Transcription Factor EB (TFEB) Expression and Localization in the Third-Trimester Placenta †
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics and Clinical Correlations
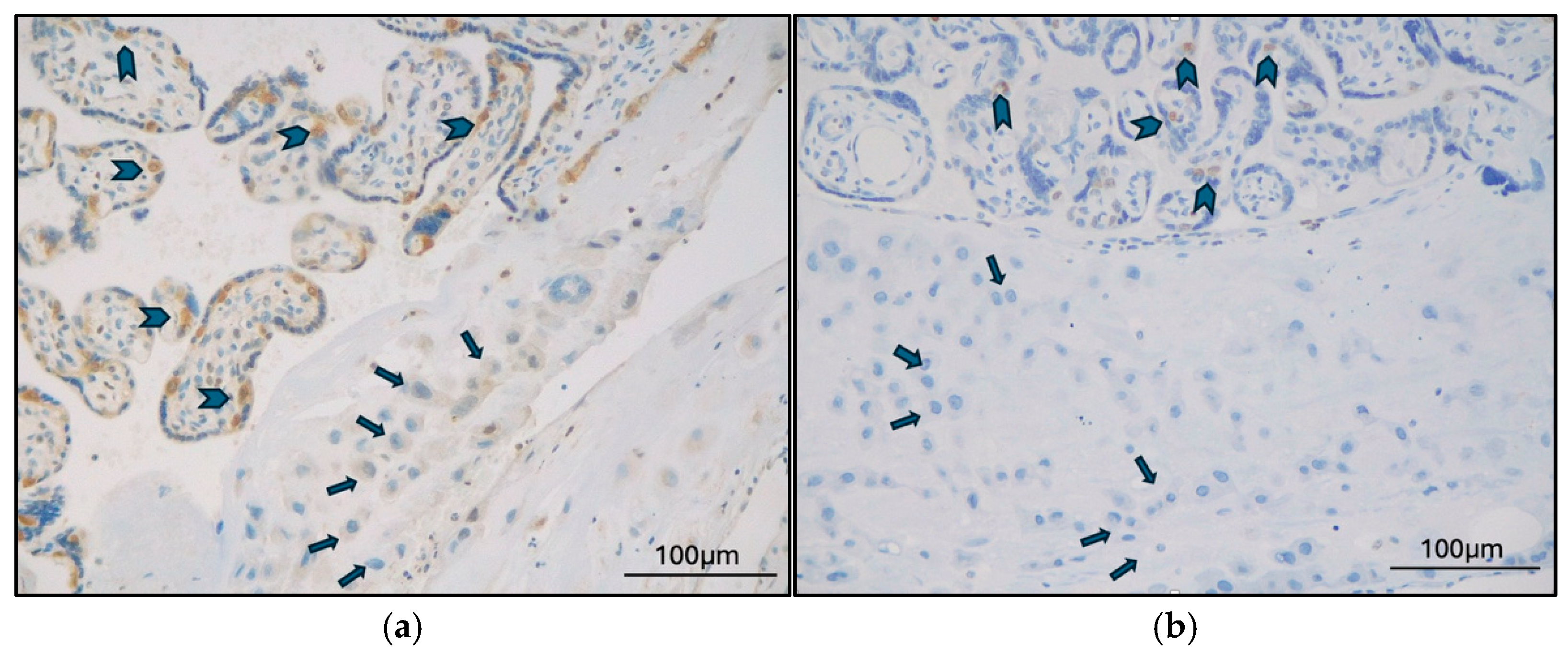
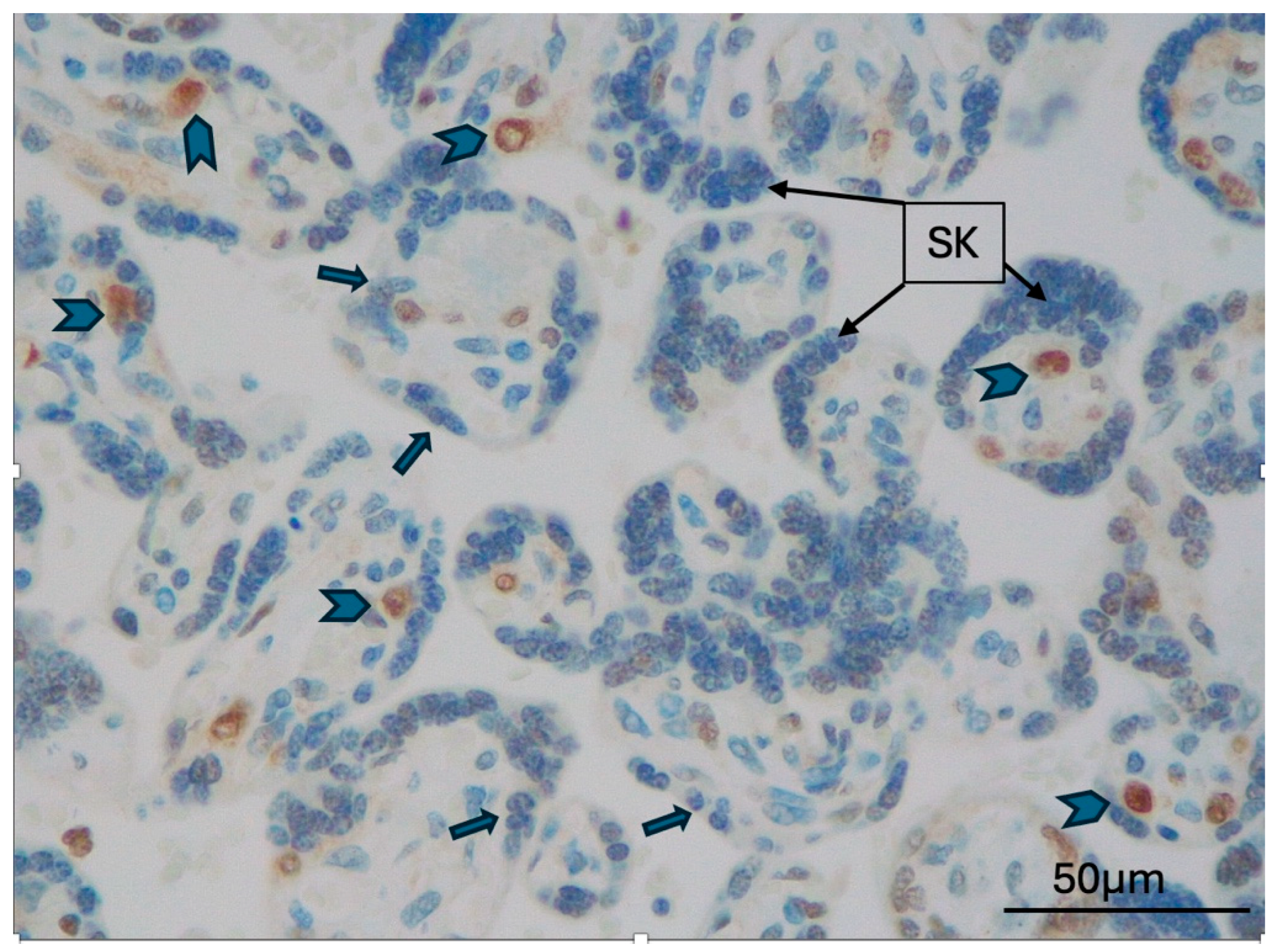
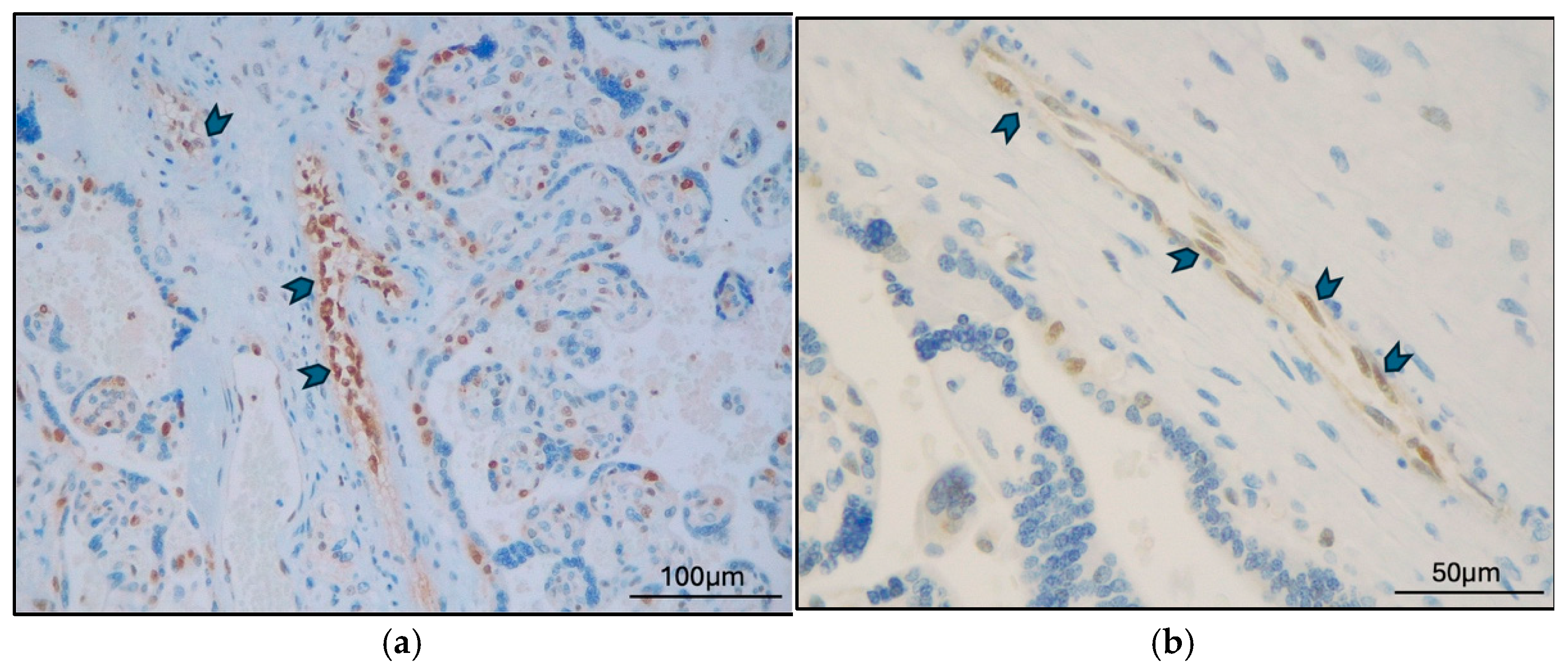
2.2. TFEB Expression and Correlations
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MVM | Maternal vascular malperfusion |
| DVM | Delayed villous maturation |
| FVM | Fetal vascular malperfusion |
| PE | Preeclampsia |
| GD | Gestational Diabetes |
| STB | Syncytiotrophoblast |
| CTB | Cytotrophoblast |
Appendix A
| Histological Diagnosis (N. of Cases) | ||||||
|---|---|---|---|---|---|---|
| Clinical Diagnosis | MVM | DVM | MVM + DVM | MVM + FVM | Total | p Value |
| Late-onset preeclampsia (N. of cases) | 9 | 0 | 0 | 0 | 9 | 0.0014 |
| Gestational diabetes (N. of cases) | 2 | 1 | 4 | 1 | 8 | |
| Total | 11 | 1 | 4 | 1 | 17 | |
| TFEB Type | Placental Districts (N. of TFEB Positive Expression/Total) | |||||
| STB | CTB | EVT | Syncytial Knots | Stem Villi Vessel | Villi Vessels | |
| TFEB-B | 3/17 | 17/17 | 0/17 | 2/17 | 10/17 | 5/17 |
| TFEB-SC | 2/17 | 17/17 | 0/17 | 0/17 | 2/17 | 1/17 |
| p value | 0.65 | 1 | 1 | 0.205 | 0.002 | 0.045 |
| Clinical Diagnosis (N. of Cases/Total) | ||||
|---|---|---|---|---|
| TFEB Type | Placental District | DG | Late-Onset PE | p Value |
| TFEB-B | STB | 3/8 | 0/9 | 0.08 |
| Syncytial knots | 2/8 | 0/9 | 0.20 | |
| Stem villi vessels | 7/8 | 3/9 | 0.049 | |
| Villi vessels | 4/8 | 1/9 | 0.13 | |
| TFEB-SC | STB | 0/8 | 2/9 | 0.47 |
| Syncytial knots | 0/8 | 0/9 | - | |
| Stem villi vessels | 1/8 | 1/9 | 1 | |
| Villi vessels | 1/8 | 0/9 | 0.47 | |
References
- Walker, J.J. Pre-eclampsia. Lancet 2000, 356, 1260–1265. [Google Scholar] [CrossRef]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Carrasco-Wong, I.; Aguilera-Olguin, M.; Escalona-Rivano, R.; Chiarello, D.I.; Barragan-Zuniga, L.J.; Sosa-Macias, M.; Galaviz-Hernandez, C.; San Martin, S.; Gutierrez, J. Syncytiotrophoblast stress in early onset preeclampsia: The issues perpetuating the syndrome. Placenta 2021, 113, 57–66. [Google Scholar] [CrossRef]
