Abstract
Major depressive disorder (MDD) is a severe mental disorder associated with significant functional impairment and decreased quality of life. Growing evidence suggests that immune-inflammatory mechanisms, particularly cytokine dysregulation, take part in its development and course. This systematic review and meta-analysis aimed to evaluate whether cytokine alterations are present in early stages of MDD, specifically in first-episode (FE) and drug-naïve (DN) patients. Following PRISMA guidelines a comprehensive search of PubMed, Scopus, and Web of Science was conducted in March 2025. Studies were eligible if they compared levels of inflammatory cytokines between adult FE or DN MDD patients and healthy controls (HCs). Meta-analyses using random-effects models were performed, including subanalyses depending on the source of the sample and the quality of the studies. In total, 17 eligible studies involving 1371 MDD patients were included. The meta-analysis showed significantly elevated levels of interleukin 6 (IL-6), interleukin 2 (IL-2), and tumor necrosis factor alfa (TNF-α) in FE patients compared to HCs. DN patients’ quantitative analysis showed increased levels of IL-6, IL-2, interleukin 4 (IL-4), interleukin 10 (IL-10), TNF-α, and interferon gamma (IFN-γ) compared to healthy individuals. Moreover, in the case of TNF-α, IL-2, interleukin 1 beta (IL-1β), and IL-4, there was a difference in results depending on the sample source (plasma/serum). Cytokine dysregulation is present in first-episode and drug-naïve MDD individuals. These findings highlight that the immune–inflammatory response exists in the early stages of this disorder. Moreover, since more cytokines were elevated in DN patients, pharmacological antidepressant treatment might be a significant factor involved in inflammatory regulation in MDD. Nonetheless, future prospective studies with standardized protocols and division by clinical subtypes are needed to better understand the dynamics and clinical relevance of cytokine alterations in depression.
1. Introduction
Major depressive disorder (MDD) is a prevalent psychiatric disorder that significantly impairs psychosocial functioning and reduces overall quality of life [1]. According to data from the World Mental Health Surveys, the estimated lifetime prevalence of MDD is 11.1%, while the annual prevalence is 5.9% [2]. Moreover, MDD ranks among the ten most disabling disorders globally [3]. The World Health Organization (WHO) projects that by 2030, MDD will become the leading contributor to the global burden of disease [4].
A substantial body of evidence has demonstrated a consistent association between depression and the activation of the inflammatory response system. It is widely hypothesized that the overexpression of pro-inflammatory cytokines contributes to the pathophysiology of depressive disorders [5,6]. Notably, interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor alpha (TNF-α) measured in the blood have been identified as the inflammatory biomarkers most consistently associated with MDD [7,8]. Moreover, a meta-analysis demonstrated elevated plasma levels of interleukin-3 (IL-3), interleukin-12 (IL-12), and interleukin-18 (IL-18) in patients with depression [9]. Cytokine level abnormalities also affect other body fluids, such as cerebrospinal fluid (CSF). The concentration of interleukin-8 (IL-8) in CSF was significantly higher in individuals with MDD compared to healthy controls (HCs) [10]. Genetic studies have also highlighted the key role of neuroinflammatory cytokines in the pathogenesis of MDD. Specifically, CRP gene methylation and polymorphisms in IL-6 (rs1800795) and interleukin-1 beta (IL-1β) (rs16944) have been identified as genetic risk loci associated with increased vulnerability to MDD [11,12,13]. Although cytokines are broadly divided into pro-inflammatory (e.g., IL-1β, IL-6, TNF-α) and anti-inflammatory (e.g., interleukin 4—IL-4, interleukin 10—IL-10, transforming growth factor β1—TGF-β1), some, including IL-6 and TGF-β1, display context-dependent activity, which may contribute to the complex and heterogeneous immune profile observed in major depressive disorder.
Chronic immune activation and elevated cytokine levels are among the most robust biological findings in depression [14]. Psychosocial stress triggers the hypothalamic–pituitary–adrenal (HPA) axis and sympathetic activation, leading to increases in glucocorticoids and catecholamines that modulate innate and adaptive immunity and promote NF-κB-mediated transcription of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α [15]. In parallel, disturbances in the gut–brain axis—including dysbiosis and increased intestinal permeability (“leaky gut”)—facilitate the translocation of pathogen-associated molecular patterns, thereby activating Toll-like receptor signaling and systemic cytokine release [16]. These converging pathways offer biologically plausible mechanisms by which environmental stressors and host vulnerability can elevate peripheral cytokines early in major depressive disorder (MDD).
Despite these findings, several uncertainties remain regarding the relationship between inflammation and depression. MDD is a chronic condition, and it is unclear whether cytokine alterations occur immediately following disease onset or emerge over the course of its progression [17]. Moreover, pharmacological treatment may influence cytokine levels, potentially confounding the relationship between inflammation and the disorder itself. Meta-analysis suggests that levels of TNF-α and IL-8 may be modulated by antidepressant therapy [18]. In recent years, two meta-analyses evaluating cytokines in drug-naïve depression patients were conducted [19,20]. However, since then, new evidence has been published that allows for the estimation of pool effects for other compounds. In addition, the studies included in this type of meta-analysis use terminology with similar meanings—drug-naïve (patients who have never taken medication), drug-free (patients who have not taken medication at least 2 weeks), and first-episode (patients with their first diagnosis of a depressive episode). Given these complexities, the present review and meta-analysis aim to evaluate whether cytokine imbalances are significant during the early stages of MDD—specifically in individuals experiencing their first episode of depression—and whether such alterations are present in drug-naïve patients. Although numerous studies have recently focused on these populations, the findings remain inconclusive.
2. Materials and Methods
This systematic review was conducted according to the PRISMA statement (Preferred reporting items for systematic review and meta-analysis).
2.1. Data Acquisition and Search Strategy
A literature search was independently conducted by two researchers, covering studies published up to 15 March 2025, using the PubMed, Scopus, and Web of Science databases, without applying any filters. This review was guided by the following search strategy: (“depression” OR “depressive” OR “major depression” OR “major depressive disorder”) AND (“first-episode” OR “first episode” OR “drug-naïve” OR “drug naïve” OR “drug-naïve” OR “drug naive” OR “medication-naïve” OR “drug-free” OR “drug free” OR “non-treated”) AND (“interleukin” OR “cytokines” OR “cytokine” OR “tumor necrosis factor” OR “interferon” OR “transforming growth factor” OR “chemokine”). In addition, references from selected articles were screened to confirm potentially related studies.
2.2. Inclusion and Exclusion Criteria
The following criteria were conditions for the inclusion of the studies in this systematic review: (1) observational study design; (2) concerning DN or FE adult patients diagnosed with MDD using any recognized diagnostic criteria (e.g., DSM-5, ICD-10) and HCs with no history of mental illness; (3) at least one of the inflammatory cytokines was measured; (4) published in English. The exclusion criteria were as follows: (1) reviews, letters, and conference abstracts; (2) non-human studies; (3) duplicate data; (4) full text was not available; (5) not in English. Two investigators worked independently to complete the preliminary screening through browsing titles and abstracts. The final decisions were made after reviewing the full texts. Disagreements between the researchers were resolved by consultation with the third author.
2.3. Definition of Study Group
As the terminology used across studies to describe patient subgroups may vary and lead to ambiguity, we provide the definitions applied in this meta-analysis for clarity. First-episode refers to cohorts sampled during the first clinically diagnosed depressive episode, regardless of prior or current antidepressant exposure at the time of blood collection. Drug-naïve denotes cohorts in which participants had never received antidepressant treatment before sampling, regardless of the episode number. Studies explicitly reporting “first-episode, drug-naïve” were marked accordingly. In the Results section, pooled analyses are presented separately for FE and DN patient data.
2.4. Data Extraction
The original data was extracted from the included studies by two of the researchers. The following information was collected: the surname of the first author, year of publication, country, population, diagnosis, sample size, MDD diagnostic criteria, sample source (serum/plasma), units of measurement, cytokine levels with mean and confidence intervals or substitute statistics, matching criteria, and results regarding outcomes of interest. Any divergences were resolved through discussion between the two investigators and consultation with a third researcher.
2.5. Quality Assessment
The quality of the included studies was independently evaluated by two authors with the Newcastle–Ottawa Scale. The studies were assessed with respect to three aspects: Selection, Comparability, and Exposure. A maximum of one star was awarded in each category for Selection and Exposure, while a maximum of two stars could be awarded for Comparability. Studies were rated from 0 to 9, with those scoring from 0 to 2 being ranked as poor0quality, 3 to 6 as fair-quality, and 7 to 9 as high-quality. Any disagreements were managed by group discussion. High-quality and fair-quality studies were included in the meta-analysis.
2.6. Statistical Analysis
The meta-analysis was conducted using RevMan 5 (version 5.4.1; Cochrane Collaboration). Continuous outcomes were pooled using standardized mean differences (SMDs) with 95% confidence intervals. However, due to variability in reporting formats across studies, several methodological adjustments were applied. On account of some cytokine concentrations being reported on the logarithmic scale, all cytokine values were converted to this scale in accordance with Higgins et al. [21], to ensure consistency in effect size estimation. Additionally, for studies reporting data as interquartile ranges (IQRs), mean and standard deviation values were estimated using validated statistical conversion tools [22,23,24]. Heterogeneity was assessed both visually using forest plots and statistically using the Chi2 test, I2 statistic, and Tau2. Where substantial heterogeneity was detected, sensitivity analyses were performed by excluding studies identified as primary contributors to heterogeneity. Thresholds from Cochrane Collaboration were consistent with the interpretation of heterogeneity: 0–40% may not be important; 30–60% may represent moderate heterogeneity; 50–90% may represent substantial heterogeneity; and 75–100% may represent a high level of heterogeneity. A random-effect model was used for analysis, and p < 0.05 was set as statistical significance. Subanalyses were also conducted, taking into account high-quality studies and dividing them according to the sources of samples (serum/plasma). No other subanalyses were permitted due to the available data. Outlier exclusion was conducted as part of the sensitivity analysis for each cytokine to evaluate the influence of individual studies on the pooled effect size. Outlier exclusion was conducted as part of the sensitivity analysis for each cytokine to evaluate the influence of individual studies on the pooled effect size.
3. Results
3.1. Study Selection
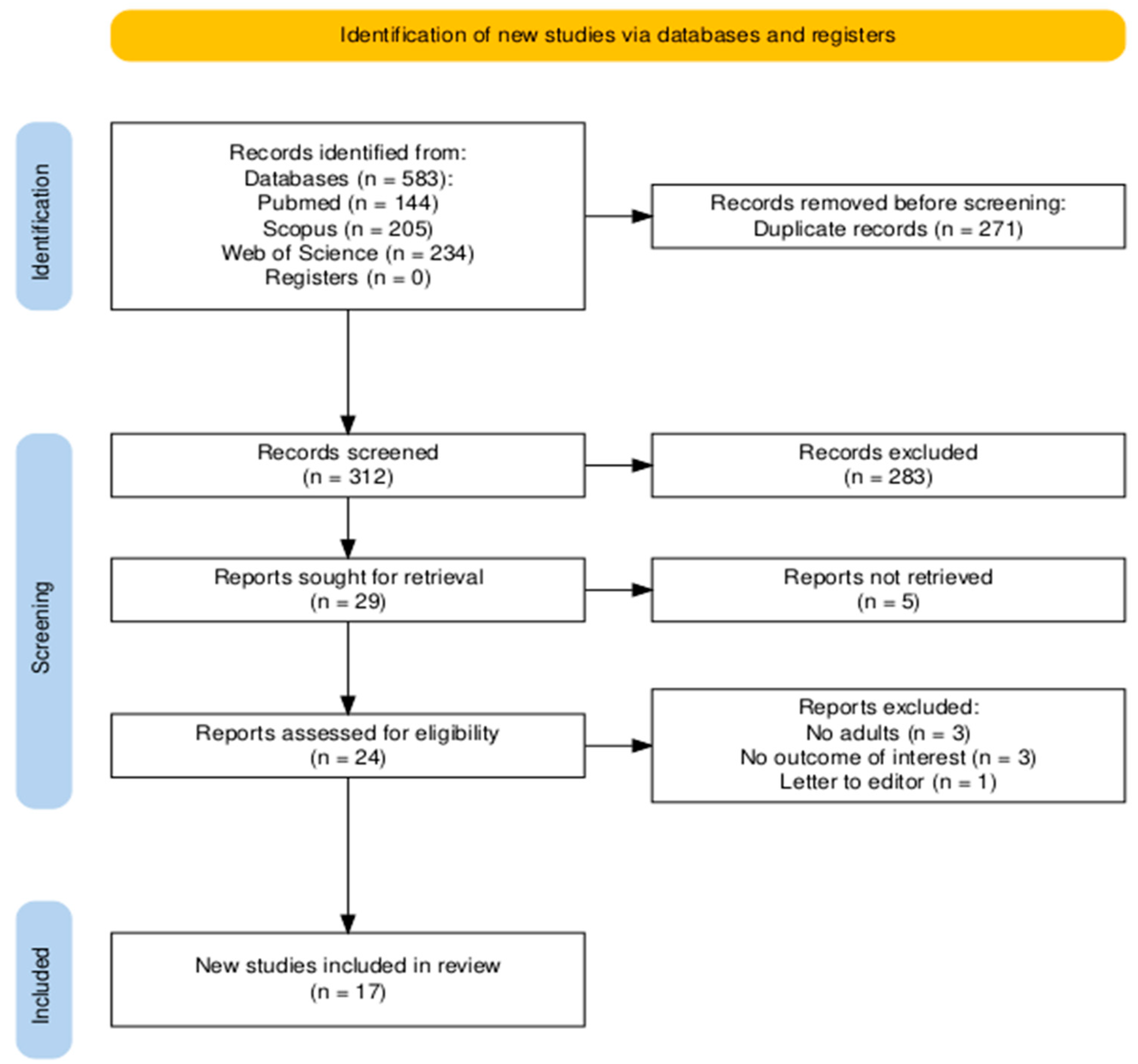
The search of three databases (PubMed, Scopus, and Web of Science) yielded a total of 583 records. After removing duplicates (n = 271) and screening titles and abstracts, 24 articles were selected for full-text review. Following a detailed eligibility assessment, 7 studies were excluded, resulting in 17 publications being included in the final systematic review and meta-analysis. All of the articles were included in the qualitative and quantitative analysis. The study selection process is illustrated in a PRISMA flow diagram (Figure 1) [25].
