Flagellin as a Versatile Adjuvant Platform: Genetic Fusion Approaches for Next-Generation Vaccines
Abstract
1. Introduction
2. Three-Dimensional Structure of Flagellin and Its Domain Organization
3. Flagellin as a Vaccine Adjuvant: Genetic Fusion Versus Co-Formulation
4. Recombinant Chimeric Proteins Combining Antigens with Flagellin Scaffolds
4.1. Proof-of Principle: Ovalbumin
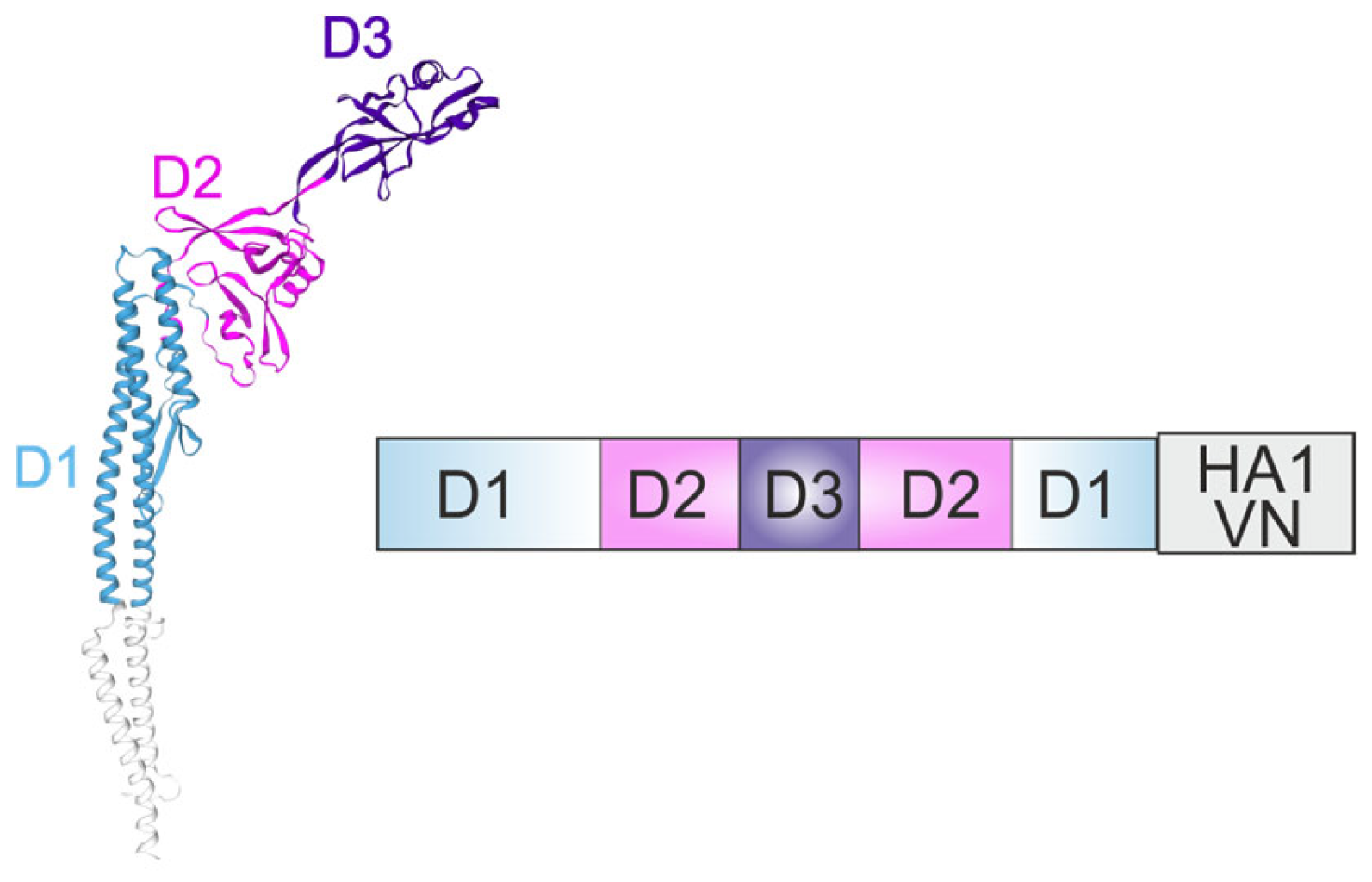
4.2. Flagellin as an Adjuvant for Recombinant Vaccines Against Influenza
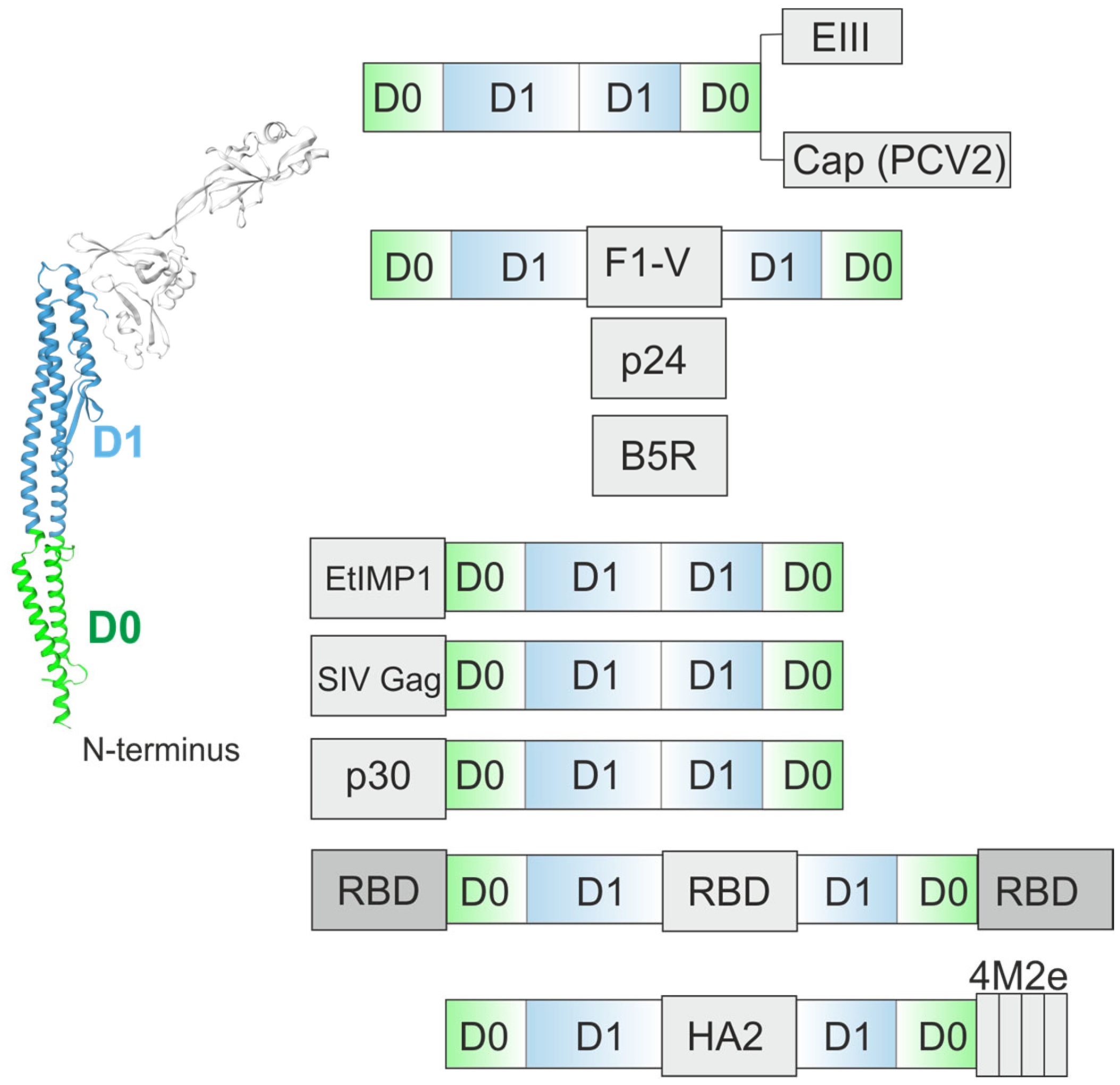
4.3. Flagellin-Based Vaccine Candidates Targeting Other Pathogens
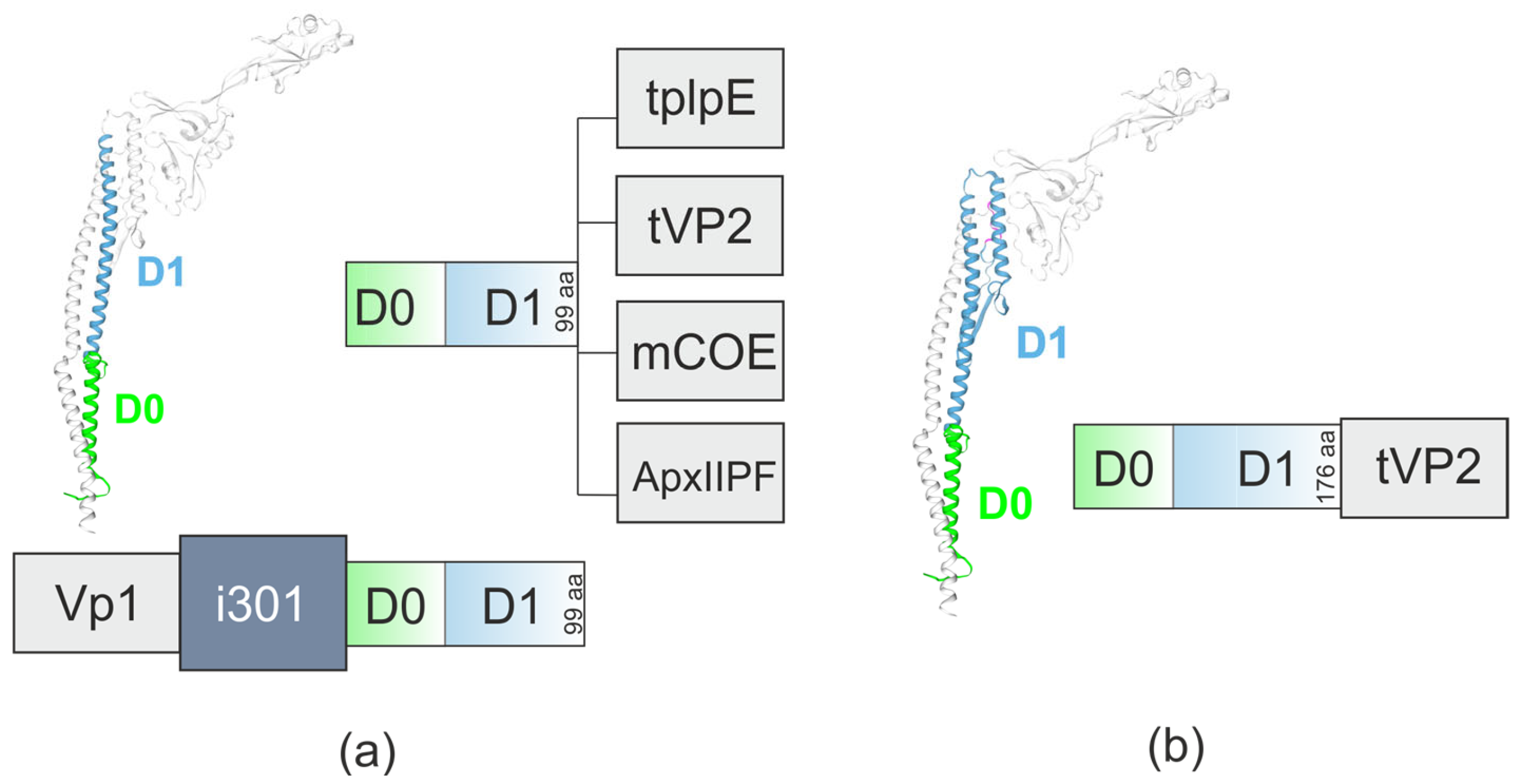
5. N-Terminal Region of Flagellin as an Adjuvant
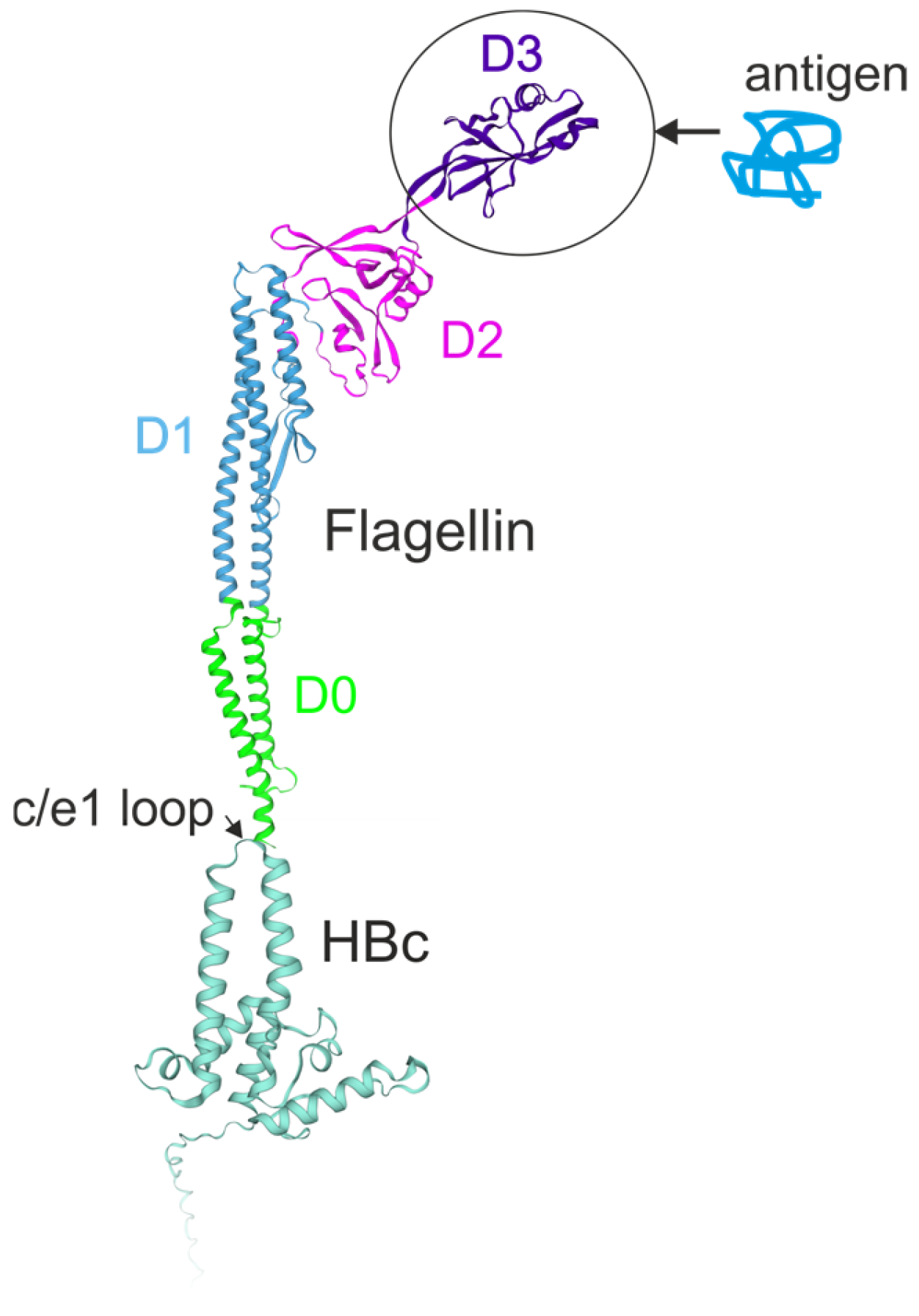
6. Flagellin-Based Peptides Incorporated into Virus-like Particles
7. Limitations and Prospects of Flagellin-Adjuvanted Vaccines
7.1. Structural Instability
7.2. The Choice of Antigen and Strategy for Its Fusion with Flagellin
7.3. Flagellin’s Own Immunogenicity and Associated Adverse Effects
8. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vijayan, A.; Rumbo, M.; Carnoy, C.; Sirard, J.-C. Compartmentalized Antimicrobial Defenses in Response to Flagellin. Trends Microbiol. 2018, 26, 423–435. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Yoon, S.; Kurnasov, O.; Natarajan, V.; Hong, M.; Gudkov, A.V.; Osterman, A.L.; Wilson, I.A. Structural Basis of TLR5-Flagellin Recognition and Signaling. Science 2012, 335, 859–864. [Google Scholar] [CrossRef]
- Rhee, J.H.; Khim, K.; Puth, S.; Choi, Y.; Lee, S.E. Deimmunization of Flagellin Adjuvant for Clinical Application. Curr. Opin. Virol. 2023, 60, 101330. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.C.; Rumbo, M.; Sirard, J.-C. Bacterial Flagellins: Mediators of Pathogenicity and Host Immune Responses in Mucosa. Trends Microbiol. 2004, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Afzal, H.; Murtaza, A.; Cheng, L.-T. Structural Engineering of Flagellin as Vaccine Adjuvant: Quest for the Minimal Domain of Flagellin for TLR5 Activation. Mol. Biol. Rep. 2025, 52, 104. [Google Scholar] [CrossRef]
- Mariathasan, S.; Newton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential Activation of the Inflammasome by Caspase-1 Adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef]
- Lightfield, K.L.; Persson, J.; Brubaker, S.W.; Witte, C.E.; von Moltke, J.; Dunipace, E.A.; Henry, T.; Sun, Y.-H.; Cado, D.; Dietrich, W.F.; et al. Critical Function for Naip5 in Inflammasome Activation by a Conserved Carboxy-Terminal Domain of Flagellin. Nat. Immunol. 2008, 9, 1171–1178. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Carvalho, F.A.; Aitken, J.D.; Fifadara, N.H.; Gewirtz, A.T. TLR5 or NLRC4 Is Necessary and Sufficient for Promotion of Humoral Immunity by Flagellin. Eur. J. Immunol. 2010, 40, 3528–3534. [Google Scholar] [CrossRef]
