Transcriptome and Targeted Hormone Metabolome Reveal the Mechanism of Flower Abscission in Soybeans Under Shade
Abstract
1. Introduction
2. Results
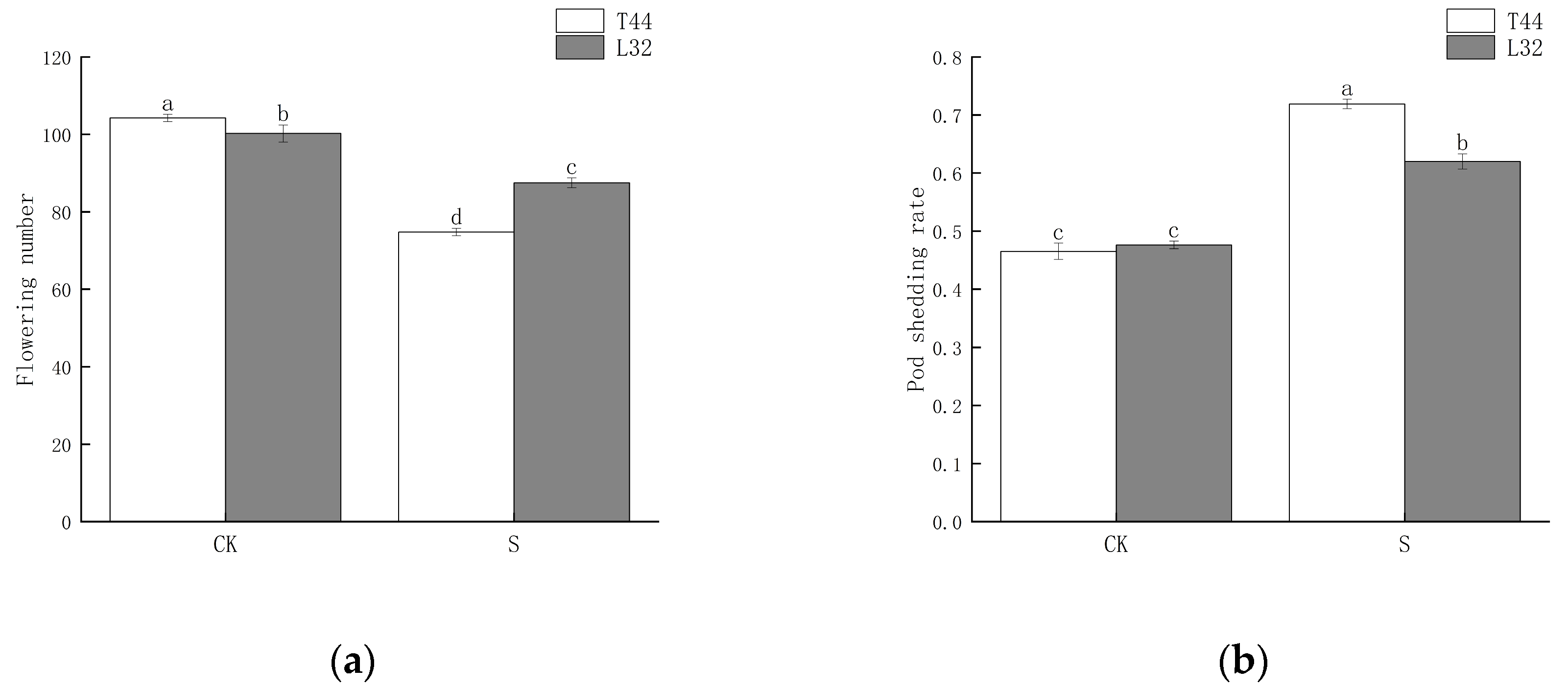
2.1. Effect of Shade Treatment on Soybean Flower and Pod Abscission
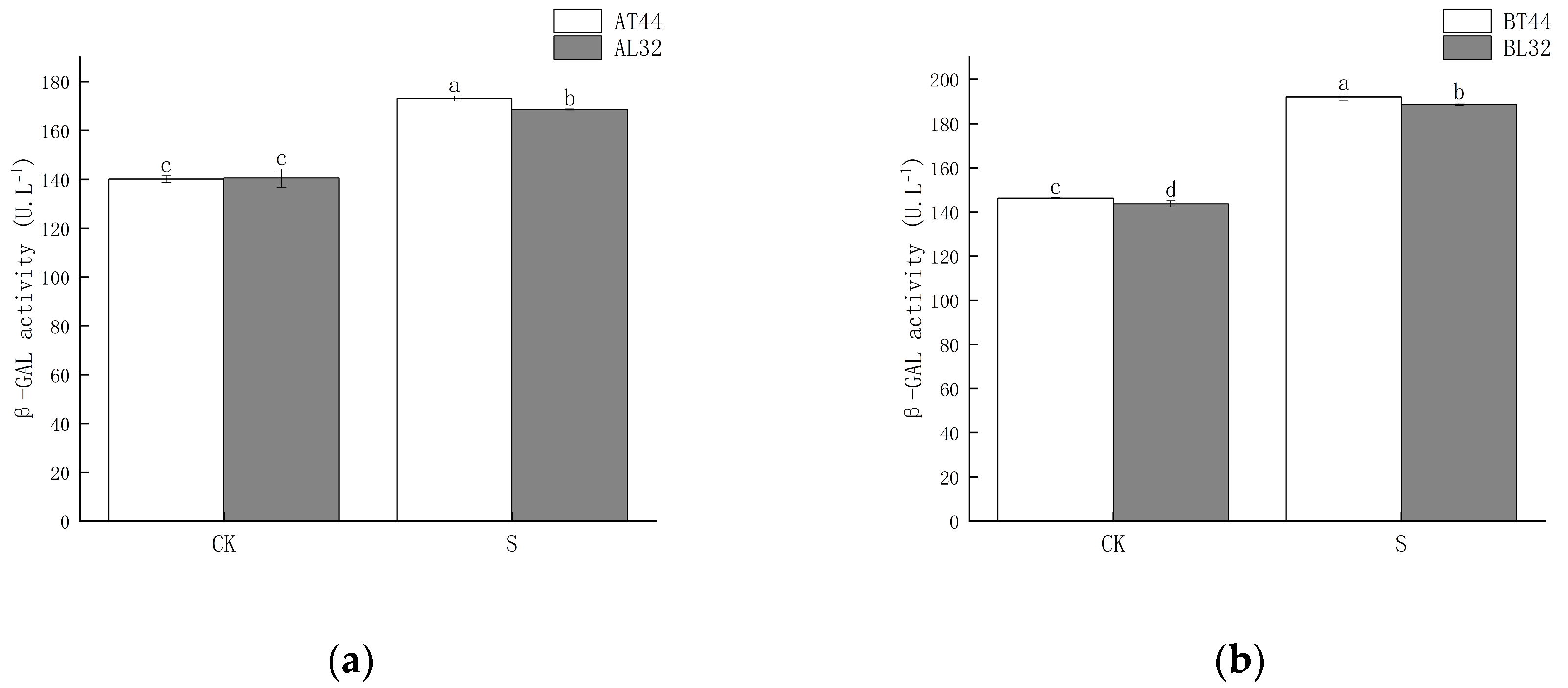
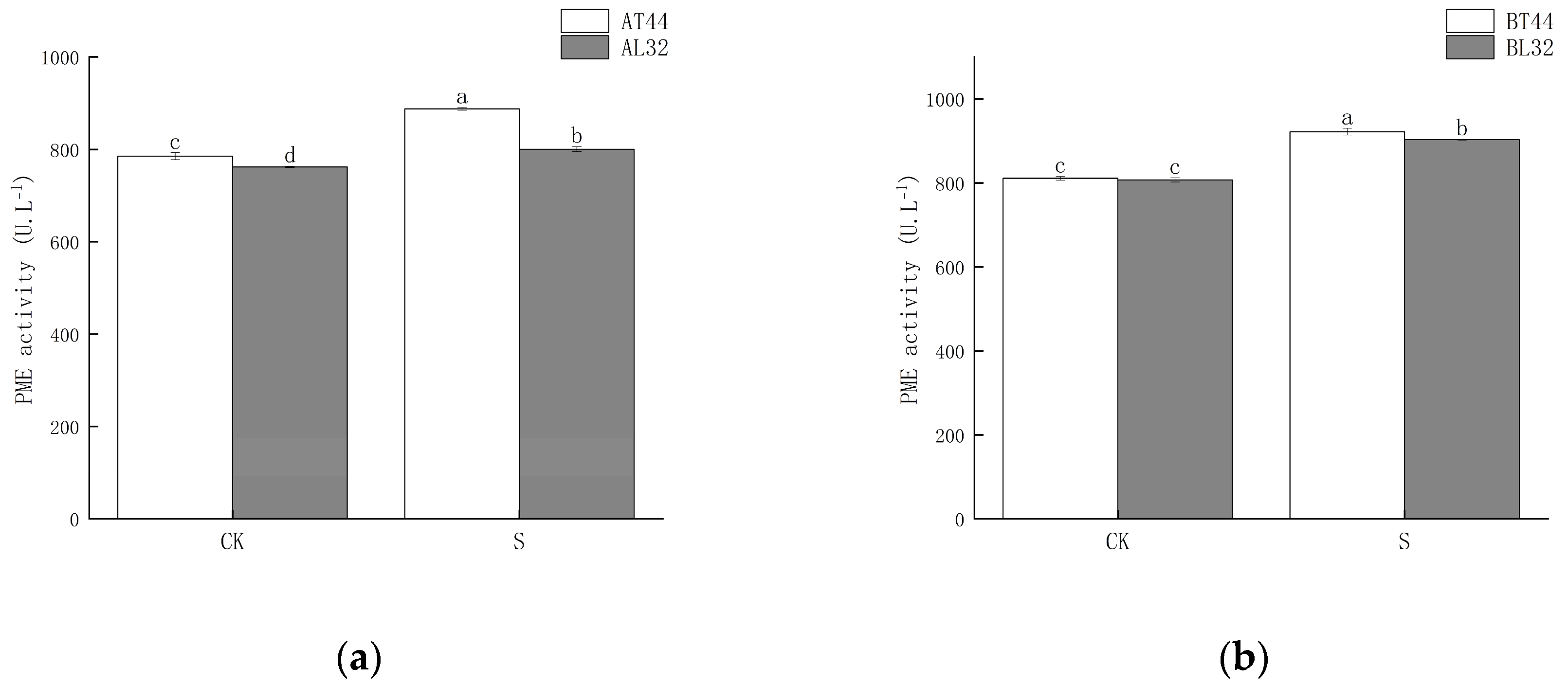
2.2. Effect of Shade Treatment on Soybean Abscission-Related Enzyme Activities
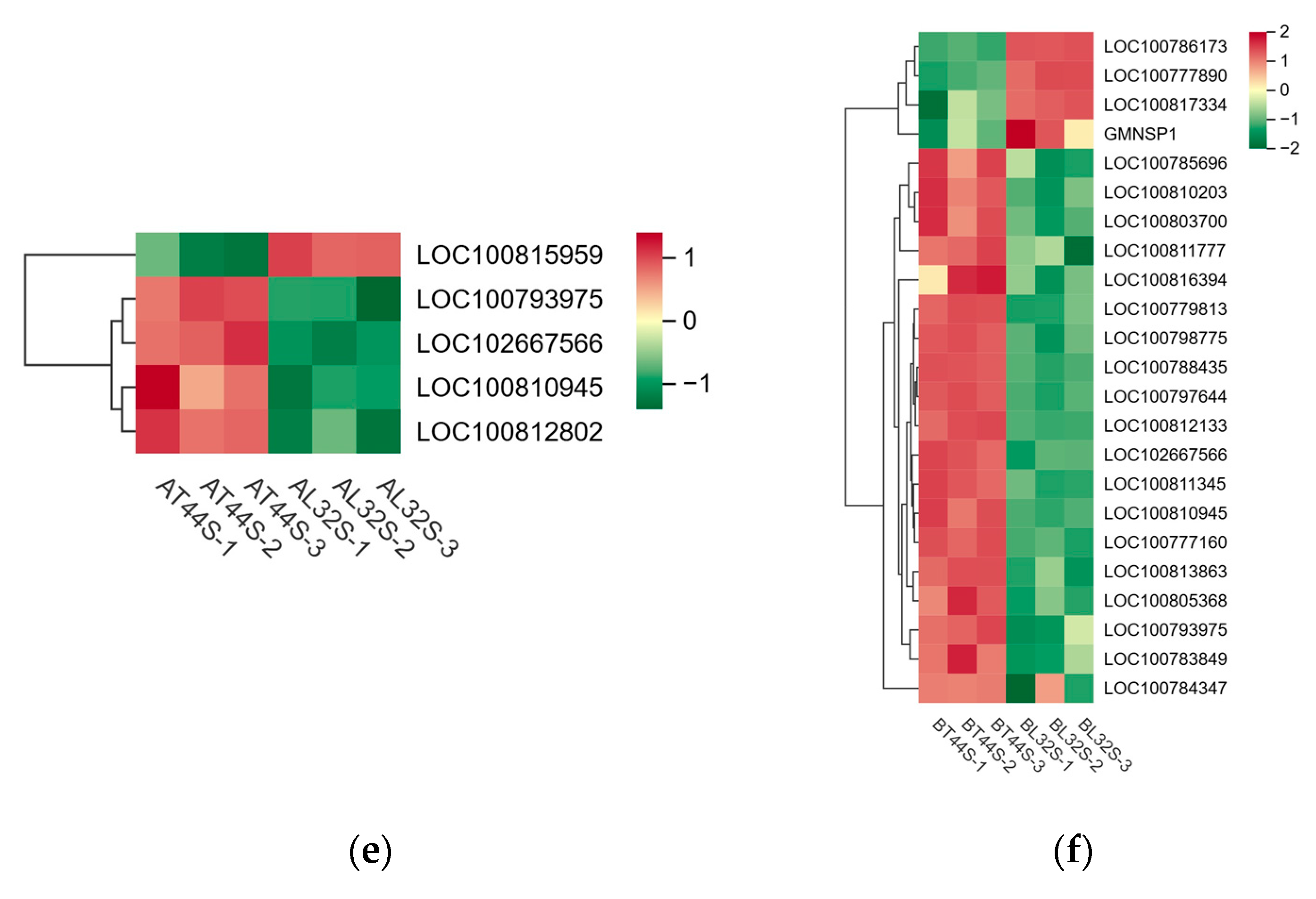
2.3. Transcriptome Difference and Enrichment Analysis
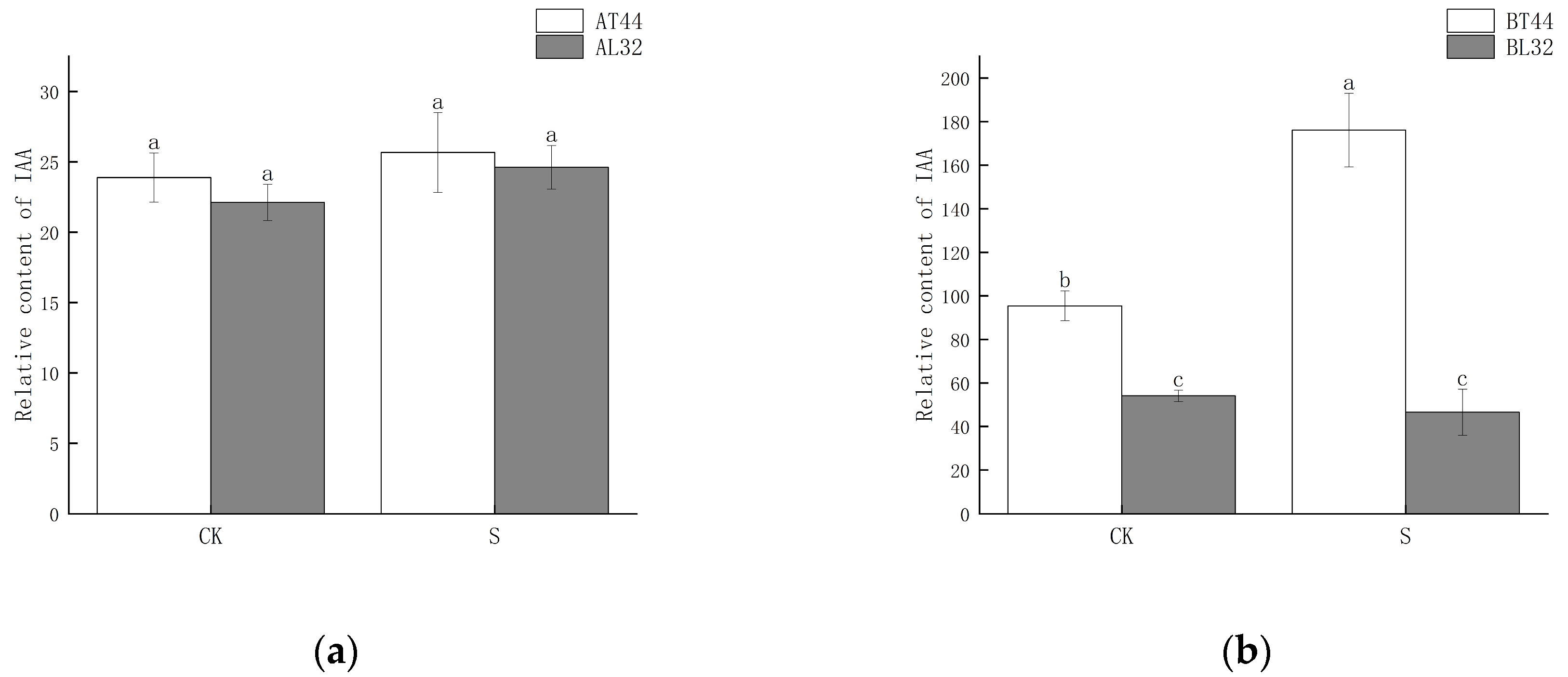
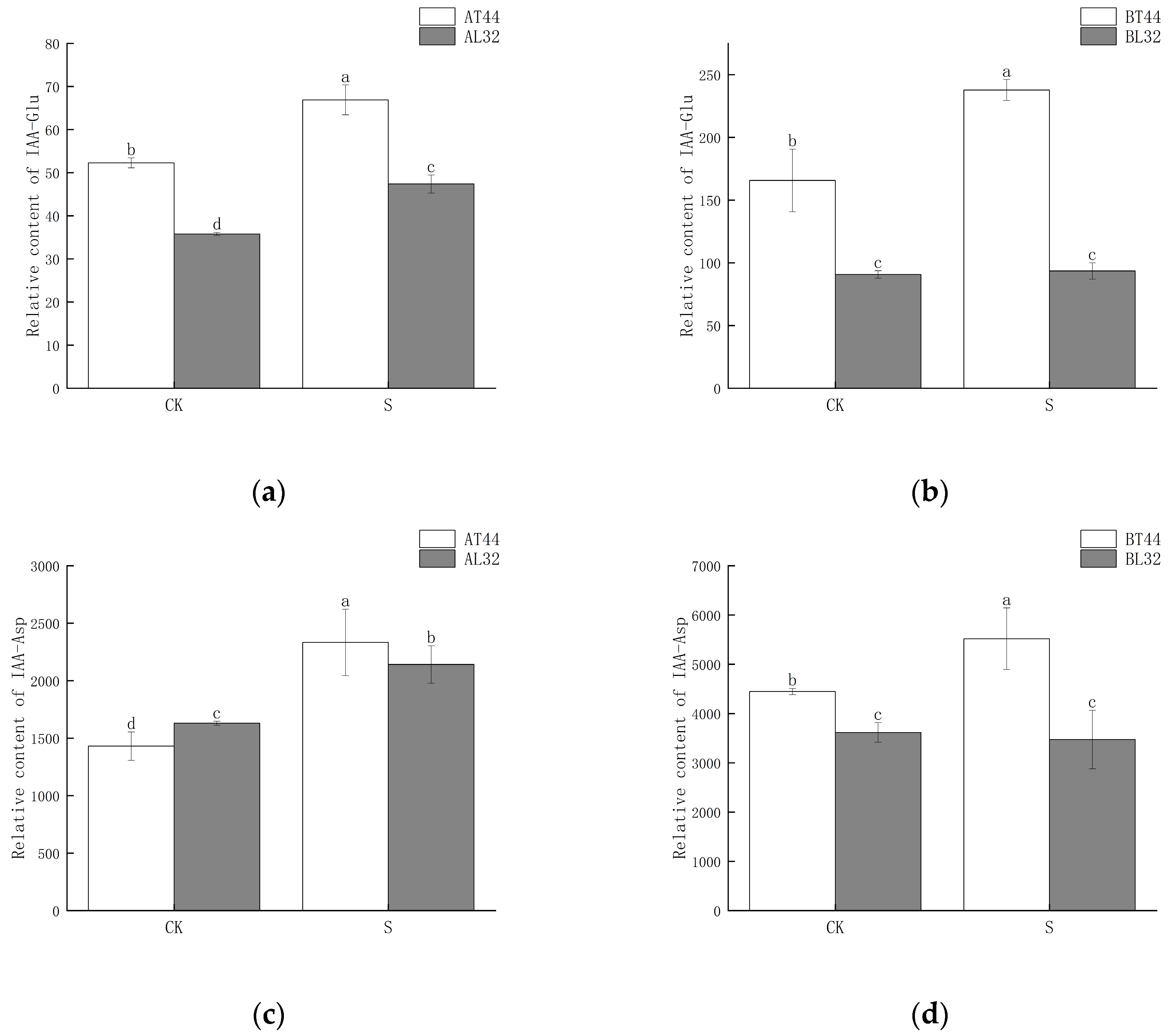
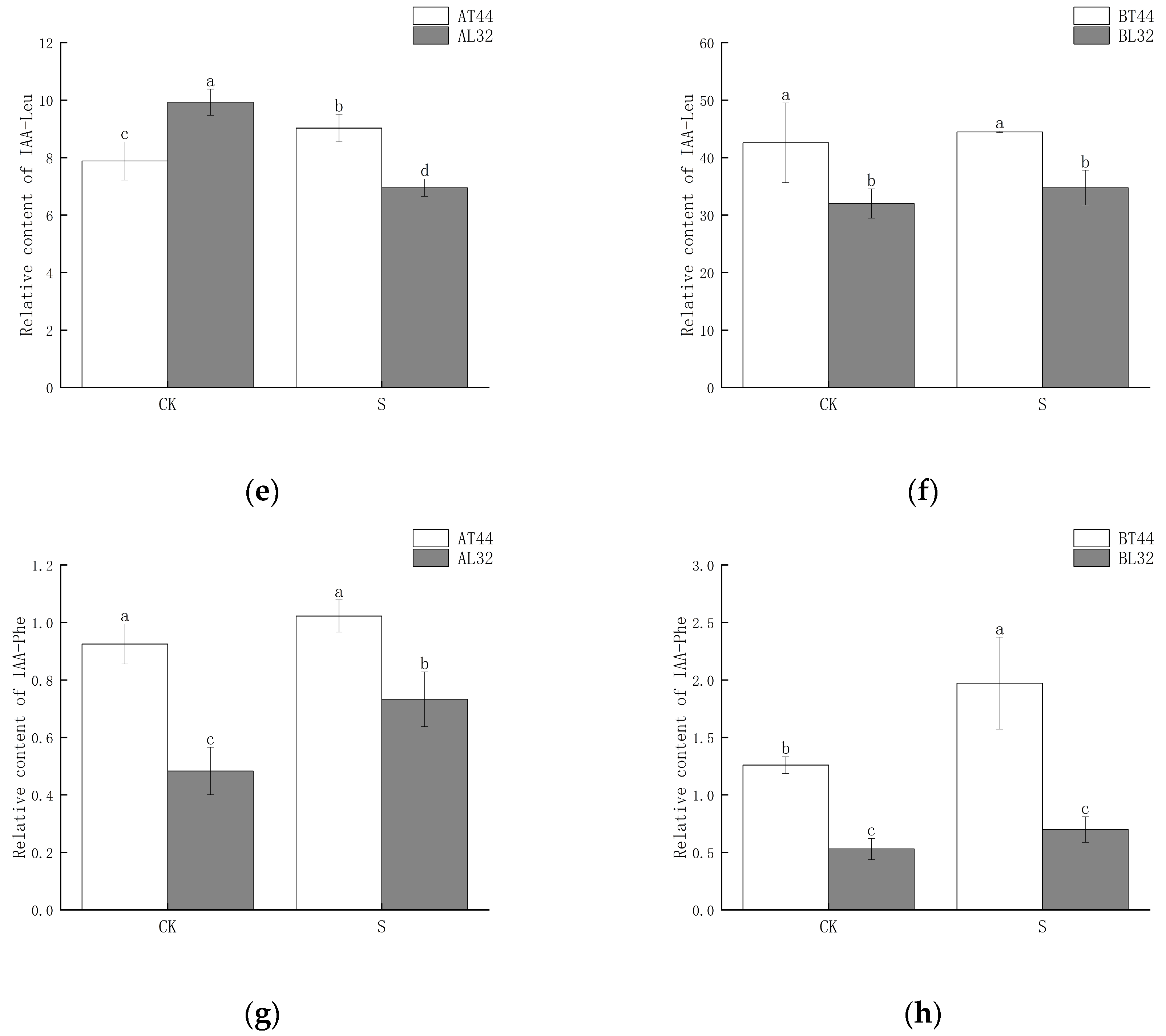
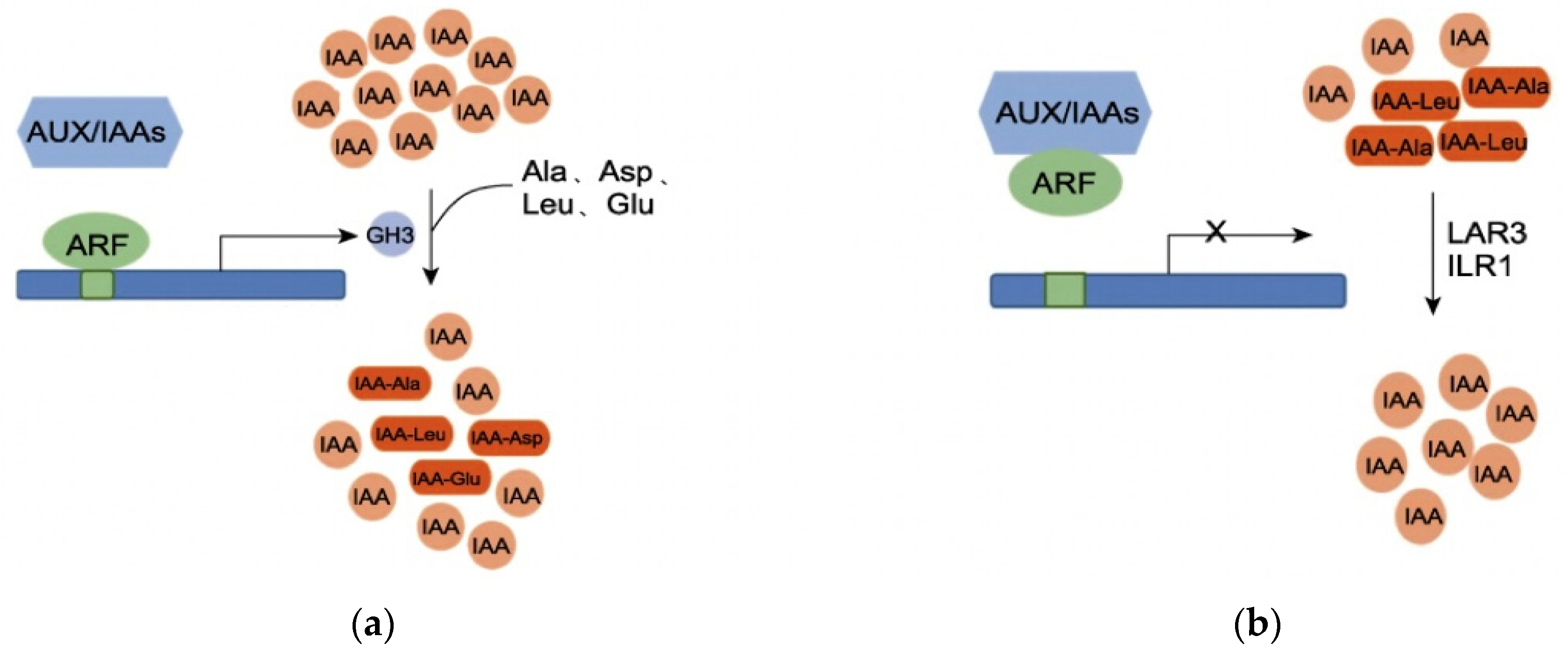
2.4. Changes in Auxin (IAA) Content and Expression of Its Response Genes
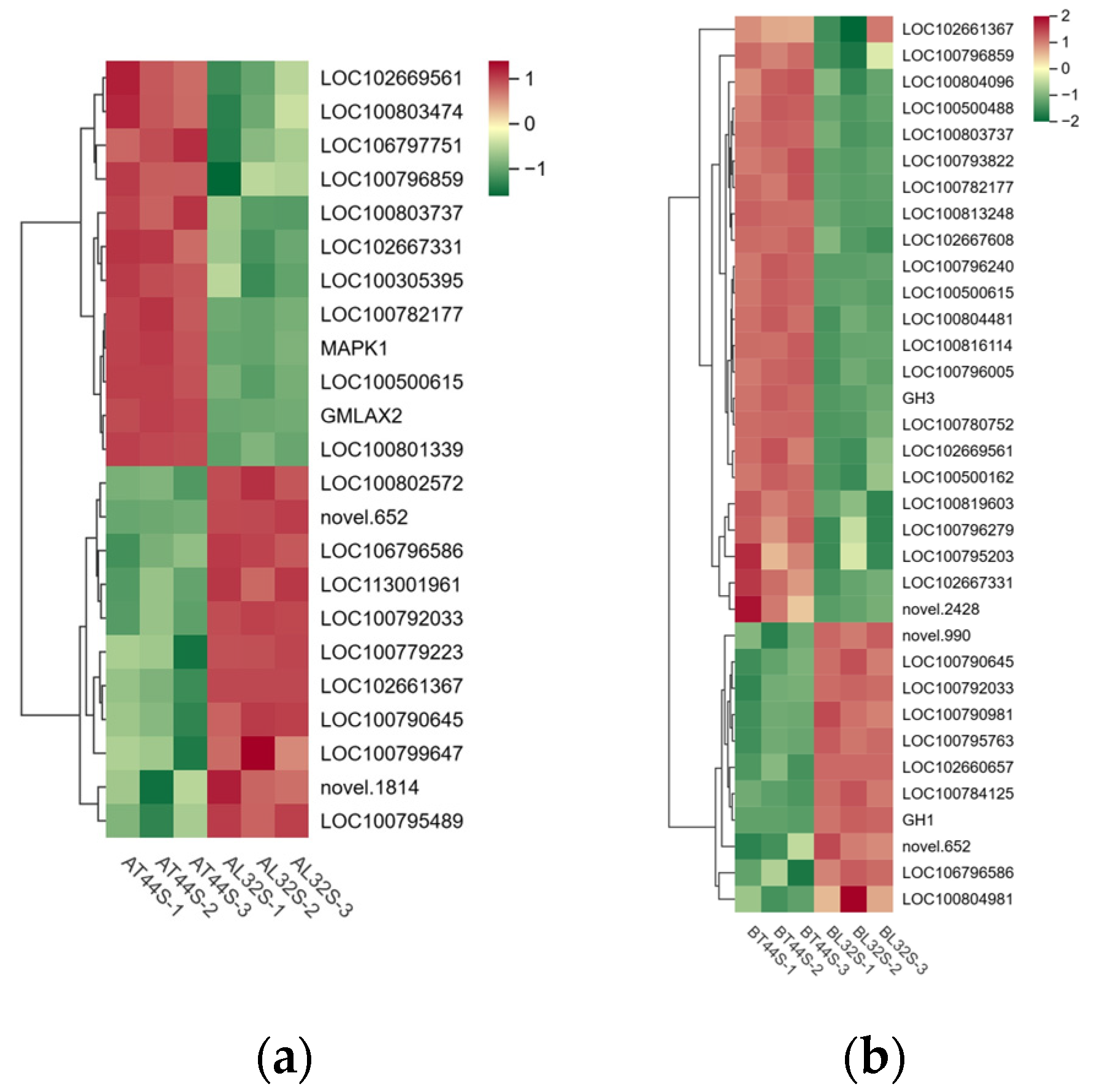
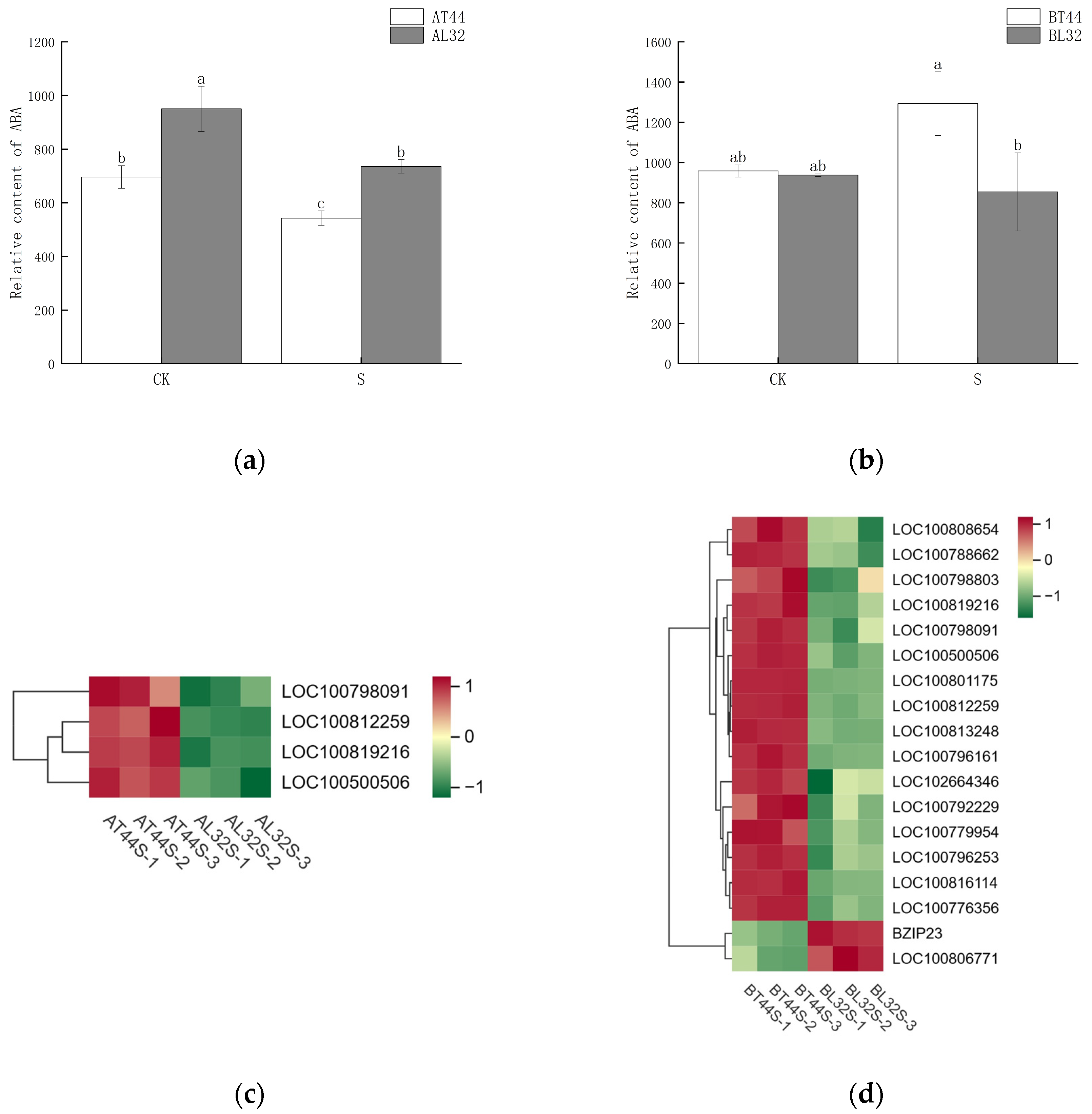
2.5. Hormone-Related Genes Involved in Soybean Flower Abscission
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sample Collection
4.2. Determination of Abscission-Related Enzyme Activities
4.3. Transcriptome Sequencing and Analysis
4.4. Analysis of Gene Expression by RT-qPCR
4.5. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Wu, C.; Hu, P.; Hou, W.; Zu, W.; Han, T. Morphological and anatomic characteristics on terminal raceme development of soybean cultivars with different stem termination types. Acta Agron. Sin. 2014, 40, 1117–1124. [Google Scholar] [CrossRef]
- Liu, B.; Qu, D.N. Effects of shading on spatial distribution of flower and flower abscission in field-grown three soybeans in Northern China. Emirates. Food Agric. 2015, 27, 629–635. [Google Scholar]
- Wang, X.; Wu, X.; Ding, G.; Yang, F.; Yong, T.; Wang, X.; Yang, W. Analysis of grain yield differences among soybean cultivars under maize–soybean intercropping. Agronomy 2020, 10, 110. [Google Scholar] [CrossRef]
