Persistent Transcriptome Alterations in Zebrafish Embryos After Discontinued Opioid Exposure
Abstract
1. Introduction
2. Results
2.1. Transcriptomic Analysis
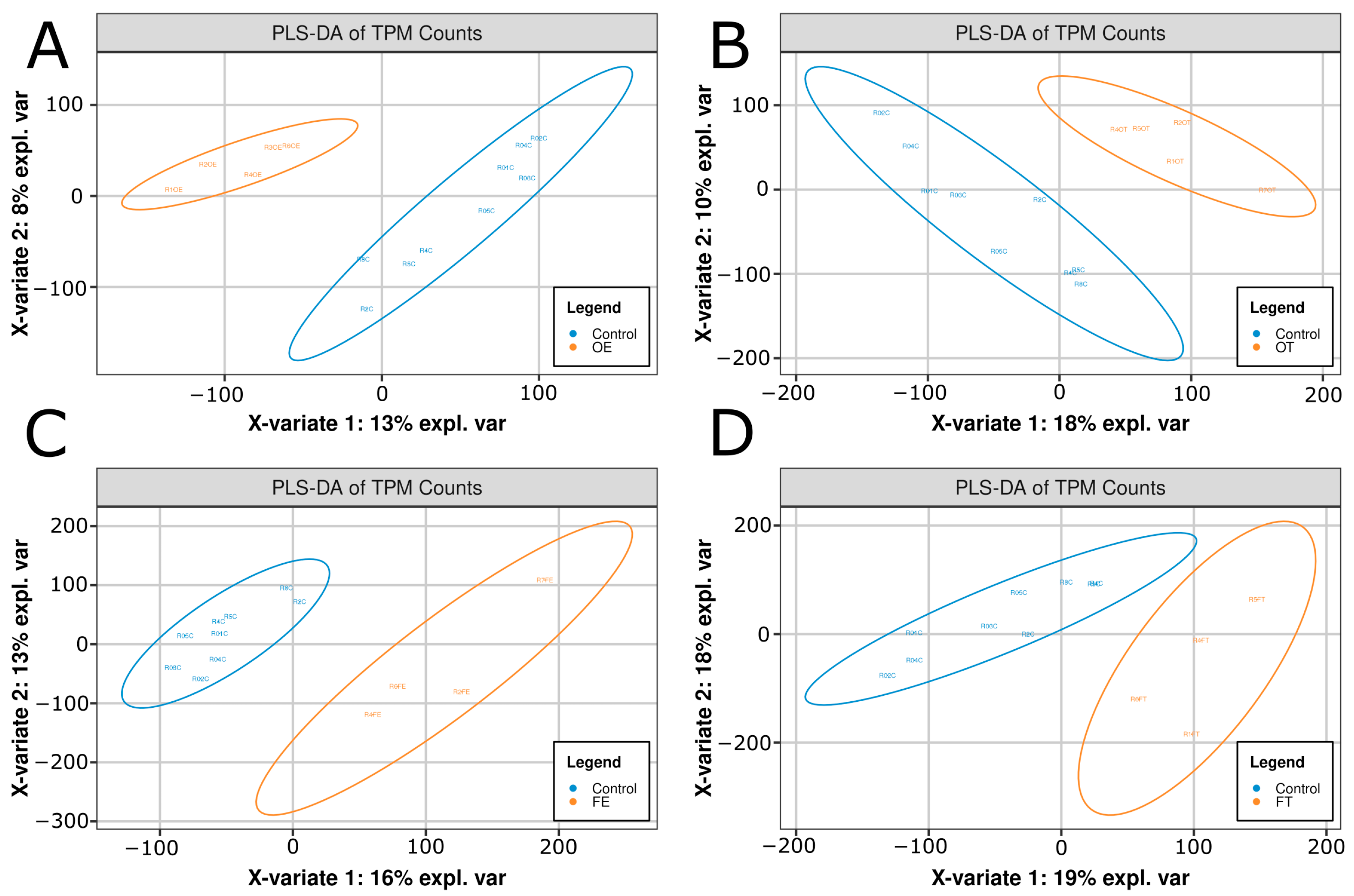
2.2. Transcriptomic Shifts Are Induced by Opioid Exposure
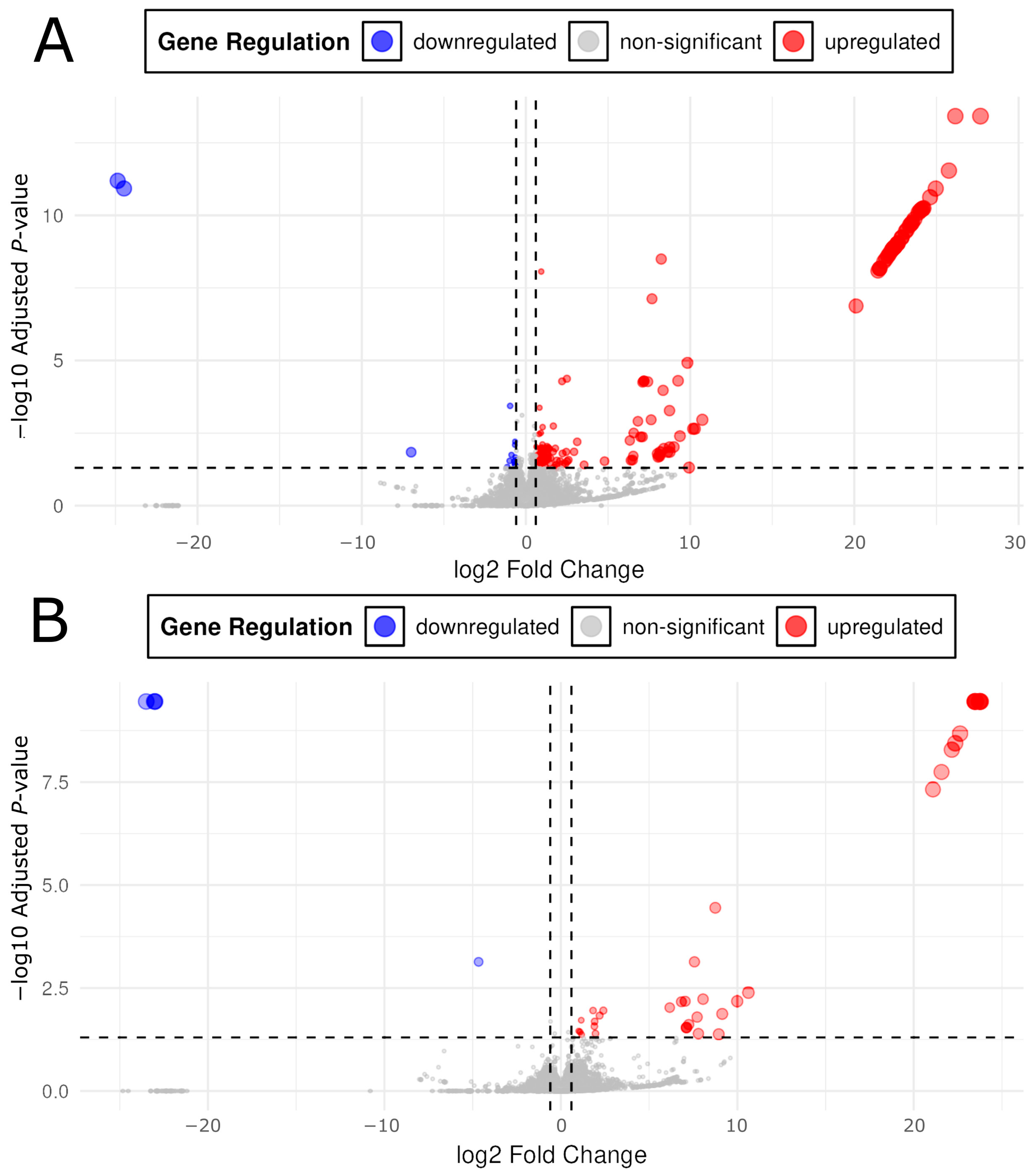
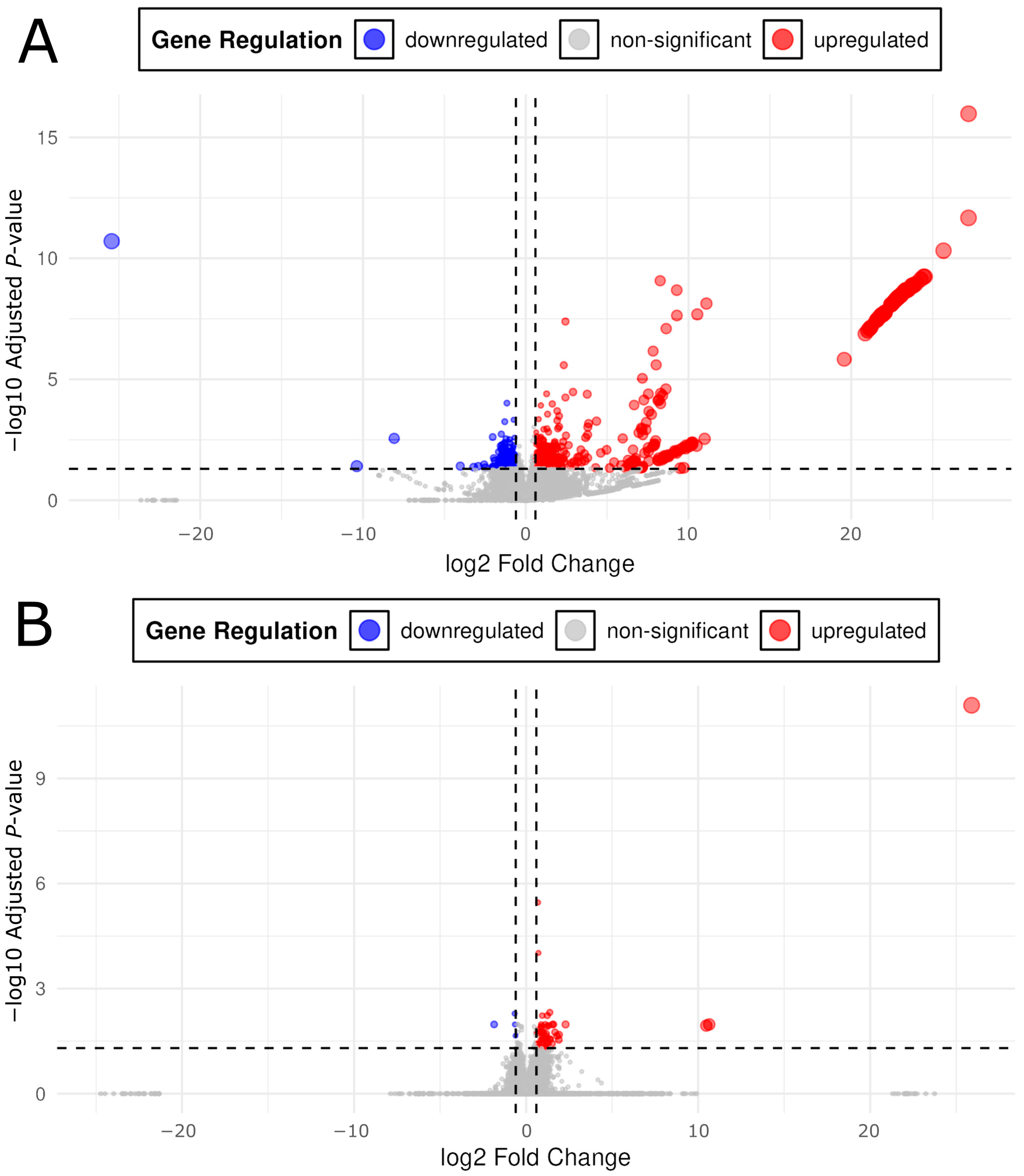
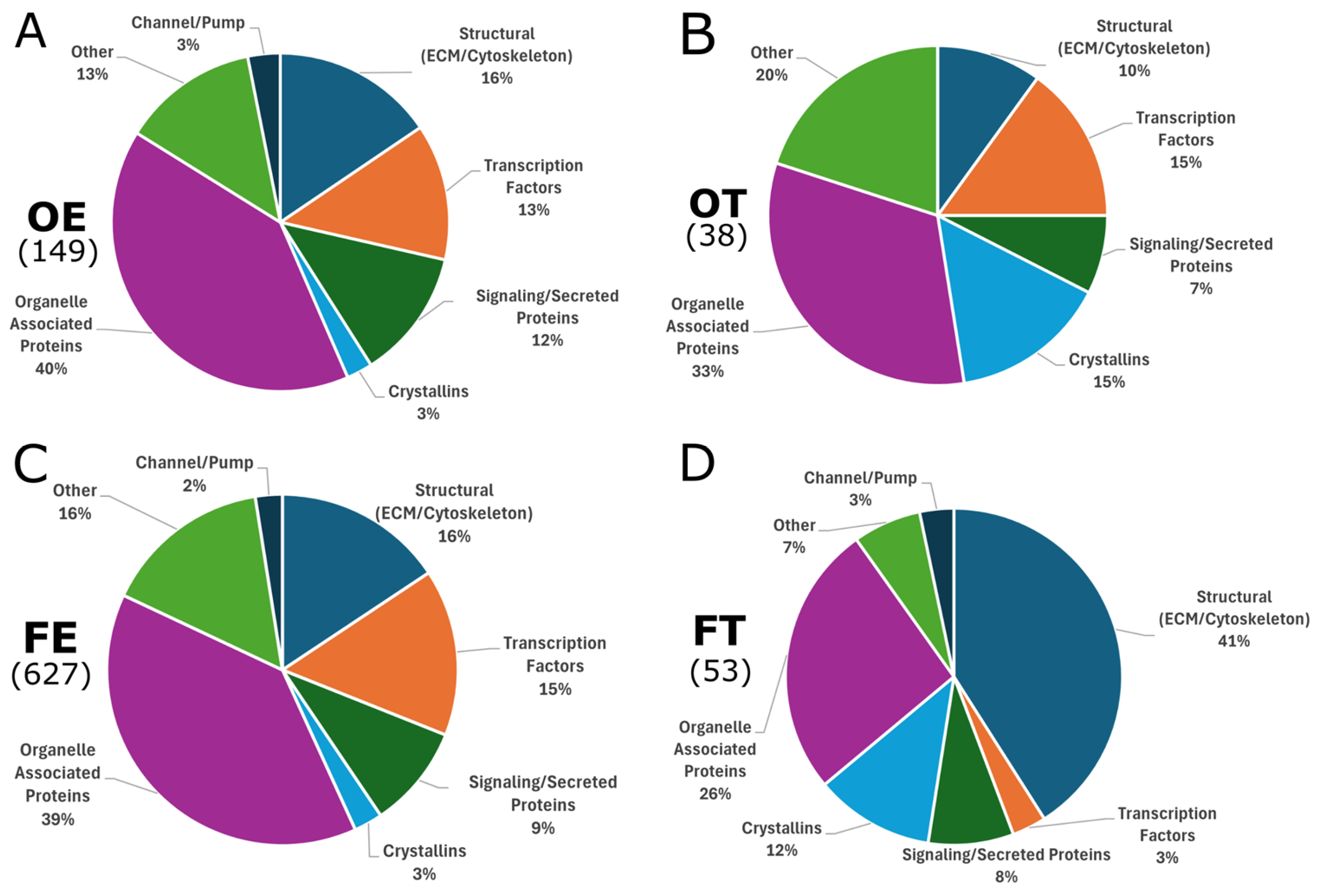
2.3. Differentially Expressed Genes
2.4. Expression of Endogenous Opioid System Genes
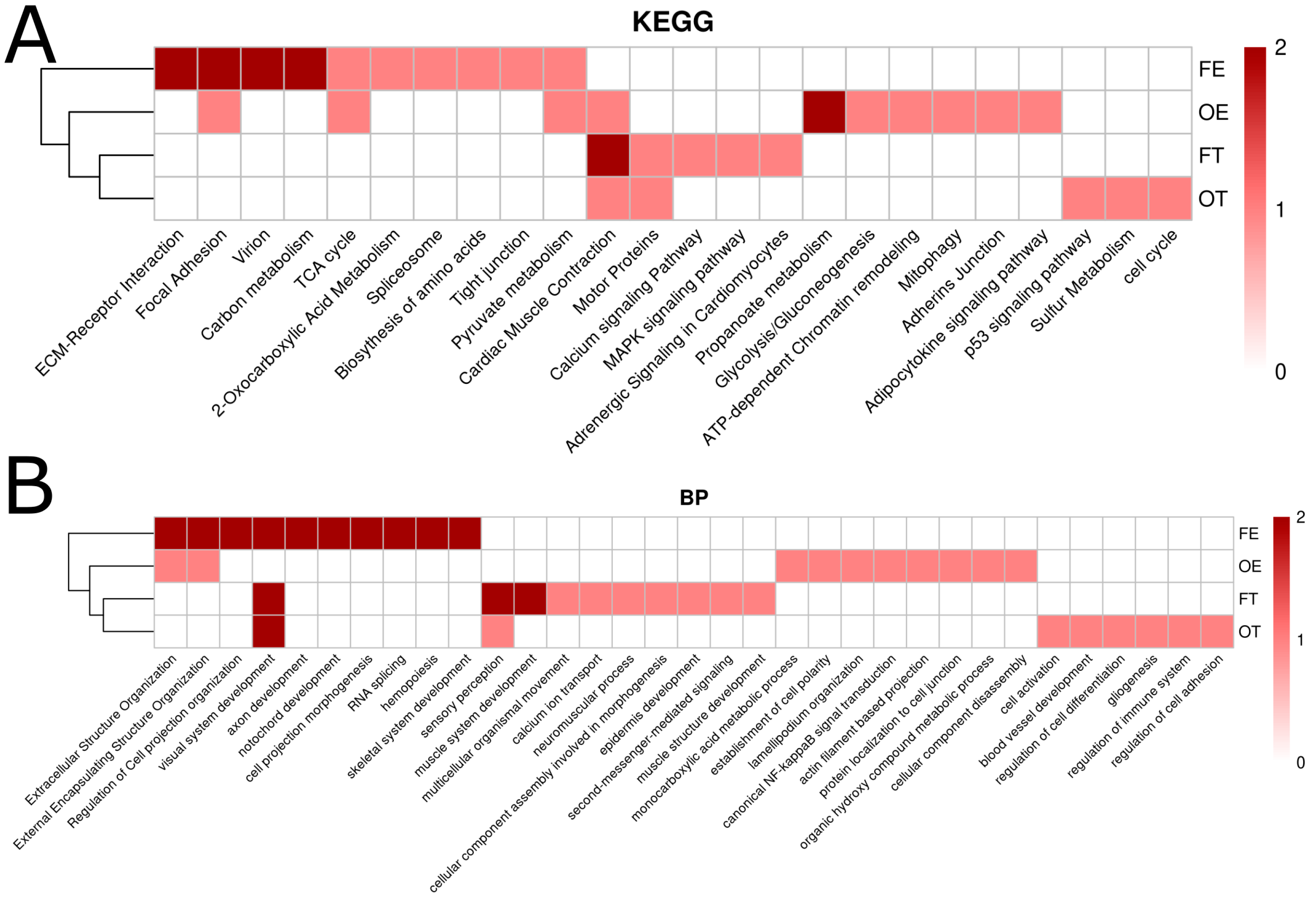
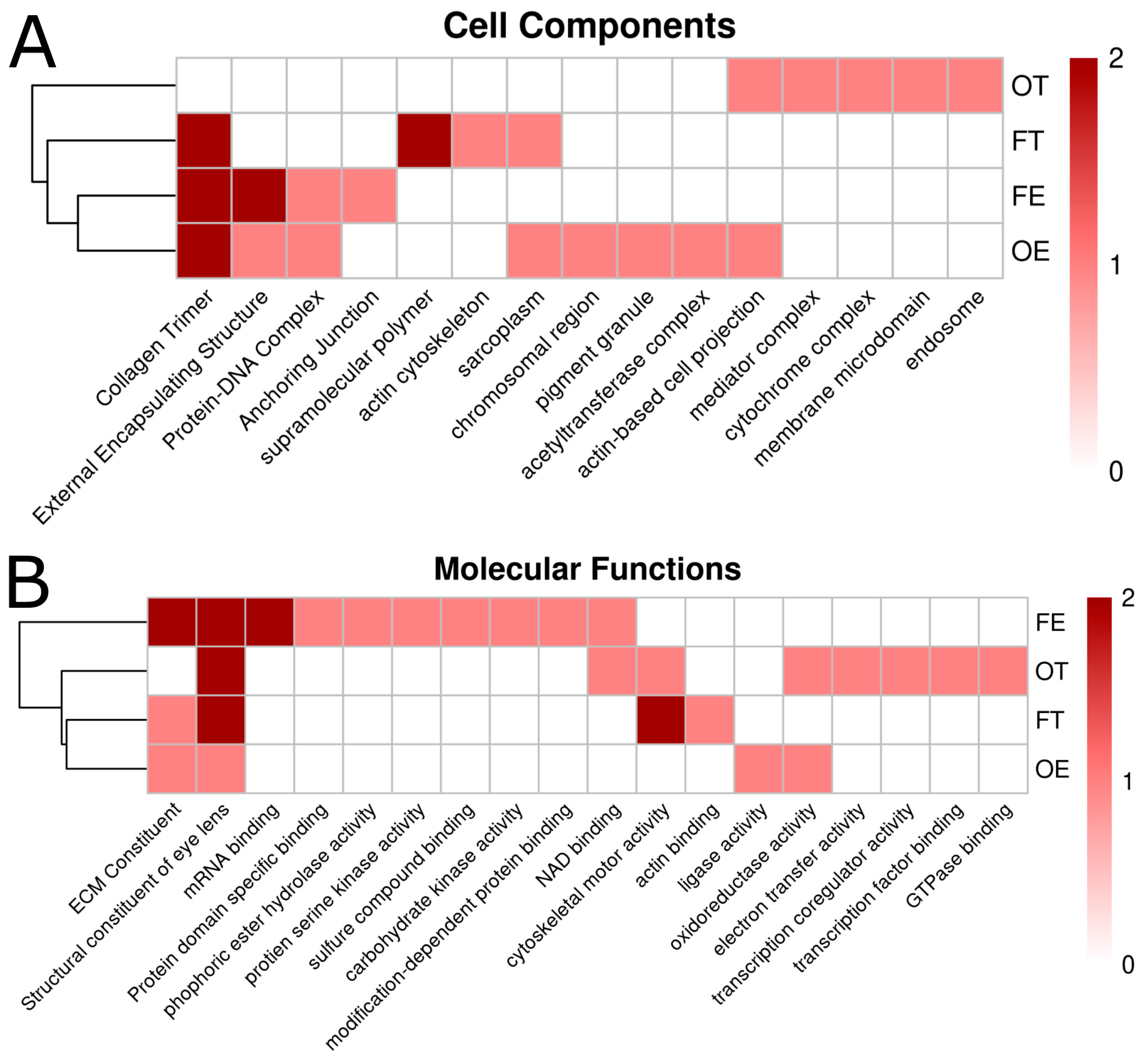
2.5. Pathway Analysis Using Kyoto Encyclopedia of Genes and Genomes/Gene Ontology (KEGG/GO) Terms
2.6. Clustering Opioid Exposures by Kyoto Encyclopedia of Genes and Genomes/Gene Ontology (KEGG/GO) Terms
3. Discussion
3.1. Lower Opioid Doses Resulted in Greater Variety of Differential Expression
3.2. Extracellular Matrix
3.3. Metalloproteinases
3.4. Cell Adhesion
3.4.1. Integrin-Mediated Adhesion and Cytoskeletal Regulation
3.4.2. Cadherin-Mediated Cell–Cell Adhesion
3.5. Opioids and Visual Development
3.6. Axon Guidance and Synapse Formation
3.7. Extracellular Matrix (ECM), Adhesion, and Visual Development
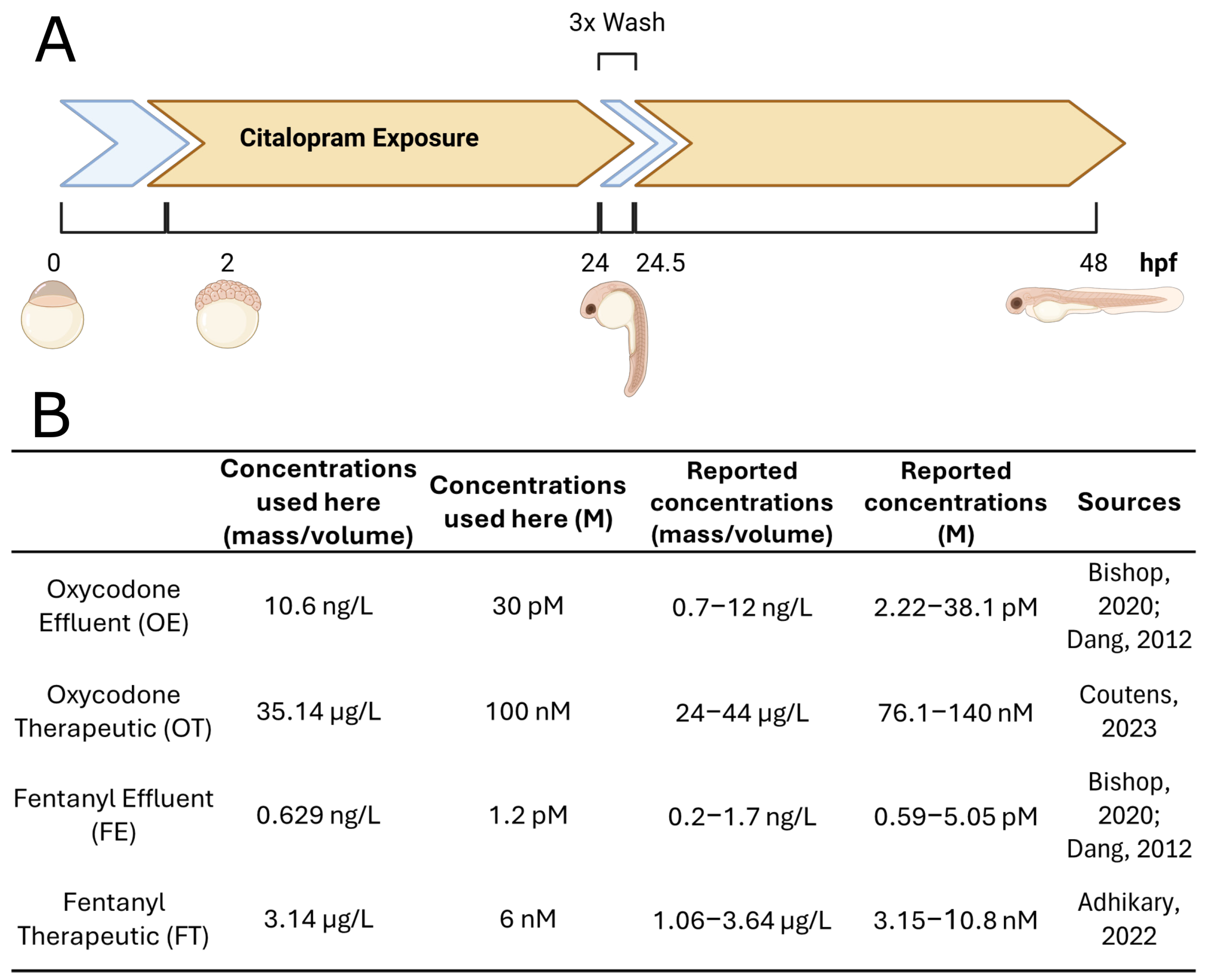
4. Materials and Methods
4.1. Zebrafish Maintenance and Husbandry
4.2. Zebrafish Embryo Treatment and Ribonucleic Acid Extraction
4.3. Software and Computational Pipeline
4.4. Kyoto Encyclopedia of Genes and Genomes/Gene Ontology (KEGG/GO) Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC About Prescription Opioids. Available online: https://www.cdc.gov/overdose-prevention/about/prescription-opioids.html (accessed on 31 January 2025).
- Desai, R.J.; Hernandez-Diaz, S.; Bateman, B.T.; Huybrechts, K.F. Increase in Prescription Opioid Use During Pregnancy Among Medicaid-Enrolled Women. Obstet. Gynecol. 2014, 123, 997. [Google Scholar] [CrossRef]
- Branum, A.M.; Ahrens, K.A. Trends in Timing of Pregnancy Awareness Among US Women. Matern. Child Health J. 2017, 21, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Yazdy, M.M.; Mitchell, A.A.; Tinker, S.C.; Parker, S.E.; Werler, M.M. Periconceptional Use of Opioids and the Risk of Neural Tube Defects. Obstet. Gynecol. 2013, 122, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, N.M.; Dice, T.J.; Mead, T. Oxycodone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Coyle, M.G.; Brogly, S.B.; Ahmed, M.S.; Patrick, S.W.; Jones, H.E. Neonatal Abstinence Syndrome. Nat. Rev. Dis. Primers 2018, 4, 47. [Google Scholar] [CrossRef]
- Skaggs, C.S.; Logue, B.A. The Prevalence of Opioids in US Drinking Water Sources Detected Using Direct-Injection High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Environ. Toxicol. Chem. 2022, 41, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Campos-Mañas, M.C.; Ferrer, I.; Thurman, E.M.; Agüera, A. Opioid Occurrence in Environmental Water Samples—A Review. Trends Environ. Anal. Chem. 2018, 20, e00059. [Google Scholar] [CrossRef]
- Bishop, N.; Jones-Lepp, T.; Margetts, M.; Sykes, J.; Alvarez, D.; Keil, D.E. Wastewater-Based Epidemiology Pilot Study to Examine Drug Use in the Western United States. Sci. Total. Environ. 2020, 745, 140697. [Google Scholar] [CrossRef]
- Stevens, C.W. The Evolution of Vertebrate Opioid Receptors. FBL 2009, 14, 1247–1269. [Google Scholar] [CrossRef] [PubMed]
- Pathan, H.; Williams, J. Basic Opioid Pharmacology: An Update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef]
- Petrocelli, G.; Pampanella, L.; Abruzzo, P.M.; Ventura, C.; Canaider, S.; Facchin, F. Endogenous Opioids and Their Role in Stem Cell Biology and Tissue Rescue. Int. J. Mol. Sci. 2022, 23, 3819. [Google Scholar] [CrossRef]
- Rullo, L.; Morosini, C.; Lacorte, A.; Cristani, M.; Coluzzi, F.; Candeletti, S.; Romualdi, P. Opioid System and Related Ligands: From the Past to Future Perspectives. J. Anesth. Analg. Crit. Care 2024, 4, 70. [Google Scholar] [CrossRef]
- Benarroch, E.E. Endogenous Opioid Systems. Neurology 2012, 79, 807–814. [Google Scholar] [CrossRef]
- Margolis, E.B.; Fujita, W.; Devi, L.A.; Fields, H.L. Two Delta Opioid Receptor Subtypes Are Functional in Single Ventral Tegmental Area Neurons, and Can Interact with the Mu Opioid Receptor. Neuropharmacology 2017, 123, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Z.; Long, S.; Li, W.; Wang, B.; Liang, N. Opioids in Cancer: The κ-Opioid Receptor (Review). Mol. Med. Rep. 2022, 25, 44. [Google Scholar] [CrossRef]
- Sah, D.; Shoffel-Havakuk, H.; Tsur, N.; Uhelski, M.L.; Gottumukkala, V.; Cata, J.P. Opioids and Cancer: Current Understanding and Clinical Considerations. Curr. Oncol. 2024, 31, 3086–3098. [Google Scholar] [CrossRef] [PubMed]
- Gumede, N.A.-C.; Khathi, A. The Role of Pro-Opiomelanocortin Derivatives in the Development of Type 2 Diabetes-Associated Myocardial Infarction: Possible Links with Prediabetes. Biomedicines 2024, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. Synthetic Opioids: A Review and Clinical Update. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221139616. [Google Scholar] [CrossRef]
- Goldfarb, S.S.; Stanwood, G.D.; Flynn, H.A.; Graham, D.L. Developmental Opioid Exposures: Neurobiological Underpinnings, Behavioral Impacts, and Policy Implications. Exp. Biol. Med. 2020, 245, 131–137. [Google Scholar] [CrossRef]