- Kofoed, E.M.; Vance, R.E. Innate Immune Recognition of Bacterial Ligands by NAIPs Determines Inflammasome Specificity. Nature 2011, 477, 592–595. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Wang, W.; Lin, G.; Hu, Z.; Han, Z.; Qi, Y.; Zhang, L.; Wang, J.; Sui, S.-F.; et al. Structural Basis for Specific Flagellin Recognition by the NLR Protein NAIP5. Cell Res. 2018, 28, 35–47. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Shi, J.; Gong, Y.-N.; Lu, Q.; Xu, H.; Liu, L.; Shao, F. The NLRC4 Inflammasome Receptors for Bacterial Flagellin and Type III Secretion Apparatus. Nature 2011, 477, 596–600. [Google Scholar] [CrossRef]
- Yang, J.-X.; Tseng, J.-C.; Yu, G.-Y.; Luo, Y.; Huang, C.-Y.F.; Hong, Y.-R.; Chuang, T.-H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [CrossRef]
- Labastida-Conde, R.G.; Ramírez-Pliego, O.; Peleteiro-Olmedo, M.; Lopez-Guerrero, D.V.; Badillo-Godinez, O.D.; Gutiérrez-Xicoténcatl, M.L.; Rosas-Salgado, G.; González-Fernández, Á.; Esquivel-Guadarrama, F.R.; Santana, M.A. Flagellin Is a Th1 Polarizing Factor for Human CD4+ T Cells and Induces Protection in a Murine Neonatal Vaccination Model of Rotavirus Infection. Vaccine 2018, 36, 4188–4197. [Google Scholar] [CrossRef]
- Firacative, C.; Gressler, A.E.; Schubert, K.; Schulze, B.; Müller, U.; Brombacher, F.; von Bergen, M.; Alber, G. Identification of T Helper (Th)1- and Th2-Associated Antigens of Cryptococcus Neoformans in a Murine Model of Pulmonary Infection. Sci. Rep. 2018, 8, 2681. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.F.; Khan, M.; Ball, J.; Toellner, K.; Serre, K.; Mohr, E.; MacLennan, I.C.M. Responses to the Soluble Flagellar Protein FliC Are Th2, While Those to FliC on Salmonella Are Th1. Eur. J. Immunol. 2004, 34, 2986–2995. [Google Scholar] [CrossRef] [PubMed]
- Tsybalova, L.M.; Stepanova, L.A.; Shuklina, M.A.; Mardanova, E.S.; Kotlyarov, R.Y.; Potapchuk, M.V.; Petrov, S.A.; Blokhina, E.A.; Ravin, N.V. Combination of M2e Peptide with Stalk HA Epitopes of Influenza A Virus Enhances Protective Properties of Recombinant Vaccine. PLoS ONE 2018, 13, e0201429. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, L.A.; Mardanova, E.S.; Shuklina, M.A.; Blokhina, E.A.; Kotlyarov, R.Y.; Potapchuk, M.V.; Kovaleva, A.A.; Vidyaeva, I.G.; Korotkov, A.V.; Eletskaya, E.I.; et al. Flagellin-Fused Protein Targeting M2e and HA2 Induces Potent Humoral and T-Cell Responses and Protects Mice against Various Influenza Viruses a Subtypes. J. Biomed. Sci. 2018, 25, 33. [Google Scholar] [CrossRef]
