulation of metformin in muscle irisin expression and function. Am. J. Transl. Res. 2015, 7, 1850–1859. [Google Scholar]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.-N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- Kang, M.J.; Moon, J.W.; Lee, J.O.; Kim, J.H.; Jung, E.J.; Kim, S.J.; Oh, J.Y.; Wu, S.W.; Lee, P.R.; Park, S.H.; et al. Metformin induces muscle atrophy by transcriptional regulation of myostatin via HDAC6 and FoxO3a. J. Cachexia Sarcopenia Muscle 2022, 13, 605–620. [Google Scholar] [CrossRef]
- Retnakaran, R.; Pu, J.; Emery, A.; Harris, S.B.; Reichert, S.M.; Gerstein, H.C.; McInnes, N.; Kramer, C.K.; Zinman, B. Determinants of sustained stabilization of beta-cell function following short-term insulin therapy in type 2 diabetes. Nat. Commun. 2023, 14, 4514. [Google Scholar] [CrossRef]
- Op den Kamp, Y.J.M.; Gemmink, A.; de Ligt, M.; Dautzenberg, B.; Kornips, E.; Jorgensen, J.A.; Schaart, G.; Esterline, R.; Pava, D.A.; Hoeks, J.; et al. Effects of SGLT2 inhibitor dapagliflozin in patients with type 2 diabetes on skeletal muscle cellular metabolism. Mol. Metab. 2022, 66, 101620. [Google Scholar] [CrossRef]
- Bamba, R.; Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Takakuwa, H.; et al. Extracellular lipidome change by an SGLT2 inhibitor, luseogliflozin, contributes to prevent skeletal muscle atrophy in db/db mice. J. Cachexia Sarcopenia Muscle 2022, 13, 574–588. [Google Scholar] [CrossRef]
- Xie, K.; Sugimoto, K.; Tanaka, M.; Akasaka, H.; Fujimoto, T.; Takahashi, T.; Onishi, Y.; Minami, T.; Yoshida, S.; Takami, Y.; et al. Effects of luseogliflozin treatment on hyperglycemia-induced muscle atrophy in rats. J. Clin. Biochem. Nutr. 2023, 72, 248–255. [Google Scholar] [CrossRef]
- Sasaki, T.; Sugawara, M.; Fukuda, M. Sodium–glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J. Diabetes Investig. 2019, 10, 108–117. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, H.; Yang, X.; Shi, J.; Sheng, X.; Wang, L.; Li, T.; Quan, H.; Zhai, X.; Li, W. Effects of dapagliflozin monotherapy and combined aerobic exercise on skeletal muscle mitochondrial quality control and insulin resistance in type 2 diabetes mellitus rats. Biomed. Pharmacother. 2023, 169, 115852. [Google Scholar] [CrossRef]
- Radlinger, B.; Ress, C.; Folie, S.; Salzmann, K.; Lechuga, A.; Weiss, B.; Salvenmoser, W.; Graber, M.; Hirsch, J.; Holfeld, J.; et al. Empagliflozin protects mice against diet-induced obesity, insulin resistance and hepatic steatosis. Diabetologia 2022, 66, 754–767. [Google Scholar] [CrossRef]
- Douros, A.; Yin, H.; Yu, O.H.Y.; Filion, K.B.; Azoulay, L.; Suissa, S. Pharmacologic Differences of Sulfonylureas and the Risk of Adverse Cardiovascular and Hypoglycemic Events. Diabetes Care 2017, 40, 1506–1513. [Google Scholar] [CrossRef]
- Tricarico, D.; Selvaggi, M.; Passantino, G.; De Palo, P.; Dario, C.; Centoducati, P.; Tateo, A.; Curci, A.; Maqoud, F.; Mele, A.; et al. Frontiers | ATP Sensitive Potassium Channels in the Skeletal Muscle Function: Involvement of the KCNJ11(Kir6.2) Gene in the Determination of Mechanical Warner Bratzer Shear Force. Front. Physiol. 2016, 7, 167. [Google Scholar] [CrossRef]
- Scala, R.; Maqoud, F.; Zizzo, N.; Mele, A.; Camerino, G.M.; Zito, F.A.; Ranieri, G.; McClenaghan, C.; Harter, T.M.; Nichols, C.G.; et al. Frontiers | Pathophysiological Consequences of KATP Channel Overactivity and Pharmacological Response to Glibenclamide in Skeletal Muscle of a Murine Model of Cantù Syndrome. Front. Pharmacol. 2020, 11, 604885. [Google Scholar] [CrossRef]