- Volpe, D.A.; Tobin, G.A.M.; Mellon, R.D.; Katki, A.G.; Parker, R.J.; Colatsky, T.; Kropp, T.J.; Verbois, S.L. Uniform Assessment and Ranking of Opioid Mu Receptor Binding Constants for Selected Opioid Drugs. Regul. Toxicol. Pharmacol. 2011, 59, 385–390. [Google Scholar] [CrossRef]
- Corbett, A.D.; Paterson, S.J.; Kosterlitz, H.W. Selectivity of Ligands for Opioid Receptors. In Opioids; Herz, A., Akil, H., Simon, E.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 645–679. ISBN 978-3-642-77460-7. [Google Scholar]
- Drewes, A.M.; Jensen, R.D.; Nielsen, L.M.; Droney, J.; Christrup, L.L.; Arendt-Nielsen, L.; Riley, J.; Dahan, A. Differences between Opioids: Pharmacological, Experimental, Clinical and Economical Perspectives. Br. J. Clin. Pharmacol. 2013, 75, 60–78. [Google Scholar] [CrossRef]
- Kalso, E. Oxycodone. J. Pain Symptom Manag. 2005, 29, 47–56. [Google Scholar] [CrossRef]
- Huddart, R.; Clarke, M.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Oxycodone Pathway, Pharmacokinetics. Pharmacogenet Genom. 2018, 28, 230–237. [Google Scholar] [CrossRef]
- Smith, H.S. Clinical Pharmacology of Oxymorphone. Pain Med. 2009, 10, S3–S10. [Google Scholar] [CrossRef]
- Olesen, A.E.; Staahl, C.; Arendt-Nielsen, L.; Drewes, A.M. Different Effects of Morphine and Oxycodone in Experimentally Evoked Hyperalgesia: A Human Translational Study. Br. J. Clin. Pharmacol. 2010, 70, 189–200. [Google Scholar] [CrossRef]
- Babalonis, S.; Comer, S.D.; Jones, J.D.; Nuzzo, P.; Lofwall, M.R.; Manubay, J.; Hatton, K.W.; Whittington, R.A.; Walsh, S.L. Relative Potency of Intravenous Oxymorphone Compared to Other μ Opioid Agonists in Humans—Pilot Study Outcomes. Psychopharmacology 2021, 238, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Wu, W.; Oleksak, P.; Jelinkova, R.; Nepovimova, E.; Spicanova, L.; Springerova, P.; Alomar, S.; Long, M.; Kuca, K. Fentanyl and Its Derivatives: Pain-Killers or Man-Killers? Heliyon 2024, 10, e28795. [Google Scholar] [CrossRef]
- Maguire, P.; Tsai, N.; Kamal, J.; Cometta-Morini, C.; Upton, C.; Loew, G. Pharmacological Profiles of Fentanyl Analogs at Mu, Delta and Kappa Opiate Receptors. Eur. J. Pharmacol. 1992, 213, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid Pharmacology. Pain Physician 2008, 11, S133–S153. [Google Scholar] [CrossRef]
- Jordan, B.A.; Cvejic, S.; Devi, L.A. Opioids and Their Complicated Receptor Complexes. Neuropsychopharmacology 2000, 23, S5–S18. [Google Scholar] [CrossRef]
- Lambert, J.E.; Peeler, C.E. Visual and Oculomotor Outcomes in Children with Prenatal Opioid Exposure. Curr. Opin. Ophthalmol. 2019, 30, 449–453. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Veldman, M.B.; Lin, S. Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatr. Res. 2008, 64, 470–476. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.; Kimelman, D. Chapter 7—Establishing The Body Plan: The First 24 Hours of Zebrafish Development. In The Zebrafish in Biomedical Research; Cartner, S.C., Eisen, J.S., Farmer, S.C., Guillemin, K.J., Kent, M.L., Sanders, G.E., Eds.; American College of Laboratory Animal Medicine: East Lansing, MI, USA; Academic Press: Cambridge, MA, USA, 2020; pp. 81–88. ISBN 978-0-12-812431-4. [Google Scholar]
- Rossant, J.; Tam, P.P.L. Early Human Embryonic Development: Blastocyst Formation to Gastrulation. Dev. Cell 2022, 57, 152–165. [Google Scholar] [CrossRef]
- Le Bras, A. Zebrafish: A New Model System for the Development of Safe Opioid Therapies. Lab Anim. 2021, 50, 123. [Google Scholar] [CrossRef]
- Sanchez-Simon, F.M.; Rodriguez, R.E. Developmental Expression and Distribution of Opioid Receptors in Zebrafish. Neuroscience 2008, 151, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kong, S.; Tang, X.; Fu, Y.; Wang, B.; Zhang, S.; Wang, H. Preimplantation Mouse Embryo Is a Target for Opioid Ligand-Receptor Signaling1. Biol. Reprod. 2014, 91, 4. [Google Scholar] [CrossRef]
- Zhu, Y.; Hsu, M.-S.; Pintar, J.E. Developmental Expression of the μ, κ, and δ Opioid Receptor mRNAs in Mouse. J. Neurosci. 1998, 18, 2538–2549. [Google Scholar] [CrossRef]
- ZFIN The Zebrafish Information Network. Available online: https://zfin.org/ (accessed on 10 December 2024).
- MGI-Mouse Genome Informatics-The International Database Resource for the Laboratory Mouse. Available online: https://www.informatics.jax.org/ (accessed on 10 December 2024).
- Herrero-Turrión, M.J.; Rodríguez-Martín, I.; López-Bellido, R.; Rodríguez, R.E. Whole-Genome Expression Profile in Zebrafish Embryos after Chronic Exposure to Morphine: Identification of New Genes Associated with Neuronal Function and Mu Opioid Receptor Expression. BMC Genom. 2014, 15, 874. [Google Scholar] [CrossRef]
- Calderon-Garcia, A.A.; Perez-Fernandez, M.; Curto-Aguilera, D.; Rodriguez-Martin, I.; Sánchez-Barba, M.; Gonzalez-Nunez, V. Exposure to Morphine and Cocaine Modify the Transcriptomic Landscape in Zebrafish Embryos. Neuroscience 2022, 507, 14–27. [Google Scholar] [CrossRef]
- Calderon-Garcia, A.A.; Sánchez-Barba, M.; Gonzalez-Nunez, V. Exposure of Zebrafish Embryos to Morphine and Cocaine Induces Changes in the Levels of Dopamine and of Proteins Related to the Reward Pathway. Fishes 2024, 9, 268. [Google Scholar] [CrossRef]