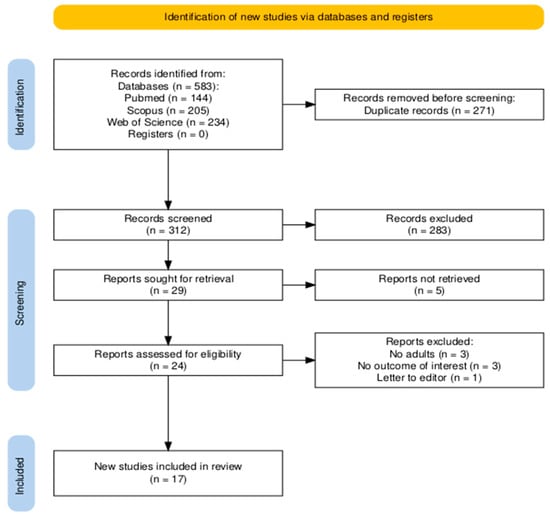
Figure 1.
PRISMA flow diagram.
3.2. Characteristics of Included Studies
A total of 17 studies were included in this review. Five studies examined the FE depression patients (n = 5) [26,27,28,29,30], nine examined DN MDD patients (n = 9) [31,32,33,34,35,36,37,38,39], and three examined first-episode drug-naïve depression patients (n = 3) [40,41,42]. The studies included, in total, 1371 MDD patients and 861 controls. Eight of the studies were conducted in China (n = 8) [27,28,30,35,36,37,38,41], two each in Turkey (n = 2) [26,29], India (n = 2) [39,40], and Iraq (n = 2) [32,33], and one each in Taiwan (n = 1) [42], Korea (n = 1) [34], and Bangladesh (n = 1) [31]. Ten of the studies conducted the measurements on serum (n = 10) [27,28,29,30,31,32,33,34,38,40], six conducted them on plasma (n = 6) [26,35,37,38,41,42], and one only reported the material as “blood” (n = 1) [39]. Fourteen studies used an Enzyme-Linked Immunosorbent Assay (ELISA) as a method for cytokine measurement (n = 14) [26,28,29,30,31,32,33,34,35,36,37,38,40,42]. A multiplexed flow cytometric assay and electrochemical luminescence were each used in one study (n = 1) [27,41]. One of the studies did not report the method used (n = 1) [39]. The most frequently investigated cytokines were IL-6, examined in fourteen studies (n = 13) [27,28,29,31,32,34,35,36,37,38,39,41], TNF-α, examined in nine studies (n = 9) [26,29,30,34,35,36,38,39,41], and both transforming growth factor-beta1 (TGF-β1) [26,32,33,38,39] and IL-1β [32,34,38,41,42], in five studies each (n = 5). The characteristics of the included studies are presented in Table 1.

Table 1.
Characteristics of the included studies.
3.3. Risk of Bias
Table 2 provides quality scores for the papers, assessing risk of bias. Most studies were rated as high-quality, and three as fair-quality. The area where points were most frequently deducted was sample size, which emphasizes that the studies conducted so far have been based on small samples.

Table 2.
Quality assessment of included studies with Newcastle–Ottawa Scale (NOS).
3.4. First Episode of Depression Versus Healthy Controls
3.4.1. IL-6
Interleukin-6 was evaluated in five studies. The mean IL-6 was significantly higher in FE than in HCs in all of them (5/5, 100%).
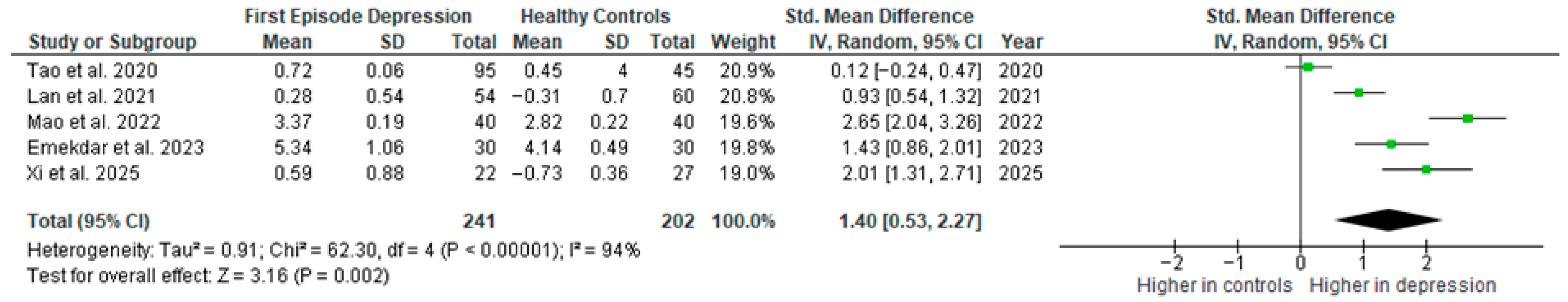
Results from five studies (n = 241/202) were included in the meta-analysis of IL-6 (Figure 2). Using the random-effect model, we did find a significantly higher level of IL-6 in FE patients than in HCs (SMD = 1.40 [0.53, 2.27], p = 0.002). Heterogeneity among studies was high (I2 = 94%). A sensitivity analysis was performed but did not result in a significant reduction in heterogeneity.
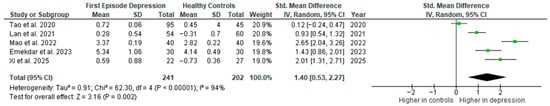
Figure 2.
First episode of depression versus healthy controls—meta-analysis of IL-6 [27,28,29,30,41].
The results obtained did not differ depending on the source of the sample between serum (SMD = 1.54 [0.31, 2.76], p = 0.01; n = 4) and plasma (according to Lan et al. [41]; n = 1).
After including only high-quality studies in the meta-analysis, the difference remains significant (SMD = 1.40 [0.33, 2.47], p = 0.01; n = 4).
3.4.2. TNF-α
Tumor necrosis factor α was evaluated in four studies. The mean TNF-α was significantly higher in FE than in HCs in all of them (4/4, 100%).
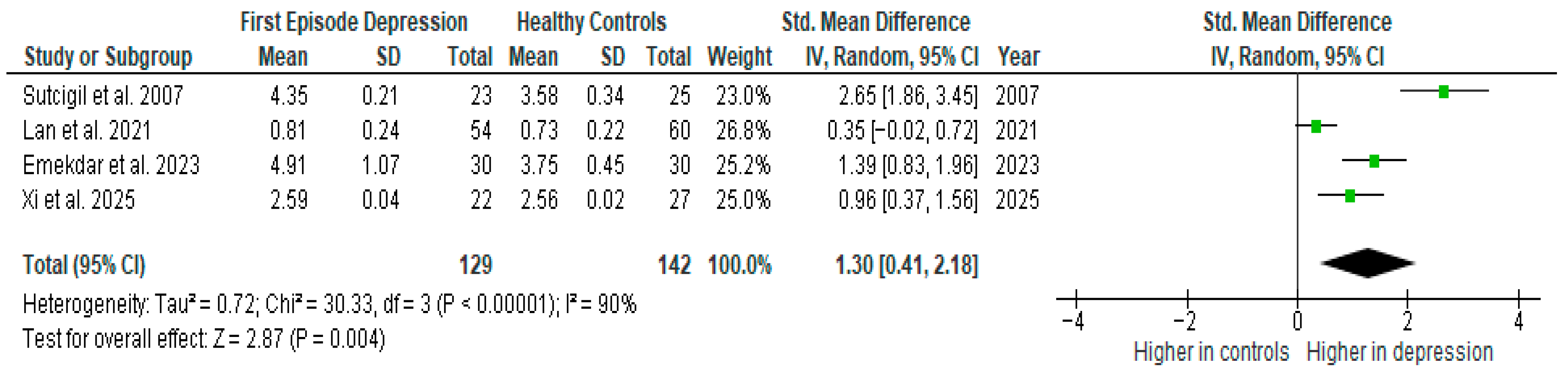
Results from four studies (n = 129/142) were included in the meta-analysis of TNF-α (Figure 3). Using the random-effect model, we did find a significantly higher level of TNF-α in FE patients than in HCs (SMD = 1.30 [0.41, 2.18] p = 0.004). Heterogeneity among studies was high (I2 = 90%). A sensitivity analysis was performed but did not result in a significant reduction in heterogeneity.
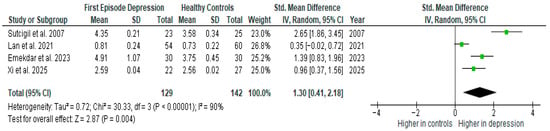
Figure 3.
First episode of depression versus healthy controls—meta-analysis of TNF-α [26,29,30,41].
The results obtained differed depending on the source of the sample between serum (SMD = 1.19 [0.77, 1.61], p < 0.00001; n = 2) and plasma (SMD = 1.47 [−0.79, 3.73], p = 0.20; n = 2).
After including only high-quality studies in the meta-analysis, the difference remains significant (SMD = 1.28 [0.07, 2.49], p = 0.04; n = 3).
3.4.3. IL-2
Interleukin-2 (IL-2) was evaluated in three studies. The mean IL-2 was significantly higher in FE than in HCs in all of them (3/3, 100%).
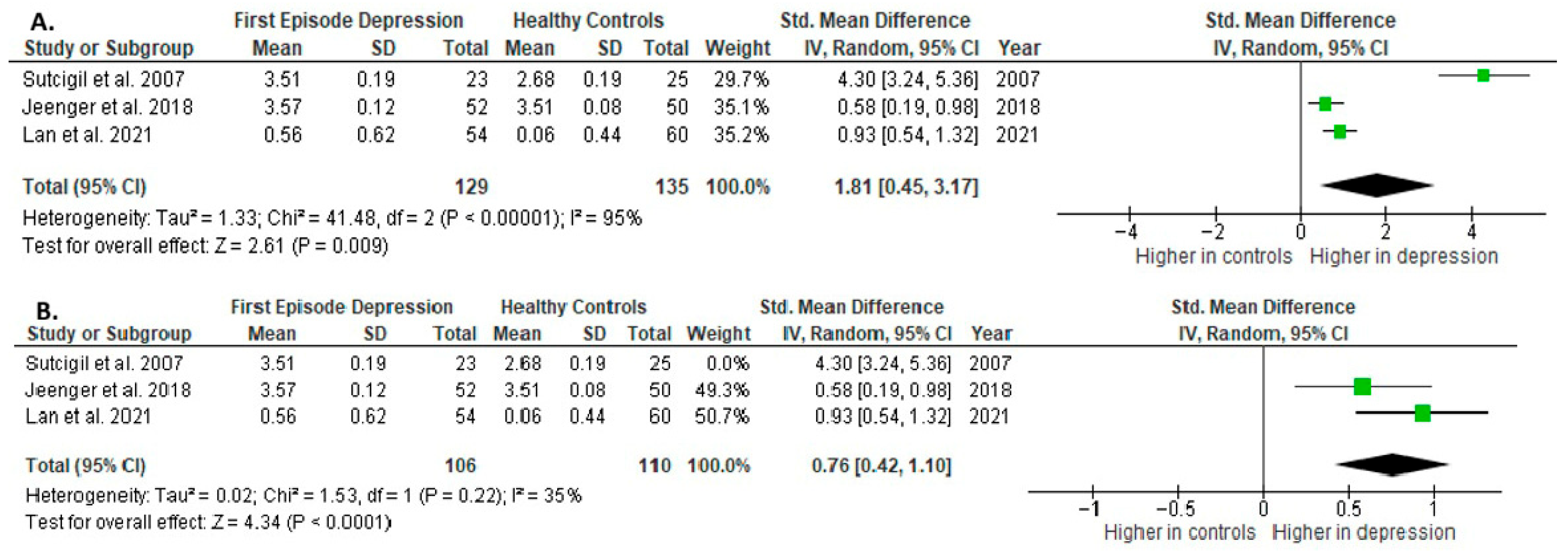
Results from three studies (n = 129/135) were included in the meta-analysis of IL-2 (Figure 4). Using the random-effect model, we did find a significantly higher level of IL-2 in FE patients than in HCs (SMD= 1.81 [0.45, 3.17], p = 0.009). Heterogeneity among studies was high (I2 = 95). A sensitivity analysis was performed, excluding a study identified as a primary contributor to heterogeneity. Following its removal, the heterogeneity was reduced to moderate (I2 = 35%), while the effect remained statistically significant (Figure 4).
