The Cytoskeleton in Adrenal Physiology and Tumours: Functional Roles and Emerging Molecular Targets
Abstract
1. Introduction
2. Role of the Cytoskeleton in Steroidogenesis and Adrenocortical Tumourigenesis
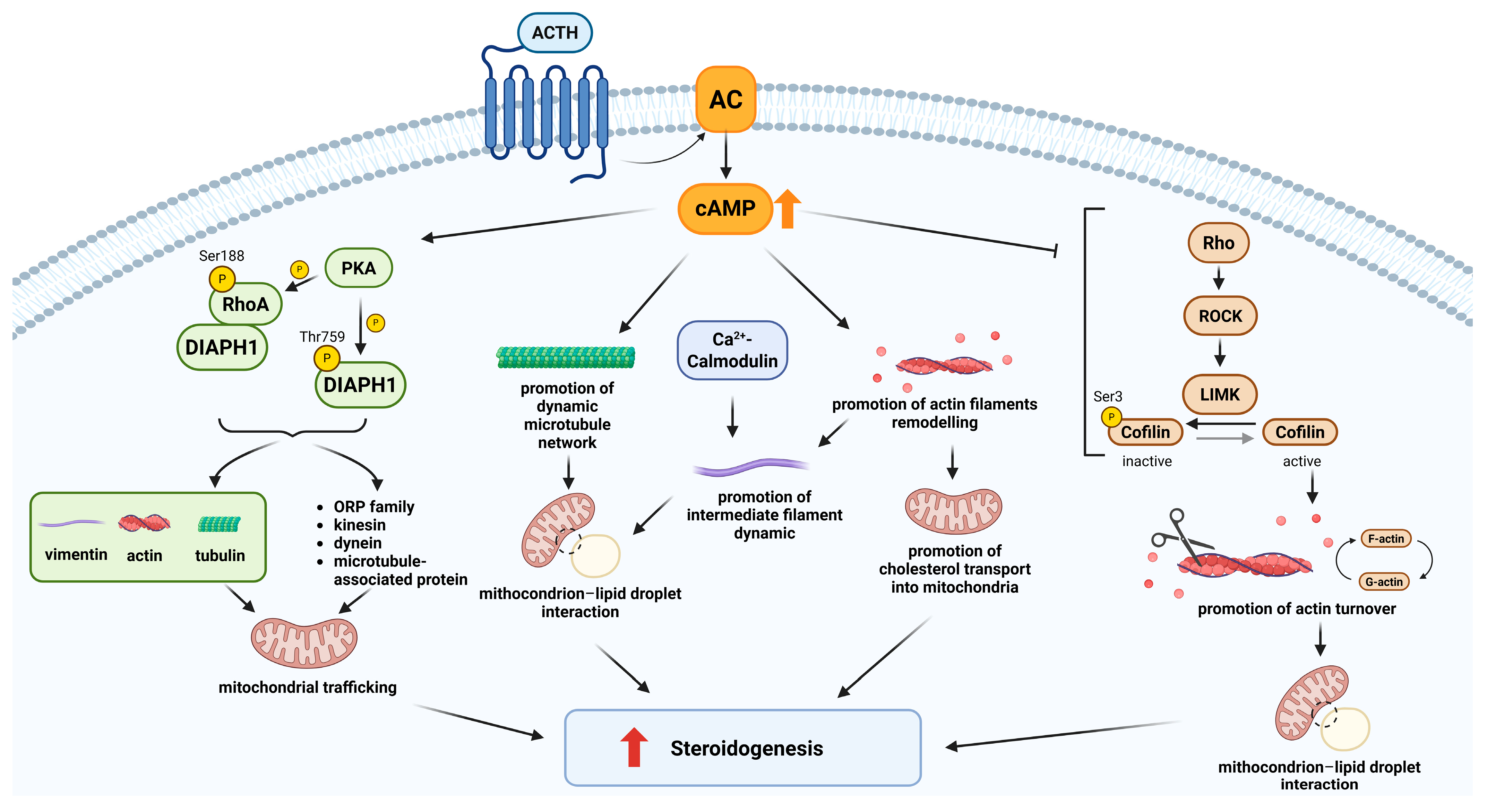
2.1. Cytoskeletal Regulation of Steroidogenesis in the Adrenal Cortex
2.2. Cytoskeleton Regulation of Oncogenic Signalling in Adrenocortical Carcinoma
2.3. Implications of Cytoskeletal Remodelling in the Aggressiveness of Adrenocortical Carcinomas
2.4. Therapeutic Approaches Targeting Cytoskeletal Functions: From Steroidogenesis to Tumour Aggressiveness
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DIAPH1 | Diaphanous-related formin 1 |
| ACC | Adrenocortical carcinoma |
| FLNA | Filamin A |
| FSCN1 | Fascin-1 |
| RASSF1A | Ras association domain family 1, isoform A |
| VAV2 | Vav guanine nucleotide exchange factor 2 |
| ACA | Adrenocortical adenoma |
| ACTH | Adrenocorticotropic hormone |
| cAMP | Cyclic AMP |
| PKA | Protein kinase A |
| CARS | Coherent Anti-Stokes Raman Scattering |
| LD | Lipid droplet |
| ATP | Adenosine triphosphate |
| RhoA | Ras homolog family member A |
| ROCK | Rho-associated kinase |
| LIMK | LIM kinase |
| CPA | Cortisol-producing adenoma |
| IGF | Insulin-like growth factor |
| NAG | Normal adrenal gland |
| IGF1R | Insulin-like growth factor 1 receptor |
| IRA | Insulin receptor isoform A |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphatidylinositol-3-kinase |
| SF-1 | Steroidogenic factor 1 |
| FAK | Focal adhesion kinase |
| DFS | Disease-free survival |
| OS | Overall survival |
| DNMT | DNA methyltransferase |
References
- Fletcher, D.; Mullins, R. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E. Structural insights into microtubule function. Annu. Rev. Biochem. 2000, 69, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Henzen-Logmans, S.C.; Stel, H.V.; van Muijen, G.N.; Mullink, H.; Meijer, C.J. Expression of intermediate filament proteins in adrenal cortex and related tumours. Histopathology 1988, 12, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Goldman, R.D. Overview of the Cytoskeleton from an Evolutionary Perspective. Cold Spring Harb. Perspect. Biol. 2018, 10, a030288. [Google Scholar] [CrossRef]
- Pimm, M.L.; Henty-Ridilla, J.L. New twists in actin-microtubule interactions. Mol. Biol. Cell 2021, 32, 211–217. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielińska, W.; Hałas-Wiśniewska, M.; Grzanka, A. Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells 2020, 9, 2245. [Google Scholar] [CrossRef]
- Patani, A.; Balram, D.; Yadav, V.K.; Lian, K.Y.; Patel, A.; Sahoo, D.K. Harnessing the power of nutritional antioxidants against adrenal hormone imbalance-associated oxidative stress. Front. Endocrinol. 2023, 14, 1271521. [Google Scholar] [CrossRef]