- Magee, L.A.; Nicolaides, K.H.; von Dadelszen, P. Preeclampsia. N. Engl. J. Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.R.; Jeong, D.H.; Lee, J.Y.; Woo, E.Y.; Shin, G.T.; Kim, S.Y. sFlt-1/PlGF ratio as a predictive and prognostic marker for preeclampsia. J. Obstet. Gynaecol. Res. 2021, 47, 2318–2323. [Google Scholar] [CrossRef]
- Chelli, D.; Hamdi, A.; Saoudi, S.; Jenayah, A.A.; Zagre, A.; Jguerim, H.; Bedis, C.; Sfar, E. Clinical Assessment of Soluble FMS-Like Tyrosine Kinase-1/Placental Growth Factor Ratio for the Diagnostic and the Prognosis of Preeclampsia in the Second Trimester. Clin. Lab. 2016, 62, 1927–1932. [Google Scholar] [CrossRef]
- Huang, Q.T.; Wang, S.S.; Zhang, M.; Huang, L.P.; Tian, J.W.; Yu, Y.H.; Wang, Z.J.; Zhong, M. Advanced oxidation protein products enhances soluble Fms-like tyrosine kinase 1 expression in trophoblasts: A possible link between oxidative stress and preeclampsia. Placenta 2013, 34, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Lewis, D.F.; Wang, Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2008, 93, 260–266. [Google Scholar] [CrossRef]
- Ahmed, A.; Dunk, C.; Ahmad, S.; Khaliq, A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen—A review. Placenta 2000, 21 (Suppl. A), S16–S24. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am. J. Obstet. Gynecol. 2022, 226, S907–S927. [Google Scholar] [CrossRef]
- Redman, C.W.; Staff, A.C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am. J. Obstet. Gynecol. 2015, 213, S9.e1–S9.e4. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Shima, T.; Aoki, A.; Kawaguchi, M.; Yasuda, I.; Tsuda, S.; Yoneda, S.; Yamaki-Ushijima, A.; Cheng, S.; Sharma, S.; et al. Placental autophagy failure: A risk factor for preeclampsia. J. Obstet. Gynaecol. Res. 2020, 46, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Longtine, M.S.; Nelson, D.M. Hypoxia induces autophagy in primary human trophoblasts. Endocrinology 2012, 153, 4946–4954. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamanaka-Tatematsu, M.; Fujita, N.; Koizumi, K.; Shima, T.; Yoshida, T.; Nikaido, T.; Okamoto, A.; Yoshimori, T.; Saito, S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy 2013, 9, 303–316. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, S.; Jash, S.; Fierce, J.; Agudelo, A.; Higashiyama, T.; Hanna, N.; Nakashima, A.; Saito, S.; Padbury, J.; et al. Exploiting sweet relief for preeclampsia by targeting autophagy-lysosomal machinery and proteinopathy. Exp. Mol. Med. 2024, 56, 1206–1220. [Google Scholar] [CrossRef]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, S.; Wen, J.; Liu, J.; Lin, X.; Zhu, C.; Cai, S.; Xie, L.; Wang, Z.; Chen, H. Universal screening for hyperglycemia in early pregnancy and the risk of adverse pregnancy outcomes. BMC Pregnancy Childbirth 2025, 25, 203. [Google Scholar] [CrossRef]
- Metzger, B.E.; Coustan, D.R.; Trimble, E.R. Hyperglycemia and Adverse Pregnancy Outcomes. Clin. Chem. 2019, 65, 937–938. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef]
- Steingrimsson, E.; Copeland, N.G.; Jenkins, N.A. Melanocytes and the microphthalmia transcription factor network. Annu. Rev. Genet. 2004, 38, 365–411. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Ballabio, A. TFEB at a glance. J. Cell Sci. 2016, 129, 2475–2481. [Google Scholar] [CrossRef]
- Tan, A.; Prasad, R.; Lee, C.; Jho, E.H. Past, present, and future perspectives of transcription factor EB (TFEB): Mechanisms of regulation and association with disease. Cell Death Differ. 2022, 29, 1433–1449. [Google Scholar] [CrossRef]
- Steingrimsson, E.; Tessarollo, L.; Reid, S.W.; Jenkins, N.A.; Copeland, N.G. The bHLH-Zip transcription factor Tfeb is essential for placental vascularization. Development 1998, 125, 4607–4616. [Google Scholar] [CrossRef]
- Doronzo, G.; Astanina, E.; Cora, D.; Chiabotto, G.; Comunanza, V.; Noghero, A.; Neri, F.; Puliafito, A.; Primo, L.; Spampanato, C.; et al. TFEB controls vascular development by regulating the proliferation of endothelial cells. EMBO J. 2019, 38, e98250. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, C.; De Marchi, F.; Ambrosi, A.; Cavaliere, S.; Caliò, A.; Mautone, D.; Martignoni, G. Transcription Factor EB (TFEB) Expression and Localization in the third-Trimester Preeclamptic and Diabetic Placenta. In Proceedings of the 114th USCAP Annual Meeting, Boston, MA, USA, 22–27 March 2025. [Google Scholar] [CrossRef]
