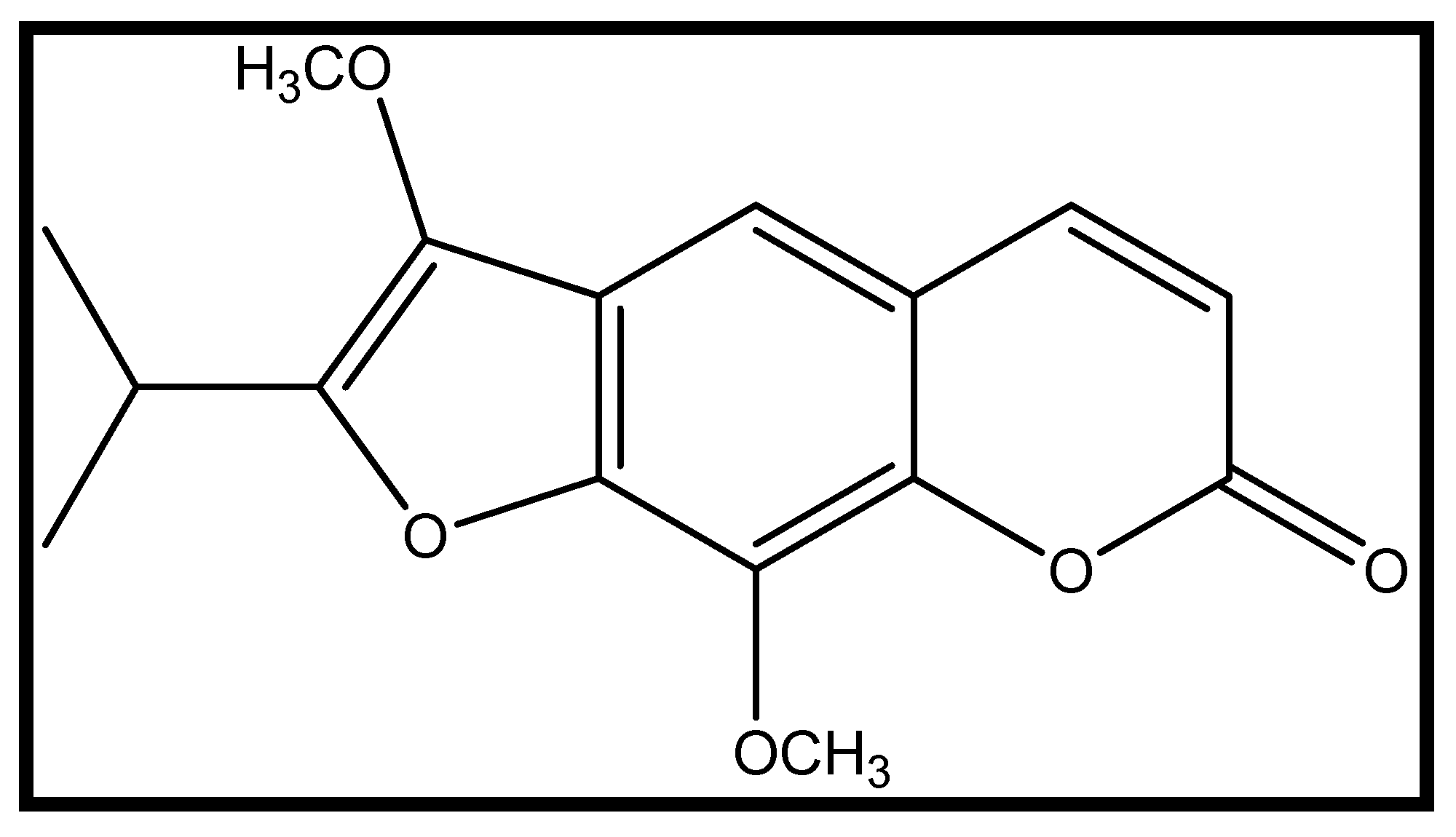
8-Methoxypeucedanin: Evaluation of Anxiolytic Effects and Modulation of Neuronal Activity Related Genes in a Zebrafish Anxiety Mode
Abstract
1. Introduction
2. Results
2.1. Evaluation of the Anxiolytic Activity
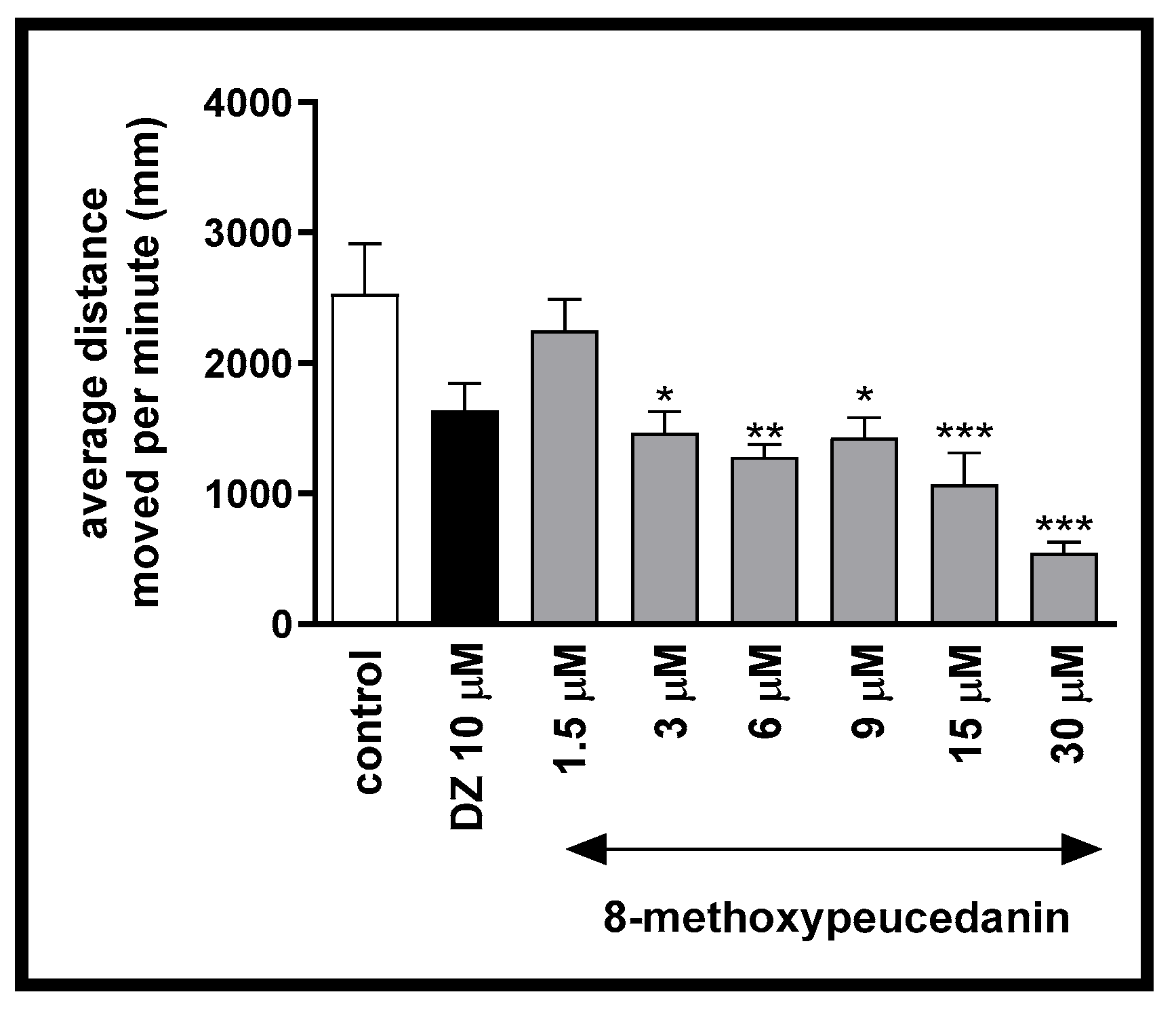
2.1.1. The Influence of 8-MP on the Spontaneous Locomotor Activity of Danio rerio Larvae
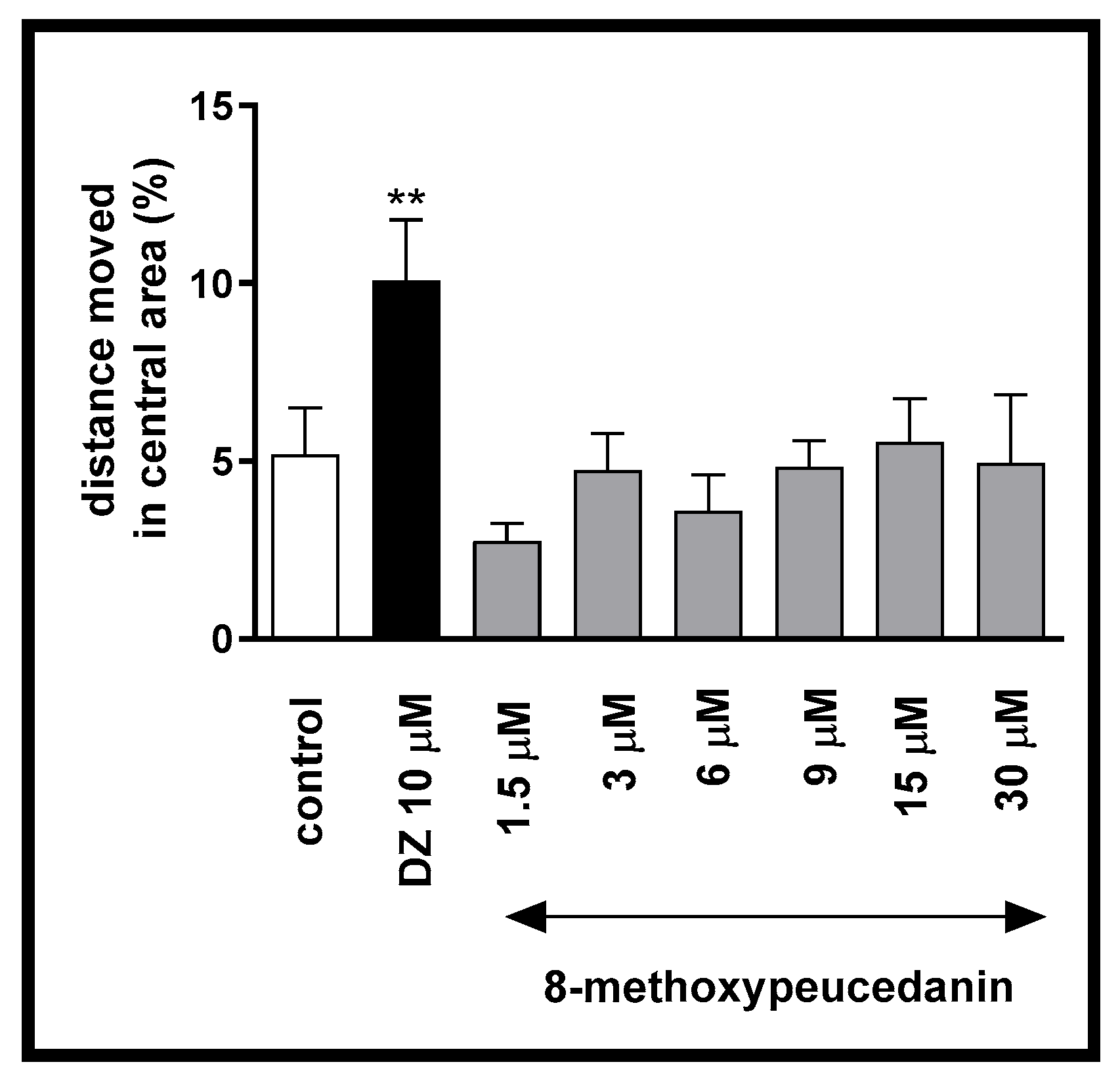
2.1.2. The Influence of 8-MP on Thigmotactic Behaviors of the Danio rerio Larvae
2.1.3. The Influence of 8-MP on the Locomotor Activity of Danio rerio Larvae During the Light–Dark Challenge
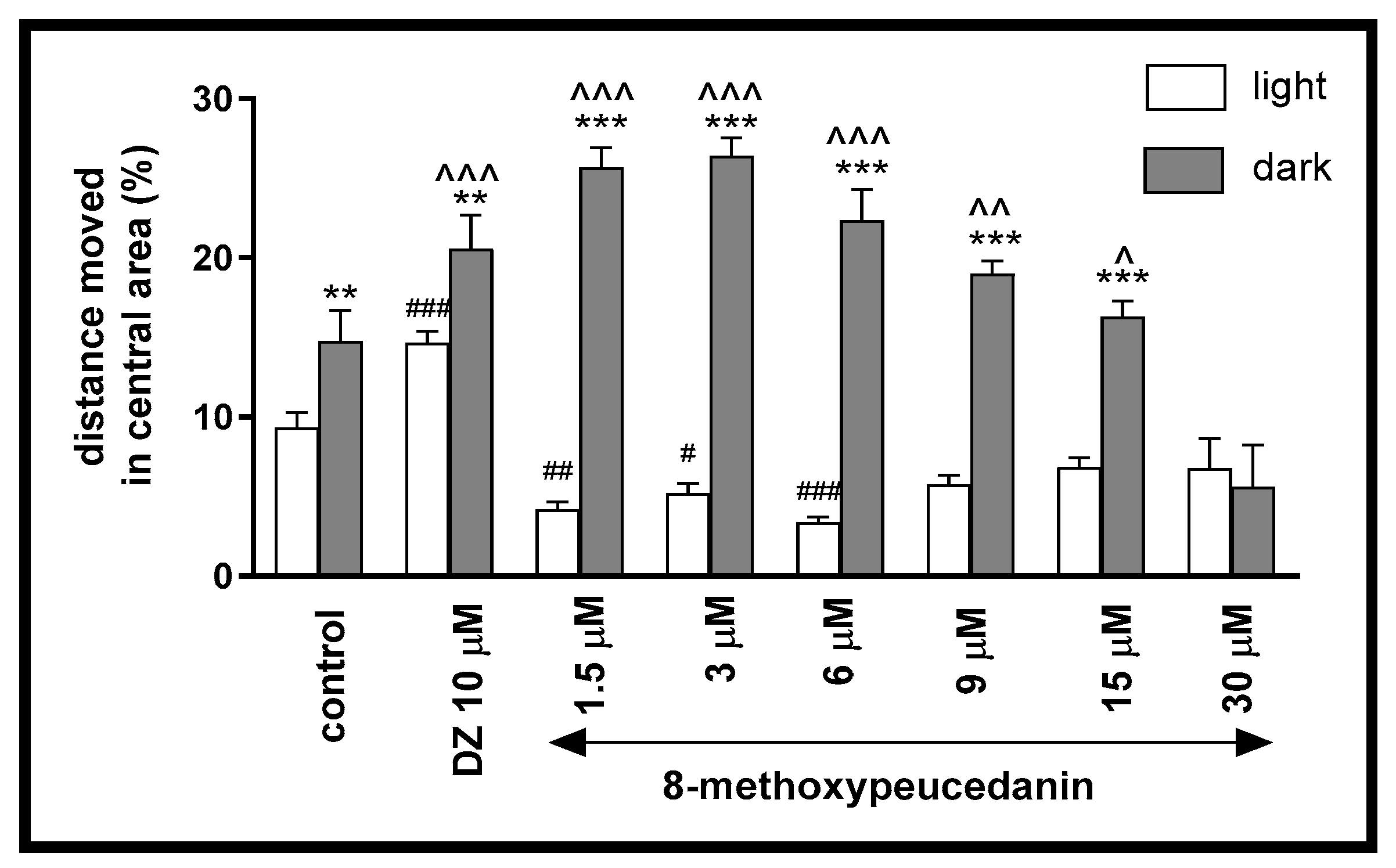
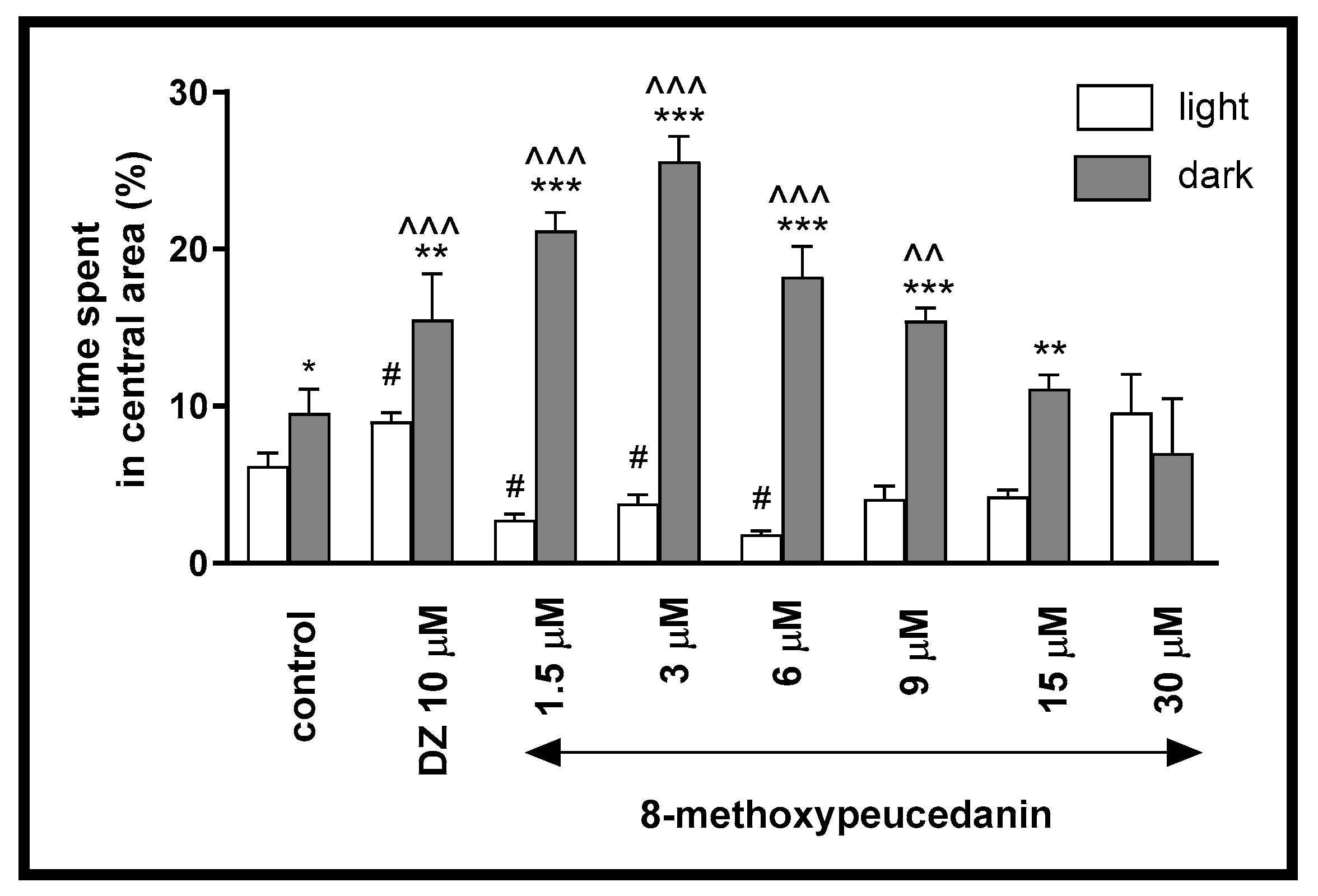
2.1.4. The Influence of 8-MP on the Thigmotactic Behavior of Danio rerio Larvae During the Light–Dark Challenge
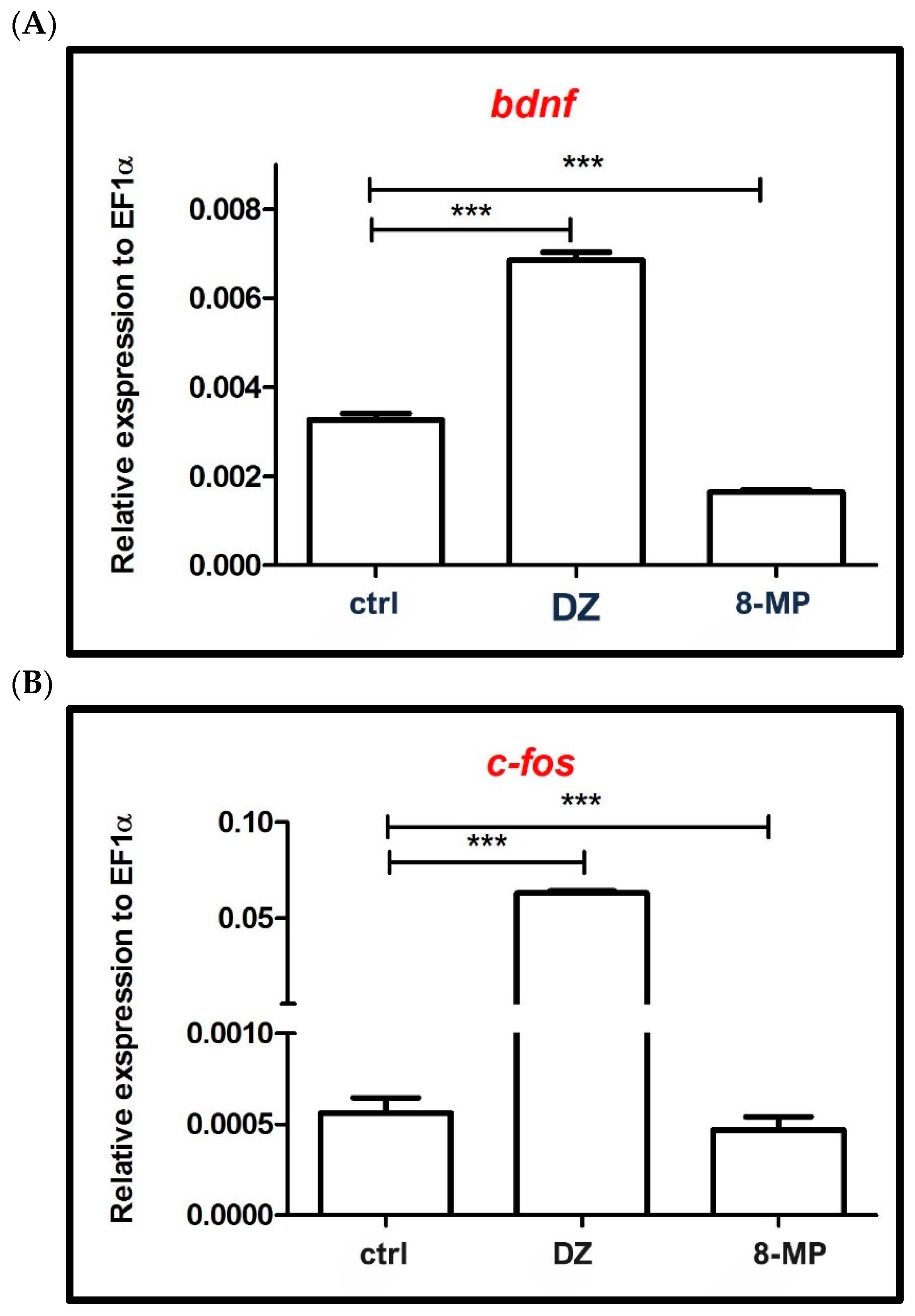
2.2. Influence of 8-MP on the Expression of Genes Associated with Neural Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.2.1. Zebrafish Experiment
4.2.2. Anxiolytic Activity Assessment
4.3. RNA Isolation and Quantitative PCR
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arya, A.; Sindhwani, G. A review on the neural circuits in anxiety disorders. Asian J. Pharm. Clin. Res. 2016, 9, 26–31. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef]
- Kessler, R.C.; McGonagle, K.A.; Zhao, S.; Nelson, C.B.; Hughes, M.; Eshleman, S.; Wittchen, H.U.; Kendler, K.S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch. Gen. Psychiatry 1994, 51, 8–19. [Google Scholar] [CrossRef]