- Luna-Marco, C.; de Marañon, A.M.; Hermo-Argibay, A.; Rodriguez-Hernandez, Y.; Hermenejildo, J.; Fernandez-Reyes, M.; Apostolova, N.; Vila, J.; Sola, E.; Morillas, C.; et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. 2023, 66, 102849. [Google Scholar] [CrossRef]
- Siddeeque, N.; Hussein, M.H.; Abdelmaksoud, A.; Bishop, J.; Attia, A.S.; Elshazli, R.M.; Fawzy, M.S.; Toraih, E.A. Neuroprotective effects of GLP-1 receptor agonists in neurodegenerative Disorders: A Large-Scale Propensity-Matched cohort study. Int. Immunopharmacol. 2024, 143, 113537. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Du, Z.; Duan, C.; Zhan, S.; Wang, T.; Zhu, M.; Shi, J.; Meng, J.; Zhang, X.; Yang, M.; et al. Beinaglutide shows significantly beneficial effects in diabetes/obesity-induced nonalcoholic steatohepatitis in ob/ob mouse model. Life Sci. 2021, 270, 118966. [Google Scholar] [CrossRef] [PubMed]
- Elbassuoni, E.A.; Ahmed, R.F. Mechanism of the neuroprotective effect of GLP-1 in a rat model of Parkinson’s with pre-existing diabetes. Neurochem. Int. 2019, 131, 104583. [Google Scholar] [CrossRef]
- Tomas, E.; Stanojevic, V.; Habener, J.F. GLP-1-derived nonapeptide GLP-1(28–36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul. Pept. 2011, 167, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Chen, S.; Chen, X.; Niu, S.; Yue, L.; Pan, X.; Li, Z.; Chen, X. An Effective Glucagon-Like Peptide-1 Receptor Agonists, Semaglutide, Improves Sarcopenic Obesity in Obese Mice by Modulating Skeletal Muscle Metabolism. Drug Des. Dev. Ther. 2022, 16, 3723–3735. [Google Scholar] [CrossRef]
- Ji, W.; Chen, X.; Lv, J.; Wang, M.; Ren, S.; Yuan, B.; Wang, B.; Chen, L. Liraglutide Exerts Antidiabetic Effect via PTP1B and PI3K/Akt2 Signaling Pathway in Skeletal Muscle of KKAy Mice. Int. J. Endocrinol. 2014, 2014, 312452. [Google Scholar] [CrossRef]
- Wu, H.; Sui, C.; Xia, F.; Zhai, H.; Zhang, H.; Xu, H.; Weng, P.; Lu, Y. Effects of exenatide therapy on insulin resistance in the skeletal muscles of high-fat diet and low-dose streptozotocin-induced diabetic rats. Endocr. Res. 2016, 41, 1–7. [Google Scholar] [CrossRef]
- Yamada, S.; Ogura, Y.; Inoue, K.; Tanabe, J.; Sugaya, T.; Ohata, K.; Nagai, Y.; Natsuki, Y.; Hoshino, S.; Watanabe, S.; et al. Effect of GLP-1 receptor agonist, liraglutide, on muscle in spontaneously diabetic torii fatty rats. Mol. Cell. Endocrinol. 2022, 539, 111472. [Google Scholar] [CrossRef]
- Dong, Z.; Chai, W.; Wang, W.; Zhao, L.; Fu, Z.; Cao, W.; Liu, Z. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E222–E228. [Google Scholar] [CrossRef]
- Koska, J.; Sands, M.; Burciu, C.; D’Souza, K.M.; Raravikar, K.; Liu, J.; Truran, S.; Franco, D.A.; Schwartz, E.A.; Schwenke, D.C.; et al. Exenatide Protects Against Glucose- and Lipid-Induced Endothelial Dysfunction: Evidence for Direct Vasodilation Effect of GLP-1 Receptor Agonists in Humans. Diabetes 2015, 64, 2624–2635. [Google Scholar] [CrossRef] [PubMed]
- Erdogdu, O.; Nathanson, D.; Sjöholm, A.; Nyström, T.; Zhang, Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol. Cell. Endocrinol. 2010, 325, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Akarte, A.S.; Srinivasan, B.P.; Gandhi, S. Vildagliptin selectively ameliorates GLP-1, GLUT4, SREBP-1c mRNA levels and stimulates β-cell proliferation resulting in improved glucose homeostasis in rats with streptozotocin-induced diabetes. J. Diabetes Complicat. 2012, 26, 266–274. [Google Scholar] [CrossRef]