- Board, J.E.; Tan, Q. Assimilatory capacity effects on soybean yield components and pod number. Crop Sci. 1995, 35, 846–851. [Google Scholar] [CrossRef]
- Du, Q.; Chen, P.; Zheng, B.; Hu, Y.; Yang, W.; Yong, T. Screening soybean for adaptation to relay intercropping systems: Associations between reproductive organ abscission and yield. Agronomy 2022, 12, 2379. [Google Scholar] [CrossRef]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–340. [Google Scholar] [CrossRef]
- Estornell, L.H.; Agustí, J.; Merelo, P.; Talón, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199, 48–60. [Google Scholar] [CrossRef]
- Burns, K.E.; Cerda-Maira, F.A.; Wang, T.; Li, H.; Bishai, W.R.; Darwin, K.H. “Depupylation” of prokaryotic ubiquitin-like protein from mycobacterial proteasome substrates. Mol. Cell 2010, 39, 821–827. [Google Scholar] [CrossRef]
- Cai, Y.; Meng, J.; Cui, Y.; Tian, M.; Shi, Z.; Wang, J. Transcriptome and targeted hormone metabolome reveal the molecular mechanisms of flower abscission in camellia. Front. Plant Sci. 2022, 13, 1076037. [Google Scholar] [CrossRef]
- Dziurka, K.; Dziurka, M.; Muszyńska, E.; Czyczyło-Mysza, I.; Warchoł, M.; Juzoń, K.; Laskoś, K.; Skrzypek, E. Anatomical and hormonal factors determining the development of haploid and zygotic embryos of oat (Avena sativa L.). Sci. Rep. 2022, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Smékalová, V.; Doskočilová, A.; Komis, G.; Šamaj, J. Crosstalk between secondary messengers, hormones and MAPK modules during abiotic stress signalling in plants. Biotechnol. Adv. 2014, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rivero, G.; Osorio-Montalvo, P.; Sánchez-Borges, R.; Us-Camas, R.; Duarte-Aké, F.; De-la-Peña, C. Plant hormone signaling in flowering: An epigenetic point of view. J. Plant Physiol. 2017, 214, 16–27. [Google Scholar] [CrossRef]
