The P2X7 Receptor Regulates IL-1β Secretion in the Human Retina
Abstract
1. Introduction
2. Results
2.1. The Profile of Cytokine and Growth Factor Secretion from BzATP-Stimulated HORCs
2.2. BzATP Induces IL-1β mRNA Expression and IL-1β Protein Release
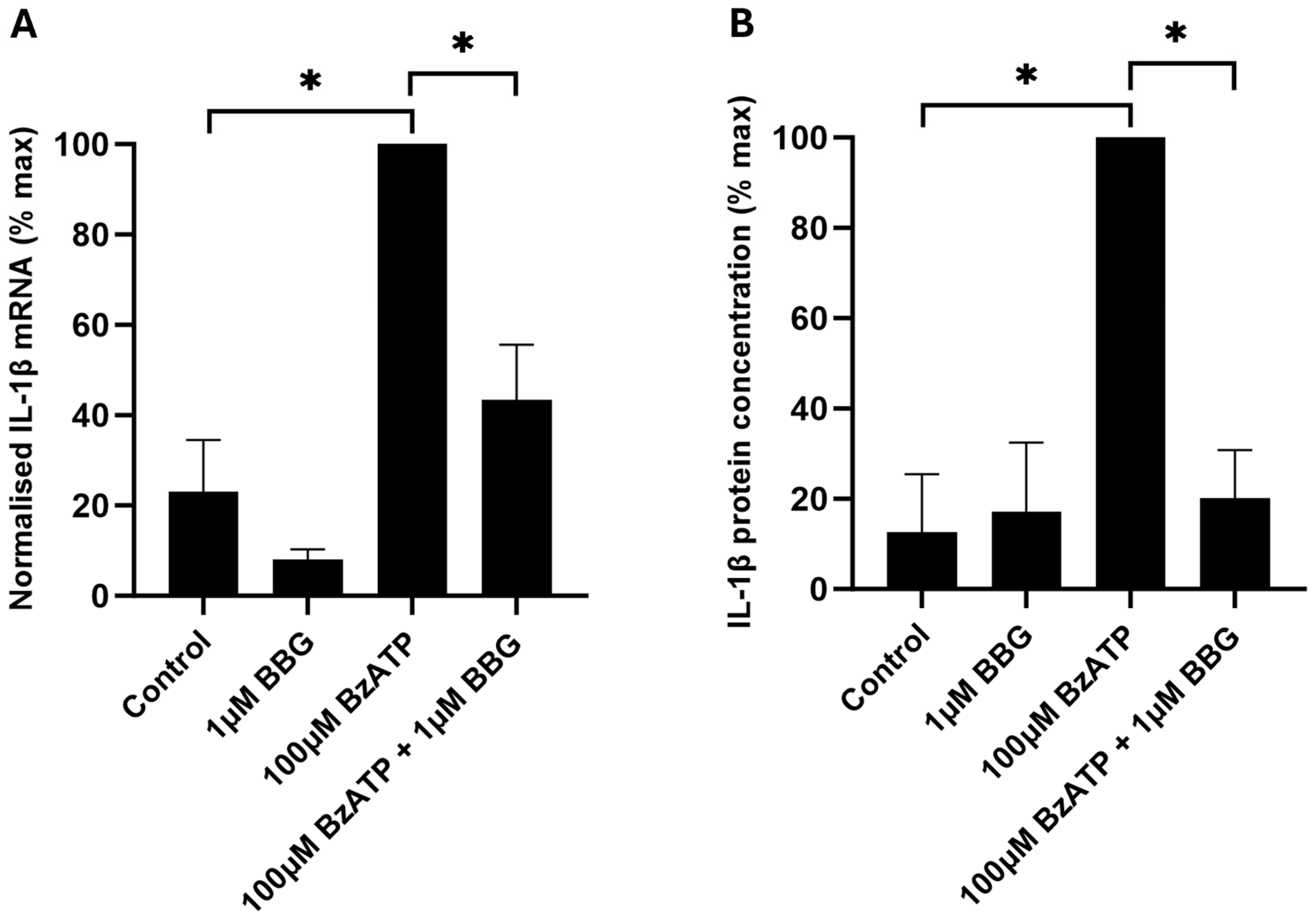
2.3. Involvement of P2X7 in IL-1β mRNA Expression and IL-1β Protein Release
2.4. IL-1β Protects RGCs from BzATP-Induced Cell Death
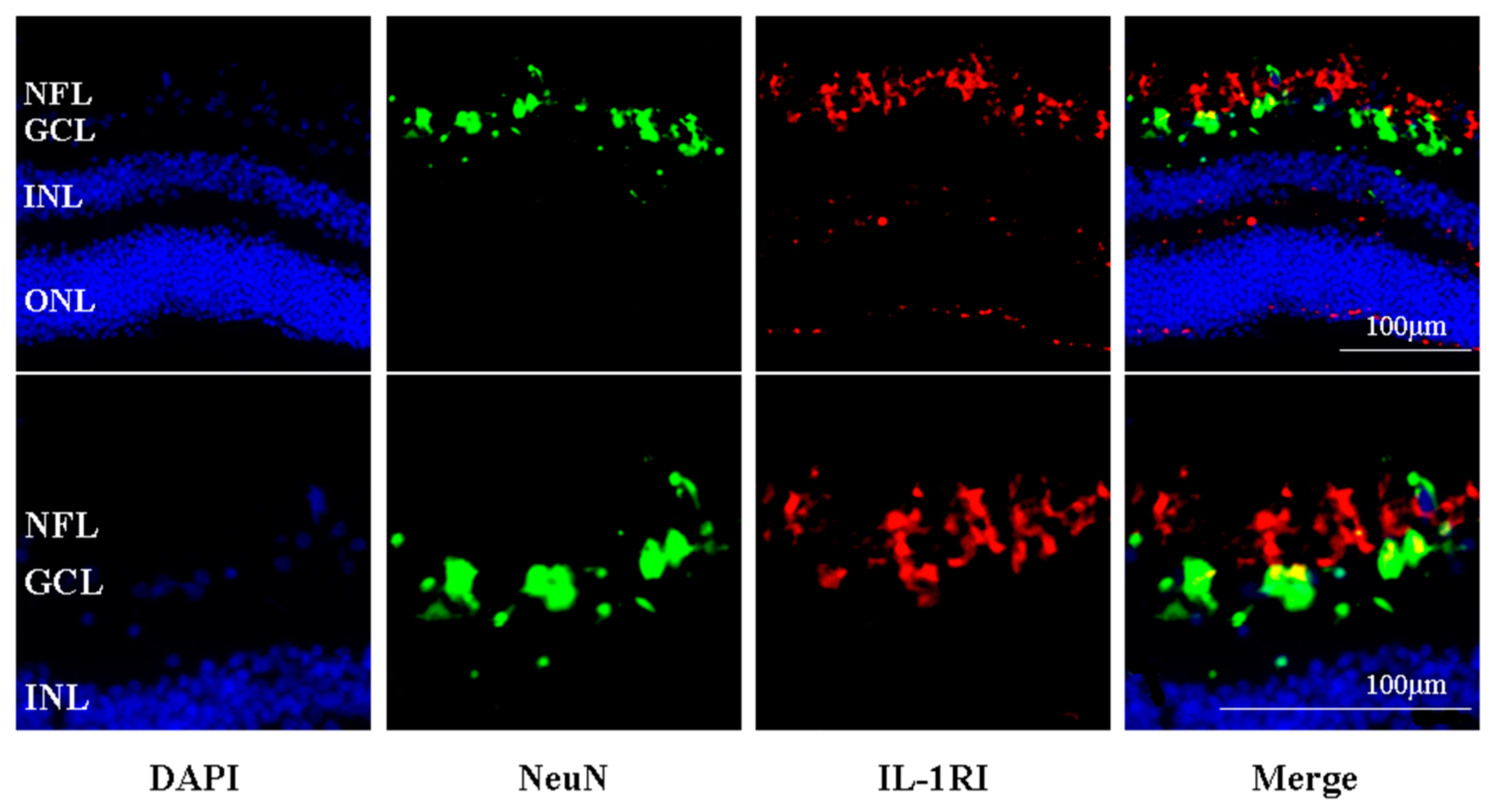
2.5. IL-R1-Immunoreactivity Was Found Principally in the Retinal Nerve Fibre and RGC Layers of Human Retina
3. Discussion
4. Materials and Methods
4.1. Human Organotypic Retinal Cultures (HORCs)
4.2. Suspended Bead Array Analysis of Cytokines and Growth Factors
4.3. Enzyme-Linked Immunosorbant Assay (ELISA)
4.4. Quantitative Real-Time Polymerase Chain Reaction (QRT-PCR)
4.5. Immunohistochemistry
4.6. Image Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar]
- Monif, M.; Reid, C.A.; Powell, K.L.; Smart, M.L.; Williams, D.A. The P2X7 Drives Microglial Activation and Proliferation: A Trophic Role for P2X7R Pore. J. Neurosci. 2009, 29, 3781–3791. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Liu, X.M.; Li, Y.W.; Huang, L.T.; Kuang, Y.Y.; Wu, X.X.; Ma, X.Q.; Zhao, B.B.; Lan, J. Unlocking the therapeutic potential of P2X7 receptor: A comprehensive review of its role in neurodegenerative disorders. Front. Pharmacol. 2024, 15, 1450704. [Google Scholar] [CrossRef]
- Ferrari, D.; Chiozzi, P.; Falzoni, S.; DalSusino, M.; Melchiorri, L.; Baricordi, O.R.; DiVirgilio, F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 1997, 159, 1451–1458. [Google Scholar] [CrossRef]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef]
- Labasi, J.M.; Petrushova, N.; Donovan, C.; McCurdy, S.; Lira, P.; Payette, M.M.; Brissette, W.; Wicks, J.R.; Audoly, L.; Gabel, C.A. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J. Immunol. 2002, 168, 6436–6445. [Google Scholar] [CrossRef]
- Ferrari, D.; Chiozzi, P.; Falzoni, S.; Hanau, S.; DiVirgilio, F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 1997, 185, 579–582. [Google Scholar] [CrossRef]
- Pelegrin, P. P2X7 receptor and the NLRP3 inflammasome: Partners in crime. Biochem. Pharmacol. 2021, 187, 114385. [Google Scholar] [CrossRef]
- Wang, X.H.; Arcuino, G.; Takano, T.; Lin, J.; Peng, W.G.; Wan, P.L.; Li, P.J.; Xu, Q.W.; Liu, Q.S.; Goldman, S.A.; et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 2004, 10, 821–827. [Google Scholar] [CrossRef]