- Ayaori, M.; Iwakami, N.; Uto-Kondo, H.; Sato, H.; Sasaki, M.; Komatsu, T.; Iizuka, M.; Takiguchi, S.; Yakushiji, E.; Nakaya, K.; et al. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J. Am. Heart Assoc. 2013, 2, e003277. [Google Scholar] [CrossRef]
- Nomoto, H.; Miyoshi, H.; Furumoto, T.; Oba, K.; Tsutsui, H.; Inoue, A.; Atsumi, T.; Manda, N.; Kurihara, Y.; Aoki, S. A Randomized Controlled Trial Comparing the Effects of Sitagliptin and Glimepiride on Endothelial Function and Metabolic Parameters: Sapporo Athero-Incretin Study 1 (SAIS1). PLoS ONE 2016, 11, e0164255. [Google Scholar] [CrossRef]
- van Poppel, P.C.; Netea, M.G.; Smits, P.; Tack, C.J. Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes Care 2011, 34, 2072–2077. [Google Scholar] [CrossRef]
- Nakamura, K.; Oe, H.; Kihara, H.; Shimada, K.; Fukuda, S.; Watanabe, K.; Takagi, T.; Yunoki, K.; Miyoshi, T.; Hirata, K.; et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc. Diabetol. 2014, 13, 110. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Therapeutic profile in the treatment of type 2 diabetes. Diabetes Obes. Metab. 2024, 26, 3–19. [Google Scholar] [CrossRef]
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef]
- Di Magno, L.; Pastena, F.D.; Bordone, R.; Coni, S.; Canettieri, G. The Mechanism of Action of Biguanides: New Answers to a Complex Question. Cancers 2022, 14, 3220. [Google Scholar] [CrossRef]
- Buczyńska, A.; Sidorkiewicz, I.; Krętowski, A.J.; Adamska, A. Examining the clinical relevance of metformin as an antioxidant intervention. Front. Pharmacol. 2024, 15, 1330797. [Google Scholar] [CrossRef]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef]
- Rena, G.; Grahame Hardie, D.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Karlsson, H.K.R.; Hällsten, K.; Björnholm, M.; Tsuchida, H.; Chibalin, A.V.; Virtanen, K.A.; Heinonen, O.J.; Lönnqvist, F.; Nuutila, P.; Zierath, J.R. Effects of Metformin and Rosiglitazone Treatment on Insulin Signaling and Glucose Uptake in Patients With Newly Diagnosed Type 2 Diabetes: A Randomized Controlled Study. Diabetes 2005, 54, 1459–1467. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An Update on SGLT2 Inhibitors for the Treatment of Diabetes Mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Wu, J.-X.; Ding, D.; Wang, M.; Chen, L. Structural Insights into the Inhibitory Mechanism of Insulin Secretagogues on the Pancreatic ATP-Sensitive Potassium Channel. Biochemistry 2020, 59, 18–25. [Google Scholar] [CrossRef]
- Guardado-Mendoza, R.; Prioletta, A.; Jiménez-Ceja, L.M.; Sosale, A.; Folli, F. The role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. Arch. Med. Sci. AMS 2013, 9, 936–943. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Manghi, F.C.P.; Landó, L.F.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Ørgaard, A.; Holst, J.J. The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia 2017, 60, 1731–1739. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Gaal, L.F.V.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- MacDonald, P.E.; El-Kholy, W.; Riedel, M.J.; Salapatek, A.M.; Light, P.E.; Wheeler, M.B. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002, 51 (Suppl. S3), S434–S442. [Google Scholar] [CrossRef]
- Old, V.J.; Davies, M.J.; Papamargaritis, D.; Choudhary, P.; Watson, E.L. The Effects of Glucagon-Like Peptide-1 Receptor Agonists on Mitochondrial Function Within Skeletal Muscle: A Systematic Review. J. Cachexia Sarcopenia Muscle 2025, 16, e13677. [Google Scholar] [CrossRef]