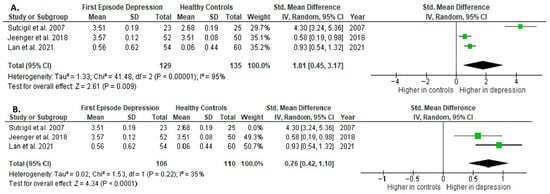
Figure 4.
First episode of depression versus healthy controls: (A) meta-analysis of IL-2. (B) Meta-analysis of IL-2 after removing the outlier [26,40,41].
The results obtained differed depending on the source of the sample between serum (SMD = 2.58 [−0.72, 5.87], p = 0.13; n = 2) and plasma (according to Jeenger et al. [40]; n = 1).
All studies included in the meta-analysis were high-quality.
3.4.4. IL-1β
Interleukin-1β was evaluated in two studies. The mean IL-1β was significantly higher in FE than in HCs in one of them (1/2, 50%). No difference was observed in one study [35].
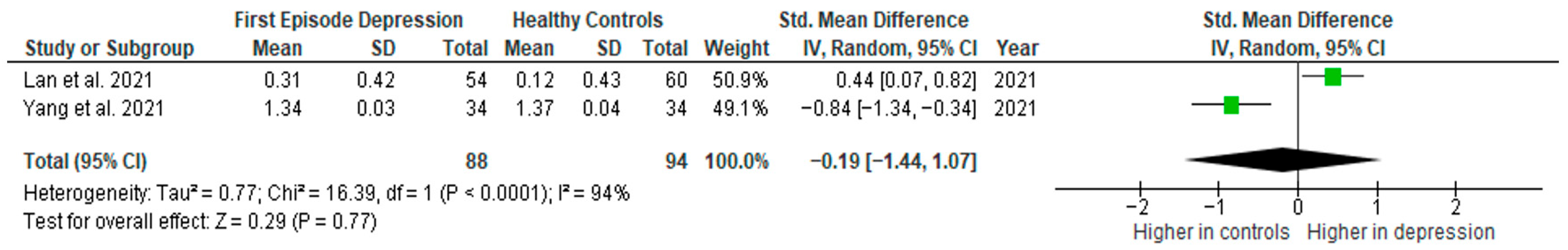
Results from two studies (n = 88/94) were included in the meta-analysis of IL-1β (Figure 5). Using the random-effect model, we did not find a significantly higher level of IL-1β in FE patients than in HCs (SMD= −0.19 [−1.44, 1.07], p = 0.77). Heterogeneity among studies was high (I2 = 94). Due to the limited number of studies, a sensitivity analysis could not be performed. All studies included in the meta-analysis were high-quality.
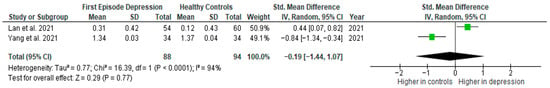
Figure 5.
First episode of depression versus healthy controls—meta-analysis of IL-1β [41,42].
3.4.5. IL-4
Interleukin-4 (IL-4) was evaluated in two studies. The mean IL-4 was not significantly higher in FE than in HCs in any of them (0/2, 0%).
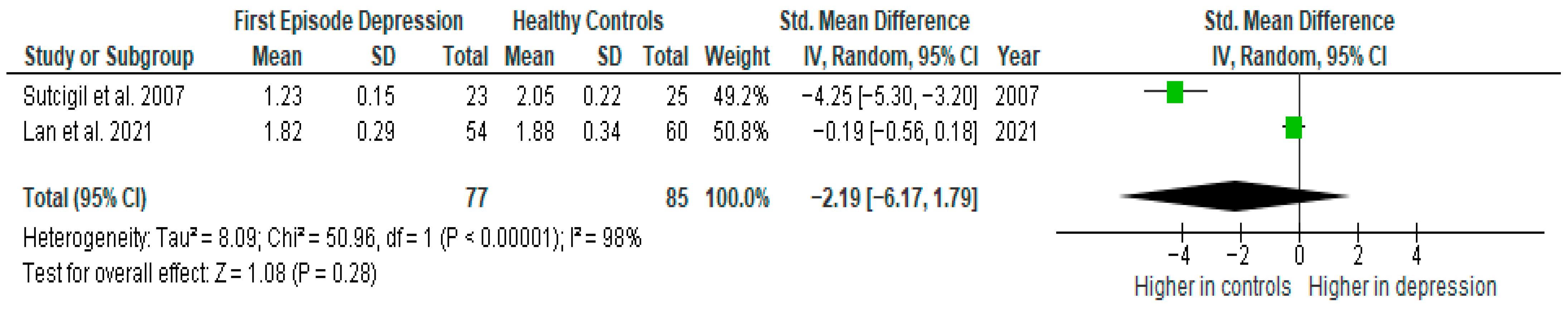
Results from two studies (n = 77/85) were included in the meta-analysis of IL-4 (Figure 6). Using the random-effect model, we did not find a significantly higher level of IL-4 in FE patients than in HCs (SMD= −2.19 [−6.17, 1.79], p = 0.28). Heterogeneity among studies was high (I2 = 98). Due to the limited number of studies, a sensitivity analysis could not be performed. All studies included in the meta-analysis were high-quality.
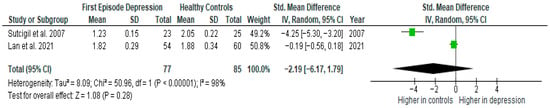
Figure 6.
First episode of depression versus healthy controls—meta-analysis of IL-4 [26,41].
3.4.6. IL-8
Interleukin-8 was evaluated in two studies. The mean IL-8 was significantly higher in FE than in HCs in one of them (1/2, 50%). No difference was observed in one study [31].
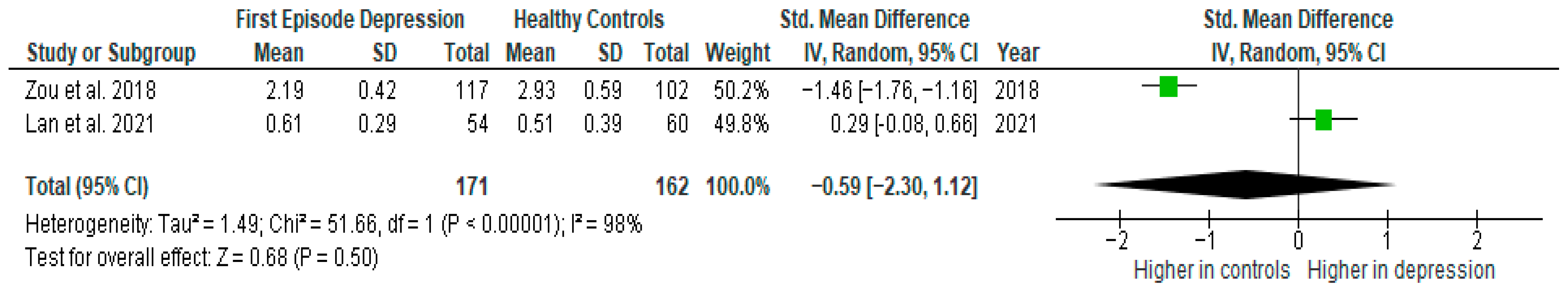
Results from two studies (n = 171/162) were included in the meta-analysis of IL-8 (Figure 7). Using a random-effect model, we did not find a significantly higher level of IL-8 in FE patients than in HCs (SMD= −0.59 [−2.30, 1.12], p = 0.50). Heterogeneity among studies was high (I2 = 98). Due to the limited number of studies, a sensitivity analysis could not be performed. All studies included in the meta-analysis were high-quality.
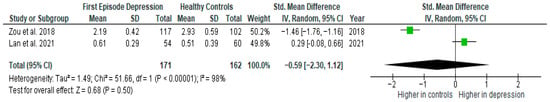
Figure 7.
First episode of depression versus healthy controls—meta-analysis of IL-8 [38,41].
3.4.7. Other Cytokines
The cytokines interleukin-5 (IL-5), interleukin-7 (IL-7), interleukin-10 (IL-10), interleukin-12, transforming growth factor beta 1 (TGF-β), interferon-gamma (IFN-γ), and interleukin-17 (IL-17) were each evaluated in a single study. Among these, the mean IL-5, IL-7 IL-10, IL-12, IFN-γ, and IL-17 were found to be significantly higher in FE patients compared to HC (1/1, 100%). The mean TGF-β1 did not show a significant difference between the FE and HC groups (0/1, 0%).
Due to the limited number of studies available for each of these cytokines, a meta-analysis could not be conducted.
3.5. Drug-Naïve Patients (DN) Versus Healthy Controls (HCs)
3.5.1. IL-6
Interleukin-6 was evaluated in nine studies. The mean IL-6 was significantly higher in DN patients than in HCs in five of them (5/9, 56%). No difference was observed in four studies [27,28,29,31].
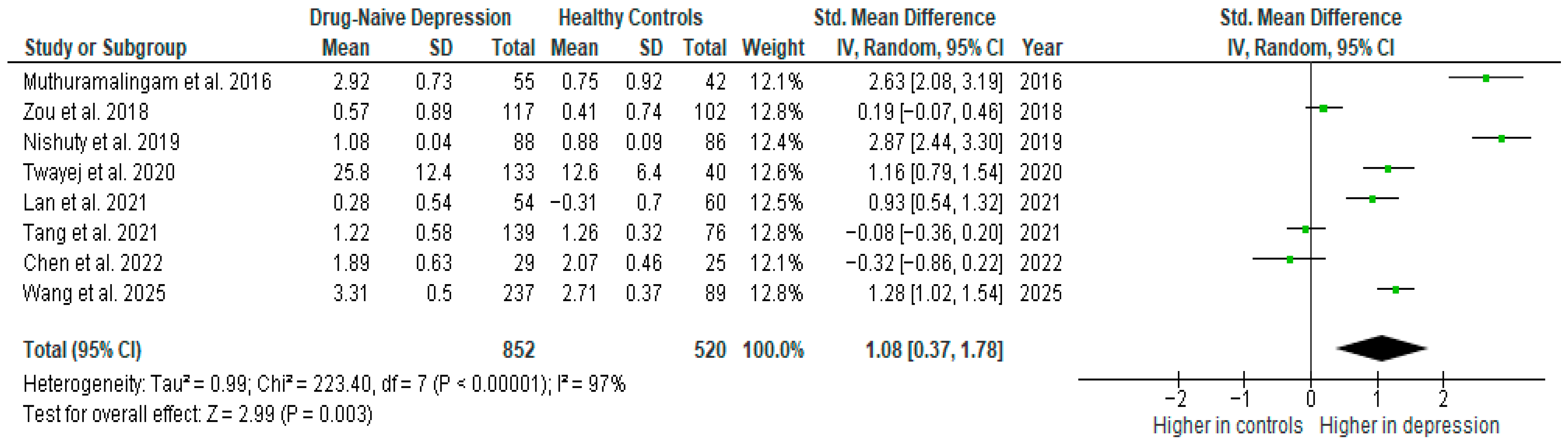
Results from eight studies (n = 852/520) were included in the meta-analysis of IL-6 (Figure 8). Using the random-effect model, we did find a significantly higher level of IL-6 in DN patients than in HCs (SMD = 1.08 [0.37, 1.78], p = 0.003). Heterogeneity among studies was high (I2 = 97). A sensitivity analysis was performed but did not result in a significant reduction in heterogeneity.
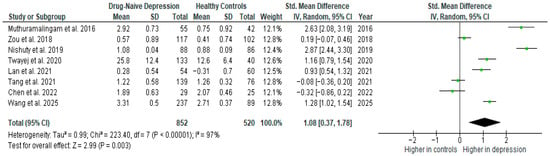
Figure 8.
Drug-naïve patients versus healthy controls—meta-analysis of IL-6 [10,31,32,35,36,38,39,41].
The results obtained did not differ depending on the source of the sample between serum (SMD = 1.40 [−0.10, 2.90], p = 0.07; n = 3) and plasma (SMD = 0.47 [−0.31, 1.25], p = 0.24; n = 4); however, analyzed separately, the differences were not statistically significant.
After including only high-quality studies in the meta-analysis, the difference was on the border of statistical significance (SMD = 0.79 [−0.04, 1.62], p = 0.06; n = 6).
3.5.2. TNF-α
Tumor necrosis factor α was evaluated in six studies. The mean TNF-α was significantly higher in DN patients than in HCs in most of them (5/6, 83%). No difference was observed in one study [27].