- Koda-Kimble, M.A.; Alldredge, B.K.; Corelli, R.L.; Ernst, M.E.; Gugliemo, B.J.; Jacobson, P.A.; Kradjan, W.A.; Williams, B.R. Koda-Kimble and Young’s Applied Therapeutics: The Clinical Use of Drugs; Lippincott Williams&Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Shader, R.I.; Greenblatt, D.J. Use of benzodiazepines in anxiety disorders. N. Engl. J. Med. 1993, 328, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Starcevic, V. The reappraisal of benzodiazepines in the treatment of anxiety and related disorders. Expert Rev. Neurother. 2014, 14, 1275–1286. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhanzes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure–Activity Relationships. Mol. Nutr. Food Res. 2018, 62, e1701073. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzyńska, B. Implication of coumarins towards central nervous system disorders. Pharmacol. Res. 2016, 103, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Maciąg, M.; Michalak, A.; Skalicka-Woźniak, K.; Zykubek, M.; Ciszewski, A.; Budzyńska, B. Zebrafish and mouse models for anxiety evaluation—A comparative study with xanthotoxin as a model compound. Brain Res. Bull. 2020, 165, 139–145. [Google Scholar] [CrossRef]
- Singewald, N. Altered brain activity processing in high-anxiety rodents revealed by challenge paradigms and functional mapping. Neurosci. Biobehav. Rev. 2007, 31, 18–40. [Google Scholar] [CrossRef]
- Champagne, D.L.; Hoefnagels, C.C.M.; de Kloet, R.E.; Richardson, M.K. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav. Brain Res. 2010, 214, 332–342. [Google Scholar] [CrossRef]