- Gatto, A.; Liu, K.; Milan, N.; Wong, S. The Effects of GLP-1 Agonists on Musculoskeletal Health and Orthopedic Care. Curr. Rev. Musculoskelet. Med. 2025, 18, 469–480. [Google Scholar] [CrossRef]
- Zinn, J.; Poretsky, L. Skeletal Muscle Mass and Body Weight Fall Proportionally With Use of Dual Glucagon-Like Peptide 1/Glucose-Dependent Insulinotropic Polypeptide Receptor Agonist Tirzepatide: Case Report and Review of Literature. AACE Clin. Case Rep. 2025, 11, 98–101. [Google Scholar] [CrossRef]
- Campbell, J.E.; Müller, T.D.; Finan, B.; DiMarchi, R.D.; Tschöp, M.H.; D’Alessio, D.A. GIPR/GLP-1R dual agonist therapies for diabetes and weight loss—Chemistry, physiology, and clinical applications. Cell Metab. 2023, 35, 1519–1529. [Google Scholar] [CrossRef]
- Nauck, M.A.; D‘Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef]
- Breitzig, M.T.; Alleyn, M.D.; Lockey, R.F.; Kolliputi, N. A mitochondrial delicacy: Dynamin-related protein 1 and mitochondrial dynamics. Am. J. Physiol. Cell Physiol. 2018, 315, C80–C90. [Google Scholar] [CrossRef]
- Sun, J.; Shao, Y.; Pei, L.; Zhu, Q.; Yu, X.; Yao, W. AKAP1 alleviates VSMC phenotypic modulation and neointima formation by inhibiting Drp1-dependent mitochondrial fission. Biomed. Pharmacother. 2024, 176, 116858. [Google Scholar] [CrossRef]
- Dickey, A.S.; Strack, S. PKA/AKAP1 and PP2A/Bβ2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 15716–15726. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Bao, L.; Shi, X.; Wang, J.; Li, Q. Harnessing exercise to combat chronic diseases: The role of Drp1-Mediated mitochondrial fission. Front. Cell Dev. Biol. 2025, 13, 1481756. [Google Scholar] [CrossRef]
- Din, S.; Mason, M.; Völkers, M.; Johnson, B.; Cottage, C.T.; Wang, Z.; Joyo, A.Y.; Quijada, P.; Erhardt, P.; Magnuson, N.S.; et al. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc. Natl. Acad. Sci. USA 2013, 110, 5969–5974. [Google Scholar] [CrossRef]
- Hall, A.R.; Burke, N.; Dongworth, R.K.; Hausenloy, D.J. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 2014, 171, 1890–1906. [Google Scholar] [CrossRef]
- McIntosh, C.H.; Widenmaier, S.; Kim, S.J. Glucose-dependent insulinotropic polypeptide signaling in pancreatic β-cells and adipocytes. J. Diabetes Investig. 2012, 3, 96–106. [Google Scholar] [CrossRef]
- Williams, G.S.; Boyman, L.; Lederer, W.J. Mitochondrial calcium and the regulation of metabolism in the heart. J. Mol. Cell. Cardiol. 2015, 78, 35–45. [Google Scholar] [CrossRef]
- Chen, J.; Mei, A.; Wei, Y.; Li, C.; Qian, H.; Min, X.; Yang, H.; Dong, L.; Rao, X.; Zhong, J. GLP-1 receptor agonist as a modulator of innate immunity. Front. Immunol. 2022, 13, 997578. [Google Scholar] [CrossRef]
- Wang, B.; Nie, J.; Wu, L.; Hu, Y.; Wen, Z.; Dong, L.; Zou, M.H.; Chen, C.; Wang, D.W. AMPKα2 Protects Against the Development of Heart Failure by Enhancing Mitophagy via PINK1 Phosphorylation. Circ. Res. 2018, 122, 712–729. [Google Scholar] [CrossRef]
- Shires, S.E.; Gustafsson, Å.B. Regulating Renewable Energy: Connecting AMPKα2 to PINK1/Parkin-Mediated Mitophagy in the Heart. Circ. Res. 2018, 122, 649–651. [Google Scholar] [CrossRef]
- Kazlauskaite, A.; Muqit, M.M. PINK1 and Parkin–mitochondrial interplay between phosphorylation and ubiquitylation in Parkinson’s disease. FEBS J. 2015, 282, 215–223. [Google Scholar]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Gegg, M.E.; Schapira, A.H. PINK1-parkin-dependent mitophagy involves ubiquitination of mitofusins 1 and 2: Implications for Parkinson disease pathogenesis. Autophagy 2011, 7, 243–245. [Google Scholar] [CrossRef]
