ZEB1 and Uveal Melanoma Invasiveness
Abstract
1. Introduction
2. Results
2.1. UM Tumor Sample Classification
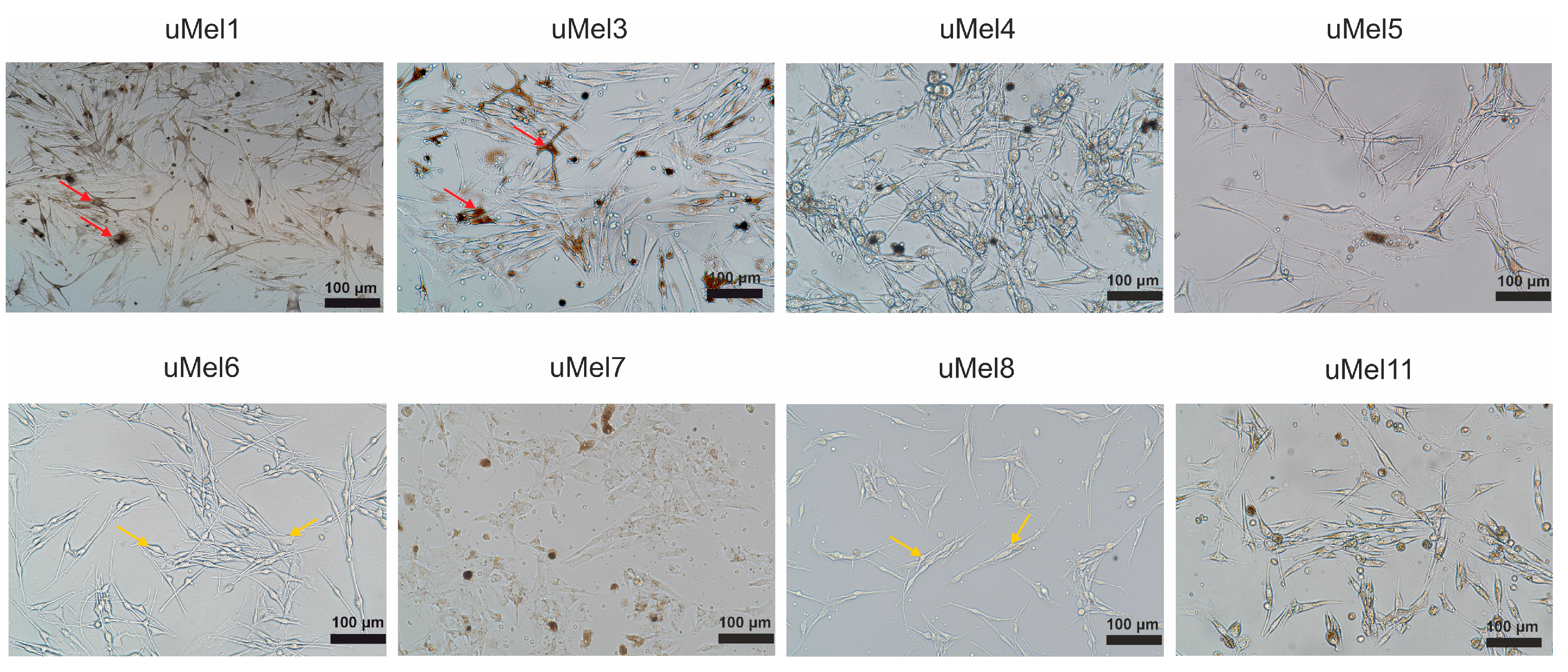
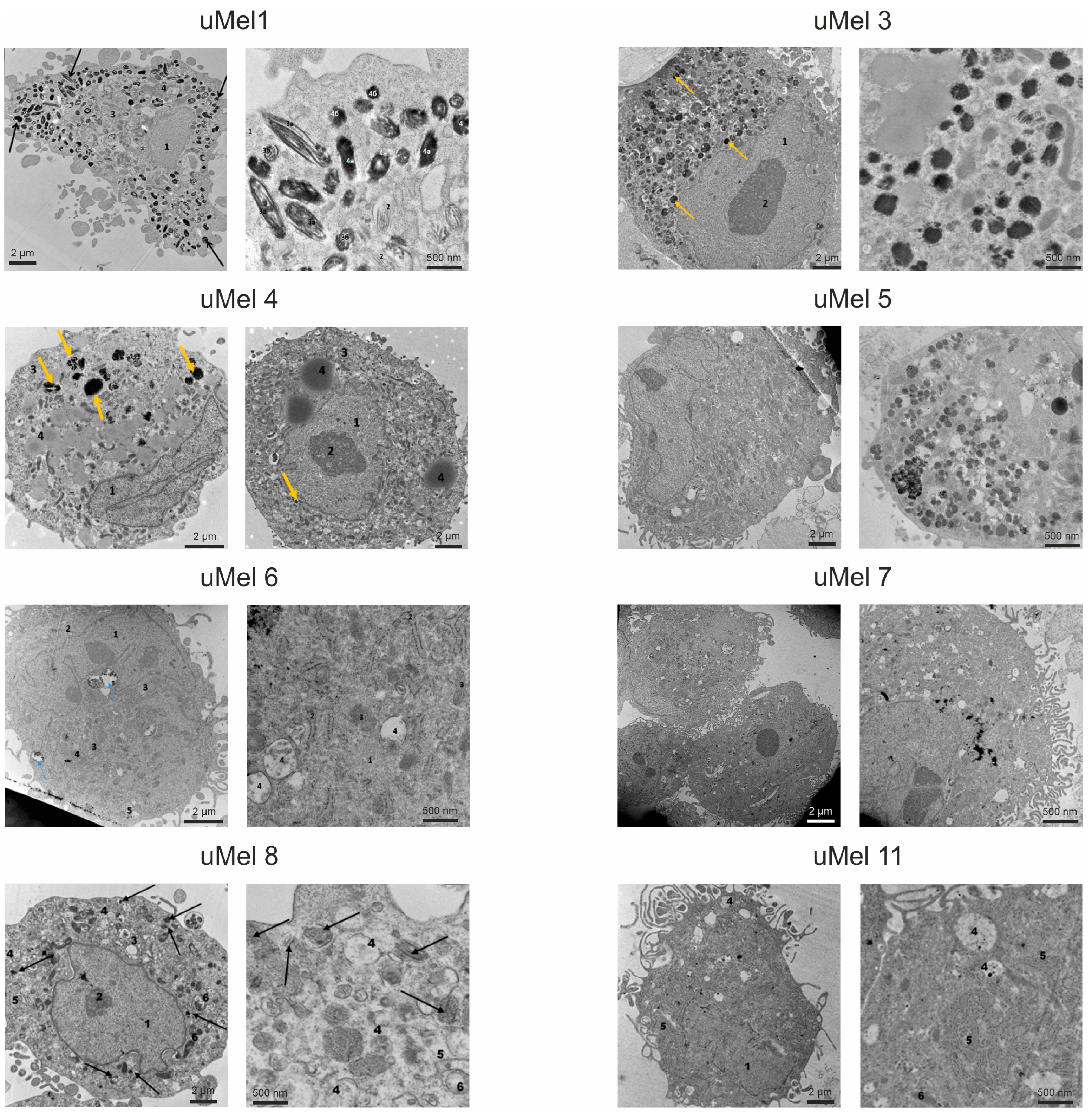
2.2. Cell Culture Morphology and Ultrastructure to Assess the Degree of Differentiation
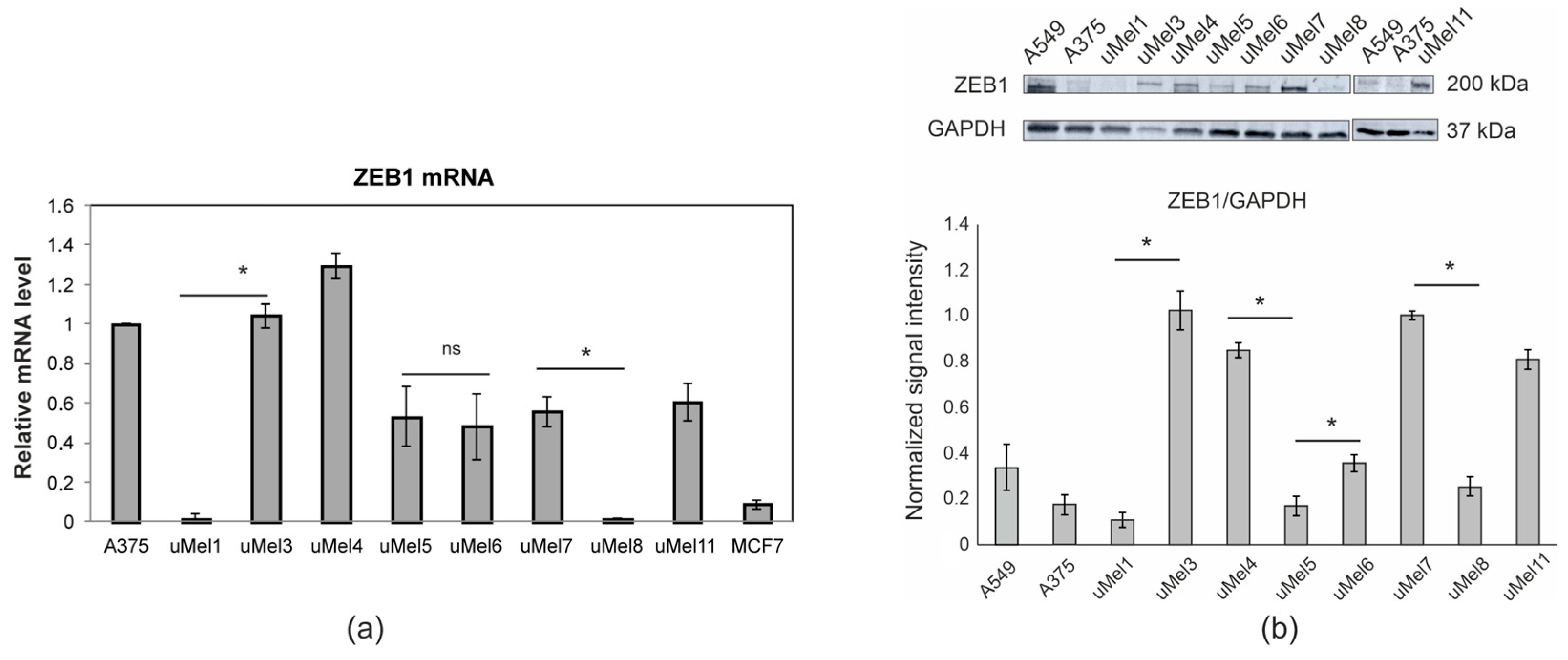
2.3. Analysis of ZEB1 Expression in UM Cells
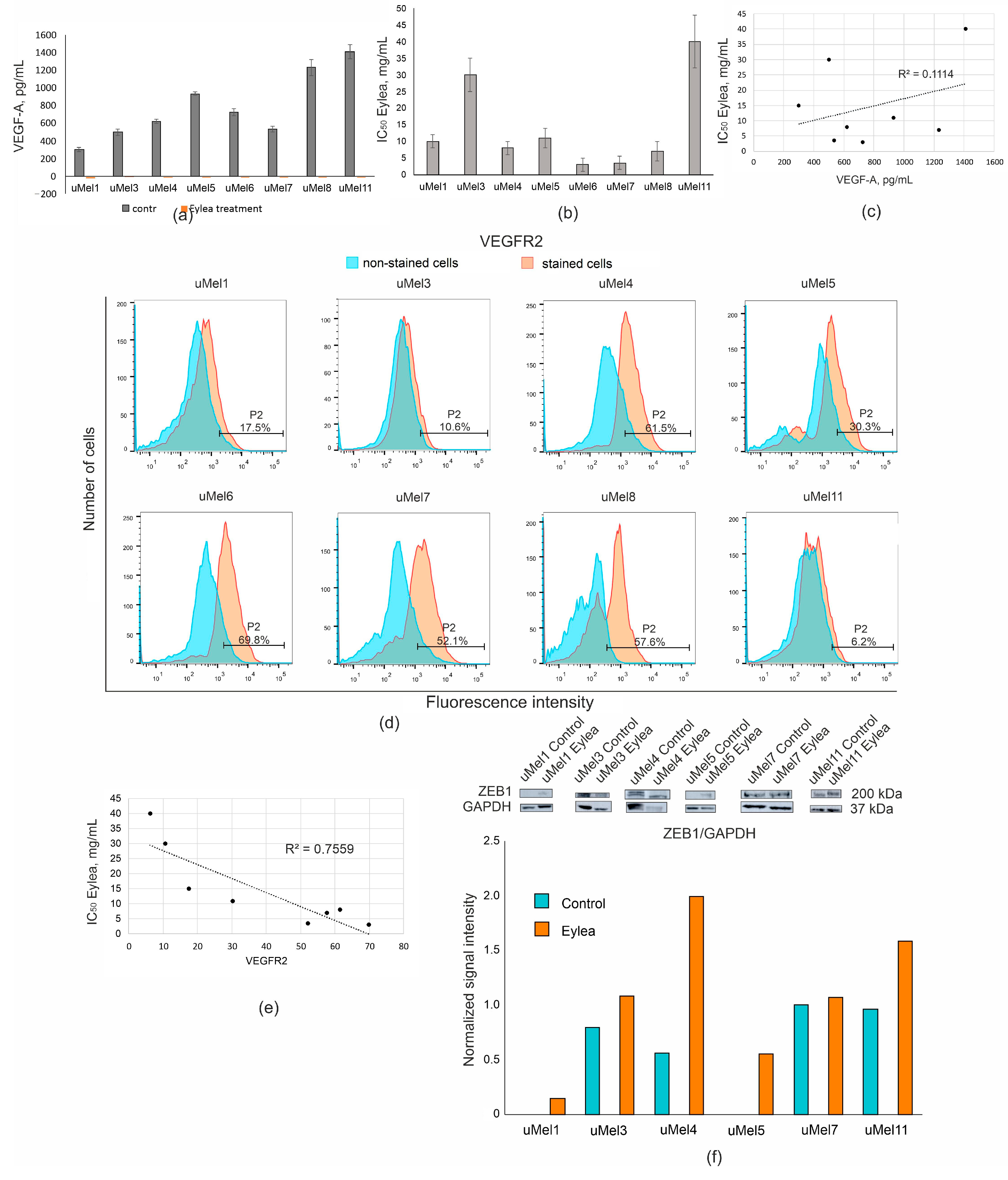
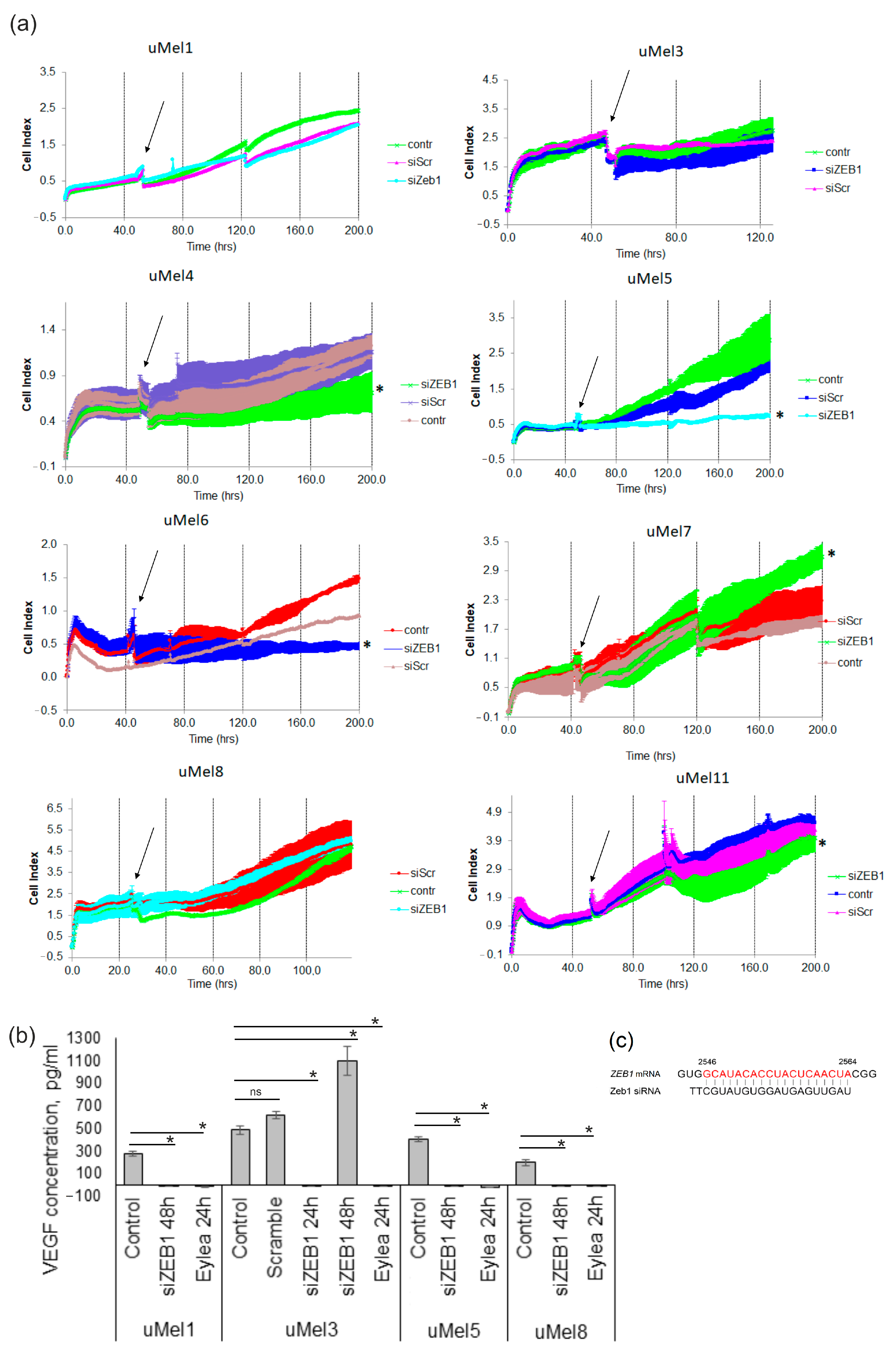
2.4. VEGF Production and the Effect of Its Inhibition in UM Cells
2.5. Effects of ZEB1 Silencing on UM Cell Proliferation and VEGF-A Synthesis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Tumors
4.3. Cell Culture Preparation
4.4. Transmission Electron Microscopy (TEM)
4.5. RNA Extraction and Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
4.6. siRNAs
4.7. Flow Cytometry
4.8. Western Blot
4.9. Cell Viability Assays
4.10. ELISA
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-MEM | Alpha-MEM medium |
| ATCC | American type culture collection |
| B2M | Beta-2-microglobulin |
| ECL | Enhanced chemiluminescence |
| EGF | Epidermal growth factor |
| ELISA | Enzyme-linked immunosorbent assay |
| EMT | Epithelial-to-mesenchymal transition |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| hnRNPA1 | Heterogenic nuclear ribonucleoprotein A1 |
| HRP | Horseradish peroxidase |
| LBD | Large basal diameter |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PBS | Phosphate-buffered saline |
| PE | Phycoerythrin |
| RT-PCR | Quantitative real-time PCR |
| SDS | Sodium dodecyl sulfate |
| siRNA | Small interfering RNA |
| TEM | Transmission electron microscopy |
| TF | Transcription factor |
| UM | Uveal melanoma |
| USP22 | Ubiquitin-specific protease 22 |
| VEGF-A | Vascular endothelial growth factor A |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| ZEB1 | Zinc finger E-box binding homeobox 1 |
References
- Naseripoor, M.; Azimi, F.; Mirshahi, R.; Khakpoor, G.; Poorhosseingholi, A.; Chaibakhsh, S. Global Incidence and Trend of Uveal Melanoma from 1943-2015: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2022, 23, 1791–1801. [Google Scholar] [CrossRef]
