Morphological Correlates of TRPV1 Agonist-Induced Activation and Defunctionalization of Nociceptor Neurons
Abstract
1. Introduction
2. Chemosensitive Primary Sensory Neurons: A Unique Class of Nociceptors
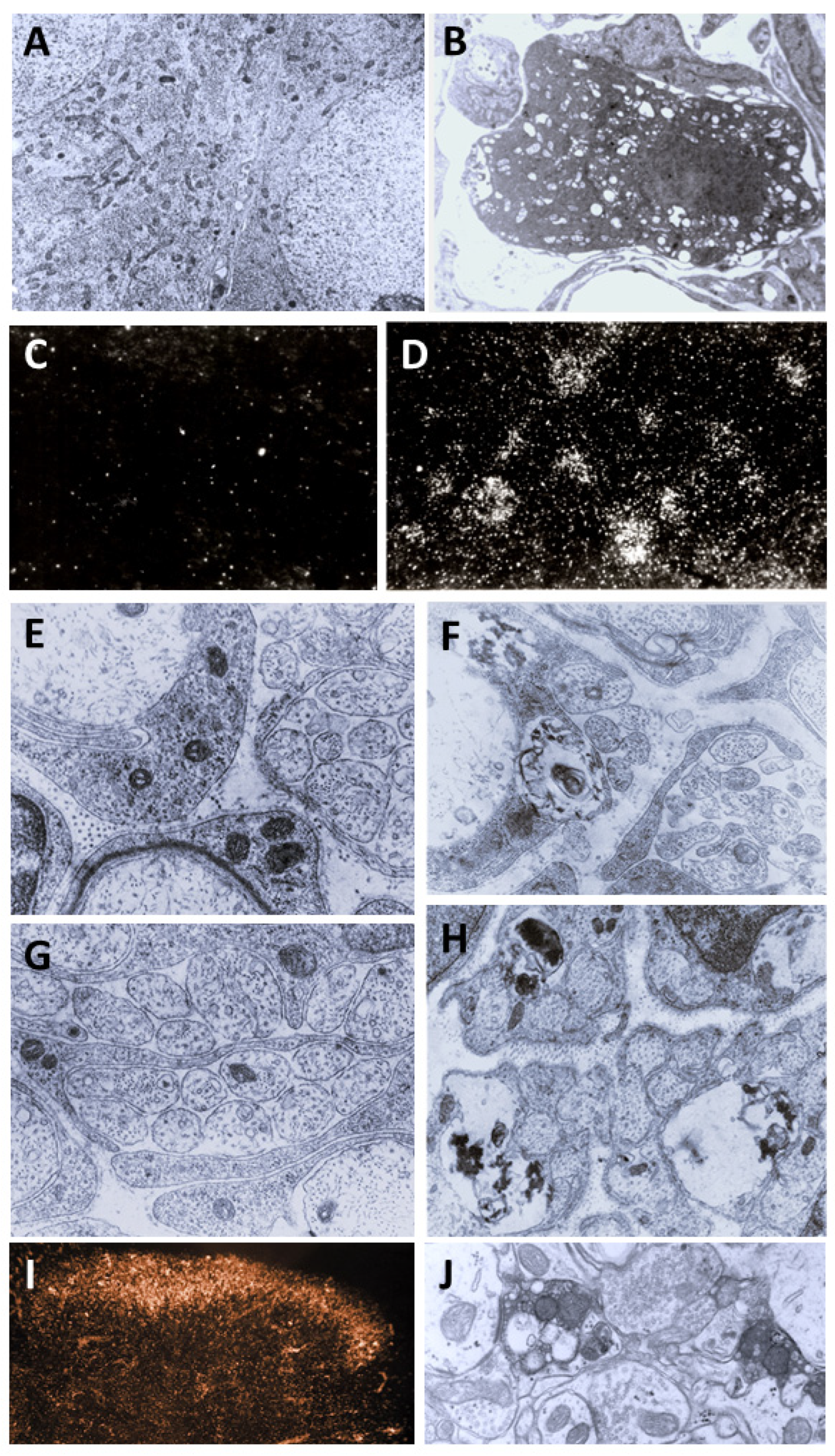
3. Morphological Correlates of Activation of CPSNs
4. Structural Changes in C-Fiber Nociceptive Afferents Underlie the Long-Lasting Analgesia Induced by Capsaicin
| Type of Treatment | Acute Changes | Chronic Changes |
|---|---|---|
Neonatal systemic | Depolarization of dorsal root and peripheral nerve axons [158,159] Ca2+ accumulation in small sensory ganglion cells [79,80] Degenerative changes in small DRG neurons [9,13,14] (including mitochondrial swelling) Degeneration of unmyelinated dorsal root and peripheral nerve axons [9,13,14] Degeneration of spinal and medullary primary afferent terminals [10,16] | Long-lasting decreased sensitivity to noxious mechanical, chemical and heat stimuli [9,12,38,160,161,162,163,164] Loss of neurogenic inflammation [9,30,165] Reduced thermal hyperalgesia [166,167] Decreased visceral sensitivity [168] Loss of B-type sensory ganglion cells (~50% of all DRG neurons) [9,169,170] Loss of C-fiber afferent axons and nerve endings (reduction by 70% and 90% of unmyelinated axons in sensory nerves and dorsal roots, respectively) [9,69,71,72,169,171] Depletion of sensory neuropeptides and specific proteins (e.g., IB4, FRAP/TMP) [56,59,60,61,172,173] Sprouting of spinal myelinated afferents [174,175,176] |
Adult systemic | Ca2+ accumulation in small sensory ganglion cells [81,82,92] Degenerative changes in small DRG neurons (including mitochondrial swelling) [3,81,130,177] Degeneration of unmyelinated dorsal root and peripheral nerve axons [130,178,179,180] Degeneration of spinal and medullary primary afferent terminals [17,20,130] | Decreased sensitivity to chemical irritants and heat [1,2,181] Reduced neurogenic inflammation [1,2,9,32,181,182,183] Decreased visceral sensitivity [99] Depletion of sensory neuropeptides and specific proteins (e.g., IB4, FRAP/TMP) [5,7,55,61,170] Degeneration of small sensory ganglion cells (~17% of all DRG neurons) [81,130,177,184] Loss of C-fiber sensory axons and nerve endings (reduction by 30–50% of unmyelinated axons in sensory nerves) [130,184] |
Local application | Burning pain, [2,185,186] Vasodilatation—Axon reflex flare [2,185,186] Hyperalgesia [2,185] Block of action potential initiation/conduction | Increased noxious heat threshold [186] Chemoanalgesia [2] Reduced neurogenic inflammation [187] Degenerative changes in peripheral C-fiber sensory axons and nerve endings (in part reversible) [132,133,187] Axoplasmatic transport block (?) Loss of thermal hyperalgesia Decreased visceral sensitivity [188] Depletion of sensory neuropeptides from sensory nerve terminals [132,133,189] Regeneration of cutaneous sensory nerves [132,133] Therapeutic effect in certain types of europathic pain (Qutenza) [190,191] |
| Intrathecal or intra- cisternal application  | Pain [192,193,194] Chemoanalgesia [193] Cutaneous vasodilatation [193,194] Mechanical allodynia [193]. | Chemoanalgesia [192,193] Increase in noxious heat threshold [192] Inhibition of heat hyperalgesia [195,196] Degeneration of spinal/medullary primary afferent terminals [193,194] Depletion of sensory neuropeptides and specific proteins (e.g., Substance P, IB4, FRAP/TMP) from central but not from peripheral branches of DRG neurons [192,194] Preserved cutaneous neurogenic inflammation [194,197] Possible therapeutic application [191,195,197,198,199] |
Perineural or local nerve application | Depolarization of C-fiber afferents [47,81,158,200,201,202] Action potential conduction block [201,202,203,204,205,206] Block of axoplasmic transport [110,111,118,143] | Selective regional chemical and thermal analgesia [110,112,206] Loss of neurogenic plasma extravasation [110,115,146,207] Reduced neurogenic sensory vasodilatation [113,114] Reduced thermal hyperalgesia [208] Reduction by >30% in C-fiber sensory axons and epidermal nerve endings [115,120,129,207,209] Loss by >30% in small DRG neurons [120] Depletion of sensory neuropeptides and specific proteins (e.g., IB4, FRAP/TMP) [118,134,135,136,137,207] Increased expression of injury peptides (galanin, VIP) and GM1 ganglioside in DRG neurons and spinal dorsal horn (phenotypic switch) [135,137,210] Transganglionic degeneration of C-fiber primary afferents [120] Inhibition of C-fiber collateral sprouting of intact cutaneous afferents [113,146] |
5. Localization of GM1 Ganglioside in CPSNs and Its Functional Significance
6. A Novel Concept of the Capsaicin-Sensitive Nociceptive Primary Sensory Neuron: The Chemosensitive Nociceptor
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANO1 | Anoctamin 1 |
| CGRP | Calcitonin gene-related peptide |
| CPSNs | Chemosensitive primary sensory neurons |
| CTB-HRP | Choleratoxin B-horseradish peroxidase conjugate |
| DRGs | Dorsal root ganglia |
| ERK 1/2 | Extracellular signal-regulated kinase 1/2 |
| FRAP | Fluoride-resistant acid phosphatase |
| GCS | Glucosylceramide synthase |
| GSL | Glycosphingolipids |
| IB4 | Bandeiraea simplicifolia isolectin B4 |
| NGF | Nerve growth factor |
| TMP | Thiamin monophosphatase |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TRPV1 | Transient receptor potential vanilloid type 1 |
| VR1 | Vanilloid receptor type 1 |
References
- Jancsó, N. Role of the Nerve Terminals in the Mechanism of Inflammatory Reactions. Bull Millard Fill. Hosp. 1960, 7, 53–77. [Google Scholar]
- Jancsó, N. Desensitization with Capsaicin and Related Acylamides as a Tool for Studying the Function of Pain Receptors. In Proceedings of the Pharmacology of Pain; Lim, R.K.S., Amstrong, D., Pardo, E.G., Eds.; Pergamon Press: Oxford, UK, 1968; pp. 33–55. [Google Scholar]
- Joó, F.; Szolcsányi, J.; Jancsó-Gábor, A. Mitochondrial Alterations in the Spinal Ganglion Cells of the Rat Accompanying the Long-Lasting Sensory Disturbance Induced by Capsaicin. Life Sci. 1969, 8, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J.; Jancsó-Gábor, A.; Joó, F. Functional and Fine Structural Characteristics of the Sensory Neuron Blocking Effect of Capsaicin. Naunyn Schmiedebergs Arch. Pharmacol. 1975, 287, 157–169. [Google Scholar] [CrossRef]
- Jancsó, G.; Knyihár, E. Functional Linkage between Nociception and Fluoride-Resistant Acid Phosphatase Activity in the Rolando Substance. Neurobiology 1975, 5, 42–43. [Google Scholar]
- Szentágothai, J. Neuronal and Synaptic Arrangement in the Substantia Gelatinosa Rolandi. J. Comp. Neurol. 1964, 122, 219–239. [Google Scholar] [CrossRef]
- Jessell, T.M.; Iversen, L.L.; Cuello, A.C. Capsaicin-Induced Depletion of Substance P from Primary Sensory Neurones. Brain Res. 1978, 152, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Gasparovic, I.; Hadzovic, S.; Hukovic, S.; Stern, P. Contribution to the Theory That Substance P Has a Transmitter Role in Sensitive Pathways. Med. Exp. Int. J. Exp. Med. 1964, 10, 303–306. [Google Scholar]
- Jancsó, G.; Király, E.; Jancsó-Gábor, A. Pharmacologically Induced Selective Degeneration of Chemosensitive Primary Sensory Neurones. Nature 1977, 270, 741–743. [Google Scholar] [CrossRef]
- Jancsó, G.; Király, E. Sensory Neurotoxins: Chemically Induced Selective Destruction of Primary Sensory Neurons. Brain Res. 1981, 210, 83–89. [Google Scholar] [CrossRef]
- Hiura, A.; Nakagawa, H.; Koshigae, Y.; Yoshizako, A.; Kubo, Y.; Ishizuka, H. Age-Related Changes in the Response to Thermal Noxious Heat and Reduction of C-Fibers by Neonatal Treatment with Capsaicin. Somatosens. Mot. Res. 1999, 16, 115–121. [Google Scholar] [CrossRef]
- Jancsó, G.; Király, E.; Such, G.; Joó, F.; Nagy, A. Neurotoxic Effect of Capsaicin in Mammals. Acta Physiol. Hung. 1987, 69, 295–313. [Google Scholar] [PubMed]
- Hiura, A.; Ishizuka, H. Changes in Features of Degenerating Primary Sensory Neurons with Time After Capsaicin Treatment. Acta Neuropathol. 1989, 78, 35–46. [Google Scholar] [CrossRef]
- Hiura, A. Neuroanatomical Effects of Capsaicin on the Primary Afferent Neurons. Arch. Histol. Cytol. 2000, 63, 199–215. [Google Scholar] [CrossRef]
- Ramon, Y.; Cajal, S. Degeneration and Regeneration of the Nervous System; Oxford University Press: London, UK, 1928. [Google Scholar]