- Gallo-Payet, N. 60 YEARS OF POMC: Adrenal and extra-adrenal functions of ACTH. J. Mol. Endocrinol. 2016, 56, T135–T156. [Google Scholar] [CrossRef]
- Mete, O.; Erickson, L.A.; Juhlin, C.C.; de Krijger, R.R.; Sasano, H.; Volante, M.; Papotti, M.G. Overview of the 2022 WHO Classification of Adrenal Cortical Tumours. Endocr. Pathol. 2022, 33, 155–196. [Google Scholar] [CrossRef]
- Vaduva, P.; Bonnet, F.; Bertherat, J. Molecular Basis of Primary Aldosteronism and Adrenal Cushing Syndrome. J. Endocr. Soc. 2020, 4, bvaa075. [Google Scholar] [CrossRef]
- Fassnacht, M.; Puglisi, S.; Kimpel, O.; Terzolo, M. Adrenocortical carcinoma: A practical guide for clinicians. Lancet Diabetes Endocrinol. 2025, 13, 438–452. [Google Scholar] [CrossRef]
- Temple, R.; Wolff, J. Stimulation of steroid secretion by antimicrotubular agents. J. Biol. Chem. 1973, 248, 2691–2698. [Google Scholar] [CrossRef]
- Hall, P.F.; Charpponnier, C.; Nakamura, M.; Gabbiani, G. The role of microfilaments in the response of adrenal tumour cells to adrenocorticotropic hormone. J. Biol. Chem. 1979, 254, 9080–9084. [Google Scholar] [CrossRef]
- Mrotek, J.J.; Hall, P.F. Response of adrenal tumour cells to adrenocorticotropin: Site of inhibition by cytochalasin B. Biochemistry 1977, 16, 3177–3181. [Google Scholar] [CrossRef]
- Osawa, S.; Betz, G.; Hall, P.F. Role of actin in the responses of adrenal cells to ACTH and cyclic AMP: Inhibition by DNase I. J. Cell Biol. 1984, 99 Pt 1, 1335–1342. [Google Scholar] [CrossRef]
- Benis, R.; Mattson, P. Microtubules, organelle transport, and steroidogenesis in cultured adrenocortical tumour cells. 1. An ultrastructural analysis of cells in which basal and ACTH-induced steroidogenesis was inhibited by taxol. Tissue Cell 1989, 21, 479–494. [Google Scholar] [CrossRef]
- Li, D.; Sewer, M.B. RhoA and DIAPH1 mediate adrenocorticotropin-stimulated cortisol biosynthesis by regulating mitochondrial trafficking. Endocrinology 2010, 151, 4313–4323. [Google Scholar] [CrossRef] [PubMed]
- Almahbobi, G.; Hall, P.F. The role of intermediate filaments in adrenal steroidogenesis. J. Cell Sci. 1990, 97 Pt 4, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Peverelli, E.; Catalano, R.; Giardino, E.; Treppiedi, D.; Morelli, V.; Ronchi, C.L.; Vaczlavik, A.; Fusco, N.; Ferrero, S.; Bertherat, J.; et al. Cofilin is a cAMP effector in mediating actin cytoskeleton reorganization and steroidogenesis in mouse and human adrenocortical tumour cells. Cancer Lett. 2017, 406, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Catalano, R.; Giardino, E.; Treppiedi, D.; Mangili, F.; Morelli, V.; Elli, F.M.; Serban, A.L.; Luconi, M.; Mannelli, M.; Spada, A.; et al. The cytoskeleton actin binding protein filamin A impairs both IGF2 mitogenic effects and the efficacy of IGF1R inhibitors in adrenocortical cancer cells. Cancer Lett. 2021, 497, 77–88. [Google Scholar] [CrossRef]