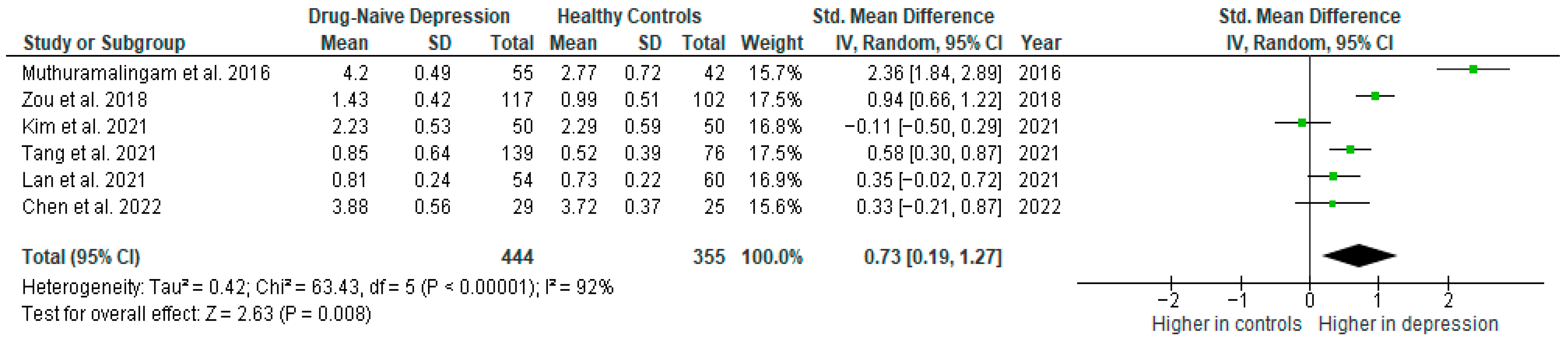
Results from six studies (n = 444/355) were included in the meta-analysis of TNF-α (Figure 9). Using the random-effect model, we did find a significantly higher level of TNF-α in DN patients than in HCs (SMD = 0.73 [0.19, 1.27], p = 0.008). Heterogeneity among studies was high (I2 = 92). The sensitivity analysis was performed but did not result in a significant reduction in heterogeneity.
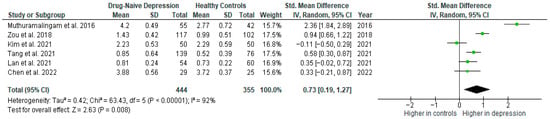
Figure 9.
Drug-naïve patients versus healthy controls—meta-analysis of TNF-α [34,35,36,38,39,41].
The results obtained differed depending on the source of the sample between serum (SMD = 0.43 [−0.60, 1.46], p = 0.41; n = 2) and plasma (SMD = 0.47 [0.26, 0.68], p < 0.0001; n = 3).
After including only high-quality studies in the meta-analysis, the difference remained significant (SMD = 0.44 [0.08, 0.80], p = 0.02; n = 5).
3.5.3. IL-1β
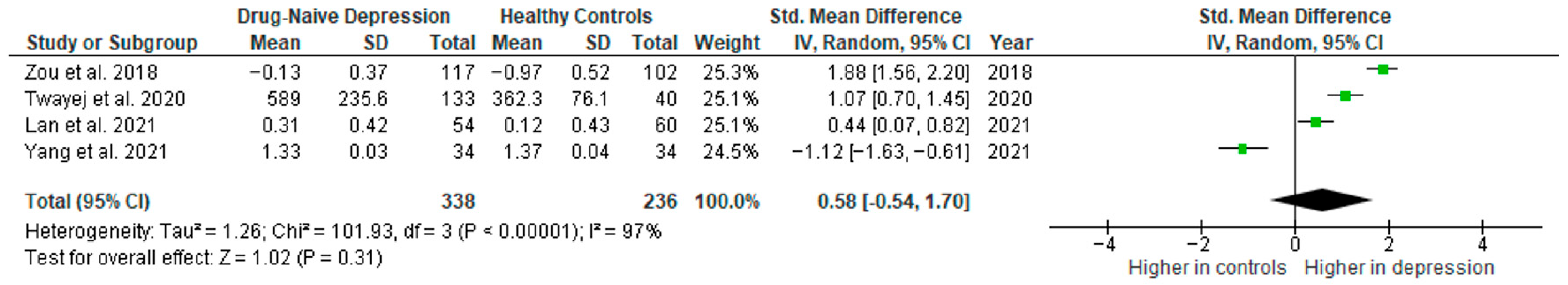
Interleukin 1β was evaluated in five studies. The mean IL-1β was significantly higher in DN patients than in HCs in four of them (4/5, 80%). No difference was observed in one study [35].
Results from four studies (n = 338/236) were included in the meta-analysis of IL-1β (Figure 10). Using the random-effect model, we did not find a significantly higher level of IL-1β in DN patients than in HCs (SMD = 0.58 [−0.54, 1.70], p = 0.31). Heterogeneity among studies was high (I2 = 97). A sensitivity analysis was performed but did not result in a significant reduction in heterogeneity.
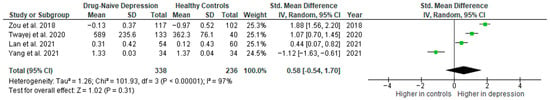
Figure 10.
Drug-naïve patients versus healthy controls—meta-analysis of IL-1β [32,38,41,42].
The results obtained differed depending on the source of the sample between serum (SMD = 1.48 [0.69, 2.27], p = 0.0002; n = 2) and plasma (SMD = −0.33 [−1.86, 1.20], p = 0.68; n = 2). All studies included in the meta-analysis were high-quality.
3.5.4. TGF-β1
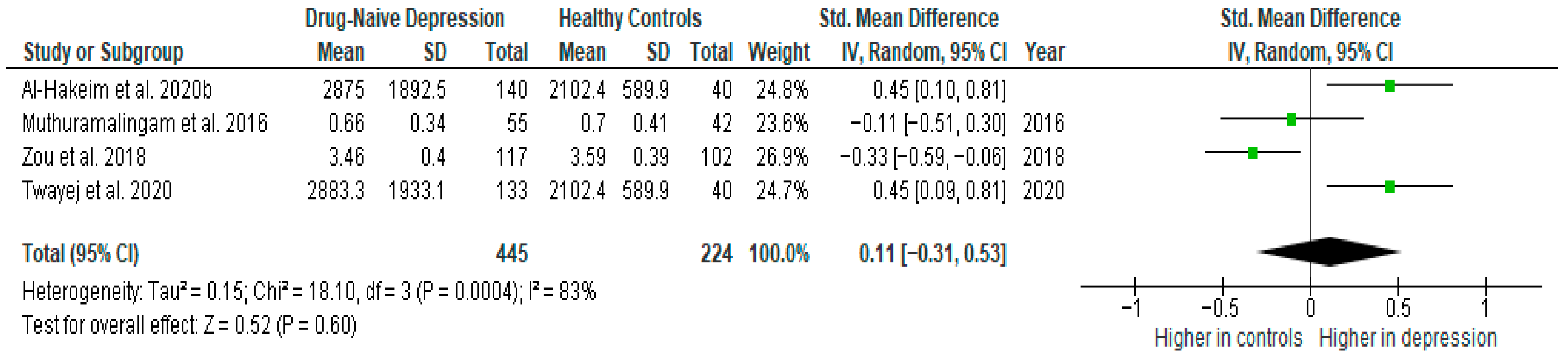
Transforming growth factor β1 was evaluated in four studies. The mean TGF-β1 was significantly higher in DN patients than in HCs in two of them (2/4, 80%). No difference was observed in two studies [38,39].
Results from four studies (n = 445/224) were included in the meta-analysis of TGF-β1 (Figure 11). Using the random-effect model, we did not find a significantly higher level of TGF-β1 in DN patients than in HCs (SMD = 0.11 [−0.31, 0.53], p = 0.60). Heterogeneity among studies was high (I2 = 83). A sensitivity analysis was performed but did not result in a significant reduction in heterogeneity.
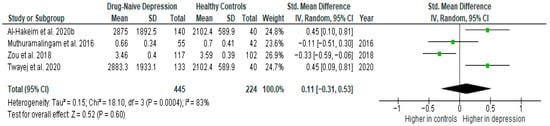
Figure 11.
Drug-naïve patients versus healthy controls—meta-analysis of TGF-β1 [32,33,38,39].
After including only high-quality studies in the meta-analysis, the difference remained not significant (SMD = 0.18 [−0.37, 0.73], p = 0.52; n = 3).
3.5.5. IL-4
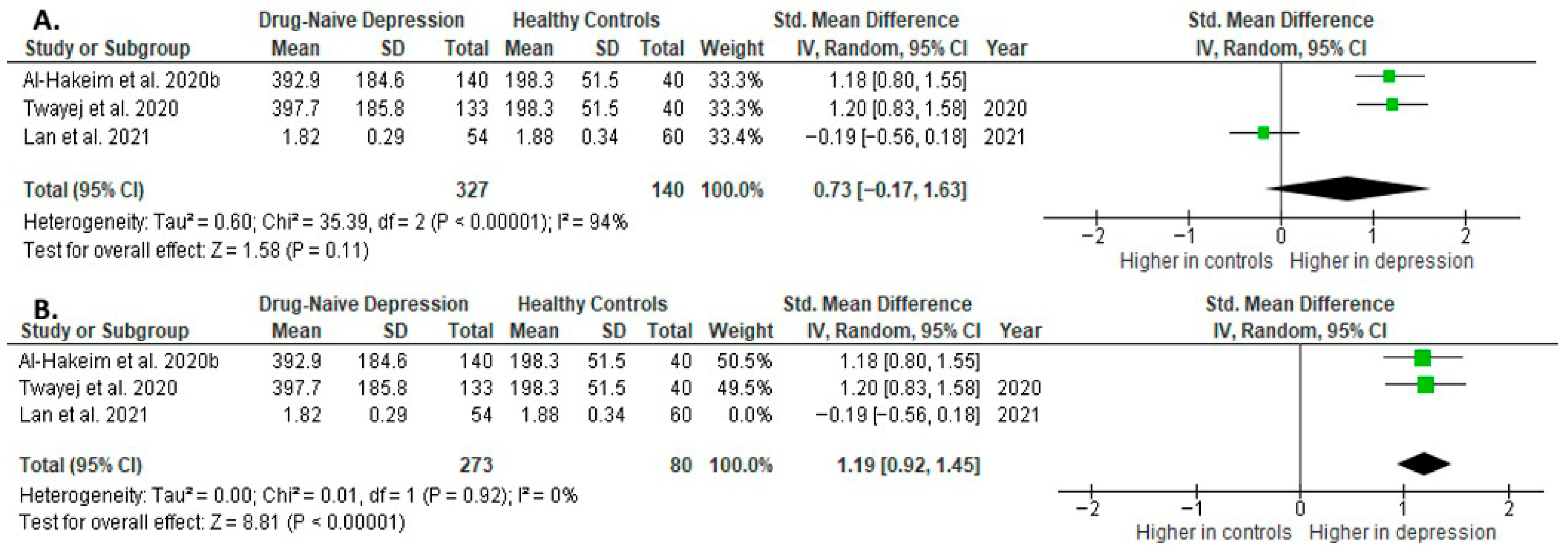
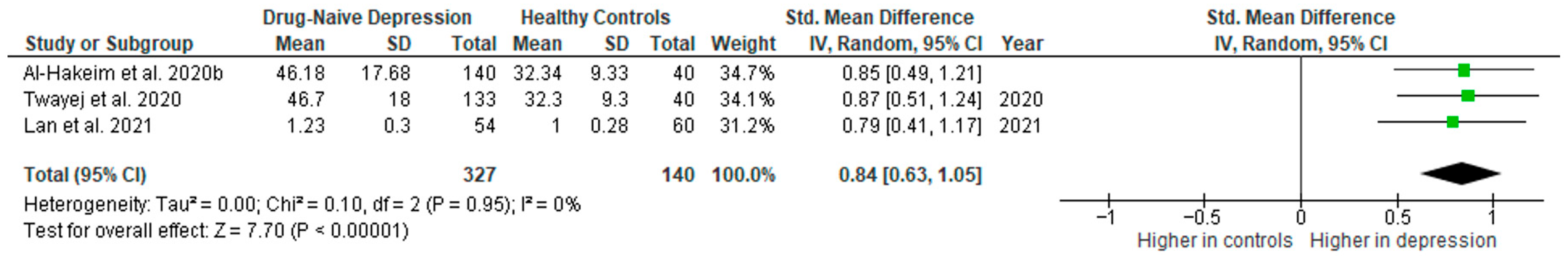
Interleukin 4 was evaluated in three studies. The mean IL-4 was significantly higher in DN patients than in HCs in two of them (2/3, 67%). No difference was observed in one study [41].
Results from three studies (n = 327/140) were included in the meta-analysis of IL-4 (Figure 12). Using the random-effect model, we did not find a significantly higher level of IL-4 in DN patients than in HCs (SMD = 0.73 [−0.17, 1.63], p = 0.11). Heterogeneity among studies was high (I2 = 94). The sensitivity analysis was performed, excluding one study identified as a primary contributor to heterogeneity. Following its removal, heterogeneity was markedly reduced (I2 = 0%), and the effect became statistically significant (SMD = 1.19 [0.92, 1.45], p < 0.00001; n = 2) (Figure 12).
