Uric Acid in Cerebral Ischemia: A Systematic Review of Its Biomarker Value and Role in Neuroprotection
Abstract
1. Introduction
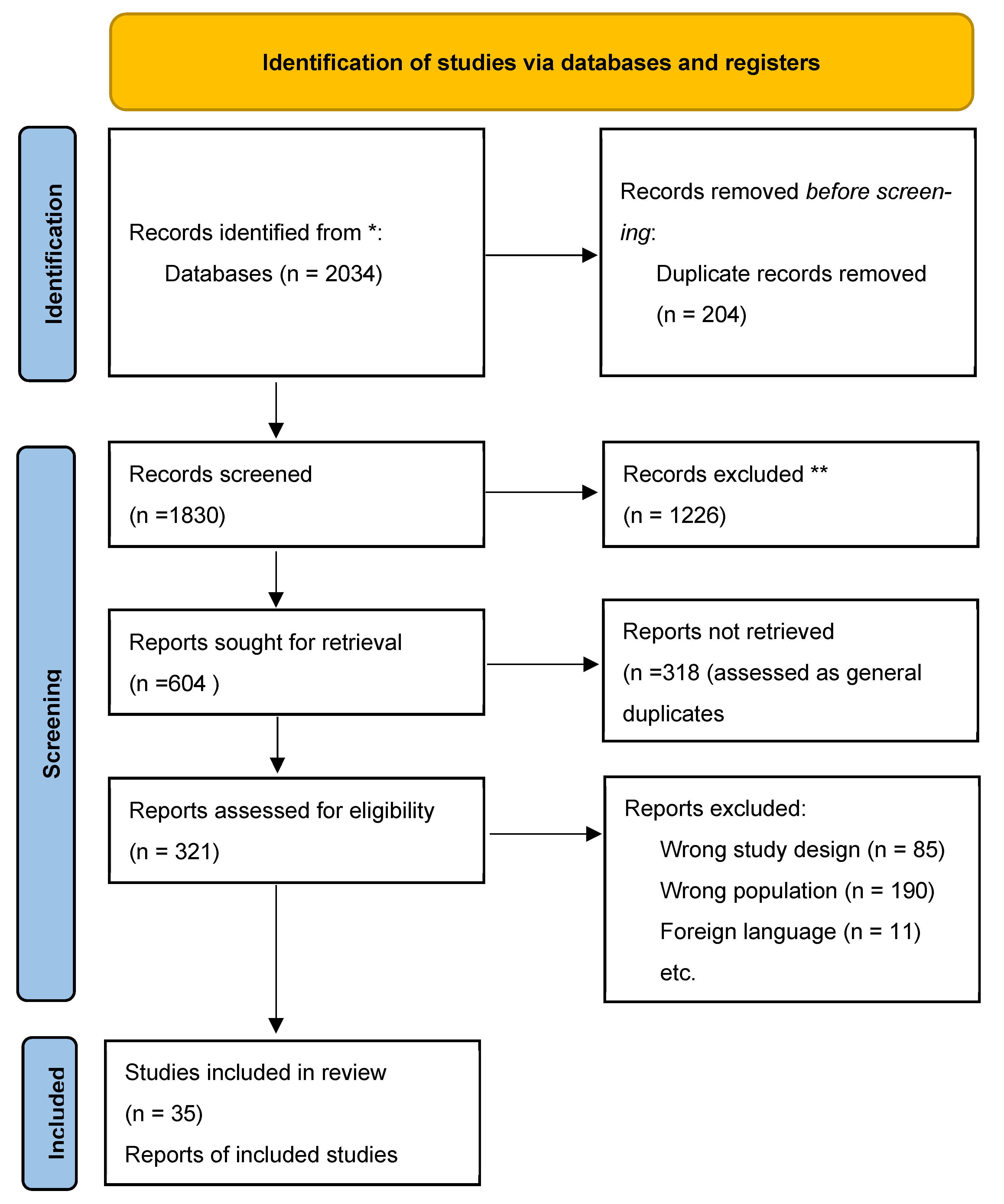
2. Methods
2.1. Protocol and Registration
- Participants: Adults (≥18 years) with acute ischemic stroke
- Exposure: Serum uric acid levels measured on admission or within 72 h
- Outcomes: Clinical endpoints including mortality, functional recovery (e.g., modified Rankin Scale), neurological deterioration
- Study Designs: Observational cohort studies (prospective or retrospective), randomized controlled trials, or meta-analyses
- Report Characteristics: Published in English between January 2010 and August 2025
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection
2.5. Data Collection Process
2.6. Data Synthesis and Statistical Analysis
2.7. Statistical Analysis
2.8. Risk of Bias
3. Clinical Background and Rationale
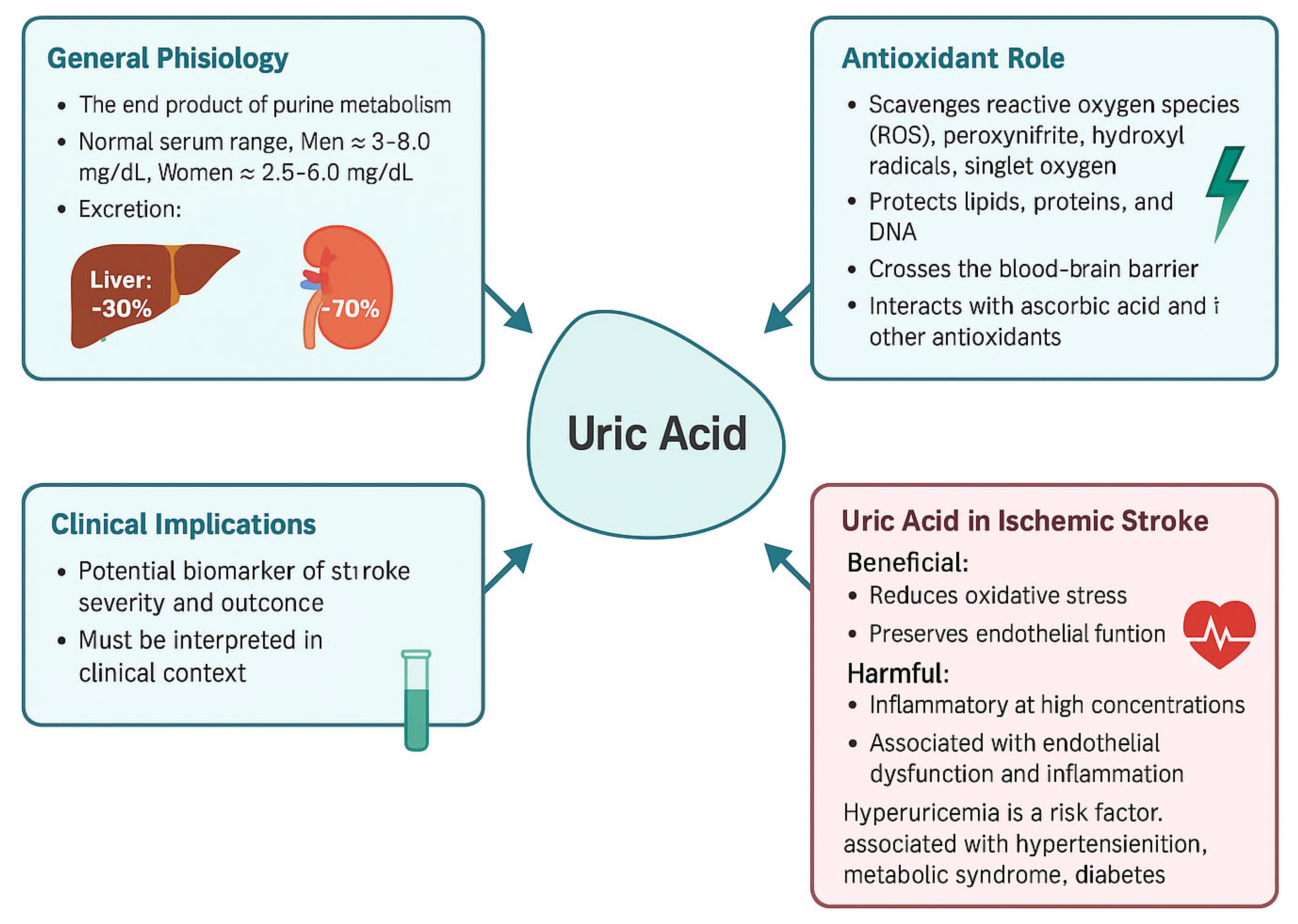
3.1. The Biochemical Pathway of Urogenesis and Its Implications in the Pathogenesis of Stroke
3.1.1. Purine Catabolism → Hypoxanthine
3.1.2. Xanthine Oxidase (XO)-Mediated Oxidation
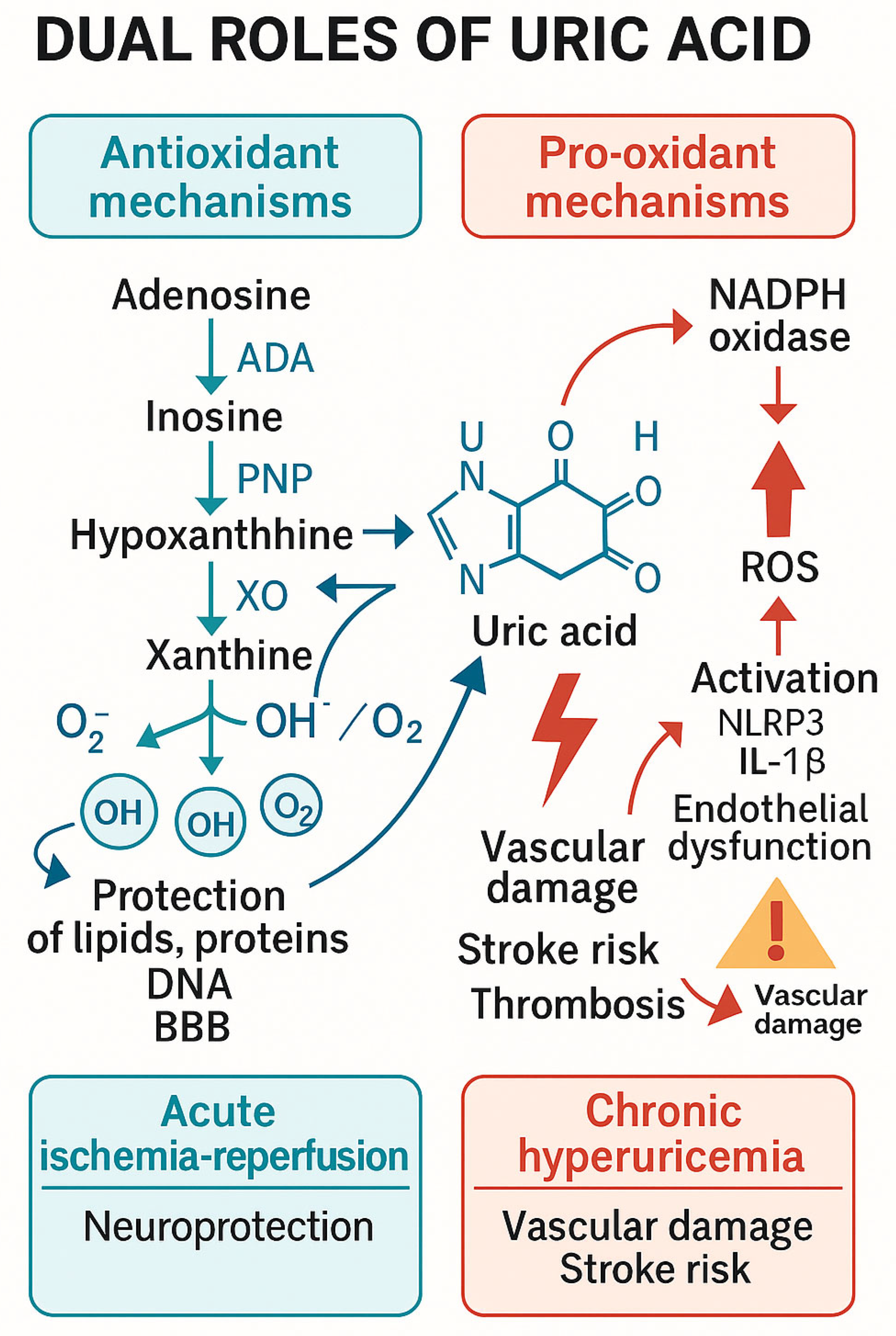
3.2. Uric Acid’s Dual Roles
3.2.1. Neuroprotection
3.2.2. Pathogenic Hyperuricemia
3.2.3. The Dual Role of Uric Acid
3.3. Exogenous Uric Acid in Stroke Therapy
3.4. XO Inhibition (e.g., Allopurinol)
3.5. Mechanistic Insights: Antioxidant and Cerebroprotective Signaling
4. Results
4.1. Results of Individual Studies
4.2. Classification of Individual Studies in Regard to Their Profiling
4.2.1. Epidemiological Studies
4.2.2. Cohort Studies in Acute Ischemic Stroke Patients
4.2.3. Clinical Trials/Acute Treatment Studies
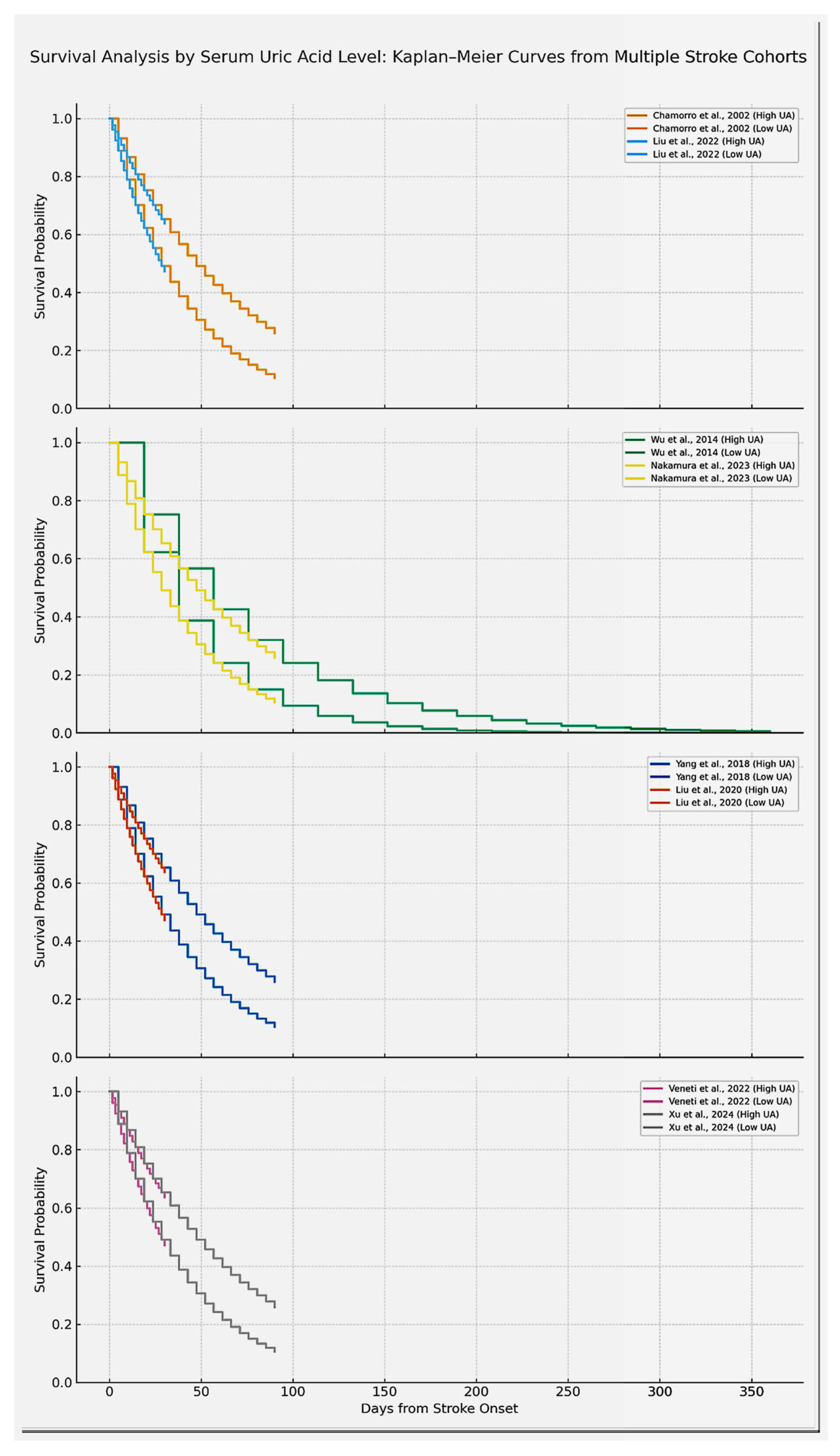
4.2.4. Comparative Findings Across Studies
4.2.5. Biological Basics
4.3. Diagnostic Value of Uric Acid
5. Discussions
5.1. Diagnostic and Prognostic Impact of Uric Acid
5.2. Discussion of Diagnostic and Prognostic Relevance
5.3. Cerebroprotective Effects of Uric Acid
5.4. Discussion of the Cerebroprotective Potential
5.5. Potential Comparison to Preclinical Data
5.6. Final Implications, Risk of Bias and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| UA | Uric Acid |
| IS | Ischemic Stroke |
| XO | Xanthine Oxidase |
| XOR | Xanthine Oxidoreductase |
| ROS | Reactive Oxygen Species |
| mRS | Modified Rankin Scale |
| SUA | Serum Uric Acid |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| tPA | Tissue Plasminogen Activator |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate (Reduced Form) |
| BBB | Blood-Brain-Barrier |
References
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Done, A.J.; Traustadóttir, T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Di Micoli, V.; Angeloni, C.; Giovannini, M.; Borghi, C. Purine Metabolism Dysfunctions: Experimental Methods of Detection and Diagnostic Potential. Int. J. Mol. Sci. 2023, 24, 7027. [Google Scholar] [CrossRef]