- Catalano, R.; Altieri, B.; Angelousi, A.; Arosio, M.; Bravi, F.; Canu, L.; Croci, G.A.; Detomas, M.; Esposito, E.; Ferrante, E.; et al. High Filamin A expression in adrenocortical carcinomas is associated with a favourable tumour behaviour: A European multicentric study. Int. J. Mol. Sci. 2023, 24, 16573. [Google Scholar] [CrossRef]
- Esposito, E.; Marra, G.; Catalano, R.; Maioli, S.; Nozza, E.; Barbieri, A.M.; Hantel, C.; Di Dalmazi, G.; Sigala, S.; Geginat, J.; et al. Therapeutic potential of targeting the FLNA-regulated Wee1 kinase in adrenocortical carcinomas. Int. J. Cancer 2025, 156, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Doghman-Bouguerra, M.; Sbiera, S.; Sbiera, I.; Parsons, M.; Ragazzon, B.; Morin, A.; Robidel, E.; Favier, J.; Bertherat, J.; et al. Dosage-dependent regulation of VAV2 expression by steroidogenic factor-1 drives adrenocortical carcinoma cell invasion. Sci. Signal. 2017, 10, eaal2464. [Google Scholar] [CrossRef]
- Sbiera, S.; Sbiera, I.; Ruggiero, C.; Doghman-Bouguerra, M.; Korpershoek, E.; de Krijger, R.R.; Ettaieb, H.; Haak, H.; Volante, M.; Papotti, M.; et al. Assessment of VAV2 expression refines prognostic prediction in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 3491–3498. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Ruggiero, C.; Cantini, G.; Canu, L.; Baroni, G.; Armignacco, R.; Jouinot, A.; Santi, R.; Ercolino, T.; Ragazzon, B.; et al. Fascin-1 Is a Novel Prognostic Biomarker Associated with Tumour Invasiveness in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2019, 104, 1712–1724. [Google Scholar] [CrossRef]
- Ruggiero, C.; Tamburello, M.; Rossini, E.; Zini, S.; Durand, N.; Cantini, G.; Cioppi, F.; Hantel, C.; Kiseljak-Vassiliades, K.; Wierman, M.E.; et al. FSCN1 as a new druggable target in adrenocortical carcinoma. Int. J. Cancer 2023, 153, 210–223. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Z.; Wei, X.; Zhou, L.; Tang, Y.; Zhou, C.; Wu, K.; Zhang, F.; Zhang, F.; Lu, Y.; et al. Expression of FSCN1 and FOXM1 are associated with poor prognosis of adrenocortical carcinoma patients. BMC Cancer 2019, 19, 1165. [Google Scholar] [CrossRef] [PubMed]
- Korah, R.; Healy, J.M.; Kunstman, J.W.; Fonseca, A.L.; Ameri, A.H.; Prasad, M.L.; Carling, T. Epigenetic silencing of RASSF1A deregulates cytoskeleton and promotes malignant behavior of adrenocortical carcinoma. Mol. Cancer 2013, 12, 87. [Google Scholar] [CrossRef]