- Jancsó, G.; Király, E. Distribution of Chemosensitive Primary Sensory Afferents in the Central Nervous System of the Rat. J. Comp. Neurol. 1980, 190, 781–792. [Google Scholar] [CrossRef]
- Ritter, S.; Dinh, T.T. Capsaicin-Induced Neuronal Degeneration: Silver Impregnation of Cell Bodies, Axons, and Terminals in the Central Nervous System of the Adult Rat. J. Comp. Neurol. 1988, 271, 79–90. [Google Scholar] [CrossRef]
- Jancsó, G.; Maggi, C.A. Distribution of Capsaicin-Sensitive Urinary-Bladder Afferents in the Rat Spinal-Cord. Brain Res. 1987, 418, 371–376. [Google Scholar] [CrossRef]
- Kim, S.H.; Hadley, S.H.; Maddison, M.; Patil, M.; Cha, B.; Kollarik, M.; Taylor-Clark, T.E. Mapping of Sensory Nerve Subsets within the Vagal Ganglia and the Brainstem Using Reporter Mice for Pirt, TRPV1, 5-HT3, and Tac1 Expression. eNeuro 2020, 7, 1–24. [Google Scholar] [CrossRef]
- Ritter, S.; Dinh, T.T. Age-Related Changes in Capsaicin-Induced Degeneration in Rat Brain. J. Comp. Neurol. 1992, 318, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Jancsó, G.; Wollemann, M. Effect of Capsaicin on Adenylate-Cyclase Activity of Rat-Brain. Brain Res. 1977, 123, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Hajós, M.; Obál, F.; Jancsó, G.; Obál, F. The Capsaicin Sensitivity of the Preoptic Region Is Preserved in Adult-Rats Pretreated as Neonates, but Lost in Rats Pretreated as Adults. Naunyn-Schmiedebergs Arch. Pharmacol. 1983, 324, 219–222. [Google Scholar] [CrossRef]
- Jancsó-Gábor, A.; Szolcsányi, J.; Jancsó, N. Irreversible Impairment of Thermoregulation Induced by Capsaicin and Similar Pungent Substances in Rats and Guinea-Pigs. J. Physiol. 1970, 206, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Hajós, M.; Jancsó, G.; Engberg, G. Capsaicin-Induced Excitation of Locus-Coeruleus Neurons. Acta Physiol. Scand. 1987, 129, 415–420. [Google Scholar] [CrossRef]
- Harada, N.; Narimatsu, N.; Kurihara, H.; Nakagata, N.; Okajima, K. Stimulation of Sensory Neurons Improves Cognitive Function by Promoting the Hippocampal Production of Insulin-like Growth Factor-I in Mice. Transl. Res. 2009, 154, 90–102. [Google Scholar] [CrossRef]
- Christianson, J.A.; McIlwrath, S.L.; Koerber, H.R.; Davis, B.M. Transient Receptor Potential Vanilloid 1-Immunopositive Neurons in the Mouse Are More Prevalent Within Colon Afferents Compared to Skin and Muscle Afferents. Neuroscience 2006, 140, 247–257. [Google Scholar] [CrossRef]
- Papka, R.E.; Furness, J.B.; Della, N.G.; Murphy, R.; Costa, M. Time Course of Effect of Capsaicin on Ultrastructure and Histochemistry of Substance P-Immunoreactive Nerves Associated with the Cardiovascular System of the Guinea-Pig. Neuroscience 1984, 12, 1277–1292. [Google Scholar] [CrossRef]
- Avelino, A.; Cruz, C.; Nagy, I.; Cruz, F. Vanilloid Receptor 1 Expression in the Rat Urinary Tract. Neuroscience 2002, 109, 787–798. [Google Scholar] [CrossRef]
- Ständer, S.; Moormann, C.; Schumacher, M.; Buddenkotte, J.; Artuc, M.; Shpacovitch, V.; Brzoska, T.; Lippert, U.; Henz, B.M.; Luger, T.A.; et al. Expression of Vanilloid Receptor Subtype 1 in Cutaneous Sensory Nerve Fibers, Mast Cells, and Epithelial Cells of Appendage Structures. Exp. Dermatol. 2004, 13, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Gamse, R.; Holzer, P.; Lembeck, F. Decrease of Substance P in Primary Afferent Neurones and Impairment of Neurogenic Plasma Extravasation by Capsaicin. Br. J. Pharmacol. 1980, 68, 207–213. [Google Scholar] [CrossRef]
- Lembeck, F.; Holzer, P. Substance P as Neurogenic Mediator of Antidromic Vasodilation and Neurogenic Plasma Extravasation. Naunyn Schmiedebergs Arch. Pharmacol. 1979, 310, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Jancsó-Gábor, A.; Szolcsányi, J. Neurogenic Inflammatory Responses. J. Dent. Res. 1972, 51, 264–269. [Google Scholar] [CrossRef]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid Receptor-1 Is Essential for Inflammatory Thermal Hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Holzer, P. Capsaicin: Cellular Targets, Mechanisms of Action, and Selectivity for Thin Sensory Neurons. Pharmacol. Rev. 1991, 43, 143–201. [Google Scholar] [CrossRef]
- Buck, S.H.; Burks, T.F. The Neuropharmacology of Capsaicin: Review of Some Recent Observations. Pharmacol. Rev. 1986, 38, 179–226. [Google Scholar] [CrossRef]
- Nagy, I.; Sántha, P.; Jancsó, G.; Urbán, L. The Role of the Vanilloid (Capsaicin) Receptor (TRPV1) in Physiology and Pathology. Eur. J. Pharmacol. 2004, 500, 351–369. [Google Scholar] [CrossRef]
- Jancsó, G.; Oszlács, O.; Sántha, P. The Capsaicin Paradox: Pain Relief by an Algesic Agent. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 10, 52–65. [Google Scholar] [CrossRef]
- Holzer, P. Local Effector Functions of Capsaicin-Sensitive Sensory Nerve Endings: Involvement of Tachykinins, Calcitonin Gene-Related Peptide and Other Neuropeptides. Neuroscience 1988, 24, 739–768. [Google Scholar] [CrossRef] [PubMed]
- Dux, M.; Sántha, P.; Jancsó, G. The Role of Chemosensitive Afferent Nerves and TRP Ion Channels in the Pathomechanism of Headaches. Pflug. Arch. Eur. J. Physiol. 2012, 464, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M. Capsaicin and Sensory Neurones—A Review. Pain 1983, 15, 109–130. [Google Scholar] [CrossRef]
- Barthó, L.; Benkó, R.; Patacchini, R.; Pethö, G.; Holzer-Petsche, U.; Holzer, P.; Lázár, Z.; Undi, S.; Illényi, L.; Antal, A.; et al. Effects of Capsaicin on Visceral Smooth Muscle: A Valuable Tool for Sensory Neurotransmitter Identification. Eur. J. Pharmacol. 2004, 500, 143–157. [Google Scholar] [CrossRef]
- Cortright, D.N.; Szallasi, A. TRP Channels and Pain. Curr. Pharm. Des. 2009, 15, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) Receptors and Mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar] [CrossRef]
- Nagy, J.J.I. Capsaicin: A Chemical Probe for Sensory Neuron Mechanisms. In Handbook of PsychophurmacoIogy; Iversen, L.L., Iversen, S., Snyder, S., Eds.; Plenum Press: New York, NY, USA, 1982; pp. 185–235. ISBN 978-1-4613-3452-1. [Google Scholar]
- Russell, L.C.; Burchiel, K.J. Neurophysiological Effects of Capsaicin. Brain Res. 1984, 320, 165–176. [Google Scholar] [CrossRef]
- Dray, A. Mechanism of Action of Capsaicin-like Molecules on Sensory Neurons. Life Sci. 1992, 51, 1759–1765. [Google Scholar] [CrossRef]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef]
- Jancsó, G.; Katona, M.; Horváth, V.J.; Sántha, P.; Nagy, J. Sensory Nerves as Modulators of Cutaneous Inflammatory Reactions in Health and Disease. In NeuroImmune Biology; Jancso, G., Ed.; Neuroimmun Biology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 8, pp. 3–36. ISBN 978-0-444-53229-9. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The Capsaicin Receptor: A Heat-Activated Ion Channel in the Pain Pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Nilius, B.; Voets, T.; Peters, J. TRP Channels in Disease. Sci. STKE 2005, 2005, re8. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M.; Nilsson, S.; Hökfelt, T.; Lundberg, J.M. Visualization by [3H]Resiniferatoxin Autoradiography of Capsaicin-Sensitive Neurons in the Rat, Pig and Man. Eur. J. Pharmacol. 1994, 264, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Simone, D.A.; Stone, L.S.; Fairbanks, C.A.; Wang, J.; Elde, R. Developmental Shift of Vanilloid Receptor 1 (VR1) Terminals into Deeper Regions of the Superficial Dorsal Horn: Correlation with a Shift from TrkA to Ret Expression by Dorsal Root Ganglion Neurons. Eur. J. Neurosci. 2001, 14, 293–304. [Google Scholar] [CrossRef]
- Priestley, J.V.; Michael, G.J.; Averill, S.; Liu, M.; Willmott, N. Regulation of Nociceptive Neurons by Nerve Growth Factor and Glial Cell Line Derived Neurotrophic Factor. Can. J. Physiol. Pharmacol. 2002, 80, 495–505. [Google Scholar] [CrossRef]
- Cuello, A.C.; Gamse, R.; Holzer, P.; Lembeck, F. Substance P Immunoreactive Neurons Following Neonatal Administration of Capsaicin. Naunyn Schmiedebergs Arch. Pharmacol. 1981, 315, 185–194. [Google Scholar] [CrossRef]