- Damato, E.M.; Damato, B.E. Detection and Time to Treatment of Uveal Melanoma in the United Kingdom: An Evaluation of 2384 Patients. Ophthalmology 2012, 119, 1582–1589. [Google Scholar] [CrossRef]
- Koval, O.; Zhilnikova, M.; Balantaeva, M.; Biryukov, M.; Atamanov, V. Cell Death of Tumor Melanocytes and Treatment Options. BIOCELL 2025, 49, 355–379. [Google Scholar] [CrossRef]
- Tran, K.; Schefler, A.C.; Chevli, N.; Hasegawa, N.; Ivey, F.; Olek, D.; Bretana, M.E.; Pino, R.; Butler, E.B.; Teh, B.S. Re-Treatment of Locally Recurrent Uveal Melanoma with Repeat Eye Plaque I-125 Brachytherapy: A Single Institution Experience. Brachytherapy 2024, 23, 604–609. [Google Scholar] [CrossRef]
- Jouhi, S.; Jager, M.J.; De Geus, S.J.R.; Desjardins, L.; Eide, N.A.; Grange, J.-D.; Kiilgaard, J.F.; Seregard, S.; Midena, E.; Parrozzani, R.; et al. The Small Fatal Choroidal Melanoma Study. A Survey by the European Ophthalmic Oncology Group. Am. J. Ophthalmol. 2019, 202, 100–108. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic Disease from Uveal Melanoma: Treatment Options and Future Prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Amaral, T.; Kähler, K.C.; Heinzerling, L.; Hassel, J.C.; Meissner, M.; Kreuzberg, N.; Loquai, C.; Reinhardt, L.; Utikal, J.; et al. Combined Immune Checkpoint Blockade for Metastatic Uveal Melanoma: A Retrospective, Multi-Center Study. J. Immunother. Cancer 2019, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Aedo-Lopez, V.; Gérard, C.L.; Boughdad, S.; Gautron Moura, B.; Berthod, G.; Digklia, A.; Homicsko, K.; Schaefer, N.; Duran, R.; Cuendet, M.A.; et al. Safety and Efficacy of Ipilimumab plus Nivolumab and Sequential Selective Internal Radiation Therapy in Hepatic and Extrahepatic Metastatic Uveal Melanoma. Cancers 2022, 14, 1162. [Google Scholar] [CrossRef]
- Kolesnikova, V.; Revishchin, A.; Fab, L.; Alekseeva, A.; Ryabova, A.; Pronin, I.; Usachev, D.Y.; Kopylov, A.; Pavlova, G. GQIcombi Application to Subdue Glioma via Differentiation Therapy. Front. Oncol. 2024, 14, 1322795. [Google Scholar] [CrossRef]