- Sales Cadena, M.R.; Cadena, P.G.; Watson, M.R.; Sarmah, S.; Boehm II, S.L.; Marrs, J.A. Zebrafish (Danio Rerio) Larvae Show Behavioral and Embryonic Development Defects When Exposed to Opioids at Embryo Stage. Neurotoxicology Teratol. 2021, 85, 106964. [Google Scholar] [CrossRef] [PubMed]
- Mech, A.M.; Merteroglu, M.; Sealy, I.M.; Teh, M.-T.; White, R.J.; Havelange, W.; Brennan, C.H.; Busch-Nentwich, E.M. Behavioral and Gene Regulatory Responses to Developmental Drug Exposures in Zebrafish. Front. Psychiatry 2022, 12, 795175. [Google Scholar] [CrossRef]
- Odegaard, K.E.; Schaal, V.L.; Clark, A.R.; Koul, S.; Sankarasubramanian, J.; Xia, Z.; Mellon, M.; Uberti, M.; Liu, Y.; Stothert, A.; et al. A Holistic Systems Approach to Characterize the Impact of Pre- and Post-Natal Oxycodone Exposure on Neurodevelopment and Behavior. Front. Cell Dev. Biol. 2021, 8, 619199. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.E.; Myers, A.L.; Lee, I.J.; Chen, H.; Coop, A.; Eddington, N.D. Regulation of Gene Expression in Brain Tissues of Rats Repeatedly Treated by the Highly Abused Opioid Agonist, Oxycodone: Microarray Profiling and Gene Mapping Analysis. Drug Metab. Dispos. 2010, 38, 157–167. [Google Scholar] [CrossRef]
- Zaig, S.; da Silveira Scarpellini, C.; Montandon, G. Respiratory Depression and Analgesia by Opioid Drugs in Freely Behaving Larval Zebrafish. eLife 2021, 10, e63407. [Google Scholar] [CrossRef] [PubMed]
- Cooman, T.; Bergeron, S.A.; Coltogirone, R.; Horstick, E.; Arroyo, L. Evaluation of Fentanyl Toxicity and Metabolism Using a Zebrafish Model. J. Appl. Toxicol. 2022, 42, 706–714. [Google Scholar] [CrossRef]
- Kirla, K.T.; Erhart, C.; Groh, K.J.; Stadnicka-Michalak, J.; Eggen, R.I.L.; Schirmer, K.; Kraemer, T. Zebrafish Early Life Stages as Alternative Model to Study ‘Designer Drugs’: Concordance with Mammals in Response to Opioids. Toxicol. Appl. Pharmacol. 2021, 419, 115483. [Google Scholar] [CrossRef]
- Lam, D.; Sebastian, A.; Bogguri, C.; Hum, N.R.; Ladd, A.; Cadena, J.; Valdez, C.A.; Fischer, N.O.; Loots, G.G.; Enright, H.A. Dose-Dependent Consequences of Sub-Chronic Fentanyl Exposure on Neuron and Glial Co-Cultures. Front. Toxicol. 2022, 4, 983415. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Sheng, Z.; Lian, W.; Wu, Y.; Liu, M. Embryonic Exposure to Fentanyl Induces Behavioral Changes and Neurotoxicity in Zebrafish Larvae. PeerJ 2022, 10, e14524. [Google Scholar] [CrossRef]
- Alipio, J.B.; Brockett, A.T.; Fox, M.E.; Tennyson, S.S.; deBettencourt, C.A.; El-Metwally, D.; Francis, N.A.; Kanold, P.O.; Lobo, M.K.; Roesch, M.R.; et al. Enduring Consequences of Perinatal Fentanyl Exposure in Mice. Addict. Biol. 2021, 26, e12895. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.C.; Christie, M.J. Mechanisms of Rapid Opioid Receptor Desensitization, Resensitization and Tolerance in Brain Neurons. Br. J. Pharmacol. 2012, 165, 1704–1716. [Google Scholar] [CrossRef]
- Adhikary, S.; Williams, J.T. Cellular Tolerance Induced by Chronic Opioids in the Central Nervous System. Front. Syst. Neurosci. 2022, 16, 937126. [Google Scholar] [CrossRef] [PubMed]
- Coutens, B.; Ingram, S.L. Key Differences in Regulation of Opioid Receptors Localized to Presynaptic Terminals Compared to Somas: Relevance for Novel Therapeutics. Neuropharmacology 2023, 226, 109408. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Li, X.; Zhao, X.; Zhao, H.; Yang, W.; Zuo, Y.; Cai, L.; Xing, Y. Temporal Dynamic Analysis of Alternative Splicing During Embryonic Development in Zebrafish. Front. Cell Dev. Biol. 2022, 10, 879795. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.T.; Ingram, S.L.; Henderson, G.; Chavkin, C.; von Zastrow, M.; Schulz, S.; Koch, T.; Evans, C.J.; Christie, M.J. Regulation of M-Opioid Receptors: Desensitization, Phosphorylation, Internalization, and Tolerance. Pharmacol. Rev. 2013, 65, 223–254. [Google Scholar] [CrossRef]
- Zuo, Z. The Role of Opioid Receptor Internalization and Beta-Arrestins in the Development of Opioid Tolerance. Anesth. Analg. 2005, 101, 728–734. [Google Scholar] [CrossRef]
- Grace, P.M.; Maier, S.F.; Watkins, L.R. Opioid-Induced Central Immune Signaling: Implications for Opioid Analgesia. Headache 2015, 55, 475–489. [Google Scholar] [CrossRef]
- Borgland, S.L.; Connor, M.; Osborne, P.B.; Furness, J.B.; Christie, M.J. Opioid Agonists Have Different Efficacy Profiles for G Protein Activation, Rapid Desensitization, and Endocytosis of Mu-Opioid Receptors. J. Biol. Chem. 2003, 278, 18776–18784. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Arttamangkul, S.; Dang, V.; Salem, A.; Whistler, J.L.; von Zastrow, M.; Grandy, D.K.; Williams, J.T. μ-Opioid Receptors: Ligand-Dependent Activation of Potassium Conductance, Desensitization, and Internalization. J. Neurosci. 2002, 22, 5769–5776. [Google Scholar] [CrossRef]
- Bao, F.; Li, C.-L.; Chen, X.-Q.; Lu, Y.-J.; Bao, L.; Zhang, X. Clinical Opioids Differentially Induce Co-Internalization of μ- and δ-Opioid Receptors. Mol. Pain 2018, 14, 1744806918769492. [Google Scholar] [CrossRef]
- Morgan, M.M.; Christie, M.J. Analysis of Opioid Efficacy, Tolerance, Addiction and Dependence from Cell Culture to Human. Br. J. Pharmacol. 2011, 164, 1322–1334. [Google Scholar] [CrossRef]