- Qian, Y.; Li, N.; Li, Y.; Tao, C.; Liu, Z.; Zhang, G.; Yang, F.; Zhang, H.; Gao, Y. Association between uric acid and the risk of hemorrhagic transformation in patients with acute ischemic stroke: A systematic review and meta-analysis. Front. Neurol. 2024, 15, 1378912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Zhang, X.; Huang, Z.-C.; Lu, T.-S.; You, S.-J.; Cao, Y.-J.; Liu, C.-F. Prognostic Significance of Uric Acid Levels in Ischemic Stroke Patients. Neurotox. Res. 2016, 29, 10–20. [Google Scholar] [CrossRef]
- Chamorro, A.; Obach, V.; Cervera, A.; Revilla, M.; Deulofeu, R.; Aponte, J.H. Prognostic Significance of Uric Acid Serum Concentration in Patients With Acute Ischemic Stroke. Stroke 2002, 33, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Pyun, S.Y.; Kim, Y.E.; Oh, M.S.; Yu, K.H.; Kim, B.; Rhee, E.; Lee, B.C. Association between baseline serum uric acid levels and functional outcomes at 3 months after acute ischemic stroke. J. Korean Neurol. Assoc. 2014, 32, 246–253. [Google Scholar]
- Liu, B.; Yang, J.; Xu, Y. Study on relationship between serum uric acid and in-hospital mortality in patients with acute cerebral infarction. J. Apoplexy Nerv. Dis. 2020, 5, 435–440. [Google Scholar]
- Bai, H.; Nie, X.; Leng, X.; Wang, D.; Pan, Y.; Yan, H.; Yang, Z.; Wen, M.; Pu, Y.; Zhang, Z.; et al. Uric acid levels and outcomes in large vessel occlusion stroke. J. Stroke Res. 2022, 34, 145–152. [Google Scholar]
- Wu, H.; Jia, Q.; Liu, G.; Liu, L.; Pu, Y.; Zhao, X.; Wang, C.; Wang, Y.; Wang, Y. Decreased Uric Acid Levels Correlate with Poor Outcomes in Acute Ischemic Stroke Patients, but Not in Cerebral Hemorrhage Patients. J. Stroke Cerebrovasc. Dis. 2014, 23, 469–475. [Google Scholar] [CrossRef]
- Nakamura, K.; Ueki, K.; Matsuo, R.; Kiyohara, T.; Irie, F.; Wakisaka, Y.; Ago, T.; Kamouchi, M.; Kitazono, T.; on Behalf of the Fukuoka Stroke Registry Investigators. Association between decreases in serum uric acid levels and unfavorable outcomes after ischemic stroke: A multicenter hospital-based observational study. PLoS ONE 2023, 18, e0287721. [Google Scholar] [CrossRef]
- Şengüldür, E.; Demir, M.C. Evaluation of the association of serum uric acid levels and stroke in emergency department patients. Düzce Tıp Fakültesi Dergisi. 2024, 26, 112–117. [Google Scholar] [CrossRef]
- Yacouba, N.M.; Ayeah, C.M.; Doualla, M.S.; Ba, H.; Ngahane, H.B.M.; Mbahe, S.; Luma, H.N. Serum Uric Acid Is Associated with Poor Outcome in Black Africans in the Acute Phase of Stroke. Stroke Res. Treat. 2017, 2017, 1935136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Li, Y.; Ding, L.; Sheng, L.; Xie, Z.; Wen, C. U-Shaped Relationship Between Functional Outcome and Serum Uric Acid in Ischemic Stroke. Cell. Physiol. Biochem. 2018, 47, 2369–2379. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Hsiao, C.-L.; Chen, P.-Y.; Tsou, A.; Tzeng, I.-S.; Lin, S.-K. J-Shaped Relationship of Serum Uric Acid with Unfavorable Short-Term Outcomes among Patients with Acute Ischemic Stroke. Biomedicines 2022, 10, 2185. [Google Scholar] [CrossRef] [PubMed]
