Repurposing HIV-Protease Inhibitor Precursors as Anticancer Agents: The Synthetic Molecule RDD-142 Delays Cell Cycle Progression and Induces Autophagy in HepG2 Cells with Enhanced Efficacy via Liposomal Formulation
Abstract
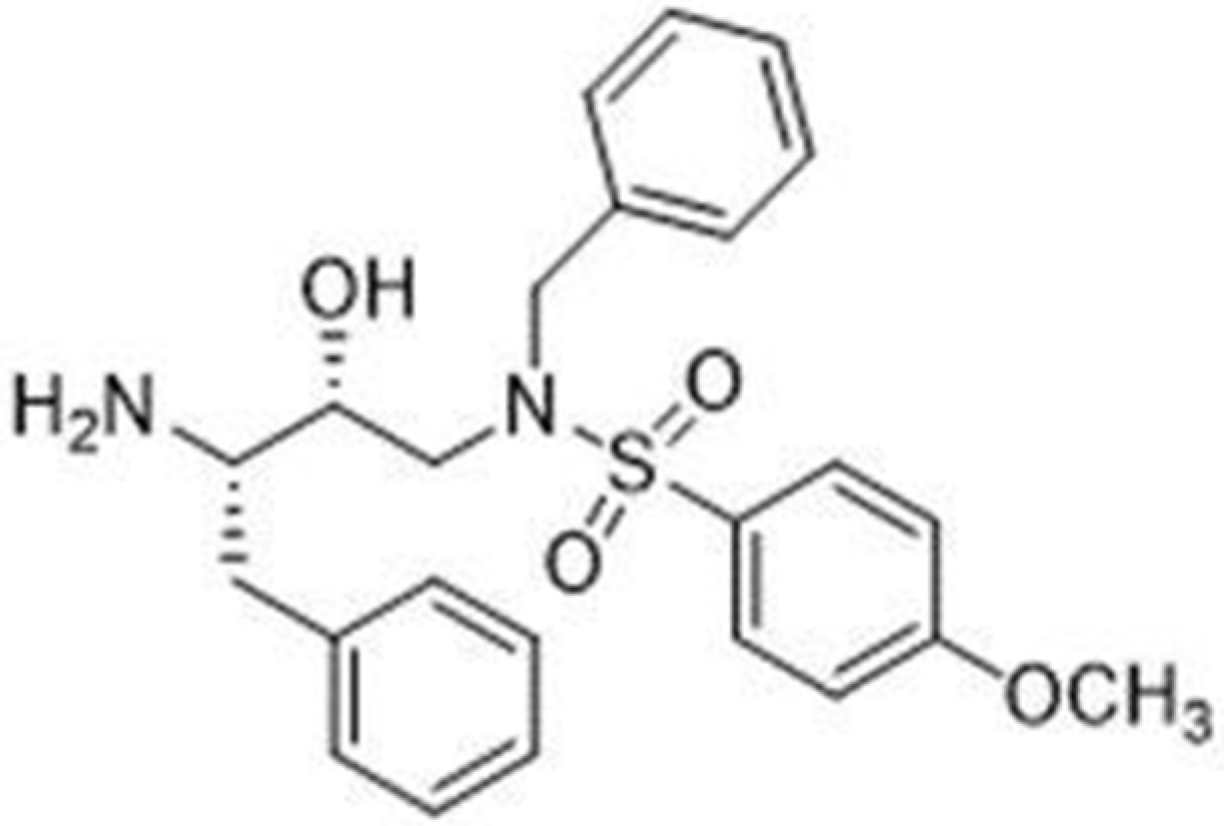
1. Introduction
2. Results
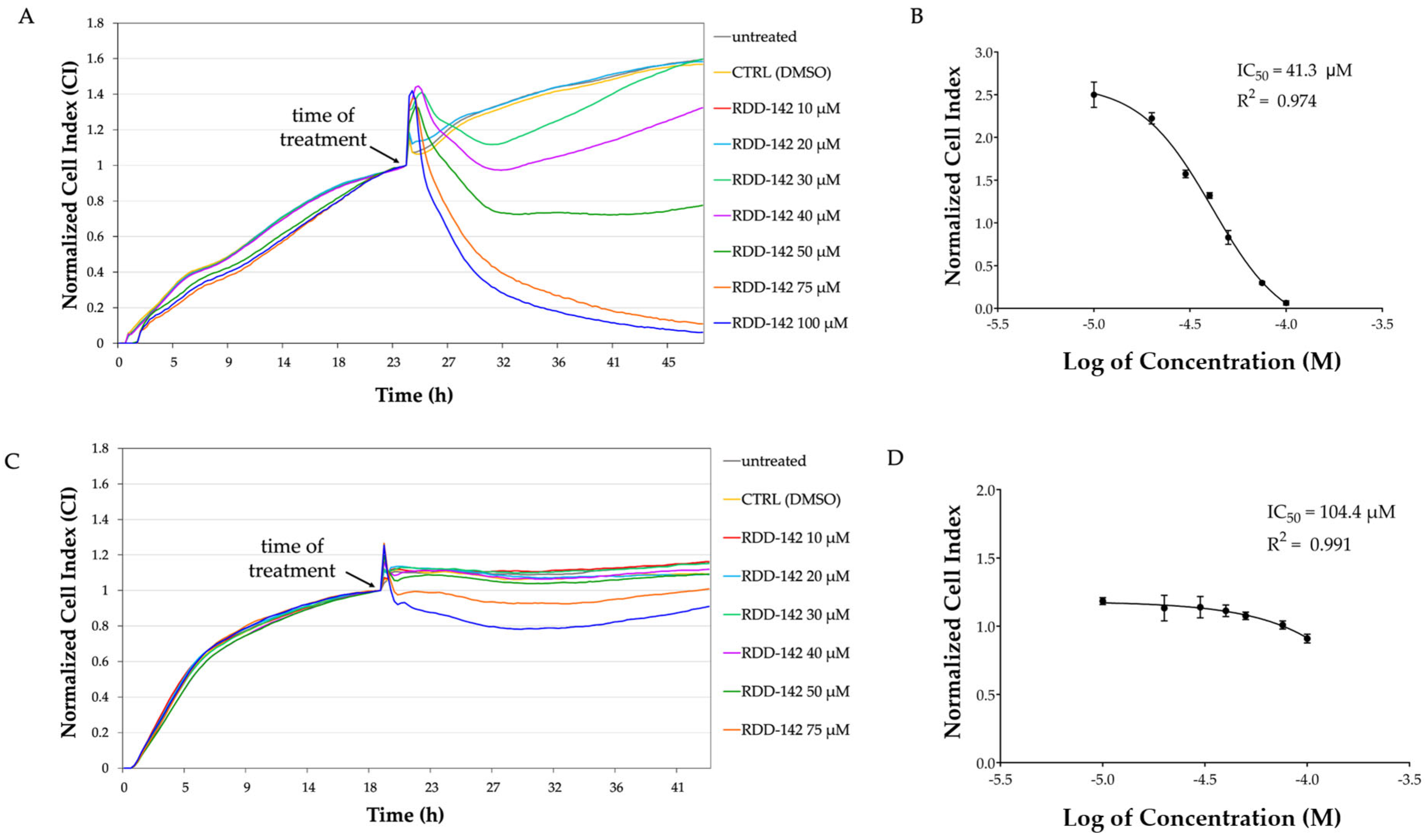
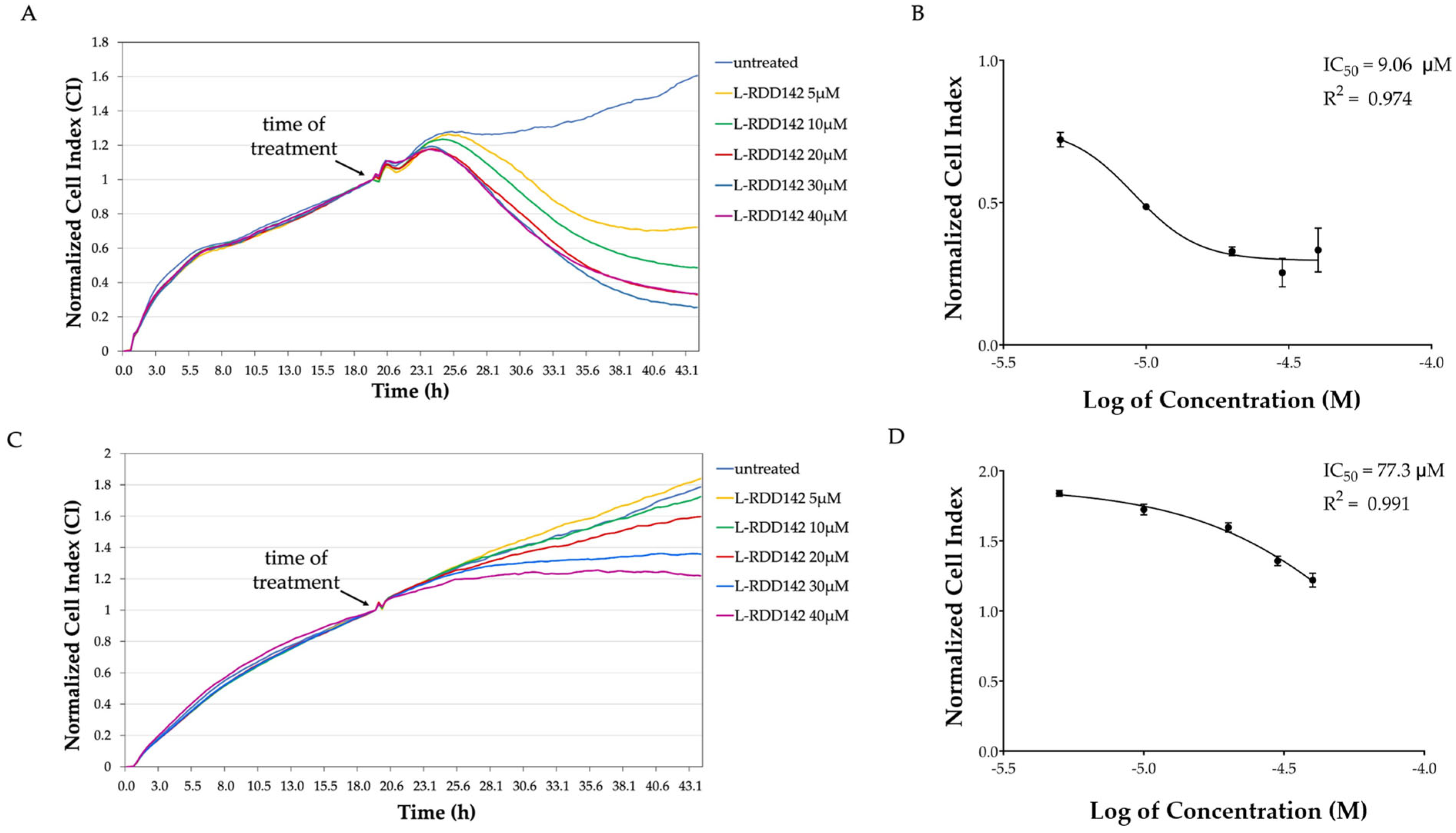
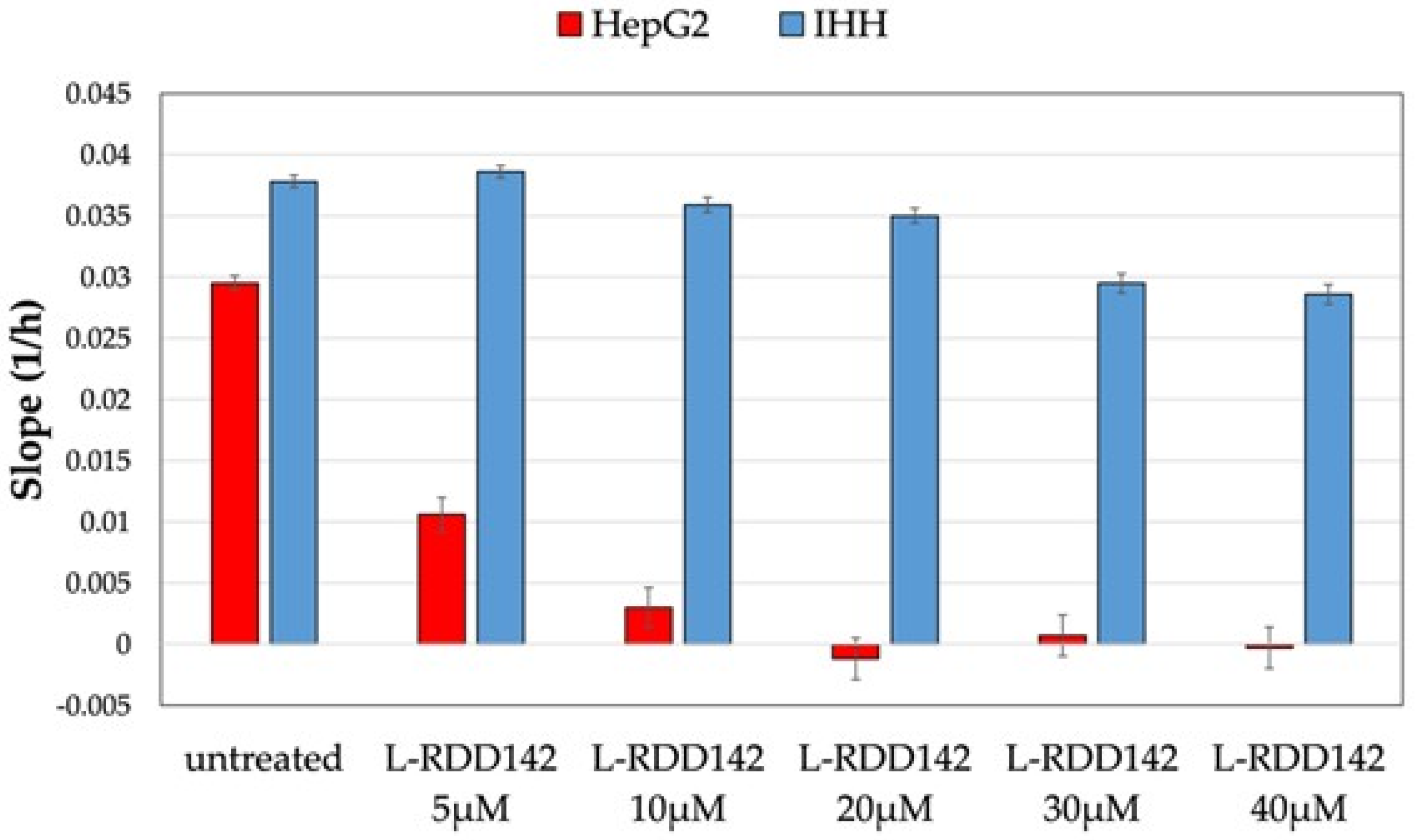
2.1. RDD-142 Negatively Influences Neoplastic Cell Proliferation
2.2. RDD-142 Attenuates Proliferation of Hepatocarcinoma Cells by Delaying Cell Cycle Progression
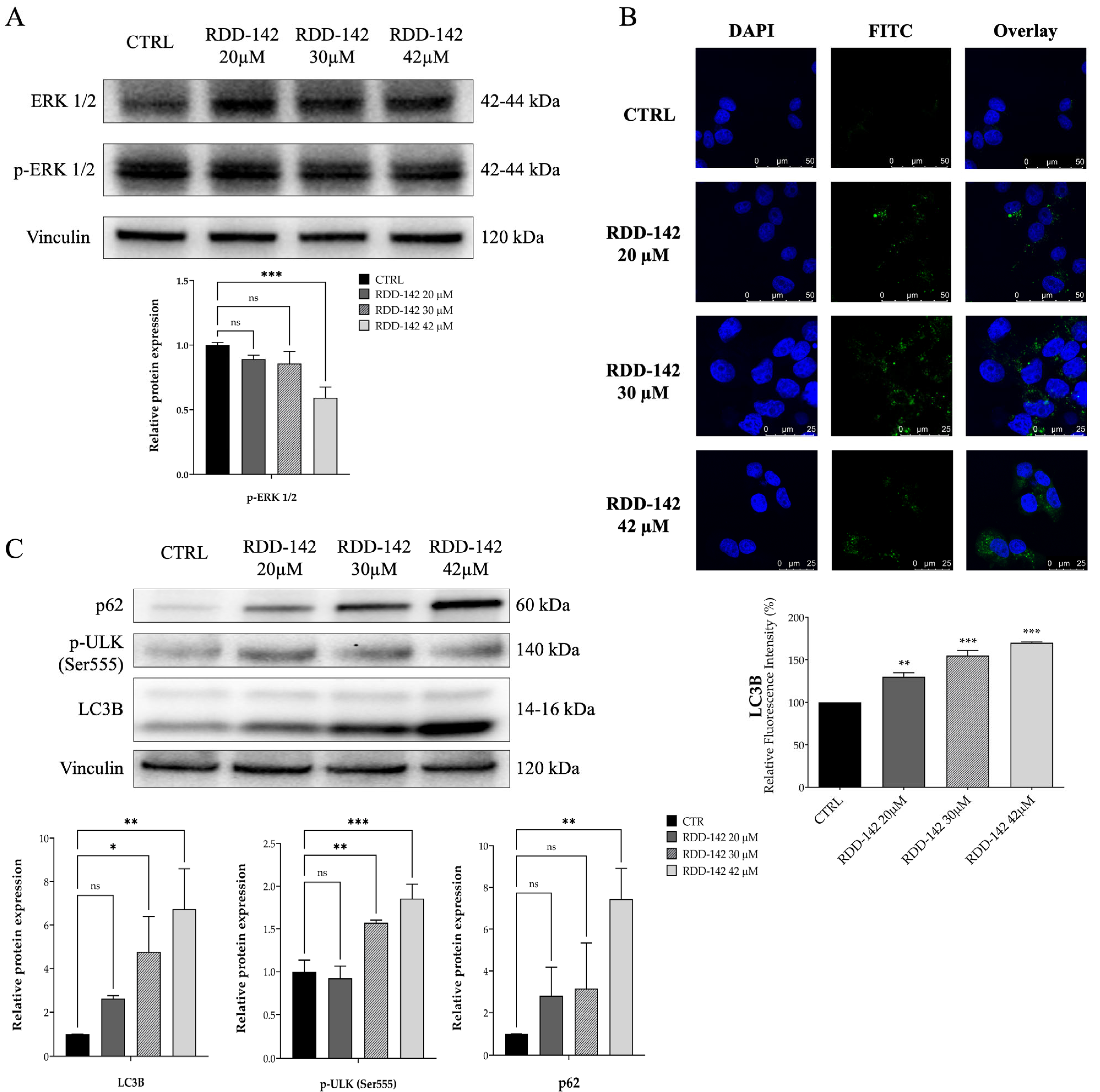
2.3. RDD-142 Induces Impairment of Cell Cycle Transit by Inactivation of ERK1/2 Signaling and Activation of Autophagy in HepG2 Cells
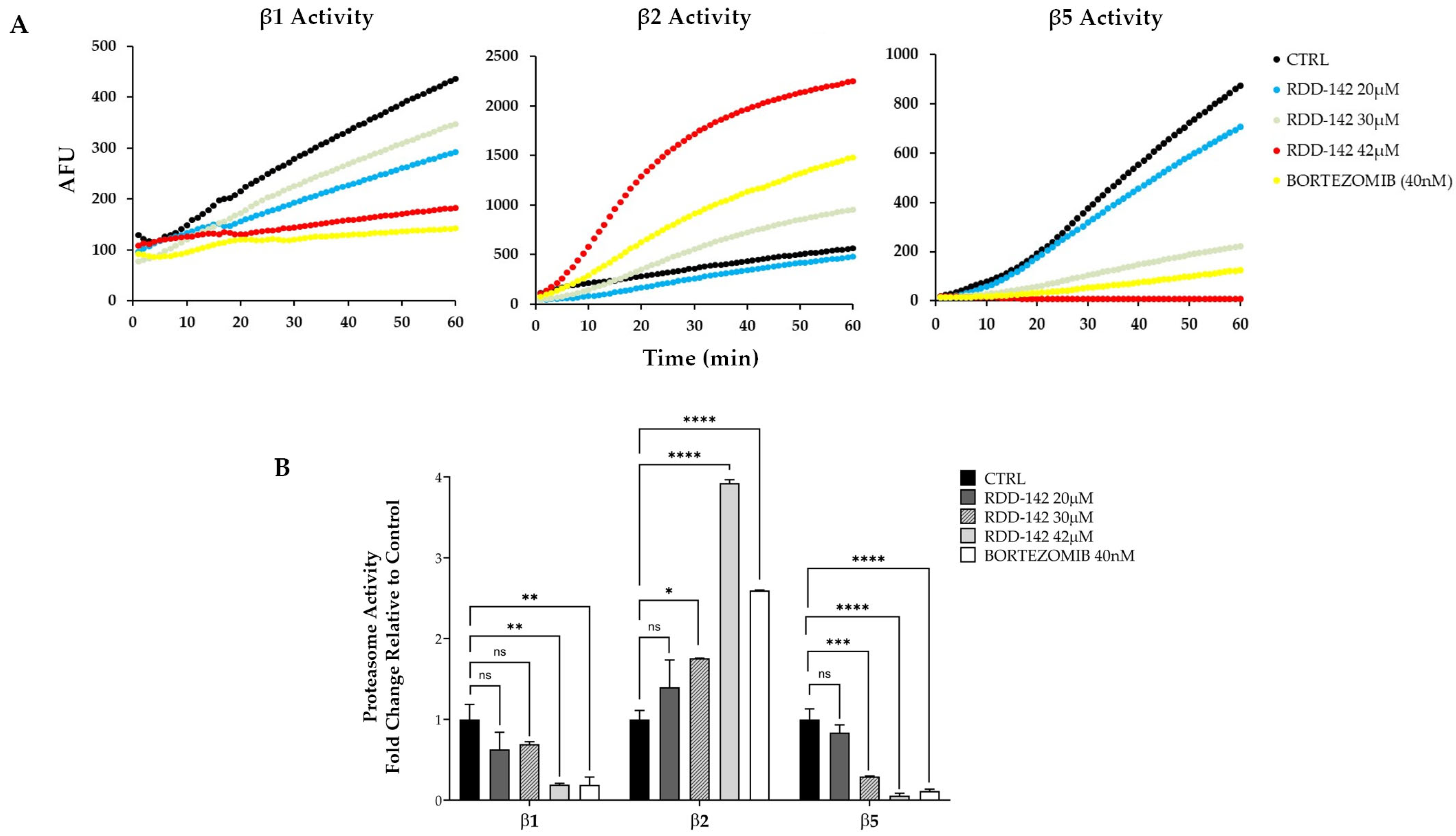
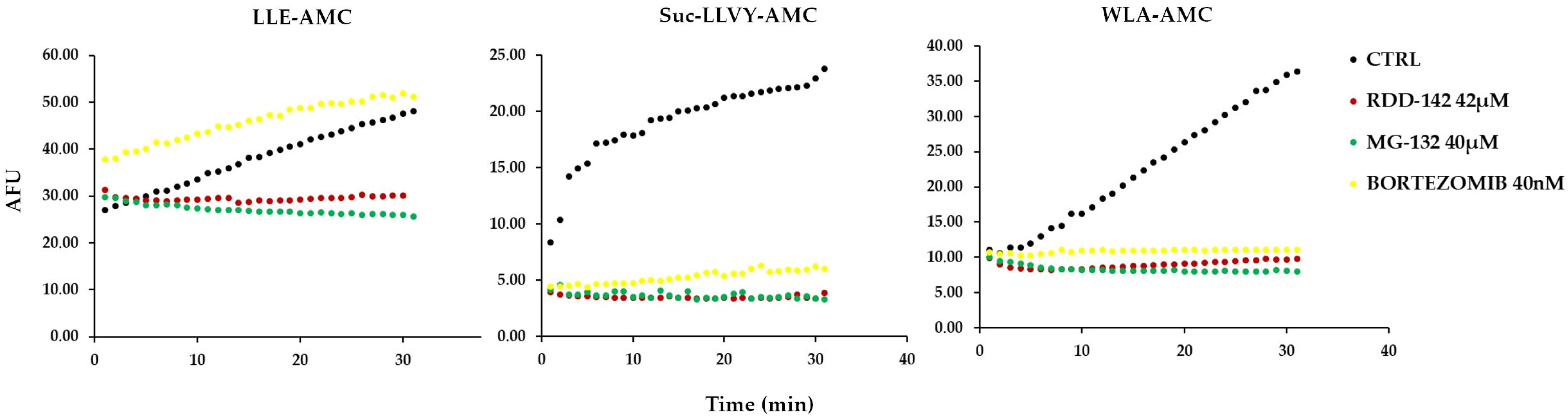
2.4. RDD-142 Inhibits Proteasome Activity
2.5. RDD-142 Inhibits Proteasome Activity In Vitro
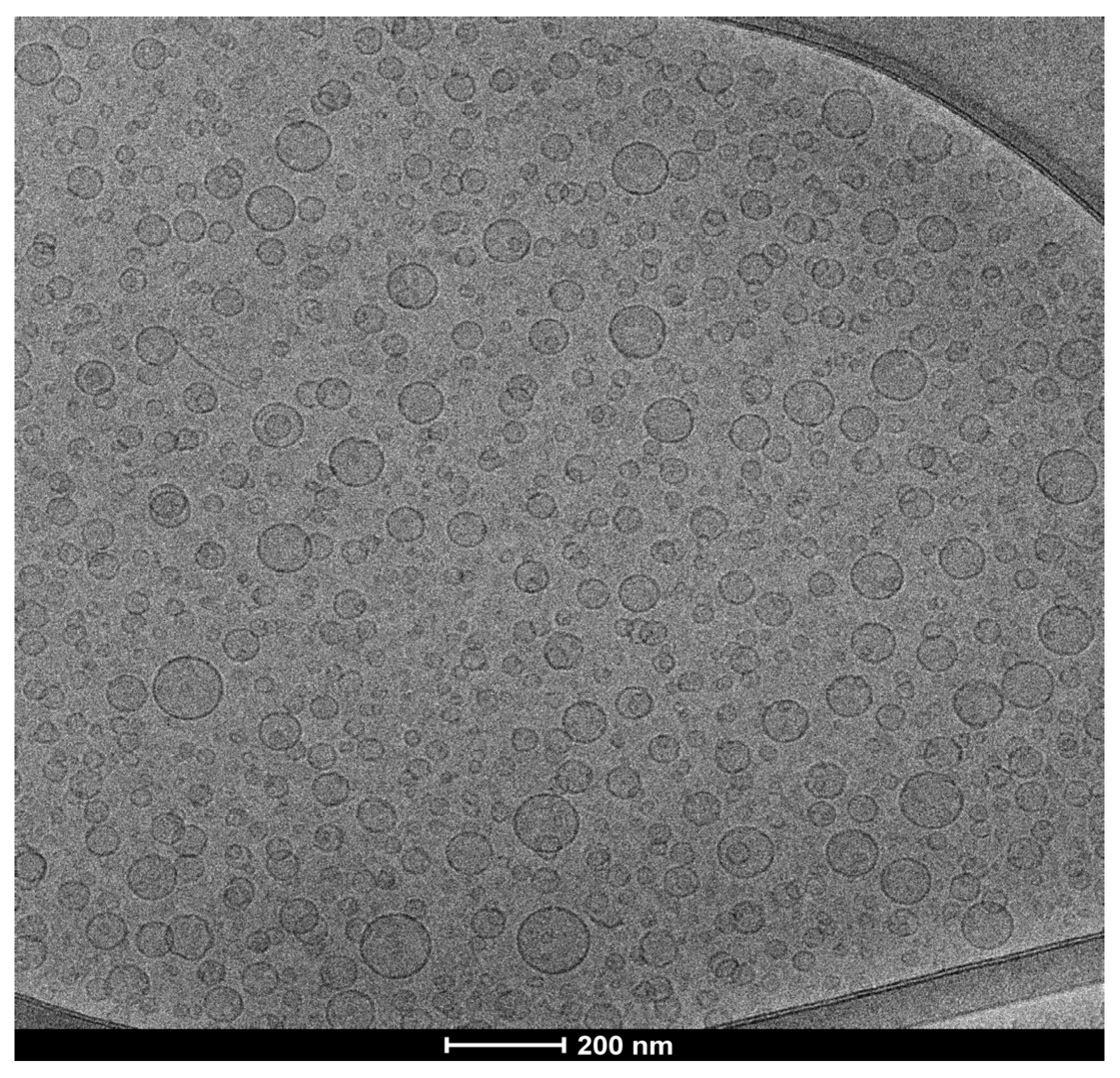
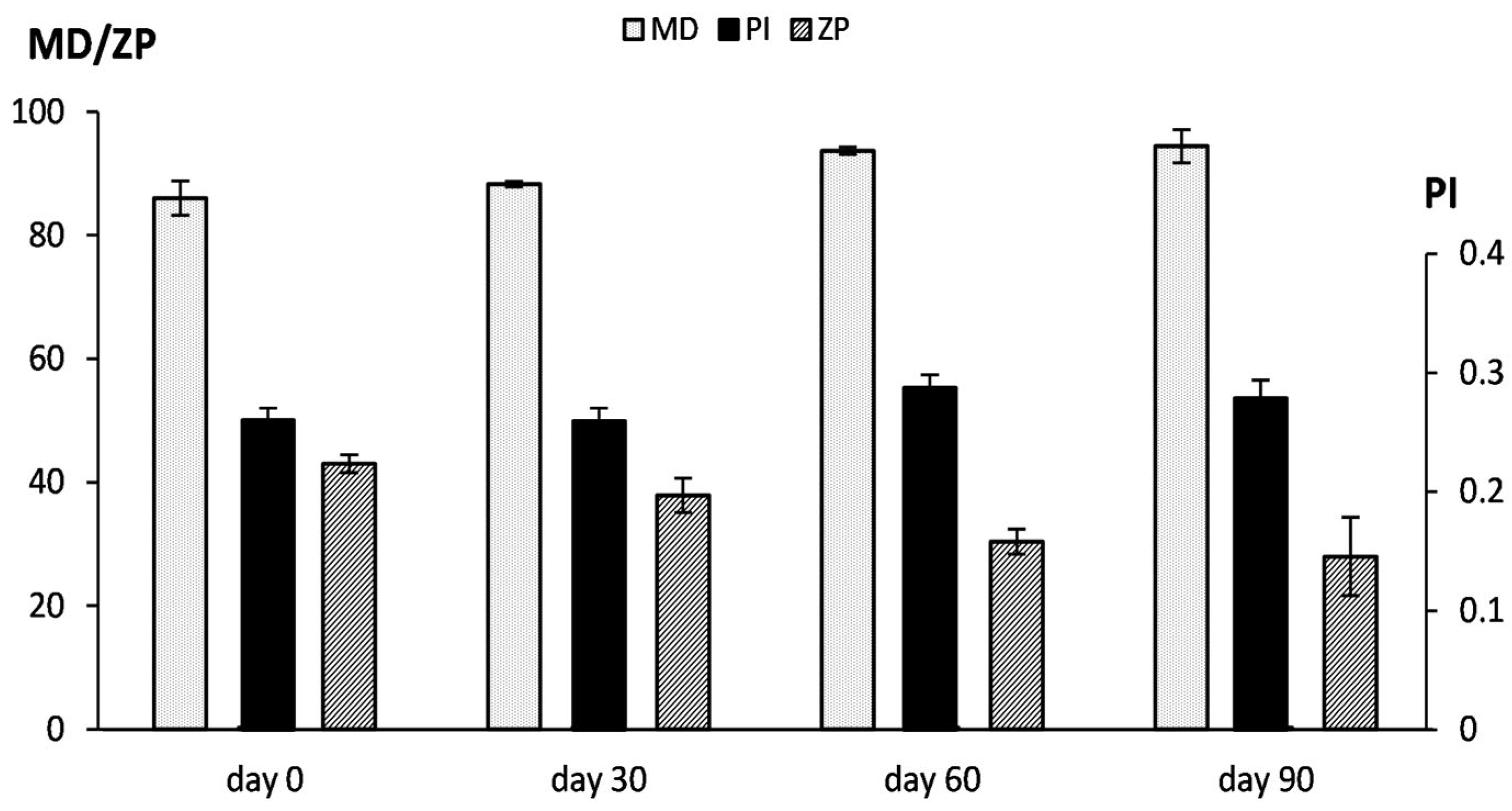
2.6. RDD-142 Formulation in PEG-Liposomes
2.7. Liposomal RDD-142 as a Novel Formulation for a More Effective Antitumor Treatment
2.8. Intracellular Intake of RDD-142
3. Discussion
4. Materials and Methods
4.1. Cell Lines: Culture Conditions and Treatments
4.2. Real-Time Cell Proliferation Monitoring by xCELLigence System
4.3. Cell Cycle Analysis
4.4. Immunoblot Analysis
4.5. Immunofluorescence and Confocal Microscopy Analysis
4.6. Proteasome Activity Assay
4.7. Analysis of Proteasome Activities In Vitro
4.8. Vesicle Preparation and Characterization
4.9. Vesicle Stability During Storage and in Simulated Biological Fluid
4.10. LC-MS/MS Analysis for the Evaluation of Cell Intake
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Amin, N.; Anwar, J.; Sulaiman, A.; Naumova, N.N.; Anwar, N. Hepatocellular Carcinoma: A Comprehensive Review. Diseases 2025, 13, 207. [Google Scholar] [CrossRef]