- D’Alba, L.; Shawkey, M.D. Melanosomes: Biogenesis, Properties, and Evolution of an Ancient Organelle. Physiol. Rev. 2019, 99, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Béliveau, A.; Bérubé, M.; Carrier, P.; Mercier, C.; Guérin, S.L. Tumorigenicity of the Mixed Spindle-Epithelioid SP6.5 and Epithelioid TP17 Uveal Melanoma Cell Lines Is Differentially Related to Alpha5beta1 Integrin Expression. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3058–3065. [Google Scholar]
- Li, F.Z.; Dhillon, A.S.; Anderson, R.L.; McArthur, G.; Ferrao, P.T. Phenotype Switching in Melanoma: Implications for Progression and Therapy. Front. Oncol. 2015, 5, 31. [Google Scholar] [CrossRef]
- Wu, H.-T.; Zhong, H.-T.; Li, G.-W.; Shen, J.-X.; Ye, Q.-Q.; Zhang, M.-L.; Liu, J. Oncogenic Functions of the EMT-Related Transcription Factor ZEB1 in Breast Cancer. J. Transl. Med. 2020, 18, 51. [Google Scholar] [CrossRef]
- Durand, S.; Tang, Y.; Pommier, R.M.; Benboubker, V.; Grimont, M.; Boivin, F.; Barbollat-Boutrand, L.; Cumunel, E.; Dupeuble, F.; Eberhardt, A.; et al. ZEB1 Controls a Lineage-Specific Transcriptional Program Essential for Melanoma Cell State Transitions. Oncogene 2024, 43, 1489–1505. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, X.; Montoya-Durango, D.E.; Liu, Y.-H.; Dean, K.C.; Darling, D.S.; Kaplan, H.J.; Dean, D.C.; Gao, L.; Liu, Y. ZEB1 Regulates Multiple Oncogenic Components Involved in Uveal Melanoma Progression. Sci. Rep. 2017, 7, 45. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, X.; Gao, L.; Dean, D.C.; Liu, Y. Spheroid-Induced Heterogeneity and Plasticity of Uveal Melanoma Cells. Cell. Oncol. 2022, 45, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, S.P.; Zhilnikova, M.V.; Biryukov, M.M.; Balantaeva, M.N.; Atamanov, V.V.; Chernykh, D.V.; Stanishevskaya, O.S.; Kononova, N.V.; Koval, O.A. MAGE Genes Expression in Human Uveal Melanoma Cells. Fyodorov J. Ophthalmic Surg. 2025, 145, 40–47. [Google Scholar] [CrossRef]
