BET Inhibitor JQ1 Attenuates Atrial Fibrillation Through Modulation of Fibrosis, Calcium Homeostasis, and Mitochondrial Function in a Murine Model
Abstract
1. Introduction
2. Results
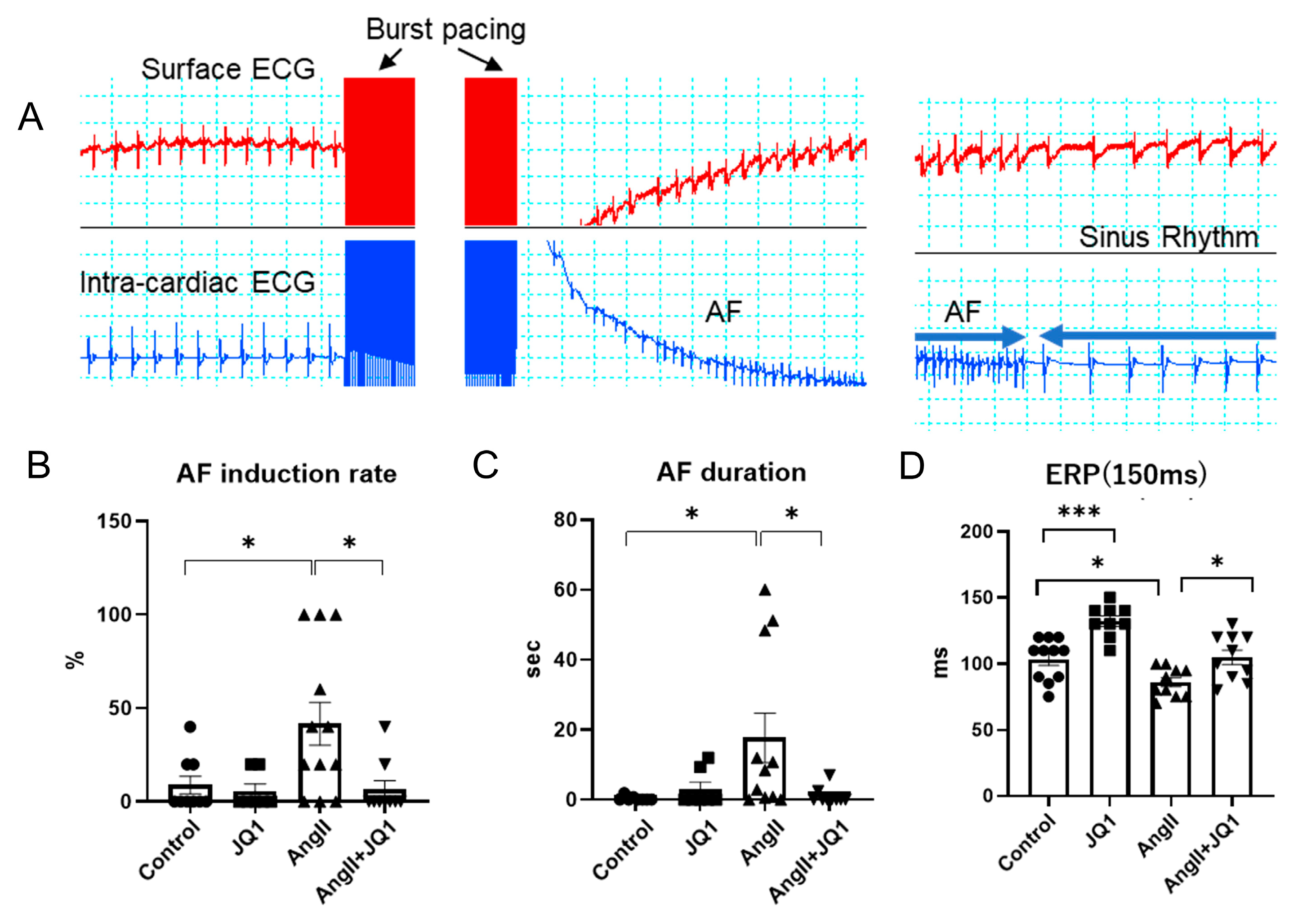
2.1. JQ1 Treatment Inhibits AngII-Induced AF Susceptibility in Mice
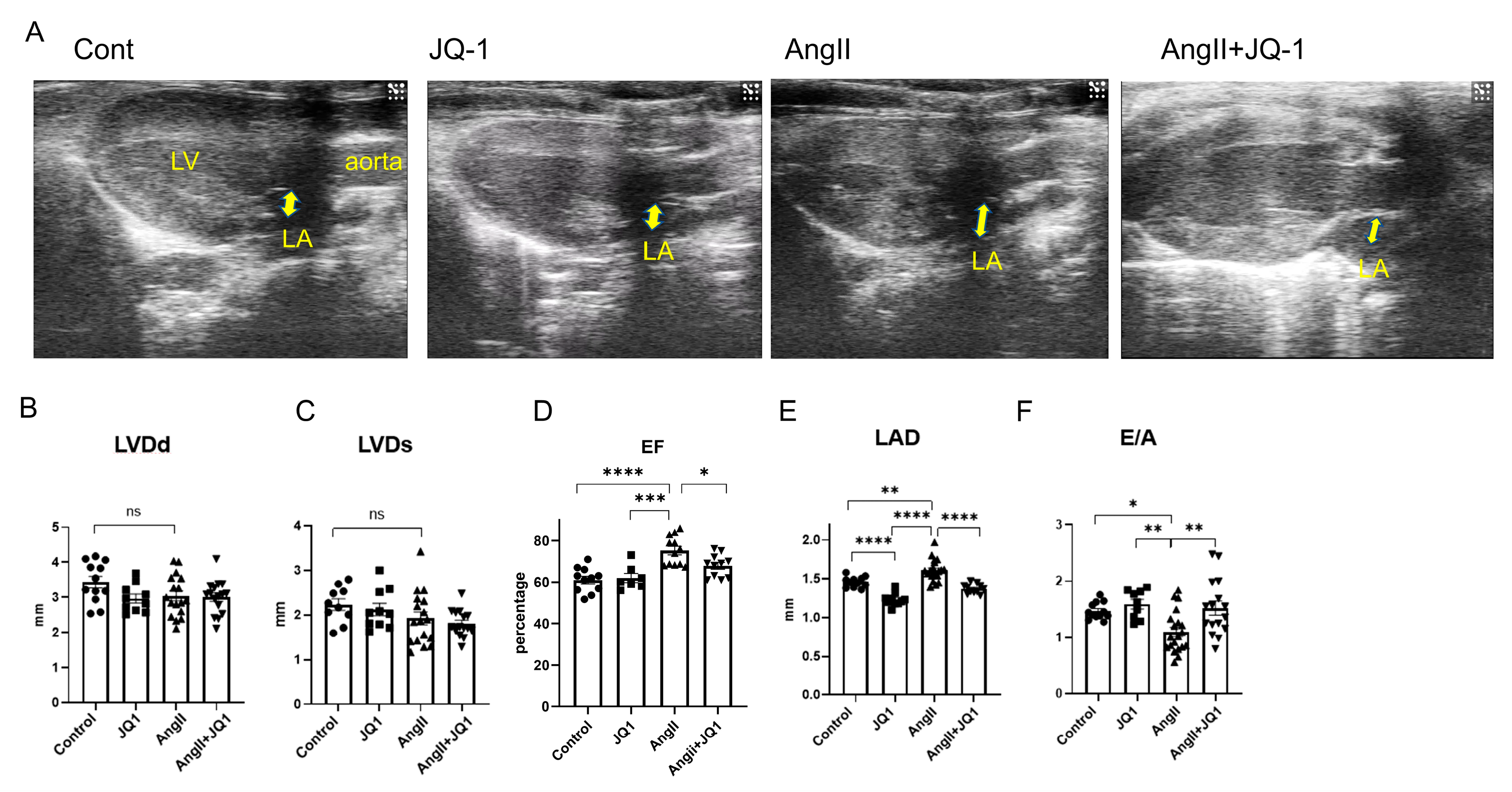
2.2. JQ1 Prevents AngII-Induced Atrial Enlargement and Diastolic Dysfunction
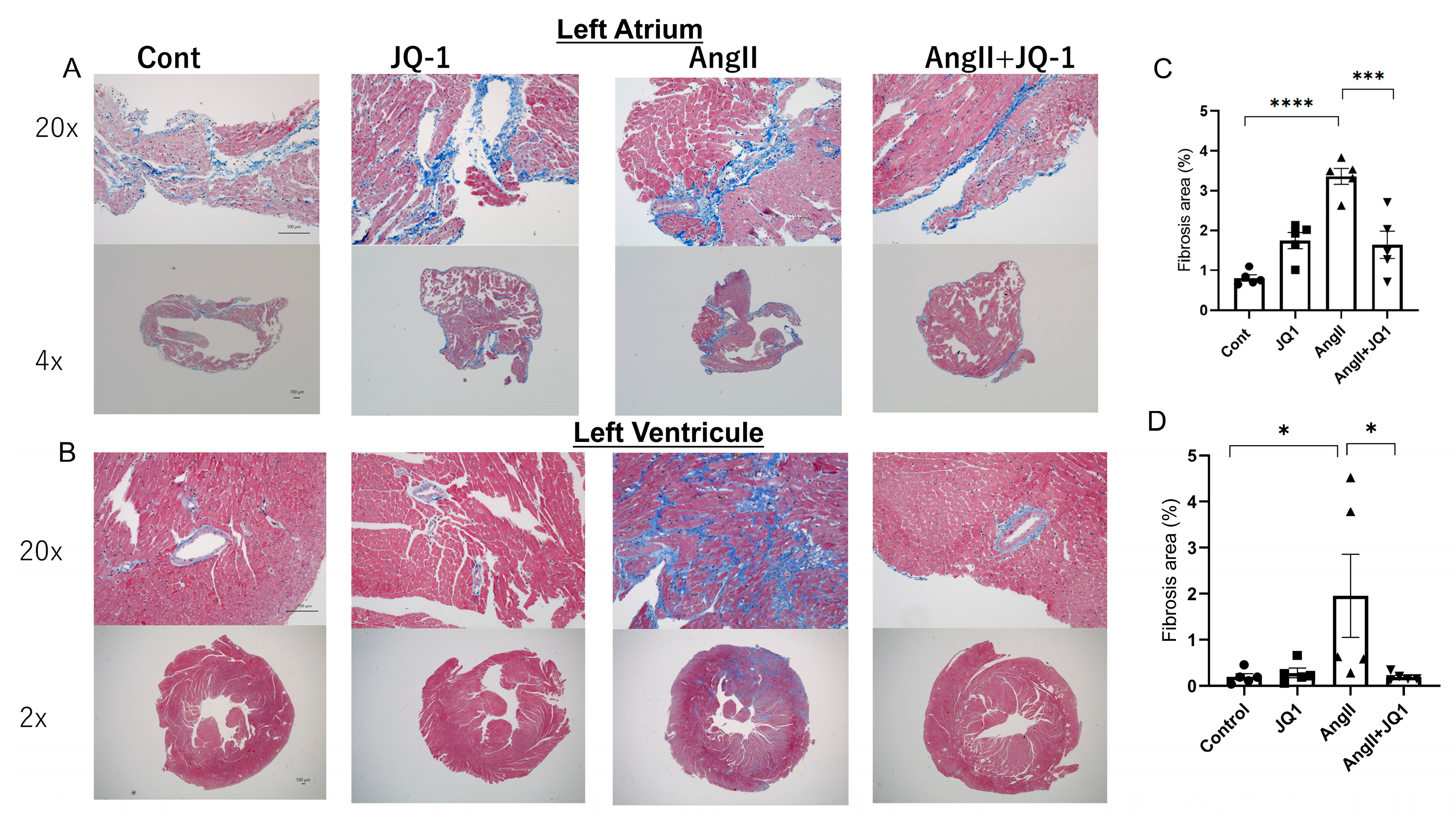
2.3. JQ1 Inhibits AngII-Induced Cardiac Fibrosis
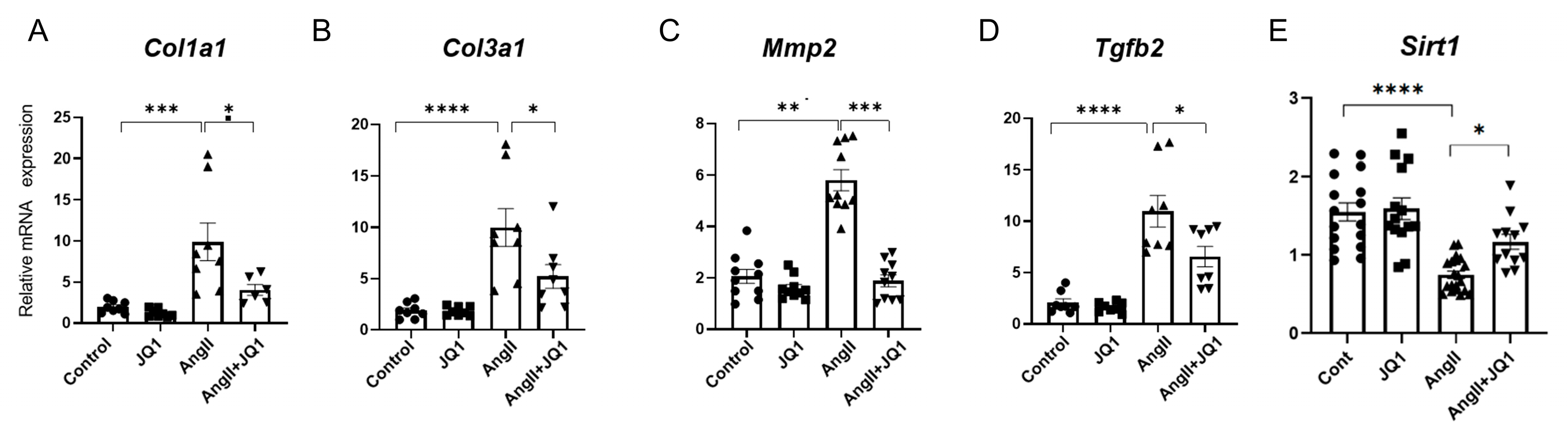
2.4. JQ1 Modulates Fibrosis-Related Gene Expression in Atrial Tissue
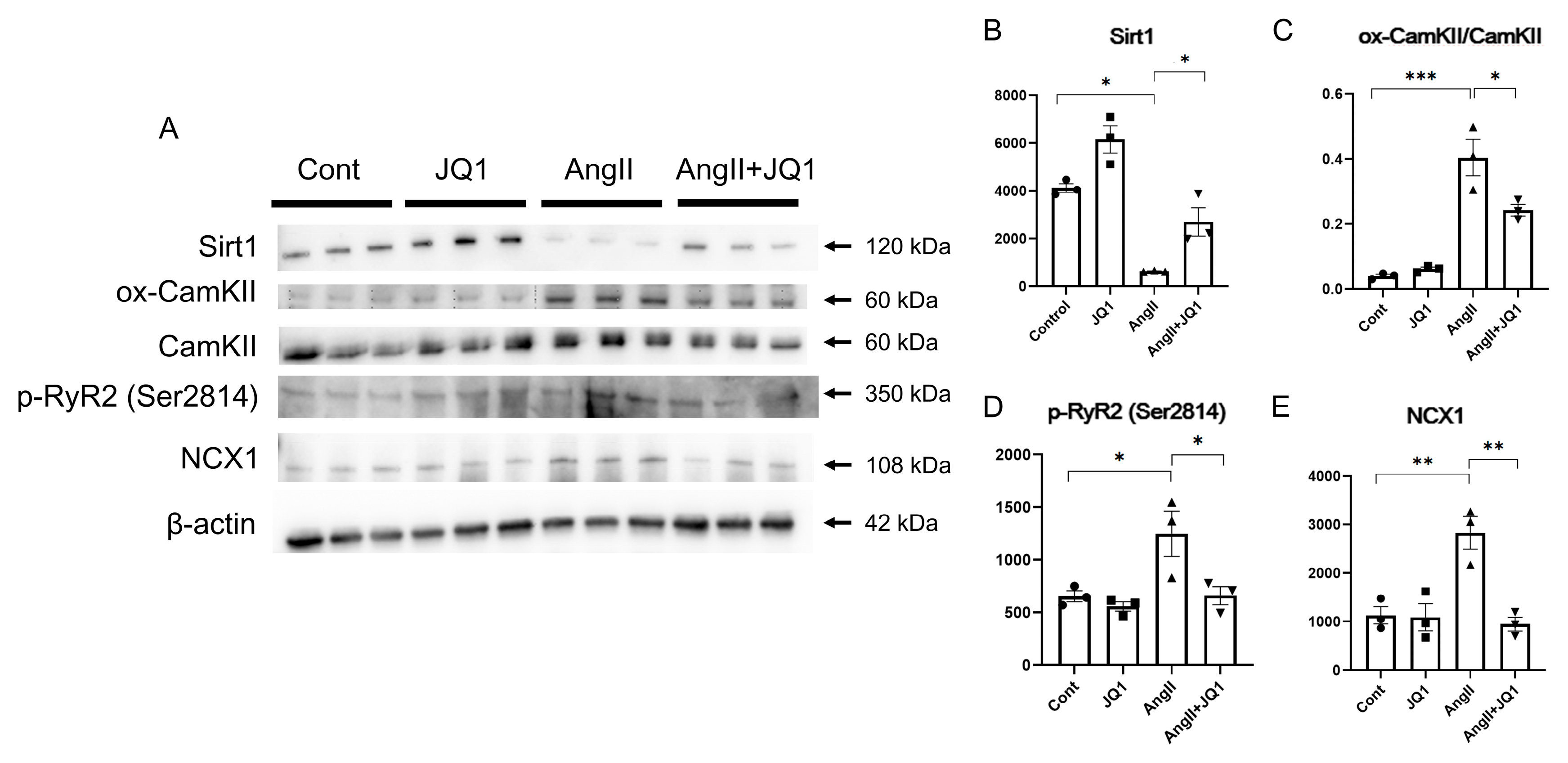
2.5. JQ1 Regulates AF-Related Protein Expression in Cardiac Tissue
2.6. JQ1 Attenuates AngII-Induced Abnormal Intracellular Ca2+ Handling in HL-1 Cells
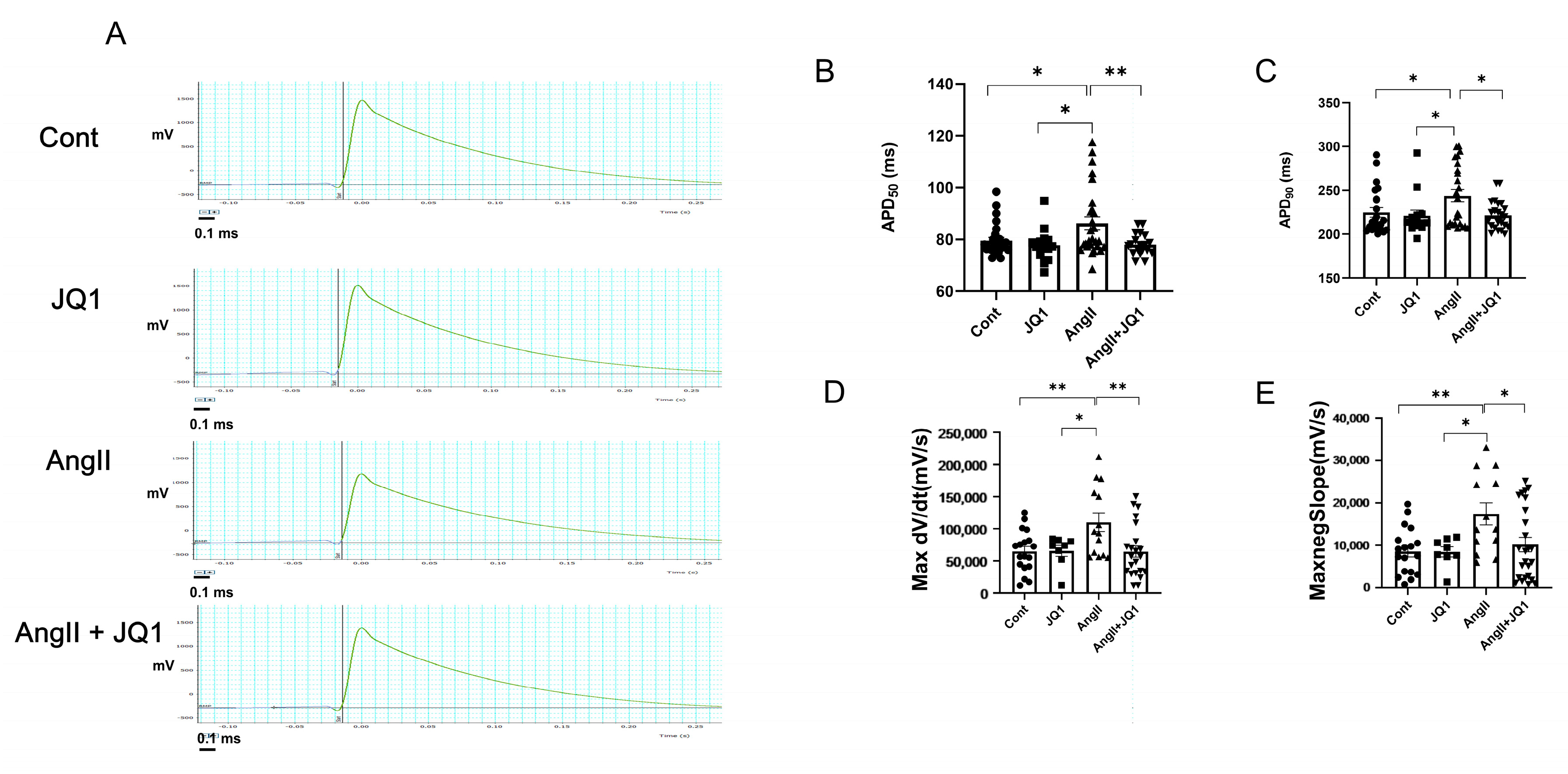
2.7. JQ1 Normalizes AngII-Induced Prolongation of APD in HL-1 Cells
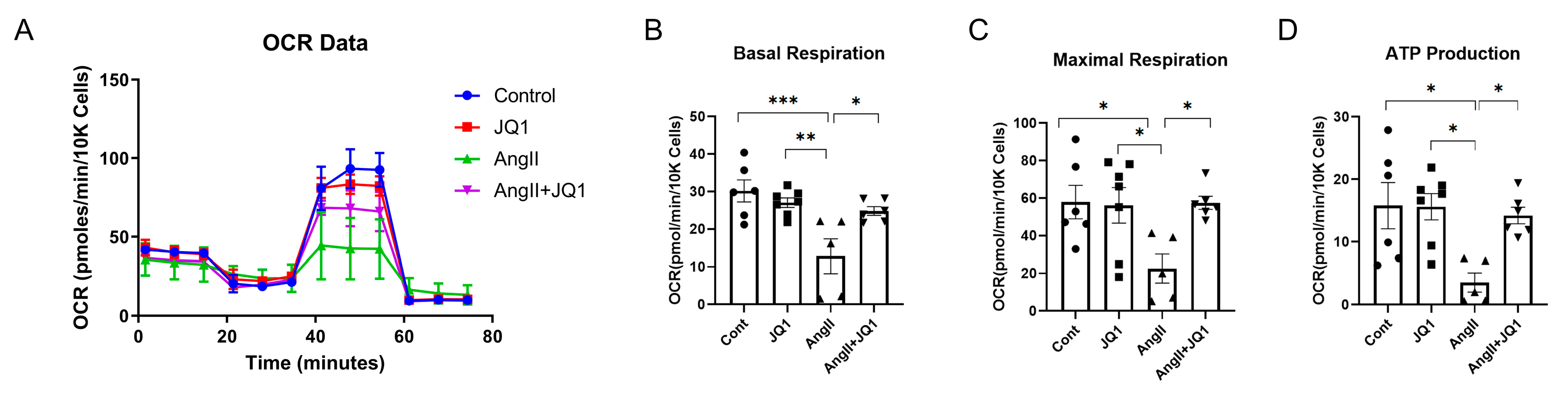
2.8. JQ1 Preserves Mitochondrial Function in AngII-Treated HL-1 Cells
3. Discussion
4. Materials and Methods
4.1. Animal Ethics and Welfare Considerations
4.2. Experimental Design
4.3. Electrophysiological Study and AF Induction
4.4. Echocardiography
4.5. HL-1 Cell Culture
4.6. Calcium Imaging of HL-1 Cells
4.7. APD Measurement in HL-1 Cells
4.8. Histological Analysis
4.9. Real-Time Quantitative Polymerase Chain Reaction (PCR)
4.10. Western Blotting
4.11. Mitochondrial Stress Test in HL-1 Cells
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AERPs | atrial effective refractory periods |