- Arbeloa, J.; Pérez-Samartín, A.; Gottlieb, M.; Matute, C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol. Dis. 2012, 45, 954–961. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, M.; Laties, A.M.; Mitchell, C.H. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2183–2191. [Google Scholar] [CrossRef]
- Niyadurupola, N.; Sidaway, P.; Ma, N.; Rhodes, J.D.; Broadway, D.C.; Sanderson, J. P2X7 Receptor Activation Mediates Retinal Ganglion Cell Death in a Human Retina Model of Ischemic Neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2163–2170. [Google Scholar] [CrossRef]
- de Lara, M.J.P.; Avilés-Trigueros, M.; Guzmán-Aránguez, A.; Valiente-Soriano, F.J.; de la Villa, P.; Vidal-Sanz, M.; Pintor, J. Potential role of P2X7 receptor in neurodegenerative processes in a murine model of glaucoma. Brain Res. Bull. 2019, 150, 61–74. [Google Scholar] [CrossRef]
- Romano, G.L.; Amato, R.; Lazzara, F.; Porciatti, V.; Chou, T.H.; Drago, F.; Bucolo, C. P2X7 receptor antagonism preserves retinal ganglion cells in glaucomatous mice. Biochem. Pharmacol. 2020, 180, 114199. [Google Scholar] [CrossRef]
- Campagno, K.E.; Lu, W.N.; Jassim, A.H.; Albalawi, F.; Cenaj, A.; Tso, H.Y.; Clark, S.P.; Sripinun, P.; Gómez, N.M.; Mitchell, C.H. Rapid morphologic changes to microglial cells and upregulation of mixed microglial activation state markers induced by P2X7 receptor stimulation and increased intraocular pressure. J. Neuroinflamm. 2021, 18, 217. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, S.Y.; Zhang, L.J.; Lei, B.; Wang, Y.C.; Wang, Z.F. Neuroprotection of the P2X7 receptor antagonist A740003 on retinal ganglion cells in experimental glaucoma. NeuroReport 2024, 35, 822–831. [Google Scholar] [CrossRef]
- Hu, H.L.; Lu, W.N.; Zhang, M.; Zhang, X.L.; Argall, A.J.; Patel, S.; Lee, G.E.; Kim, Y.C.; Jacobson, K.A.; Laties, A.M.; et al. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp. Eye Res. 2010, 91, 425–432. [Google Scholar] [CrossRef]
- Sugiyama, T.; Lee, S.Y.; Horie, T.; Oku, H.; Takai, S.; Tanioka, H.; Kuriki, Y.; Kojima, S.; Ikeda, T. P2X7 receptor activation may be involved in neuronal loss in the retinal ganglion cell layer after acute elevation of intraocular pressure in rats. Mol. Vis. 2013, 19, 2080–2090. [Google Scholar]
- Zheng, H.Y.; Liu, Q.; Zhou, S.W.; Luo, H.L.; Zhang, W.J. Role and therapeutic targets of P2X7 receptors in neurodegenerative diseases. Front. Immunol. 2024, 15, 1345625. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Drago, F.; Bucolo, C. The P2X7 receptor as a new pharmacological target for retinal diseases. Biochem. Pharmacol. 2022, 198, 114942. [Google Scholar] [CrossRef]