- Guo, S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes Brain Behav. 2004, 3, 63–74. [Google Scholar] [CrossRef]
- Lin, C.; Chiang, C.; Tsai, H. Zebrafish and medaka: New model organisms for modern biomedical research. J. Biomed. Sci. 2016, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.D.; Soanes, K.H. A larval zebrafish model of bipolar disorder as a screening platform for neuro-therapeutics. Behav. Brain Res. 2012, 233, 450–457. [Google Scholar] [CrossRef]
- Schnorr, S.J.; Steenberger, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain. Res. 2012, 228, 367–374. [Google Scholar] [CrossRef]
- Widelski, J.; Luca, S.V.; Skiba, A.; Chinou, I.; Marcourt, L.; Wolfender, J.; Skalicka-Wozniak, K. Isolation and antimicrobial activity of coumarin derivatives from fruits of Peucedanum luxurians tamamsch. Molecules 2018, 23, 1222. [Google Scholar] [CrossRef]
- Widelski, J.; Kukula-Koch, W.; Baj, T.; Kedzierski, B.; Fokialakis, N.; Magiatis, P.; Pozarowski, P.; Rolinski, J.; Graikou, K.; Chinou, I.; et al. Rare coumarins induce apoptosis, G1 cell block and reduce RNA content in HL60 cells. Open Chem. 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Markowski, W.; Świeboda, R.; Głowniak, K. Computer-assisted searching for coumarins in Peucedanum alsaticum L. and Peucedanum cervaria (L.) lap. Acta Chromatogr. 2009, 21, 531–546. [Google Scholar] [CrossRef]
- Urbain, A.; Marston, A.; Hostettmann, K. Coumarins from Peucedanum ostruthium as inhibitors of acetylcholinesterase. Pharm. Biol. 2005, 43, 647–650. [Google Scholar] [CrossRef]