- Esbin, M.N.; Dahal, L.; Fan, V.B.; McKenna, J.; Yin, E.; Darzacq, X.; Tjian, R. TFEB controls expression of human syncytins during cell-cell fusion. Genes. Dev. 2024, 38, 718–737. [Google Scholar] [CrossRef] [PubMed]
- Li, H.P.; Chen, X.; Li, M.Q. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int. J. Clin. Exp. Pathol. 2013, 6, 650–659. [Google Scholar] [PubMed]
- Bellissimo, C.J.; Ribeiro, T.A.; Yeo, E.; Jazwiec, P.A.; Luo, H.; Bains, J.; Bowdish, D.M.E.; Sloboda, D.M. Maternal high-fat, high-sucrose diet-induced excess adiposity is linked to placental hypoxia and disruption of fetoplacental immune homeostasis in late gestation. bioRxiv 2025. [Google Scholar] [CrossRef]
- Cetin, I.; Abati, I. The hypoxic fetus as a patient: Adaptations to the intrauterine environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2025, 380, 20240181. [Google Scholar] [CrossRef]
- Cesana, M.; Tufano, G.; Panariello, F.; Zampelli, N.; Soldati, C.; Mutarelli, M.; Montefusco, S.; Grieco, G.; Sepe, L.V.; Rossi, B.; et al. TFEB controls syncytiotrophoblast formation and hormone production in placenta. Cell Death Differ. 2024, 31, 1439–1451. [Google Scholar] [CrossRef]
- Staff, A.C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 2019, 134–135, 1–10. [Google Scholar] [CrossRef]
- Fogarty, N.M.; Ferguson-Smith, A.C.; Burton, G.J. Syncytial knots (Tenney-Parker changes) in the human placenta: Evidence of loss of transcriptional activity and oxidative damage. Am. J. Pathol. 2013, 183, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Heazell, A.E.; Moll, S.J.; Jones, C.J.; Baker, P.N.; Crocker, I.P. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta 2007, 28 (Suppl. A), S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Shao, L.; Luo, X.; Mu, Y.; Xu, W.; Gao, L.; Xu, H.; Cui, Y. Expressions of VEGF-A and VEGFR-2 in placentae from GDM pregnancies. Reprod. Biol. Endocrinol. 