- Peng, M.; Ying, P.; Liu, X.; Li, C.; Xia, R.; Li, J.; Zhao, M. Genome-wide identification of histone modifiers and their expression patterns during fruit abscission in litchi. Front. Plant Sci. 2017, 8, 639. [Google Scholar] [CrossRef]
- Cui, H.; Feng, N.; Sun, F.; Liu, T.; Li, J.; Du, J.; Han, Y.; Zheng, D. Regulation of DTA-6 by abscission cellulase and GmAC gene expression in flowers and pods of soybean. Acta Agron. Sin. 2016, 42, 51–57. [Google Scholar] [CrossRef]
- Dal Cin, V.; Danesin, M.; Botton, A.; Boschetti, A.; Dorigoni, A.; Ramina, A. Ethylene and preharvest drop: The effect of AVG and NAA on fruit abscission in apple (Malus domestica L. Borkh). Plant Growth Regul. 2008, 56, 317–325. [Google Scholar] [CrossRef]
- Aziz, A. Spermidine and related-metabolic inhibitors modulate sugar and amino acid levels in Vitis vinifera L.: Possible relationships with initial fruitlet abscission. J. Exp. Bot. 2003, 54, 355–363. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Han, S.; Zhu, X.; Wang, Y.; Pan, M.; Zhang, W. Research progress on regulatory. Chin. Bull. Bot. 2025, 60, 472–482. [Google Scholar]
- Kućko, A.; Wilmowicz, E.; Pokora, W.; Alché, J.D.D. Disruption of the auxin gradient in the abscission zone area evokes asymmetrical changes leading to flower separation in yellow lupine. Int. J. Mol. Sci. 2020, 21, 3815. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Gurung, G.; Yadav, A.; Laha, R.; Mishra, V.K. Factors associated with citrus fruit abscission and management strategies developed so far: A review. N. Z. J. Crop Hortic. Sci. 2023, 51, 467–488. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Huang, X.; Li, J.; Wang, H.; Li, J. De novo assembly and characterization of fruit transcriptome in Litchi chinensis Sonn and analysis of differentially regulated genes in fruit in response to shading. BMC Genom. 2013, 14, 552. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, D.F.; Li, C.Y.; Luo, J.H.; Zhu, B.F.; Zhu, J.J.; Shangguan, Y.Y.; Wang, Z.X.; Sang, T.; Zhou, B.; et al. Genetic control of seed shattering in rice by the APETALA2 transcription factor SHATTERING ABORTION1. Plant Cell 2012, 24, 1034–1048. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Ma, X.; Zhao, S.S.; Tang, Y.Y.; Liu, F.X.; Gu, P.; Fu, Y.C.; Zhu, Z.F.; Cai, H.W.; Sun, C.Q.; et al. The APETALA2-like transcription factor SUPERNUMERARY BRACT controls rice seed shattering and seed size. Plant Cell 2019, 31, 17–36. [Google Scholar] [CrossRef]
- Lewis, M.W.; Leslie, M.E.; Liljegren, S.J. Plant separation: 50 ways to leave your mother. Curr. Opin. Plant Biol. 2006, 9, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.J.; Roeder, A.H.K.; Ditta, G.S.; Yanofsky, M.F. A novel role for the floral homeotic gene APETALA2 during Arabidopsis fruit development. Development 2011, 138, 5167–5176. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Kimbara, J.; Fujisawa, M.; Kitagawa, M.; Ihashi, N.; Maeda, H.; Kasumi, T.; Ito, Y. MACROCALYX and JOINTLESS interact in the transcriptional regulation of tomato fruit abscission zone development. Plant Physiol. 2012, 158, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Wang, D.; Qin, Z.R.; Zhang, D.D.; Yin, L.J.; Wu, L.; Colasanti, J.; Li, A.L.; Mao, L. The SEPALLATA MADS-box protein SLMBP21 forms protein complexes with JOINTLESS and MACROCALYX as a transcription activator for development of the tomato flower abscission zone. Plant J. 2014, 77, 284–296. [Google Scholar] [CrossRef]