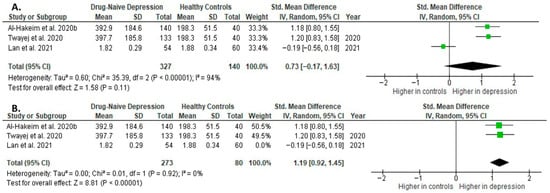
Figure 12.
Drug-naïve patients versus healthy controls: (A) meta-analysis of IL-4. (B) Meta-analysis of IL-4 after removing the outlier [32,33,41].
The results obtained differed depending on the source of the sample between serum (SMD = 1.19 [0.92, 1.45], p < 0.00001; n = 2) and plasma (according to Lan et al. [41]; n = 1).
After including only high-quality studies in the meta-analysis, the difference was not significant (SMD = 0.73 [−0.17, 1.63], p = 0.11; n = 3).
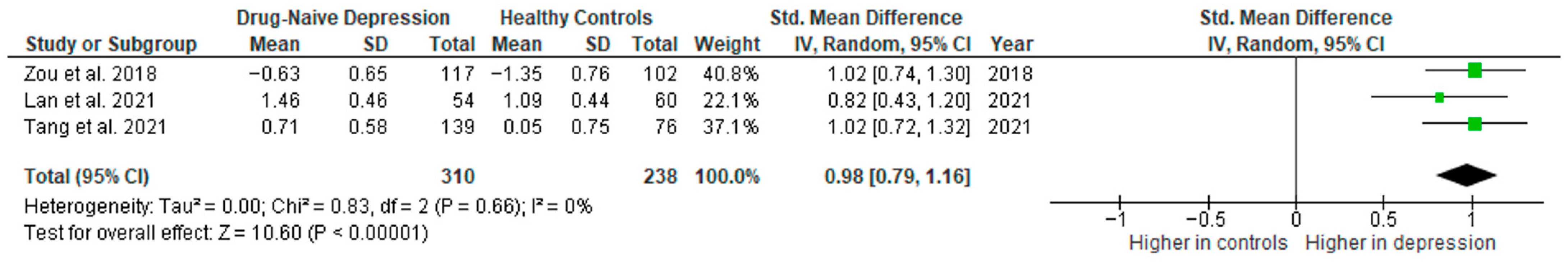
3.5.6. IL-10
Interleukin 10 was evaluated in three studies. The mean IL-10 was significantly higher in DN patients than in HCs in all of them (3/3, 100%).
Results from three studies (n = 310/238) were included in the meta-analysis of IL-10 (Figure 13). Using the random-effect model, we did find a significantly higher level of IL-10 in DN patients than in HCs (SMD = 0.98 [0.79, 1.16], p < 0.00001). Heterogeneity among studies was low (I2 = 0).
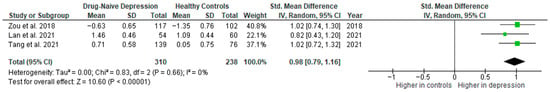
Figure 13.
Drug-naïve patients versus healthy controls—meta-analysis of IL-10 [35,38,41].
The results obtained did not differ depending on the source of the sample between serum (according to Zou et al. [38]; n = 1) and plasma (SMD = 0.94 [0.71, 1.18], p < 0.00001; n = 2). All studies included in the meta-analysis were high-quality.
3.5.7. IFN-γ
Interferon γ was evaluated in three studies. The mean IFN-γ was significantly higher in DN patients than in HCs in all of them (3/3, 100%).
Results from three studies (n = 327/140) were included in the meta-analysis of IFN-γ (Figure 14). Using the random-effect model, we did find a significantly higher level of IFN-γ in DN patients than in HCs (SMD = 0.84 [0.63, 1.05], p < 0.00001). Heterogeneity among studies was low (I2 = 0).
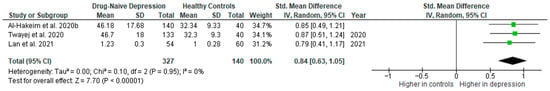
Figure 14.
Drug-naïve patients versus healthy controls—meta-analysis of IFN-γ [32,33,41].
The results obtained did not differ depending on the source of the sample between serum (SMD = 0.86 [0.60, 1.12], p < 0.00001; n = 2) and plasma (according to Lan et al. [41]; n = 1). All studies included in the meta-analysis were high-quality.
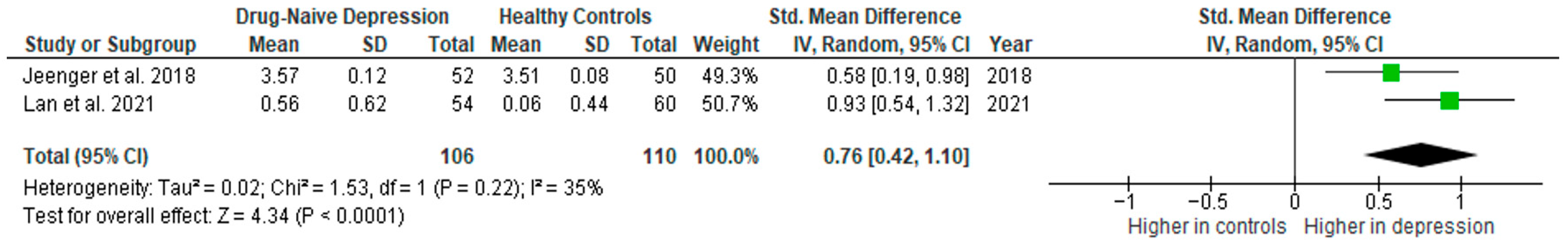
3.5.8. IL-2
Interleukin 2 was evaluated by two studies. The mean IL-2 was significantly higher in DN patients than in HCs in both of them (2/2, 100%).
Results from two studies (n = 106/110) were included in the meta-analysis of IL-2 (Figure 15). Using the random-effect model, we did find a significantly higher level of IL-2 in DN patients than in HCs (SMD = 0.76 [0.42, 1.10], p < 0.0001). Heterogeneity among studies was moderate (I2 = 35). Due to the limited number of studies, a sensitivity analysis could not be performed.
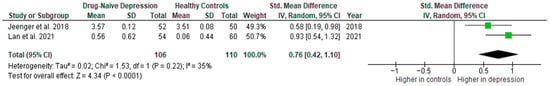
Figure 15.
Drug-naïve patients versus healthy controls—meta-analysis of IL-2 [40,41].
The results obtained did not differ depending on the source of the sample between serum (according to Jeenger et al. [40]; n = 1) and plasma (according to Lan et al. [41]; n = 1). All studies included in the meta-analysis were high-quality.
3.5.9. Other Cytokines
The cytokines interleukin 5, interleukin 7, interleukin 8, interleukin 17, and interleukin 18 were each evaluated in a single study. The mean IL-5, IL-7, IL-17, and IL-18 were significantly higher in DN patients than in HCs (1/1, 100%). The mean IL-8 did not show a significant difference between the FED and HC groups (0/1, 0%).
4. Discussion
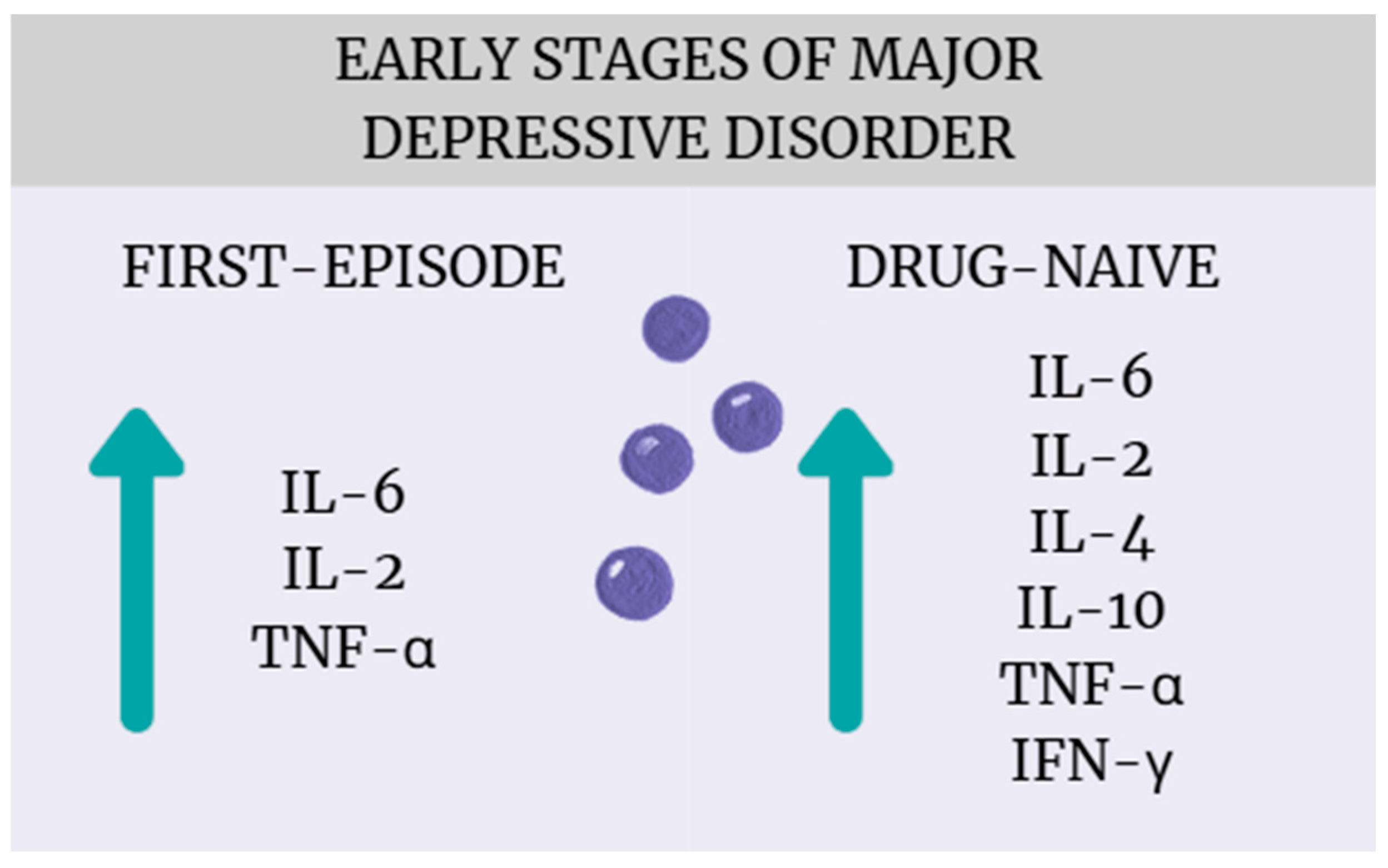
The most important finding of this systematic review and meta-analysis is that patients with early-stage MDD, particularly those who are drug-naïve or experiencing their first episode of major depression, exhibit significant alterations in cytokine levels compared to healthy controls (Figure 16). These results align with a growing body of literature suggesting that immune–inflammatory mechanisms play an important role in the pathophysiology of MDD [43,44,45]. While many studies to date, including meta-analyses, have shown that patients with depression experience cytokine dysregulation, most of these studies include patients with many years of illness who have received numerous pharmacotherapies [8,46]. Considering that both the progression of depression and antidepressant treatment can affect inflammation in different ways, our meta-analysis provides new findings confirming that cytokine dysregulation is already present at the early stages of illness and without drug treatment. From a clinical perspective, this suggests potential diagnostic and therapeutic implications, such as the need to develop reliable biomarkers capable of identifying an “inflammatory subtype” of depression, which could facilitate patient stratification and personalized treatment approaches [47] (Miller). Moreover, the early presence of immune activation supports the potential utility of anti-inflammatory or immune-modulating interventions—either as adjunctive strategies or as preventive approaches aimed at mitigating disease progression and improving treatment response (Molero, Du) [48,49]. Notably, a broader range of cytokines was found to be elevated in drug-naïve patients (IL-6, TNF-α, IL-4, IL-2, IL-10, INF-γ) compared to those experiencing a first episode (IL-6, TNF-α, IL-2), suggesting that antidepressant treatment possibly affects inflammatory regulation.
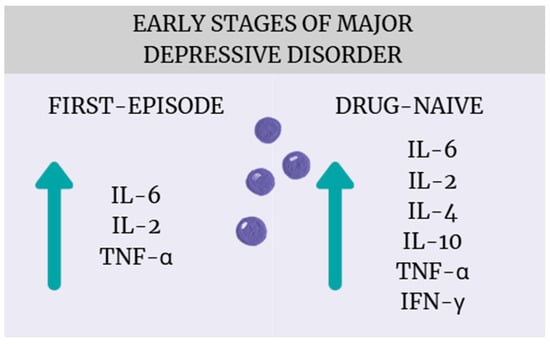
Figure 16.