2016, 14, 61. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, H.; Liang, W.; Garcia-Barrio, M.T.; Guo, Y.; Zhang, J.; Zhu, T.; Hao, Y.; Zhang, J.; Chen, Y.E. Endothelial TFEB (Transcription Factor EB) Positively Regulates Postischemic Angiogenesis. Circ. Res. 2018, 122, 945–957. [Google Scholar] [CrossRef]
- Villalobos-Labra, R.; Silva, L.; Subiabre, M.; Araos, J.; Salsoso, R.; Fuenzalida, B.; Saez, T.; Toledo, F.; Gonzalez, M.; Quezada, C.; et al. Akt/mTOR Role in Human Foetoplacental Vascular Insulin Resistance in Diseases of Pregnancy. J. Diabetes Res. 2017, 2017, 5947859. [Google Scholar] [CrossRef] [PubMed]
- Leiva, A.; Pardo, F.; Ramirez, M.A.; Farias, M.; Casanello, P.; Sobrevia, L. Fetoplacental vascular endothelial dysfunction as an early phenomenon in the programming of human adult diseases in subjects born from gestational diabetes mellitus or obesity in pregnancy. Exp. Diabetes Res. 2011, 2011, 349286. [Google Scholar] [CrossRef]
- Subiabre, M.; Silva, L.; Villalobos-Labra, R.; Toledo, F.; Paublo, M.; Lopez, M.A.; Salsoso, R.; Pardo, F.; Leiva, A.; Sobrevia, L. Maternal insulin therapy does not restore foetoplacental endothelial dysfunction in gestational diabetes mellitus. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2987–2998. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet. Gynecol. 2020, 135, 1492–1495. [CrossRef]
- Giacometti, C.; Ludwig, K.; Guidi, M.; Colantuono, E.; Coracina, A.; Rigano, M.; Cassaro, M.; Ambrosi, A. Gestational Diabetes-Placental Expression of Human Equilibrative Nucleoside Transporter 1 (hENT1): Is Delayed Villous Maturation an Adaptive Pattern? Diagnostics 2023, 13, 2034. [Google Scholar] [CrossRef]
- Langston, C.; Kaplan, C.; Macpherson, T.; Manci, E.; Peevy, K.; Clark, B.; Murtagh, C.; Cox, S.; Glenn, G. Practice guideline for examination of the placenta: Developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Arch. Pathol. Lab. Med. 1997, 121, 449–476. [Google Scholar]

| Mothers and Deliveries | Number/Total (%) | Mean ± SD | Range |