- Sawicki, M.; Aït Barka, E.; Clément, C.; Vaillant-Gaveau, N.; Jacquard, C. Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J. Exp. Bot. 2015, 66, 1707–1719. [Google Scholar] [CrossRef]
- Liu, F.; Jensen, C.R.; Andersen, M.N. Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: Its implication in altering pod set. Field Crops Res. 2004, 86, 1–13. [Google Scholar] [CrossRef]
- Jiang, H.; Egli, D. Shade induced changes in flower and pod number and flower and fruit abscission on in soybean. Agron. J. 1993, 85, 221–225. [Google Scholar] [CrossRef]
- Heindl, J.C.; Brun, W.A. Light and shade effects on abscission and 14C-photoassimilate partitioning among reproductive structures in soybean. Plant Physiol. 1983, 73, 434–439. [Google Scholar] [CrossRef]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Clements, J.C.; Atkins, C.A. Characterization of a Non-abscission Mutant in Lupinus angustifolius L. Physiological Aspects. Ann. Bot. 2001, 88, 629–635. [Google Scholar] [CrossRef][Green Version]
- Bonghi, C.; Rascio, N.; Ramina, A.; Casadoro, G. Cellulase and polygalacturonase involvement in the abscission of leaf and fruit explants of peach. Plant Mol. Biol. 1992, 20, 839–848. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef]
- Kacprzyk, J.; Burke, R.; Schwarze, J.; McCabe, P.F. Plant programmed cell death meets auxin signalling. FEBS J. 2022, 289, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ma, C.; Xu, T.; Reid, M.S.; Jiang, C.Z.; Li, T. Auxin response and transport during induction of pedicel abscission in tomato. Hortic. Res. 2021, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, L.; Wei, S.; Wei, D.; Qin, L.; Li, C. Niujiaojiao and Guijiao No.6, the mechanism of endogenous hormones and main enzymes regulating style shedding. J. South China Agric. 2018, 49, 1351–1357. [Google Scholar]
