Beyond Folding: Expanding the Functional Landscape of Hsp90 Chaperone Machinery in Health and Disease
Abstract
1. Introduction
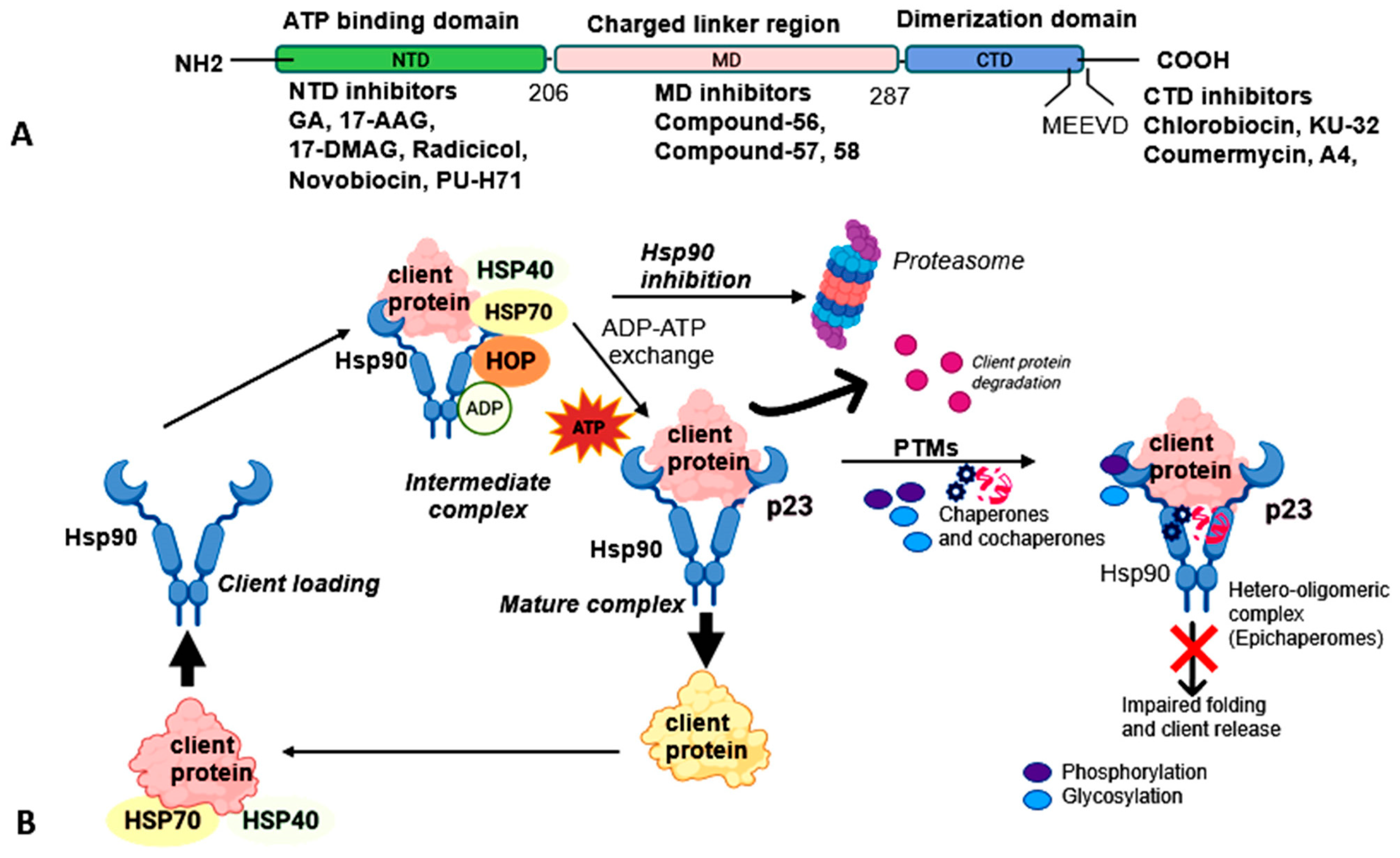
1.1. Hsp90 Structure and Co-Chaperone and Client Interactions
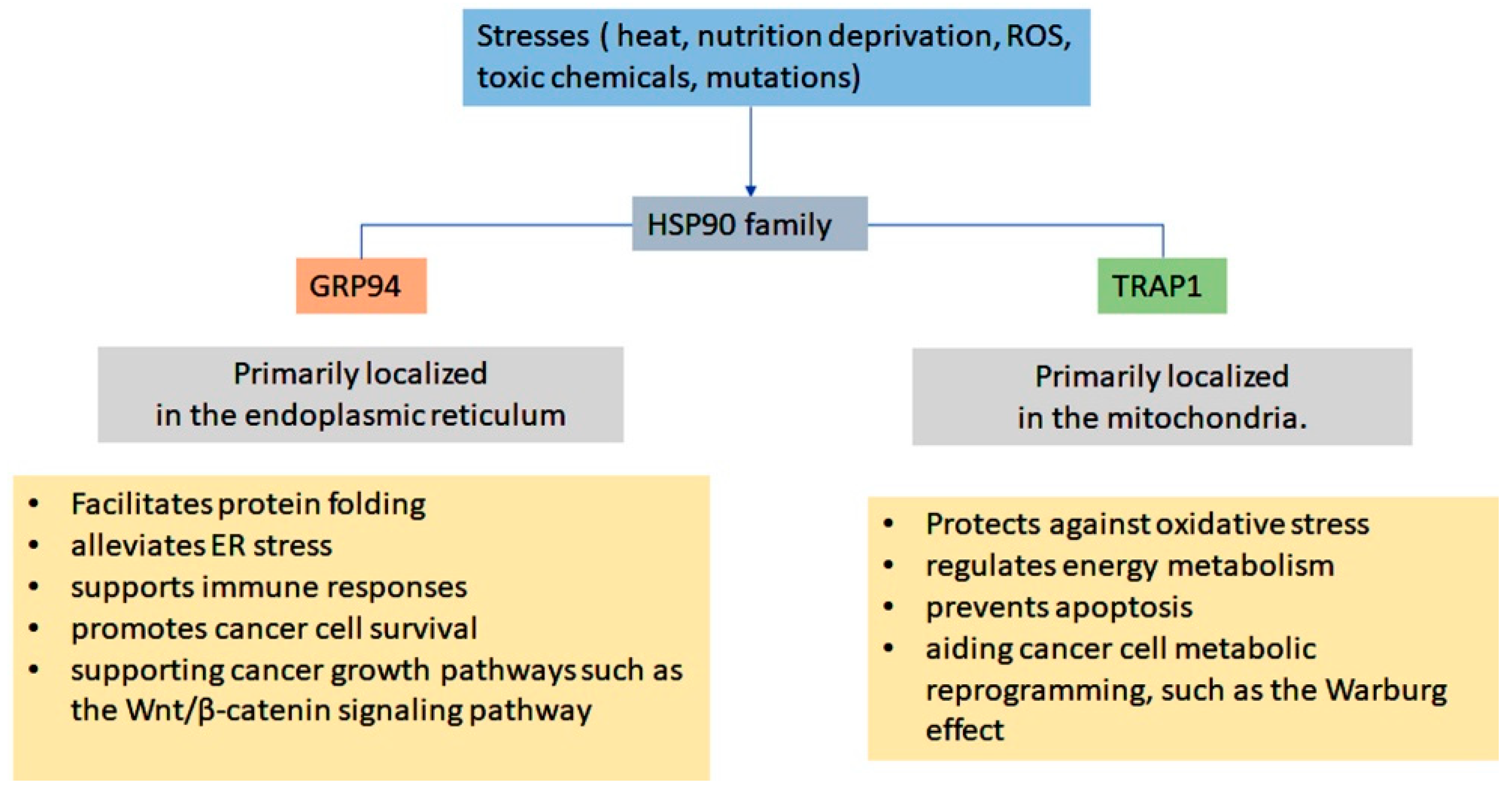
1.2. ER-Resident Hsp90 (Grp94)
1.3. Mitochondrial Hsp90 (TRAP1)
1.4. Post Translational Modifications (PTMs)
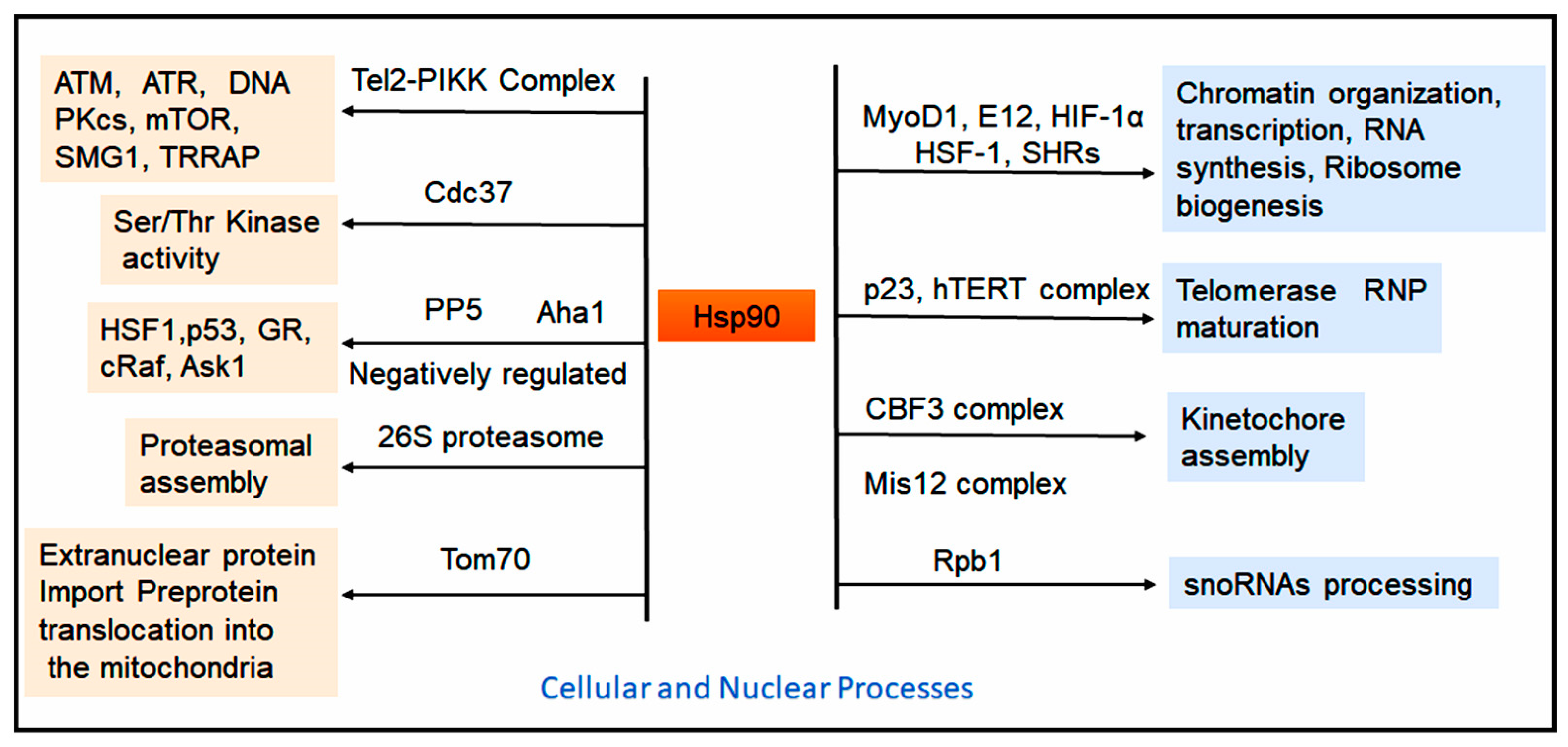
1.5. Role of Hsp90 in Cellular Processs and Protein Homeostasis
2. Implications of Hsp90 in Health and Diseases
2.1. Hsp90, ER-Localized Grp94, and Mitochondrial Hsp90 in Cancers
2.2. Grp94
2.3. Mitochondrial Chaperone TRAP1
2.4. Clinical Applications of Hsp90 Inhibitors in Cancer
2.5. Curcumin-Based Hsp90 Inhibitors
2.6. Geldanamycin and Derivatives
2.7. Purine-Based Hsp90 Inhibitors
2.8. Small Molecule Inhibitors of Hsp90
| S. No. | Hsp90 Inhibitors | Derivatives | Binding Sites | Clinical Phase/Trails | Action Mechanism | Diseases | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Curcumin and derivatives | C212 | Reduces Hsp90 level | Preclinical | Targets client protein | Leukemia cells | [197] |
| C1206 | MD | In vivo/in vitro | Inhibits ATPase activity | CML | [169] | ||
| C0818 | CTD | In vivo/in vitro | Inhibits ATP hydrolysis | In vivo and in vitro tumor models | [170] | ||
| CUR3d | Reduces Hsp90 expression | Phase I | Modulates multiple signaling pathways and client proteins | HepG2, hepatocellular cancer | [171] | ||
| 2. | Geldanamycin and derivatives | 17-AAG | NTD | Phase III | Inhibits ATPase activity | Breast, Leukemia, Lung; thyroid cancer | [177,179] |
| 17-DMAG | NTD | Phase I | Targets client proteins | HCC, HepG2, AML | [180,181] | ||
| IPI-504 | NTD | Phase III | Inhibits ATPase activity | GIST | [198] | ||
| IPI493 | Impairs signaling protein kinases | Phase III | Acts as tyrosine kinase inhibitor | GIST | [199] | ||
| 3. | Purine-based inhibitors | CUDC-305 | NTD | Phase I | Tyrosine kinase inhibition | Breast cancer, NSCLC | [187] |
| PU-H71 | NTD | Phase I | Targets client proteins | TNBC, myeloma; HCC | [200] | ||
| PU3 | NTD | In vivo/in vitro | Cell cycle inhibition | Breast cancer | [201] | ||
| MPC-3100 | NTD | Phase I | Inhibits apoptosis | Breast cancer | [202] | ||
| BIIB021 | NTD | Phase II | Inhibits ATPase activity | Xenograft models | [188] | ||
| 4. | Small-molecule inhibitors/benzamide-scaffold inhibitors | Novobiocin (KU135; A4) | CTD | Phase I | Disrupts the dimerization and promotes release of client proteins | Breast cancer | [203] |
| Epigallocatechin gallate (EGCG) | CTD | Phase IV | Targets XAP2-bound Hsp90 complex | Pancreatic, prostate cancer | [191,204] | ||
| SNX-2112, SNX-5422 | NTD | Phase I | Induces cell cycle arrest; inhibits eNOS and AKT signaling | NSCLC, SCLC, multiple myeloma | [194] | ||
| AT13387 (Onalespib) | NTD | Phase I/II | Depletes Hsp90 client proteins | Prostate cancer | [205] | ||
| 5. | Tryptophan (TRP) analogs | XL888 | NTD | Phase I | Inhibits cell cycle; targets client proteins | Colorectal, pancreatic cancer | [206] |
| San A-amide | N-middle domain | Phase I/II | Allosterically disrupts CTD protein binding | Pancreatic, breast, colon cancers | [192] | ||
| 6. | Resorcinol-based inhibitors | NVP-AUY922 (Luminespib) | NTD | Phase I/II | Inactivates apoptotic pathways | Multiple myeloma, solid tumors | [207] |
| Ganetespib | N-terminal ATP-binding site | Phase I/II | Induces cell cycle arrest and apoptosis | Ovarian, breast cancer | [193] | ||
| Pimitespib (TAS-116) | NTD | Phase III | Inhibits growth and induces apoptosis | GIST, colorectal cancer | [195] |
3. Hsp90 in Alzheimer’s Disease (AD) and Neurodegeneration
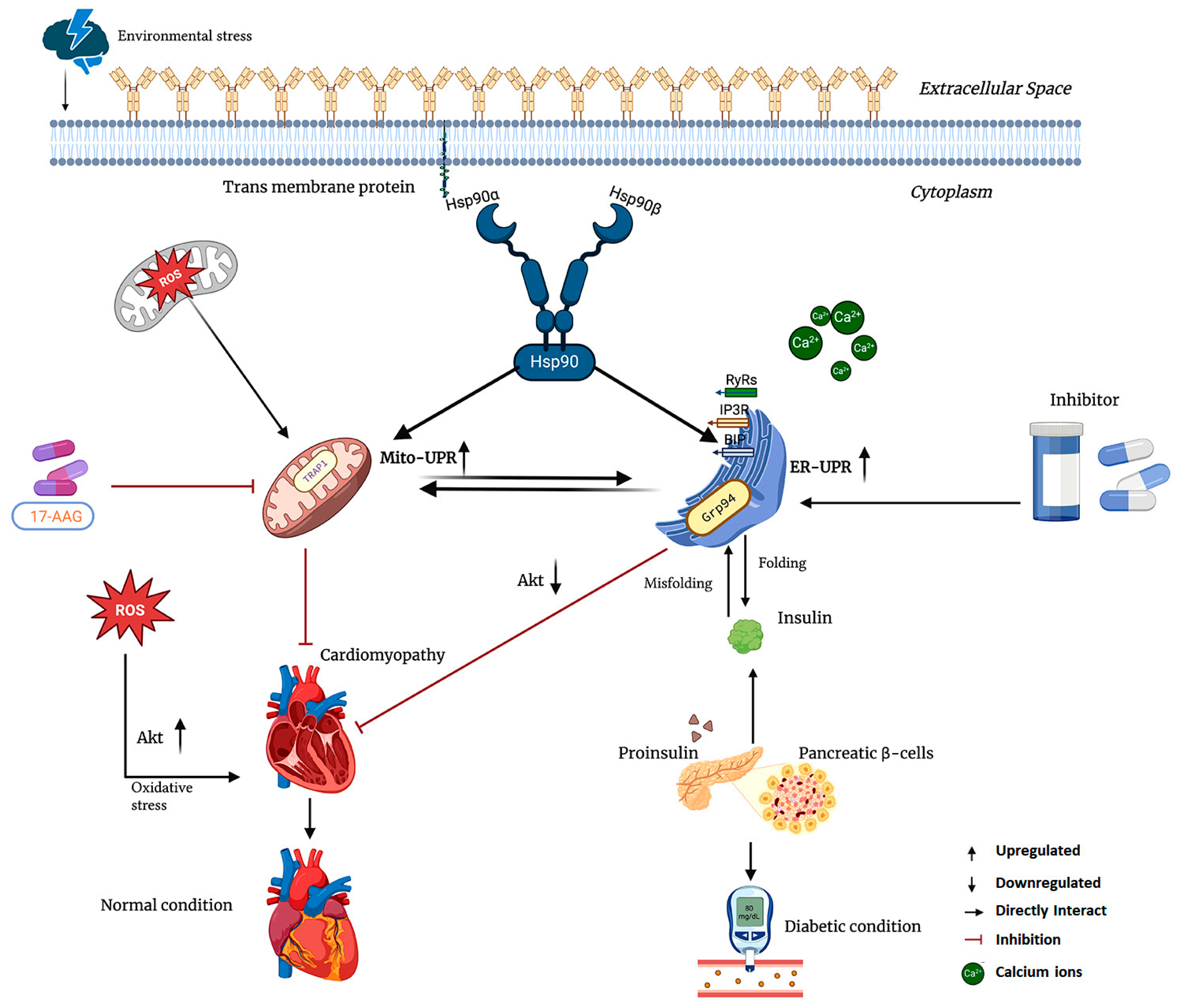
4. Hsp90 in Cardiac Disease and Diabetes
4.1. Grp94 in Metabolic Disease and Cardiac Hypertrophy
4.2. Hsp90 Inhibitors and Their Role in Cardiac Disorder and Diabetes
4.3. Geldanamycin (GA) and Its Derivatives Role in Cardiac Disorders and Diabetes
4.4. Mitochondria-Directed and Extracellular Hsp90 Inhibition
5. Conclusions and Future Direction
- Designing small molecules or peptide analogs of the TRP domain that disrupt Hsp90 complex assembly by competitively binding the CTD.
- Targeting PTMs that regulate Hsp90-client or Hsp90–co-chaperone interactions, either genetically or pharmacologically, to enhance the effectiveness of existing inhibitors.
- Co-inhibiting HSF1 to prevent stress-induced feedback responses that attenuate the effects of Hsp90 blockade.
- Developing isoform-specific inhibitors, particularly targeting Hsp90α, which has been associated with cardiac hypertrophy and heart failure due to pressure overload.
- Integration of organelle-specific targeting motifs into pan-Hsp90 inhibitors to promote their selective accumulation in target organelles, including mitochondria and the ER.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 17-AAG | 17-allylamino-17-demethoxygeldanamycin |
| 17DMAG | 17-dimethylaminoethylamino-17-demethoxygeldanamycin |
| Aβ | Amyloid-β |
| AD | Alzheimer’s disease |
| AHA1 | Activator of Hsp90 ATPase homolog 1 |
| AML | Acute Myeloid Leukemia |
| AGO | Argonaute |
| Ask1 | Apoptosis signal–regulating kinase 1 |
| CTD | C-terminal dimerization domain |
| COXII | Cytochrome c oxidase subunit II |
| ER | Endoplasmic Reticulum |
| eHsp90 | Extracellular Hsp90 |
| FGFR3 | Fibroblast growth factor receptor 3 |
| GARP | Glycoprotein A repetitions predominant |
| GIST | Gastrointestinal stromal tumors |
| GR | Glucocorticoid receptor |
| Grp94 | Glucose-regulated protein 94 |
| HCC | Hepatocellular carcinoma |
| HCM | Hypertrophic cardiomyopathy |
| HD | Huntington’s disease |
| HIF-1α | Hypoxia-inducible factor 1α |
| Hch1 | High copy Hsp90 suppressor 1 |
| HIP | Hsp70-interacting proteins |
| HSPs | Heat shock proteins |
| Hsp90 | Heat shock protein 90 |
| IGF | Insulin-like growth factors |
| JNK | c-Jun N-terminal kinase |
| LRP1 | Low-density lipoprotein receptor-related protein-1 |
| MD | Middle domain |
| MDR | Multidrug resistance |
| NTD | N-terminal nucleotide-binding domain |
| PD | Parkinson’s disease |
| PP5 | Protein phosphatase 5 |
| PTMs | Post-translational modifications |
| PPP | Pentose phosphate pathway |
| PPIase | Peptidyl-prolyl cis-trans isomerase |
| PIKK | Phosphatidylinositol-3 kinase–related kinase |
| PR | Progesterone receptor |
| PTP | Mitochondrial permeability transition pore |
| ROS | Reactive oxygen species |
| SREBPs | sterol regulatory element-binding proteins |
| SDH | Succinate dehydrogenase |
| SHR | Steroid hormone receptor |
| TAC | Transverse aortic constriction |
| TCA | Tricarboxylic acid |
| TRAP1 | Tumor necrosis factor receptor-associated protein |
| TPR | Tetratricopeptide repeat |
| TLR | Toll-like receptors |
| UPR | Unfolded protein response |
| VEGFRs | Vascular endothelial growth factor receptors |
References
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef]
- Maiti, S.; Picard, D. Cytosolic Hsp90 isoform-specific functions and clinical significance. Biomolecules 2022, 12, 1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhong, D.; Monteiro, A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genom. 2006, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Li, M.; Schnell, D.J. An essential role for chloroplast heat shock protein 90 (Hsp90C) in protein import into chloroplasts. Proc. Natl. Acad. Sci. USA 2013, 110, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Ostrovsky, O.; Makarewich, C.A.; Wanderling, S.; Gidalevitz, T.; Argon, Y. The peptide-binding activity of GRP94 is regulated by calcium. Biochem. J. 2007, 405, 233–241. [Google Scholar] [CrossRef]
- Schleiff, E.; Becker, T. Common ground for protein translocation: Access control for mitochondria and chloroplasts. Nat. Rev. Mol. Cell Biol. 2011, 12, 48–59. [Google Scholar] [CrossRef]
- Kang, B.H. TRAP1 regulation of mitochondrial life or death decision in cancer cells and mitochondria-targeted TRAP1 inhibitors. BMB Rep. 2012, 45, 1–6. [Google Scholar] [CrossRef]
- Kang, B.H.; Plescia, J.; Dohi, T.; Rosa, J.; Doxsey, S.J.; Altieri, D.C. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell 2007, 131, 257–270. [Google Scholar] [CrossRef]
- Li, W.; Sahu, D.; Tsen, F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 730–741. [Google Scholar] [CrossRef]
- Chaudhury, S.; D’Amico, T.; Blagg, B.S. The Hsp90β Isoform: An Attractive Target for Drug Development. Med. Res. Rev. 2025, 45, 1452–1465. [Google Scholar] [CrossRef]
- Wegele, H.; Müller, L.; Buchner, J. Hsp70 and Hsp90—A relay team for protein folding. Rev. Physiol. Biochem. Pharmacol. 2004, 151, 1–44. [Google Scholar] [PubMed]