| AF | atrial fibrillation |
| AngII | angiotensin II |
| APD | action potential duration |
| ATP | adenosine triphosphate |
| BD1 | bromodomain 1 |
| BD2 | bromodomain 2 |
| BET | bromodomain and extraterminal domain |
| BRD2 | bromodomain-containing protein 2 |
| BRD3 | bromodomain-containing protein 3 |
| BRD4 | bromodomain-containing protein 4 |
| BRDT | bromodomain testis-specific protein |
| CamKII | calcium/calmodulin-dependent protein kinase II |
| Col1a1 | collagen type I alpha I chain |
| Col3a1 | collagen type III alpha I chain |
| DT | decay time |
| E/A | early-to-atrial filling velocity ratio |
| LA | left atrial |
| LAD | left atrial-diameter |
| LV | left ventricular |
| LVDd | left ventricular end-diastolic diameter |
| LVDs | left ventricular end-systolic diameter |
| LVEF | left ventricular ejection fraction |
| MEA | multi-electrode array |
| MMP2 | matrix metalloproteinase-2 |
| NAD+ | nicotinamide adenine dinucleotide |
| NCX1 | sodium/calcium exchanger |
| OCR | oxygen consumption rate |
| RyR2 | ryanodine receptors 2 |
| Sirt1 | sirtuin 1 |
| Tgfb2 | transforming growth factor beta-2 |
| TP | time to peak |
References
- Williams, B.A.; Chamberlain, A.M.; Blankenship, J.C.; Hylek, E.M.; Voyce, S. Trends in atrial fibrillation incidence rates within an integrated health care delivery system, 2006 to 2018. JAMA Netw. Open 2020, 3, e2014874. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef]