- Meng, S.; Xiang, H.; Yang, X.; Ye, Y.; Han, L.; Xu, T.; Yufeng, L.; Feng, W.; Tan, C.; Qi, M. Effects of low temperature on pedicel abscission and auxin synthesis key genes of tomato. Int. J. Mol. Sci. 2023, 24, 9186. [Google Scholar] [CrossRef]
- Qin, G.; Gu, H.; Zhao, Y.; Ma, Z.; Shi, G.; Yang, Y.; Pichersky, E.; Chen, H.; Liu, M.; Chen, Z.; et al. An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell 2005, 17, 2693–2704. [Google Scholar] [CrossRef]
- Yuan, Y.; Enhebayaer; Qi, Y. Research progress on biological function of plant GH3 gene family. Acta Bot. 2023, 58, 770–782. [Google Scholar]
- Westfall, C.S.; Herrmann, J.; Chen, Q.; Wang, S.; Jez, J.M. Modulating plant hormones by enzyme action: The GH3 family of acyl acid amido synthetases. Plant Signal. Behav. 2010, 5, 1607–1612. [Google Scholar] [CrossRef]
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Huang, X.; Li, J.; Wang, H.; Li, J. An improved fruit transcriptome and the identification of the candidate genes involved in fruit abscission induced by carbohydrate stress in litchi. Front. Plant Sci. 2015, 6, 439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Dardick, C.D.; Beers, E.P.; Callanhan, A.M.; Xia, R.; Yuan, R. Transcriptomics of shading-induced and NAA-induced abscission in apple (Malus domestica) reveals a shared pathway involving reduced photosynthesis, alterations in carbohydrate transport and signaling and hormone crosstalk. BMC Plant Biol. 2011, 11, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zeng, L.; Xiao, Q.; Shi, S. Changes of fruit abscission and carbohydrate, ABA and related genes expression in the pericarp and fruit abscission zone of longan under starvation stress. Acta Hortic. Sin. 2021, 48, 1457–1469. [Google Scholar]