- Crivello, J.F.; Jefcoate, C.R. Mechanisms of corticotropin action in rat adrenal cells. I. The effects of inhibitors of protein synthesis and of microfilament formation on corticosterone synthesis. Biochim. Biophys. Acta 1978, 542, 315–329. [Google Scholar] [CrossRef]
- Cortese, F.; Wolf, J. Cytochalasin-stimulated steroidogenesis from high density lipoproteins. J. Cell Biol. 1978, 77, 507–516. [Google Scholar] [CrossRef]
- Nan, X.; Potma, E.O.; Xie, X.S. Nonperturbative chemical imaging of organelle transport in living cells with coherent anti-stokes Raman scattering microscopy. Biophys. J. 2006, 91, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Chen, J.S.; Ko, T.L.; Wang, S.M. Mechanism of colchicine-induced steroidogenesis in rat adrenocortical cells. J. Cell. Biochem. 2001, 81, 162–171. [Google Scholar] [CrossRef]
- Almahbobi, G.; Williams, L.J.; Hall, P.F. Attachment of steroidogenic lipid droplets to intermediate filaments in adrenal cells. J. Cell Sci. 1992, 101 Pt 2, 383–393. [Google Scholar] [CrossRef]
- Hall, P.F. The roles of calmodulin, actin, and vimentin in steroid synthesis by adrenal cells. Steroids 1997, 62, 185–189. [Google Scholar] [CrossRef]
- Li, D.; Dammer, E.B.; Lucki, N.C.; Sewer, M.B. cAMP-stimulated phosphorylation of diaphanous 1 regulates protein stability and interaction with binding partners in adrenocortical cells. Mol. Biol. Cell 2013, 24, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Relav, L.; Doghman-Bouguerra, M.; Ruggiero, C.; Muzzi, J.C.D.; Figueiredo, B.C.; Lalli, E. Steroidogenic factor 1, a Goldilocks transcription factor from adrenocortical organogenesis to malignancy. Int. J. Mol. Sci. 2023, 24, 3585. [Google Scholar] [CrossRef]
- Whitehouse, B.J.; Gyles, S.L.; Squires, P.E.; Sayed, S.B.; Burns, C.J.; Persaud, S.J.; Jones, P.M. Interdependence of steroidogenesis and shape changes in Y1 adrenocortical cells: Studies with inhibitors of phosphoprotein phosphatases. J. Endocrinol. 2002, 172, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, F.B.; Khor, V.K.; Shen, W.J.; Azhar, S. Cholesterol ester droplets and steroidogenesis. Mol. Cell. Endocrinol. 2013, 371, 15–19. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Moraczewska, J. Cofilin—A protein controlling dynamics of actin filaments. Postępy Hig. Med. Dośw. 2017, 71, 339–351. [Google Scholar] [CrossRef]