- de Torre-Minguela, C.; Barberà-Cremades, M.; Gómez, A.I.; Martín-Sánchez, F.; Pelegrín, P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci. Rep. 2016, 6, 22586. [Google Scholar] [CrossRef]
- Vlachogiannis, N.I.; Legaki, A.I.; Chatzigeorgiou, A. Hepatic pannexin-1 controls interleukin-33 synthesis and release via the ATP-P2X7 pathway with clinical implications for the resolution of hyperinflammation in sepsis. Clin. Transl. Discov. 2022, 2, e94. [Google Scholar] [CrossRef]
- Townsend, E.A.; Guadarrama, A.; Shi, L.; Roti, E.R.; Denlinger, L.C. P2X7 signaling influences the production of pro-resolving and pro-inflammatory lipid mediators in alveolar macrophages derived from individuals with asthma. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2023, 325, L399–L410. [Google Scholar] [CrossRef]
- Ptito, M.; Bleau, M.; Bouskila, J. The Retina: A Window into the Brain. Cells 2021, 10, 3269. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma A Review. JAMA-J. Am. Med. Assoc. 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Sommer, A.; Tielsch, J.M.; Katz, J.; Quigley, H.A.; Gottsch, J.D.; Javitt, J.; Singh, K. Relationship between Intraocular-Pressure and Primary Open Angle Glaucoma amongt White and Black-Americans—The Baltimore Eye Survey. Arch. Ophthalmol. 1991, 109, 1090–1095. [Google Scholar] [CrossRef]
- Xue, B.; Xie, Y.T.; Xue, Y.; Hu, N.; Zhang, G.W.; Guan, H.J.; Ji, M. Involvement of P2X7 receptors in retinal ganglion cell apoptosis induced by activated Muller cells. Exp. Eye Res. 2016, 153, 42–50. [Google Scholar] [CrossRef]
- Dong, L.D.; Hu, Y.H.; Zhou, L.; Cheng, X.L. P2X7 receptor antagonist protects retinal ganglion cells by inhibiting microglial activation in a rat chronic ocular hypertension model. Mol. Med. Rep. 2018, 17, 2289–2296. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xu, Y.; Sun, Q.; Xue, S.D.; Guan, H.J.; Ji, M. Activation of P2X7R-NLRP3 pathway in Retinal microglia contribute to Retinal Ganglion Cells death in chronic ocular hypertension (COH). Exp. Eye Res. 2019, 188, 107771. [Google Scholar] [CrossRef]
- Lu, W.N.; Hu, H.L.; Sévigny, J.; Gabelt, B.T.; Kaufman, P.L.; Johnson, E.C.; Morrison, J.C.; Zode, G.S.; Sheffield, V.C.; Zhang, X.L.; et al. Rat, Mouse, and Primate Models of Chronic Glaucoma Show Sustained Elevation of Extracellular ATP and Altered Purinergic Signaling in the Posterior Eye. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3075–3083. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Models Mech. 2015, 8, 443–455. [Google Scholar] [CrossRef]