- Shin, H.; Yu, S.J. A Concise Review of Updated Global Guidelines for the Management of Hepatocellular Carcinoma: 2017–2024. J. Liver Cancer 2025, 25, 19–30. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Danpanichkul, P.; Agopian, V.; Mehta, N.; Parikh, N.D.; Abou-Alfa, G.K.; Singal, A.G.; Yang, J.D. Hepatocellular Carcinoma: Updates on Epidemiology, Surveillance, Diagnosis and Treatment. Clin. Mol. Hepatol. 2025, 31, S228–S254. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, N.; Grimshaw, A.A.; Axson, S.A.; Choe, S.H.; Miller, J.E. Drug Repurposing: A Systematic Review on Root Causes, Barriers and Facilitators. BMC Health Serv. Res. 2022, 22, 970. [Google Scholar] [CrossRef] [PubMed]
- Al Khzem, A.H.; Gomaa, M.S.; Alturki, M.S.; Tawfeeq, N.; Sarafroz, M.; Alonaizi, S.M.; Al Faran, A.; Alrumaihi, L.A.; Alansari, F.A.; Alghamdi, A.A. Drug Repurposing for Cancer Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 12441. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.L. Drug Repurposing for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 92. [Google Scholar] [CrossRef]
- Bernstein, W.B.; Dennis, P.A. Repositioning HIV Protease Inhibitors as Cancer Therapeutics. Curr. Opin. HIV AIDS 2008, 3, 666–675. [Google Scholar] [CrossRef]
- Chow, W.A.; Jiang, C.; Guan, M. Anti-HIV Drugs for Cancer Therapeutics: Back to the Future? Lancet Oncol. 2009, 10, 61–71. [Google Scholar] [CrossRef]
- Subeha, M.R.; Telleria, C.M. The Anti-Cancer Properties of the Hiv Protease Inhibitor Nelfinavir. Cancers 2020, 12, 3437. [Google Scholar] [CrossRef]
- Pereira, M.; Vale, N. Exploring Darunavir, Rilpivirine and Etravirine as Potential Therapies for Bladder Cancer: Efficacy and Synergistic Effects. Biomedicines 2024, 12, 647. [Google Scholar] [CrossRef]
- Pyrko, P.; Kardosh, A.; Wang, W.; Xiong, W.; Schönthal, A.H.; Chen, T.C. HIV-1 Protease Inhibitors Nelfinavir and Atazanavir Induce Malignant Glioma Death by Triggering Endoplasmic Reticulum Stress. Cancer Res. 2007, 67, 10920–10928. [Google Scholar] [CrossRef]