- Nattel, S.; Sager, P.T.; Hüser, J.; Heijman, J.; Dobrev, D. Why translation from basic discoveries to clinical applications is so difficult for atrial fibrillation and possible approaches to improving it. Cardiovasc. Res. 2021, 117, 1616–1631. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chiang, C.M. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem. 2007, 282, 13141–13145. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W.B.; Fedorov, O.; Morse, E.M.; Keates, T.; Hickman, T.T.; Felletar, I.; et al. Selective inhibition of BET bromodomains. Nature 2010, 468, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Borck, P.C.; Guo, L.-W.; Plutzky, J. BET epigenetic reader proteins in cardiovascular transcriptional programs. Circ. Res. 2020, 126, 1190–1208. [Google Scholar] [CrossRef] [PubMed]
- Auguste, G.; Rouhi, L.; Matkovich, S.J.; Coarfa, C.; Robertson, M.J.; Czernuszewicz, G.; Gurha, P.; Marian, A.J. BET bromodomain inhibition attenuates cardiac phenotype in myocyte-specific lamin A/C–deficient mice. J. Clin. Investig. 2020, 130, 4740–4758. [Google Scholar] [CrossRef]
- Song, S.; Liu, L.; Yu, Y.; Zhang, R.; Li, Y.; Cao, W.; Xiao, Y.; Fang, G.; Li, Z.; Wang, X.; et al. Inhibition of BRD4 attenuates transverse aortic constriction- and TGF-β-induced endothelial-mesenchymal transition and cardiac fibrosis. J. Mol. Cell. Cardiol. 2019, 127, 83–96. [Google Scholar] [CrossRef]
- Alexanian, M.; Przytycki, P.F.; Micheletti, R.; Padmanabhan, A.; Ye, L.; Travers, J.G.; Gonzalez-Teran, B.; Silva, A.C.; Duan, Q.; Ranade, S.S.; et al. A transcriptional switch governs fibroblast activation in heart disease. Nature 2021, 595, 438–443. [Google Scholar] [CrossRef]