- Rios, E.I.; Hunsberger, I.L.; Johnson, J.L. Insights into Hsp90 mechanism and In Vivo functions learned from studies in the yeast, Saccharomyces cerevisiae. Front. Mol. Biosci. 2024, 11, 1325590. [Google Scholar] [CrossRef]
- Nemoto, T.; Matsusaka, T.; Ota, M.; Takagi, T.; Collinge, D.B.; Walther-Larsen, H. Dimerization characteristics of the 94-kDa glucose-regulated protein. J. Biochem. 1996, 120, 249–256. [Google Scholar] [CrossRef]
- Henot, F.; Rioual, E.; Favier, A.; Macek, P.; Crublet, E.; Josso, P.; Brutscher, B.; Frech, M.; Gans, P.; Loison, C. Visualizing the transiently populated closed-state of human HSP90 ATP binding domain. Nat. Commun. 2022, 13, 7601. [Google Scholar] [CrossRef]
- Schulze, A.; Beliu, G.; Helmerich, D.A.; Schubert, J.; Pearl, L.H.; Prodromou, C.; Neuweiler, H. Cooperation of local motions in the Hsp90 molecular chaperone ATPase mechanism. Nat. Chem. Biol. 2016, 12, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Roe, S.M.; Prodromou, C.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999, 42, 260–266. [Google Scholar] [CrossRef]
- Meyer, P.; Prodromou, C.; Hu, B.; Vaughan, C.; Roe, S.M.; Panaretou, B.; Piper, P.W.; Pearl, L.H. Structural and functional analysis of the middle segment of hsp90: Implications for ATP hydrolysis and client protein and cochaperone interactions. Mol. Cell 2003, 11, 647–658. [Google Scholar] [CrossRef]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef]
- Müller, L.; Schaupp, A.; Walerych, D.; Wegele, H.; Buchner, J. Hsp90 regulates the activity of wild type p53 under physiological and elevated temperatures. J. Biol. Chem. 2004, 279, 48846–48854. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; Fulton, D.; Chen, Y.; Fairchild, T.A.; McCabe, T.J.; Fujita, N.; Tsuruo, T.; Sessa, W.C. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 2002, 90, 866–873. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Mollapour, M.; Graf, C.; Lee, C.-T.; Scroggins, B.T.; Xu, W.; Haslerova, L.; Hessling, M.; Konstantinova, A.A.; Trepel, J.B. Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain. Nat. Struct. Mol. Biol. 2009, 16, 1141–1147. [Google Scholar] [CrossRef]
- Riedl, S.; Bilgen, E.; Agam, G.; Hirvonen, V.; Jussupow, A.; Tippl, F.; Riedl, M.; Maier, A.; Becker, C.F.; Kaila, V.R. Evolution of the conformational dynamics of the molecular chaperone Hsp90. Nat. Commun. 2024, 15, 8627. [Google Scholar] [CrossRef]
- Röhl, A.; Rohrberg, J.; Buchner, J. The chaperone Hsp90: Changing partners for demanding clients. Trends Biochem. Sci. 2013, 38, 253–262. [Google Scholar] [CrossRef]
- Gracia, L.; Lora, G.; Blair, L.J.; Jinwal, U.K. Therapeutic potential of the Hsp90/Cdc37 interaction in neurodegenerative diseases. Front. Neurosci. 2019, 13, 1263. [Google Scholar] [CrossRef]
- Taipale, M.; Krykbaeva, I.; Koeva, M.; Kayatekin, C.; Westover, K.D.; Karras, G.I.; Lindquist, S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 2012, 150, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P. Structural basis for recruitment of the ATPase activator Aha1 to the Hsp90 chaperone machinery. EMBO J. 2004, 23, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.H.; Smith, H.W.; Jackson, S.E. Stimulation of the weak ATPase activity of human hsp90 by a client protein. J. Mol. Biol. 2002, 315, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Dahiya, V.; Delhommel, F.; Freiburger, L.; Stehle, R.; Asami, S.; Rutz, D.; Blair, L.; Buchner, J.; Sattler, M. Client binding shifts the populations of dynamic Hsp90 conformations through an allosteric network. Sci. Adv. 2021, 7, eabl7295. [Google Scholar] [CrossRef]
- Li, J.; Richter, K.; Buchner, J. Mixed Hsp90–cochaperone complexes are important for the progression of the reaction cycle. Nat. Struct. Mol. Biol. 2011, 18, 61–66. [Google Scholar] [CrossRef]
- Soroka, J.; Wandinger, S.K.; Mäusbacher, N.; Schreiber, T.; Richter, K.; Daub, H.; Buchner, J. Conformational switching of the molecular chaperone Hsp90 via regulated phosphorylation. Mol. Cell 2012, 45, 517–528. [Google Scholar] [CrossRef]
- Weaver, A.J.; Sullivan, W.P.; Felts, S.J.; Owen, B.A.; Toft, D.O. Crystal structure and activity of human p23, a heat shock protein 90 co-chaperone. J. Biol. Chem. 2000, 275, 23045–23052. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Kobayashi, M.; Yoda, M.; Sakaguchi, Y.; Katsuma, S.; Suzuki, T.; Tomari, Y. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol. Cell 2010, 39, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef]
- Lanneau, D.; Brunet, M.; Frisan, E.; Solary, E.; Fontenay, M.; Garrido, C. Heat shock proteins: Essential proteins for apoptosis regulation. J. Cell. Mol. Med. 2008, 12, 743–761. [Google Scholar] [CrossRef]
- Liu, K.; Jin, H.; Guo, Y.; Liu, Y.; Wan, Y.; Zhao, P.; Zhou, Z.; Wang, J.; Wang, M.; Zou, C. CFTR interacts with Hsp90 and regulates the phosphorylation of AKT and ERK1/2 in colorectal cancer cells. FEBS Open Bio 2019, 9, 1119–1127. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Yuan, Z.; Li, Y.; Li, X.; Gong, Z.; Peng, Y. Heat shock protein 90β inhibits apoptosis of intestinal epithelial cells induced by hypoxia through stabilizing phosphorylated Akt. BMB Rep. 2013, 46, 47. [Google Scholar] [CrossRef]
- Silbermann, L.-M.; Vermeer, B.; Schmid, S.; Tych, K. The known unknowns of the Hsp90 chaperone. eLife 2024, 13, e102666. [Google Scholar] [CrossRef]
- Cintron, N.S.; Toft, D. Defining the requirements for Hsp40 and Hsp70 in the Hsp90 chaperone pathway. J. Biol. Chem. 2006, 281, 26235–26244. [Google Scholar] [CrossRef]
- Chadli, A.; Bouhouche, I.; Sullivan, W.; Stensgard, B.; McMahon, N.; Catelli, M.G.; Toft, D.O. Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90. Proc. Natl. Acad. Sci. USA 2000, 97, 12524–12529. [Google Scholar] [CrossRef]
- Hernández, M.P.; Chadli, A.; Toft, D.O. HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor. J. Biol. Chem. 2002, 277, 11873–11881. [Google Scholar] [CrossRef] [PubMed]
- Ricketson, D.; Hostick, U.; Fang, L.; Yamamoto, K.; Darimont, B. A conformational switch in the ligand-binding domain regulates the dependence of the glucocorticoid receptor on Hsp90. J. Mol. Biol. 2007, 368, 729–741. [Google Scholar] [CrossRef]
- Wang, R.Y.-R.; Noddings, C.M.; Kirschke, E.; Myasnikov, A.G.; Johnson, J.L.; Agard, D.A. Structure of Hsp90–Hsp70–Hop–GR reveals the Hsp90 client-loading mechanism. Nature 2022, 601, 460–464. [Google Scholar] [CrossRef]
- Biebl, M.M.; Lopez, A.; Rehn, A.; Freiburger, L.; Lawatscheck, J.; Blank, B.; Sattler, M.; Buchner, J. Structural elements in the flexible tail of the co-chaperone p23 coordinate client binding and progression of the Hsp90 chaperone cycle. Nat. Commun. 2021, 12, 828. [Google Scholar] [CrossRef]
- Noddings, C.M.; Wang, R.Y.-R.; Johnson, J.L.; Agard, D.A. Structure of Hsp90–p23–GR reveals the Hsp90 client-remodelling mechanism. Nature 2022, 601, 465–469. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Krukenberg, K.A.; Street, T.O.; Lavery, L.A.; Agard, D.A. Conformational dynamics of the molecular chaperone Hsp90. Q. Rev. Biophys. 2011, 44, 229–255. [Google Scholar] [CrossRef]
- Gupta, R.S. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol. Biol. Evol. 1995, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Huck, J.D.; Que, N.L.; Hong, F.; Li, Z.; Gewirth, D.T. Structural and functional analysis of GRP94 in the closed state reveals an essential role for the pre-N domain and a potential client-binding site. Cell Rep. 2017, 20, 2800–2809. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E. Hsp90: Structure and function. In Molecular Chaperones; Springer: Berlin/Heidelberg, Germany, 2012; pp. 155–240. [Google Scholar]
- Csermely, P.; Schnaider, T.; So, C.; Prohászka, Z.; Nardai, G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998, 79, 129–168. [Google Scholar] [CrossRef]
- Li, X.; Yin, M.-Y.; Zhang, S.-T.; Xie, S.-A. The role of canopy family proteins: Biological mechanism and disease function. Mol. Biol. Rep. 2025, 52, 164. [Google Scholar] [CrossRef]
- Amankwah, Y.S.; Fleifil, Y.; Unruh, E.; Collins, P.; Wang, Y.; Vitou, K.; Bates, A.; Obaseki, I.; Sugoor, M.; Alao, J.P. Structural transitions modulate the chaperone activities of Grp94. Proc. Natl. Acad. Sci. USA 2024, 121, e2309326121. [Google Scholar] [CrossRef]
- Amankwah, Y.S.; Collins, P.; Fleifil, Y.; Unruh, E.; Marquez, K.J.R.; Vitou, K.; Kravats, A.N. Grp94 works upstream of BiP in protein remodeling under heat stress. J. Mol. Biol. 2022, 434, 167762. [Google Scholar] [CrossRef]
- Boczek, E.E.; Reefschläger, L.G.; Dehling, M.; Struller, T.J.; Häusler, E.; Seidl, A.; Kaila, V.R.; Buchner, J. Conformational processing of oncogenic v-Src kinase by the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. USA 2015, 112, E3189–E3198. [Google Scholar] [CrossRef]