|---|---|---|---|
| Age (years) | 34.76 ± 5.09 | 29–45 | |
| Clinical diagnosis | |||
| Late-onset PE | 9/17 (53%) | ||
| GD | 8/17 (47%) | ||
| Histological diagnosis | |||
| MVM | 11/17 (65%) | ||
| DVM | 1/17 (6%) | ||
| MVM + DVM | 4/17 (23%) | ||
| MVM + FVM | 1/17 (6%) | ||
| Type of delivery | |||
| Caesarian | 15/17 (88%) | ||
| Vaginal, induced/operative | 1/17 (6%) | ||
| Vaginal, spontaneous | 1/17 (6%) | ||
| Newborns and Placentas | Mean ± SD | Range | |
| Gestational age (weeks ± days) | 37.00 ± 14.70 | 31.57–40 | |
| Birth weight (grams) | 2703.23 ± 550.81 | 1500–3650 | |
| APGAR score (1 min) | 8.76 ± 1.30 | 6–10 | |
| APGAR score (5 min) | 9.41 ± 0.93 | 7–10 | |
| Umbilical cord (arterial) pH | 7.27 ± 0.09 | 7.07–7.4 | |
| Base excess (arterial) | −2.24 ± 4.32 | −10.6–4.8 | |
| Placental weight (grams) | 364.11 ± 82.58 | 210–470 | |
| Placental Weight Ratio | 7.57 ± 1.40 | 5.62–10.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacometti, C.; Ambrosi, A.; Cavaliere, S.; Caliò, A.; Mautone, D.; Martignoni, G. Transcription Factor EB (TFEB) Expression and Localization in the Third-Trimester Placenta. Int. J. Mol. Sci. 2025, 26, 10294. https://doi.org/10.3390/ijms262110294
Giacometti C, Ambrosi A, Cavaliere S, Caliò A, Mautone D, Martignoni G. Transcription Factor EB (TFEB) Expression and Localization in the Third-Trimester Placenta. International Journal of Molecular Sciences. 2025; 26(21):10294. https://doi.org/10.3390/ijms262110294
Chicago/Turabian StyleGiacometti, Cinzia, Alessandro Ambrosi, Serena Cavaliere, Anna Caliò, Daniele Mautone, and Guido Martignoni. 2025. "Transcription Factor EB (TFEB) Expression and Localization in the Third-Trimester Placenta" International Journal of Molecular Sciences 26, no. 21: 10294. https://doi.org/10.3390/ijms262110294
APA StyleGiacometti, C., Ambrosi, A., Cavaliere, S., Caliò, A., Mautone, D., & Martignoni, G. (2025). Transcription Factor EB (TFEB) Expression and Localization in the Third-Trimester Placenta. International Journal of Molecular Sciences, 26(21), 10294. https://doi.org/10.3390/ijms262110294