- Yang, M.; Wang, T.; Shao, J.; Ran, X.; Xiao, R.; Zhao, R.; Li, C.; Liu, J.; Zhang, Q.; Hu, L.; et al. (+)-JQ-1 alleviates cardiac injury in myocardial infarction by inhibiting ferroptosis through the NAMPT/SIRT1 pathway. Cell Death Dis. 2025, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Gou, R.; Wang, Y.; Shi, X.; Zhang, Y.; Zhang, J.; Li, Y.; Li, W.; Li, X.; et al. Ameliorative role of SIRT1 in peritoneal fibrosis: An in vivo and in vitro study. Cell Biosci. 2021, 11, 105. [Google Scholar] [CrossRef]
- Wang, M.; Yang, L.; Yang, J.; Wang, C. Shen Shuai II recipe attenuates renal injury and fibrosis in chronic kidney disease by regulating NLRP3 inflammasome and Sirt1/Smad3 deacetylation pathway. BMC Complement. Altern. Med. 2019, 19, 107. [Google Scholar] [CrossRef]
- Yang, L.; Ao, Q.; Zhong, Q.; Li, W.; Li, W.; Li, Y.; Zhang, H.; Wang, C.; Chen, X.; Liu, H.; et al. SIRT1/IGFBPrP1/TGF-β1 axis involved in cucurbitacin B ameliorating concanavalin A-induced mice liver fibrosis. Basic Clin. Pharmacol. Toxicol. 2020, 127, 371–379. [Google Scholar] [CrossRef]
- Mansour, H.H.; Omran, M.M.; Hasan, H.F.; El Kiki, S.M. Modulation of bleomycin-induced oxidative stress and pulmonary fibrosis by N-acetylcysteine in rats via AMPK/SIRT1/NF-κB. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1943–1952. [Google Scholar] [CrossRef]
- Zhang, D.; Li, B.; Li, B.; Tang, Y.; Zhou, J.; Wang, H.; Chen, X.; Li, W.; Liu, H.; Zhang, Y.; et al. Regulation of left atrial fibrosis induced by mitral regurgitation by SIRT1. Sci. Rep. 2020, 10, 64308. [Google Scholar] [CrossRef]