- Tikhonoff, V.; Casiglia, E.; Spinella, P.; Barbagallo, C.M.; Bombelli, M.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; Desideri, G.; D’elia, L.; et al. Serum uric acid and cerebrovascular events: A prospective study. Eur. J. Prev. Cardiol. 2022, 29, 1001–1010. [Google Scholar]
- Wajid, M.; Rathore, R.; Butt, N.F.; Iqbal, A.; Alatif, H.; Abbas, M. Association between high serum uric acid level and good outcome in patients of ischemic stroke. Pak. J. Med. Health Sci. 2023, 17, 43–45. [Google Scholar] [CrossRef]
- Das, A.; Rahman, M.M.; Baker, I.M.; Talukder, S.; Najnin, F.; Akhter, R. Serum Uric Acid Level in Acute Stroke Patient: Study of 100 Cases. Sch. J. Appl. Med. Sci. 2022, 10, 222–227. [Google Scholar] [CrossRef]
- Tsankof, A.; Valanikas, E.; Papathanasiou, E.; Polychronopoulos, G.; Papadopoulos, A.; Tzavelas, M.; Satsoglou, S.; Veneti, S.; Ztriva, E.; Tziomalos, K. SERUM URIC ACID LEVELS APPEAR TO PREDICT IN-HOSPITAL MORTALITY IN PATIENTS ADMITTED WITH ACUTE ISCHEMIC STROKE. J. Hypertens. 2022, 40, e146–e147. [Google Scholar] [CrossRef]
- Zhong, J.; Cai, H.; Zhang, Z.; Wang, J.; Xiao, L.; Zhang, P.; Xu, Y.; Tu, W.; Zhu, W.; Liu, X.; et al. Serum uric acid and prognosis of ischemic stroke: Cohort study, meta-analysis and Mendelian randomization study. Eur. Stroke J. 2024, 9, 235–243. [Google Scholar] [CrossRef]
- Tsai, P.-H.; Kuo, C.-F.; See, L.-C.; Li, P.-R.; Chen, J.-S.; Tseng, W.-Y. Stroke Risk in Patients with Gout: A Nationwide Retrospective Cohort Study in Taiwan. J. Clin. Med. 2022, 11, 3779. [Google Scholar] [CrossRef]
- Chiquete, E.; Ruiz-Sandoval, J.L.; Murillo-Bonilla, L.M.; Arauz, A.; Orozco-Valera, D.R.; Ochoa-Guzmán, A.; Villarreal-Careaga, J.; León-Jiménez, G.; Barinagarrementeria, F.; Ramos-Moreno, A. Serum uric acid levels and outcomes after ischemic stroke. J. Neurol. Sci. 2013, 328, 12–17. [Google Scholar]
- Sun, Z.; Feng, J.; He, M.; Wang, M.; Zhang, Y.; Wang, N.; Liu, T.; Zhang, G. Higher uric acid is associated with better discharge recovery and short-term outcome in stroke patients treated with thrombolysis. Neurol. Sci. 2020, 42, 3225–3231. [Google Scholar] [CrossRef]
- Browne, L.D.; Jaouimaa, F.-Z.; Walsh, C.; Perez-Ruiz, F.; Richette, P.; Burke, K.; Stack, A.G. Serum uric acid and mortality thresholds among men and women in the Irish health system: A cohort study. Eur. J. Intern. Med. 2021, 84, 46–55. [Google Scholar] [CrossRef]
- Tong, X.; Lyu, C.; Guo, M.; Gu, J.; Zhao, Y. Serum uric acid as a predictor of mortality in patients with stroke: Results from National Health and Nutrition Examination Survey 2007–2016. Front. Neurol. 2024, 15, 1383300. [Google Scholar] [CrossRef]