- Yan, P.; Patel, H.J.; Sharma, S.; Corben, A.; Wang, T.; Panchal, P.; Yang, C.; Sun, W.; Araujo, T.L.; Rodina, A. Molecular stressors engender protein connectivity dysfunction through aberrant N-glycosylation of a chaperone. Cell Rep. 2020, 31, 107840. [Google Scholar] [CrossRef] [PubMed]
- Mirikar, D.; Bushman, Y.; Truman, A.W. Structural transitions modulate the chaperone activities of Grp94. Trends Biochem. Sci. 2024, 49, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Xu, J.; Liu, Y.; Li, Y.; He, T.; Ding, L.; He, Y.; Yi, Y.; Li, F.; Guo, R. FBXL2 counteracts Grp94 to destabilize EGFR and inhibit EGFR-driven NSCLC growth. Nat. Commun. 2021, 12, 5919. [Google Scholar] [CrossRef]
- Ghosh, S.; Shinogle, H.E.; Galeva, N.A.; Dobrowsky, R.T.; Blagg, B.S. Endoplasmic reticulum-resident heat shock protein 90 (HSP90) isoform glucose-regulated protein 94 (GRP94) regulates cell polarity and cancer cell migration by affecting intracellular transport. J. Biol. Chem. 2016, 291, 8309–8323. [Google Scholar] [CrossRef]
- Zuehlke, A.; Johnson, J.L. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolym. Orig. Res. Biomol. 2010, 93, 211–217. [Google Scholar] [CrossRef]
- Kang, S.; Kang, B.H. Structure, function, and inhibitors of the mitochondrial chaperone TRAP1. J. Med. Chem. 2022, 65, 16155–16172. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Park, H.-K.; Jeong, H.; Lim, J.; Lee, A.-J.; Cheon, K.Y.; Kim, C.-S.; Thomas, A.P.; Bae, B.; Kim, N.D. Development of a mitochondria-targeted Hsp90 inhibitor based on the crystal structures of human TRAP1. J. Am. Chem. Soc. 2015, 137, 4358–4367. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Siegelin, M.D.; Plescia, J.; Raskett, C.M.; Garlick, D.S.; Dohi, T.; Lian, J.B.; Stein, G.S.; Languino, L.R.; Altieri, D.C. Preclinical characterization of mitochondria-targeted small molecule hsp90 inhibitors, gamitrinibs, in advanced prostate cancer. Clin. Cancer Res. 2010, 16, 4779–4788. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Hong, J.-H.; Oh, Y.T.; Kim, S.S.; Yin, J.; Lee, A.-J.; Chae, Y.C.; Kim, J.H.; Park, S.-H.; Park, C.-K. Interplay between TRAP1 and sirtuin-3 modulates mitochondrial respiration and oxidative stress to maintain stemness of glioma stem cells. Cancer Res. 2019, 79, 1369–1382. [Google Scholar] [CrossRef]
- Ramos Rego, I.; Santos Cruz, B.; Ambrósio, A.F.; Alves, C.H. TRAP1 in oxidative stress and neurodegeneration. Antioxidants 2021, 10, 1829. [Google Scholar] [CrossRef]
- Gutierrez, A.; Archdeacon, J.; Blagg, B.S. TRAP1 and its therapeutic potential. Bioorg. Med. Chem. Lett. 2025, 129, 130395. [Google Scholar] [CrossRef]
- Dharaskar, S.P.; Subbarao, S.A. The mitochondrial chaperone TRAP-1 regulates the glutamine metabolism in tumor cells. Mitochondrion 2023, 69, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, G.; Sciacovelli, M.; Bernardi, P.; Rasola, A. Inhibition of succinate dehydrogenase by the mitochondrial chaperone TRAP1 has anti-oxidant and anti-apoptotic effects on tumor cells. Oncotarget 2014, 5, 11897. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, M.; Amoroso, M.R.; Piscazzi, A.; Esposito, F. Heat shock proteins, cell survival and drug resistance: The mitochondrial chaperone TRAP1, a potential novel target for ovarian cancer therapy. Gynecol. Oncol. 2010, 117, 177–182. [Google Scholar] [CrossRef]
- Hu, K.; Babapoor-Farrokhran, S.; Rodrigues, M.; Deshpande, M.; Puchner, B.; Kashiwabuchi, F.; Hassan, S.J.; Asnaghi, L.; Handa, J.T.; Merbs, S. Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget 2016, 7, 7816, Erratum in Oncotarget 2016, 12, 519–520. [Google Scholar] [CrossRef]
- Sisinni, L.; Maddalena, F.; Condelli, V.; Pannone, G.; Simeon, V.; Li Bergolis, V.; Lopes, E.; Piscazzi, A.; Matassa, D.S.; Mazzoccoli, C. TRAP1 controls cell cycle G2–M transition through the regulation of CDK1 and MAD2 expression/ubiquitination. J. Pathol. 2017, 243, 123–134. [Google Scholar] [CrossRef]
- Mathieu, C.; Chamayou, Q.; Luong, T.T.H.; Naud, D.; Mahuteau-Betzer, F.; Alami, M.; Fattal, E.; Messaoudi, S.; Vergnaud-Gauduchon, J. Synthesis and antiproliferative activity of 6BrCaQ-TPP conjugates for targeting the mitochondrial heat shock protein TRAP1. Eur. J. Med. Chem. 2022, 229, 114052. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-targeted triphenylphosphonium-based compounds: Syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Sanchez-Martin, C.; Menon, D.; Moroni, E.; Ferraro, M.; Masgras, I.; Elsey, J.; Arbiser, J.L.; Colombo, G.; Rasola, A. Honokiol bis-dichloroacetate is a selective allosteric inhibitor of the mitochondrial chaperone TRAP1. Antioxid. Redox Signal. 2021, 34, 505–516. [Google Scholar] [CrossRef]
- Zhang, L.; Pang, E.; Loo, R.R.O.; Rao, J.; Go, V.L.W.; Loo, J.A.; Lu, Q.Y. Concomitant inhibition of HSP90, its mitochondrial localized homologue TRAP1 and HSP27 by green tea in pancreatic cancer HPAF-II cells. Proteomics 2011, 11, 4638–4647. [Google Scholar] [CrossRef]
- Backe, S.J.; Sager, R.A.; Woodford, M.R.; Makedon, A.M.; Mollapour, M. Post-translational modifications of Hsp90 and translating the chaperone code. J. Biol. Chem. 2020, 295, 11099–11117. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Sharma, S.; Ginsberg, S.D.; Chiosis, G. PTMs as molecular encoders: Reprogramming chaperones into epichaperomes for network control in disease. Trends Biochem. Sci. 2025, 50, 892–905. [Google Scholar] [CrossRef]
- Wang, X.; Song, X.; Zhuo, W.; Fu, Y.; Shi, H.; Liang, Y.; Tong, M.; Chang, G.; Luo, Y. The regulatory mechanism of Hsp90α secretion and its function in tumor malignancy. Proc. Natl. Acad. Sci. USA 2009, 106, 21288–21293. [Google Scholar] [CrossRef]
- Gajsek, O.; Becker, C.F.; Conibear, A.C. Site-specifically Phosphorylated Hsp90C-terminal Domain Variants Provide Access to Deciphering the Chaperone Code. Chem. Eur. J. 2025, 31, e202403676. [Google Scholar] [CrossRef]
- Jakob, U.; Meyer, I.; Bügl, H.; André, S.; Bardwell, J.C.; Buchner, J. Structural organization of procaryotic and eucaryotic Hsp90. Influence of divalent cations on structure and function. J. Biol. Chem. 1995, 270, 14412–14419. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, T.; McNutt, S.W.; Pasala, C.; Nguyen, H.T.; Thornton, D.T.; Sharma, S.; Botticelli, L.; Digwal, C.S.; Joshi, S.; Yang, N. Phosphorylation-driven epichaperome assembly is a regulator of cellular adaptability and proliferation. Nat. Commun. 2024, 15, 8912. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, T.; Andresen, C.; Unger, A.; Just, S.; Rottbauer, W.; Linke, W.A. Lysine methyltransferase Smyd2 regulates Hsp90-mediated protection of the sarcomeric titin springs and cardiac function. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 812–822. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Miao, Q.; Shi, Z.; Hu, L.; Liu, S.; Gao, J.; Zhao, S.; Chen, H.; Huang, Z. Inhibition of HSP90 S-nitrosylation alleviates cardiac fibrosis via TGFβ/SMAD3 signalling pathway. Br. J. Pharmacol. 2021, 178, 4608–4625. [Google Scholar] [CrossRef]
- Shelton, L.B. Targeting the Hsp90/Aha1 Complex for the Treatment of Tauopathies. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2018. [Google Scholar]
- Ginsberg, S.D.; Sharma, S.; Norton, L.; Chiosis, G. Targeting stressor-induced dysfunctions in protein–protein interaction networks via epichaperomes. Trends Pharmacol. Sci. 2023, 44, 20–33. [Google Scholar] [CrossRef]
- Magni, A.; Sciva, C.; Castelli, M.; Digwal, C.S.; Rodina, A.; Sharma, S.; Ochiana, S.; Patel, H.J.; Shah, S.; Chiosis, G. N-Glycosylation-Induced Pathologic Protein Conformations as a Tool to Guide the Selection of Biologically Active Small Molecules. Chem. Eur. J. 2024, 30, e202401957. [Google Scholar] [CrossRef]
- Pasala, C.; Digwal, C.S.; Sharma, S.; Wang, S.; Bubula, A.; Chiosis, G. Epichaperomes: Redefining chaperone biology and therapeutic strategies in complex diseases. RSC Chem. Biol. 2025, 6, 678–698. [Google Scholar] [CrossRef]
- Young, J.C.; Moarefi, I.; Hartl, F.U. Hsp90: A specialized but essential protein-folding tool. J. Cell Biol. 2001, 154, 267. [Google Scholar] [CrossRef]
- Echeverría, P.C.; Bernthaler, A.; Dupuis, P.; Mayer, B.; Picard, D. An interaction network predicted from public data as a discovery tool: Application to the Hsp90 molecular chaperone machine. PLoS ONE 2011, 6, e26044. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Dittmar, K.D. Studies with purified chaperones advance the understanding of the mechanism of glucocorticoid receptor–hsp90 heterocomplex assembly. Trends Endocrinol. Metab. 1998, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.F.; Vos, M.H.; Lindquist, S. In Vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc. Natl. Acad. Sci. USA 1997, 94, 12949–12956. [Google Scholar] [CrossRef]