- Faivre, E.J.; McDaniel, K.F.; Albert, D.H.; Mantena, S.R.; Plotnik, J.P.; Wilcox, D.; Zhang, L.; Bui, M.H.; Sheppard, G.; Wang, L.; et al. Selective inhibition of the BD2 bromodomain of BET proteins in prostate cancer. Nature 2020, 578, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Rao, S.V.; Sutton, J.; Cheeseman, D.; Dunn, S.; Papachristou, E.K.; Gonzalez Prada, J.-E.; Couturier, D.-L.; Kumar, S.; Kishore, K.; et al. ARID1A influences HDAC1/BRD4 activity, intrinsic proliferative capacity and breast cancer treatment response. Nat. Genet. 2020, 52, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Lin, C.Y.; Duan, Q.; Griffin, G.; Federation, A.; Paranal, R.M.; Bair, S.; Newton, G.; Lichtman, A.; Kung, A.; et al. NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol. Cell 2014, 56, 219–231. [Google Scholar] [CrossRef]
- Albrecht, B.K.; Gehling, V.S.; Hewitt, M.C.; Vaswani, R.G.; Côté, A.; Leblanc, Y.; Nasveschuk, C.G.; Bellon, S.; Bergeron, L.; Campbell, R.; et al. Identification of a Benzoisoxazoloazepine inhibitor (CPI-0610) of the bromodomain and extra-terminal (BET) family as a candidate for human clinical trials. J. Med. Chem. 2016, 59, 1330–1339. [Google Scholar] [CrossRef]
- Stratton, M.S.; Bagchi, R.A.; Felisbino, M.B.; Hirsch, R.A.; Smith, H.E.; Riching, A.S.; La, H.J.; Graves, B.J.; Rothbart, S.B.; Strahl, B.D.; et al. Dynamic chromatin targeting of BRD4 stimulates cardiac fibroblast activation. Circ. Res. 2019, 125, 662–677. [Google Scholar] [CrossRef] [PubMed]