- Wei, X.; Cho, K.S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and microglia in glaucoma: Time for a paradigm shift. J. Neurosci. Res. 2019, 97, 70–76, Erratum in J. Neurosci. Res. 2019, 97, 374. [Google Scholar] [CrossRef]
- Yuan, L.Y.; Neufeld, A.H. Activated microglia in the human glaucomatous optic nerve head. J. Neurosci. Res. 2001, 64, 523–532. [Google Scholar] [CrossRef]
- Yoneda, S.; Tanihara, H.; Kido, N.; Honda, Y.; Goto, W.; Hara, H.; Miyawaki, N. Interleukin-1β mediates ischemic injury in the rat retina. Exp. Eye Res. 2001, 73, 661–667. [Google Scholar] [CrossRef]
- Kido, N.; Inatani, M.; Honjo, M.; Yoneda, S.; Hara, H.; Miyawaki, N.; Honda, Y.; Tanihara, H. Dual effects of interleukin-1β on N-methyl-D-aspartate-induced retinal neuronal death in rat eyes. Brain Res. 2001, 910, 153–162. [Google Scholar] [CrossRef]
- Zhang, X.; Chintala, S.K. Influence of interleukin-1 beta induction and mitogen-activated protein kinase phosphorylation on optic nerve ligation-induced matrix metalloproteinase-9 activation in the retina. Exp. Eye Res. 2004, 78, 849–860. [Google Scholar] [CrossRef]
- Chi, W.; Li, F.; Chen, H.R.; Wang, Y.D.; Zhu, Y.T.; Yang, X.J.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Munemasa, Y.; Nakazawa, T.; Ueno, S. NMDA-induced interleukin-1β expression is mediated by nuclear factor-kappa B p65 in the retina. Brain Res. 2007, 1142, 247–255. [Google Scholar] [CrossRef]
- Sivakumar, V.; Foulds, W.S.; Luu, C.D.; Ling, E.A.; Kaur, C. Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J. Pathol. 2011, 224, 245–260. [Google Scholar] [CrossRef]
- Albalawi, F.; Lu, W.N.; Beckel, J.M.; Lim, J.C.; McCaughey, S.A.; Mitchell, C.H. The P2X7 Receptor Primes IL-1β and the NLRP3 Inflammasome in Astrocytes Exposed to Mechanical Strain. Front. Cell Neurosci. 2017, 11, 227. [Google Scholar] [CrossRef]
- Diem, R.; Hobom, M.; Grötsch, P.; Kramer, B.; Bähr, M. Interleukin-1β protects neurons via the interleukin-1 (IL-1) receptor-mediated Akt pathway and by IL-1 receptor-independent decrease of transmembrane currents in vivo. Mol. Cell. Neurosci. 2003, 22, 487–500. [Google Scholar] [CrossRef]
- Namekata, K.; Harada, C.; Kohyama, K.; Matsumoto, Y.; Harada, T. Interleukin-1 stimulates glutamate uptake in glial cells by accelerating membrane trafficking of Na+/K+-ATPase via actin depolymerization. Mol. Cell. Biol. 2008, 28, 3273–3280. [Google Scholar] [CrossRef]
