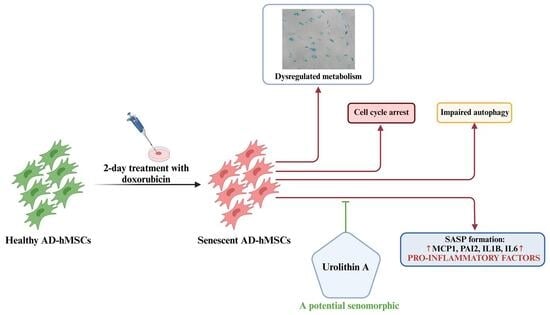
Urolithin A Alleviates Doxorubicin-Induced Senescence in Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results
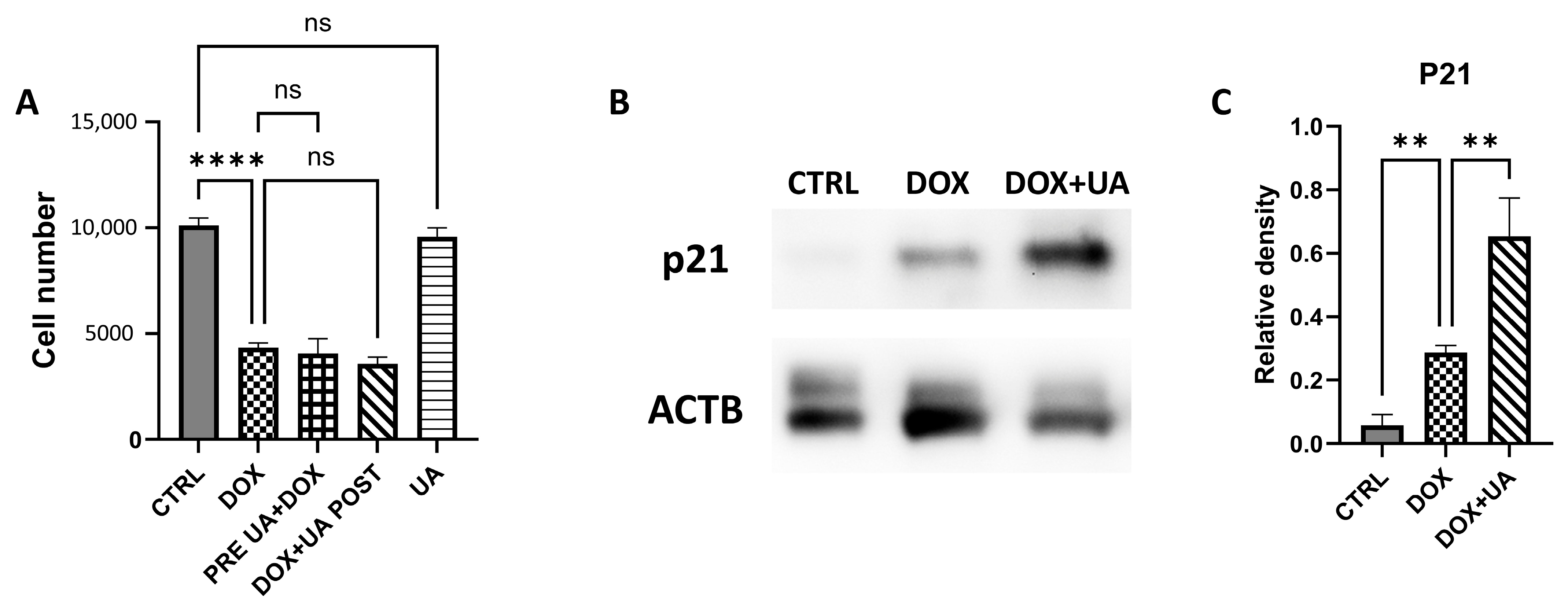
2.1. Urolithin A Does Not Affect Proliferation of AD-hMSCs
2.2. Urolithin A Does Not Alleviate Senescence-Associated Lysosomal Dysfunction
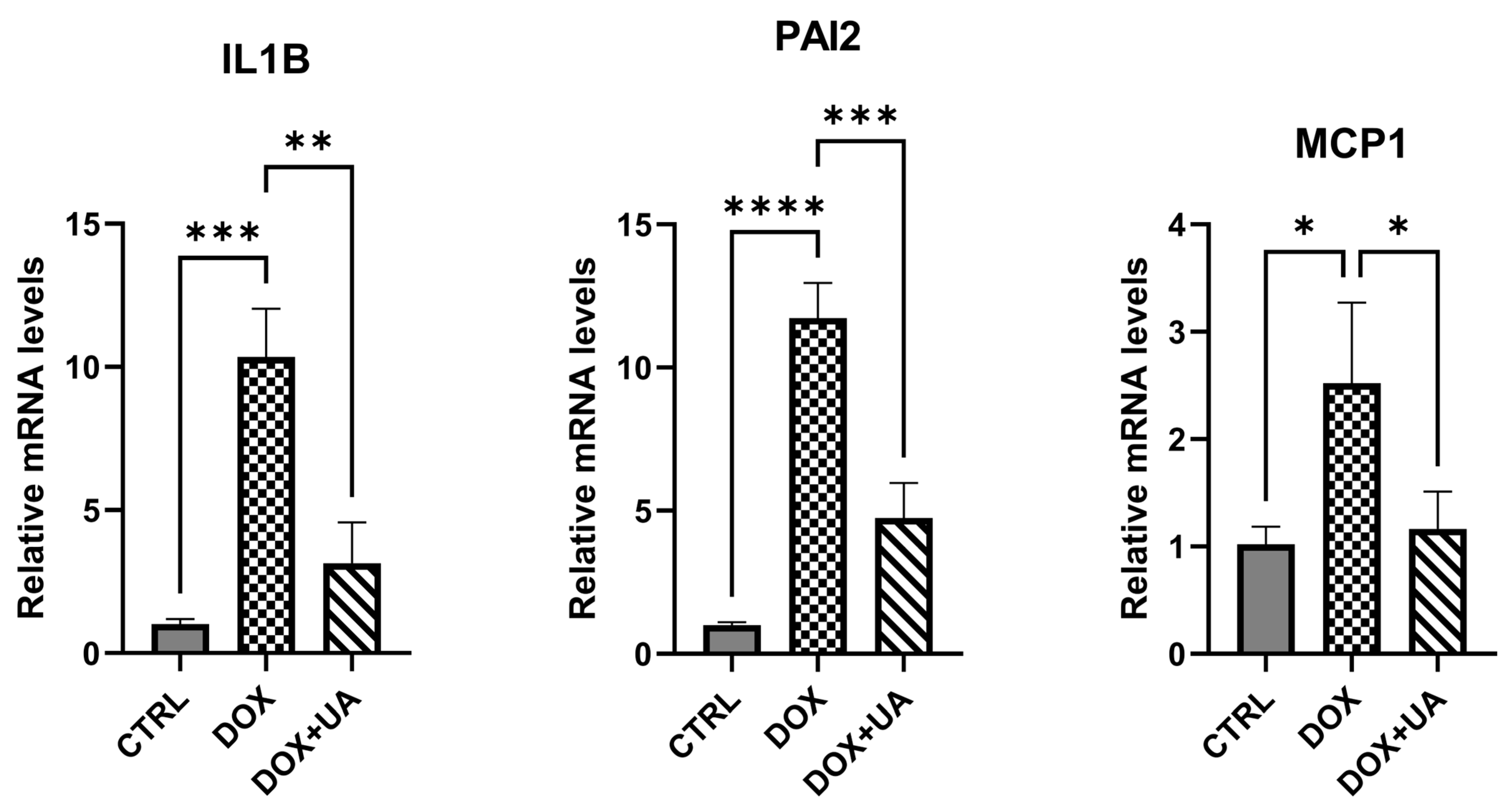
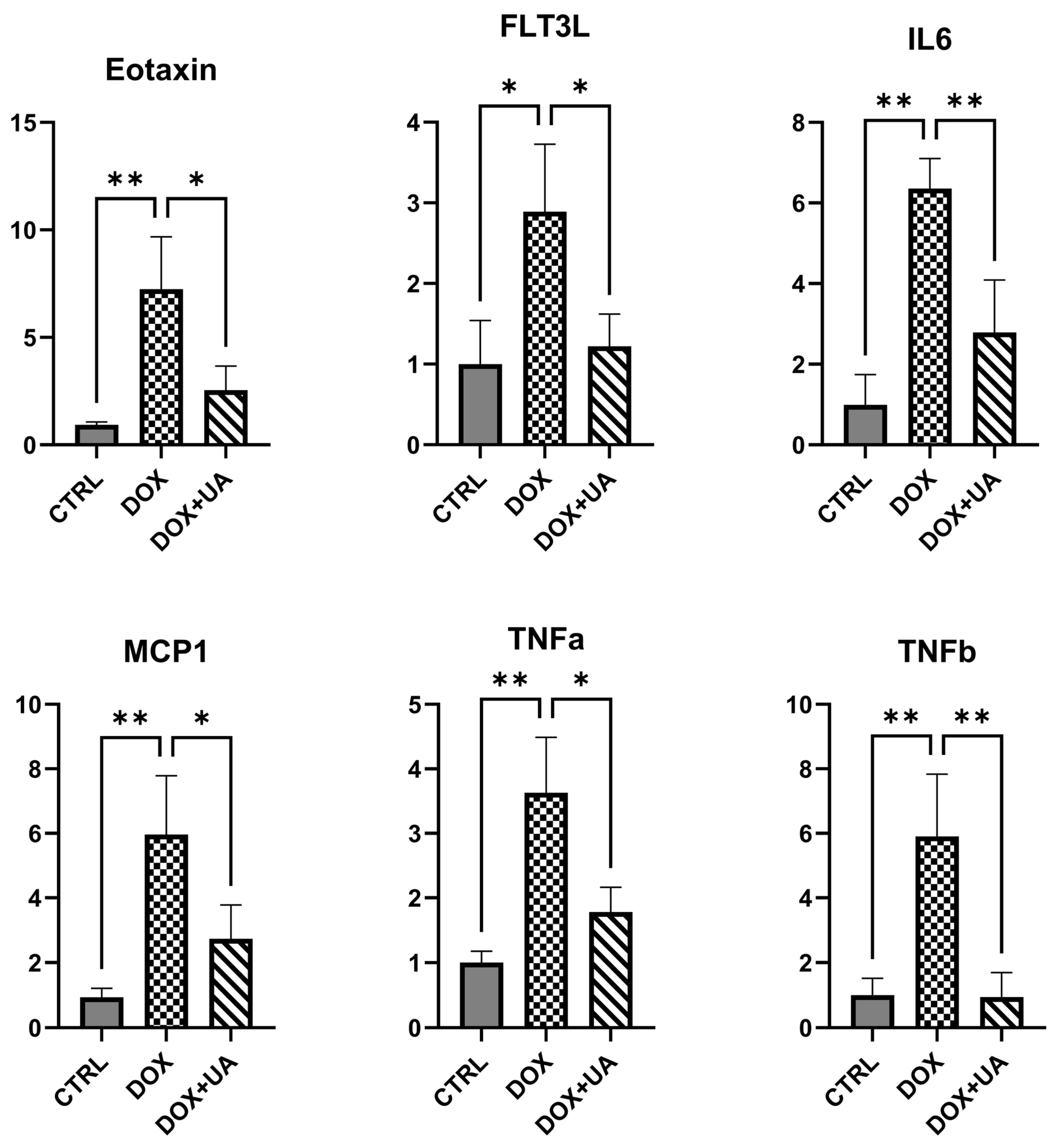
2.3. Urolithin A Suppresses the Secretory Phenotype of Senescent AD-hMSCs
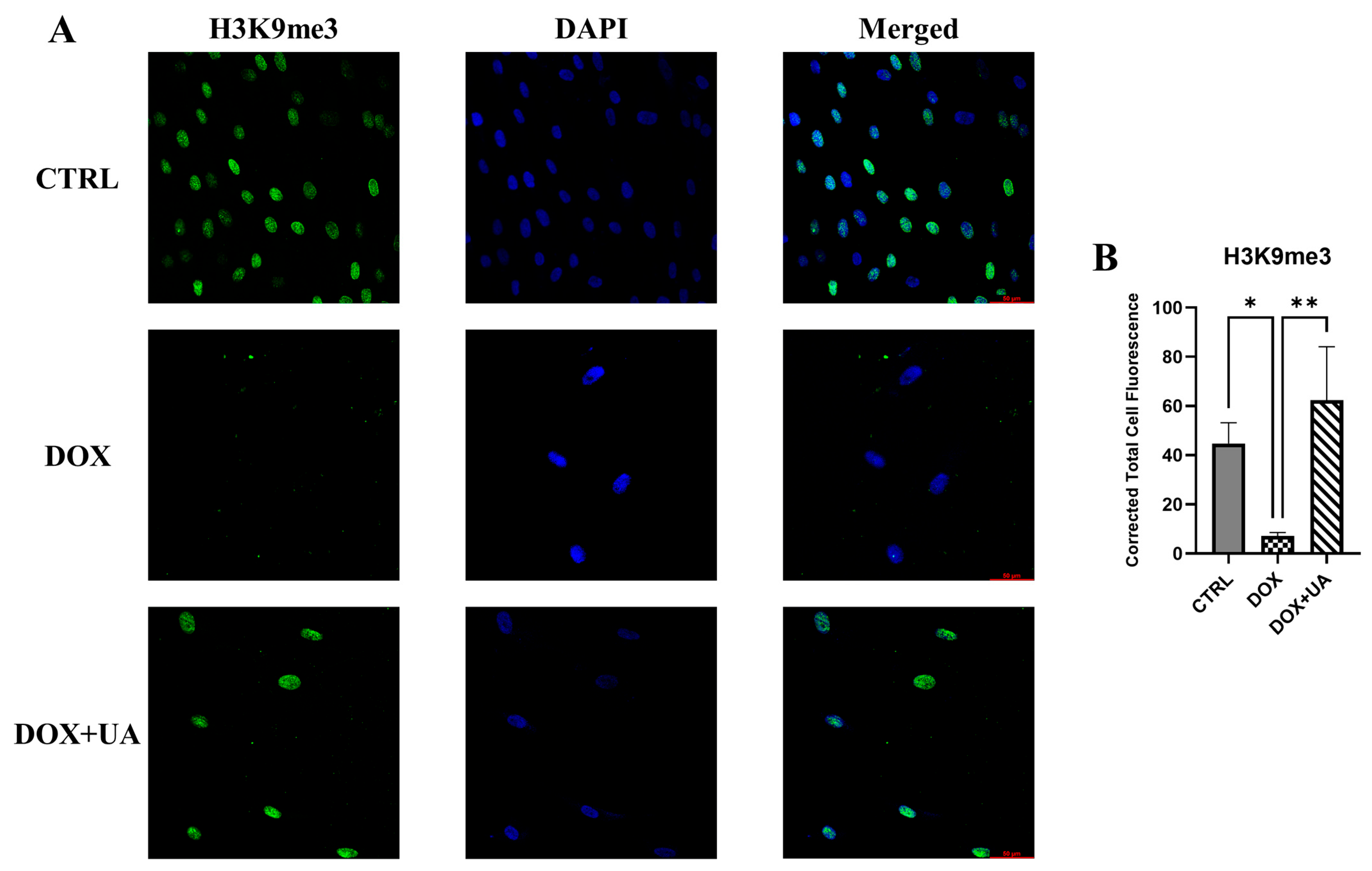
2.4. Urolithin A Restores Levels of H3K9me3 in Senescent AD-hMSCs
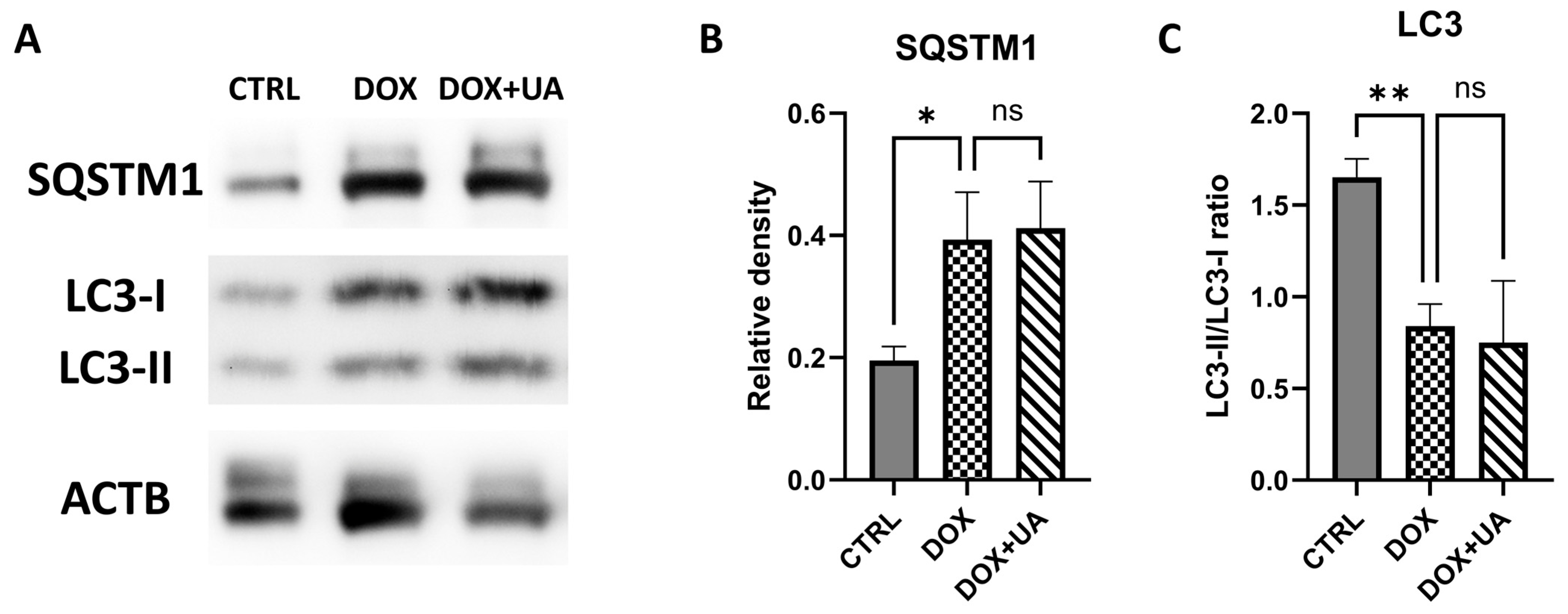
2.5. Doxorubicin-Induced Senescence in AD-hMSCs Is Characterized by Decreased Autophagy
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Cell Viability Assay
4.3. Senescence-Associated Beta-Galactosidase (SA-βGal) Staining
4.4. Western Blot Analysis
4.5. Real-Time Polymerase Chain Reaction (RT-qPCR)
4.6. Multiplex Immunoassay (Magpix)
4.7. Immunofluorescent Staining of AD-hMSCs
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD-hMSCs | Adipose-derived human mesenchymal stem cells |
| CCK8 | Cell counting kit-8 |
| CTRL | Control |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified eagle medium |
| DOX | Doxorubicin |
| ER-stress | Endoplasmic reticulum stress |
| FLT3L | FMS-Like Tyrosine kinase 3 Ligand |
| H3K9me3 | Trimethylated histone H3 Lysine 9 |