- Kozioł, E.; Deniz, F.S.S.; Orhan, I.E.; Marcourt, L.; Budzyńska, B.; Wolfender, J.; Crawford, A.D.; Skalicka-Woźniak, K. High-performance counter-current chromatography isolation and initial neuroactivity characterization of furanocoumarin derivatives from Peucedanum alsaticum L (apiaceae). Phytomedicine 2019, 54, 259–264. [Google Scholar] [CrossRef]
- Budzyńska, B.; Kruk-Slomka, M.; Skalicka-Wozniak, K.; Biala, G.; Głowniak, K. The effects of imperatorin on anxiety and memory-related behavior in male swiss mice. Exp. Clin. Psychopharmacol. 2012, 20, 325–332. [Google Scholar] [CrossRef]
- Sternbach, L.H. The benzodiazepine story. Prog. Drug Res. 1978, 22, 229–266. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, S.S. Structure-dependent activity of natural GABA(A) receptor modulators. Molecules 2018, 23, 1512. [Google Scholar] [CrossRef]
- Łuszczki, J.J.; Andres-Mach, M.; Gleńsk, M.; Skalicka-Woźniak, K. Anticonvulsant effects of four linear furanocoumarins, bergapten, imperatorin, oxypeucedanin, and xanthotoxin, in the mouse maximal electroshock-induced seizure model: A comparative study. Pharmacol. Rep. 2010, 62, 1231–1236. [Google Scholar] [CrossRef]
- Pearl, P.L.; Gibson, K.M. Clinical aspects off the disorders of GABA metabolism in children. Curr. Opin. Neurol. 2004, 17, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Awad, R.; Ahmed, F.; Bourbonnais-Spear, N.; Mullally, M.; Ta, C.A.; Tang, A.; Merali, Z.; Maquin, P.; Caal, F.; Cal, V.; et al. Ethnopharmacology of Q’eqchi’ maya antiepileptic and anxiolytic plants: Effects on the GABAergic system. J. Ethnopharmacol. 