- Caplan, A.J. Hsp90’s secrets unfold: New insights from structural and functional studies. Trends Cell Biol. 1999, 9, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Von Kriegsheim, A.; Pitt, A.; Grindlay, G.J.; Kolch, W.; Dhillon, A.S. Regulation of the Raf–MEK–ERK pathway by protein phosphatase 5. Nat. Cell Biol. 2006, 8, 1011–1016. [Google Scholar] [CrossRef]
- Morita, K.-i.; Saitoh, M.; Tobiume, K.; Matsuura, H.; Enomoto, S.; Nishitoh, H.; Ichijo, H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001, 20, 6028–6036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bao, S.; Furumai, R.; Kucera, K.S.; Ali, A.; Dean, N.M.; Wang, X.-F. Protein phosphatase 5 is required for ATR-mediated checkpoint activation. Mol. Cell. Biol. 2005, 25, 9910–9919. [Google Scholar] [CrossRef]
- Amable, L.; Grankvist, N.; Largen, J.W.; Ortsäter, H.; Sjöholm, Å.; Honkanen, R.E. Disruption of serine/threonine protein phosphatase 5 (PP5: PPP5c) in mice reveals a novel role for PP5 in the regulation of ultraviolet light-induced phosphorylation of serine/threonine protein kinase Chk1 (CHEK1). J. Biol. Chem. 2011, 286, 40413–40422. [Google Scholar] [CrossRef]
- Li, J.; Soroka, J.; Buchner, J. The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 624–635. [Google Scholar] [CrossRef]
- Zuo, Z.; Urban, G.; Scammell, J.G.; Dean, N.M.; McLean, T.K.; Aragon, I.; Honkanen, R.E. Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry 1999, 38, 8849–8857. [Google Scholar] [CrossRef]
- Zuo, Z.; Dean, N.M.; Honkanen, R.E. Serine/threonine protein phosphatase type 5 acts upstream of p53 to regulate the induction of p21WAF1/Cip1 and mediate growth arrest. J. Biol. Chem. 1998, 273, 12250–12258. [Google Scholar] [CrossRef]
- Jaime-Garza, M.; Nowotny, C.A.; Coutandin, D.; Wang, F.; Tabios, M.; Agard, D.A. Hsp90 provides a platform for kinase dephosphorylation by PP5. Nat. Commun. 2023, 14, 2197. [Google Scholar] [CrossRef] [PubMed]
- Gasc, J.-M.; Renoir, J.-M.; Faber, L.E.; Delahaye, F.; Baulieu, E.-E. Nuclear localization of two steroid receptor-associated proteins, hsp90 and p59. Exp. Cell Res. 1990, 186, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Nin, G.; Echeverria, O.; Carbajal, M.; Tanguay, R.; Diez, J.; Fakan, S. Immunoelectron microscope localization of Mr 90000 heat shock protein and Mr 70000 heat shock cognate protein in the salivary glands of Chironomus thummi. Chromosoma 1992, 102, 50–59. [Google Scholar] [CrossRef]
- Schlatter, L.K.; Howard, K.J.; Parker, M.G.; Distelhorst, C.W. Comparison of the 90-kilodalton heat shock protein interaction with In Vitro translated glucocorticoid and estrogen receptors. Mol. Endocrinol. 1992, 6, 132–140. [Google Scholar]
- Chambraud, B.; Berry, M.; Redeuilh, G.; Chambon, P.; Baulieu, E. Several regions of human estrogen receptor are involved in the formation of receptor-heat shock protein 90 complexes. J. Biol. Chem. 1990, 265, 20686–20691. [Google Scholar] [CrossRef]
- Sweitzer, T.D.; Hanover, J.A. Calmodulin activates nuclear protein import: A link between signal transduction and nuclear transport. Proc. Natl. Acad. Sci. USA 1996, 93, 14574–14579. [Google Scholar] [CrossRef]
- Defranco, D.B. Role of molecular chaperones in subnuclear trafficking of glucocorticoid receptors. Kidney Int. 2000, 57, 1241–1249. [Google Scholar] [CrossRef]
- Santoro, M.G. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 2000, 59, 55–63. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Guettouche, T.; Smith, D.F.; Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 1998, 94, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, H.; Langer, T.; Rosmus, S.; Onneken, M.-L.; Fasold, H. A novel function for the 90 kDa heat-shock protein (Hsp90): Facilitating nuclear export of 60 S ribosomal subunits. Biochem. J. 2002, 362, 675–684. [Google Scholar] [CrossRef]
- Keppler, B.R.; Grady, A.T.; Jarstfer, M.B. The biochemical role of the heat shock protein 90 chaperone complex in establishing human telomerase activity. J. Biol. Chem. 2006, 281, 19840–19848. [Google Scholar] [CrossRef] [PubMed]
- Gray Jr, P.J.; Prince, T.; Cheng, J.; Stevenson, M.A.; Calderwood, S.K. Targeting the oncogene and kinome chaperone CDC37. Nat. Rev. Cancer 2008, 8, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Boter, M.; Li, K.; Kadota, Y.; Panaretou, B.; Prodromou, C.; Shirasu, K.; Pearl, L.H. Structural and functional coupling of Hsp90-and Sgt1-centred multi-protein complexes. EMBO J. 2008, 27, 2789–2798. [Google Scholar] [CrossRef]
- Davies, A.E.; Kaplan, K.B. Hsp90–Sgt1 and Skp1 target human Mis12 complexes to ensure efficient formation of kinetochore–microtubule binding sites. J. Cell Biol. 2010, 189, 261–274. [Google Scholar] [CrossRef]
- Fan, A.C.; Bhangoo, M.K.; Young, J.C. Hsp90 functions in the targeting and outer membrane translocation steps of Tom70-mediated mitochondrial import. J. Biol. Chem. 2006, 281, 33313–33324. [Google Scholar] [CrossRef]
- Imai, J.; Maruya, M.; Yashiroda, H.; Yahara, I.; Tanaka, K. The molecular chaperone Hsp90 plays a role in the assembly and maintenance of the 26S proteasome. EMBO J. 2003, 22, 3557–3567. [Google Scholar] [CrossRef]
- Martins, C.D.; Da Pieve, C.; Burley, T.A.; Smith, R.; Ciobota, D.M.; Allott, L.; Harrington, K.J.; Oyen, W.J.; Smith, G.; Kramer-Marek, G. HER3-mediated resistance to Hsp90 inhibition detected in breast cancer xenografts by affibody-based PET imaging. Clin. Cancer Res. 2018, 24, 1853–1865. [Google Scholar] [CrossRef]
- Makhnevych, T.; Houry, W.A. The role of Hsp90 in protein complex assembly. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 674–682. [Google Scholar] [CrossRef]
- Boulon, S.; Pradet-Balade, B.; Verheggen, C.; Molle, D.; Boireau, S.; Georgieva, M.; Azzag, K.; Robert, M.-C.; Ahmad, Y.; Neel, H. HSP90 and its R2TP/Prefoldin-like cochaperone are involved in the cytoplasmic assembly of RNA polymerase II. Mol. Cell 2010, 39, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Sun, W.; Taldone, T.; Rodina, A.; Chiosis, G. Heat shock protein 90 in neurodegenerative diseases. Mol. Neurodegener. 2010, 5, 24. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Blagg, B.S.; Kerr, T.D. Hsp90 inhibitors: Small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med. Res. Rev. 2006, 26, 310–338. [Google Scholar] [CrossRef]
- Powers, M.V.; Workman, P. Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr. Relat. Cancer 2006, 13 (Suppl. S1), S125–S135. [Google Scholar] [CrossRef] [PubMed]
- Barrott, J.J.; Haystead, T.A. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013, 280, 1381–1396. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef]
- Cheng, Q.; Chang, J.T.; Geradts, J.; Neckers, L.M.; Haystead, T.; Spector, N.L.; Lyerly, H.K. Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 2012, 14, R62. [Google Scholar] [CrossRef]
- Calderwood, S.K. Heat shock proteins and cancer: Intracellular chaperones or extracellular signalling ligands? Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160524. [Google Scholar] [CrossRef]
- Fusella, F.; Seclì, L.; Busso, E.; Krepelova, A.; Moiso, E.; Rocca, S.; Conti, L.; Annaratone, L.; Rubinetto, C.; Mello-Grand, M. The IKK/NF-κB signaling pathway requires Morgana to drive breast cancer metastasis. Nat. Commun. 2017, 8, 1636. [Google Scholar] [CrossRef]
- Seclì, L.; Avalle, L.; Poggio, P.; Fragale, G.; Cannata, C.; Conti, L.; Iannucci, A.; Carrà, G.; Rubinetto, C.; Miniscalco, B. Targeting the extracellular HSP90 co-chaperone morgana inhibits cancer cell migration and promotes anticancer immunity. Cancer Res. 2021, 81, 4794–4807. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, C.; Chen, S.; Liu, J.; Fu, Y.; Luo, Y. Extracellular Hsp90α and clusterin synergistically promote breast cancer epithelial-to-mesenchymal transition and metastasis via LRP1. J. Cell Sci. 2019, 132, jcs228213. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yang, Y.; Zhu, W.; Xian, Y.; Han, Z.; Huang, H.; Peng, L.; Zhang, K.; Zhao, Y. Heat shock protein 90 triggers multi-drug resistance of ovarian cancer via AKT/GSK3β/β-catenin signaling. Front. Oncol. 2021, 11, 620907. [Google Scholar] [CrossRef]
- Fernández, J.G.; Rodríguez, D.A.; Valenzuela, M.; Calderon, C.; Urzúa, U.; Munroe, D.; Rosas, C.; Lemus, D.; Díaz, N.; Wright, M.C. Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced β-catenin/Tcf-Lef dependent transcription. Mol. Cancer 2014, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Swords, R.; Carew, J.S.; Nawrocki, S.; Bhalla, K.; Giles, F. Targeting HSP90 for cancer therapy. Br. J. Cancer 2009, 100, 1523–1529. [Google Scholar] [CrossRef]
- Nimmanapalli, R.; O’Bryan, E.; Bhalla, K. Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res. 2001, 61, 1799–1804. [Google Scholar]