- Boulle, N.; Logié, A.; Gicquel, C.; Perin, L.; Le Bouc, Y. Increased levels of insulin-like growth factor II (IGF-II) and IGF-binding protein-2 are associated with malignancy in sporadic adrenocortical tumours. J. Clin. Endocrinol. Metab. 1998, 83, 1713–1720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giordano, T.J.; Thomas, D.G.; Kuick, R.; Lizyness, M.; Misek, D.E.; Smith, A.L.; Sanders, D.; Aljundi, R.T.; Gauger, P.G.; Thompson, N.W.; et al. Distinct transcriptional profiles of adrenocortical tumours uncovered by DNA microarray analysis. Am. J. Pathol. 2003, 162, 521–531. [Google Scholar] [CrossRef]
- Almeida, M.Q.; Fragoso, M.C.; Lotfi, C.F.; Santos, M.G.; Nishi, M.Y.; Costa, M.H.; Lerario, A.M.; Maciel, C.C.; Mattos, G.E.; Jorge, A.A.; et al. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumours. J. Clin. Endocrinol. Metab. 2008, 93, 3524–3531. [Google Scholar] [CrossRef]
- De Martino, M.C.; van Koetsveld, P.M.; Feelders, R.A.; de Herder, W.W.; Dogan, F.; Janssen, J.A.M.J.L.; Hofste Op Bruinink, D.; Pivonello, C.; Waaijers, A.M.; Colao, A.; et al. IGF and mTOR pathway expression and in vitro effects of linsitinib and mTOR inhibitors in adrenocortical cancer. Endocrine 2019, 64, 673–684. [Google Scholar] [CrossRef]
- Blyth, A.J.; Kirk, N.S.; Forbes, B.E. Understanding IGF-II Action through Insights into Receptor Binding and Activation. Cells 2020, 9, 2276. [Google Scholar] [CrossRef]
- Treppiedi, D.; Catalano, R.; Mangili, F.; Mantovani, G.; Peverelli, E. Role of filamin A in the pathogenesis of neuroendocrine tumours and adrenal cancer. Endocr. Oncol. 2022, 2, R143–R152. [Google Scholar] [CrossRef] [PubMed]
- Stossel, T.P.; Condeelis, J.; Cooley, L.; Hartwig, J.H.; Noegel, A.; Schleicher, M.; Shapiro, S.S. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2001, 2, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Stossel, T.P.; Hartwig, J.H. The filamins: Organizers of cell structure and function. Cell Adh. Migr. 2011, 5, 160–169. [Google Scholar] [CrossRef]
- Lian, G.; Lu, J.; Hu, J.; Zhang, J.; Cross, S.H.; Ferland, R.J.; Sheen, V.L. Filamin A regulates neural progenitor proliferation and cortical size through Wee1-dependent Cdk1 phosphorylation. J. Neurosci. 2012, 32, 7672–7684. [Google Scholar] [CrossRef]
- Ghelli Luserna di Rorà, A.; Cerchione, C.; Martinelli, G.; Simonetti, G. A WEE1 family business: Regulation of mitosis, cancer progression, and therapeutic target. J. Hematol. Oncol. 2020, 13, 126. [Google Scholar] [CrossRef]
- Doghman, M.; Lalli, E. A matter of dosage: SF-1 in adrenocortical development and cancer. Ann. Endocrinol. 2009, 70, 148–152. [Google Scholar] [CrossRef]
- Doghman, M.; Karpova, T.; Rodrigues, G.A.; Arhatte, M.; De Moura, J.; Cavalli, L.R.; Virolle, V.; Barbry, P.; Zambetti, G.P.; Figueiredo, B.C.; et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol. Endocrinol. 2007, 21, 2968–2987. [Google Scholar] [CrossRef]