- Madia, P.A.; Dighe, S.V.; Sirohi, S.; Walker, E.A.; Yoburn, B.C. Dosing Protocol and Analgesic Efficacy Determine Opioid Tolerance in the Mouse. Psychopharmacology 2009, 207, 413–422. [Google Scholar] [CrossRef]
- Calabrese, E.J. Opiates: Biphasic Dose Responses. Crit. Rev. Toxicol. 2001, 31, 585–604. [Google Scholar] [CrossRef]
- Calabrese, E.; Iavicoli, I.; Calabrese, V. Hormesis: Its Impact on Medicine and Health. Hum. Exp. Toxicol. 2013, 32, 120–152. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J.; Cowan, A.; Raffa, R.B. On Deriving the Dose–Effect Relation of an Unknown Second Component: An Example Using Buprenorphine Preclinical Data. Drug Alcohol Depend. 2010, 109, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Dudekula, N.; Arora, V.; Callaerts-Vegh, Z.; Bond, R.A. The Temporal Hormesis of Drug Therapies. Dose Response 2006, 3, 414–424. [Google Scholar] [CrossRef]
- Calabrese, E.J. The Maturing of Hormesis as a Credible Dose-Response Model. Nonlinearity Biol. Toxicol. Med. 2003, 1, 319–343. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, J.; Mai, Y.; Hong, Y.; Jia, Z.; Tian, G.; Liu, Y.; Liang, H.; Liu, J. Current Advances and Future Trends of Hormesis in Disease. npj Aging 2024, 10, 26. [Google Scholar] [CrossRef]
- Cooper, G.; North, R.; Hunt-Smith, T.; Larson, J.; Rennie, M.; Bailey, M.L.; Scarlata, S.; Merzdorf, C.S.; Bothner, B. Persistent Metabolic Changes Are Induced by 24 h Low-Dose Lead (Pb) Exposure in Zebrafish Embryos. Int. J. Mol. Sci. 2025, 26, 1050. [Google Scholar] [CrossRef]
- Berger, A.C.; Whistler, J.L. How to Design an Opioid Drug That Causes Reduced Tolerance and Dependence. Ann. Neurol. 2010, 67, 559–569. [Google Scholar] [CrossRef]
- Ray, M.H.; Williams, B.R.; Kuppe, M.K.; Bryant, C.D.; Logan, R.W. A Glitch in the Matrix: The Role of Extracellular Matrix Remodeling in Opioid Use Disorder. Front. Integr. Neurosci. 2022, 16, 899637. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, D.J.; Mareel, M.M.; Bracke, M.E. Opioids Affect Focal Contact-Mediated Cell–Substrate Adhesion. Eur. J. Cancer Prev. 2010, 19, 227–238. [Google Scholar] [CrossRef]
- Pello, O.M.; Duthey, B.; García-Bernal, D.; Rodríguez-Frade, J.M.; Stein, J.V.; Teixido, J.; Martínez-A, C.; Mellado, M. Opioids Trigger A5β1 Integrin-Mediated Monocyte Adhesion1. J. Immunol. 2006, 176, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Sampieri, L.; Di Giusto, P.; Alvarez, C. CREB3 Transcription Factors: ER-Golgi Stress Transducers as Hubs for Cellular Homeostasis. Front. Cell Dev. Biol. 2019, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.J.; Kudo, A. The Role of Periostin in Tissue Remodeling across Health and Disease. Cell. Mol. Life. Sci. 2013, 71, 1279–1288. [Google Scholar] [CrossRef]
- Exposito, J.-Y.; Valcourt, U.; Cluzel, C.; Lethias, C. The Fibrillar Collagen Family. Int. J. Mol. Sci. 2010, 11, 407–426. [Google Scholar] [CrossRef]
- Chute, M.; Aujla, P.; Jana, S.; Kassiri, Z. The Non-Fibrillar Side of Fibrosis: Contribution of the Basement Membrane, Proteoglycans, and Glycoproteins to Myocardial Fibrosis. J. Cardiovasc. Dev. Dis. 2019, 6, 35. [Google Scholar] [CrossRef]
- Abreu-Velez, A.M.; Howard, M.S. Collagen IV in Normal Skin and in Pathological Processes. N. Am. J. Med. Sci. 2012, 4, 1–8. [Google Scholar] [CrossRef]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV Is Essential for Basement Membrane Stability but Dispensable for Initiation of Its Assembly During Early Development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef]
- Wenstrup, R.J.; Florer, J.B.; Brunskill, E.W.; Bell, S.M.; Chervoneva, I.; Birk, D.E. Type V Collagen Controls the Initiation of Collagen Fibril Assembly *. J. Biol. Chem. 2004, 279, 53331–53337. [Google Scholar] [CrossRef]
- Chang, P.-J.; Chen, M.-Y.; Huang, Y.-S.; Lee, C.-H.; Huang, C.-C.; Lam, C.-F.; Tsai, Y.-C. Morphine Enhances Tissue Content of Collagen and Increases Wound Tensile Strength. J. Anesth. 2010, 24, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Uitto, J. Type VII Collagen: The Anchoring Fibril Protein at Fault in Dystrophic Epidermolysis Bullosa. Dermatol. Clin. 2010, 28, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimkhani, M.R.; Kiani, S.; Oakley, F.; Kendall, T.; Shariftabrizi, A.; Tavangar, S.M.; Moezi, L.; Payabvash, S.; Karoon, A.; Hoseininik, H.; et al. Naltrexone, an Opioid Receptor Antagonist, Attenuates Liver Fibrosis in Bile Duct Ligated Rats. Gut 2006, 55, 1606–1616. [Google Scholar] [CrossRef]
- Gu, C.; Shi, X.; Dang, X.; Chen, J.; Chen, C.; Chen, Y.; Pan, X.; Huang, T. Identification of Common Genes and Pathways in Eight Fibrosis Diseases. Front. Genet. 2021, 11, 627396. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, Q.-N.; Wu, J.-F.; Ai, W.-B.; Ma, L. Analysis of Key Genes and Related Transcription Factors in Liver Fibrosis Based on Bioinformatic Technology. Int. J. Clin. Exp. Pathol. 2021, 14, 444–454. [Google Scholar]
- Odegaard, K.E.; Gallegos, G.; Koul, S.; Schaal, V.L.; Vellichirammal, N.N.; Guda, C.; Dutoit, A.P.; Lisco, S.J.; Yelamanchili, S.V.; Pendyala, G. Distinct Synaptic Vesicle Proteomic Signatures Associated with Pre- and Post-Natal Oxycodone-Exposure. Cells 2022, 11, 1740. [Google Scholar] [CrossRef]