- McLean, K.; VanDeVen, N.A.; Sorenson, D.R.; Daudi, S.; Liu, J.R. The HIV Protease Inhibitor Saquinavir Induces Endoplasmic Reticulum Stress, Autophagy, and Apoptosis in Ovarian Cancer Cells. Gynecol. Oncol. 2009, 112, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Auyeung, A.; Lee, D.L.; Lambert, P.F.; Carchman, E.H.; Sherer, N.M. HIV-1 Protease Inhibitors Slow Hpv16-driven Cell Proliferation through Targeted Depletion of Viral E6 and E7 Oncoproteins. Cancers 2021, 13, 949. [Google Scholar] [CrossRef] [PubMed]
- Lopiccolo, J.; Kawabata, S.; Gills, J.J.; Dennis, P.A. Combining Nelfinavir with Chloroquine Inhibits In Vivo Growth of Human Lung Cancer Xenograft Tumors. Vivo 2021, 35, 141–145. [Google Scholar] [CrossRef]
- Yu, L.; Han, W.; Zhang, J.; Liu, G.; Li, H.; Xu, Y.; Liu, F.; Sun, S. Repurposing HIV Protease Inhibitors as Senotherapeutic Agents in Cervical Cancer: Dual Targeting of CDK1/6-Cell Cycle Arrest and P53/P21/P16 Signaling Axis. Biochem. Biophys. Res. Commun. 2025, 771, 152040. [Google Scholar] [CrossRef]
- Narayanan, S.; Cai, C.Y.; Assaraf, Y.G.; Guo, H.Q.; Cui, Q.; Wei, L.; Huang, J.J.; Ashby, C.R.; Chen, Z.S. Targeting the Ubiquitin-Proteasome Pathway to Overcome Anti-Cancer Drug Resistance. Drug Resist. Updates 2020, 48, 100663. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical Liposomal Drug Delivery: A Review of New Delivery Systems and a Look at the Regulatory Landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Jensen, G.M.; Hodgson, D.F. Opportunities and Challenges in Commercial Pharmaceutical Liposome Applications. Adv. Drug Deliv. Rev. 2020, 154–155, 2–12. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of Liposomes in Medicine and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Hamidi, M. Passive and Active Targeting in Cancer Therapy by Liposomes and Lipid Nanoparticles. Drug Metab. Pers. Ther. 2019, 34. [Google Scholar] [CrossRef]
- Lee, M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, R.; Funicello, M.; Laurita, T.; Lupattelli, P.; Berti, F.; Chiummiento, L. The Pseudo-Symmetric N-benzyl Hydroxyethylamine Core in a New Series of Heteroarylcarboxyamide HIV-1 Pr Inhibitors: Synthesis, Molecular Modelling and Biological Evaluation. Biomolecules 2021, 11, 1584. [Google Scholar] [CrossRef]