- Zhilnikova, M.V.; Atamanov, V.V.; Zvereva, S.P.; Gayner, T.A.; Boyarskikh, U.A.; Stanishevskaya, O.M.; Chernykh, D.V.; Kononova, N.V.; Koval, O.A. Molecular and Genetic Analysis of Personal Cultures of Uveal Melanoma Cells. Fyodorov J. Ophthalmic Surg. 2024, 140, 63–71. [Google Scholar] [CrossRef]
- Zhilnikova, M.V.; Novak, D.D.; Troitskaya, O.S.; Nushtaeva, A.A.; Biryukov, M.M.; Zvereva, S.P.; Varlamov, M.E.; Koval, V.V.; Stanishevskaya, O.M.; Chernikh, D.V.; et al. A New Human Uveal Melanoma Cell Line: Melanin Production and Molecular Markers for Targeted Therapy. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2023, 17, 165–171. [Google Scholar] [CrossRef]
- Schweigert, I.; Biryukov, M.; Polyakova, A.; Krychkova, N.; Gorbunova, E.; Epanchintseva, A.; Pyshnaya, I.; Zakrevsky, D.; Milakhina, E.; Koval, O. Pulsed Voltage Cold Atmospheric Plasma Jet and Gold Nanoparticles Enhance Cytotoxic Anticancer Effect. J. Phys. Appl. Phys. 2024, 57, 255205. [Google Scholar] [CrossRef]
- Missotten, G.S.O. Vascular Endothelial Growth Factor A in Eyes With Uveal Melanoma. Arch. Ophthalmol. 2006, 124, 1428. [Google Scholar] [CrossRef]
- El Filali, M.; Missotten, G.S.O.A.; Maat, W.; Ly, L.V.; Luyten, G.P.M.; Van Der Velden, P.A.; Jager, M.J. Regulation of VEGF-A in Uveal Melanoma. Investig. Opthalmology Vis. Sci. 2010, 51, 2329. [Google Scholar] [CrossRef]
- Srivastava, O.; Weis, E. Outcomes of Second-Line Intravitreal Anti-VEGF Switch Therapy in Radiation Retinopathy Secondary to Uveal Melanoma: Moving from Bevacizumab to Aflibercept. Ocul. Oncol. Pathol. 2022, 8, 230–235. [Google Scholar] [CrossRef]
- Li, M.; Knapp, S.K.; Iden, S. Mechanisms of Melanocyte Polarity and Differentiation: What Can We Learn from Other Neuroectoderm-Derived Lineages? Curr. Opin. Cell Biol. 2020, 67, 99–108. [Google Scholar] [CrossRef]
- Jager, M.J.; Magner, J.A.B.; Ksander, B.R.; Dubovy, S.R. Uveal Melanoma Cell Lines: Where Do They Come from? (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2016, 114, T5. [Google Scholar]