- Colige, A.; Ruggiero, F.; Vandenberghe, I.; Dubail, J.; Kesteloot, F.; Van Beeumen, J.; Beschin, A.; Brys, L.; Lapière, C.M.; Nusgens, B. Domains and Maturation Processes That Regulate the Activity of ADAMTS-2, a Metalloproteinase Cleaving the Aminopropeptide of Fibrillar Procollagens Types I-III and V. J. Biol. Chem. 2005, 280, 34397–34408. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Kashimoto, T.; Yonezawa, T.; Sano, N.; Saitoh, R.; Igarashi, S.; Chikazawa, S.; Kanai, K.; Hoshi, F.; Itoh, N.; et al. Matrix Metalloproteinase-2 Stimulates Collagen-I Expression through Phosphorylation of Focal Adhesion Kinase in Rat Cardiac Fibroblasts. Am. J. Physiol.-Cell Physiol. 2012, 303, C947–C953. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hirata, M.; Saito, T.; Itoh, S.; Chung, U.; Kawaguchi, H. Transcriptional Induction of ADAMTS5 Protein by Nuclear Factor-κB (NF-κB) Family Member RelA/P65 in Chondrocytes during Osteoarthritis Development. J. Biol. Chem. 2013, 288, 28620–28629. [Google Scholar] [CrossRef]
- Wu, K.-I.S.; Schmid-Schönbein, G.W. NF Kappa B and Matrix Metalloproteinase Induced Receptor Cleavage in the Spontaneously Hypertensive Rat. Hypertension 2011, 57, 261–268. [Google Scholar] [CrossRef]
- Paciolla, M.; Boni, R.; Fusco, F.; Pescatore, A.; Poeta, L.; Ursini, M.V.; Lioi, M.B.; Miano, M.G. Nuclear Factor-Kappa-B-Inhibitor Alpha (NFKBIA) Is a Developmental Marker of NF-κB/P65 Activation During in Vitro Oocyte Maturation and Early Embryogenesis. Hum. Reprod. 2011, 26, 1191–1201. [Google Scholar] [CrossRef]
- Halsey, T.A.; Yang, L.; Walker, J.R.; Hogenesch, J.B.; Thomas, R.S. A Functional Map of NFκB Signaling Identifies Novel Modulators and Multiple System Controls. Genome. Biol. 2007, 8, R104. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.; Chase, A.J.; Baker, A.H.; Newby, A.C. Inhibition of Transcription Factor NF-kappaB Reduces Matrix Metalloproteinase-1, -3 and -9 Production by Vascular Smooth Muscle Cells. Cardiovasc. Res. 2001, 50, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Mui, K.L.; Chen, C.S.; Assoian, R.K. The Mechanical Regulation of Integrin–Cadherin Crosstalk Organizes Cells, Signaling and Forces. J. Cell Sci. 2016, 129, 1093–1100. [Google Scholar] [CrossRef]
- Choi, Y.E.; Song, M.J.; Hara, M.; Imanaka-Yoshida, K.; Lee, D.H.; Chung, J.H.; Lee, S.-T. Effects of Tenascin C on the Integrity of Extracellular Matrix and Skin Aging. Int. J. Mol. Sci. 2020, 21, 8693. [Google Scholar] [CrossRef] [PubMed]
- Chetty, A.K.; Ha, B.H.; Boggon, T.J. Rho Family GTPase Signaling through Type II P21-Activated Kinases. Cell. Mol. Life. Sci. 2022, 79, 598. [Google Scholar] [CrossRef]
- Crespo, C.L.; Vernieri, C.; Keller, P.J.; Garrè, M.; Bender, J.R.; Wittbrodt, J.; Pardi, R. The PAR Complex Controls the Spatiotemporal Dynamics of F-Actin and the MTOC in Directionally Migrating Leukocytes. J. Cell Sci. 2014, 127, 4381–4395. [Google Scholar] [CrossRef]
- Wenzel, J.; Rose, K.; Haghighi, E.B.; Lamprecht, C.; Rauen, G.; Freihen, V.; Kesselring, R.; Boerries, M.; Hecht, A. Loss of the Nuclear Wnt Pathway Effector TCF7L2 Promotes Migration and Invasion of Human Colorectal Cancer Cells. Oncogene 2020, 39, 3893–3909. [Google Scholar] [CrossRef]
- Burridge, K.; Wennerberg, K. Rho and Rac Take Center Stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Pullikuth, A.K.; Catling, A.D. Scaffold Mediated Regulation of MAPK Signaling and Cytoskeletal Dynamics: A Perspective. Cell Signal. 2007, 19, 1621–1632. [Google Scholar] [CrossRef]
- Calabro, N.E.; Kristofik, N.J.; Kyriakides, T.R. Thrombospondin-2 and Extracellular Matrix Assembly. Biochim. Biophys. Acta 2014, 1840, 2396–2402. [Google Scholar] [CrossRef]
- Belcheva, M.M.; Clark, A.L.; Haas, P.D.; Serna, J.S.; Hahn, J.W.; Kiss, A.; Coscia, C.J. Mu and Kappa Opioid Receptors Activate ERK/MAPK via Different Protein Kinase C Isoforms and Secondary Messengers in Astrocytes. J. Biol. Chem. 2005, 280, 27662–27669. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Tsapara, A.; Kampa, M.; Papakonstanti, E.A.; Krasagakis, K.; Castanas, E.; Stournaras, C. Distinct Signaling Pathways Regulate Differential Opioid Effects on Actin Cytoskeleton in Malignant MCF7 and Nonmalignant MCF12A Human Breast Epithelial Cells. Exp. Cell Res. 2003, 288, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Papakonstanti, E.A.; Bakogeorgou, E.; Castanas, E.; Emmanouel, D.S.; Hartig, R.; Stournaras, C. Early Alterations of Actin Cytoskeleton in OK Cells by Opioids. J. Cell. Biochem. 1998, 70, 60–69. [Google Scholar] [CrossRef]
- Ru, Q.; Wang, Y.; Zhou, E.; Chen, L.; Wu, Y. The Potential Therapeutic Roles of Rho GTPases in Substance Dependence. Front. Mol. Neurosci. 2023, 16, 1125277. [Google Scholar] [CrossRef]
- Zagon, I.S.; Rahn, K.A.; McLaughlin, P.J. Opioids and Migration, Chemotaxis, Invasion, and Adhesion of Human Cancer Cells. Neuropeptides 2007, 41, 441–452. [Google Scholar] [CrossRef]
- Erskine, L.; Herrera, E. Connecting the Retina to the Brain. ASN Neuro. 2014, 6, 1759091414562107. [Google Scholar] [CrossRef]