- Yonekura, K.; Maki-Yonekura, S.; Namba, K. Complete Atomic Model of the Bacterial Flagellar Filament by Electron Cryomicroscopy. Nature 2003, 424, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Samatey, F.A.; Imada, K.; Nagashima, S.; Vonderviszt, F.; Kumasaka, T.; Yamamoto, M.; Namba, K. Structure of the Bacterial Flagellar Protofilament and Implications for a Switch for Supercoiling. Nature 2001, 410, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling Protein Tertiary and Quaternary Structure Using Evolutionary Information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, K.; Maki-Yonekura, S.; Namba, K. Building the Atomic Model for the Bacterial Flagellar Filament by Electron Cryomicroscopy and Image Analysis. Structure 2005, 13, 407–412. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Z.; Kang, X.; Yang, Y.; Kang, H.; Zhang, N.; Rosati, J.M.; Jiao, X. Amino Acids 89–96 of Salmonella Typhimurium Flagellin Represent the Major Domain Responsible for TLR5-Independent Adjuvanticity in the Humoral Immune Response. Cell Mol. Immunol. 2015, 12, 625–632. [Google Scholar] [CrossRef]
- Song, W.S.; Jeon, Y.J.; Namgung, B.; Hong, M.; Yoon, S. A Conserved TLR5 Binding and Activation Hot Spot on Flagellin. Sci. Rep. 2017, 7, 40878. [Google Scholar] [CrossRef]
- Biedma, M.E.; Cayet, D.; Tabareau, J.; Rossi, A.H.; Ivičak-Kocjan, K.; Moreno, G.; Errea, A.; Soulard, D.; Parisi, G.; Jerala, R.; et al. Recombinant Flagellins with Deletions in Domains D1, D2, and D3: Characterization as Novel Immunoadjuvants. Vaccine 2019, 37, 652–663. [Google Scholar] [CrossRef]
- Pang, S.; Wang, L.; Liu, M.; Shao, M.; Zhu, G.; Duan, Q. Truncated Flagellin Lacking the Hypervariable Region: A Structural Basis for Improved Immune Responses and Adjuvanticity. Int. J. Biol. Macromol. 2025, 308, 142742. [Google Scholar] [CrossRef]
- Talukder, A.; Rahman, M.M.; Rahi, M.S.; Pountney, D.L.; Wei, M.Q. Flagellins as Vaccine Adjuvants and Cancer Immunotherapy: Recent Advances and Future Prospects. Immunology 2025, 176, 277–303. [Google Scholar] [CrossRef]
- Cui, B.; Liu, X.; Fang, Y.; Zhou, P.; Zhang, Y.; Wang, Y. Flagellin as a Vaccine Adjuvant. Expert. Rev. Vaccines 2018, 17, 335–349. [Google Scholar] [CrossRef]