Cytokine dysregulation in the early stages of MDD.
Another important observation of this meta-analysis is the substantial heterogeneity obtained across most analyses. Although sensitivity analyses were performed, they were largely ineffective in reducing heterogeneity. While this warrants interpreting the pooled results with caution, it also reflects the considerable methodological and clinical variability among studies on depression. Despite the use of strict inclusion and exclusion criteria, the populations of patients with MDD remain highly heterogeneous in terms of illness duration, symptom profile, and comorbid conditions, which complicates the generalization of findings. Additional factors—such as differences in cytokine assessment methods, assay sensitivity, and biological sample type (serum vs. plasma)—likely contributed to the observed variability. In this meta-analysis, we observed different results depending on the source of collection for several cytokines (TNF-α, IL-2, IL-1 β, and IL-4). Furthermore, when restricted to high-quality studies, cytokine patterns differed between groups. In the FE group, results remained unchanged, while in the DN group, IL-4 became non-significant and IL-6 decreased to borderline significance. Future studies should address these issues by including larger and more homogeneous patient samples, clearly defined clinical subgroups, and standardized laboratory procedures. Comparative studies measuring cytokine levels in both serum and plasma would also be valuable to determine how the sample source influences biomarker estimates and between-study variability. While being aware of the limitations of the present analysis, we demonstrated that FE patients with MDD have higher IL-6, TNF-α, and IL-2 levels relative to HCs. In the case of DN patients with MDD, quantitative analysis showed higher IL-6, TNF-α, IL-4, IL-2, IL-10, and INF-γ levels compared to healthy individuals. These results are discussed below in the context of particular cytokines.
IL-6 was elevated in both FE (SMD = 1.40 [0.53, 2.27], p = 0.002) and DN (SMD = 1.08 [0.37, 1.78], p = 0.003) patients. Previous research has consistently implicated IL-6 in the pathophysiology of MDD [50]. Elevated IL-6 concentrations have been observed in individuals with depression [51,52,53], and some studies have additionally reported increased levels of IL-6 and its receptor antagonist in patients with treatment-resistant MDD [54,55]. Moreover, serum and plasma IL-6 levels were found to be significantly higher in FE DN individuals and medication-free MDD patients, compared to HCs [56,57], and a previous meta-analysis on the FE DN group confirmed these findings (g = 0.62; 95% CI, 0.17 to 1.06; p = 0.007; I2 = 85%) [17]. What is more, a positive correlation between serum IL-6 levels and Hamilton Depression Rating Scale (HAM-D) scores has also been documented, suggesting a link between IL-6 concentration and symptom severity in depression [51,58,59].
However, there is also evidence against the link between IL-6 and MDD. One meta-analysis has shown that IL-6 levels may decrease or remain unchanged following antidepressant treatment [60]. Another investigation including nine studies reported no significant differences in IL-6 concentrations between depressed adolescents and healthy controls [61]. Furthermore, a recent meta-analysis incorporating seven papers in the same age group did not find significantly elevated IL-6 levels in DN MDD patients compared to HCs [62]. These results indicate a probable complex contribution of IL-6 to depression, susceptible to the involvement of multiple factors such as differences in sample characteristics or the confounding effects of pharmacological interventions on cytokine expression.
The combined data revealed elevated levels of IL-2 in both FE (SMD = 1.81 [0.45, 3.17], p = 0.009) and DN (SMD = 0.76 [0.42, 1.10], p < 0.0001) patients. These findings are consistent with the previous literature, which has reported increased serum IL-2 concentrations in individuals with MDD [63]. Furthermore, one study identified an association between IL-2 levels and suicidal ideation in DN FE MDD patients, suggesting a potential link between IL-2 dysregulation and the severity or clinical features of depressive episodes [64]. Moreover, a previous meta-analysis on FE DN patients also confirmed elevated levels of this cytokine compared to HCs (g = 4.41; 95% CI, 0.13 to 8.69; p = 0.04; I2 = 98%) [20]. These findings indicate that IL-2 plays a significant role in the onset and development of depression.
FE and DN patients showed higher TNF-α levels compared to controls (SMD= 1.30 [0.41, 2.18] p = 0.004 and SMD = 0.73 [0.19, 1.27], p = 0.008, respectively). Interestingly, the previous literature presents mixed findings regarding this cytokine. For instance, one study reported significantly higher plasma TNF-α levels in medication-free FE patients compared to HCs [57]. Additionally, a previous meta-analysis confirmed elevated TNF-α concentrations in DN MDD patients relative to controls (SMD 1.04, 95% CI: 0.69–1.39, z = 5.84, p < 0.001) [19] and a meta-analysis on FE DN individuals also showed similar results (g = 1.21; 95% CI, 0.57 to 1.85; p < 0.001; I2 = 89%) [20]. However, other studies have found no significant differences in TNF-α levels between MDD patients and healthy individuals [46,65]. Crucially, a quantitative analysis showed that there was a difference in TNF-α concentrations regarding the source of the sample, reaching a significant difference in serum in the first episode of depression (SMD = 1.19 [0.77, 1.61], p < 0.00001; n = 2) and plasma in drug-naïve patients (SMD = 0.47 [0.26, 0.68], p < 0.0001; n = 3). These findings suggest that TNF-α might play a role in the pathology of depression, but methodological limitations and differences in sample source may hinder the recognition of this process.
The synthesized results demonstrated elevated IL-4 in DN patients (SMD = 1.19 [0.92, 1.45], p < 0.00001; n = 2). Notably, statistical significance was achieved only after excluding an outlier that contributed substantially to heterogeneity. The existing literature also provides conflicting results regarding this cytokine. A recent study reported increased IL-4 concentrations in MDD patients relative to controls, along with a positive correlation between IL-4 serum levels and HAM-D scores [66]. In contrast, Köhler et al. did not observe significant differences in IL-4 levels between MDD patients and healthy individuals [46], while Osimo et al. even observed a reduction in IL-4 concentrations among MDD patients [9]. Moreover, a previous meta-analysis failed to identify significantly elevated IL-4 levels in DN MDD patients compared to HCs [62], and a meta-analysis on FE DN individuals did not find higher levels of IL-4 in the patient group compared to HCs (g = −1.71; 95% CI, −4.73 to 1.31; p = 0.27; I2 = 97%) [20]. Importantly, in our analysis, IL-4 levels were not elevated in patients experiencing an FE (SMD = 0.73 [−0.17, 1.63], p = 0.11). Furthermore, we showed that IL-4 concentrations differ depending on the source—plasma/serum. These mixed findings highlight the need for further investigation into the role of IL-4 in MDD pathology, particularly in relation to treatment status and sample source.
Levels of IL-10 were higher in DN patients compared to HCs (SMD = 0.98 [0.79, 1.16], p < 0.00001). This finding aligns with previous research, including a recent study that reported increased serum IL-10 concentrations in drug-free MDD patients relative to HCs, along with a positive correlation between IL-10 levels and HAM-D scores [67]. Interestingly, Gazal et al. found no significant difference in IL-10 levels between MDD patients and controls [68]. However, within that study, higher IL-10 concentrations were observed in patients with later-onset depression compared to both early-onset patients and healthy individuals. In contrast, a previous meta-analysis did not find higher levels of IL-4 in FE DN patients compared to HCs (g = −0.75; 95% CI, −2.77 to 1.28; p = 0.47; I2 = 98%) [20]. Based on these results, IL-10 might be an important factor in MDD progression, but its role should be studied further, especially in terms of DN patients.
Meta-analytic integration revealed elevated levels of IFN-γ in DN patients (SMD = 0.84 [0.63, 1.05], p < 0.00001). Similar findings in medication-free MDD patients have been previously reported [69]; however, this is not without conflicting results. Previous meta-analysis did not identify significantly higher IFN-γ levels in DN MDD patients relative to HCs (SMD −0.05, 95% CI: −2.72–2.62, z = 0.03, p = 0.97) [19]. Moreover, one study reported significantly decreased serum IFN-γ levels in MDD patients compared to controls, along with a negative correlation between IFN-γ concentrations and HAM-D scores [70]. Importantly, a meta-analysis on DN FE MDD patients showed no significant rise in IFN-γ in this group (g = −0.33; 95% CI, −1.31 to 0.66; p = 0.51; I2 = 87%) [20]. These differences appear to result from the proportion of pharmacological treatment and indicate the need for further research with a better identification of the DN subgroup.
The combined data did not reveal elevated levels of IL-1β in either FE (SMD= −0.19 [−1.44, 1.07], p = 0.77) or DN (SMD = 0.58 [−0.54, 1.70], p = 0.31) patients. The findings of previous studies are contradictory. Two recent meta-analysis reported elevated levels of IL-1β in depressed individuals [9,71], suggesting a potential role in the disorder’s inflammatory profile. Furthermore, a previous meta-analysis on FE DN patients found significantly higher levels of this cytokine in depressed individuals (g = 1.52; 95% CI, 0.38 to 2.66; p = 0.009; I2 = 96%) [20]. In contrast, Haapakoski et al. did not find evidence for an association between IL-1β levels and MDD [53]. Moreover, a meta-analysis on DN MDD patients also did not find elevated IL-1β compared to HCs [62]. Importantly, this paper demonstrated difference in IL-1β levels between serum (SMD = 1.48 [0.69, 2.27], p = 0.0002; n = 2) and plasma (SMD = −0.33 [−1.86, 1.20], p = 0.68; n = 2) samples in DN individuals. This result may explain the differences observed in the above studies and indicate directions for future research.
Quantitative synthesis did not reveal higher levels of TGF-β1 in DN patients (SMD = 0.11 [−0.31, 0.53], p = 0.60). A previous meta-analysis also did not report significantly elevated levels of TGF-β1 in depression [9]. What is more, a meta-analysis on FE DN patients observed a trend of decreased TGF-β1 in depressed individuals (g = −0.91; 95% CI, −1.98 to 0.16; p = 0.10; I2 = 95%) [20]. Moreover, a genetic study also did not find any meaningful differences among MDD patients and healthy controls [72]. These findings suggest that TGF-β1 may not be a consistent indicator of inflammatory processes in MDD.
In addition to elevated levels of IL-6 and TNF-α in depression, these cytokines have been proven to correlate with symptoms of MDD [73]. This may suggest the potential utility of IL-6 and TNF-α as therapeutic targets. However, studies on the effect of antidepressants on these cytokines remain inconclusive [60,74]. On the one hand, future meta-analyses on large sample sizes with pre–post assessment are needed to evaluate how antidepressants affect IL-6 and TNF-α. On the other hand, anti-inflammatory treatment for mood disorders is developing [75,76], and there is evidence that anti-cytokine therapy may be effective in major depression [77]. This research indicates that it may be helpful to develop a treatment that will normalize the levels of these cytokines.
Although the precise mechanisms by which inflammatory cytokines take part in the pathogenesis of MDD remain to be fully elucidated, several biological pathways have been proposed to explain their role in the development and persistence of depressive symptoms.
Pro-inflammatory cytokines such as IL-1β and TNF-α are known to activate the hypothalamic–pituitary–adrenal axis, resulting in elevated levels of adrenocorticotropic hormone (ACTH) and cortisol [73,74,75]. This hyperactivation may impair the function of glucocorticoid receptors (GRs), thereby diminishing the anti-inflammatory effects of cortisol and perpetuating a state of chronic inflammation [76]. Notably, HPA axis dysregulation is one of the most consistently replicated biological abnormalities observed in patients with clinical depression [50,77,78]. In addition, cytokines including IFN-γ, IL-1β, IL-6, and TNF-α can induce the expression of indoleamine 2,3-dioxygenase (IDO), an enzyme that catalyzes the conversion of tryptophan into kynurenine [79,80,81,82]. This metabolic shift reduces the availability of tryptophan for serotonin (5-HT) synthesis and promotes the accumulation of neurotoxic metabolites such as quinolinic acid, which enhances glutamate release and excitotoxicity—mechanisms implicated in the neurobiology of depression [83,84]. Furthermore, elevated cytokine levels have been shown to modulate intracellular signaling pathways, leading to increased oxidative stress and neuronal apoptosis [85]. These processes may disrupt neurotransmitter signaling in key brain regions such as the prefrontal cortex and hippocampus, contributing to the emergence of affective and cognitive symptoms [86]. The activation of neuroglial cells, particularly microglia and astrocytes, by pro-inflammatory cytokines may further exacerbate neuroinflammation and synaptic dysfunction [87]. Finally, pro-inflammatory cytokines have been associated with reduced expression of brain-derived neurotrophic factor (BDNF) and its receptor—tropomyosin receptor kinase B (TrkB)—in the hippocampus [83]. This downregulation impairs neurogenesis and synaptic plasticity, both of which are critical for mood regulation and cognitive function, and may represent a key mechanism linking inflammation to the pathophysiology of MDD [88].