- Rinaldi, R.; Miglionico, R.; Nigro, I.; D’Orsi, R.; Chiummiento, L.; Funicello, M.; Lupattelli, P.; Laurenzana, I.; Sgambato, A.; Monné, M.; et al. Two Novel Precursors of the Hiv-1 Protease Inhibitor Darunavir Target the Upr/Proteasome System in Human Hepatocellular Carcinoma Cell Line Hepg2. Cells 2021, 10, 3052. [Google Scholar] [CrossRef]
- Dan, S.; Yoshimi, H.; Okamura, M.; Mukai, Y.; Yamori, T. Inhibition of PI3K by ZSTK474 Suppressed Tumor Growth Not via Apoptosis but G0/G1 Arrest. Biochem. Biophys. Res. Commun. 2009, 379, 104–109. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Ochocka, J.R. Real-time cell analysis system in cytotoxicity applications: Usefulness and comparison with tetrazolium salt assays. Toxicol. Rep. 2020, 7, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Guégan, J.P.; Frémin, C.; Baffet, G. The MAPK MEK1/2-ERK1/2 Pathway and Its Implication in Hepatocyte Cell Cycle Control. Int. J. Hepatol. 2012, 2012, 328372. [Google Scholar] [CrossRef]
- Ko, J.K.; Choi, C.H.; Kim, Y.K.; Kwon, C.H. The Proteasome Inhibitor Mg-132 Induces Aif Nuclear Translocation through down-Regulation of ERK and Akt/MTOR Pathway. Neurochem. Res. 2011, 36, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Su, T.; Lv, D.; Xie, F.; Liu, W.; Cao, J.; Sheikh, I.A.; Qin, X.; Li, L.; Chen, L. ERK1/2 Mediates Lung Adenocarcinoma Cell Proliferation and Autophagy Induced by Apelin-13. Acta Biochim. Biophys. Sin. 2014, 46, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhang, H.; Yu, Q.; Liu, G.; Long, M.; Zhang, K.; Liu, W.; Song, R.; Bian, J.; Gu, J.; et al. ERK1/2 MAPK Promotes Autophagy to Suppress ER Stress-Mediated Apoptosis Induced by Cadmium in Rat Proximal Tubular Cells. Toxicol. Vitr. 2018, 52, 60–69. [Google Scholar] [CrossRef]
- Laplante, G.; Zhang, W. Targeting the Ubiquitin-Proteasome System for Cancer Therapeutics by Small-Molecule Inhibitors. Cancers 2021, 13, 3079. [Google Scholar] [CrossRef]