- Nempont, C.; Cayet, D.; Rumbo, M.; Bompard, C.; Villeret, V.; Sirard, J.-C. Deletion of Flagellin’s Hypervariable Region Abrogates Antibody-Mediated Neutralization and Systemic Activation of TLR5-Dependent Immunity. J. Immunol. 2008, 181, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Huleatt, J.W.; Jacobs, A.R.; Tang, J.; Desai, P.; Kopp, E.B.; Huang, Y.; Song, L.; Nakaar, V.; Powell, T.J. Vaccination with Recombinant Fusion Proteins Incorporating Toll-like Receptor Ligands Induces Rapid Cellular and Humoral Immunity. Vaccine 2007, 25, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-S.; Webby, R.J. Traditional and New Influenza Vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Palese, P. Is a Universal Influenza Virus Vaccine Possible? Annu. Rev. Med. 2020, 71, 315–327. [Google Scholar] [CrossRef]
- Huleatt, J.W.; Nakaar, V.; Desai, P.; Huang, Y.; Hewitt, D.; Jacobs, A.; Tang, J.; McDonald, W.; Song, L.; Evans, R.K.; et al. Potent Immunogenicity and Efficacy of a Universal Influenza Vaccine Candidate Comprising a Recombinant Fusion Protein Linking Influenza M2e to the TLR5 Ligand Flagellin. Vaccine 2008, 26, 201–214. [Google Scholar] [CrossRef]
- Talbot, H.K.; Rock, M.T.; Johnson, C.; Tussey, L.; Kavita, U.; Shanker, A.; Shaw, A.R.; Taylor, D.N. Immunopotentiation of Trivalent Influenza Vaccine When Given with VAX102, a Recombinant Influenza M2e Vaccine Fused to the TLR5 Ligand Flagellin. PLoS ONE 2010, 5, e14442. [Google Scholar] [CrossRef]
- Song, L.; Nakaar, V.; Kavita, U.; Price, A.; Huleatt, J.; Tang, J.; Jacobs, A.; Liu, G.; Huang, Y.; Desai, P.; et al. Efficacious Recombinant Influenza Vaccines Produced by High Yield Bacterial Expression: A Solution to Global Pandemic and Seasonal Needs. PLoS ONE 2008, 3, e2257. [Google Scholar] [CrossRef]
- Taylor, D.N.; Treanor, J.J.; Strout, C.; Johnson, C.; Fitzgerald, T.; Kavita, U.; Ozer, K.; Tussey, L.; Shaw, A. Induction of a Potent Immune Response in the Elderly Using the TLR-5 Agonist, Flagellin, with a Recombinant Hemagglutinin Influenza–Flagellin Fusion Vaccine (VAX125, STF2.HA1 SI). Vaccine 2011, 29, 4897–4902. [Google Scholar] [CrossRef]
- Song, L.; Zhang, Y.; Yun, N.E.; Poussard, A.L.; Smith, J.N.; Smith, J.K.; Borisevich, V.; Linde, J.J.; Zacks, M.A.; Li, H.; et al. Superior Efficacy of a Recombinant Flagellin:H5N1 HA Globular Head Vaccine Is Determined by the Placement of the Globular Head within Flagellin. Vaccine 2009, 27, 5875–5884. [Google Scholar] [CrossRef]
- Taylor, D.N.; Treanor, J.J.; Sheldon, E.A.; Johnson, C.; Umlauf, S.; Song, L.; Kavita, U.; Liu, G.; Tussey, L.; Ozer, K.; et al. Development of VAX128, a Recombinant Hemagglutinin (HA) Influenza-Flagellin Fusion Vaccine with Improved Safety and Immune Response. Vaccine 2012, 30, 5761–5769. [Google Scholar] [CrossRef]