- Cioni, J.-M.; Wong, H.H.-W.; Bressan, D.; Kodama, L.; Harris, W.A.; Holt, C.E. Axon-Axon Interactions Regulate Topographic Optic Tract Sorting via CYFIP2-Dependent WAVE Complex Function. Neuron 2018, 97, 1078–1093.e6. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Wald, J.H.; Rafidi, H.; Cuevas, A.; Sweeney, C.; Carraway, K.L. The ER Structural Protein Rtn4A Stabilizes and Enhances Signaling through the Receptor Tyrosine Kinase ErbB3. Sci. Signal. 2016, 9, ra65. [Google Scholar] [CrossRef]
- Blasky, A.J.; Pan, L.; Moens, C.B.; Appel, B. Pard3 Regulates Contact between Neural Crest Cells and the Timing of Schwann Cell Differentiation but Is Not Essential for Neural Crest Migration or Myelination. Dev. Dyn. 2014, 243, 1511–1523. [Google Scholar] [CrossRef]
- Navickas, S.M.; Giles, K.A.; Brettingham-Moore, K.H.; Taberlay, P.C. The Role of Chromatin Remodeler SMARCA4/BRG1 in Brain Cancers: A Potential Therapeutic Target. Oncogene 2023, 42, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Chaya, T.; Ishikane, H.; Varner, L.R.; Sugita, Y.; Maeda, Y.; Tsutsumi, R.; Motooka, D.; Okuzaki, D.; Furukawa, T. Deficiency of the Neurodevelopmental Disorder-Associated Gene Cyfip2 Alters the Retinal Ganglion Cell Properties and Visual Acuity. Hum. Mol. Genet. 2022, 31, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Cheng, Y.; Luo, Y.; Shi, X.; Nelson, S.; Hyde, D.R. The Zebrafish Pard3 Ortholog Is Required for Separation of the Eye Fields and Retinal Lamination. Dev. Biol. 2004, 269, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Marcos, S.; González-Lázaro, M.; Beccari, L.; Carramolino, L.; Martin-Bermejo, M.J.; Amarie, O.; Mateos-San Martín, D.; Torroja, C.; Bogdanović, O.; Doohan, R.; et al. Meis1 Coordinates a Network of Genes Implicated in Eye Development and Microphthalmia. Development 2015, 142, 3009–3020. [Google Scholar] [CrossRef]
- Baek, C.; Freem, L.; Goïame, R.; Sang, H.; Morin, X.; Tozer, S. Mib1 Prevents Notch Cis-Inhibition to Defer Differentiation and Preserve Neuroepithelial Integrity during Neural Delamination. PLoS Biol. 2018, 16, e2004162. [Google Scholar] [CrossRef]
- Karaca, E.; Li, X.; Lewicki, J.; Neofytou, C.; Guérout, N.; Barnabé-Heider, F.; Hermanson, O. The Corepressor CtBP2 Is Required for Proper Development of the Mouse Cerebral Cortex. Mol. Cell. Neurosci. 2020, 104, 103481. [Google Scholar] [CrossRef]
- Rowan, S.; Conley, K.W.; Le, T.T.; Donner, A.L.; Maas, R.L.; Brown, N.L. Notch Signaling Regulates Growth and Differentiation in the Mammalian Lens. Dev. Biol. 2008, 321, 111–122. [Google Scholar] [CrossRef]
- Holdhof, D.; Schoof, M.; Al-Kershi, S.; Spohn, M.; Kresbach, C.; Göbel, C.; Hellwig, M.; Indenbirken, D.; Moreno, N.; Kerl, K.; et al. Brahma-Related Gene 1 Has Time-Specific Roles during Brain and Eye Development. Development 2021, 148, dev196147. [Google Scholar] [CrossRef]
- The Nuclear Receptor NR2E3 Plays a Role in Human Retinal Photoreceptor Differentiation and Degeneration|PNAS. Available online: https://www.pnas.org/doi/10.1073/pnas.022533099 (accessed on 7 January 2025).
- Zhang, L.; Cho, J.; Ptak, D.; Leung, Y.F. The Role of Egr1 in Early Zebrafish Retinogenesis. PLoS ONE 2013, 8, e56108. [Google Scholar] [CrossRef]
- Auger, N.; Rhéaume, M.-A.; Low, N.; Lee, G.E.; Ayoub, A.; Luu, T.M. Impact of Prenatal Exposure to Opioids, Cocaine, and Cannabis on Eye Disorders in Children. J. Addict. Med. 2020, 14, 459. [Google Scholar] [CrossRef]
- Andley, U.P. Crystallins in the Eye: Function and Pathology. Prog. Retin. Eye Res. 2007, 26, 78–98. [Google Scholar] [CrossRef]
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. Prog. Mol. Biol. Transl. Sci. 2015, 134, 119–127. [Google Scholar] [CrossRef]
- Gestri, G.; Link, B.A.; Neuhauss, S.C. The Visual System of Zebrafish and Its Use to Model Human Ocular Diseases. Dev. Neurobiol. 2012, 72, 302–327. [Google Scholar] [CrossRef]
- Drastichova, Z.; Skrabalova, J.; Jedelsky, P.; Neckar, J.; Kolar, F.; Novotny, J. Global Changes in the Rat Heart Proteome Induced by Prolonged Morphine Treatment and Withdrawal. PLoS ONE 2012, 7, e47167. [Google Scholar] [CrossRef]
- Farnsworth, D.; Posner, M.; Miller, A. Single Cell Transcriptomics of the Developing Zebrafish Lens and Identification of Putative Controllers of Lens Development. Exp. Eye Res. 2021, 206, 108535. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Feder, M.E.; Kang, L. Evolution of Heat-Shock Protein Expression Underlying Adaptive Responses to Environmental Stress. Mol. Ecol. 2018, 27, 3040–3054. [Google Scholar] [CrossRef]
- Massaquoi, M.S.; Kong, G.L.; Chilin-Fuentes, D.; Ngo, J.S.; Horve, P.F.; Melancon, E.; Hamilton, M.K.; Eisen, J.S.; Guillemin, K. Cell-Type-Specific Responses to the Microbiota across All Tissues of the Larval Zebrafish. Cell Rep. 2023, 42, 112095. [Google Scholar] [CrossRef] [PubMed]
- Ammon, S.; Mayer, P.; Riechert, U.; Tischmeyer, H.; Höllt, V. Microarray Analysis of Genes Expressed in the Frontal Cortex of Rats Chronically Treated with Morphine and after Naloxone Precipitated Withdrawal. Brain Res. Mol. Brain Res. 2003, 112, 113–125. [Google Scholar] [CrossRef]
- Oral Morphine Consumption Reduces Lens Development in Rat Embryos. Available online: https://www.researchgate.net/publication/258769688_Oral_Morphine_Consumption_Reduces_Lens_Development_in_Rat_Embryos (accessed on 11 December 2024).