- Aughton, K.; Shahidipour, H.; Djirackor, L.; Coupland, S.E.; Kalirai, H. Characterization of Uveal Melanoma Cell Lines and Primary Tumor Samples in 3D Culture. Transl. Vis. Sci. Technol. 2020, 9, 39. [Google Scholar] [CrossRef]
- Puig, I.; Chicote, I.; Pálmer, H.G. Identifying Cell Differentiation in Colorectal Cancer. In Intestinal Differentiated Cells; Ordóñez-Morán, P., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2023; Volume 2650, pp. 227–233. ISBN 978-1-07-163075-4. [Google Scholar]
- Castro-Pérez, E.; Singh, M.; Sadangi, S.; Mela-Sánchez, C.; Setaluri, V. Connecting the Dots: Melanoma Cell of Origin, Tumor Cell Plasticity, Trans-differentiation, and Drug Resistance. Pigment Cell Melanoma Res. 2023, 36, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Shakhova, O. Neural Crest Stem Cells in Melanoma Development. Curr. Opin. Oncol. 2014, 26, 215–221. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Chen, A.T.; Gao, X.; Himes, B.T.; Zhang, H.; Chen, Z.; Wang, J.; Sheu, W.C.; Deng, G.; et al. ZNF117 Regulates Glioblastoma Stem Cell Differentiation towards Oligodendroglial Lineage. Nat. Commun. 2022, 13, 2196. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Deng, W.; Weng, J.; Li, M.; Lan, G.; Li, X.; Ye, L.; Wang, Y.; Liu, F.; Ou, H.; et al. Epithelial-Mesenchymal Transition Classification of Circulating Tumor Cells Predicts Clinical Outcomes in Progressive Nasopharyngeal Carcinoma. Front. Oncol. 2022, 12, 988458. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.K.; Madany, M.; Tay, A.S.-M.S.; Edwards, L.A.; Kim, S.; Michael, J.S.; Nuno, M.; Thomas, T.; Li, A.; Berel, D.; et al. ZEB1 Loss Increases Glioma Stem Cell Tumorigenicity and Resistance to Chemoradiation. J. Neurosurg. 2023, 138, 1313–1324. [Google Scholar] [CrossRef]
- Kumar, K.; Chow, C.R.; Ebine, K.; Arslan, A.D.; Kwok, B.; Bentrem, D.J.; Eckerdt, F.D.; Platanias, L.C.; Munshi, H.G. Differential Regulation of ZEB1 and EMT by MAPK-Interacting Protein Kinases (MNK) and eIF4E in Pancreatic Cancer. Mol. Cancer Res. 2016, 14, 216–227. [Google Scholar] [CrossRef]