- Todd, L.; Palazzo, I.; Suarez, L.; Liu, X.Y.; Volkov, L.; Hoang, T.V.; Campbell, W.A.; Blackshaw, S.; Quan, N.; Fischer, A.J. Reactive microglia and IL1/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J. Neuroinflamm. 2019, 16, 118. [Google Scholar] [CrossRef]
- Niyadurupola, N.; Sidaway, P.; Osborne, A.; Broadway, D.C.; Sanderson, J. The development of human organotypic retinal cultures (HORCs) to study retinal neurodegeneration. Brit J. Ophthalmol. 2011, 95, 720–726. [Google Scholar] [CrossRef][Green Version]
- Osborne, A.; Hopes, M.; Wright, P.; Broadway, D.C.; Sanderson, J. Human organotypic retinal cultures (HORCs) as a chronic experimental model for investigation of retinal ganglion cell degeneration. Exp. Eye Res. 2016, 143, 28–38. [Google Scholar] [CrossRef]
- Jiang, L.H.; Mackenzie, A.B.; North, R.A.; Surprenant, A. Brilliant blue G selectively blocks ATP-gated rat P2X7 receptors. Mol. Pharmacol. 2000, 58, 82–88. [Google Scholar] [CrossRef]
- Boyd, Z.S.; Kriatchko, A.; Yang, J.J.; Agarwal, N.; Wax, M.B.; Patil, R.V. Interleukin-10 receptor signaling through STAT-3 regulates the apoptosis of retinal ganglion cells in response to stress. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5206–5211. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Gauldie, J.; Ball, A.K. Effects of adenoviral-mediated gene transfer of interleukin-10, interleukin-4, and transforming growth factor-β on the survival of axotomized retinal ganglion cells. Neuroscience 2004, 125, 903–920. [Google Scholar] [CrossRef]
- Markiewicz, L.; Pytel, D.; Mucha, B.; Szymanek, K.; Szaflik, J.; Szaflik, J.P.; Ajsterek, I. Altered Expression Levels of MMP1, MMP9, MMP12, TIMP1, and IL-1β as a Risk Factor for the Elevated IOP and Optic Nerve Head Damage in the Primary Open-Angle Glaucoma Patients. Biomed. Res. Int. 2015, 2015, 812503. [Google Scholar] [CrossRef]
- Oliveira, M.B.; de Vasconcellos, O.P.C.; Ananina, G.; Costa, V.P.; de Melo, M.B. Association between IL-1A and IL-1 polymorphisms and primary open angle glaucoma in a Brazilian population. Exp. Biol. Med. 2018, 243, 1083–1091. [Google Scholar] [CrossRef]
- Sluyter, R.; Shemon, A.N.; Wiley, J.S. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1β release from human monocytes. J. Immunol. 2004, 172, 3399–3405. [Google Scholar] [CrossRef]
- Rampe, D.; Wang, L.; Ringheim, G.E. P2X7 receptor modulation of β-amyloid- and LPS-induced cytokine secretion from human macrophages and microglia. J. Neuroimmunol. 2004, 147, 56–61. [Google Scholar] [CrossRef]