| IL1B | Interleukin 1 Beta |
| IL6 | Interleukin 6 |
| LC3-I | Light Chain protein 3 form 1 |
| LC3-II | Light Chain protein 3 form 2 |
| MCP1 | Monocyte chemoattractant protein-1 |
| MSCs | Mesenchymal stem cells |
| ns | not significant |
| PAI2 | Plasminogen Activator Inhibitor 2 |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| RIPA | Radioimmunoprecipitation assay |
| RT | Room temperature |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SASP | Senescence-associated secretory phenotype |
| SA-βGal | Senescence-associated beta-galactosidase |
| SD | Standard seviation |
| SQSTM1 | Sequestosome 1 |
| TNFa | Tumor necrosis factor alpha |
| TNFb | Tumor necrosis factor beta |
| UA | Urolithin A |
References
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and Cancer—Role and Therapeutic Opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Saito, Y.; Yamamoto, S.; Chikenji, T.S. Role of Cellular Senescence in Inflammation and Regeneration. Inflamm. Regen. 2024, 44, 28. [Google Scholar] [CrossRef]
- Lee, K.-A.; Flores, R.R.; Jang, I.H.; Saathoff, A.; Robbins, P.D. Immune Senescence, Immunosenescence and Aging. Front. Aging 2022, 3, 900028. [Google Scholar] [CrossRef]
- Kim, E.-C.; Kim, J.-R. Senotherapeutics: Emerging Strategy for Healthy Aging and Age-Related Disease. BMB Rep. 2019, 52, 47–55. [Google Scholar] [CrossRef]