- Tussey, L.; Strout, C.; Davis, M.; Johnson, C.; Lucksinger, G.; Umlauf, S.; Song, L.; Liu, G.; Abraham, K.; White, C.J. Phase 1 Safety and Immunogenicity Study of a Quadrivalent Seasonal Flu Vaccine Comprising Recombinant Hemagglutinin-Flagellin Fusion Proteins. Open Forum Infect. Dis. 2016, 3, ofw015. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Kotlyarov, R.Y.; Kuprianov, V.V.; Stepanova, L.A.; Tsybalova, L.M.; Lomonosoff, G.P.; Ravin, N.V. Rapid High-Yield Expression of a Candidate Influenza Vaccine Based on the Ectodomain of M2 Protein Linked to Flagellin in Plants Using Viral Vectors. BMC Biotechnol. 2015, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Kotlyarov, R.Y.; Ravin, N.V. Rapid Transient Expression of Receptor-Binding Domain of SARS-CoV-2 and the Conserved M2e Peptide of Influenza A Virus Linked to Flagellin in Nicotiana Benthamiana Plants Using Self-Replicating Viral Vector. Plants 2022, 11, 3425. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, E.A.; Mardanova, E.S.; Stepanova, L.A.; Tsybalova, L.M.; Ravin, N.V. Plant-Produced Recombinant Influenza A Virus Candidate Vaccine Based on Flagellin Linked to Conservative Fragments of M2 Protein and Hemagglutintin. Plants 2020, 9, 162. [Google Scholar] [CrossRef]
- Liu, G.; Song, L.; Beasley, D.W.C.; Putnak, R.; Parent, J.; Misczak, J.; Li, H.; Reiserova, L.; Liu, X.; Tian, H.; et al. Immunogenicity and Efficacy of Flagellin-Envelope Fusion Dengue Vaccines in Mice and Monkeys. Clin. Vaccine Immunol. 2015, 22, 516–525. [Google Scholar] [CrossRef]
- Bargieri, D.Y.; Rosa, D.S.; Braga, C.J.M.; Carvalho, B.O.; Costa, F.T.M.; Espíndola, N.M.; Vaz, A.J.; Soares, I.S.; Ferreira, L.C.S.; Rodrigues, M.M. New Malaria Vaccine Candidates Based on the Plasmodium Vivax Merozoite Surface Protein-1 and the TLR-5 Agonist Salmonella Typhimurium FliC Flagellin. Vaccine 2008, 26, 6132–6142. [Google Scholar] [CrossRef]
- Asadi Karam, M.R.; Oloomi, M.; Mahdavi, M.; Habibi, M.; Bouzari, S. Vaccination with Recombinant FimH Fused with Flagellin Enhances Cellular and Humoral Immunity against Urinary Tract Infection in Mice. Vaccine 2013, 31, 1210–1216. [Google Scholar] [CrossRef]
- Delaney, K.N.; Phipps, J.P.; Johnson, J.B.; Mizel, S.B. A Recombinant Flagellin-Poxvirus Fusion Protein Vaccine Elicits Complement-Dependent Protection Against Respiratory Challenge with Vaccinia Virus in Mice. Viral Immunol. 2010, 23, 201–210. [Google Scholar] [CrossRef]
- Kalnin, K.; Tibbitts, T.; Yan, Y.; Stegalkina, S.; Shen, L.; Costa, V.; Sabharwal, R.; Anderson, S.F.; Day, P.M.; Christensen, N.; et al. Low Doses of Flagellin-L2 Multimer Vaccines Protect against Challenge with Diverse Papillomavirus Genotypes. Vaccine 2014, 32, 3540–3547. [Google Scholar] [CrossRef]