- Atta, H.; Alzahaby, N.; Hamdy, N.M.; Emam, S.H.; Sonousi, A.; Ziko, L. New Trends in Synthetic Drugs and Natural Products Targeting 20S Proteasomes in Cancers. Bioorg. Chem. 2023, 133, 106427. [Google Scholar] [CrossRef]
- Chen, X.; Dou, Q.P.; Liu, J.; Tang, D. Targeting Ubiquitin–Proteasome System with Copper Complexes for Cancer Therapy. Front. Mol. Biosci. 2021, 8, 649151. [Google Scholar] [CrossRef]
- Oerlemans, R.; Franke, N.E.; Assaraf, Y.G.; Cloos, J.; Van Zantwijk, I.; Berkers, C.R.; Scheffer, G.L.; Debipersad, K.; Vojtekova, K.; Lemos, C.; et al. Molecular Basis of Bortezomib Resistance: Proteasome Subunit 25 (PSMB5) Gene Mutation and Overexpression of PSMB5 Protein. Blood 2008, 112, 2489–2499. [Google Scholar] [CrossRef]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, Biocompatibility and Antioxidant Activity of PEG-Modified Liposomes Containing Resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef]
- Caddeo, C.; Miglionico, R.; Rinaldi, R.; Nigro, I.; Lamorte, D.; Chiummiento, L.; Lupattelli, P.; Funicello, M.; D’Orsi, R.; Valenti, D.; et al. PEGylated Liposomes Loaded with Carbamate Inhibitor ANP0903 Trigger Apoptosis by Enhancing ER Stress in HepG2 Cancer Cells. Int. J. Mol. Sci. 2023, 24, 4552. [Google Scholar] [CrossRef]
- Maksimovic-Ivanic, D.; Fagone, P.; McCubrey, J.; Bendtzen, K.; Mijatovic, S.; Nicoletti, F. HIV-Protease Inhibitors for the Treatment of Cancer: Repositioning HIV Protease Inhibitors While Developing More Potent NO-Hybridized Derivatives? Int. J. Cancer 2017, 140, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Du, L.; Pham, P.; Zhu, B.; Jiang, S. Nelfinavir, an HIV Protease Inhibitor, Induces Apoptosis and Cell Cycle Arrest in Human Cervical Cancer Cells via the ROS-Dependent Mitochondrial Pathway. Cancer Lett. 2015, 364, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Besse, A.; Sedlarikova, L.; Buechler, L.; Kraus, M.; Yang, C.H.; Strakova, N.; Soucek, K.; Navratil, J.; Svoboda, M.; Welm, A.L.; et al. HIV-Protease Inhibitors Potentiate the Activity of Carfilzomib in Triple-Negative Breast Cancer. Br. J. Cancer 2024, 131, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Small, G.W.; Shi, Y.Y. Evidence That Inhibition of P44/42 Mitogen-Activated Protein Kinase Signaling is a Factor in Proteasome Inhibitor-Mediated Apoptosis. J. Biol. Chem. 2002, 277, 27864–27871. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, B.H.; Ahn, S.G.; Oh, S.H. Proteasome Inhibition-Induced P38 MAPK/ERK Signaling Regulates Autophagy and Apoptosis through the Dual Phosphorylation of Glycogen Synthase Kinase 3β. Biochem. Biophys. Res. Commun. 2012, 418, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 Links the Autophagy Pathway and the Ubiqutin-Proteasome System upon Ubiquitinated Protein Degradation. Cell Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Athonvarangkul, D.; Mishall, P.; Sahu, S.; Singh, R. Autophagy Proteins Regulate ERK Phosphorylation. Nat. Commun. 2013, 4, 2799. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, S.K.; Wu, P.K.; Richards, A.L.; Jackson, W.T.; Park, J.I. Raf/MEK/ERK Can Regulate Cellular Levels of LC3B and SQSTM1/P62 at Expression Levels. Exp. Cell Res. 2014, 327, 340. [Google Scholar] [CrossRef]
- Coppola, F.; Monaci, S.; Falsini, A.; Aldinucci, C.; Filippi, I.; Rossi, D.; Carraro, F.; Naldini, A. SQSTM1/P62 Inhibition Impairs pro-Survival Signaling in Hypoxic Human Dendritic Cells. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2024, 1871, 119625. [Google Scholar] [CrossRef]
- Della Sala, G.; Pacelli, C.; Agriesti, F.; Laurenzana, I.; Tucci, F.; Tamma, M.; Capitanio, N.; Piccoli, C. Unveiling Metabolic Vulnerability and Plasticity of Human Osteosarcoma Stem and Differentiated Cells to Improve Cancer Therapy. Biomedicines 2022, 10, 28. [Google Scholar] [CrossRef]
- Mazzoccoli, C.; Crispo, F.; Laurenzana, I.; Pietrafesa, M.; Sisinni, L.; Lerose, R.; Telesca, D.; Milella, M.R.; Liu, T.; Della Sala, G.; et al. Biological Evaluation of [4-(4-Aminophenyl)-1-(4-Fluorophenyl)-1H-Pyrrol-3-Yl](3,4,5-Trimethoxyphenyl)Methanone as Potential Antineoplastic Agent in 2D and 3D Breast Cancer Models. Arch. Pharm. 2023, 356, e2300354. [Google Scholar] [CrossRef]