- Yi, G.; He, Z.; Zhou, X.; Xian, L.; Yuan, T.; Jia, X.; Hong, J.; He, L.; Liu, J. Low Concentration of Metformin Induces a P53-Dependent Senescence in Hepatoma Cells via Activation of the AMPK Pathway. Int. J. Oncol. 2013, 43, 1503–1510. [Google Scholar] [CrossRef]
- Cheng, F.-F.; Liu, Y.-L.; Du, J.; Lin, J.-T. Metformin’s Mechanisms in Attenuating Hallmarks of Aging and Age-Related Disease. Aging Dis. 2022, 13, 970–986. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- Cho, S.I.; Jo, E.-R.; Song, H. Urolithin A Attenuates Auditory Cell Senescence by Activating Mitophagy. Sci. Rep. 2022, 12, 7704. [Google Scholar] [CrossRef]
- Shi, P.-Z.; Wang, J.-W.; Wang, P.-C.; Han, B.; Lu, X.-H.; Ren, Y.-X.; Feng, X.-M.; Cheng, X.-F.; Zhang, L. Urolithin a Alleviates Oxidative Stress-Induced Senescence in Nucleus Pulposus-Derived Mesenchymal Stem Cells through SIRT1/PGC-1α Pathway. World J. Stem Cells 2021, 13, 1928–1946. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Zhang, Z.; Qiu, L.; Ding, S.; Xue, J.; Zhao, G.; Li, J. Antiaging Effects of Urolithin A on Replicative Senescent Human Skin Fibroblasts. Rejuvenation Res. 2019, 22, 191–200. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Senolytics and Senomorphics. FEBS J. 2022, 290, 1–22. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A New Gene Set Identifies Senescent Cells and Predicts Senescence-Associated Pathways across Tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef]