- Spiltoir, J.I.; Stratton, M.S.; Cavasin, M.A.; Demos-Davies, K.; Reid, B.G.; Qi, J.; Bradner, J.E.; McKinsey, T.A. BET acetyl-lysine binding proteins control pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2013, 63, 175–179. [Google Scholar] [CrossRef]
- Anand, P.; Brown, J.D.; Lin, C.Y.; Qi, J.; Zhang, R.; Calderon Artero, P.; Alaiti, M.A.; Bullard, J.; Alazem, K.; Margulies, K.B.; et al. BET bromodomains mediate transcriptional pause release in heart failure. Cell 2013, 154, 569–582. [Google Scholar] [CrossRef]
- Dutzmann, J.; Haertlé, M.; Daniel, J.-M.; Kloss, F.; Musmann, R.-J.; Kalies, K.; Knöpp, K.; Pilowski, C.; Sirisko, M.; Sieweke, J.-T.; et al. BET bromodomain-containing epigenetic reader proteins regulate vascular smooth muscle cell proliferation and neointima formation. Cardiovasc. Res. 2021, 117, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yuan, J.; Fang, G.; Li, Y.; Ding, S.; Wang, Y.; Wang, Q. BRD4 as a therapeutic target for atrial fibrosis and atrial fibrillation. Eur. J. Pharmacol. 2024, 977, 176714. [Google Scholar] [CrossRef]
- Doñate Puertas, R.; Arora, R.; Rome, S.; Asatryan, B.; Roderick, H.L.; Chevalier, P. Epigenetics in atrial fibrillation: A reappraisal. Heart Rhythm 2021, 18, 824–832. [Google Scholar] [CrossRef]
- Feng, D.; Xu, D.Z.; Murakoshi, N.; Tajiri, K.; Qin, R.; Yonebayashi, S.; Okabe, Y.; Li, S.; Yuan, Z.; Aonuma, K.; et al. Nicotinamide phosphoribosyltransferase (Nampt)/nicotinamide adenine dinucleotide (NAD) axis suppresses atrial fibrillation by modulating the calcium handling pathway. Int. J. Mol. Sci. 2020, 21, 4655. [Google Scholar] [CrossRef]