- Landriscina, M.; Laudiero, G.; Maddalena, F.; Amoroso, M.R.; Piscazzi, A.; Cozzolino, F.; Monti, M.; Garbi, C.; Fersini, A.; Pucci, P.; et al. Mitochondrial Chaperone Trap1 and the Calcium Binding Protein Sorcin Interact and Protect Cells against Apoptosis Induced by Antiblastic Agents. Cancer Res. 2010, 70, 6577–6586. [Google Scholar] [CrossRef] [PubMed]
- Kisselev, A.F.; Goldberg, A.L. Monitoring Activity and Inhibition of 26S Proteasomes with Fluorogenic Peptide Substrates. Methods Enzymol. 2005, 398, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yang, H.; Yang, K.; Zhang, B. Pitting Corrosion Resistance of La Added 316L Stainless Steel in Simulated Body Fluids. Mater. Lett. 2007, 61, 1154–1157. [Google Scholar] [CrossRef]
- Pappalardo, I.; Santarsiero, A.; De Luca, M.; Acquavia, M.A.; Todisco, S.; Caddeo, C.; Bianco, G.; Infantino, V.; Martelli, G.; Vassallo, A. Exploiting the Anti-Inflammatory Potential of White Capsicum Extract by the Nanoformulation in Phospholipid Vesicles. Antioxidants 2021, 10, 1683. [Google Scholar] [CrossRef] [PubMed]


| Formulation | MD nm ± SD | PI ± SD | ZP mV ± SD |
|---|---|---|---|
| Empty PEG-liposomes | 91 ± 4.6 | 0.28 ± 0.03 | −25 ± 4.1 |
| RDD-142 PEG-liposomes | * 86 ± 3.2 | 0.26 ± 0.01 | ** +43 ± 2.5 |
| Empty liposomes | §§§ 82 ± 1.5 | 0.29 ± 0.01 | §§§ −6 ± 0.5 |
| RDD-142 liposomes | ###° 76 ± 3.2 | ###°° 0.32 ± 0.01 | ##°° +49 ± 2.5 |
| Formulation | Time | MD (nm ± SD) | PI ± SD | ZP (mV ± SD) |
|---|---|---|---|---|
| Empty PEG-liposomes | t0 | 87 ± 0.9 | 0.21 ± 0.01 | −2.4 ± 0.5 |
| t24 | 86 ± 0.7 | 0.21 ± 0.01 | −2.2 ± 0.2 | |
| RDD-142 PEG-liposomes | t0 | 85 ± 2.2 | 0.22 ± 0.02 | −0.2 ± 0.03 |
| t24 | 83 ± 2.5 | 0.20 ± 0.01 | −0.2 ± 0.06 |
| Formulation | P90G | DPPC | MPEG-5000-DPPE | RDD142 | H2O |
|---|---|---|---|---|---|
| Empty PEG-liposomes | 40 mg | 20 mg | 2 mg | -- | 1 mL |
| RDD-142 PEG-liposomes | 40 mg | 20 mg | 2 mg | 5 mM | 1 mL |
| Empty liposomes | 40 mg | 20 mg | -- | -- | 1 mL |