- Chinnammanavar, P.K.B.; Somannavar, V.G.; Mohan, P.B. Role of serum calcium, serum albumin, and serum uric acid as markers of initial neurological severity and short-term outcome indicators in acute ischemic stroke. J. Assoc. Physicians India 2024, 72, 41–44. [Google Scholar] [CrossRef]
- Tahir, M.; Ul Haq, Z.; Mahboob, Q.; Khan, S.N.; Zarrar, M. Association between Hyperuricemia and Ischemic Stroke: A Case–Control Study. Ann. Pak. Inst. Med. Sci. (APIMS) 2024, 20 (Suppl. S1), 1229. [Google Scholar] [CrossRef]
- Konta, T.; Ichikawa, K.; Kawasaki, R.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Narita, I.; Kondo, M.; et al. Association between Serum Uric Acid Levels and Mortality: A Nationwide Community-Based Cohort Study. Sci. Rep. 2020, 10, 6066. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.H.; Kim, D.; Kesavan, R.; Brown, H.; Dey, T.; Soflaee, M.H.; Vu, H.S.; Tasdogan, A.; Guo, J.; Bezwada, D.; et al. De novo and Salvage Purine Synthesis Pathways Across Tissues and Tumors. Cell 2024, 187, 3602–3618.e20. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, C.; Ipata, P.L. Metabolic regulation of ATP breakdown and of adenosine production in rat brain extracts. Int. J. Biochem. Cell Biol. 2004, 36, 2214–2225. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Nesterowicz, M.; Zalewska, A.; Biedrzycki, G.; Gerreth, P.; Hojan, K.; Gerreth, K. Salivay Xanthine Oxidase as a Potential Biomarker in Stroke Diagnostics. Front. Immunol. 2022, 13, 897413. [Google Scholar] [CrossRef]
- Manful, C.F.; Fordjour, E.; Subramaniam, D.; Sey, A.A.; Abbey, L.; Thomas, R. Antioxidants and Reactive Oxygen Species: Shaping Human Health and Disease Outcomes. Int. J. Mol. Sci. 2025, 26, 7520. [Google Scholar] [CrossRef] [PubMed]
- Aliena-Valero, A.; Rius-Pérez, S.; Baixauli-Martín, J.; Torregrosa, G.; Chamorro, Á.; Pérez, S.; Salom, J.B. Uric Acid Neuroprotection Associated to IL-6/STAT3 Signaling Pathway Activation in Rat Ischemic Stroke. Mol. Neurobiol. 2021, 58, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Amaro, S.; Laredo, C.; Renú, A.; Llull, L.; Rudilosso, S.; Obach, V.; Urra, X.; Planas, A.M.; Chamorro, Á.; URICO-ICTUS Investigators. Uric acid therapy prevents early ischemic stroke progression: A tertiary analysis of the URICO-ICTUS trial (efficacy study of combined treatment with uric acid and r-tPA in acute ischemic stroke). Stroke 2016, 47, 2874–2876. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.A.; Shamim, S.A.; Rana, K.F.; Saeed, A.; Malik, B.H. Serum Uric Acid—Risk Factor for Acute Ischemic Stroke and Poor Outcomes. Cureus 2019, 11, e6007. [Google Scholar] [CrossRef]
- Yu, W.; Cheng, J.D. Uric Acid and Cardiovascular Disease: An Update From Molecular Mechanism to Clinical Perspective. Front. Pharmacol. 2020, 11, 582680. [Google Scholar] [CrossRef]