- McDonald, W.F.; Huleatt, J.W.; Foellmer, H.G.; Hewitt, D.; Tang, J.; Desai, P.; Price, A.; Jacobs, A.; Takahashi, V.N.; Huang, Y.; et al. A West Nile Virus Recombinant Protein Vaccine That Coactivates Innate and Adaptive Immunity. J. Infect. Dis. 2007, 195, 1607–1617. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, S.; Wei, L.; Yan, X.; Wang, J.; Quan, R.; She, R.; Hu, F.; Liu, J. Recombinant Flagellin-Porcine Circovirus Type 2 Cap Fusion Protein Promotes Protective Immune Responses in Mice. PLoS ONE 2015, 10, e0129617. [Google Scholar] [CrossRef] [PubMed]
- Mizel, S.B.; Graff, A.H.; Sriranganathan, N.; Ervin, S.; Lees, C.J.; Lively, M.O.; Hantgan, R.R.; Thomas, M.J.; Wood, J.; Bell, B. Flagellin-F1-V Fusion Protein Is an Effective Plague Vaccine in Mice and Two Species of Nonhuman Primates. Clin. Vaccine Immunology 2009, 16, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Deere, J.D.; Chang, W.L.W.; Castillo, L.D.; Schmidt, K.A.; Kieu, H.T.; Renzette, N.; Kowalik, T.; Barthold, S.W.; Shacklett, B.L.; Barry, P.A.; et al. Utilizing a TLR5-Adjuvanted Cytomegalovirus as a Lentiviral Vaccine in the Nonhuman Primate Model for AIDS. PLoS ONE 2016, 11, e0155629. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhong, M.; Zhang, Y.; Zhang, E.; Sun, Y.; Cao, Y.; Li, Y.; Zhou, D.; He, B.; Chen, Y.; et al. Antigen Replacement of Domains D2 and D3 in Flagellin Promotes Mucosal IgA Production and Attenuates Flagellin-Induced Inflammatory Response after Intranasal Immunization. Hum. Vaccin. Immunother. 2013, 9, 1084–1092. [Google Scholar] [CrossRef]
- Yin, G.; Qin, M.; Liu, X.; Suo, J.; Tang, X.; Tao, G.; Han, Q.; Suo, X.; Wu, W. An Eimeria Vaccine Candidate Based on Eimeria Tenella Immune Mapped Protein 1 and the TLR-5 Agonist Salmonella Typhimurium FliC Flagellin. Biochem. Biophys. Res. Commun. 2013, 440, 437–442. [Google Scholar] [CrossRef]
- Huang, X.; Kang, X.; Han, S.; Meng, C.; Song, H.; Jiao, X.; Pan, Z. Recombinant African Swine Fever Virus P30–Flagellin Fusion Protein Promotes P30-Specific Humoral and Cellular Immune Responses in Mice. Vet. Immunol. Immunopathol. 2025, 279, 110864. [Google Scholar] [CrossRef]
- Yang, J.; Liu, M.-Q.; Liu, L.; Li, X.; Xu, M.; Lin, H.; Liu, S.; Hu, Y.; Li, B.; Liu, B.; et al. A Triple-RBD-Based Mucosal Vaccine Provides Broad Protection against SARS-CoV-2 Variants of Concern. Cell Mol. Immunol. 2022, 19, 1279–1289. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Yang, J.; Zhou, L.; Liu, L.; Li, M.; Wang, S.; Liu, M.-Q.; Huang, Z.; Zhang, Z.; et al. Nasal Vaccination of Triple-RBD Scaffold Protein with Flagellin Elicits Long-Term Protection against SARS-CoV-2 Variants Including JN.1. Signal Transduct. Target. Ther. 2024, 9, 114. [Google Scholar] [CrossRef]