- Mezey, E.; Toth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of MRNA for Vanilloid Receptor Subtype 1 (VR1), and VR1-like Immunoreactivity, in the Central Nervous System of the Rat and Human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Toth, A.; Boczan, J.; Kedei, N.; Lizanecz, E.; Bagi, Z.; Papp, Z.; Edes, I.; Csiba, L.; Blumberg, P.M. Expression and Distribution of Vanilloid Receptor 1 (TRPV1) in the Adult Rat Brain. Brain Res. Mol. Brain Res. 2005, 135, 162–168. [Google Scholar] [CrossRef]
- Jancsó, G.; Hökfelt, T.; Lundberg, J.M.; Király, E.; Halász, N.; Nilsson, G.; Terenius, L.; Rehfeld, J.; Steinbusch, H.; Verhofstad, A.; et al. Immunohistochemical Studies on the Effect of Capsaicin on Spinal and Medullary Peptide and Monoamine Neurons Using Antisera to Substance-P, Gastrin-Cck, Somatostatin, Vip, Enkephalin, Neurotensin and 5-Hydroxytryptamine. J. Neurocytol. 1981, 10, 963–980. [Google Scholar] [CrossRef]
- Skofitsch, G.; Jacobowitz, D.M. Calcitonin Gene-Related Peptide Coexists with Substance P in Capsaicin Sensitive Neurons and Sensory Ganglia of the Rat. Peptides 1985, 6, 747–754. [Google Scholar] [CrossRef]
- Priestley, J.V.; Bramwell, S.; Butcher, L.L.; Cuello, A.C. Effect of Capsaicin on Neuropeptides in Areas of Termination of Primary Sensory Neurones. Neurochem. Int. 1982, 4, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cuello, A.C.; Del Fiacco, M.; Paxinos, G. The Central and Peripheral Ends of the Substance P-Containing Sensory Neurones in the Rat Trigeminal System. Brain Res. 1978, 152, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Kashiba, H.; Ueda, Y.; Senba, E. Systemic Capsaicin in the Adult Rat Differentially Affects Gene Expression for Neuropeptides and Neurotrophin Receptors in Primary Sensory Neurons. Neuroscience 1997, 76, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggström, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased Classification of Sensory Neuron Types by Large-Scale Single-Cell RNA Sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Li, C.L.; Li, K.C.; Wu, D.; Chen, Y.; Luo, H.; Zhao, J.R.; Wang, S.S.; Sun, M.M.; Lu, Y.J.; Zhong, Y.Q.; et al. Somatosensory Neuron Types Identified by High-Coverage Single-Cell RNA-Sequencing and Functional Heterogeneity. Cell Res. 2016, 26, 83–102, Erratum in Cell Res. 2016, 26, 967. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, P.; Bai, L.; Trimmer, J.S.; Bean, B.P.; Ginty, D.D. Deep Sequencing of Somatosensory Neurons Reveals Molecular Determinants of Intrinsic Physiological Properties. Neuron 2019, 103, 598–616.e7. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular Mechanisms of Nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Emery, E.C.; Ernfors, P. Dorsal Root Ganglion Neuron Types and Their Functional Specialization. In The Oxford Handbook of the Neurobiology of Pain; Oxford Academic: Oxford, UK, 2018; pp. 129–155. [Google Scholar] [CrossRef]
- Nagy, J.I.; Iversen, L.L.; Goedert, M.; Chapman, D.; Hunt, S.P. Dose-Dependent Effects of Capsaicin on Primary Sensory Neurons in the Neonatal Rat. J. Neurosci. 1983, 3, 399–406. [Google Scholar] [CrossRef]
- Holje, L.; Hildebrand, C.; Fried, K. Proportion of Unmyelinated Axons in the Rat Inferior Alveolar Nerve and Mandibular Molar Pulps after Neonatal Administration of Capsaicin. Brain Res. 1983, 266, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Chad, D.; Rasool, C.; Bradley, W.G.; Good, P.; Reichlin, S. Unmyelinated Axon Subpopulations in the Rat Peripheral Nervous System. Neurology 1983, 33, 848–852. [Google Scholar] [CrossRef]
- Scadding, J.W. The Permanent Anatomical Effects of Neonatal Capsaicin on Somatosensory Nerves. J. Anat. 1980, 131, 471–482. [Google Scholar]
- Chad, D.; Bradley, W.G.; Rasool, C.; Good, P.; Reichlin, S.; Zivin, J. Sympathetic Postganglionic Unmyelinated Axons in the Rat Peripheral Nervous System. Neurology 1983, 33, 841–847. [Google Scholar] [CrossRef]
- Hiura, A.; Sakamoto, Y. Quantitative Estimation of the Effects of Capsaicin on the Mouse Primary Sensory Neurons. Neurosci. Lett. 1987, 76, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Shiers, S.; Klein, R.M.; Price, T.J. Quantitative Differences in Neuronal Subpopulations Between Mouse and Human Dorsal Root Ganglia Demonstrated with RNAscope in Situ Hybridization. Pain 2020, 161, 2410–2424. [Google Scholar] [CrossRef]
- Zylka, M.J.; Dong, X.; Southwell, A.L.; Anderson, D.J. Atypical Expansion in Mice of the Sensory Neuron-Specific Mrg G Protein-Coupled Receptor Family. Proc. Natl. Acad. Sci. USA 2003, 100, 10043–10048. [Google Scholar] [CrossRef]
- Rostock, C.; Schrenk-Siemens, K.; Pohle, J.; Siemens, J. Human vs. Mouse Nociceptors—Similarities and Differences. Neuroscience 2018, 387, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Dourado, M.; Maksymetz, J.; Jacobson, A.; Laufer, B.I.; Baca, M.; Foreman, O.; Hackos, D.H.; Riol-Blanco, L.; Kaminker, J.S. Cross-Species Transcriptomic Atlas of Dorsal Root Ganglia Reveals Species-Specific Programs for Sensory Function. Nat. Commun. 2023, 14, 366. [Google Scholar] [CrossRef]
- Jancsó, G.; Sávay, G.; Király, E. Appearance of Histochemically Detectable Ionic Calcium in Degenerating Primary Sensory Neurons. Acta Histochem. 1978, 62, 165–169. [Google Scholar] [CrossRef]
- Jancsó, G.; Karcsu, S.; Király, E.; Szebeni, A.; Tóth, L.; Bácsy, E.; Joó, F.; Párducz, A. Neurotoxin Induced Nerve Cell Degeneration: Possible Involvement of Calcium. Brain Res. 1984, 295, 211–216. [Google Scholar] [CrossRef]
- Marsh, S.J.; Stansfeld, C.E.; Brown, D.A.; Davey, R.; McCarthy, D. The Mechanism of Action of Capsaicin on Sensory C-Type Neurons and Their Axons In Vitro. Neuroscience 1987, 23, 275–289. [Google Scholar] [CrossRef]
- Pecze, L.; Blum, W.; Schwaller, B. Mechanism of Capsaicin Receptor TRPV1-Mediated Toxicity in Pain-Sensing Neurons Focusing on the Effects of Na(+)/Ca(2+) Fluxes and the Ca(2+)-Binding Protein Calretinin. Biochim. Biophys. Acta 2013, 1833, 1680–1691. [Google Scholar] [CrossRef]
- Wood, J.N.; Winter, J.; James, I.F.; Rang, H.P.; Yeats, J.; Bevan, S. Capsaicin-Induced Ion Fluxes in Dorsal Root Ganglion Cells in Culture. J. Neurosci. 1988, 8, 3208–3220. [Google Scholar] [CrossRef]
- Hogan, P.G. Expression of Markers for Pain Sensory Neurons in Cell Culture; Harvard University: Cambridge, MA, USA, 1983. [Google Scholar]
- Chard, P.S.; Bleakman, D.; Savidge, J.R.; Miller, R.J. Capsaicin-Induced Neurotoxicity in Cultured Dorsal Root Ganglion Neurons: Involvement of Calcium-Activated Proteases. Neuroscience 1995, 65, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Kulik, A.; Polgar, E.; Matesz, C.; Szucs, P.; Kothalawala, S.; Nagy, I. Sub-Population of Capsaicin Sensitive Primary Afferent Neurons in Thoracic, Lumbar and Sacral Dorsal Root Ganglion in Young Rats Revealed by Stimulated Cobalt Uptake. Acta Biol. Hung. 1996, 47, 251–259. [Google Scholar] [PubMed]
- Dedov, V.N.; Roufogalis, B.D. Mitochondrial Calcium Accumulation Following Activation of Vanilloid (VR1) Receptors by Capsaicin in Dorsal Root Ganglion Neurons. Neuroscience 2000, 95, 183–188. [Google Scholar] [CrossRef]
- Shin, C.Y.; Shin, J.; Kim, B.M.; Wang, M.H.; Jang, J.H.; Surh, Y.J.; Oh, U. Essential Role of Mitochondrial Permeability Transition in Vanilloid Receptor 1-Dependent Cell Death of Sensory Neurons. Mol. Cell Neurosci. 2003, 24, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Wang, S.S.; Asgar, J.; Joseph, J.; Ro, J.Y.; Wei, F.; Campbell, J.N.; Chung, M.K. Ca2+ and Calpain Mediate Capsaicin-Induced Ablation of Axonal Terminals Expressing Transient Receptor Potential Vanilloid 1. J. Biol. Chem. 2017, 292, 8291–8303. [Google Scholar] [CrossRef]
- Jin, H.W.; Ichikawa, H.; Fujita, M.; Yamaai, T.; Mukae, K.; Nomura, K.; Sugimoto, T. Involvement of Caspase Cascade in Capsaicin-Induced Apoptosis of Dorsal Root Ganglion Neurons. Brain Res. 2005, 1056, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Xiao, C.; Ichikawa, H. Neonatal Primary Neuronal Death Induced by Capsaicin and Axotomy Involves an Apoptotic Mechanism. Brain Res. 1998, 807, 147–154. [Google Scholar] [CrossRef]
- Yuan, L.; Chandel, N.S.; Julius, D. Mitochondrial Activity Tunes Nociceptor Resilience to Excitotoxicity. Cell 2025, 188, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Barrantes, R.; Córdova, C.; Gatica, S.; Rodriguez, B.; Lozano, C.; Marchant, I.; Echeverria, C.; Simon, F.; Olivero, P. Transient Receptor Potential Vanilloid 1 Expression Mediates Capsaicin-Induced Cell Death. Front. Physiol. 2018, 9, 682. [Google Scholar] [CrossRef]