- Erskine, L.; Herrera, E. The Retinal Ganglion Cell Axon’s Journey: Insights into Molecular Mechanisms of Axon Guidance. Dev. Biol. 2007, 308, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lwigale, P.Y.; Bronner-Fraser, M. Lens-Derived Semaphorin3A Regulates Sensory Innervation of the Cornea. Dev. Biol. 2007, 306, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, T.; Zhang, H.; Kumanogoh, A.; Takegahara, N.; Yabuki, M.; Harada, K.; Hori, M.; Kikutani, H. Guidance of Myocardial Patterning in Cardiac Development by Sema6D Reverse Signalling. Nat. Cell. Biol. 2004, 6, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Fischer von Mollard, G.; Mignery, G.A.; Baumert, M.; Perin, M.S.; Hanson, T.J.; Burger, P.M.; Jahn, R.; Südhof, T.C. Rab3 Is a Small GTP-Binding Protein Exclusively Localized to Synaptic Vesicles. Proc. Natl. Acad. Sci. USA 1990, 87, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, H.; Aggarwal, M.; Pan, P.-Y. Mini-Review: Synaptojanin 1 and Its Implications in Membrane Trafficking. Neurosci. Lett. 2021, 765, 136288. [Google Scholar] [CrossRef]
- Fjorback, A.W.; Müller, H.K.; Wiborg, O. Membrane Glycoprotein M6B Interacts with the Human Serotonin Transporter. J. Mol. Neurosci. 2009, 37, 191–200. [Google Scholar] [CrossRef]
- Formoso, K.; Garcia, M.D.; Frasch, A.C.; Scorticati, C. Evidence for a Role of Glycoprotein M6a in Dendritic Spine Formation and Synaptogenesis. Mol. Cell. Neurosci. 2016, 77, 95–104. [Google Scholar] [CrossRef]
- Cosker, K.E.; Fenstermacher, S.J.; Pazyra-Murphy, M.F.; Elliott, H.L.; Segal, R.A. The RNA-Binding Protein SFPQ Orchestrates an RNA Regulon to Promote Axon Viability. Nat. Neurosci. 2016, 19, 690–696. [Google Scholar] [CrossRef]
- Lebrand, C.; Dent, E.W.; Strasser, G.A.; Lanier, L.M.; Krause, M.; Svitkina, T.M.; Borisy, G.G.; Gertler, F.B. Critical Role of Ena/VASP Proteins for Filopodia Formation in Neurons and in Function Downstream of Netrin-1. Neuron 2004, 42, 37–49. [Google Scholar] [CrossRef]
- Kunda, P.; Paglini, G.; Quiroga, S.; Kosik, K.; Caceres, A. Evidence for the Involvement of Tiam1 in Axon Formation. J. Neurosci. 2001, 21, 2361–2372. [Google Scholar] [CrossRef]
- Perälä, N.; Peitsaro, N.; Sundvik, M.; Koivula, H.; Sainio, K.; Sariola, H.; Panula, P.; Immonen, T. Conservation, Expression, and Knockdown of Zebrafish Plxnb2a and Plxnb2b. Dev. Dyn. 2010, 239, 2722–2734. [Google Scholar] [CrossRef]
- Kaur, N.; Han, W.; Li, Z.; Madrigal, M.P.; Shim, S.; Pochareddy, S.; Gulden, F.O.; Li, M.; Xu, X.; Xing, X.; et al. Neural Stem Cells Direct Axon Guidance via Their Radial Fiber Scaffold. Neuron 2020, 107, 1197–1211.e9. [Google Scholar] [CrossRef]
- Nitzan, A.; Corredor-Sanchez, M.; Galron, R.; Nahary, L.; Safrin, M.; Bruzel, M.; Moure, A.; Bonet, R.; Pérez, Y.; Bujons, J.; et al. Inhibition of Sema-3A Promotes Cell Migration, Axonal Growth, and Retinal Ganglion Cell Survival. Transl. Vis. Sci. Technol. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Duwe, L.; Fouassier, L.; Lafuente-Barquero, J.; Andersen, J.B. Unraveling the Actin Cytoskeleton in the Malignant Transformation of Cholangiocyte Biology. Transl. Oncol. 2022, 26, 101531. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Bu, L.; Zhang, J.; Liu, F.-Y.; Sall, J.; Liang, F.-X.; Furley, A.J.; Fishman, G.I. Neural Cell Adhesion Molecule Is Required for Ventricular Conduction System Development. Development 2021, 148, dev199431. [Google Scholar] [CrossRef]
- Erdmann, B.; Kirsch, F.-P.; Rathjen, F.G.; Moré, M.I. N-Cadherin Is Essential for Retinal Lamination in the Zebrafish. Dev. Dyn. 2003, 226, 570–577. [Google Scholar] [CrossRef]
- László, Z.I.; Lele, Z. Flying under the Radar: CDH2 (N-Cadherin), an Important Hub Molecule in Neurodevelopmental and Neurodegenerative Diseases. Front. Neurosci. 2022, 16, 972059. [Google Scholar] [CrossRef]
- Helmbacher, F. Fat1 Regulates Astrocyte Maturation and Angiogenesis in the Retina. bioRxiv 2020. [Google Scholar] [CrossRef]
- Forecki, J.; Morales, C.; Merzdorf, C. Trails to Research: An Inquiry-Based Course Using Zebrafish To Provide Research Experience to Tribal College Students. J. Microbiol. Biol. Educ. 2023, 24, e00243-22. [Google Scholar] [CrossRef]
- Baker, D.R.; Kasprzyk-Hordern, B. Multi-Residue Analysis of Drugs of Abuse in Wastewater and Surface Water by Solid-Phase Extraction and Liquid Chromatography–Positive Electrospray Ionisation Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 1620–1631. [Google Scholar] [CrossRef]
- Andresen, H.; Gullans, A.; Veselinovic, M.; Anders, S.; Schmoldt, A.; Iwersen-Bergmann, S.; Mueller, A. Fentanyl: Toxic or Therapeutic? Postmortem and Antemortem Blood Concentrations After Transdermal Fentanyl Application. J. Anal. Toxicol. 2012, 36, 182–194. [Google Scholar] [CrossRef]
- Kinnunen, M.; Piirainen, P.; Kokki, H.; Lammi, P.; Kokki, M. Updated Clinical Pharmacokinetics and Pharmacodynamics of Oxycodone. Clin. Pharmacokinet. 2019, 58, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.; Freeman, J. RNA Isolation from Embryonic Zebrafish and cDNA Synthesis for Gene Expression Analysis. J. Vis. Exp. 2009, 7, 1470. [Google Scholar] [CrossRef]
- Song, L.; Florea, L. Rcorrector: Efficient and Accurate Error Correction for Illumina RNA-Seq Reads. GigaScience 2015, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Danio Rerio Genome Assembly GRCz11. Available online: https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000002035.6/ (accessed on 10 December 2024).
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De Novo Transcript Sequence Reconstruction from RNA-Seq: Reference Generation and Analysis with Trinity. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. G: Profiler—Interoperable Web Service for Functional Enrichment Analysis and Gene Identifier Mapping (2023 Update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]
- Elizarraras, J.M.; Liao, Y.; Shi, Z.; Zhu, Q.; Pico, A.R.; Zhang, B. WebGestalt 2024: Faster Gene Set Analysis and New Support for Metabolomics and Multi-Omics. Nucleic Acids Res. 2024, 52, W415–W421. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 10 December 2024).