- Giulia, E.; Alessandro, B.; Mariano, D.; Andrea, B.; Benedetto, R.; Angelo, R. Early induction of apple fruitlet abscission is characterized by an increase of both isoprene emission and abscisic acid content. Plant Physiol. 2013, 161, 112–120. [Google Scholar] [CrossRef]
- Xu, D.; Pan, H.; Yao, J.; Feng, Y.; Wu, P.; Shao, K. Stress responses and biological residues of sulfanilamide antibiotics in Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2020, 199, 110727. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Li, D.; Liu, L.; Zhao, Z.; Li, J. Changes of physiological and biochemical indexes during abscission of young areca nut. J. Plant Physiol. 2024, 60, 451–460. [Google Scholar]
- Iglesias, D.J.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Carbohydrate and ethylene levels related to fruitlet drop through abscission zone A in citrus. Trees 2006, 20, 348–355. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Mehouachi, J.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Hormonal regulation of fruitlet abscission induced by carbohydrate shortage in citrus. Planta 2000, 210, 636–643. [Google Scholar] [CrossRef]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R.; Ruperti, B. Signalling pathways mediating the induction of apple fruitlet abscission. Plant Physiol. 2011, 155, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Gundesli, M.; Kafkas, S.; Guney, M.; Kafkas, N.E. Identification of the profile of endogenous cytokinin-like compounds during different plant growth stages and their effects on flower bud abscission in pistachio (Pistacia vera L.). Folia Hortic. 2020, 32, 21–35. [Google Scholar] [CrossRef]