- Winter, J. Characterization of Capsaicin-Sensitive Neurones in Adult Rat Dorsal Root Ganglion Cultures. Neurosci. Lett. 1987, 80, 134–140. [Google Scholar] [CrossRef]
- Bráz, J.M.; Basbaum, A.I. Differential ATF3 Expression in Dorsal Root Ganglion Neurons Reveals the Profile of Primary Afferents Engaged by Diverse Noxious Chemical Stimuli. Pain 2010, 150, 290. [Google Scholar] [CrossRef]
- Gover, T.D.; Kao, J.P.Y.; Weinreich, D. Calcium Signaling in Single Peripheral Sensory Nerve Terminals. J. Neurosci. 2003, 23, 4793–4797. [Google Scholar] [CrossRef]
- Gershon, D.; Negev-Goldstein, R.H.; Abd al Razzaq, L.; Lev, S.; Binshtok, A.M. In Vivo Optical Recordings of Ion Dynamics in Mouse Corneal Primary Nociceptive Terminals. STAR Protoc. 2022, 3, 101224. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Katz, B.; Lev, S.; Binshtok, A.M. Ultrafast Optical Recording Reveals Distinct Capsaicin-Induced Ion Dynamics along Single Nociceptive Neurite Terminals In Vitro. J. Biomed. Opt. 2017, 22, 076010. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Meli, A. The Sensory-Efferent Function of Capsaicin-Sensitive Sensory Neurons. Gen. Pharmacol. 1988, 19, 1–43. [Google Scholar] [CrossRef]
- Hegarty, D.M.; Hermes, S.M.; Yang, K.; Aicher, S.A. Select Noxious Stimuli Induce Changes on Corneal Nerve Morphology. J. Comp. Neurol. 2017, 525, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Goldstein, D.; Park, S.B.; Krishnan, A.V.; Markoulli, M. Corneal Nerve Changes Following Treatment with Neurotoxic Anticancer Drugs. Ocul. Surf. 2021, 21, 221–237. [Google Scholar] [CrossRef]
- Cosmo, E.; Midena, G.; Frizziero, L.; Bruno, M.; Cecere, M.; Midena, E. Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. J. Clin. Med. 2022, 11, 5130. [Google Scholar] [CrossRef] [PubMed]
- Donnerer, J.; Liebmann, I. A Fluorescence-Immunohistochemical Study on Phosphorylation of ERK1/2, P38, and STAT3 in Rat Dorsal Root Ganglia Following Noxious Stimulation of Hind Paw Sensory Neurons. Tissue Cell 2011, 43, 178–189. [Google Scholar] [CrossRef]
- Dai, Y.; Iwata, K.; Fukuoka, T.; Kondo, E.; Tokunaga, A.; Yamanaka, H.; Tachibana, T.; Liu, Y.; Noguchi, K. Phosphorylation of Extracellular Signal-Regulated Kinase in Primary Afferent Neurons by Noxious Stimuli and Its Involvement in Peripheral Sensitization. J. Neurosci. 2002, 22, 7737. [Google Scholar] [CrossRef]
- Torres-Pérez, J.V.V.; Sántha, P.; Varga, A.; Szucs, P.; Sousa-Valente, J.; Gaal, B.; Sivadó, M.; Andreou, A.P.P.; Beattie, S.; Nagy, B.; et al. Phosphorylated Histone 3 at Serine 10 Identifies Activated Spinal Neurons and Contributes to the Development of Tissue Injury-Associated Pain. Sci. Rep. 2017, 7, srep41221. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 Ion Channel Determined by Electron Cryo-Microscopy. Nature 2013, 504, 107. [Google Scholar] [CrossRef]
- Moiseenkova-Bell, V.Y.; Stanciu, L.A.; Serysheva, I.I.; Tobe, B.J.; Wensel, T.G. Structure of TRPV1 Channel Revealed by Electron Cryomicroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 7451–7455. [Google Scholar] [CrossRef]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 Structures in Distinct Conformations Reveal Mechanisms of Activation. Nature 2013, 504, 113. [Google Scholar] [CrossRef]
- Jancsó, G.; Dux, M.; Oszlács, O.; Sántha, P. Activation of the Transient Receptor Potential Vanilloid-1 (TRPV1) Channel Opens the Gate for Pain Relief. Br. J. Pharmacol. 2008, 155, 1139–1141. [Google Scholar] [CrossRef]
- Jancsó, G.; Király, E.; Jancsó-Gábor, A. Direct Evidence for an Axonal Site of Action of Capsaicin. Naunyn Schmiedebergs Arch. Pharmacol. 1980, 313, 91–94. [Google Scholar] [CrossRef]
- Jancsó, G.; Such, G.; Rödel, C. A New Approach to Selective Regional Analgesia. In Trends in cluster headache; Sicuteri, F., Vecchiet, L., Fanciullacci, M., Eds.; Excerpta Medica: Amsterdam, NY, USA, 1987; pp. 59–68. [Google Scholar]
- Fitzgerald, M.; Woolf, C.J. The Time Course and Specificity of the Changes in the Behavioural and Dorsal Horn Cell Responses to Noxious Stimuli Following Peripheral Nerve Capsaicin Treatment in the Rat. Neuroscience 1982, 7, 2051–2056. [Google Scholar] [CrossRef]
- Sántha, P.; Lakatos, S.; Horváth, Á.; Dux, M.; Jancsó, G. Perineural Capsaicin Treatment Inhibits Collateral Sprouting of Intact Cutaneous Nociceptive Afferents. Biomedicines 2022, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Domoki, F.; Sántha, P.; Bari, F.; Jancsó, G. Perineural Capsaicin Treatment Attenuates Reactive Hyperaemia in the Rat Skin. Neurosci. Lett. 2003, 341, 127–130. [Google Scholar] [CrossRef]
- Dux, M.; Sann, H.; Jancsó, G. Changes in Fibre Populations of the Rat Hairy Skin after Selective Chemodenervation by Capsaicin. Eur. J. Neurosci. 1998, 10, 299. [Google Scholar]
- Sann, H.; Mccarthy, P.W.; Jancsó, G.; Pierau, F.K. Rt97—A Marker for Capsaicin-Insensitive Sensory Endings in the Rat Skin. Cell Tissue Res. 1995, 282, 155–161. [Google Scholar] [CrossRef]
- Jancsó, G.; Ferencsik, M.; Such, G.; Király, E.; Nagy, A.; Bujdosó, M. Morphological Effects of Capsaicin and and Its Analogues in New- Born and Adult Animals. In Tachykinin Antagonists; Hakanson, R., Sundler, F., Eds.; Elsevier: Amsterdam, NY, USA; Oxford, UK, 1985; pp. 35–44. [Google Scholar]
- Gamse, R.; Petsche, U.; Lembeck, F.; Jancsó, G. Capsaicin Applied to Peripheral-Nerve Inhibits Axoplasmic-Transport of Substance-P and Somatostatin. Brain Res. 1982, 239, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.; Jessell, T.M. Lactoseries Carbohydrates Specify Subsets of Dorsal Root Ganglion Neurons Projecting to the Superficial Dorsal Horn of Rat Spinal Cord. J. Neurosci. 1985, 5, 3278–3294. [Google Scholar] [CrossRef]
- Jancsó, G.; Lawson, S.N. Transganglionic Degeneration of Capsaicin-Sensitive C-Fiber Primary Afferent Terminals. Neuroscience 1990, 39, 501–511. [Google Scholar] [CrossRef]
- Schaefer, I.; Prato, V.; Arcourt, A.; Taberner, F.J.; Lechner, S.G. Differential Modulation of Voltage-Gated Sodium Channels by Nerve Growth Factor in Three Major Subsets of TrkA-Expressing Nociceptors. Mol. Pain 2018, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Michael, G.J.; Priestley, J.V. Differential Expression of the MRNA for the Vanilloid Receptor Subtype 1 in Cells of the Adult Rat Dorsal Root and Nodose Ganglia and Its Downregulation by Axotomy. J. Neurosci. 1999, 19, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Bevan, S.; Campbell, E.A. Capsaicin and Pain Mechanisms. Br. J. Anaesth. 1995, 75, 157–168. [Google Scholar] [CrossRef]
- Szigeti, C.; Sántha, P.; Körtvély, E.; Nyári, T.; Horváth, V.J.; Deák, T.; Dux, M.; Gulya, K.; Jancsó, G. Disparate Changes in the Expression of Transient Receptor Potential Vanilloid Type 1 Receptor MRNA and Protein in Dorsal Root Ganglion Neurons Following Local Capsaicin Treatment of the Sciatic Nerve in the Rat. Neuroscience 2012, 201, 320–330. [Google Scholar] [CrossRef]
- Jancsó, G.; Lynn, B. Possible Use of Capsaicin in Pain Therapy. Clin. J. Pain 1987, 3, 123–126. [Google Scholar] [CrossRef]
- Kissin, I. Vanilloid-Induced Conduction Analgesia: Selective, Dose-Dependent, Long-Lasting, with a Low Level of Potential Neurotoxicity. Anesth. Analg. 2008, 107, 271–281. [Google Scholar] [CrossRef]
- Kissin, I.; Freitas, C.F.; Bradley, E.L., Jr. Perineural Resiniferatoxin Prevents the Development of Hyperalgesia Produced by Loose Ligation of the Sciatic Nerve in Rats. Anesth. Analg. 2007, 104, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Jancsó, G.; Sántha, P.; Gecse, K. Peripheral nerve lesion-induced uptake and transport of choleragenoid by capsaicin-sensitive C-fibre spinal ganglion neurons. Acta Biol. Hun. 2002, 53, 77–84. [Google Scholar] [CrossRef]
- Pini, A.; Baranowski, R.; Lynn, B. Long-Term Reduction in the Number of C-Fibre Nociceptors Following Capsaicin Treatment of a Cutaneous Nerve in Adult Rats. Eur. J. Neurosci. 1990, 2, 89–97. [Google Scholar] [CrossRef]