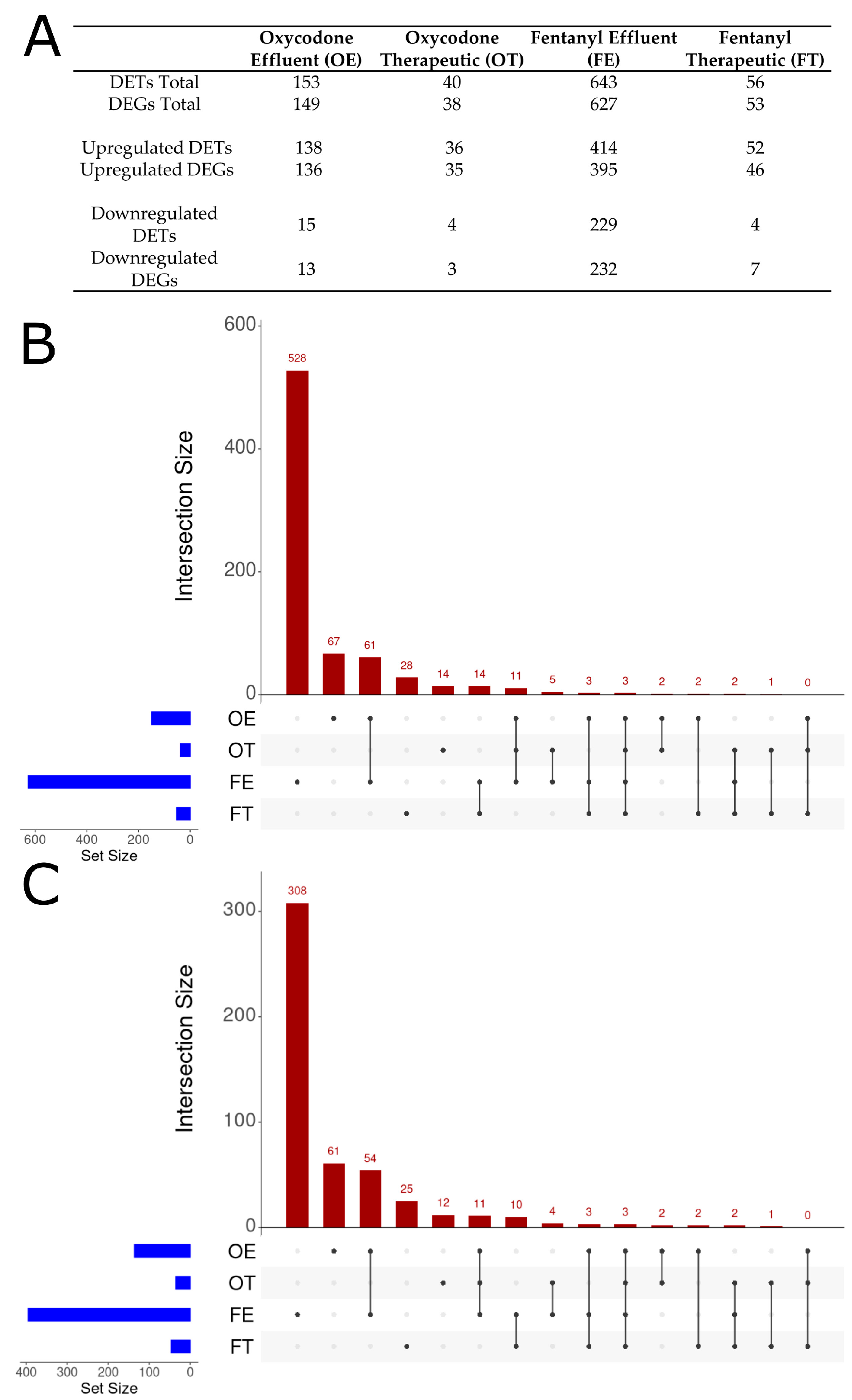

| A | Paired Reads Content Surviving Post-Trim (i) | Total Paired Sequences Post-Trim (ii) | % Successfully Mapped (iii) | |
|---|---|---|---|---|
| Control | 92.94% | 113,705,405 | 60.57% | |
| Oxycodone Effluent (OE) | 93.69% | 109,595,918 | 66.62% | |
| Oxycodone Therapeutic (OT) | 93.86% | 113,560,151 | 65.99% | |
| Fentanyl Effluent (FE) | 92.48% | 97,358,043 | 70.8% | |
| Fentanyl Therapeutic (FT) | 92.68% | 123,727,632 | 69.24% | |
| B | Oxycodone Effluent (OE) | Oxycodone Therapeutic (OT) | Fentanyl Effluent (FE) | Fentanyl Therapeutic (FT) |
| Total number of upregulated DETs | 138 | 36 | 414 | 52 |
| Average upregulated log2FC | 6.75 ± 8.72 | 10.47 ± 8.68 | 4.84 ± 6.92 | 4.37 ± 7.91 |
| Total number of downregulated DETs | 15 | 4 | 229 | 4 |
| Average downregulated log2FC | −2.88 ± 7.02 | −18.54 ± 9.25 | −1.08 ± 1.72 | −0.61 ± 0.75 |
| Total number of DETs compared to control | 153 | 40 | 643 | 56 |
| Overall average log2FC | 5.63 ± 9.05 | 7.29 ± 12.07 | 2.6 ± 6.25 | 4.13 ± 7.77 |
| Gene Name | Description | Condition |
|---|---|---|
| ECM-Related | ||
| col I, II, IV, V VII, IX, XI, XII, XVII, XXVII, XXVIII | See Table S1A | FE |
| col IV, V, VII, XXVIII, | See Table S1A | OE |
| col VII, XVII, XXVIII | See Table S1A | FT |
| adamts2 | disintegrin and metalloproteinase; exercises N-propeptide of fibrillar procollagens type I, II, III, and V | FE |
| VIT | vitrin; ECM protein associated with cell adhesion and migration; expressed highly in the brain | FE |
| postn | periostin; ECM protein associated with regeneration, tissue development, binds to integrins | FE |
| si:ch211-196i2.1 | predicted ECM structural protein; predicted involvement in ECM organization | FE |
| zgc:113232 | predicted ECM structural protein; predicted involvement in ECM organization | FE |
| creb3l2 | cAMP responsive element binding protein 3-like 2; transcriptional activator | FE, OE |
| nfkbiaa | NFKB inhibitor alpha; interacts with REL dimers to inhibit NFKB/REL complex | FE |
| nfkbib | NFKB inhibitor beta; complexes with NFKB and sequesters to cytoplasm | FE |
| nlrx1 | NLR family member X1; enhances NFKB kinase dependent signaling | FE, OE |
| traf2b | TNF Receptor Associated Factor; regulates activation of NFKA bad JNK signaling | OE |
| Cell Adhesion | ||
| actb | Actin beta; forms actin filaments essential for cytoskeletal function | FE, OE |
| nexn | Nexilin F-actin binding protein; binds filamentous actin; involved in cell migration and adhesion; important in muscle tissue | OE |
| mapk8 and 14 | Mitogen-activated protein kinase; cellular kinase important for proliferation, differentiation, apoptosis | FE |
| pak6b | P21 (RAC1) activated kinase 6; broad cellular function important to cytoskeletal rearrangement, apoptosis, MAPK signaling, and integrin signaling | OE |
| pak2b | P21 (RAC1) activated kinase 2; effector kinase linking Rho GTPases to cytoskeleton reorganization | FE |
| prkcba | Protein kinase C beta a; broad cellular function involved in B cell activation, apoptosis, endothelial cell proliferation, and metabolism | FE |
| pxn | Paxillin; cytoskeletal protein involved in actin-membrane attachment sites of cell adhesion to ECM | FE |
| rac2 | Rac family small GTPase 2; GTP metabolizing protein important to secretion, phagocytosis, cell polarization | OE |
| thbs2b | Thrombospondin 2; glycoprotein that mediates cell–cell and cell–ECM interactions | FE |
| tncb | tenascin Cb; acts upstream of axon development, synaptic assembly, collagen ECM regulation, and neuron projection | OE |
| pard3ab | Par-3 Family Cell Polarity regulator alpha; important for asymmetrical cell division, polarized cell growth, tight junction assembly | FE, OE |
| tcf7l2 | Transcription factor 7 like 2; key role in Wnt signaling pathway and blood homeostasis | FE, OE |
| ctnna2 | Catenin alpha 2; enables actin filament binding and regulation of Arp2/3 complex function; implicated in nervous system function | FE |
| ncam1a | Neural cell adhesion molecule 1; cell adhesion protein involved in cell–cell and cell–ECM interactions; implicated in development of nervous system and nervous system function | FE |
| cdh2 | Cadherin 2 (N-type); calcium dependent cell adhesion molecule and glycoprotein; important for left-right axis, nervous system, and cartilage development | FE |
| prcdh1g30 | Protocadherin 1; membrane protein found at cell–cell boundaries; involved in neural cell adhesion and development | OE |
| fat1a | FAT atypical cadherin 1; adhesion/signaling molecule implicated in cell proliferation | FE, FT, OE |
| Lens and Visual Development | ||
| crygm2d 2, 3, 10, 12, 17 | See Table S1B | OT |
| crygm2d 2, 3, 12, 17 | See Table S1B | OE |
| crygm2d 1, 2, 5, 9, 12, 14, 16 | See Table S1B | FT |
| cryba2b, crygm2d 1, 2, 3, 4, 5, 7, 9, 12, 13, 14, 15, 16, 17, 20 | See Table S1B | FE |
| cyfip2 | Cytoplasmic FMR1 interacting protein 2; cell–cell adhesion and post-synapse assembly | FE |
| rtn4a | Reticulon 4; neuroendocrine secretion and membrane trafficking in neuroendocrine cells; neurite outgrowth inhibitor; regeneration of CNS | FE |
| smarca4 | SWI/SNF-related BAF chromatin remodeling complex subunit ATPase 4; helicase and ATPase activities implicated in neural stem cell renewal and proliferation as well as broad neuronal function | FE |
| insm1a | INSM transcriptional repressor 1a; neuroendocrine differentiation and promotes neuronal basal progenitor cells | FE |
| meis1a | Meis homeobox 1a; nervous system development and vascular patterning | FE |
| mib1 | MIB E3 ubiquitin protein ligase 1; positively regulates NOTCH signaling; important for ubiquitination and apoptosis | FE |
| Axon Guidance and Synapse Formation | ||
| plxnb2a | Plexin B2a; transmembrane receptors important for axon guidance and cell migration; responsive to semaphorins | FE |
| rabgap1l | RAB GTPase activation protein 1-like; enables GTPase activator activity and GTPase binding activity; implicated in cell migration | FE |
| rab33a | RAB33A, member RAS Oncogene Family; small GTPase superfamily that binds GTP and is involved in vesicle transport | FE |
| rab3aa | RAB3A, Member RAS oncogene family; neurotransmitter release cycle, exocytosis, and plasma membrane repair | FE |
| gpm6bb | Glycoprotein M6B; membrane glycoprotein expressed in the brain; involved in membrane trafficking and cell–cell communication | FE |
| sfpq | Splicing Factor Proline and Glutamine Rich; broad cellular functions relating to gene expression; binds histone deacetylases and spliceosome binding | FE |
| tiam1 | TIAM Rac1 Associated GEF 1; mediates exchange of GDP for GTP implicated in broad functions including cell shape, migration, adhesion, growth, and polarity | FE |
| vasp | Vasodilator Stimulated Phosphoprotein; implicated in focal adhesion, F-actin formation, cell adhesion, and motility | FE |
| synj1 | Synaptojanin 1; regulates levels of membrane phosphatidylinositol-4,5-bisphosphate; implicated in synaptic transmission and membrane trafficking | FE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
North, R.J.; Cooper, G.; Mears, L.; Bothner, B.; Dlakić, M.; Merzdorf, C.S. Persistent Transcriptome Alterations in Zebrafish Embryos After Discontinued Opioid Exposure. Int. J. Mol. Sci. 2025, 26, 4840. https://doi.org/10.3390/ijms26104840
North RJ, Cooper G, Mears L, Bothner B, Dlakić M, Merzdorf CS. Persistent Transcriptome Alterations in Zebrafish Embryos After Discontinued Opioid Exposure. International Journal of Molecular Sciences. 2025; 26(10):4840. https://doi.org/10.3390/ijms26104840
Chicago/Turabian StyleNorth, Ryan J., Gwendolyn Cooper, Lucas Mears, Brian Bothner, Mensur Dlakić, and Christa S. Merzdorf. 2025. "Persistent Transcriptome Alterations in Zebrafish Embryos After Discontinued Opioid Exposure" International Journal of Molecular Sciences 26, no. 10: 4840. https://doi.org/10.3390/ijms26104840
APA StyleNorth, R. J., Cooper, G., Mears, L., Bothner, B., Dlakić, M., & Merzdorf, C. S. (2025). Persistent Transcriptome Alterations in Zebrafish Embryos After Discontinued Opioid Exposure. International Journal of Molecular Sciences, 26(10), 4840. https://doi.org/10.3390/ijms26104840