- Wang, C.; Xu, T.; Wang, J.; Li, F.; Guan, Y.; Dong, L.; Wang, Y.; Meng, W.; Tian, F.; Wei, F. Development of a Novel Nanobody-Fused Flagellin Adjuvant to Enhance Immunogenicity in a PCV2 Subunit Vaccine. Microb. Pathog. 2025, 207, 107912. [Google Scholar] [CrossRef]
- Khim, K.; Bang, Y.J.; Puth, S.; Choi, Y.; Lee, Y.S.; Jeong, K.; Lee, S.E.; Rhee, J.H. Deimmunization of Flagellin for Repeated Administration as a Vaccine Adjuvant. NPJ Vaccines 2021, 6, 116. [Google Scholar] [CrossRef]
- Afzal, H.; Hoa, N.-T.; Murtaza, A.; Doan, T.-D.; Chung, Y.-C.; Ke, G.-M.; Cheng, L.-T. Conserved Domains of Flagellin as Adjuvants for an Inactivated Vaccine against Porcine Epidemic Diarrhea Virus. Vet. J. 2025, 313, 106393. [Google Scholar] [CrossRef]
- Ajerloo, M.B.; Hajizade, A.; Nazarian, S.; Khalili, K. Design and in Silico Evaluation of a Novel Chimeric Protein Vaccine Candidate against Salmonella Typhi. J. Microbiol. Methods 2025, 238, 107268. [Google Scholar] [CrossRef]
- Doan, T.-D.; Wang, H.-Y.; Ke, G.-M.; Cheng, L.-T. N-Terminus of Flagellin Fused to an Antigen Improves Vaccine Efficacy against Pasteurella Multocida Infection in Chickens. Vaccines 2020, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, A.; Afzal, H.; Doan, T.-D.; Ke, G.-M.; Cheng, L.-T. Flagellin Improves the Immune Response of an Infectious Bursal Disease Virus (IBDV) Subunit Vaccine. Vaccines 2022, 10, 1780. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, A.; Hoa, N.-T.; Dieu-Huong, D.; Afzal, H.; Tariq, M.H.; Cheng, L.-T.; Chung, Y.-C. Advancing PEDV Vaccination: Comparison between Inactivated and Flagellin N-Terminus-Adjuvanted Subunit Vaccines. Vaccines 2024, 12, 139. [Google Scholar] [CrossRef]
- Chuekwon, K.; Chu, C.-Y.; Cheng, L.-T. N-Terminus of Flagellin Enhances Vaccine Efficacy against Actinobacillus Pleuropneumoniae. BMC Vet. Res. 2022, 18, 279. [Google Scholar] [CrossRef]
- Kheirvari, M.; Liu, H.; Tumban, E. Virus-like Particle Vaccines and Platforms for Vaccine Development. Viruses 2023, 15, 1109. [Google Scholar] [CrossRef]
- Pei, C.; Dong, H.; Teng, Z.; Wei, S.; Zhang, Y.; Yin, S.; Tang, J.; Sun, S.; Guo, H. Self−Assembling Nanovaccine Fused with Flagellin Enhances Protective Effect against Foot−and−Mouth Disease Virus. Vaccines 2023, 11, 1675. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Zhang, Y.; Zhang, F.; Du, E. Incorporation of a Truncated Form of Flagellin (TFlg) into Porcine Circovirus Type 2 Virus-like Particles Enhances Immune Responses in Mice. BMC Vet. Res. 2020, 16, 45. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Voyer, J.; Li, Y.; Chen, X. Flagellin/Virus-like Particle Hybrid Platform with High Immunogenicity, Safety, and Versatility for Vaccine Development. ACS Appl. Mater. Interfaces 2022, 14, 21872–21885. [Google Scholar] [CrossRef]