- Hooper, D.C.; Spitsin, S.; Kean, R.B.; Champion, J.M.; Dickson, G.M.; Chaudhry, I.; Koprowski, H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA 1998, 95, 675–680. [Google Scholar] [CrossRef]
- Llull, L.; Laredo, C.; Renú, A.; Pérez, B.; Vila, E.; Obach, V.; Urra, X.; Amaro, S.; Chamorro, Á. Uric acid therapy improves clinical outcomes in patients with acute ischemic stroke receiving reperfusion therapies. Stroke 2015, 46, 2162–2167. [Google Scholar] [CrossRef]
- Xu, L.; Li, C.; Wan, T.; Sun, X.; Lin, X.; Yan, D.; Li, J.; Wei, P. Targeting uric acid: A promising intervention against oxidative stress and neuroinflammation in neurodegenerative diseases. Cell Commun. Signal. 2025, 23, 4. [Google Scholar] [CrossRef]
- Ciarambino, T.; Crispino, P.; Giordano, M. Hyperuricemia and Endothelial Function: Is It a Simple Association or Do Gender Differences Play a Role in This Binomial? Biomedicines 2022, 10, 3067. [Google Scholar] [CrossRef]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef]
- Otani, N.; Hoshiyama, E.; Ouchi, M.; Takekawa, H.; Suzuki, K. Uric acid and neurological disease: A narrative review. Front. Neurol. 2023, 14, 1164756. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Roman-Filip, C.; Catană, M.G.; Bereanu, A.; Lăzăroae, A.; Gligor, F.; Sava, M. Therapeutic Plasma Exchange and Double Filtration Plasmapheresis in Severe Neuroimmune Disorders. Acta Clin. Croat. 2019, 58, 621–625. [Google Scholar] [CrossRef]
- Ndrepepa, G. Uric acid and cardiovascular disease—Recent evidence on the association and underlying mechanisms. J. Lab. Precis. Med. 2025, 10, 8. [Google Scholar] [CrossRef]
- Gherghina, M.-E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress—Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Muir, S.W.; Harrow, C.; Dawson, J.; Lees, K.R.; Weir, C.J.; Sattar, N.; Walters, M.R. Allopurinol Use Yields Potentially Beneficial Effects on Inflammatory Indices in Those with Recent Ischemic Stroke. Stroke 2008, 39, 3303–3307. [Google Scholar] [CrossRef]
- Roman-Filip, I.; Morosanu, V.; Bajko, Z.; Roman-Filip, C.; Balasa, I.R.-F. Non-Aneurysmal Perimesencephalic Subarachnoid Hemorrhage: A Literature Review. Diagnostics 2023, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Filip, I.R.; Morosanu, V.; Spinu, D.; Motoc, C.; Bajko, Z.; Sarmasan, E.; Roman, C.; Balasa, R. Cervical Artery Dissections—A Demographical Analysis of Risk Factors, Clinical Characteristics Treatment Procedures, and Outcomes—A Single Centre Study of 54 Consecutive Cases. J. Pers. Med. 2023, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, J.; Xu, J.; He, M. Serum uric acid is associated with shunt dependent hydrocephalus of aneurysmal subarachnoid hemorrhage patients. Neurosurg. Rev. 2025, 48, 424. [Google Scholar] [CrossRef]