2009, 125, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Bergendorff, O.; Dekermendjian, K.; Nielsen, M.; Shan, R.; Witt, R.; Ai, J.; Sterner, O. Furanocoumarins with affinity to brain benzodiazepine receptors in vitro. Phytochemistry 1997, 44, 1121–1124. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ahn, E.; Song, M.; Kim, D.W.; Kang, J.H.; Kwon, O.; Kang, T.-C.; Baek, N. In vitro GABA-transaminase inhibitory compounds from the root of Angelica dahurica. Phytother. Res. 2005, 19, 839–845. [Google Scholar] [CrossRef]
- Matosiuk, D.; Gralak, D.; Ryznar, M. Lipophilicity of natural coumarins. Ann. Univ. Mariae Curie Sklodowska Pharm. 2011, 24, 19–25. [Google Scholar]
- Huong, D.T.L.; Choi, H.C.; Rho, T.C.; Lee, H.S.; Lee, M.K.; Kim, Y.H. Inhibitory activity of monoamine oxidase by coumarins from Peucedanum japonicum. Arch. Pharm. Res. 1999, 22, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; Briley, M. The noradrenergic symptom cluster: Clinical expression and neuropharmacology. Neuropsychiatr. Dis. Treat. 2011, 7, 15–20. [Google Scholar] [CrossRef]
- Roy-Byrne, P. Treatment in nonresponsive patients with social anxiety: Back to the future with benzodiazepines. Am. J. Psychiatry 2014, 171, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Yoshida, M.; Emoto, H.; Ishii, H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: Basic studies. Eur. J. Pharmacol. 2000, 405, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Itoi, K.; Sugimoto, N. The brainstem noradrenergic systems in stress, anxiety and depression. J. Neuroendocrinol. 2010, 22, 355–361. [Google Scholar] [CrossRef]