- Zeng, K.; Xie, W.; Wang, C.; Wang, S.; Liu, W.; Su, Y.; Lin, L.; Zou, R.; Sun, G.; Zhou, B.; et al. USP22 Upregulates ZEB1-Mediated VEGFA Transcription in Hepatocellular Carcinoma. Cell Death Dis. 2023, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Asnaghi, L.; Gezgin, G.; Tripathy, A.; Handa, J.T.; Merbs, S.L.; van der Velden, P.A.; Jager, M.J.; Harbour, J.W.; Eberhart, C.G. EMT-Associated Factors Promote Invasive Properties of Uveal Melanoma Cells. Mol. Vis. 2015, 21, 919–929. [Google Scholar] [PubMed]
- Sharma, T.; Dhingra, R.; Singh, S.; Sharma, S.; Tomar, P.; Malhotra, M.; R. Bhardwaj, T. Aflibercept: A Novel VEGF Targeted Agent to Explore the Future Perspectives of Anti-Angiogenic Therapy for the Treatment of Multiple Tumors. Mini-Rev. Med. Chem. 2013, 13, 530–540. [Google Scholar] [CrossRef]
- Logan, P.; Burnier, J.; Burnier, M.N. Vascular Endothelial Growth Factor Expression and Inhibition in Uveal Melanoma Cell Lines. Ecancermedicalscience 2013, 7, 336. [Google Scholar] [CrossRef]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 Signaling Network in Endothelial Cells Relevant to Angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef]
- Title, A.C.; Hong, S.-J.; Pires, N.D.; Hasenöhrl, L.; Godbersen, S.; Stokar-Regenscheit, N.; Bartel, D.P.; Stoffel, M. Genetic Dissection of the miR-200–Zeb1 Axis Reveals Its Importance in Tumor Differentiation and Invasion. Nat. Commun. 2018, 9, 4671. [Google Scholar] [CrossRef]
- Lee, J.; You, J.H.; Kim, M.-S.; Roh, J.-L. Epigenetic Reprogramming of Epithelial-Mesenchymal Transition Promotes Ferroptosis of Head and Neck Cancer. Redox Biol. 2020, 37, 101697. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tong, Q.; Liu, S.; Cui, J.; Zhang, Q.; Sun, W.; Yang, S. ZEB1 Upregulates VEGF Expression and Stimulates Angiogenesis in Breast Cancer. PLoS ONE 2016, 11, e0148774. [Google Scholar] [CrossRef]
- Lan, H.; Zou, M.; Zhu, F.; Chen, H.; Wang, T.; Huang, X. Pro-angiogenic Role of ZEB1 in Skin Wound Healing by Upregulating VEGFA via MICRORNA-206 Suppression. Exp. Dermatol. 2022, 31, 1392–1401. [Google Scholar] [CrossRef]