- Wilson, H.L.; Varcoe, R.W.; Stokes, L.; Holland, K.L.; Francis, S.E.; Dower, S.K.; Surprenant, A.; Crossman, D.C. P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells. Brit. J. Pharmacol. 2007, 151, 96–108. [Google Scholar] [CrossRef]
- Pizzirani, C.; Ferrari, D.; Chiozzi, P.; Adinolfi, E.; Sandonà, D.; Savaglio, E.; Di Virgilio, F. Stimulation of P2 receptors causes release of IL-1β-loaded microvesicles from human dendritic cells. Blood 2007, 109, 3856–3864. [Google Scholar] [CrossRef]
- Kanjanamekanant, K.; Luckprom, P.; Pavasant, P. Mechanical stress-induced interleukin-1beta expression through adenosine triphosphate/P2X7 receptor activation in human periodontal ligament cells. J. Periodontal Res. 2013, 48, 169–176. [Google Scholar] [CrossRef]
- Franceschini, A.; Capece, M.; Chiozzi, P.; Falzoni, S.; Sanz, J.M.; Sarti, A.C.; Bonora, M.; Pinton, P.; Di Virgilio, F. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. Faseb J. 2015, 29, 2450–2461. [Google Scholar] [CrossRef]
- Campagno, K.E.; Lu, W.N.; Sripinun, P.; Albalawi, F.; Cenaj, A.; Mitchell, C.H. Priming and release of cytokine IL-1β in microglial cells from the retina. Exp. Eye Res. 2025, 252, 110246. [Google Scholar] [CrossRef]
- Morigiwa, K.; Quan, M.Z.; Murakami, M.; Yamashita, M.; Fukuda, Y. P2 purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neurosci. Lett. 2000, 282, 153–156. [Google Scholar] [CrossRef]
- Hangai, M.; Yoshimura, N.; Yoshida, M.; Yabuuchi, K.; Honda, Y. Interleukin-1 Gene-Expression in Transient Retinal Ischemia in the Rat. Investig. Ophthalmol. Vis. Sci. 1995, 36, 571–578. [Google Scholar]
- Pronin, A.; Pham, D.; An, W.J.; Dvoriantchikova, G.; Reshetnikova, G.; Qiao, J.Z.; Kozhekbaeva, Z.; Reiser, A.E.; Slepak, V.Z.; Shestopalov, V.I. Inflammasome Activation Induces Pyroptosis in the Retina Exposed to Ocular Hypertension Injury. Front. Mol. Neurosci. 2019, 12, 36. [Google Scholar] [CrossRef]
- Pringle, A.K.; Niyadurupola, N.; Johns, P.; Anthony, D.C.; Iannotti, F. Interleukin-1β exacerbates hypoxia-induced neuronal damage, but attenuates toxicity produced by simulated ischaemia and excitotoxicity in rat organotypic hippocampal slice cultures. Neurosci. Lett. 2001, 305, 29–32. [Google Scholar] [CrossRef]
- Coyle, S.; Khan, M.N.; Chemaly, M.; Callaghan, B.; Doyle, C.; Willoughby, C.E.; Atkinson, S.D.; Gregory-Ksander, M.; McGilligan, V. Targeting the NLRP3 Inflammasome in Glaucoma. Biomolecules 2021, 11, 1239. [Google Scholar] [CrossRef]