- Aonuma, K.; Xu, D.Z.; Murakoshi, N.; Tajiri, K.; Okabe, Y.; Yuan, Z.; Li, S.; Murakata, Y.; Tominaga, K.; Nogami, A.; et al. Novel preventive effect of isorhamnetin on electrical and structural remodeling in atrial fibrillation. Clin. Sci. 2022, 136, 1831–1849. [Google Scholar] [CrossRef] [PubMed]
- Murakata, Y.; Yamagami, F.; Murakoshi, N.; Xu, D.Z.; Song, Z.; Li, S.; Okabe, Y.; Aonuma, K.; Yuan, Z.; Mori, H.; et al. Electrical, structural, and autonomic atrial remodeling underlies atrial fibrillation in inflammatory atrial cardiomyopathy. Front. Cardiovasc. Med. 2023, 9, 1075358. [Google Scholar] [CrossRef]
- Chen, M.; Xu, D.; Wu, A.Z.; Kranias, E.; Lin, S.-F.; Chen, P.-S.; Chen, Z. Phospholamban regulates nuclear Ca2+ stores and inositol 1,4,5-trisphosphate mediated nuclear Ca2+ cycling in cardiomyocytes. J. Mol. Cell. Cardiol. 2018, 123, 185–197. [Google Scholar] [CrossRef]
- Chen, M.; Xu, D.-Z.; Wu, A.Z.; Guo, S.; Wan, J.; Yin, D.; Lin, S.-F.; Chen, Z.; Rubart-von der Lohe, M.; Everett, T.H., 4th; et al. Concomitant SK current activation and sodium current inhibition cause J wave syndrome. JCI Insight 2018, 3, e122329. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Xu, D.Z.; Shimoda, Y.; Yuan, Z.; Murakata, Y.; Xi, B.; Sato, K.; Yamamoto, M.; Tajiri, K.; Ishizu, T.; et al. Metabolic remodeling and calcium handling abnormality in induced pluripotent stem cell-derived cardiomyocytes in dilated phase of hypertrophic cardiomyopathy with MYBPC3 frameshift mutation. Sci. Rep. 2024, 14, 15422. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Murakoshi, N.; Xu, D.; Xi, B.; Murakata, Y.; Aonuma, K.; Tajiri, K.; Ishizu, T. BET Inhibitor JQ1 Attenuates Atrial Fibrillation Through Modulation of Fibrosis, Calcium Homeostasis, and Mitochondrial Function in a Murine Model. Int. J. Mol. Sci. 2025, 26, 10363. https://doi.org/10.3390/ijms262110363
Song Z, Murakoshi N, Xu D, Xi B, Murakata Y, Aonuma K, Tajiri K, Ishizu T. BET Inhibitor JQ1 Attenuates Atrial Fibrillation Through Modulation of Fibrosis, Calcium Homeostasis, and Mitochondrial Function in a Murine Model. International Journal of Molecular Sciences. 2025; 26(21):10363. https://doi.org/10.3390/ijms262110363
Chicago/Turabian StyleSong, Zonghu, Nobuyuki Murakoshi, Dongzhu Xu, Binyang Xi, Yoshiko Murakata, Kazuhiro Aonuma, Kazuko Tajiri, and Tomoko Ishizu. 2025. "BET Inhibitor JQ1 Attenuates Atrial Fibrillation Through Modulation of Fibrosis, Calcium Homeostasis, and Mitochondrial Function in a Murine Model" International Journal of Molecular Sciences 26, no. 21: 10363. https://doi.org/10.3390/ijms262110363
APA StyleSong, Z., Murakoshi, N., Xu, D., Xi, B., Murakata, Y., Aonuma, K., Tajiri, K., & Ishizu, T. (2025). BET Inhibitor JQ1 Attenuates Atrial Fibrillation Through Modulation of Fibrosis, Calcium Homeostasis, and Mitochondrial Function in a Murine Model. International Journal of Molecular Sciences, 26(21), 10363. https://doi.org/10.3390/ijms262110363