- Zhou, J.; Sittmann, J.; Guo, L.; Xiao, Y.; Huang, X.; Pulapaka, A.; Liu, Z. Gibberellin and auxin signaling genes RGA1 and ARF8 repress accessory fruit initiation in diploid strawberry. Plant Physiol. 2021, 185, 1059–1075. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lu, X.; Li, Q.T. soybean seeds reveals the genetic basis of seed trait adaptation during domestication. Plant J. 2016, 86, 530–544. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Han, W.; Mao, W.; Wang, X.; Li, S.; Luan, X.; Yao, X.; Guo, K.; Xie, F. Transcriptome and Targeted Hormone Metabolome Reveal the Mechanism of Flower Abscission in Soybeans Under Shade. Int. J. Mol. Sci. 2025, 26, 10303. https://doi.org/10.3390/ijms262110303
Tan Z, Han W, Mao W, Wang X, Li S, Luan X, Yao X, Guo K, Xie F. Transcriptome and Targeted Hormone Metabolome Reveal the Mechanism of Flower Abscission in Soybeans Under Shade. International Journal of Molecular Sciences. 2025; 26(21):10303. https://doi.org/10.3390/ijms262110303
Chicago/Turabian StyleTan, Zhuorui, Wenhui Han, Wanmin Mao, Xiang Wang, Shijun Li, Xinyang Luan, Xingdong Yao, Kai Guo, and Futi Xie. 2025. "Transcriptome and Targeted Hormone Metabolome Reveal the Mechanism of Flower Abscission in Soybeans Under Shade" International Journal of Molecular Sciences 26, no. 21: 10303. https://doi.org/10.3390/ijms262110303
APA StyleTan, Z., Han, W., Mao, W., Wang, X., Li, S., Luan, X., Yao, X., Guo, K., & Xie, F. (2025). Transcriptome and Targeted Hormone Metabolome Reveal the Mechanism of Flower Abscission in Soybeans Under Shade. International Journal of Molecular Sciences, 26(21), 10303. https://doi.org/10.3390/ijms262110303