- Ozin, A.J.; Claret, L.; Auvray, F.; Hughes, C. The FliS Chaperone Selectively Binds the Disordered Flagellin C-Terminal D0 Domain Central to Polymerisation. FEMS Microbiol. Lett. 2003, 219, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Swartz, J.R. Functional Properties of Flagellin as a Stimulator of Innate Immunity. Sci. Rep. 2016, 6, 18379. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, G.; Umlauf, S.; Liu, X.; Li, H.; Tian, H.; Reiserova, L.; Hou, F.; Bell, R.; Tussey, L. A Rationally Designed Form of the TLR5 Agonist, Flagellin, Supports Superior Immunogenicity of Influenza B Globular Head Vaccines. Vaccine 2014, 32, 4317–4323. [Google Scholar] [CrossRef]
- Turley, C.B.; Rupp, R.E.; Johnson, C.; Taylor, D.N.; Wolfson, J.; Tussey, L.; Kavita, U.; Stanberry, L.; Shaw, A. Safety and Immunogenicity of a Recombinant M2e–Flagellin Influenza Vaccine (STF2.4xM2e) in Healthy Adults. Vaccine 2011, 29, 5145–5152. [Google Scholar] [CrossRef]
- Côté-Cyr, M.; Gauthier, L.; Zottig, X.; Bourgault, S.; Archambault, D. Recombinant Bacillus Subtilis Flagellin Hag Is a Potent Immunostimulant with Reduced Proinflammatory Properties Compared to Salmonella Enterica Serovar Typhimurium FljB. Vaccine 2022, 40, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.; Lim, M.Y. Balancing Harm and Harmony: Evolutionary Dynamics between Gut Microbiota-Derived Flagellin and TLR5-Mediated Host Immunity and Metabolism. Virulence 2025, 16, 2512035. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardanova, E.S.; Ravin, N.V. Flagellin as a Versatile Adjuvant Platform: Genetic Fusion Approaches for Next-Generation Vaccines. Int. J. Mol. Sci. 2025, 26, 10295. https://doi.org/10.3390/ijms262110295
Mardanova ES, Ravin NV. Flagellin as a Versatile Adjuvant Platform: Genetic Fusion Approaches for Next-Generation Vaccines. International Journal of Molecular Sciences. 2025; 26(21):10295. https://doi.org/10.3390/ijms262110295
Chicago/Turabian StyleMardanova, Eugenia S., and Nikolai V. Ravin. 2025. "Flagellin as a Versatile Adjuvant Platform: Genetic Fusion Approaches for Next-Generation Vaccines" International Journal of Molecular Sciences 26, no. 21: 10295. https://doi.org/10.3390/ijms262110295
APA StyleMardanova, E. S., & Ravin, N. V. (2025). Flagellin as a Versatile Adjuvant Platform: Genetic Fusion Approaches for Next-Generation Vaccines. International Journal of Molecular Sciences, 26(21), 10295. https://doi.org/10.3390/ijms262110295