| RDD-142 liposomes | 40 mg | 20 mg | -- | 5 mM | 1 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crispo, F.; Vassallo, A.; Faraone, I.; Santarsiere, A.; Chiummiento, L.; Martinelli, M.; Cascelli, N.; Fernàndez-Busquets, X.; Miglionico, R.; Nigro, I.; et al. Repurposing HIV-Protease Inhibitor Precursors as Anticancer Agents: The Synthetic Molecule RDD-142 Delays Cell Cycle Progression and Induces Autophagy in HepG2 Cells with Enhanced Efficacy via Liposomal Formulation. Int. J. Mol. Sci. 2025, 26, 10305. https://doi.org/10.3390/ijms262110305
Crispo F, Vassallo A, Faraone I, Santarsiere A, Chiummiento L, Martinelli M, Cascelli N, Fernàndez-Busquets X, Miglionico R, Nigro I, et al. Repurposing HIV-Protease Inhibitor Precursors as Anticancer Agents: The Synthetic Molecule RDD-142 Delays Cell Cycle Progression and Induces Autophagy in HepG2 Cells with Enhanced Efficacy via Liposomal Formulation. International Journal of Molecular Sciences. 2025; 26(21):10305. https://doi.org/10.3390/ijms262110305
Chicago/Turabian StyleCrispo, Fabiana, Antonio Vassallo, Immacolata Faraone, Alessandro Santarsiere, Lucia Chiummiento, Mara Martinelli, Nicoletta Cascelli, Xavier Fernàndez-Busquets, Rocchina Miglionico, Ilaria Nigro, and et al. 2025. "Repurposing HIV-Protease Inhibitor Precursors as Anticancer Agents: The Synthetic Molecule RDD-142 Delays Cell Cycle Progression and Induces Autophagy in HepG2 Cells with Enhanced Efficacy via Liposomal Formulation" International Journal of Molecular Sciences 26, no. 21: 10305. https://doi.org/10.3390/ijms262110305
APA StyleCrispo, F., Vassallo, A., Faraone, I., Santarsiere, A., Chiummiento, L., Martinelli, M., Cascelli, N., Fernàndez-Busquets, X., Miglionico, R., Nigro, I., Caddeo, C., & Armentano, M. F. (2025). Repurposing HIV-Protease Inhibitor Precursors as Anticancer Agents: The Synthetic Molecule RDD-142 Delays Cell Cycle Progression and Induces Autophagy in HepG2 Cells with Enhanced Efficacy via Liposomal Formulation. International Journal of Molecular Sciences, 26(21), 10305. https://doi.org/10.3390/ijms262110305