- Boncela, J.; Przygodzka, P.; Papiewska-Pajak, I.; Wyroba, E.; Cierniewski, C.S. Association of Plasminogen Activator Inhibitor Type 2 (PAI-2) with Proteasome within Endothelial Cells Activated with Inflammatory Stimuli. J. Biol. Chem. 2011, 286, 43164–43171. [Google Scholar] [CrossRef]
- Hsieh, H.-H.; Chen, Y.-C.; Jhan, J.-R.; Lin, J.-J. The Serine Protease Inhibitor SerpinB2 Binds and Stabilizes P21 in Senescent Cells. J. Cell Sci. 2017, 130, 3272–3281. [Google Scholar] [CrossRef]
- Lavandoski, P.; Pierdoná, V.; Maurmann, R.M.; Grun, L.K.; Guma, F.T.C.R.; Barbé-Tuana, F.M. Eotaxin-1/CCL11 Promotes Cellular Senescence in Human-Derived Fibroblasts through pro-Oxidant and pro-Inflammatory Pathways. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Guermonprez, P.; Helft, J.; Claser, C.; Deroubaix, S.; Karanje, H.; Gazumyan, A.; Darasse-Jèze, G.; Telerman, S.B.; Breton, G.; Schreiber, H.A.; et al. Inflammatory Flt3l Is Essential to Mobilize Dendritic Cells and for T Cell Responses during Plasmodium Infection. Nat. Med. 2013, 19, 730–738. [Google Scholar] [CrossRef]
- Dasgupta, N.; Arnold, R.; Equey, A.; Gandhi, A.; Adams, P.D. The Role of the Dynamic Epigenetic Landscape in Senescence: Orchestrating SASP Expression. npj Aging 2024, 10, 48. [Google Scholar] [CrossRef]