- Bremer, K.; Kocha, K.M.; Snider, T.; Moyes, C.D. Sensing and responding to energetic stress: The role of the AMPK-PGC1α-NRF1 axis in control of mitochondrial biogenesis in fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 199, 4–12. [Google Scholar] [CrossRef]
- Fischhuber, K.; Matzinger, M.; Heiss, E.H. AMPK Enhances Transcription of Selected Nrf2 Target Genes via Negative Regulation of Bach1. Front. Cell Dev. Biol. 2020, 8, 628. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef]
- Olmos, Y.; Valle, I.; Borniquel, S.; Tierrez, A.; Soria, E.; Lamas, S.; Monsalve, M. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J. Biol. Chem. 2009, 284, 14476–14484. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Chrissobolis, S.; Faraci, F.M. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol. Med. 2008, 14, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Jih, P.J.; Lin, H.H.; Lin, J.S.; Chang, L.L.; Shen, Y.H.; Jeng, S.T. Nitric oxide activates superoxide dismutase and ascorbate peroxidase to repress the cell death induced by wounding. Plant Mol. Biol. 2011, 77, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Wu, D.; Wang, Y.; Zhao, Y.; Shen, H.; Yao, J.; Huang, X.; Li, X.; Zhou, Z.; Wang, Z.; et al. The mitochondrial calcium uniporter engages UCP1 to form a thermoporter that promotes thermogenesis. Cell Metab. 2022, 34, 1325–1341.e6. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Lee, G.; Yoon, S.; Min, C.K.; Kim, T.G.; Yamamoto, T.; Kim, D.H.; Lee, K.W.; Eom, S.H. S92 phosphorylation induces structural changes in the N-terminus domain of human mitochondrial calcium uniporter. Sci. Rep. 2020, 10, 9131. [Google Scholar] [CrossRef]
- Makrilakis, K. The Role of DPP-4 Inhibitors in the Treatment Algorithm of Type 2 Diabetes Mellitus: When to Select, What to Expect. Int. J. Environ. Res. Public Health 2019, 16, 2720. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Gallwitz, B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 479–486. [Google Scholar] [CrossRef]
- Foley, J.E.; Jordan, J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: Mechanistic basis and clinical experience. Vasc. Health Risk Manag. 2010, 6, 541–548. [Google Scholar] [CrossRef]
- Cao, F.; Wu, K.; Zhu, Y.Z.; Bao, Z.W. Roles and Mechanisms of Dipeptidyl Peptidase 4 Inhibitors in Vascular Aging. Front. Endocrinol. 2021, 12, 731273. [Google Scholar] [CrossRef]
- Bartoloni, B.; Mannelli, M.; Gamberi, T.; Fiaschi, T. The Multiple Roles of Lactate in the Skeletal Muscle. Cells 2024, 13, 1177. [Google Scholar] [CrossRef]
- Nikooie, R.; Moflehi, D.; Zand, S. Lactate regulates autophagy through ROS-mediated activation of ERK1/2/m-TOR/p-70S6K pathway in skeletal muscle. J. Cell Commun. Signal. 2021, 15, 107–123. [Google Scholar] [CrossRef]
- Love, K.M.; Barrett, E.J.; Malin, S.K.; Reusch, J.E.B.; Regensteiner, J.G.; Liu, Z. Diabetes pathogenesis and management: The endothelium comes of age. J. Mol. Cell Biol. 2021, 13, 500–512. [Google Scholar] [CrossRef]
- Chantarasakha, K.; Yangchum, A.; Isaka, M.; Tepaamorndech, S. Nidulin stimulates glucose uptake in myotubes through the IRS-AKT pathway and alters redox balance and intracellular calcium. Nat. Prod. Bioprospecting 2025, 15, 63. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, P.; Verma, S.K.; Ratre, P.; Mishra, P.K. Omics-based cutting-edge technologies for identifying predictive biomarkers to measure the impact of air borne particulate matter exposure on male reproductive health. J. Reprod. Healthc. Med. 2024, 5, 2. [Google Scholar] [CrossRef]