- Lim, J.C.; Lu, W.A.; Beckel, J.M.; Mitchell, C.H. Neuronal Release of Cytokine IL-3 Triggered by Mechanosensitive Autostimulation of the P2X7 Receptor Is Neuroprotective. Front. Cell Neurosci. 2016, 10, 270. [Google Scholar] [CrossRef]
- Lu, W.N.; Albalawi, F.; Beckel, J.M.; Lim, J.C.; Laties, A.M.; Mitchell, C.H. The P2X7 receptor links mechanical strain to cytokine IL-6 up-regulation and release in neurons and astrocytes. J. Neurochem. 2017, 141, 436–448. [Google Scholar] [CrossRef]
- Shao, X.L.; Guha, S.; Lu, W.N.; Campagno, K.E.; Beckel, J.M.; Mills, J.A.; Yang, W.L.; Mitchell, C.H. Polarized Cytokine Release Triggered by P2X7 Receptor from Retinal Pigmented Epithelial Cells Dependent on Calcium Influx. Cells 2020, 9, 2537. [Google Scholar] [CrossRef]
- Mori, T.; Nagaraj, N.R.; Surico, P.L.; Zhou, W.J.; Parmar, U.P.S.; D’Esposito, F.; Gagliano, C.; Musa, M.; Zeppieri, M. The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review. Open Life Sci. 2024, 19, 20221023. [Google Scholar] [CrossRef]
- Brändle, U.; Kohler, K.; Wheeler-Schilling, T.H. Expression of the P2X7-receptor subunit in neurons of the rat retina. Mol. Brain Res. 1998, 62, 106–109. [Google Scholar] [CrossRef]
- Ishii, K.; Kaneda, M.; Li, H.B.; Rockland, K.S.; Hashikawa, T. Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. J. Comp. Neurol. 2003, 459, 267–277. [Google Scholar] [CrossRef]
- Bartlett, R.; Yerbury, J.J.; Sluyter, R. P2X7 Receptor Activation Induces Reactive Oxygen Species Formation and Cell Death in Murine EOC13 Microglia. Mediat. Inflamm. 2013, 2013, 271813. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J. Neurosci. 2001, 21, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Beckel, J.M.; Argall, A.J.; Lim, J.C.; Xia, J.S.; Lu, W.N.; Coffey, E.E.; Macarak, E.J.; Shahidullah, M.; Delamere, N.A.; Zode, G.S.; et al. Mechanosensitive Release of Adenosine 5′-triphosphate Through Pannexin Channels and Mechanosensitive Upregulation of Pannexin Channels in Optic Nerve Head Astrocytes: A Mechanism for Purinergic Involvement in Chronic Strain. Glia 2014, 62, 1486–1501. [Google Scholar] [CrossRef]
- Loiola, E.C.; Ventura, A.L. Release of ATP from Muller Cells of the Embryonic Chick Retina in Culture. J. Neurochem. 2011, 118, 76. [Google Scholar]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]



| Cytokine or Growth Factor | Mean (± S.E.M.) Protein Concentration in Control Medium (pg/mL) | Mean (± S.E.M.) Protein Concentration in 100 μM BzATP-Treated Medium (pg/mL) | Significance * p < 0.05 |