- Gadecka, A.; Koblowska, M.; Kossowska, H.; Iwanicka-Nowicka, R.; Janiszewska, D.; Mosieniak, G.; Bojakowski, K.; Goryca, K.; Bielak-Zmijewska, A. Senescence-Associated Alterations in Histone H3 Modifications, HP1 Alpha Levels and Distribution, and in the Transcriptome of Vascular Smooth Muscle Cells in Different Types of Senescence. Cell Commun. Signal. 2025, 23, 321. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, J.W.; Jeoung, J.A.; Kim, M.-S.; Kang, C. Autophagy Is Pro-Senescence When Seen in Close-Up, but Anti-Senescence in Long-Shot. Mol. Cells 2017, 40, 607–612. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The Mitophagy Activator Urolithin A Is Safe and Induces a Molecular Signature of Improved Mitochondrial and Cellular Health in Humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- El-Wetidy, M.S.; Ahmad, R.; Rady, I.; Helal, H.; Rady, M.I.; Vaali-Mohammed, M.-A.; Al-Khayal, K.; Traiki, T.B.; Abdulla, M.-H. Urolithin A Induces Cell Cycle Arrest and Apoptosis by Inhibiting Bcl-2, Increasing P53-P21 Proteins and Reactive Oxygen Species Production in Colorectal Cancer Cells. Cell Stress Chaperones 2021, 26, 473–493. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Ciudad, C.J.; Izquierdo-Pulido, M.; Noé, V. Urolithin A Causes P21 Up-Regulation in Prostate Cancer Cells. Eur. J. Nutr. 2016, 55, 1099–1112. [Google Scholar] [CrossRef]