- Leira, E.C.; Planas, A.M.; Chauhan, A.K.; Chamorro, A. Uric Acid: A Translational Journey in Cerebroprotection That Spanned Preclinical and Human Data. Neurology 2023, 101, 1068–1074. [Google Scholar] [CrossRef]
- Zhen, H.; Gui, F. The role of hyperuricemia on vascular endothelium dysfunction. Biomed. Rep. 2017, 7, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Silva, G.S.; Selim, M.; Kasner, S.E.; Aziz, Y.; Sutherland, J.; Jauch, E.C.; Adeoye, O.M.; Hill, M.D.; Mistry, E.A.; et al. Enhancing enrollment in acute stroke trials: Current state and consensus recommendations. Stroke 2023, 54, 2698–2708. [Google Scholar] [CrossRef]


| Study Type | Tool Applied | Overall Risk of Bias | Notes |
|---|---|---|---|
| Observational cohort studies (n = 29) | Newcastle–Ottawa Scale (NOS) | Low to Moderate | Most studies scored well for selection and outcome; some lacked adjustment for confounders. |
| Randomized controlled trials (n = 2) | Cochrane RoB 2.0 | Low | Randomization and blinding adequately reported (e.g., URICO-ICTUS trial). |
| Registry-based studies (n = 4) | Adapted NOS | Moderate | Large datasets but often limited detail on follow-up completeness or subtype classification. |
| Study | Year | Participants | Study Type | Prognostic Impact |
|---|---|---|---|---|
| Xia Zhang et al. [6] | 2015 | 303 | Observational | Negative |
| Chamorro et al. [7] | 2002 | 881 | Prospective Cohort | Positive |
| Pyun et al. [8] | 2014 | 204 | Cohort | Negative |
| Liu et al. [9] | 2020 | 275 | Observational | Negative |
| Haiwei Bai et al. [10] | 2022 | 780 | Cohort | Negative |
| Wu et al. [11] | 2014 | 1832 | Cohort | Negative |
| Nakamura et al. [12] | 2023 | 4621 | Registry-Based Cohort | Negative |
| Senguldur et al. [13] | 2024 | 1186 | Observational | Positive |
| Yacouba et al. [14] | 2017 | 480 | Prospective | Negative |
| Yimin Yang et al. [15] | 2018 | 710 | Cohort | Negative |
| Liu et al. [16] | 2022 | 3370 | Retrospective Cohort | Negative |
| Tikhonoff et al. [17] | 2022 | 14,588 | Prospective Cohort | Negative |
| Wajid et al. [18] | 2023 | 230 | Cohort | Positive |
| Das et al. [19] | 2022 | 100 | Cohort | Negative |
| Tsankof et al. [20] | 2022 | 1107 | Prospective Cohort | Negative |
| Xu et al. [21] | 2024 | 5631 | Observational | Negative |
| Tsai et al. [22] | 2022 | 3370 | Retrospective | Negative |
| Chiquete et al. [23] | 2013 | 463 | Prospective | Positive |
| Sun et al. [24] | 2021 | 321 | Observational | Positive |
| Liu Cy et al. [16] | 2022 | 3370 | Retrospective | Negative |
| Browne et al. [25] | 2021 | 2000 | Retrospective | Negative |
| Tong et al. [26] | 2024 | 395 | Retrospective | Negative |
| Chinnammanavar et al. [27] | 2023 | 110 | Cohort | Neutral |
| Tahir et al. [28] | 2024 | 120 | Case–Control | Positive |
| Konta et al. [29] | 2023 | 500,000 | Prospective | Negative |
| Part 1 | |||||