|---|---|---|---|
| IL-2 | 0 | 0 | |
| IL-1β | 95.7 ± 20.0 | 295.3 ± 55.3 | * |
| IL-1ra | 663.7 ± 133.5 | 526.0 ± 28.7 | |
| IL-4 | 26.6 ± 10.5 | 14.3 ± 5.2 | |
| IL-5 | 0 | 0 | |
| IL-6 | 5101.5 ± 965.3 | 6253.7 ± 1124.3 | |
| IL-7 | 7.6 ± 3.9 | 6.6 ± 2.7 | |
| IL-8 | 10,040.7 ± 1029.8 | 16,879.0 ± 10,321.1 | |
| IL-9 | 213.4 ± 43.1 | 154.7 ± 13.7 | |
| IL-10 | 33.9 ± 12.6 | 118.0 ± 17.0 | * |
| IL-12 | 94.3 ± 10.1 | 126.7 ± 19.5 | |
| IL-13 | 14.8 ± 4.9 | 43.2 ± 14.3 | |
| IL-15 | 239.3 ± 52.5 | 196.2 ± 24.8 | |
| IL-17 | 0 | 0 | |
| TNF-α | 738.7 ± 212.1 | 536.3 ± 60.0 | |
| Interferon-γ (gamma) | 1906.4 ± 465.0 | 1369.2 ± 131.6 | |
| Interferon-Inducible Protein 10 | 56,210.7 ± 9465.3 | 31,524.1 ± 2788.1 | |
| Granulocyte-Colony Stimulating Factor | 2460.8 ± 682.6 | 3650.4 ± 424.9 | |
| Granulocyte Monocyte-Colony Stimulating Factor | 76.3 ± 54.8 | 0 | |
| Monocyte Chemoattractant Protein-1 (Monocyte Chemoattractant and Activating Factor) | 4697.6 ± 628.1 | 4093.5 ± 444.3 | |
| Macrophage Inflammatory Protein-1alpha | 1174.9 ± 96.9 | 1212.3 ± 54.1 | |
| Macrophage Inflammatory Protein-1beta | 6353.2 ± 456.7 | 5588.0 ± 201.1 | |
| Eotaxin | 448.3 ± 93.0 | 319.6 ± 28.1 | |
| Regulated on Activation, Normal T Expressed and Secreted (RANTES) | 390.9 ± 97.8 | 161.5 ± 21.4 | |
| Vascular Endothelial Growth Factor | 149.8 ± 55.5 | 629.4 ± 329.1 | |
| Platelet Derived Growth Factor-BB | 95.0 ± 59.6 | 83.9 ± 48.7 | |
| Fibroblast Growth Factor-Basic | 55.7 ± 24.7 | 39.0 ± 17.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niyadurupola, N.; Sidaway, P.; Broadway, D.C.; Sanderson, J. The P2X7 Receptor Regulates IL-1β Secretion in the Human Retina. Int. J. Mol. Sci. 2025, 26, 10345. https://doi.org/10.3390/ijms262110345
Niyadurupola N, Sidaway P, Broadway DC, Sanderson J. The P2X7 Receptor Regulates IL-1β Secretion in the Human Retina. International Journal of Molecular Sciences. 2025; 26(21):10345. https://doi.org/10.3390/ijms262110345
Chicago/Turabian StyleNiyadurupola, Nuwan, Peter Sidaway, David C. Broadway, and Julie Sanderson. 2025. "The P2X7 Receptor Regulates IL-1β Secretion in the Human Retina" International Journal of Molecular Sciences 26, no. 21: 10345. https://doi.org/10.3390/ijms262110345
APA StyleNiyadurupola, N., Sidaway, P., Broadway, D. C., & Sanderson, J. (2025). The P2X7 Receptor Regulates IL-1β Secretion in the Human Retina. International Journal of Molecular Sciences, 26(21), 10345. https://doi.org/10.3390/ijms262110345