- Jancsó, G.; Király, E.; Joó, F.; Such, G.; Nagy, A. Selective Degeneration by Capsaicin of A Subpopulation of Primary Sensory Neurons in the Adult-Rat. Neurosci. Lett. 1985, 59, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Specific Binding of Resiniferatoxin, an Ultrapotent Capsaicin Analog, by Dorsal Root Ganglion Membranes. Brain Res. 1990, 524, 106–111. [Google Scholar] [CrossRef]
- Nolano, M.; Simone, D.A.; Wendelschafer-Crabb, G.; Johnson, T.; Hazen, E.; Kennedy, W.R. Topical Capsaicin in Humans: Parallel Loss of Epidermal Nerve Fibers and Pain Sensation. Pain 1999, 81, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Simone, D.A.; Nolano, M.; Johnson, T.; Wendelschafer-Crabb, G.; Kennedy, W.R. Intradermal Injection of Capsaicin in Humans Produces Degeneration and Subsequent Reinnervation of Epidermal Nerve Fibers: Correlation with Sensory Function. J. Neurosci. 1998, 18, 8947–8954. [Google Scholar] [CrossRef]
- Ainsworth, A.; Hall, P.; Wall, P.D.; Allt, G.; MacKenzie, M.L.; Gibson, S.; Polak, J.M. Effects of Capsaicin Applied Locally to Adult Peripheral Nerve. II. Anatomy and Enzyme and Peptide Chemistry of Peripheral Nerve and Spinal Cord. Pain 1981, 11, 379–388. [Google Scholar] [CrossRef]
- Oszlács, O.; Jancsó, G.; Kis, G.; Dux, M.; Sántha, P. Perineural Capsaicin Induces the Uptake and Transganglionic Transport of Choleratoxin b Subunit by Nociceptive C-Fiber Primary Afferent Neurons. Neuroscience 2015, 311, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Szeredi, I.D.; Jancsó, G.; Oszlács, O.; Sántha, P. Prior Perineural or Neonatal Treatment with Capsaicin Does Not Alter the Development of Spinal Microgliosis Induced by Peripheral Nerve Injury Ivett. Cell Tissue Res. 2021, 383, 677–692. [Google Scholar] [CrossRef]
- Wall, P.D. The Central Consequences of the Application of Capsaicin to One Peripheral Nerve in Adult Rat. Acta Physiol. Hung. 1987, 69, 275–286. [Google Scholar]
- Sann, H.; Jancso, G.; Rossler, W.; Pierau, F.K. Reduction of Substance P Binding Sites in the Spinal Dorsal Horn after Perineural Capsaicin Treatment in the Rat. Neurosci. Lett. 1995, 190, 151–154. [Google Scholar] [CrossRef]
- Helliwell, R.J.A.; McLatchie, L.M.; Clarke, M.; Winter, J.; Bevan, S.; McIntyre, P. Capsaicin Sensitivity Is Associated with the Expression of the Vanilloid (Capsaicin) Receptor (VR1) MRNA in Adult Rat Sensory Ganglia. Neurosci. Lett. 1998, 250, 177–180. [Google Scholar] [CrossRef]
- Dickenson, A.H. Capsaicin: Gaps in Our Knowledge Start to Be Filled. Trends Neurosci. 1991, 14, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.S.; Buck, S.H.; Sipes, I.G.; Yamamura, H.I.; Burks, T.F. Regulation of Substance P by Nerve Growth Factor: Disruption by Capsaicin. Brain Res. 1982, 250, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Jancsó, G.; Ambrus, A. Capsaicin Sensitivity of Primary Sensory Neurones and Its Regulation. In Peripheral Neurons in Nociception: Physio-Pharmacological Aspects; Besson, J.M., Guilbaud, G., Ollat, H., Eds.; John Libbey Eurotext: Paris, France, 1994; Volume 1, pp. 71–87. [Google Scholar]
- Taylor, D.C.; Pierau, F.K.; Szolcsányi, J. Capsaicin-Induced Inhibition of Axoplasmic Transport Is Prevented by Nerve Growth Factor. Cell Tissue Res. 1985, 240, 569–573. [Google Scholar] [CrossRef]
- Hu-Tsai, M.; Woolf, C.; Winter, J. Influence of Inflammation or Disconnection from Peripheral Target Tissue on the Capsaicin Sensitivity of Rat Dorsal Root Ganglion Sensory Neurones. Neurosci. Lett. 1996, 203, 119–122. [Google Scholar] [CrossRef]
- Devor, M.; Schonfeld, D.; Seltzer, Z.; Wall, P.D. Two Modes of Cutaneous Reinnervation Following Peripheral Nerve Injury. J. Comp. Neurol. 1979, 185, 211–220. [Google Scholar] [CrossRef]
- Pertovaara, A. Collateral Sprouting of Nociceptive C-Fibers after Cut or Capsaicin Treatment of the Sciatic Nerve in Adult Rats. Neurosci. Lett. 1988, 90, 248–253. [Google Scholar] [CrossRef]
- Cobianchi, S.; de Cruz, J.; Navarro, X. Assessment of Sensory Thresholds and Nociceptive Fiber Growth after Sciatic Nerve Injury Reveals the Differential Contribution of Collateral Reinnervation and Nerve Regeneration to Neuropathic Pain. Exp. Neurol. 2014, 255, 1–11. [Google Scholar] [CrossRef]
- Leibovich, H.; Buzaglo, N.; Tsuriel, S.; Peretz, L.; Caspi, Y.; Katz, B.; Lev, S.; Lichtstein, D.; Binshtok, A.M. Abnormal Reinnervation of Denervated Areas Following Nerve Injury Facilitates Neuropathic Pain. Cells 2020, 9, 1007. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, V.; Zheng, H.; Taberner, F.J.; Landry, J.; Nees, T.A.; Pistolic, J.; Agarwal, N.; Männich, D.; Benes, V.; Helmstaedter, M.; et al. Neuropathic Pain Caused by Miswiring and Abnormal End Organ Targeting. Nature 2022, 606, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Evison, C.J.; O’Brien, C.; Benowitz, L.; Lindsay, R.M.; Mulderry, P.; Woolf, C. Neurotoxic Damage Evokes Regenerative Responses from Adult Rat Sensory Neurones. Neurosci. Lett. 1992, 146, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Frey, E.; Karney-Grobe, S.; Krolak, T.; Milbrandt, J.; DiAntonio, A. TRPV1 Agonist, Capsaicin, Induces Axon Outgrowth After Injury via Ca2+/PKA Signaling. eNeuro 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Gilmore, S.A.; Skinner, R.D. Intraspinal Non-Neuronal Cellular Responses to Peripheral Nerve Injury. Anat. Rec. 1979, 194, 369–387. [Google Scholar] [CrossRef]
- Eriksson, N.P.; Persson, J.K.; Svensson, M.; Arvidsson, J.; Molander, C.; Aldskogius, H. A Quantitative Analysis of the Microglial Cell Reaction in Central Primary Sensory Projection Territories Following Peripheral Nerve Injury in the Adult Rat. Exp. Brain Res. 1993, 96, 19–27. [Google Scholar] [CrossRef]
- Colburn, R.W.; Rickman, A.J.; Deleo, J.A. The Effect of Site and Type of Nerve Injury on Spinal Glial Activation and Neuropathic Pain Behavior. Exp. Neurol. 1999, 157, 289–304. [Google Scholar] [CrossRef]
- Gu, N.; Peng, J.; Murugan, M.; Wang, X.; Eyo, U.; Sun, D.; Ren, Y.; DiCicco-Bloom, E.; Young, W.; Dong, H.; et al. Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity After Peripheral Nerve Injury. Cell Rep. 2016, 16, 605–614. [Google Scholar] [CrossRef]
- Suter, M.R.; Berta, T.; Gao, Y.J.; Decosterd, I.; Ji, R.R. Large A-Fiber Activity Is Required for Microglial Proliferation and P38 MAPK Activation in the Spinal Cord: Different Effects of Resiniferatoxin and Bupivacaine on Spinal Microglial Changes after Spared Nerve Injury. Mol. Pain 2009, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Matsumoto, Y.; Kohno, K.; Nakashima, Y.; Tsuda, M. Microgliosis in the Spinal Dorsal Horn Early After Peripheral Nerve Injury Is Associated with Damage to Primary Afferent Aβ-Fibers. Cells 2025, 14, 666. [Google Scholar] [CrossRef] [PubMed]
- Ault, B.; Evans, R. Depolarizing Action of Capsaicin on Isolated Dorsal Root Fibres of the Rat. J. Physiol. 1980, 306, 22–23. [Google Scholar]
- Bell, J.A.; Jaffe, J.H. Electrophysiological Evidence for a Presynaptic Mechanism of Morphine Withdrawal in the Neonatal Rat Spinal Cord. Brain Res. 1986, 382, 299–304. [Google Scholar] [CrossRef]
- Holzer, P.; Jurna, I.; Gamse, R.; Lembeck, F. Nociceptive Threshold after Neonatal Capsaicin Treatment. Eur. J. Pharmacol. 1979, 58, 511–514. [Google Scholar] [CrossRef]
- Hammond, D.L.; Ruda, M.A. Developmental Alterations in Nociceptive Threshold, Immunoreactive Calcitonin Gene-Related Peptide and Substance P, and Fluoride-Resistant Acid Phosphatase in Neonatally Capsaicin-Treated Rats. J. Comp. Neurol. 1991, 312, 436–450. [Google Scholar] [CrossRef]
- Cervero, F.; McRitchie, H.A. Neonatal Capsaicin and Thermal Nociception: A Paradox. Brain Res. 1981, 215, 414–418. [Google Scholar] [CrossRef]
- Hayes, A.G.; Scadding, J.W.; Skingle, M.; Tyers, M.B. Effects of Neonatal Administration of Capsaicin on Nociceptive Thresholds in the Mouse and Rat. J. Pharm. Pharmacol. 1981, 33, 183–185. [Google Scholar] [CrossRef]
- Nagy, J.I.; van der Kooy, D. Effects of Neonatal Capsaicin Treatment on Nociceptive Thresholds in the Rat. J. Neurosci. 1983, 3, 1145–1150. [Google Scholar] [CrossRef]
- Jancsó, G.; Király, E.; Jancsó-Gábor, A. Chemosensitive Pain Fibres and Inflammation. Int. J. Tissue React. 1980, 2, 57–66. [Google Scholar]