| Author | Country | Sample Size | Stroke | UA Level | Outcome & FU |
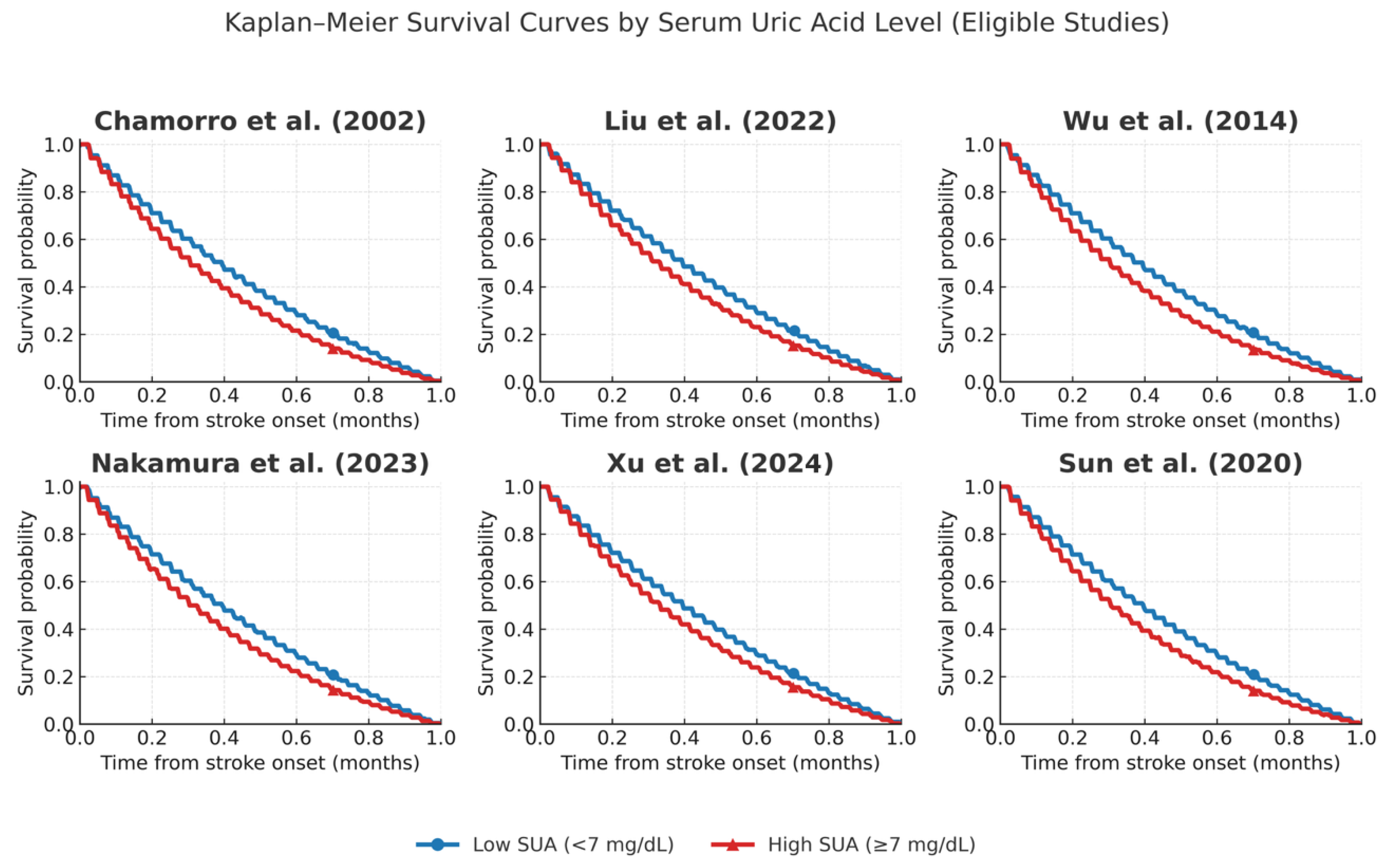
| Chamorro et al. (2002) [7] | Spain | 881 | Acute Ischemic | Mean: varies by group | Mathew score > 75 (functional outcome); At discharge |
| Liu et al. (2020) [9] | China | 275 | Acute Ischemic | Hyperuricemia vs. normal | In-hospital mortality; In-hospital |
| Haiwei Bai et al. (2022) [10] | China | 780 | Large Vessel Occlusion (LVO) | Quartiles | mRS 0–1 at 90 days; 90 days |
| Wu et al. (2014)[11] | China | 1452 ischemic, 380 hemorrhagic | Acute Ischemic | Median: 303 µmol/L | mRS > 2, vascular events, death; 1 year |
| Nakamura et al. (2023) [12] | Japan | 4621 | Acute Ischemic | Decrease rate from baseline | mRS ≥ 3 and 3–5 at 3 months; 3 months |
| Pyun et al. (2014) [8] | South Korea | Not clearly stated | Acute Ischemic | Within 48 h of onset | mRS at 3 months, ENI; 3 months |
| Senguldur et al. (2024) [13] | Turkey | 1186 | Ischemic and Hemorrhagic | Hyperuricemia ≥ 7 mg/dL, Hypouricemia ≤ 2.8 mg/dL | Stroke incidence; ED presentation |
| Xia Zhang et al. (2015) [6] | China | 303 | Acute Ischemic | Measured on 2nd day | mRS at 90 days; 90 days |
| Yacouba et al. (2017) [14] | Cameroon | 480 | Acute Ischemic | Mean: 71.1 mg/dL | 3-month mortality and functional outcome; 3 months |
| Part 2 | |||||
| Author | Country | Sample Size | Stroke | UA Level | Outcome & FU |
| Yimin Yang et al. (2018) [15] | China | 710 | Acute Ischemic | Measured at admission | mRS > 2 at 3 months; 3 months |
| Tikhonoff et al. (2022) [17] | Italy | 14,588 | Cerebrovascular Events (Fatal + Non-fatal) | Cut-off: ~5.6 mg/dL (women), ~6.0 mg/dL (men) | Combined cerebrovascular events; 10.1 ± 5.1 years |
| Chinnammanavar et al. (2023) [27] | India | 110 | Acute Ischemic Stroke | Mean 6.2 mg/dL; elevated in 27.3% | Association with gender, age; N/A |
| Liu et al. (2022)[16] | Taiwan | 3370 | Acute Ischemic Stroke | Quartiles < 4.1 mg/dL to >6.5 mg/dL | Unfavorable outcomes (mRS > 2), death; Short-term (discharge) |
| Wajid et al. (2023) [18] | Pakistan | 230 | Acute Ischemic Stroke | Group A: ≥7 mg/dL vs. Group B: <7 mg/dL | mRS at 5 days; 5 days |
| Tahir et al. (2024) [28] | Pakistan | 120 (60 cases, 60 controls) | Acute Ischemic Stroke | Hyperuricemia > 7 mg/dL | Prevalence of hyperuricemia; N/A |
| Das et al. (2022) [19] | Bangladesh | 100 | Acute Stroke (Ischemic + Hemorrhagic) | Mean 7.11 mg/dL | Stroke incidence & mortality; N/A |
| Tsankof et al. (2022) [20] | Greece | 1107 | Acute Ischemic Stroke | Measured day 2, fasting | mRS at discharge, in-hospital mortality; In-hospital |
| Xu et al. [21] | China | 5631 | Acute Ischemic | Baseline serum UA | 3-month mRS; 3 months |
| Part 3 | |||||
| Author | Country | Sample Size | Stroke | UA Level | Outcome & FU |
| Tsai et al. (2022)[22] | Taiwan | 621,640 | Ischemic & Hemorrhagic | Gout diagnosis (proxy) | Stroke incidence; Up to 18 years |