- Lewis, J.; Devin, A.; Miller, A.; Lin, Y.; Rodriguez, Y.; Neckers, L.; Liu, Z.-g. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-κB activation. J. Biol. Chem. 2000, 275, 10519–10526. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Whitesell, L.; Sutphin, P.D.; Pulcini, E.J.; Martinez, J.D.; Cook, P.H. The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an hsp90-binding agent. Mol. Cell. Biol. 1998, 18, 1517–1524. [Google Scholar] [CrossRef]
- Muise-Helmericks, R.C.; Grimes, H.L.; Bellacosa, A.; Malstrom, S.E.; Tsichlis, P.N.; Rosen, N. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 1998, 273, 29864–29872. [Google Scholar] [CrossRef]
- Srethapakdi, M.; Liu, F.; Tavorath, R.; Rosen, N. Inhibition of Hsp90 function by ansamycins causes retinoblastoma gene product-dependent G1 arrest. Cancer Res. 2000, 60, 3940–3946. [Google Scholar]
- Miyata, Y.; Nakamoto, H.; Neckers, L. The therapeutic target Hsp90 and cancer hallmarks. Curr. Pharm. Des. 2013, 19, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Toogun, O.A.; DeZwaan, D.C.; Freeman, B.C. The hsp90 molecular chaperone modulates multiple telomerase activities. Mol. Cell. Biol. 2008, 28, 457–467. [Google Scholar] [CrossRef]
- Laederich, M.B.; Degnin, C.R.; Lunstrum, G.P.; Holden, P.; Horton, W.A. Fibroblast growth factor receptor 3 (FGFR3) is a strong heat shock protein 90 (Hsp90) client: Implications for therapeutic manipulation. J. Biol. Chem. 2011, 286, 19597–19604. [Google Scholar] [CrossRef]
- Fu, Y.; Lee, A.S. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol. Ther. 2006, 5, 741–744. [Google Scholar] [CrossRef]
- Reimold, A.M.; Iwakoshi, N.N.; Manis, J.; Vallabhajosyula, P.; Szomolanyi-Tsuda, E.; Gravallese, E.M.; Friend, D.; Grusby, M.J.; Alt, F.; Glimcher, L.H. Plasma cell differentiation requires the transcription factor XBP-1. Nature 2001, 412, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, A.; Shapiro-Shelef, M.; Iwakoshi, N.N.; Lee, A.-H.; Qian, S.-B.; Zhao, H.; Yu, X.; Yang, L.; Tan, B.K.; Rosenwald, A. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 2004, 21, 81–93. [Google Scholar] [CrossRef]
- Duan, X.; Iwanowycz, S.; Ngoi, S.; Hill, M.; Zhao, Q.; Liu, B. Molecular chaperone GRP94/GP96 in cancers: Oncogenesis and therapeutic target. Front. Oncol. 2021, 11, 629846. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, Y.; Liu, C.-G.; Mikhail, S.; Yang, C.S. Overexpression of glucose-regulated protein 94 (Grp94) in esophageal adenocarcinomas of a rat surgical model and humans. Carcinogenesis 2002, 23, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; An, L.; Chen, Y.; Yue, S. Expression of endoplasmic reticulum molecular chaperon GRP94 in human lung cancer tissues and its clinical significance. Chin. Med. J. 2002, 115, 1615–1619. [Google Scholar]
- Wang, X.-P.; Qiu, F.-R.; Liu, G.-Z.; Chen, R.-F. Correlation between clinicopathology and expression of heat shock protein 70 and glucose-regulated protein 94 in human colonic adenocarcinoma. World J. Gastroenterol. WJG 2005, 11, 1056. [Google Scholar] [CrossRef]
- Wang, X.-P.; Wang, Q.-X.; Ying, X.-P. Correlation between clinicopathology and expression of heat shock protein 72 and glycoprotein 96 in human gastric adenocarcinoma. Tohoku J. Exp. Med. 2007, 212, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bini, L.; Magi, B.; Marzocchi, B.; Arcuri, F.; Tripodi, S.; Cintorino, M.; Sanchez, J.C.; Frutiger, S.; Hughes, G.; Pallini, V. Protein expression profiles in human breast ductal carcinoma and histologically normal tissue. Electrophoresis 1997, 18, 2832–2841. [Google Scholar] [CrossRef]
- Bagratuni, T.; Wu, P.; Gonzalez de Castro, D.; Davenport, E.L.; Dickens, N.J.; Walker, B.A.; Boyd, K.; Johnson, D.C.; Gregory, W.; Morgan, G.J. XBP1s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood J. Am. Soc. Hematol. 2010, 116, 250–253. [Google Scholar] [CrossRef]
- Davenport, E.L.; Moore, H.E.; Dunlop, A.S.; Sharp, S.Y.; Workman, P.; Morgan, G.J.; Davies, F.E. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood J. Am. Soc. Hematol. 2007, 110, 2641–2649. [Google Scholar] [CrossRef]
- White-Gilbertson, S.; Hua, Y.; Liu, B. The role of endoplasmic reticulum stress in maintaining and targeting multiple myeloma: A double-edged sword of adaptation and apoptosis. Front. Genet. 2013, 4, 109. [Google Scholar] [CrossRef]
- Dejeans, N.; Glorieux, C.; Guenin, S.; Beck, R.; Sid, B.; Rousseau, R.; Bisig, B.; Delvenne, P.; Calderon, P.B.; Verrax, J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: Implications for tumor recurrence. Free Radic. Biol. Med. 2012, 52, 993–1002. [Google Scholar] [CrossRef]
- Pan, Z.; Erkan, M.; Streit, S.; Friess, H.; Kleeff, J. Silencing of GRP94 expression promotes apoptosis in pancreatic cancer cells. Int. J. Oncol. 2009, 35, 823–828. [Google Scholar] [CrossRef]
- Smid, M.; Wang, Y.; Zhang, Y.; Sieuwerts, A.M.; Yu, J.; Klijn, J.G.; Foekens, J.A.; Martens, J.W. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008, 68, 3108–3114. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Muixí, L.; Foj, R.; Sanz-Pamplona, R.; Badia-Villanueva, M.; Abramowicz, A.; Marcé-Grau, A.; Cosialls, A.M.; Gil, J.; Archilla, I. GRP94 promotes brain metastasis by engaging pro-survival autophagy. Neuro-Oncology 2020, 22, 652–664. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Hou, J.; Gui, M.; Ying, J.; Zhao, H.; Lv, N.; Meng, S. Cell membrane gp96 facilitates HER 2 dimerization and serves as a novel target in breast cancer. Int. J. Cancer 2015, 137, 512–524. [Google Scholar] [CrossRef]
- Liu, B.; Staron, M.; Hong, F.; Wu, B.X.; Sun, S.; Morales, C.; Crosson, C.E.; Tomlinson, S.; Kim, I.; Wu, D. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 6877–6882. [Google Scholar] [CrossRef]
- Morales, C.; Rachidi, S.; Hong, F.; Sun, S.; Ouyang, X.; Wallace, C.; Zhang, Y.; Garret-Mayer, E.; Wu, J.; Liu, B. Immune chaperone gp96 drives the contributions of macrophages to inflammatory colon tumorigenesis. Cancer Res. 2014, 74, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, J.; Huang, Z.; Wei, P.; Liu, Y.; Hao, J.; Zhao, L.; Zhang, F.; Tu, Y.; Wei, T. Aberrantly upregulated TRAP1 is required for tumorigenesis of breast cancer. Oncotarget 2015, 6, 44495. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, K.W.; Kim, J.-E.; Hwang, S.W.; Park, J.H.; Kim, S.-H.; Ji, J.H.; Kim, T.G.; Nam, H.-Y.; Roh, M.S. Differential expression of heat shock protein 90 isoforms in small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9487. [Google Scholar] [PubMed]
- Masgras, I.; Laquatra, C.; Cannino, G.; Serapian, S.A.; Colombo, G.; Rasola, A. The molecular chaperone TRAP1 in cancer: From the basics of biology to pharmacological targeting. Semin. Cancer Biol. 2021, 76, 45–53. [Google Scholar] [CrossRef]
- Wengert, L.A.; Backe, S.J.; Bourboulia, D.; Mollapour, M.; Woodford, M.R. TRAP1 chaperones the metabolic switch in cancer. Biomolecules 2022, 12, 786. [Google Scholar] [CrossRef]
- Xiang, F.; Ma, S.-y.; Lv, Y.-l.; Zhang, D.-x.; Song, H.-p.; Huang, Y.-s. Tumor necrosis factor receptor-associated protein 1 regulates hypoxia-induced apoptosis through a mitochondria-dependent pathway mediated by cytochrome c oxidase subunit II. Burn. Trauma 2019, 7, 16. [Google Scholar] [CrossRef]
- Xiang, F.; Ma, S.-Y.; Zhang, D.-X.; Zhang, Q.; Huang, Y.-S. Tumor necrosis factor receptor-associated protein 1 improves hypoxia-impaired energy production in cardiomyocytes through increasing activity of cytochrome c oxidase subunit II. Int. J. Biochem. Cell Biol. 2016, 79, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Tsutsumi, S.; Muhlebach, G.; Sourbier, C.; Lee, M.-J.; Lee, S.; Vartholomaiou, E.; Tatokoro, M.; Beebe, K.; Miyajima, N. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc. Natl. Acad. Sci. USA 2013, 110, E1604–E1612. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.A.; Guzzo, G.; Morandi, A.; Perra, A.; Menegon, S.; Masgras, I.; Trevisan, E.; Angioni, M.M.; Fornari, F.; Quagliata, L. Metabolic reprogramming identifies the most aggressive lesions at early phases of hepatic carcinogenesis. Oncotarget 2016, 7, 32375. [Google Scholar] [CrossRef]
- Amoroso, M.R.; Matassa, D.S.; Laudiero, G.; Egorova, A.V.; Polishchuk, R.S.; Maddalena, F.; Piscazzi, A.; Paladino, S.; Sarnataro, D.; Garbi, C. TRAP1 and the proteasome regulatory particle TBP7/Rpt3 interact in the endoplasmic reticulum and control cellular ubiquitination of specific mitochondrial proteins. Cell Death Differ. 2012, 19, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-j.; Zhou, Y.-x.; Zhang, L.-r.; Lin, Q.-f.; Gao, P.-z.; Cai, F.; Zhu, L.-p.; Liu, B.; Xu, J.-h. C1206, a novel curcumin derivative, potently inhibits Hsp90 and human chronic myeloid leukemia cells In Vitro. Acta Pharmacol. Sin. 2018, 39, 649–658. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Zhang, L.; Cai, F.; Zhu, L.; Xu, J. C0818, a novel curcumin derivative, interacts with Hsp90 and inhibits Hsp90 ATPase activity. Acta Pharm. Sin. B 2017, 7, 91–96. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Jha, A.; Rupasinghe, H.V. Novel carbocyclic curcumin analog CUR3d modulates genes involved in multiple apoptosis pathways in human hepatocellular carcinoma cells. Chem. Biol. Interact. 2015, 242, 107–122. [Google Scholar] [CrossRef]