- Montoya, A.; Bruins, R.; Katzman, M.A.; Blier, P. The noradrenergic paradox: Implications in the management of depression and anxiety. Neuropsychiatr. Dis. Treat. 2016, 12, 541–557. [Google Scholar] [CrossRef]
- Zarrindast, M.; Khakpai, F. The modulatory role of dopamine in anxiety-like behavior. Arch. Iran. Med. 2015, 18, 591–603. [Google Scholar]
- Lin, S.; Lee, L.; Yang, Y.K. Serotonin and mental disorders: A concise review on molecular neuroimaging evidence. Clin. Psychopharmacol. Neurosci. 2014, 12, 196–202. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Zhang, Y.; Yang, X. Simultaneous assessment of absorption characteristics of coumarins from Angelicae Pubescentis Radix: In vitro transport across caco-2 cell and in vivo pharmacokinetics in rats after oral administration. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1060, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.; Vijayan, M.M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R711–R719. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.Y.B.; Mathur, P.; Gould, G.G.; Guo, S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, S.; Liu, S.; Zhang, C.; Peng, G. Effects of lorazepam and WAY-200070 in larval zebrafish light/dark choice test. Neuropharmacology 2015, 95, 226–233. [Google Scholar] [CrossRef]
- Almeida, E.R.; Lima-Rezende, C.A.; Schneider, S.E.; Garbinato, C.; Pedroso, J.; Decui, L.; Aguiar, G.P.S.; Müller, L.G.; Oliveira, J.V.; Siebel, A.M. Micronized resveratrol shows anticonvulsant properties in pentylenetetrazole-induced seizure model in adult zebrafish. Neurochem. Res. 2021, 46, 241–251. [Google Scholar] [CrossRef]