Antidepressant treatment may counteract these cytokine-induced neuroimmune and neuroendocrine disturbances through several complementary mechanisms. By attenuating systemic inflammation, antidepressants can normalize HPA axis function and suppress IDO activity, thereby restoring serotonergic neurotransmission and neuroplasticity [89]. In addition, several antidepressant classes, particularly selective serotonin reuptake inhibitors, have been shown to downregulate NF-κB signaling, reduce circulating concentrations of IL-6, TNF-α, and CRP, and enhance BDNF expression in the hippocampus [90]. These immunomodulatory and neurotrophic effects suggest that the therapeutic action of antidepressants extends beyond monoamine restoration, encompassing broader regulation of immune–neuroendocrine interactions implicated in the pathophysiology of major depressive disorder.
Limitations
This study has several limitations that should be acknowledged. Firstly, substantial heterogeneity was observed in the analysis of most cytokines, likely attributable to differences in study populations, diagnostic criteria, cytokine measurement techniques, and units of quantification. Such methodological variability may have influenced the pooled effect sizes and limited the interpretability of subgroup comparisons. Secondly, for several cytokines, the number of available studies was small (often limited to two or three), which reduces the statistical power and limits the reliability and generalizability of pooled estimates. Additionally, most of the included studies were based on relatively small sample sizes, which further reduces the accuracy of subgroup analysis. Thirdly, a potential limitation of this study is the absence of multivariate analysis, which could have allowed for a more accurate estimation of the relationships between variables; however, the available data and sample size did not permit such an approach. Due to incomplete or inconsistent reporting of clinical and methodological variables across studies, it was not possible to perform additional subgroup analyses beyond sample source and study quality. Additionally, the severity of depressive symptoms was not consistently reported, preventing adjustment for this potentially important confounding factor. Fourthly, sex-related differences could not be assessed, as the majority of the included studies did not report cytokine levels separately for male and female participants. Future research should incorporate sex-stratified analyses to clarify the influence of biological sex in immune–inflammatory alterations in MDD. Another limitation is that the analyses were restricted to peripheral blood cytokines, which may not fully reflect immune processes occurring within the central nervous system. Finally, the cross-sectional nature of the included studies precludes causal inference and limits conclusions about the temporal relationship between immune alterations and the onset or progression of depression.
5. Conclusions
This systematic review and meta-analysis demonstrated elevated levels of inflammatory cytokines in individuals diagnosed with MDD, particularly within FE and DN subgroups, when compared to healthy controls. Specifically, increased concentrations of IL-6, IL-2, and TNF-α were observed in FE patients, while DN patients exhibited elevated levels of IL-6, IL-2, IL-4, IL-10, TNF-α, and IFN-γ. These findings reinforce the hypothesis that immune–inflammatory mechanisms, particularly cytokine-mediated pathways, play a meaningful role in the pathogenesis of MDD. Moreover, since more cytokines were elevated in DN patients, it is likely that pharmacological treatment plays a major role in inflammatory regulation. Furthermore, several methodological conclusions have also emerged. The high heterogeneity despite selectively chosen inclusion and exclusion criteria shows that populations of patients with depression can be highly heterogeneous, which should be considered in future research. Another methodological conclusion of this research is the potential difference in the value of TNF-α, IL-2, IL-1β, and IL-4 depending on the sample source—plasma or serum. These results provide direction for future prospective large-scale studies incorporating rigorous designs, harmonized measurement protocols, unified sample sources, and stratification by treatment status.
Author Contributions
Conceptualization, A.G. and S.M.; methodology, A.G. and S.M.; data curation, A.G., S.M. and M.M.; writing—original draft preparation, A.G. and S.M.; writing—review and editing, A.G., S.M., A.W., M.I. and M.D.; visualization, A.G. and S.M.; supervision, A.W. and M.D.; project administration, M.D.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical concerns.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-HT | Serotonin |
| ACTH | Adrenocorticotropic hormone |
| BDNF | Brain-derived neurotrophic factor |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| DN | Drug-naïve |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders |
| ELISA | Enzyme-linked immunosorbent assay |
| FE | First-episode |
| GR | Glucocorticoid receptors |
| HAM-D | Hamilton Depression Rating Scale |
| HC | Healthy controls |
| HPA | Hypothalamic–pituitary–adrenal axis |
| ICD-10 | International Classification of Diseases, tenth revision |
| IDO | Indoleamine-2,3-dioxygenase |
| IFN-γ | Interferon γ |
| IL-1β | Interleukin 1β |
| IL-2 | Interleukin 2 |
| IL-3 | Interleukin 3 |
| IL-4 | Interleukin 4 |
| IL-5 | Interleukin 5 |
| IL-6 | Interleukin 6 |
| IL-7 | Interleukin 7 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IL-17 | Interleukin 17 |
| IL-18 | Interleukin 18 |
| IQR | Interquartile ranges |
| MDD | Major depressive disorder |
| NOS | Newcastle–Ottawa Scale |
| PRISMA | Preferred reporting items for systematic review and meta-analysis |
| SMD | Standardized mean difference |
| TGF-β1 | Transforming growth factor β1 |
| TNF-α | Tumor necrosis factor α |
| TrkB | Tropomyosin receptor kinase B |
| WHO | World Health Organization |
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major Depressive Disorder: New Clinical, Neurobiological, and Treatment Perspectives. Lancet 2012, 379, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Lépine, J.P.; Briley, M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011, 7, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Bekhbat, M.; Mehta, N.D.; Felger, J.C. Inflammation-Related Functional and Structural Dysconnectivity as a Pathway to Psychopathology. Biol. Psychiatry 2023, 93, 405–418. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of Low-Grade Inflammation in Depression: A Systematic Review and Meta-Analysis of CRP Levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory Markers in Depression: A Meta-Analysis of Mean Differences and Variability in 5166 Patients and 5083 Controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef]
- Wang, A.K.; Miller, B.J. Meta-Analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr. Bull. 2018, 44, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Shen, X.; Stevenson, A.J.; Conole, E.L.S.; Harris, M.A.; Barbu, M.C.; Hawkins, E.L.; Adams, M.J.; Hillary, R.F.; Lawrie, S.M.; et al. Structural Brain Correlates of Serum and Epigenetic Markers of Inflammation in Major Depressive Disorder. Brain Behav. Immun. 2021, 92, 39–48. [Google Scholar] [CrossRef]
- Tartter, M.; Hammen, C.; Bower, J.E.; Brennan, P.A.; Cole, S. Effects of Chronic Interpersonal Stress Exposure on Depressive Symptoms Are Moderated by Genetic Variation at IL6 and IL1β in Youth. Brain Behav. Immun. 2015, 46, 104–111. [Google Scholar] [CrossRef]
- Udina, M.; Moreno-España, J.; Navinés, R.; Giménez, D.; Langohr, K.; Gratacòs, M.; Capuron, L.; de la Torre, R.; Solà, R.; Martín-Santos, R. Serotonin and Interleukin-6: The Role of Genetic Polymorphisms in IFN-Induced Neuropsychiatric Symptoms. Psychoneuroendocrinology 2013, 38, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic Stress, Neuroinflammation, and Depression: An Overview of Pathophysiological Mechanisms and Emerging Anti-Inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2022, 24, 578. [Google Scholar] [CrossRef] [PubMed]
- Eltokhi, A.; Sommer, I.E. A Reciprocal Link Between Gut Microbiota, Inflammation and Depression: A Place for Probiotics? Front. Neurosci. 2022, 16, 852506. [Google Scholar] [CrossRef]
- Belmaker, R.H.; Agam, G.; Bains, N.; Abdijadid, S. Major Depressive Disorder. N. Engl. J. Med. 2025, 358, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Wei, Y.B.; Strawbridge, R.; Bao, Y.; Chang, S.; Shi, L.; Que, J.; Gadad, B.S.; Trivedi, M.H.; Kelsoe, J.R.; et al. Peripheral Cytokine Levels and Response to Antidepressant Treatment in Depression: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2020, 25, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Sohan, M.; Daria, S.; Masud, A.A.; Ahmed, M.U.; Roy, A.; Shahriar, M. Evaluation of Inflammatory Cytokines in Drug-Naïve Major Depressive Disorder: A Systematic Review and Meta-Analysis. Int. J. Immunopathol. Pharmacol. 2023, 37, 03946320231198828. [Google Scholar] [CrossRef] [PubMed]
- Çakici, N.; Sutterland, A.L.; Penninx, B.; Dalm, V.A.; de Haan, L.; van Beveren, N.J.M. Altered Peripheral Blood Compounds in Drug-Naive First-Episode Patients with Either Schizophrenia or Major Depressive Disorder: A Meta-Analysis. Brain Behav. Immun. 2020, 88, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; White, I.R.; Anzures-Cabrera, J. Meta-Analysis of Skewed Data: Combining Results Reported on Log-Transformed or Raw Scales. Stat. Med. 2008, 27, 6072–6092. [Google Scholar] [CrossRef] [PubMed]
- University, H.K.B. Mean Variance Estimation Calculator. Available online: https://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html (accessed on 31 March 2025).
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Sutcigil, L.; Oktenli, C.; Musabak, U.; Bozkurt, A.; Cansever, A.; Uzun, O.; Sanisoglu, S.Y.; Yesilova, Z.; Ozmenler, N.; Ozsahin, A.; et al. Pro- and Anti-Inflammatory Cytokine Balance in Major Depression: Effect of Sertraline Therapy. Clin. Dev. Immunol. 2007, 2007, 76396. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Chen, X.; Zhou, H.F.; Fu, J.H.; Yu, Q.; Liu, Y. Changes of Serum Melatonin, Interleukin-6, Homocysteine, and Complement C3 and C4 Levels in Patients with Depression. Front. Psychol. 2020, 11, 1271. [Google Scholar] [CrossRef]
- Mao, L.; Ren, X.; Wang, X.; Tian, F. Associations between Autoimmunity and Depression: Serum IL-6 and IL-17 Have Directly Impact on the HAMD Scores in Patients with First-Episode Depressive Disorder. J. Immunol. Res. 2022, 2022, 6724881. [Google Scholar] [CrossRef]
- Emekdar, G.; Tas, H.I.; Sehitoglu, H. Investigation of the Relationship between Inflammation and Oxidative Stress Markers and Treatment Response in First-Attack Major Depression Patients: A Follow-Up Study. Turk. Psikiyatr. Derg. 2023, 34, 89–99. [Google Scholar] [CrossRef]
- Xi, Y.Q.; Wang, Z.Q.; Li, G.J.; Hao, Z.Q.; Nie, J.H.; Li, J.X.; Tan, Y.T.; Hu, X.D.; Wang, G.W.; Liu, S.; et al. Association of Inflammation Cytokines with Cognitive Function in First-Episode Major Depressive Disorder. Front. Psychiatry 2025, 15, 1473418. [Google Scholar] [CrossRef]
- Nishuty, N.L.; Khandoker, M.M.H.; Karmoker, T.R.; Ferdous, S.; Shahriar, M.; Qusar, M.; Islam, M.S.; Kadir, M.F.; Islam, M.R. Evaluation of Serum Interleukin-6 and C-Reactive Protein Levels in Drug-Naive Major Depressive Disorder Patients. Cureus J. Med. Sci. 2019, 11, e3868. [Google Scholar] [CrossRef] [PubMed]
- Twayej, A.J.; Al-Hakeim, H.K.; Al-Dujaili, A.H.; Maes, M. Lowered Zinc and Copper Levels in Drug-Naive Patients with Major Depression: Effects of Antidepressants, Ketoprofen and Immune Activation. World J. Biol. Psychiatry 2020, 21, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Twayej, A.J.; Al-Dujaili, A.H.; Maes, M. Plasma Indoleamine-2,3-Dioxygenase (IDO) Is Increased in Drug-Naive Major Depressed Patients and Treatment with Sertraline and Ketoprofen Normalizes IDO in Association with Pro-Inflammatory and Immune-Regulatory Cytokines. CNS Neurol. Disord.-Drug Targets 2020, 19, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.H.; Chang, K.A. Sex Difference in Peripheral Inflammatory Biomarkers in Drug-Naive Patients with Major Depression in Young Adulthood. Biomedicines 2021, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, H.Y.; Chen, L.X.; Zhao, K.; Zhang, Y.Y.; Zheng, K.; Zhu, C.; Zheng, T.S.; Liu, J.H.; Wang, D.D.; et al. Inflammatory Cytokines, Complement Factor H and Anhedonia in Drug-Naive Major Depressive Disorder. Brain Behav. Immun. 2021, 95, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.P.; Zeng, X.L.; Zhou, S.J.; Gu, Z.W.; Pan, J.Y. Correlation Between Serum High-Sensitivity C-Reactive Protein, Tumor Necrosis Factor-Alpha, Serum Interleukin-6 and White Matter Integrity Before and After the Treatment of Drug-Naive Patients with Major Depressive Disorder. Front. Neurosci. 2022, 16, 948637. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Xie, J.; Yang, Y.; Li, M.J.; Li, G.; Zhang, X.; Li, J. The Relationship Between Plasma Interleukin-6 and Cognition Based on Curve Correlation in Drug-Naïve Patients with Major Depressive Disorder. J. Affect. Disord. 2025, 369, 211–217. [Google Scholar] [CrossRef]