- Giommarelli, C.; Zuco, V.; Favini, E.; Pisano, C.; Dal Piaz, F.; De Tommasi, N.; Zunino, F. The enhancement of antiproliferative and proapoptotic activity of HDAC inhibitors by curcumin is mediated by Hsp90 inhibition. Cell. Mol. Life Sci. 2010, 67, 995–1004. [Google Scholar] [CrossRef]
- Liew, H.Y.; Tan, X.Y.; Chan, H.H.; Khaw, K.Y.; Ong, Y.S. Natural HSP90 inhibitors as a potential therapeutic intervention in treating cancers: A comprehensive review. Pharmacol. Res. 2022, 181, 106260. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.P.; Guida, T.; Alfano, L.; Avilla, E.; Santoro, M.; Carlomagno, F.; Melillo, R.M. Molecular mechanism of 17-allylamino-17-demethoxygeldanamycin (17-AAG)-induced AXL receptor tyrosine kinase degradation. J. Biol. Chem. 2013, 288, 17481–17494. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Kim, G.P.; Foster, N.R.; Wang-Gillam, A.; Erlichman, C.; McWilliams, R.R. Phase II trial of gemcitabine and tanespimycin (17AAG) in metastatic pancreatic cancer: A Mayo Clinic Phase II Consortium study. Investig. New Drugs 2015, 33, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Okuda, K.; Zheng, W.; Butrynski, J.; Capelletti, M.; Wang, L.; Gray, N.S.; Wilner, K.; Christensen, J.G.; Demetri, G. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010, 70, 10038–10043. [Google Scholar] [CrossRef]
- Braga-Basaria, M.; Hardy, E.; Gottfried, R.; Burman, K.D.; Saji, M.; Ringel, M.D. 17-Allylamino-17-demethoxygeldanamycin activity against thyroid cancer cell lines correlates with heat shock protein 90 levels. J. Clin. Endocrinol. Metab. 2004, 89, 2982–2988. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Z.; Zhao, Y.; Zhang, T.; Gu, X.; Yang, W. The heat shock protein 90 inhibitor 17-AAG suppresses growth and induces apoptosis in human cholangiocarcinoma cells. Clin. Exp. Med. 2013, 13, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.N.; Roychowdhury, S.; Kini, A.R.; Alkan, S. HSP90 inhibitor 17AAG causes apoptosis in ATRA-resistant acute promyelocytic leukemia cells. Leuk. Res. 2008, 32, 143–149. [Google Scholar] [CrossRef]
- Egorin, M.J.; Lagattuta, T.F.; Hamburger, D.R.; Covey, J.M.; White, K.D.; Musser, S.M.; Eiseman, J.L. Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD2F1 mice and Fischer 344 rats. Cancer Chemother. Pharmacol. 2002, 49, 7–19. [Google Scholar] [CrossRef]
- Panarsky, R.; Reichert, F.; Ben-Ishay, Z. Effectiveness of 17DMAG, a geldanamycin derivative, in murine acute myeloid leukemia. Acta Haematol. 2009, 121, 32–36. [Google Scholar] [CrossRef]
- Hollingshead, M.; Alley, M.; Burger, A.M.; Borgel, S.; Pacula-Cox, C.; Fiebig, H.-H.; Sausville, E.A. In Vivo antitumor efficacy of 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride), a water-soluble geldanamycin derivative. Cancer Chemother. Pharmacol. 2005, 56, 115–125. [Google Scholar] [CrossRef]
- Niu, G.; Li, Z.; Cao, Q.; Chen, X. Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with 64Cu-DOTA-trastuzumab. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1510–1519. [Google Scholar] [CrossRef]
- Leng, A.-m.; Liu, T.; Yang, J.; Cui, J.-f.; Li, X.-h.; Zhu, Y.-n.; Xiong, T.; Zhang, G.; Chen, Y. The apoptotic effect and associated signalling of HSP90 inhibitor 17-DMAG in hepatocellular carcinoma cells. Cell Biol. Int. 2012, 36, 893–899. [Google Scholar] [CrossRef]
- Mellatyar, H.; Talaei, S.; Pilehvar-Soltanahmadi, Y.; Barzegar, A.; Akbarzadeh, A.; Shahabi, A.; Barekati-Mowahed, M.; Zarghami, N. Targeted cancer therapy through 17-DMAG as an Hsp90 inhibitor: Overview and current state of the art. Biomed. Pharmacother. 2018, 102, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Sugita, M.; Wilkes, D.C.; Bareja, R.; Eng, K.W.; Nataraj, S.; Jimenez-Flores, R.A.; Yan, L.; De Leon, J.P.; Croyle, J.A.; Kaner, J. Targeting the epichaperome as an effective precision medicine approach in a novel PML-SYK fusion acute myeloid leukemia. npj Precis. Oncol. 2021, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Lai, C.-J.; Wang, D.-G.; Qu, H.; Yin, L.; Zifcak, B.; Tao, X.; Wang, J.; Atoyan, R.; Samson, M. Targeting heat shock protein 90 with CUDC-305 overcomes erlotinib resistance in non–small cell lung cancer. Mol. Cancer Ther. 2009, 8, 3296–3306. [Google Scholar] [CrossRef]
- Lundgren, K.; Biamonte, M.A. The Discovery of BIIB021 and BIIB028; Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Kim, J.Y.; Cho, T.-M.; Park, J.M.; Park, S.; Park, M.; Nam, K.D.; Ko, D.; Seo, J.; Kim, S.; Jung, E. A novel HSP90 inhibitor SL-145 suppresses metastatic triple-negative breast cancer without triggering the heat shock response. Oncogene 2022, 41, 3289–3297. [Google Scholar] [CrossRef]
- Kang, B.H.; Tavecchio, M.; Goel, H.; Hsieh, C.; Garlick, D.S.; Raskett, C.; Lian, J.; Stein, G.; Languino, L.R.; Altieri, D.C. Targeted inhibition of mitochondrial Hsp90 suppresses localised and metastatic prostate cancer growth in a genetic mouse model of disease. Br. J. Cancer 2011, 104, 629–634. [Google Scholar] [CrossRef]
- Palermo, C.M.; Westlake, C.A.; Gasiewicz, T.A. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry 2005, 44, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Vasko, R.C.; Rodriguez, R.A.; Cunningham, C.N.; Ardi, V.C.; Agard, D.A.; McAlpine, S.R. Mechanistic studies of Sansalvamide A-amide: An allosteric modulator of Hsp90. ACS Med. Chem. Lett. 2010, 1, 4–8. [Google Scholar] [CrossRef]
- Ying, W.; Du, Z.; Sun, L.; Foley, K.P.; Proia, D.A.; Blackman, R.K.; Zhou, D.; Inoue, T.; Tatsuta, N.; Sang, J. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol. Cancer Ther. 2012, 11, 475–484. [Google Scholar] [CrossRef]
- Okawa, Y.; Hideshima, T.; Steed, P.; Vallet, S.; Hall, S.; Huang, K.; Rice, J.; Barabasz, A.; Foley, B.; Ikeda, H. SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood J. Am. Soc. Hematol. 2009, 113, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Honma, Y.; Sawaki, A.; Naito, Y.; Iwagami, S.; Komatsu, Y.; Takahashi, T.; Nishida, T.; Doi, T. Pimitespib in patients with advanced gastrointestinal stromal tumor (CHAPTER-GIST-301): A randomized, double-blind, placebo-controlled phase III trial. Ann. Oncol. 2022, 33, 959–967. [Google Scholar] [CrossRef]
- Brough, P.A.; Barril, X.; Borgognoni, J.; Chene, P.; Davies, N.G.; Davis, B.; Drysdale, M.J.; Dymock, B.; Eccles, S.A.; Garcia-Echeverria, C. Combining hit identification strategies: Fragment-based and in silico approaches to orally active 2-aminothieno [2, 3-d] pyrimidine inhibitors of the Hsp90 molecular chaperone. J. Med. Chem. 2009, 52, 4794–4809. [Google Scholar] [CrossRef]
- Liu, B.; Shen, Y.; Huang, H.; Croce, K.D.; Wu, M.; Fan, Y.; Liu, Y.; Xu, J.; Yao, G. Curcumin derivative C212 inhibits Hsp90 and eliminates both growing and quiescent leukemia cells in deep dormancy. Cell Commun. Signal. 2020, 18, 159. [Google Scholar] [CrossRef]
- Wagner, A.J.; Chugh, R.; Rosen, L.S.; Morgan, J.A.; George, S.; Gordon, M.; Dunbar, J.; Normant, E.; Grayzel, D.; Demetri, G.D. A phase I study of the HSP90 inhibitor retaspimycin hydrochloride (IPI-504) in patients with gastrointestinal stromal tumors or soft-tissue sarcomas. Clin. Cancer Res. 2013, 19, 6020–6029. [Google Scholar] [CrossRef]
- Floris, G.; Sciot, R.; Wozniak, A.; Van Looy, T.; Wellens, J.; Faa, G.; Normant, E.; Debiec-Rychter, M.; Schöffski, P. The Novel HSP90 inhibitor, IPI-493, is highly effective in human gastrostrointestinal stromal tumor xenografts carrying heterogeneous KIT mutations. Clin. Cancer Res. 2011, 17, 5604–5614. [Google Scholar] [CrossRef]
- Saber, S.; Abdelhady, R.; Elhemely, M.A.; Elmorsy, E.A.; Hamad, R.S.; Abdel-Reheim, M.A.; El-Kott, A.F.; AlShehri, M.A.; Morsy, K.; AlSheri, A.S. PU-H71 (NSC 750424): A molecular masterpiece that targets HSP90 in cancer and beyond. Front. Pharmacol. 2024, 15, 1475998. [Google Scholar] [CrossRef]
- Taldone, T.; Chiosis, G. Purine-scaffold Hsp90 inhibitors. Curr. Top. Med. Chem. 2009, 9, 1436–1446. [Google Scholar] [CrossRef]