- Liu, H.; Xue, X.; Shi, H.; Qi, L.; Gong, D. Osthole upregulates BDNF to enhance adult hippocampal neurogenesis in APP/PS1 transgenic mice. Biol. Pharm. Bull. 2015, 38, 1439–1449. [Google Scholar] [CrossRef]
- Miyazaki, S.; Oikawa, H.; Takekoshi, H.; Hoshizaki, M.; Ogata, M.; Fujikawa, T. Anxiolytic effects of Acanthopanax senticosus HARMS occur via regulation of autonomic function and activate hippocampal BDNF-TrkB signaling. Molecules 2019, 24, 132. [Google Scholar] [CrossRef]
- Maffioli, E.; Angiulli, E.; Nonnis, S.; Grassi Scalvini, F.; Negri, A.; Tedeschi, G.; Arisi, I.; Frabetti, F.; D’aniello, S.; Alleva, E.; et al. Brain proteome and behavioural analysis in wild type, BDNF+/− and BDNF−/− adult zebrafish (danio rerio) exposed to two different temperatures. Int. J. Mol. Sci. 2022, 23, 5606. [Google Scholar] [CrossRef]
- Widelski, J.; Luca, S.V.; Skiba, A.; Maciąg, M.; Budzyńska, B.; Marcourt, L.; Wolfender, J.; Skalicka-Wozniak, K. Coumarins from Seseli devenyense Simonkai.: Isoaltion by liquid-liquid chromatography and potential anxiolytic activity using an in vivo zebrafish larvae model. Int. J. Mol. Sci. 2021, 22, 1829. [Google Scholar] [CrossRef]
- Kasica-Jarosz, N.; Podlasz, P.; Kaleczyc, J. Pituitary adenylate cyclase–activating polypeptide (PACAP-38) plays an inhibitory role against inflammation induced by chemical damage to zebrafish hair cells. PLoS ONE 2018, 13, e0198180. [Google Scholar] [CrossRef]


| Gene | Forward 5′-3′ | Reverse 5′-3′ | Accession No/Source |
|---|---|---|---|
| c-fos | GTGCAGCACGGCTTCACCGA | TTGAGCTGCGCCGTTGGAGG | NM_205569.1 |