- Meller, S.T.; Gebhart, G.F.; Maves, T.J. Neonatal Capsaicin Treatment Prevents the Development of the Thermal Hyperalgesia Produced in a Model of Neuropathic Pain in the Rat. Pain 1992, 51, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Shir, Y.; Seltzer, Z. A-Fibers Mediate Mechanical Hyperesthesia and Allodynia and C-Fibers Mediate Thermal Hyperalgesia in a New Model of Causalgiform Pain Disorders in Rats. Neurosci. Lett. 1990, 115, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A. The Dual Function of Capsaicin-Sensitive Sensory Nerves in the Bladder and Urethra. Ciba Found. Symp. 1990, 151, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.N. The Morphological Consequences of Neonatal Treatment with Capsaicin on Primary Afferent Neurones in Adult Rats. Acta Physiol. Hung. 1987, 69, 315–321. [Google Scholar]
- McDougal, D.B.; McDougal, S.H.; Johnson, E.M. Effect of Capsaicin upon Fluoride Sensitive Acid Phosphatases in Selected Ganglia and Spinal Cord and upon Neuronal Size and Number in Dorsal Root Ganglion. Brain Res. 1985, 331, 63–70. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Toscano-Márquez, B.; Gutiérrez-Ospina, G. Long-Term Effects of Neonatal Capsaicin Treatment on Intraepidermal Nerve Fibers and Keratinocyte Proliferation in Rat Glabrous Skin. Anat. Rec. 2011, 294, 173–184. [Google Scholar] [CrossRef]
- Carr, P.A.; Yamamoto, T.; Nagy, J.I. Calcitonin Gene-Related Peptide in Primary Afferent Neurons of Rat: Co-Existence with Fluoride-Resistant Acid Phosphatase and Depletion by Neonatal Capsaicin. Neuroscience 1990, 36, 751–760. [Google Scholar] [CrossRef]
- Inomata, K.; Nasu, F. Effects of Neonatal Capsaicin Treatment on Thiamine Monophosphatase (TMPase) Activity in the Substantia Gelatinosa of the Rat Spinal Cord. Int. J. Dev. Neurosci. 1984, 2, 307–311. [Google Scholar] [CrossRef]
- Nagy, J.I.; Hunt, S.P. The Termination of Primary Afferents within the Rat Dorsal Horn: Evidence for Rearrangement Following Capsaicin Treatment. J. Comp. Neurol. 1983, 218, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Réthelyi, M.; Salim, M.Z.; Jancsó, G. Altered Distribution of Dorsal-Root Fibers in the Rat Following Neonatal Capsaicin Treatment. Neuroscience 1986, 18, 749–761. [Google Scholar] [CrossRef]
- Beal, J.A.; Knight, D.S. Classification of Aberrant Primary Afferents in the Substantia Gelatinosa of the Rat Following Neonatal Capsaicin Treatment. Neurosci. Lett. 1987, 74, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Czaja, K.; Burns, G.A.; Ritter, R.C. Capsaicin-Induced Neuronal Death and Proliferation of the Primary Sensory Neurons Located in the Nodose Ganglia of Adult Rats. Neuroscience 2008, 154, 621–630. [Google Scholar] [CrossRef][Green Version]
- Király, E.; Jancsó, G.; Hajós, M. Possible Morphological Correlates of Capsaicin Desensitization. Brain Res. 1991, 540, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Dux, M.; Sántha, P.; Jancsó, G. Capsaicin-Sensitive Neurogenic Sensory Vasodilatation in the Dura Mater of the Rat. J. Physiol. 2003, 552, 859–867. [Google Scholar] [CrossRef]
- Hoyes, A.D.; Barber, P.; Jagessar, H. Effect of Capsaicin on the Intraperitoneal Axons of the Rat Trachea. Neurosci. Lett. 1981, 26, 329–334. [Google Scholar] [CrossRef]
- Jancsó, N. Speicherung. Stoffanreichung Im Retikuloendothel Und in Der Niere; Akadémiai Kiadó: Budapest, Hungary, 1955. [Google Scholar]
- Jancsó, N.; Jancsó-Gábor, A.; Szolcsányi, J. Direct Evidence for Neurogenic Inflammation and Its Prevention by Denervation and by Pretreatment with Capsaicin. Br. J. Pharmacol. Chemother. 1967, 31, 138–151. [Google Scholar] [CrossRef]
- Jancsó, N.; Jancsó-Gábor, A.; Szolcsányi, J. The Role of Sensory Nerve Endings in Neurogenic Inflammation Induced in Human Skin and in the Eye and Paw of the Rat. Br. J. Pharmacol. Chemother. 1968, 33, 32–41. [Google Scholar] [CrossRef]
- Hiura, A.; Lopez, V.E.; Ishizuka, H. Age-Dependent Attenuation of the Decrease of C Fibers by Capsaicin and Its Effects on Responses to Nociceptive Stimuli. Somatosens. Mot. Res. 1992, 9, 37–43. [Google Scholar] [CrossRef]
- LaMotte, R.H.; Lundberg, L.E.; Torebjörk, H.E. Pain, Hyperalgesia and Activity in Nociceptive C Units in Humans after Intradermal Injection of Capsaicin. J. Physiol. 1992, 448, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Toth Kasa, I.; Jancso, G.; Obal, F.; Tóth-Kása, I.; Husz, S.; Simon, N. Involvement of Sensory Nerve Endings in Cold and Heat Urticaria. J. Invest. Dermatol. 1983, 80, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Orosz, K.; Jancsó, G.; Dux, M.; Erős, I. Cutaneous Innervation and Vascular Reactions After Topical Capsaicin Treatment. Eur. J. Pharm. Sci. 1998, 6, 220. [Google Scholar] [CrossRef]
- Cruz, F.; Avelino, A.; Coimbra, A. Desensitization Follows Excitation of Bladder Primary Afferents by Intravesical Capsaicin, as Shown by c-Fos Activation in the Rat Spinal Cord. Pain 1996, 64, 553–557. [Google Scholar] [CrossRef]
- Avelino, A.; Cruz, F. Peptide Immunoreactivity and Ultrastructure of Rat Urinary Bladder Nerve Fibers After Topical Desensitization by Capsaicin or Resiniferatoxin. Auton. Neurosci. 2000, 86, 37–46. [Google Scholar] [CrossRef]
- Anand, P.; Bley, K. Topical Capsaicin for Pain Management: Therapeutic Potential and Mechanisms of Action of the New High-Concentration Capsaicin 8% Patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef]
- Chung, M.K.; Campbell, J.N. Use of Capsaicin to Treat Pain: Mechanistic and Therapeutic Considerations. Pharmaceuticals 2016, 9, 66. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Farb, D.H.; Leeman, S.E.; Jessell, T.M. Intrathecal Capsaicin Depletes Substance P in the Rat Spinal Cord and Produces Prolonged Thermal Analgesia. Science 1979, 206, 481–483. [Google Scholar] [CrossRef]
- Jancsó, G. Intracisternal Capsaicin—Selective Degeneration of Chemosensitive Primary Sensory Afferents in the Adult-Rat. Neurosci. Lett. 1981, 27, 41–45. [Google Scholar] [CrossRef]
- Gamse, R.; Jancsó, G.; Király, E. Intracisternal Capsaicin: A Novel Approach for Studying Nociceptive Sensory Neurons. In Neurogenic Inflammation and Antidromic Vasodilatation; Chahl, J., Szolcsanyi, J., Lembeck, F., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1984; pp. 93–110. [Google Scholar]
- Zhang, K.; Ramamurthy, S.; Prihoda, T.J.; Eckmann, M.S. Effect of Delayed Intrathecal Administration of Capsaicin on Neuropathic Pain Induced by Chronic Constriction Injury of the Sciatic Nerve in Rats. J. Pain Res. 2014, 7, 547–554. [Google Scholar] [CrossRef][Green Version]
- Leo, M.; Schulte, M.; Schmitt, L.I.; Schäfers, M.; Kleinschnitz, C.; Hagenacker, T. Intrathecal Resiniferatoxin Modulates TRPV1 in DRG Neurons and Reduces TNF-Induced Pain-Related Behavior. Mediat. Inflamm. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sapio, M.R.; Neubert, J.K.; Lapaglia, D.M.; Maric, D.; Keller, J.M.; Raithel, S.J.; Rohrs, E.L.; Anderson, E.M.; Butman, J.A.; Caudle, R.M.; et al. Pain Control Through Selective Chemo-Axotomy of Centrally Projecting TRPV1+ Sensory Neurons. J. Clin. Investig. 2018, 128, 1657–1670. [Google Scholar] [CrossRef]
- Brown, D.C.; Agnello, K.; Iadarola, M.J. Intrathecal Resiniferatoxin in a Dog Model: Efficacy in Bone Cancer Pain. Pain 2015, 156, 1018–1024. [Google Scholar] [CrossRef]
- Brown, D.C. Resiniferatoxin: The Evolution of the “Molecular Scalpel” for Chronic Pain Relief. Pharmaceuticals 2016, 9, 47. [Google Scholar] [CrossRef]
- Hayes, A.G.; Hawcock, A.B.; Hill, R.G. The Depolarising Action of Capsaicin on Rat Isolated Sciatic Nerve. Life Sci. 1984, 35, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Jancsó, G.; Such, G. Effects of Capsaicin Applied Perineurally to the Vagus Nerve on Cardiovascular and Respiratory Functions in the Cat. J. Physiol. 1983, 341, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Such, G.; Jancsó, G. Axonal Effects of Capsaicin—An Electrophysiological Study. Acta Physiol. Hung. 1986, 67, 53–63. [Google Scholar]
- Baranowski, R.; Lynn, B.; Pini, A. The Effects of Locally Applied Capsaicin on Conduction in Cutaneous Nerves in Four Mammalian Species. Br. J. Pharmacol. 1986, 89, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Petsche, U.; Fleischer, E.; Lembeck, F.; Handwerker, H.O. The Effect of Capsaicin Application to a Peripheral Nerve on Impulse Conduction in Functionally Identified Afferent Nerve Fibres. Brain Res. 1983, 265, 233–240. [Google Scholar] [CrossRef]