- Richard, G.; Dalle, S.; Monet, M.; Ligier, M.; Boespflug, A.; Pommier, R.M.; De La Fouchardière, A.; Perier-Muzet, M.; Depaepe, L.; Barnault, R.; et al. ZEB 1-mediated Melanoma Cell Plasticity Enhances Resistance to MAPK Inhibitors. EMBO Mol. Med. 2016, 8, 1143–1161. [Google Scholar] [CrossRef]
- Nushtaeva, A.A.; Stepanov, G.A.; Semenov, D.V.; Juravlev, E.S.; Balahonova, E.A.; Gerasimov, A.V.; Sidorov, S.V.; Savelyev, E.I.; Kuligina, E.V.; Richter, V.A.; et al. Characterization of Primary Normal and Malignant Breast Cancer Cell and Their Response to Chemotherapy and Immunostimulatory Agents. BMC Cancer 2018, 18, 728. [Google Scholar] [CrossRef] [PubMed]
- Biryukov, M.; Semenov, D.; Kryachkova, N.; Polyakova, A.; Patrakova, E.; Troitskaya, O.; Milakhina, E.; Poletaeva, J.; Gugin, P.; Ryabchikova, E.; et al. The Molecular Basis for Selectivity of the Cytotoxic Response of Lung Adenocarcinoma Cells to Cold Atmospheric Plasma. Biomolecules 2023, 13, 1672. [Google Scholar] [CrossRef] [PubMed]
- Novak, D.D.; Troitskaya, O.S.; Nushtaeva, A.A.; Zhilnikova, M.V.; Richter, V.A.; Meschaninova, M.I.; Koval, O.A. EGFR Suppression Inhibits the Sphere Formation of MCF7 Cells Overexpressing EGFR. Acta Naturae 2023, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Troitskaya, O.; Golubitskaya, E.; Biryukov, M.; Varlamov, M.; Gugin, P.; Milakhina, E.; Richter, V.; Schweigert, I.; Zakrevsky, D.; Koval, O. Non-Thermal Plasma Application in Tumor-Bearing Mice Induces Increase of Serum HMGB1. Int. J. Mol. Sci. 2020, 21, 5128. [Google Scholar] [CrossRef]
- Koval, O.; Kochneva, G.; Tkachenko, A.; Troitskaya, O.; Sivolobova, G.; Grazhdantseva, A.; Nushtaeva, A.; Kuligina, E.; Richter, V. Recombinant Vaccinia Viruses Coding Transgenes of Apoptosis-Inducing Proteins Enhance Apoptosis But Not Immunogenicity of Infected Tumor Cells. BioMed Res. Int. 2017, 2017, 3620510. [Google Scholar] [CrossRef]

| Tumor (Cells) | Invasiveness | Tumor Type | LBD *, mm | Level of Differentiation |
|---|---|---|---|---|
| uMel1 | - | spindle B | 15.9 | high |
| uMel3 | sprouting into the sclera | spindle B | 20.9 | high |