- Wheelock, C.E.; Goss, V.M.; Balgoma, D.; Nicholas, B.; Brandsma, J.; Skipp, P.J.; Snowden, S.; Burg, D.; D’Amico, A.; Horvath, I.; et al. Application of ’omics technologies to biomarker discovery in inflammatory lung diseases. Eur. Respir. J. 2013, 42, 802–825. [Google Scholar] [CrossRef]
- Chen, S.; Sun, J.; Wen, W.; Chen, Z.; Yu, Z. Integrative multi-omics summary-based mendelian randomization identifies key oxidative stress-related genes as therapeutic targets for atrial fibrillation and flutter. Front. Genet. 2024, 15, 1447872. [Google Scholar] [CrossRef]


| Redox Modification | Mechanism | Action on Skeletal Muscle | Reference(s) |
|---|---|---|---|
| Protein carbonylation | Oxidation of amino acid side chains to introduce carbonyl groups | Loss of enzyme activity and structural protein function; carbonylation of GLUT4 impairs activity | [106,108] |
| Lipid peroxidation | Hydroxyl radical attack on PUFAs that generate aldehydes (4-HNE, 4-ONE, 4-HHE) | Akt2 modification, inhibition → reduced GLUT4 function and glucose uptake; increased carbonylation and GSH depletion | [107,108,109,110] |
| S-nitrosylation | Covalent atachment of NO to protein thiols | Modification of IR, IRS, Akt inhibiting insulin signaling; Akt/PKB inactivation | [119,120] |
| S-glutathionylation | Reversible addition of GSH to cysteine residues under oxidative distress | Excess modification impairs Ca2+ uptake into SR via SERCA inhibition → muscle fatigue | [121,122] |
| Mitochondrial ROS production | Nutrient overload → higher electron flux through ETC → enhanced ROS generation | mtDNA and respiratory chain damage → reduced oxidative capacity; incomplete fatty acid oxidation with acylcarnitine accumulation → impaired Akt phosphorylation | [37,123,124,125] |
| Lipid accumulation | Ectopic lipid (DAGs, ceramides) deposition in myocytes during caloric surplus | DAG synthesis → PKC activation → decreased IRS-1 activity, impaired Akt activation, increased NF-κB activity; Ceramide activation of phosphatases → Akt dephosphorylation and apoptosis; Ceramide-induced depletion of CoQ → reduced mitochondrial respiratory complexes → mitochondrial dysfunction and oxidant production | [132,133,135,136,137,138,139,140] |
| Antioxidant defense depletion | Oxidative overload → reduced SOD and GPX activity | Over-oxidation of redox-sensitive proteins → accumulation of oxidative damage → insulin resistance | [151,152,153] |
| Drug Class | Primary Glucose-Lowering Mechanism | Known/Proposed Effects on Redox Homeostasis in Skeletal Muscle | Types of Evidence | References |
|---|---|---|---|---|
| Biguanides (Metformin) | ↓ hepatic glucose production, intestinal glucose absorption ↑ peripheral glucose uptake | Modulates complex I and AMPK activation ↓ mitochondrial ROS ↑ antioxidant defenses | In vitro—isolated rat liver mitochondria; C2C12 myoblasts; primary human myotubes. In vivo—HCR/LCR rats; ob/ob mice; db/db mice. | [177,178,179,180,181,182] |
| Insulin | Activates insulin receptor signaling (PI3K-Akt) to ↑ GLUT4-mediated uptake | Mitigates hyperglycemia-induced oxidative distress ↓ mitochondrial ROS and AGEs ↑ eNOS-mediated NO signaling | In vitro—C2C12 myoblasts; primary human myotubes. Indirect—RCT; animal models. | [67,92,183] |
| SGLT2 Inhibitors | Inhibit renal SGLT2 transporter in proximal tubules | ↓ lipid-induced oxidative distress ↑ mitochondrial quality/antioxidant defenses | In vivo—STZ/HFD rats; db/db mice. Ex vivo—skeletal muscle fibers. Indirect—cohort study, cross-over study. | [184,185,186,187,188,189] |