- Wall, P.D.; Fitzgerald, M. Effects of Capsaicin Applied Locally to Adult Peripheral Nerve. I. Physiology of Peripheral Nerve and Spinal Cord. Pain 1981, 11, 363–377. [Google Scholar] [CrossRef]
- Chung, J.M.; Lee, K.H.; Hori, Y.; Willis, W.D. Effects of Capsaicin Applied to a Peripheral Nerve on the Responses of Primate Spinothalamic Tract Cells. Brain Res. 1985, 329, 27–38. [Google Scholar] [CrossRef]
- Sann, H.; Jancsó, G.; Ambrus, A.; Pierau, F.K. Capsaicin Treatment Induces Selective Sensory Degeneration and Increased Sympathetic Innervation in the Rat Ureter. Neuroscience 1995, 67, 953–966. [Google Scholar] [CrossRef]
- Neubert, J.K.; Mannes, A.J.; Karai, L.J.; Jenkins, A.C.; Zawatski, L.; Abu-Asab, M.; Iadarola, M.J. Perineural Resiniferatoxin Selectively Inhibits Inflammatory Hyperalgesia. Mol. Pain 2008, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheepudomwit, T.; Güzelsu, E.; Zhou, C.; Griffin, J.W.; Höke, A. Comparison of Cytokine Expression Profile during Wallerian Degeneration of Myelinated and Unmyelinated Peripheral Axons. Neurosci. Lett. 2008, 430, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Mannion, R.J.; Doubell, T.P.; Coggeshall, R.E.; Woolf, C.J. Collateral Sprouting of Uninjured Primary Afferent A-Fibers into the Superficial Dorsal Horn of the Adult Rat Spinal Cord after Topical Capsaicin Treatment to the Sciatic Nerve. J. Neurosci. 1996, 16, 5189–5195. [Google Scholar] [CrossRef]
- Robertson, B.; Grant, G. Immunocytochemical Evidence for the Localization of the GM1 Ganglioside in Carbonic Anhydrase-Containing and RT 97-Immunoreactive Rat Primary Sensory Neurons. J. Neurocytol. 1989, 18, 77–86. [Google Scholar] [CrossRef]
- Woolf, C.J.; Shortland, P.; Coggeshall, R.E. Peripheral Nerve Injury Triggers Central Sprouting of Myelinated Afferents. Nature 1992, 355, 75–78. [Google Scholar] [CrossRef]
- Lekan, H.A.; Carlton, S.M.; Coggeshall, R.E. Sprouting of Aβ Fibers into Lamina II of the Rat Dorsal Horn in Peripheral Neuropathy. Neurosci. Lett. 1996, 208, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.P.; Tian, L. Cholera Toxin B Subunit Labeling in Lamina II of Spinal Cord Dorsal Horn Following Chronic Inflammation in Rats. Neurosci. Lett. 2002, 327, 161–164. [Google Scholar] [CrossRef]
- Bridges, D.; Thompson, S.W.; Rice, A.S. Mechanisms of Neuropathic Pain. Br. J. Anaesth. 2001, 87, 12–26. [Google Scholar] [CrossRef]
- Tong, Y.G.; Wang, H.F.; Ju, G.; Grant, G.; Hökfelt, T.; Zhang, X. Increased Uptake and Transport of Cholera Toxin B-Subunit in Dorsal Root Ganglion Neurons after Peripheral Axotomy: Possible Implications for Sensory Sprouting. J. Comp. Neurol. 1999, 404, 143–158. [Google Scholar] [CrossRef]
- Bao, L.; Wang, H.F.; Cai, H.J.; Tong, Y.G.; Jin, S.X.; Lu, Y.J.; Grant, G.; Hökfelt, T.; Zhang, X. Peripheral Axotomy Induces Only Very Limited Sprouting of Coarse Myelinated Afferents into Inner Lamina II of Rat Spinal Cord. Eur. J. Neurosci. 2002, 16, 175–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Liedtke, W.; Wang, F. Lack of Evidence for Ectopic Sprouting of Genetically Labeled Aβ Touch Afferents in Inflammatory and Neuropathic Trigeminal Pain. Mol. Pain 2015, 11, 1–9. [Google Scholar] [CrossRef]
- Hughes, D.I.; Scott, D.T.; Todd, A.J.; Riddell, J.S. Lack of Evidence for Sprouting of Abeta Afferents into the Superficial Laminas of the Spinal Cord Dorsal Horn After Nerve Section. J. Neurosci. 2003, 23, 9491–9499. [Google Scholar] [CrossRef]
- Sántha, P.; Jancsó, G. Capsaicin-Sensitive Primary Afferent Neurons Play an Essential Role in the Structural Reorganization of the Spinal Dorsal Horn Following Peripheral Nerve Injury. In Proceedings of the PAIN in Europe III, Nice, France, 26–29 September 2000. [Google Scholar]
- Sántha, P.; Jancsó, G. Transganglionic Transport of Choleragenoid by Capsaicin-Sensitive C-Fibre Afferents to the Substantia Gelatinosa of the Spinal Dorsal Horn After Peripheral Nerve Section. Neuroscience 2003, 116, 621–627. [Google Scholar] [CrossRef]
- Jancsó, G.; Sántha, P. Transganglionic Transport of Choleragenoid by Injured C Fibres to the Substantia Gelatinosa: Relevance to Neuropathic Pain and Hyperalgesia. In Hyperalgesia: Molecular Mechanisms and Clinical Implications; Brune, K., Handwerker, H.O., Eds.; Progress in Pain Research and Management; IASP Press: Seattle, WA, USA, 2004; Volume 1, pp. 143–156. [Google Scholar]
- Jancsó, G.; Sántha, P.; Szigeti, C.; Dux, M. Selective C-Fiber Deafferentation of the Spinal Dorsal Horn Prevents Lesion-Induced Transganglionic Transport of Choleragenoid to the Substantia Gelatinosa in the Rat. Neurosci. Lett. 2004, 361, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.M.; Paik, K.S.; Kim, J.S.; Nam, S.C.; Kim, K.J.; Oh, U.T.; Hasegawa, T.; Chung, K.; Willis, W.D. Chronic Effects of Topical Application of Capsaicin to the Sciatic Nerve on Responses of Primate Spinothalamic Neurons. Pain 1993, 53, 311–321. [Google Scholar] [CrossRef]
- Cuatrecasas, P. Gangliosides and Membrane Receptors for Cholera Toxin. Biochemistry 1973, 12, 3558–3566. [Google Scholar] [CrossRef]
- Stoeckel, K.; Schwab, M.; Thoenen, H. Role of Gangliosides in the Uptake and Retrograde Axonal Transport of Cholera and Tetanus Toxin as Compared to Nerve Growth Factor and Wheat Germ Agglutinin. Brain Res. 1977, 132, 273–285. [Google Scholar] [CrossRef]
- Nehr-Majoros, A.K.; Király, Á.; Helyes, Z.; Szőke, É. Lipid Raft Disruption as an Opportunity for Peripheral Analgesia. Curr. Opin. Pharmacol. 2024, 75, 102432. [Google Scholar] [CrossRef]
- Sántha, P.; Oszlács, O.; Dux, M.; Dobos, I.; Jancsó, G. Inhibition of Glucosylceramide Synthase Reversibly Decreases the Capsaicin-Induced Activation and TRPV1 Expression of Cultured Dorsal Root Ganglion Neurons. Pain 2010, 150, 103–112. [Google Scholar] [CrossRef]
- Sántha, P.; Dobos, I.; Kis, G.; Jancsó, G. Role of Gangliosides in Peripheral Pain Mechanisms. Int. J. Mol. Sci. 2020, 21, 1005. [Google Scholar] [CrossRef]
- Mutoh, T.; Tokuda, A.; Inokuchi, J.; Kuriyama, M. Glucosylceramide Synthase Inhibitor Inhibits the Action of Nerve Growth Factor in PC12 Cells. J.Biol. Chem. 1998, 273, 26001–26007. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Fukumoto, S.; Furukawa, K.; Ichimura, A.; Miyazaki, H.; Kusunoki, S.; Urano, T.; Furukawa, K. Overexpressed GM1 Suppresses Nerve Growth Factor (NGF) Signals by Modulating the Intracellular Localization of NGF Receptors and Membrane Fluidity in PC12 Cells. J. Biol. Chem. 2004, 279, 33368–33378. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, T.; Huang, Y. Insights into the Pathobiology of GM1 Gangliosidosis from Single-Nucleus Transcriptomic Analysis of CNS Cells in a Mouse Model. Int. J. Mol. Sci. 2024, 25, 9712. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Itokazu, Y.; Yu, R.K. GM1 Ganglioside Is Involved in Epigenetic Activation Loci of Neuronal Cells. Neurochem. Res. 2016, 41, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Gyányi, B.; Varga, M.; Szekers, A.; Jancsó, G.; Sántha, P. Nerve Injury-Induced Alterations of Ganglioside Levels in Rat Peripheral Nerves Assessed by HPLC-HRMS. In Proceedings of the Pain in Europe XIV, Lyon, France, 24–26 April 2025; p. 683. [Google Scholar]
- Frey, M.V. Untersuchungen Über Die Sinnesfunctionen Der Menschlichen Haut. In Erste Abhandlung: Drucke; Leipzig, S. Hirzel: Leipzig, Germany, 1896. [Google Scholar]
- Adrian, E.D. The Impulses Produced by Sensory Nerve-endings: Part 4. Impulses from Pain Receptors. J. Physiol. 1926, 62, 33–51. [Google Scholar] [CrossRef]
- Lynn, B. Somatosensory Receptors and Their CNS Connections. Annu. Rev. Physiol. 1975, 37, 105–127. [Google Scholar] [CrossRef]
- Burgess, P.R.; Perl, E.R. Cutaneous Mechanoreceptors and Nociceptors. In Somatosensory System; Springer: Berlin/Heidelberg, Germany, 1973; Volume 2, pp. 29–78. [Google Scholar] [CrossRef]
- Perl, E.R. Ideas About Pain, a Historical View. Nat. Rev. Neurosci. 2007, 8, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Julius, D. From Peppers to Peppermints: Insights into Thermosensation and Pain. Available online: https://www.nobelprize.org/prizes/medicine/2021/julius/lecture/ (accessed on 15 October 2025).