- Zou, W.; Feng, R.J.; Yang, Y. Changes in the Serum Levels of Inflammatory Cytokines in Antidepressant Drug-Naïve Patients with Major Depression. PLoS ONE 2018, 13, e0197267. [Google Scholar] [CrossRef]
- Muthuramalingam, A.; Menon, V.; Rajkumar, R.P.; Negi, V. Is Depression an Inflammatory Disease? Findings from a Cross-Sectional Study at a Tertiary Care Center. Indian J. Psychol. Med. 2016, 38, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Jeenger, J.; Singroha, V.; Sharma, M.; Mathur, D.M. C-Reactive Protein, Brain-Derived Neurotrophic Factor, Interleukin-2, and Stressful Life Events in Drug-Naive First-Episode and Recurrent Depression: A Cross-Sectional Study. Indian. J. Psychiatry 2018, 60, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.F.; Zhou, Y.L.; Wu, F.C.; Wu, K.; Zhan, Y.N.; Wang, C.Y.; Zheng, W.; Yu, M.; Deng, X.R.; Ning, Y.P. The Relationship between Plasma Cytokine Levels and Antidepressant Response in Patients with First-Episode Major Depressive Disorder. J. Affect. Disord. 2021, 287, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Liu, M.N.; Liou, Y.J.; Hu, L.Y.; Yang, B.H.; Chou, Y.H. Interleukin-1 Family and Serotonin Transporter in First-Episode, Drug-Naive Major Depressive Disorder: A Pilot Study. J. Psychiatr. Res. 2021, 135, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kućma, M.; Legutko, B.; Szewczyk, B.; Novak, K.; Znojek, P.; Poleszak, E.; Papp, M.; Pilc, A.; Nowak, G. Antidepressant-like Activity of Zinc: Further Behavioral and Molecular Evidence. J. Neural Transm. 2008, 115, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Carvalho, A.F. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol. Neurobiol. 2018, 55, 8885–8903. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral Cytokine and Chemokine Alterations in Depression: A Meta-Analysis of 82 Studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H. Advancing an Inflammatory Subtype of Major Depression. Am. J. Psychiatry 2025, 182, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Molero, P.; De Lorenzi, F.; Gędek, A.; Strater, C.; Popescu, E.; Ortuño, F.; Van Der Does, W.; Martínez-González, M.A.; Molendijk, M.L. Diet Quality and Depression Risk: A Systematic Review and Meta-Analysis of Prospective Studies. J. Affect. Disord. 2025, 382, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Dou, Y.; Wang, M.; Wang, Y.; Yan, Y.; Fan, H.; Fan, N.; Yang, X.; Ma, X. Efficacy and Acceptability of Anti-Inflammatory Agents in Major Depressive Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2024, 15, 1407529. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, W.; Yang, Y. Effects of IL-6 and Cortisol Fluctuations in Post-Stroke Depression. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2016, 36, 732–735. [Google Scholar] [CrossRef]
- Fan, N.; Luo, Y.; Ou, Y.; He, H. Altered Serum Levels of TNF-α, IL-6, and IL-18 in Depressive Disorder Patients. Hum. Psychopharmacol. 2017, 32, e2588. [Google Scholar] [CrossRef]
- Ye, G.; Yin, G.Z.; Tang, Z.; Fu, J.L.; Chen, J.; Chen, S.S.; Li, J.; Fu, T.; Yu, X.; Xu, D.W.; et al. Association between Increased Serum Interleukin-6 Levels and Sustained Attention Deficits in Patients with Major Depressive Disorder. Psychol. Med. 2018, 48, 2508–2514. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative Meta-Analysis of Interleukins 6 and 1β, Tumour Necrosis Factor α and C-Reactive Protein in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef]
- Maes, M.; Meltzer, H.Y.; Bosmans, E.; Bergmans, R.; Vandoolaeghe, E.; Ranjan, R.; Desnyder, R. Increased Plasma Concentrations of Interleukin-6, Soluble Interleukin-6, Soluble Interleukin-2 and Transferrin Receptor in Major Depression. J. Affect. Disord. 1995, 34, 301–309. [Google Scholar] [CrossRef]
- Maes, M.; Bosmans, E.; De Jongh, R.; Kenis, G.; Vandoolaeghe, E.; Neels, H. INCREASED SERUM IL-6 AND IL-1 RECEPTOR ANTAGONIST CONCENTRATIONS IN MAJOR DEPRESSION AND TREATMENT RESISTANT DEPRESSION. Cytokine 1997, 9, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Kakeda, S.; Watanabe, K.; Katsuki, A.; Sugimoto, K.; Igata, N.; Ueda, I.; Igata, R.; Abe, O.; Yoshimura, R.; Korogi, Y. Relationship between Interleukin (IL)-6 and Brain Morphology in Drug-Naive, First-Episode Major Depressive Disorder Using Surface-Based Morphometry. Sci. Rep. 2018, 8, 10054. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jinxiang, T.; Shu, Y.; Yadong, P.; Ying, L.; Meng, Y.; Ping, Z.; Xiao, H.; Yixiao, F. Childhood Trauma and the Plasma Levels of IL-6, TNF-α Are Risk Factors for Major Depressive Disorder and Schizophrenia in Adolescents: A Cross-Sectional and Case-Control Study. J. Affect. Disord. 2022, 305, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Lavebratt, C.; Herring, M.P.; Liu, J.J.; Wei, Y.B.; Bossoli, D.; Hallgren, M.; Forsell, Y. Interleukin-6 and Depressive Symptom Severity in Response to Physical Exercise. Psychiatry Res. 2017, 252, 270–276. [Google Scholar] [CrossRef]
- Karlović, D.; Serretti, A.; Vrkić, N.; Martinac, M.; Marčinko, D. Serum Concentrations of CRP, IL-6, TNF-α and Cortisol in Major Depressive Disorder with Melancholic or Atypical Features. Psychiatry Res. 2012, 198, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The Effect of Antidepressant Medication Treatment on Serum Levels of Inflammatory Cytokines: A Meta-Analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Yang, N.; Du, J.; Liu, P.; Dai, W.; Dong, Q. Differences Between Adolescent Depression and Healthy Controls in Biomarkers Associated with Immune or Inflammatory Processes: A Systematic Review and Meta-Analysis. Psychiatry Investig. 2025, 22, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.K.; Daouk, J.; Kurkinen, K.; Kraav, S.L.; Eriksson, P.; Tolmunen, T.; Kanninen, K.M. Blood Cytokines in Major Depressive Disorder in Drug-Naïve Adolescents: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2025, 372, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Suhee, F.I.; Shahriar, M.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Elevated Serum IL-2 Levels Are Associated with Major Depressive Disorder: A Case-Control Study. Clin. Pathol. 2023, 16, 2632010X231180797. [Google Scholar] [CrossRef]
- Tian, X.; Dong, Y.Q.; Yuan, J.Y.; Gao, Y.; Zhang, C.H.; Li, M.J.; Li, J. Association between Peripheral Plasma Cytokine Levels and Suicidal Ideation in First-Episode, Drug-Naïve Major Depressive Disorder. Psychoneuroendocrinology 2024, 165, 107042. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef]
- Sarmin, N.; Roknuzzaman, A.S.M.; Mouree, T.Z.; Islam, M.R.; Al Mahmud, Z. Evaluation of Serum Interleukin-12 and Interleukin-4 as Potential Biomarkers for the Diagnosis of Major Depressive Disorder. Sci. Rep. 2024, 14, 1652. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Qusar, M.; Shahriar, M.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Altered Serum Interleukin-7 and Interleukin-10 Are Associated with Drug-Free Major Depressive Disorder. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320916655. [Google Scholar] [CrossRef] [PubMed]
- Gazal, M.; Jansen, K.; Souza, L.D.; Oses, J.P.; Magalhães, P.V.; Pinheiro, R.; Ghisleni, G.; Quevedo, L.; Kaster, M.P.; Kapczinski, F.; et al. Association of Interleukin-10 Levels with Age of Onset and Duration of Illness in Patients with Major Depressive Disorder. Braz. J. Psychiatry 2015, 37, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Ormstad, H.; Aass, H.C.D.; Malt, U.F.; Bendz, L.T.; Sandvik, L.; Brundin, L.; Andreassen, O.A. The Plasma Levels of Various Cytokines Are Increased during Ongoing Depression and Are Reduced to Normal Levels after Recovery. Psychoneuroendocrinology 2014, 45, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Daria, S.; Proma, M.A.; Shahriar, M.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Serum Interferon-Gamma Level Is Associated with Drug-Naive Major Depressive Disorder. SAGE Open Med. 2020, 8, 2050312120974169. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF-α and CRP in Elderly Patients with Depression or Alzheimer’s Disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Kim, Y.-K. Transforming Growth Factor-Β1 and Major Depressive Disorder with and without Attempted Suicide: Preliminary Study. Psychiatry Res. 2010, 178, 92–96. [Google Scholar] [CrossRef]
- Elgellaie, A.; Thomas, S.J.; Kaelle, J.; Bartschi, J.; Larkin, T. Pro-Inflammatory Cytokines IL-1α, IL-6 and TNF-α in Major Depressive Disorder: Sex-Specific Associations with Psychological Symptoms. Eur. J. Neurosci. 2023, 57, 1913–1928. [Google Scholar] [CrossRef]
- Yin, M.; Zhou, H.; Li, J.; Wang, L.; Zhu, M.; Wang, N.; Yang, P.; Yang, Z. The Change of Inflammatory Cytokines after Antidepressant Treatment and Correlation with Depressive Symptoms. J. Psychiatr. Res. 2025, 184, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Gędek, A.; Szular, Z.; Antosik, A.Z.; Mierzejewski, P.; Dominiak, M. Celecoxib for Mood Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 3497. [Google Scholar] [CrossRef]
- Dominiak, M.; Gędek, A.; Sikorska, M.; Mierzejewski, P.; Wojnar, M.; Antosik-Wójcińska, A.Z. Acetylsalicylic Acid and Mood Disorders: A Systematic Review. Pharmaceuticals 2022, 16, 67. [Google Scholar] [CrossRef]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.B.; Khandaker, G.M. Antidepressant Activity of Anti-Cytokine Treatment: A Systematic Review and Meta-Analysis of Clinical Trials of Chronic Inflammatory Conditions. Mol. Psychiatry 2018, 23, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bosmans, E.; Suy, E.; Vandervorst, C.; DeJonckheere, C.; Raus, J. Depression-Related Disturbances in Mitogen-Induced Lymphocyte Responses and Interleukin-1β and Soluble Interleukin-2 Receptor Production. Acta Psychiatr. Scand. 1991, 84, 379–386. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Scott, L.V.; Dinan, T.G. Cytokines: Abnormalities in Major Depression and Implications for Pharmacological Treatment. Hum. Psychopharmacol. Clin. Exp. 2004, 19, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.; Mathias, C. Inflammation and Its Relevance to Psychiatry. Adv. Psychiatr. Treat. 2008, 14, 248–255. [Google Scholar] [CrossRef][Green Version]
- de Kloet, E.R.; Vreugdenhil, E.; Oitzl, M.S.; Joёls, M. Brain Corticosteroid Receptor Balance in Health and Disease. Endocr. Rev. 1998, 19, 269–301. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA Axis in Major Depression: Classical Theories and New Developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Stetler, C.; Miller, G.E. Depression and Hypothalamic-Pituitary-Adrenal Activation: A Quantitative Summary of Four Decades of Research. Biopsychosoc. Sci. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- Connor, T.J.; Starr, N.; O’Sullivan, J.B.; Harkin, A. Induction of Indolamine 2,3-Dioxygenase and Kynurenine 3-Monooxygenase in Rat Brain Following a Systemic Inflammatory Challenge: A Role for IFN-Gamma? Neurosci. Lett. 2008, 441, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Galecki, P.; Verkerk, R.; Rief, W. Somatization, but Not Depression, Is Characterized by Disorders in the Tryptophan Catabolite (TRYCAT) Pathway, Indicating Increased Indoleamine 2,3-Dioxygenase and Lowered Kynurenine Aminotransferase Activity. Neuro Endocrinol. Lett. 2011, 32, 264–273. [Google Scholar] [PubMed]
- Lichtblau, N.; Schmidt, F.M.; Schumann, R.; Kirkby, K.C.; Himmerich, H. Cytokines as Biomarkers in Depressive Disorder: Current Standing and Prospects. Int. Rev. Psychiatry 2013, 25, 592–603. [Google Scholar] [CrossRef]
- Mechawar, N.; Savitz, J. Neuropathology of Mood Disorders: Do We See the Stigmata of Inflammation? Transl. Psychiatry 2016, 6, e946. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, W.; Liu, W.; Wang, C.; Zhan, Y.; Li, H.; Chen, L.; Ning, Y. Cross-Sectional Relationship between Kynurenine Pathway Metabolites and Cognitive Function in Major Depressive Disorder. Psychoneuroendocrinology 2019, 101, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Nava, R.G.; Adri, A.S.; Filgueiras, I.S.; Nóbile, A.L.; Barcelos, P.M.; Corrêa, Y.L.G.; de Oliveira, S.F.; Cabral-Miranda, G.; Dias, H.D.; Schimke, L.F.; et al. Modulation of Neuroimmune Cytokine Networks by Antidepressants: Implications in Mood Regulation. Transl. Psychiatry 2025, 15, 314. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).