- Gökşen Tosun, N. Enhancing therapeutic efficacy in breast cancer: A study on the combined cytotoxic effects of doxorubicin and MPC-3100. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3249–3259. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.G.; Schulte, T.W.; Neckers, L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J. Natl. Cancer Inst. 2000, 92, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Jiang, Y.; Lee, H.-F.; Schwartz, S.J.; Sun, D. (−)-Epigallocatechin-3-gallate inhibits Hsp90 function by impairing Hsp90 association with cochaperones in pancreatic cancer cell line Mia Paca-2. Mol. Pharm. 2009, 6, 1152–1159. [Google Scholar] [CrossRef]
- Smyth, T.; Paraiso, K.H.; Hearn, K.; Rodriguez-Lopez, A.M.; Munck, J.M.; Haarberg, H.E.; Sondak, V.K.; Thompson, N.T.; Azab, M.; Lyons, J.F. Inhibition of HSP90 by AT13387 delays the emergence of resistance to BRAF inhibitors and overcomes resistance to dual BRAF and MEK inhibition in melanoma models. Mol. Cancer Ther. 2014, 13, 2793–2804. [Google Scholar] [CrossRef] [PubMed]
- Haarberg, H.E.; Paraiso, K.H.; Wood, E.; Rebecca, V.W.; Sondak, V.K.; Koomen, J.M.; Smalley, K.S. Inhibition of Wee1, AKT, and CDK4 underlies the efficacy of the HSP90 inhibitor XL888 in an In Vivo model of NRAS-mutant melanoma. Mol. Cancer Ther. 2013, 12, 901–912. [Google Scholar] [CrossRef]
- Yeramian, A.; Vea, A.; Benítez, S.; Ribera, J.; Domingo, M.; Santacana, M.; Martinez, M.; Maiques, O.; Valls, J.; Dolcet, X. 2-phenylethynesulphonamide (PFT-μ) enhances the anticancer effect of the novel hsp90 inhibitor NVP-AUY922 in melanoma, by reducing GSH levels. Pigment Cell Melanoma Res. 2016, 29, 352–371. [Google Scholar] [CrossRef]
- Batko, J.; Antosz, K.; Miśków, W.; Pszczołowska, M.; Walczak, K.; Leszek, J. Chaperones—A new class of potential therapeutic targets in Alzheimer’s disease. Int. J. Mol. Sci. 2024, 25, 3401. [Google Scholar] [CrossRef] [PubMed]
- Wasik, U.; Schneider, G.; Mietelska-Porowska, A.; Mazurkiewicz, M.; Fabczak, H.; Weis, S.; Zabke, C.; Harrington, C.R.; Filipek, A.; Niewiadomska, G. Calcyclin binding protein and Siah-1 interacting protein in Alzheimer’s disease pathology: Neuronal localization and possible function. Neurobiol. Aging 2013, 34, 1380–1388. [Google Scholar] [CrossRef]
- Smith, J.R.; Workman, P. Targeting CDC37: An alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle 2009, 8, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Dou, F.; Rodina, A.; Chip, S.; Kim, J.; Zhao, Q.; Moulick, K.; Aguirre, J.; Wu, N.; Greengard, P. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc. Natl. Acad. Sci. USA 2007, 104, 9511–9516. [Google Scholar] [CrossRef]
- Cruz, J.C.; Tseng, H.-C.; Goldman, J.A.; Shih, H.; Tsai, L.-H. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 2003, 40, 471–483. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Yu, Y.; Zou, P.; Jiang, Y.; Sun, D. Characterization of celastrol to inhibit hsp90 and cdc37 interaction. J. Biol. Chem. 2009, 284, 35381–35389. [Google Scholar] [CrossRef]
- Grelle, G.; Kostka, S.; Otto, A.; Kersten, B.; Genser, K.F.; Müller, E.-C.; Wälter, S.; Böddrich, A.; Stelzl, U.; Hänig, C. Identification of VCP/p97, carboxyl terminus of Hsp70-interacting protein (CHIP), and amphiphysin II interaction partners using membrane-based human proteome arrays. Mol. Cell. Proteom. 2006, 5, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Ambegaokar, S.S.; Jackson, G.R. Functional genomic screen and network analysis reveal novel modifiers of tauopathy dissociated from tau phosphorylation. Hum. Mol. Genet. 2011, 20, 4947–4977. [Google Scholar] [CrossRef]
- Blair, L.J.; Sabbagh, J.J.; Dickey, C.A. Targeting Hsp90 and its co-chaperones to treat Alzheimer’s disease. Expert Opin. Ther. Targets 2014, 18, 1219–1232. [Google Scholar] [CrossRef]
- Ma, J.; Farmer, K.L.; Pan, P.; Urban, M.J.; Zhao, H.; Blagg, B.S.; Dobrowsky, R.T. Heat shock protein 70 is necessary to improve mitochondrial bioenergetics and reverse diabetic sensory neuropathy following KU-32 therapy. J. Pharmacol. Exp. Ther. 2014, 348, 281–292. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Liu, D.; Li, J.J.; Xue, Y.; Sakata, K.; Zhu, L.-q.; Heldt, S.A.; Xu, H.; Liao, F.-F. Hsp90 chaperone inhibitor 17-AAG attenuates Aβ-induced synaptic toxicity and memory impairment. J. Neurosci. 2014, 34, 2464–2470. [Google Scholar] [CrossRef]
- Pillay, I.; Nakano, H.; Sharma, S. Radicicol inhibits tyrosine phosphorylation of the mitotic Src substrate Sam68 and retards subsequent exit from mitosis of Src-transformed cells. Cell Cycle 1996, 20, 21. [Google Scholar]
- Blair, L.J.; Nordhues, B.A.; Hill, S.E.; Scaglione, K.M.; O’Leary, J.C.; Fontaine, S.N.; Breydo, L.; Zhang, B.; Li, P.; Wang, L. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Investig. 2013, 123, 4158–4169. [Google Scholar] [CrossRef]
- Lackie, R.E.; de Miranda, A.S.; Lim, M.P.; Novikov, V.; Madrer, N.; Karunatilleke, N.C.; Rutledge, B.S.; Tullo, S.; Brickenden, A.; Maitland, M.E. Stress-inducible phosphoprotein 1 (HOP/STI1/STIP1) regulates the accumulation and toxicity of α-synuclein In Vivo. Acta Neuropathol. 2022, 144, 881–910. [Google Scholar] [CrossRef] [PubMed]
- K Jinwal, U.; Koren III, J.; A Dickey, C. Reconstructing the Hsp90/tau machine. Curr. Enzym. Inhib. 2013, 9, 41–45. [Google Scholar] [CrossRef]
- McFarland, N.R.; Dimant, H.; Kibuuka, L.; Ebrahimi-Fakhari, D.; Desjardins, C.A.; Danzer, K.M.; Danzer, M.; Fan, Z.; Schwarzschild, M.A.; Hirst, W. Chronic treatment with novel small molecule Hsp90 inhibitors rescues striatal dopamine levels but not α-synuclein-induced neuronal cell loss. PLoS ONE 2014, 9, e86048. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Raffaele, I.; Mazzon, E. Modulating stress proteins in response to therapeutic interventions for Parkinson’s disease. Int. J. Mol. Sci. 2023, 24, 16233. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, C.; Song, C. Pan-and isoform-specific inhibition of Hsp90: Design strategy and recent advances. Eur. J. Med. Chem. 2022, 238, 114516. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Maiti, S.; Zahoran, S.; Weidenauer, L.; Hany, D.; Wider, D.; Bernasconi, L.; Quadroni, M.; Collart, M.; Picard, D. Translational reprogramming in response to accumulating stressors ensures critical threshold levels of Hsp90 for mammalian life. Nat. Commun. 2022, 13, 6271. [Google Scholar] [CrossRef]
- Qi, S.; Yi, G.; Yu, K.; Feng, C.; Deng, S. The role of HSP90 inhibitors in the treatment of cardiovascular diseases. Cells 2022, 11, 3444. [Google Scholar] [CrossRef]
- Pan, L.; Huang, C.; Jin, X.; Wu, J.; Jin, K.; Lin, J.; Wang, Y.; Li, J.; Yin, C.; Wang, X. Cardiac secreted HSP90α exacerbates pressure overload myocardial hypertrophy and heart failure. Redox Biol. 2025, 79, 103466. [Google Scholar] [CrossRef]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.-J. Fibroblast-specific TGF-β–Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; An, Y.S.; Kim, M.R.; Kim, Y.A.; Lee, J.K.; Hwang, C.S.; Chung, E.; Park, I.C.; Yi, J.Y. Heat shock protein 90 regulates subcellular localization of smads in Mv1Lu cells. J. Cell. Biochem. 2016, 117, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, M.; Tsuchiya, Y.; Nakao, Y.; Suzuki, T.; Motohashi, H.; Yamamoto, M.; Kamata, H. Structural instability of IκB kinase β promotes autophagic degradation through enhancement of Keap1 binding. PLoS ONE 2018, 13, e0203978. [Google Scholar] [CrossRef]
- Thangjam, G.S.; Dimitropoulou, C.; Joshi, A.D.; Barabutis, N.; Shaw, M.C.; Kovalenkov, Y.; Wallace, C.M.; Fulton, D.J.; Patel, V.; Catravas, J.D. Novel mechanism of attenuation of LPS-induced NF-κB activation by the heat shock protein 90 inhibitor, 17-N-allylamino-17-demethoxygeldanamycin, in human lung microvascular endothelial cells. Am. J. Respir. Cell Mol. Biol. 2014, 50, 942–952. [Google Scholar] [CrossRef]
- Zhu, W.S.; Guo, W.; Zhu, J.N.; Tang, C.M.; Fu, Y.H.; Lin, Q.X.; Tan, N.; Shan, Z.X. Hsp90aa1: A novel target gene of miR-1 in cardiac ischemia/reperfusion injury. Sci. Rep. 2016, 6, 24498. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.; Chung, J.H. Heat shock protein 90 regulates IκB kinase complex and NF-κB activation in angiotensin II-induced cardiac cell hypertrophy. Exp. Mol. Med. 2010, 42, 703–711. [Google Scholar] [CrossRef]
- Lee, J.-H.; Gao, J.; Kosinski, P.A.; Elliman, S.J.; Hughes, T.E.; Gromada, J.; Kemp, D.M. Heat shock protein 90 (HSP90) inhibitors activate the heat shock factor 1 (HSF1) stress response pathway and improve glucose regulation in diabetic mice. Biochem. Biophys. Res. Commun. 2013, 430, 1109–1113. [Google Scholar] [CrossRef]
- Archer, A.E.; Von Schulze, A.T.; Geiger, P.C. Exercise, heat shock proteins and insulin resistance. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160529. [Google Scholar] [CrossRef]
- Rowles, J.E.; Keane, K.N.; Gomes Heck, T.; Cruzat, V.; Verdile, G.; Newsholme, P. Are Heat Shock Proteins an Important Link between Type 2 Diabetes and Alzheimer Disease? Int. J. Mol. Sci. 2020, 21, 8204. [Google Scholar] [CrossRef]