- Gatto, G.; Smith, K.M.; Ross, S.E.; Goulding, M. Neuronal Diversity in the Somatosensory System: Bridging the Gap Between Cell Type and Function. Curr. Opin. Neurobiol. 2019, 56, 167–174. [Google Scholar] [CrossRef]
- Messlinger, K. Functional Morphology of Nociceptive and Other Fine Sensory Endings (Free Nerve Endings) in Different Tissues. Prog. Brain Res. 1996, 113, 273–298. [Google Scholar] [CrossRef]
- Boros, K.; Jancsó, G.; Dux, M.; Fekécs, Z.; Bencsik, P.; Oszlács, O.; Katona, M.; Ferdinandy, P.; Nógrádi, A.; Sántha, P.; et al. Multiple Impairments of Cutaneous Nociceptor Function Induced by Cardiotoxic Doses of Adriamycin in the Rat. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 1009–1020. [Google Scholar] [CrossRef]
- Axelsson, H.E.; Minde, J.K.; Sonesson, A.; Toolanen, G.; Högestätt, E.D.; Zygmunt, P.M. Transient Receptor Potential Vanilloid 1, Vanilloid 2 and Melastatin 8 Immunoreactive Nerve Fibers in Human Skin from Individuals with and Without Norrbottnian Congenital Insensitivity to Pain. Neuroscience 2009, 162, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Morbin, M.; Lombardi, R.; Capobianco, R.; Camozzi, F.; Pareyson, D.; Manconi, M.; Geppetti, P. Expression of Capsaicin Receptor Immunoreactivity in Human Peripheral Nervous System and in Painful Neuropathies. J. Peripher. Nerv. Syst. 2006, 11, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Klein, C.M.; Coggeshall, R.E. The Receptive Part of the Primary Afferent Axon Is Most Vulnerable to Systemic Capsaicin in Adult Rats. Brain Res. 1990, 511, 222–226. [Google Scholar] [CrossRef]
- Duggan, A.W.; Furmidge, L.J. Probing the Brain and Spinal Cord with Neuropeptides in Pathways Related to Pain and Other Functions. Front. Neuroendocrinol. 1994, 15, 275–300. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin Gene-Related Peptide: Physiology and Pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The Role of Calcitonin Gene-Related Peptide in Peripheral and Central Pain Mechanisms Including Migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef]
- Király, E.; Joó, F.; Jancsó, G. Changes in Fibre Populations of Peripheral Nerves after Capsaicin Treatment of Newborn and Adult Rats. Verh. Anat. Ges. 1986, 80, 269–270. [Google Scholar]
- Isensee, J.; Wenzel, C.; Buschow, R.; Weissmann, R.; Kuss, A.W.; Hucho, T. Subgroup-Elimination Transcriptomics Identifies Signaling Proteins That Define Subclasses of TRPV1-Positive Neurons and a Novel Paracrine Circuit. PLoS ONE 2014, 9, e115731. [Google Scholar] [CrossRef] [PubMed]
- Waddell, P.J.; Lawson, S.N. The C-Fibre Conduction Block Caused by Capsaicin on Rat Vagus Nerve In Vitro. Pain 1989, 39, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Heyman, I.; Rang, H.P. Depolarizing Responses to Capsaicin in a Subpopulation of Rat Dorsal Root Ganglion Cells. Neurosci. Lett. 1985, 56, 69–75. [Google Scholar] [CrossRef]
- Anand, U.; Otto, W.R.; Facer, P.; Zebda, N.; Selmer, I.; Gunthorpe, M.J.; Chessell, I.P.; Sinisi, M.; Birch, R.; Anand, P. TRPA1 Receptor Localisation in the Human Peripheral Nervous System and Functional Studies in Cultured Human and Rat Sensory Neurons. Neurosci. Lett. 2008, 438, 221–227. [Google Scholar] [CrossRef]
- Bernardini, N.; Neuhuber, W.; Reeh, P.W.; Sauer, S.K. Morphological Evidence for Functional Capsaicin Receptor Expression and Calcitonin Gene-Related Peptide Exocytosis in Isolated Peripheral Nerve Axons of the Mouse. Neuroscience 2004, 126, 585–590. [Google Scholar] [CrossRef]
- Guo, A.; Vulchanova, L.; Wang, J.; Li, X.; Elde, R. Immunocytochemical Localization of the Vanilloid Receptor 1 (VR1): Relationship to Neuropeptides, the P2X(3) Purinoceptor and IB4 Binding Sites. Eur. J. Neurosci. 1999, 11, 946–958. [Google Scholar] [CrossRef]
- Szallasi, A.; Nilsson, S.; Farkas-Szallasi, T.; Blumberg, P.M.; Hökfelt, T.; Lundberg, J.M. Vanilloid (Capsaicin) Receptors in the Rat: Distribution in the Brain, Regional Differences in the Spinal Cord, Axonal Transport to the Periphery, and Depletion by Systemic Vanilloid Treatment. Brain Res. 1995, 703, 175–183. [Google Scholar] [CrossRef]
- Devesa, I.; Ferrándiz-Huertas, C.; Mathivanan, S.; Wolf, C.; Luján, R.; Changeux, J.P.; Ferrer-Montiel, A. ACGRP Is Essential for Algesic Exocytotic Mobilization of TRPV1 Channels in Peptidergic Nociceptors. Proc. Natl. Acad. Sci. USA 2014, 111, 18345–18350. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.M.; Reeh, P.W. Sensitization to Heat through G-Protein-Coupled Receptor Pathways in the Isolated Sciatic Mouse Nerve. Eur. J. Neurosci. 2007, 25, 3570–3575. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.K.; Reeh, P.W. Inflammation and Hypersensitivity in the Context of the Sensory Functions of Axonal Membranes: What Are the Molecular Mechanisms? Dig. Dis. 2009, 27, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.P.; Yang, K.; Li, Y.Q. Activation of Capsaicin Receptors on the Sciatic Nerve Induces FOS Expression in the Spinal Dorsal Horn of Adult Rats. Neurosignals 2002, 11, 151–157. [Google Scholar] [CrossRef]
- Palermo, N.N.; Brown, H.K.; Smith, D.L. Selective Neurotoxic Action of Capsaicin on Glomerular C-Type Terminals in Rat Substantia Gelatinosa. Brain Res. 1981, 208, 506–510. [Google Scholar] [CrossRef]
- Acs, G.; Palkovits, M.; Blumberg, P.M. Comparison of [3H]Resiniferatoxin Binding by the Vanilloid (Capsaicin) Receptor in Dorsal Root Ganglia, Spinal Cord, Dorsal Vagal Complex, Sciatic and Vagal Nerve and Urinary Bladder of the Rat. Life Sci. 1994, 55, 1017–1026. [Google Scholar] [CrossRef]
- Hoffmann, T.; Sauer, S.K.; Horch, R.E.; Reeh, P.W. Sensory Transduction in Peripheral Nerve Axons Elicits Ectopic Action Potentials. J. Neurosci. 2008, 28, 6281–6284. [Google Scholar] [CrossRef]
- Hoffmann, T.; Sauer, S.K.; Horch, R.E.; Reeh, P.W. Projected Pain from Noxious Heat Stimulation of an Exposed Peripheral Nerve—A Case Report. Eur. J. Pain 2009, 13, 35–37. [Google Scholar] [CrossRef]
- Takayama, Y. Interaction between Thermosensitive TRP Channels and Anoctamin 1. J. Physiol. Sci. 2025, 75, 35–37. [Google Scholar] [CrossRef]
- Cho, H.; Yang, Y.D.; Lee, J.; Lee, B.; Kim, T.; Jang, Y.; Back, S.K.; Na, H.S.; Harfe, B.D.; Wang, F.; et al. The Calcium-Activated Chloride Channel Anoctamin 1 Acts as a Heat Sensor in Nociceptive Neurons. Nat. Neurosci. 2012, 15, 1015–1021. [Google Scholar] [CrossRef]
- Kimourtzis, G.; Rangwani, N.; Jenkins, B.J.; Jani, S.; McNaughton, P.A.; Raouf, R. Prostaglandin E2 Depolarises Sensory Axons In Vitro in an ANO1 and Nav1.8 Dependent Manner. Sci. Rep. 2024, 14, 17360. [Google Scholar] [CrossRef]
- Catalano, M.; Landini, L.; Nozzoli, F.; Nassini, R.; Roviello, G.; De Logu, F. Unraveling the Role of Perineural Invasion in Cancer-Associated Pain: Insights and Treatment Strategies. Curr. Res. Biotechnol. 2025, 10, 100305. [Google Scholar] [CrossRef]
- Szallasi, A. Targeting TRPV1 for Cancer Pain Relief: Can It Work? Cancers 2024, 16, 648. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Wu, F.; Qian, J.; Ochiai, Y.; Lian, G.; Malagola, E.; Zheng, B.; Tu, R.; Zeng, Y.; Kobayashi, H.; et al. Nociceptive Neurons Promote Gastric Tumour Progression via a CGRP-RAMP1 Axis. Nature 2025, 640, 802–810. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jancsó, G.; Dux, M.; Sántha, P. Morphological Correlates of TRPV1 Agonist-Induced Activation and Defunctionalization of Nociceptor Neurons. Int. J. Mol. Sci. 2025, 26, 10350. https://doi.org/10.3390/ijms262110350
Jancsó G, Dux M, Sántha P. Morphological Correlates of TRPV1 Agonist-Induced Activation and Defunctionalization of Nociceptor Neurons. International Journal of Molecular Sciences. 2025; 26(21):10350. https://doi.org/10.3390/ijms262110350
Chicago/Turabian StyleJancsó, Gábor, Mária Dux, and Péter Sántha. 2025. "Morphological Correlates of TRPV1 Agonist-Induced Activation and Defunctionalization of Nociceptor Neurons" International Journal of Molecular Sciences 26, no. 21: 10350. https://doi.org/10.3390/ijms262110350
APA StyleJancsó, G., Dux, M., & Sántha, P. (2025). Morphological Correlates of TRPV1 Agonist-Induced Activation and Defunctionalization of Nociceptor Neurons. International Journal of Molecular Sciences, 26(21), 10350. https://doi.org/10.3390/ijms262110350