- Sbiera, S.; Schmull, S.; Assie, G.; Voelker, H.U.; Kraus, L.; Beyer, M.; Ragazzon, B.; Beuschlein, F.; Willenberg, H.S.; Hahner, S.; et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumours. J. Clin. Endocrinol. Metab. 2010, 95, E161–E171. [Google Scholar] [CrossRef]
- Ruggiero, C.; Lalli, E. VAV2: A novel prognostic marker and a druggable target for adrenocortical carcinoma. Oncotarget 2017, 8, 88257–88258. [Google Scholar] [CrossRef]
- Tan, V.Y.; Lewis, S.J.; Adams, J.C.; Martin, R.M. Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: A systematic review and meta-analysis. BMC Med. 2013, 11, 52. [Google Scholar] [CrossRef]
- Sarantelli, E.; Mourkakis, A.; Zacharia, L.C.; Stylianou, A.; Gkretsi, V. Fascin-1 in Cancer Cell Metastasis: Old Target-New Insights. Int. J. Mol. Sci. 2023, 24, 11253. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kim, D.J.; Adams, J.C. The roles of fascins in health and disease. J. Pathol. 2011, 224, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.Q.; Zhang, T.P.; Zhao, W.J.; Liu, Z.W.; You, L.; Zhou, L.; Guo, J.C.; Zhao, Y.P. Filamin A: Insights into its exact role in cancers. Pathol. Oncol. Res. 2016, 22, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lalli, E.; Luconi, M. The next step: Mechanisms driving adrenocortical carcinoma metastasis. Endocr. Relat. Cancer 2018, 25, R31–R48. [Google Scholar] [CrossRef] [PubMed]
- Cantini, G.; Fei, L.; Canu, L.; De Filpo, G.; Ercolino, T.; Nesi, G.; Mannelli, M.; Luconi, M. Circulating Fascin 1 as a promising prognostic marker in adrenocortical cancer. Front. Endocrinol. 2021, 12, 698862. [Google Scholar] [CrossRef]
- Esposito, F.; Giuffrida, R.; Raciti, G.; Puglisi, C.; Forte, S. Wee1 kinase: A potential target to overcome tumour resistance to therapy. Int. J. Mol. Sci. 2021, 22, 10689. [Google Scholar] [CrossRef]
- Montalvo-Ortiz, B.L.; Castillo-Pichardo, L.; Hernández, E.; Humphries-Bickley, T.; De la Mota-Peynado, A.; Cubano, L.A.; Vlaar, C.P.; Dharmawardhane, S. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J. Biol. Chem. 2012, 287, 13228–13238. [Google Scholar] [CrossRef] [PubMed]
- Lalli, E.; Doghman, M.; Latre de Late, P.; El Wakil, A.; Mus-Veteau, I. Beyond steroidogenesis: Novel target genes for SF-1 discovered by genomics. Mol. Cell. Endocrinol. 2013, 371, 154–159. [Google Scholar] [CrossRef]
- Ruggiero, C.; Lalli, E. Targeting the cytoskeleton against metastatic dissemination. Cancer Metast. Rev. 2021, 40, 89–140. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Chan, M.H.; Liang, S.M.; Chang, Y.C.; Hsiao, M. Fascin-1: Updated biological functions and therapeutic implications in cancer biology. BBA Adv. 2022, 2, 100052. [Google Scholar] [CrossRef]