- Chandra, A.; Lagnado, A.B.; Farr, J.N.; Doolittle, M.; Tchkonia, T.; Kirkland, J.L.; LeBrasseur, N.K.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. Targeted Clearance of P21-but Not P16-positive Senescent Cells Prevents Radiation-induced Osteoporosis and Increased Marrow Adiposity. Aging Cell 2022, 21, e13602. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, T.; Huang, X.; Lin, Y.; Chen, B.; Pang, J.; Li, G.; Wang, Q.; Zohrabian, S.; Duan, C.; et al. Astragalus Polysaccharide Restores Autophagic Flux and Improves Cardiomyocyte Function in Doxorubicin-Induced Cardiotoxicity. Oncotarget 2017, 8, 4837–4848. [Google Scholar] [CrossRef]
- Kim, D.-G.; Jung, K.H.; Lee, D.-G.; Yoon, J.-H.; Choi, K.S.; Kwon, S.W.; Shen, H.-M.; Morgan, M.J.; Hong, S.-S.; Kim, Y.-S. 20(S)-Ginsenoside Rg3 Is a Novel Inhibitor of Autophagy and Sensitizes Hepatocellular Carcinoma to Doxorubicin. Oncotarget 2014, 5, 4438–4451. [Google Scholar] [CrossRef]
- Li, D.L.; Wang, Z.V.; Ding, G.; Tan, W.; Luo, X.; Criollo, A.; Xie, M.; Jiang, N.; May, H.; Kyrychenko, V.; et al. Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification. Circulation 2016, 133, 1668–1687. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to Detect Senescence-Associated Beta-Galactosidase (SA-Βgal) Activity, a Biomarker of Senescent Cells in Culture and in Vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Degasperi, A.; Birtwistle, M.R.; Volinsky, N.; Rauch, J.; Kolch, W.; Kholodenko, B.N. Evaluating Strategies to Normalise Biological Replicates of Western Blot Data. PLoS ONE 2014, 9, e87293. [Google Scholar] [CrossRef]