- Jing, E.; Sundararajan, P.; Majumdar, I.D.; Hazarika, S.; Fowler, S.; Szeto, A.; Gesta, S.; Mendez, A.J.; Vishnudas, V.K.; Sarangarajan, R.; et al. Hsp90β knockdown in DIO mice reverses insulin resistance and improves glucose tolerance. Nutr. Metab. 2018, 15, 11. [Google Scholar] [CrossRef]
- Ghiasi, S.M.; Dahlby, T.; Hede Andersen, C.; Haataja, L.; Petersen, S.; Omar-Hmeadi, M.; Yang, M.; Pihl, C.; Bresson, S.E.; Khilji, M.S.; et al. Endoplasmic Reticulum Chaperone Glucose-Regulated Protein 94 Is Essential for Proinsulin Handling. Diabetes 2019, 68, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cui, J.; He, Q.; Chen, Z.; Arvan, P.; Liu, M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes. Mol. Asp. Med. 2015, 42, 105–118. [Google Scholar] [CrossRef]
- Kim, D.-S.; Song, L.; Gou, W.; Kim, J.; Liu, B.; Wei, H.; Muise-Helmericks, R.C.; Li, Z.; Wang, H. GRP94 is an IGF-1R chaperone and regulates beta cell death in diabetes. Cell Death Dis. 2024, 15, 374. [Google Scholar] [CrossRef]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Vitadello, M.; Penzo, D.; Petronilli, V.; Michieli, G.; Gomirato, S.; Menabò, R.; Di Lisa, F.; Gorza, L. Overexpression of the stress-protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Ruiz-Velasco, A.; Raja, R.; Howell, G.; Miller, J.M.; Abouleisa, R.R.E.; Ou, Q.; Mace, K.; Hille, S.S.; Frey, N.; et al. Paracrine signal emanating from stressed cardiomyocytes aggravates inflammatory microenvironment in diabetic cardiomyopathy. iScience 2022, 25, 103973. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef]
- Ding, L.; Li, J.; Song, B.; Xiao, X.; Zhang, B.; Qi, M.; Huang, W.; Yang, L.; Wang, Z. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol. Appl. Pharmacol. 2016, 304, 99–109. [Google Scholar] [CrossRef]
- Kim, Y.; Rouse, M.; González-Mariscal, I.; Egan, J.M.; O’Connell, J.F. Dietary curcumin enhances insulin clearance in diet-induced obese mice via regulation of hepatic PI3K-AKT axis and IDE, and preservation of islet integrity. Nutr. Metab. 2019, 16, 48. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, Y.; Gao, J.; Zheng, Z.; Zhang, Z.; Yao, L.; Li, D. Curcumin improves insulin sensitivity in high-fat diet-fed mice through gut microbiota. Nutr. Metab. 2022, 19, 76. [Google Scholar] [CrossRef]
- Datta, R.; Bansal, T.; Rana, S.; Datta, K.; Datta Chaudhuri, R.; Chawla-Sarkar, M.; Sarkar, S. Myocyte-derived Hsp90 modulates collagen upregulation via biphasic activation of STAT-3 in fibroblasts during cardiac hypertrophy. Mol. Cell. Biol. 2017, 37, e00611-16. [Google Scholar] [CrossRef]
- Fukuyo, Y.; Hunt, C.R.; Horikoshi, N. Geldanamycin and its anti-cancer activities. Cancer Lett. 2010, 290, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Marunouchi, T.; Ito, T.; Onda, S.; Kyo, L.; Takahashi, K.; Uchida, M.; Yano, E.; Tanonaka, K. Effects of 17-AAG on the RIP1/RIP3/MLKL pathway during the development of heart failure following myocardial infarction in rats. J. Pharmacol. Sci. 2021, 147, 192–199. [Google Scholar] [CrossRef]
- Kim, J.; Jang, S.-W.; Park, E.; Oh, M.; Park, S.; Ko, J. The role of heat shock protein 90 in migration and proliferation of vascular smooth muscle cells in the development of atherosclerosis. J. Mol. Cell. Cardiol. 2014, 72, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Shen, H.-H.; Cheng, P.-Y.; Chu, Y.-J.; Hwang, H.-R.; Lam, K.-K.; Lee, Y.-M. 17-DMAG, an HSP90 inhibitor, ameliorates multiple organ dysfunction syndrome via induction of HSP70 in endotoxemic rats. PLoS ONE 2016, 11, e0155583. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Matute, J.; Fernandez-Garcia, C.E.; Gomez-Guerrero, C.; Lopez-Franco, O.; Munoz-Garcia, B.; Egido, J.; Blanco-Colio, L.M.; Martin-Ventura, J.L. HSP90 inhibition by 17-DMAG attenuates oxidative stress in experimental atherosclerosis. Cardiovasc. Res. 2012, 95, 116–123. [Google Scholar] [CrossRef]
- Pant, A.; Krishnakumar, K.C.; Dileep, N.C.; Yamana, M.; Alamelu, N.M.; Paithankar, K.; Amash, V.; Subbarao, S.A. Hsp90 and its mitochondrial homologue TRAP-1 independently regulate hypoxia adaptations in Caenorhabditis elegans. Mitochondrion 2021, 60, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, I.; Oguiza, A.; Recio, C.; Mallavia, B.; Madrigal-Matute, J.; Blanco, J.; Egido, J.; Martin-Ventura, J.-L.; Gomez-Guerrero, C. Targeting HSP90 Ameliorates Nephropathy and Atherosclerosis Through Suppression of NF-κB and STAT Signaling Pathways in Diabetic Mice. Diabetes 2015, 64, 3600–3613. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, I.; Oguiza, A.; Recio, C.; Lopez-Sanz, L.; Bernal, S.; Egido, J.; Gomez-Guerrero, C. Interplay between HSP90 and Nrf2 pathways in diabetes-associated atherosclerosis. Clínica Investig. Arterioscler. (Engl. Ed.) 2017, 29, 51–59. [Google Scholar]
- Neckers, L.; Blagg, B.; Haystead, T.; Trepel, J.B.; Whitesell, L.; Picard, D. Methods to validate Hsp90 inhibitor specificity, to identify off-target effects, and to rethink approaches for further clinical development. Cell Stress Chaperones 2018, 23, 467–482. [Google Scholar] [CrossRef]
- Boucherat, O.; Peterlini, T.; Bourgeois, A.; Nadeau, V.; Breuils-Bonnet, S.; Boilet-Molez, S.; Potus, F.; Meloche, J.; Chabot, S.; Lambert, C. Mitochondrial HSP90 accumulation promotes vascular remodeling in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 90–103. [Google Scholar] [CrossRef]
- Aceros, H.; Der Sarkissian, S.; Borie, M.; Stevens, L.-M.; Mansour, S.; Noiseux, N. Celastrol-type HSP90 modulators allow for potent cardioprotective effects. Life Sci. 2019, 227, 8–19. [Google Scholar] [CrossRef]
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, P.C.; Picard, D. Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2010, 1803, 641–649. [Google Scholar] [CrossRef]

| Localization | Gene/Protein | Characteristics | Homology | Functions | References |
|---|---|---|---|---|---|
| Cytosol | Hsp90α (Hsp90AA1) | Stress inducible | ~86% amino acid sequence similarity between Hsp90α and Hsp90β; >95% homology within N-terminal and ATP-binding domains | Chaperoning and maturation of client proteins; regulation of steroid hormone receptor signaling; facilitation of protein trafficking; cell-cycle control | [2] |
| Hsp90β (Hsp90 AB1) | Constitutively expressed | ||||
| Endoplasmic reticulum (ER) | Grp94/Gp96 (Hsp90B1) | Ca2+-binding chaperone; ATP hydrolysis triggers conformational activation | >50% amino acid sequence homology with cytosolic Hsp90; possesses a KDEL retention motif; lacks MEEVD sequence | Folding and maturation of secretory and membrane proteins; quality control of ER clients such as TLR, insulin-like growth factors | [5] |
| Mitochondria | TRAP1 (TNF receptor-associated protein 1) | Cytoplasmic and imported into mitochondria; mitochondrial targeting sequence cleaved upon import | >85% identity with N-terminal ATP-binding domain of cytosolic Hsp90; lacks charged linker region and MEEVD motif | Maintains mitochondrial integrity; protects against ROS and oxidative stress; overexpressed in several cancers | [6,7,8] |
| Extracellular medium | eHsp90α (plasma and serum); eHsp90β (exosome secretion dependent on p53 activation in cancer cells) | Cell surface-associated and secreted forms of Hsp90 | High homology with cytosolic Hsp90; contains a distinct 115-amino-acid middle and charged linker binding domain | Facilitates tumor cell invasion, migration, and extracellular matrix remodeling | [9,10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.K.; Ranbhise, J.S.; Fu, M.; Ju, S.; Han, S.; Yun, H.R.; Choe, W.; Kim, S.S.; Kang, I. Beyond Folding: Expanding the Functional Landscape of Hsp90 Chaperone Machinery in Health and Disease. Int. J. Mol. Sci. 2025, 26, 10279. https://doi.org/10.3390/ijms262110279
Singh MK, Ranbhise JS, Fu M, Ju S, Han S, Yun HR, Choe W, Kim SS, Kang I. Beyond Folding: Expanding the Functional Landscape of Hsp90 Chaperone Machinery in Health and Disease. International Journal of Molecular Sciences. 2025; 26(21):10279. https://doi.org/10.3390/ijms262110279
Chicago/Turabian StyleSingh, Manish Kumar, Jyotsna S. Ranbhise, Minghao Fu, Songhyun Ju, Sunhee Han, Hyeong Rok Yun, Wonchae Choe, Sung Soo Kim, and Insug Kang. 2025. "Beyond Folding: Expanding the Functional Landscape of Hsp90 Chaperone Machinery in Health and Disease" International Journal of Molecular Sciences 26, no. 21: 10279. https://doi.org/10.3390/ijms262110279
APA StyleSingh, M. K., Ranbhise, J. S., Fu, M., Ju, S., Han, S., Yun, H. R., Choe, W., Kim, S. S., & Kang, I. (2025). Beyond Folding: Expanding the Functional Landscape of Hsp90 Chaperone Machinery in Health and Disease. International Journal of Molecular Sciences, 26(21), 10279. https://doi.org/10.3390/ijms262110279