| uMel4 | - | mixed | 18.9 | med |
| uMel5 | sprouting of the ciliary body, eye membranes, the orbital fiber and the optic nerve | spindle | 21.0 | low |
| uMel6 | - | mixed | 9.1 | low |
| uMel7 | - | spindle | 15.8 | low |
| uMel8 | - | spindle | 16.8 | high |
| uMel11 | sprouting into the sclera | mixed | 19.7 | non-differentiated |
| Gene | Primer Sequence | Reference Sequence Positions Matching Primer Sequence | Exons of a Reference Sequence | NCBI Reference Sequence |
|---|---|---|---|---|
| B2M | For: 5′-TGGGTTTCATCCATCCGACA-3′ | 174–193 | 2 | NM_004048.4 |
| Rev: 5′-CGGCATCTTCAAACCTCCAT-3′ | 418–399 | 3/4 * | ||
| ZEB1 | For: 5′-GCAGTCCAAGAACCACCCTT-3′ | 2576–2595 | 7 | NM_001174096.2 |
| Rev: 5′-CATGAGGTCTTTTACCTGTGTGT-3′ | 2819–2797 | 8/9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhilnikova, M.; Balantaeva, M.; Zvereva, S.; Biryukov, M.; Atamanov, V.; Poletaeva, J.; Ryabchikova, E.; Stanishevskaya, O.; Chernykh, D.; Kononova, N.; et al. ZEB1 and Uveal Melanoma Invasiveness. Int. J. Mol. Sci. 2025, 26, 10346. https://doi.org/10.3390/ijms262110346
Zhilnikova M, Balantaeva M, Zvereva S, Biryukov M, Atamanov V, Poletaeva J, Ryabchikova E, Stanishevskaya O, Chernykh D, Kononova N, et al. ZEB1 and Uveal Melanoma Invasiveness. International Journal of Molecular Sciences. 2025; 26(21):10346. https://doi.org/10.3390/ijms262110346
Chicago/Turabian StyleZhilnikova, Maria, Maria Balantaeva, Sofia Zvereva, Mikhail Biryukov, Vasiliy Atamanov, Julia Poletaeva, Elena Ryabchikova, Olga Stanishevskaya, Dmitryi Chernykh, Natalia Kononova, and et al. 2025. "ZEB1 and Uveal Melanoma Invasiveness" International Journal of Molecular Sciences 26, no. 21: 10346. https://doi.org/10.3390/ijms262110346
APA StyleZhilnikova, M., Balantaeva, M., Zvereva, S., Biryukov, M., Atamanov, V., Poletaeva, J., Ryabchikova, E., Stanishevskaya, O., Chernykh, D., Kononova, N., & Koval, O. (2025). ZEB1 and Uveal Melanoma Invasiveness. International Journal of Molecular Sciences, 26(21), 10346. https://doi.org/10.3390/ijms262110346