| Chiquete et al. (2013) [23] | Mexico | 463 | Acute Ischemic | Admission SUA (mean 6.1) | mRS at 30 days; 30 days |
| Sun et al. (2021) [24] | Taiwan | Not stated | Acute Ischemic (thrombolysis) | Admission SUA | Discharge outcome; Discharge |
| Bai et al. (2022)[10] | China | 780 | Large Vessel Occlusion | Quartiles | mRS 0–1; 90 days |
| Konta et al. [29] | Japan | ~500,000 | CVD events incl. stroke | ≥7 mg/dL men, ≥5 mg/dL women | Stroke mortality; 7 years |
| Author | Year | Country | Stroke Type | Outcome | Sample Size | OR | Lower CI | Upper CI |
|---|---|---|---|---|---|---|---|---|
| Chamorro et al. [7] | 2002 | Spain | Acute Ischemic | Favorable outcome | 881 | 1.2 | 1.01 | 1.45 |
| Liu et al. [9] | 2020 | China | Acute Ischemic | In-hospital mortality | 275 | 1.35 | 1.12 | 1.62 |
| Bai et al. [10] | 2022 | China | LVO | mRS 0–1 at 90 d | 780 | 0.88 | 0.7 | 1.1 |
| Wu et al. [11] | 2014 | China | Mixed | Events & mortality | 1832 | 1.4 | 1.2 | 1.65 |
| Nakamura et al. [12] | 2023 | Japan | Acute Ischemic | Poor outcome (mRS ≥ 3) | 4621 | 1.1 | 1.01 | 1.21 |
| Yang et al. [15] | 2018 | China | Acute Ischemic | mRS > 2 | 710 | 1.18 | 1.02 | 1.35 |
| Liu et al. [16] | 2022 | Taiwan | Acute Ischemic | mRS > 2 or death | 3370 | 1.22 | 1.05 | 1.42 |
| Tsankof et al. [20] | 2022 | Greece | Acute Ischemic | mRS and in-hospital mortality | 1107 | 1.3 | 1.1 | 1.52 |
| Xu et al. [21] | 2021 | China | Acute Ischemic | 3-month mRS | 5631 | 1.15 | 1.04 | 1.28 |
| Tikhonoff et al. [17] | 2022 | Italy | CVD | Cerebrovascular events | 14,588 | 1.05 | 1.01 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman-Filip, I.; Roman-Filip, C.; Morosanu, V.; Andone, S.; Bajko, Z.; Balasa, R. Uric Acid in Cerebral Ischemia: A Systematic Review of Its Biomarker Value and Role in Neuroprotection. Int. J. Mol. Sci. 2025, 26, 10268. https://doi.org/10.3390/ijms262110268
Roman-Filip I, Roman-Filip C, Morosanu V, Andone S, Bajko Z, Balasa R. Uric Acid in Cerebral Ischemia: A Systematic Review of Its Biomarker Value and Role in Neuroprotection. International Journal of Molecular Sciences. 2025; 26(21):10268. https://doi.org/10.3390/ijms262110268
Chicago/Turabian StyleRoman-Filip, Iulian, Corina Roman-Filip, Valentin Morosanu, Sebastian Andone, Zoltan Bajko, and Rodica Balasa. 2025. "Uric Acid in Cerebral Ischemia: A Systematic Review of Its Biomarker Value and Role in Neuroprotection" International Journal of Molecular Sciences 26, no. 21: 10268. https://doi.org/10.3390/ijms262110268
APA StyleRoman-Filip, I., Roman-Filip, C., Morosanu, V., Andone, S., Bajko, Z., & Balasa, R. (2025). Uric Acid in Cerebral Ischemia: A Systematic Review of Its Biomarker Value and Role in Neuroprotection. International Journal of Molecular Sciences, 26(21), 10268. https://doi.org/10.3390/ijms262110268