| Insulin Secretagogues | Stimulate insulin secretion by closing β-cell KATP channels | Indirect through changes in systemic insulin and glucose | In vivo—Kir6.1[V65M] mice. Indirect—cohort study. | [190,191,192] |
| GLP-1 Receptor Agonists Dual GLP-1/GIP Agonists | Activate GLP-1 and GIP receptors ⟶ increased insulin, ↓ glucagon, ↓ gastric emptying, ↓ appetite | ↑ Mitochondrial biogenesis, mitophagy, mitochondrial fusion, antioxidant expression ↑ Gαs-coupled adenylate cyclase stimulation ⟶ increased cAMP ⟶ increased PKA activation ⟶ increased calcium-dependent dehydrogenase activity ⟶ increased substrate flux ↓ mitochondrial electron leak ⟶ decreased ROS ↑ eNOS ⟶ NO ⟶ improved perfusion ↓NOX-mediated ROS production | In vivo—C57BL/6, KKAy mice; ob/ob mice; Sprague-Dawley rats; albino rats. In vitro—mouse hepatocytes. Indirect—RCT; cohort study; cross sectional study. | [193,194,195,196,197,198,199,200,201,202,203,204,205] |
| DPP-4 Inhibitors | Inhibit DPP-4 enzyme ⟶ prolong GLP-1 and GIP activity, ↑ insulin, ↓ glucagon | ↑ Incretin-mediated responses Direct antioxidant and vascular effects (↓ NO, ↓ vascular oxidative distress, ↑ perfusion) | In vivo—STZ rats. Indirect—cohort study; RCT(s); cross-over study. | [206,207,208,209,210] |
| Mechanism of Action of Redox Impacts | T2DM Drug Classes | References |
|---|---|---|
| Inhibition of mitochondrial complex I | Biguanides | [177] |
| PI3K-Akt signaling | Insulin Incretin Therapies | [113,204,246] |
| AMPK-PGC-1α | Biguanides SGLT2 Inhibitors Incretin therapies | [180,181,182,231] |
| Enhancement of mitochondrial quality (dynamics, mitophagy, etc.) | SGLT2 Inhibitors Incretin therapies | [189,194,195,196,197,198,199,200,201,233] |
| Regulation of vascular tone through NO signaling | Insulin Incretin therapies | [202,203,204,218,231] |
| Reduction in substrate overload and lipid-induced oxidative distress | Biguanides SGLT2 Inhibitors | [181,185] |
| Inhibition of β-cell KATP Channels | Insulin Secretagogues | [191,221,222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkinson, M.S.; Rollin, T.A.; Kuriakose, M.; Haggerty-Goede, R.A.L.; Miller, D.M.; Dunham-Snary, K.J. Redox Homeostasis in Metabolic Syndrome and Type II Diabetes: Role of Skeletal Muscle and Impact of Gold-Standard Treatments. Int. J. Mol. Sci. 2025, 26, 10370. https://doi.org/10.3390/ijms262110370
Wilkinson MS, Rollin TA, Kuriakose M, Haggerty-Goede RAL, Miller DM, Dunham-Snary KJ. Redox Homeostasis in Metabolic Syndrome and Type II Diabetes: Role of Skeletal Muscle and Impact of Gold-Standard Treatments. International Journal of Molecular Sciences. 2025; 26(21):10370. https://doi.org/10.3390/ijms262110370
Chicago/Turabian StyleWilkinson, Mia S., Thomas A. Rollin, Michelle Kuriakose, Roan A. L. Haggerty-Goede, Dalia M. Miller, and Kimberly J. Dunham-Snary. 2025. "Redox Homeostasis in Metabolic Syndrome and Type II Diabetes: Role of Skeletal Muscle and Impact of Gold-Standard Treatments" International Journal of Molecular Sciences 26, no. 21: 10370. https://doi.org/10.3390/ijms262110370
APA StyleWilkinson, M. S., Rollin, T. A., Kuriakose, M., Haggerty-Goede, R. A. L., Miller, D. M., & Dunham-Snary, K. J. (2025). Redox Homeostasis in Metabolic Syndrome and Type II Diabetes: Role of Skeletal Muscle and Impact of Gold-Standard Treatments. International Journal of Molecular Sciences, 26(21), 10370. https://doi.org/10.3390/ijms262110370