| bdnf | GGCGAAGAGCGGACGAATATC | AAGGAGACCATTCAGCAGGACAG | NM_131595.2 |
| Ef1α | CTGGAGGCCAGCTCAAACAT | ATCAAGAAGAGTAGTACCGCTAGCATTAC | NM_131263.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widelski, J.; Maciąg, M.; Kasica, N.; Budzyńska, B.; Podlasz, P.; Luca, S.V.; Fondai, D.; Skalicka-Woźniak, K. 8-Methoxypeucedanin: Evaluation of Anxiolytic Effects and Modulation of Neuronal Activity Related Genes in a Zebrafish Anxiety Mode. Int. J. Mol. Sci. 2025, 26, 10259. https://doi.org/10.3390/ijms262110259
Widelski J, Maciąg M, Kasica N, Budzyńska B, Podlasz P, Luca SV, Fondai D, Skalicka-Woźniak K. 8-Methoxypeucedanin: Evaluation of Anxiolytic Effects and Modulation of Neuronal Activity Related Genes in a Zebrafish Anxiety Mode. International Journal of Molecular Sciences. 2025; 26(21):10259. https://doi.org/10.3390/ijms262110259
Chicago/Turabian StyleWidelski, Jarosław, Monika Maciąg, Natalia Kasica, Barbara Budzyńska, Piotr Podlasz, Simon Vlad Luca, Dafina Fondai, and Krystyna Skalicka-Woźniak. 2025. "8-Methoxypeucedanin: Evaluation of Anxiolytic Effects and Modulation of Neuronal Activity Related Genes in a Zebrafish Anxiety Mode" International Journal of Molecular Sciences 26, no. 21: 10259. https://doi.org/10.3390/ijms262110259
APA StyleWidelski, J., Maciąg, M., Kasica, N., Budzyńska, B., Podlasz, P., Luca, S. V., Fondai, D., & Skalicka-Woźniak, K. (2025). 8-Methoxypeucedanin: Evaluation of Anxiolytic Effects and Modulation of Neuronal Activity Related Genes in a Zebrafish Anxiety Mode. International Journal of Molecular Sciences, 26(21), 10259. https://doi.org/10.3390/ijms262110259