- Gavet, O.; Pines, J. Progressive Activation of CyclinB1-Cdk1 Coordinates Entry to Mitosis. Dev. Cell 2010, 18, 533–543. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Forward | Reverse |
|---|---|---|
| ACTB | CCTGGCACCCAGCACAAT | GGGCCGGACTCGTCATAC |
| IL1B | ACAGATGAAGTGCTCCTTCCA | GTCGGAGATTCGTAGCTGGAT |
| MCP1 | CAGCCAGATGCAATCAATGCC | TGGAATCCTGAACCCACTTCT |
| PAI2 | CATGGAGCATCTCGTCCAC | ACTGCATTGGCTCCCACTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinin, A.; Zubkova, E.; Menshikov, M.; Parfyonova, Y. Urolithin A Alleviates Doxorubicin-Induced Senescence in Mesenchymal Stem Cells. Int. J. Mol. Sci. 2025, 26, 10257. https://doi.org/10.3390/ijms262110257
Kalinin A, Zubkova E, Menshikov M, Parfyonova Y. Urolithin A Alleviates Doxorubicin-Induced Senescence in Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2025; 26(21):10257. https://doi.org/10.3390/ijms262110257
Chicago/Turabian StyleKalinin, Alexander, Ekaterina Zubkova, Mikhail Menshikov, and Yelena Parfyonova. 2025. "Urolithin A Alleviates Doxorubicin-Induced Senescence in Mesenchymal Stem Cells" International Journal of Molecular Sciences 26, no. 21: 10257. https://doi.org/10.3390/ijms262110257
APA StyleKalinin, A., Zubkova, E., Menshikov, M., & Parfyonova, Y. (2025). Urolithin A Alleviates Doxorubicin-Induced Senescence in Mesenchymal Stem Cells. International Journal of Molecular Sciences, 26(21), 10257. https://doi.org/10.3390/ijms262110257