- Zeinali, T.; Mansoori, B.; Mohammadi, A.; Baradaran, B. Regulatory mechanisms of miR-145 expression and the importance of its function in cancer metastasis. Biomed. Pharmacother. 2019, 109, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Lee, T.C.; Cheung, K.F.; Fan, D.S.; Lo, K.W.; Beaverson, K.L.; Abramson, D.H.; Lam, D.S.; Yu, C.B.; Pang, C.P. Clinical implications of promoter hypermethylation in RASSF1A and MGMT in retinoblastoma. Neoplasia 2005, 7, 200–206. [Google Scholar] [CrossRef]
- Shen, W.J.; Dai, D.Q.; Teng, Y.; Liu, H.B. Regulation of demethylation and re-expression of RASSF1A gene in gastric cancer cell lines by combined treatment of 5-Aza-CdR and NaB. World J. Gastroenterol. 2008, 14, 595–600. [Google Scholar] [CrossRef]


| Protein | Level of Expression | Role | Clinical Significance | References |
|---|---|---|---|---|
| Actin filaments | / |
| Modulation of steroidogenesis | [14,15,16] |
| Microtubules | / |
| Modulation of steroidogenesis | [17,18,32,33] |
| Intermediate filaments | / |
| Modulation of steroidogenesis | [19,34,35] |
| DIAPH1 | / |
| Modulation of steroidogenesis | [18,36] |
| Cofilin | Upregulated in cortisol-producing adenomas |
| Modulation of steroidogenesis | [20] |
| FLNA | Downregulated in ACC |
| Anti-tumourigenic | [21,22,23] |
| VAV2 | Upregulated in ACC |
| Pro-tumourigenic and metastatic | [24,25,37] |
| FSCN1 | Upregulated in ACC |
| Pro-tumourigenic and metastatic | [26,27,28] |
| RASSF1A | Downregulated in ACC |
| Anti-tumourigenic | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, R.; Nozza, E.; Esposito, E.; Di Bari, S.; Mantovani, G.; Peverelli, E. The Cytoskeleton in Adrenal Physiology and Tumours: Functional Roles and Emerging Molecular Targets. Int. J. Mol. Sci. 2025, 26, 10348. https://doi.org/10.3390/ijms262110348
Catalano R, Nozza E, Esposito E, Di Bari S, Mantovani G, Peverelli E. The Cytoskeleton in Adrenal Physiology and Tumours: Functional Roles and Emerging Molecular Targets. International Journal of Molecular Sciences. 2025; 26(21):10348. https://doi.org/10.3390/ijms262110348
Chicago/Turabian StyleCatalano, Rosa, Emma Nozza, Emanuela Esposito, Sonia Di Bari, Giovanna Mantovani, and Erika Peverelli. 2025. "The Cytoskeleton in Adrenal Physiology and Tumours: Functional Roles and Emerging Molecular Targets" International Journal of Molecular Sciences 26, no. 21: 10348. https://doi.org/10.3390/ijms262110348
APA StyleCatalano, R., Nozza, E., Esposito, E., Di Bari, S., Mantovani, G., & Peverelli, E. (2025). The Cytoskeleton in Adrenal Physiology and Tumours: Functional Roles and Emerging Molecular Targets. International Journal of Molecular Sciences, 26(21), 10348. https://doi.org/10.3390/ijms262110348






