Adipose Tissue Dysfunction and Metabolic Diseases: The Role of Vitamin D/Vitamin D Receptor Axis
Abstract
1. Introduction
2. Adipose Tissue and Metabolic Diseases
2.1. The Adipose Tissue as an Endocrine Organ
2.2. Sick Fat and Metabolic Impairment
3. The Vitamin D/Vitamin D Receptor Axis in Metabolic Regulation
3.1. Vitamin D and VDR: General Overview
3.2. VD/VDR in Metabolic Diseases: Experimental Evidence
3.3. Local Regulation of Vitamin D Metabolism Within Adipose Tissue
4. VD/VDR Axis and the Adipose Tissue
4.1. Pathways Involved in Adipose Tissue Homeostasis
4.2. From Physiology to Metabolic Impairment
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AT | Adipose Tissue |
| ATEs | Adipose Tissue Eosinophils |
| BMI | Body Mass Index |
| CoAs | Co-activators |
| CoRs | Co-repressors |
| CYP | Cytochrome |
| DBD | DNA-Binding Domain |
| ECM | Extracellular Matrix |
| FA | Fatty Acid |
| FABP4 | Fatty Acid Binding Protein 4 |
| HF | High-Fat |
| IL | Interleukin |
| ILC2s | Group 2 Innate Lymphoid Cells |
| LBD | Ligand-Binding Domain |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MSC | Mesenchymal Stem Cells |
| nVDRE | Negative Vitamin D Response Element |
| RXR | Retinoid X Receptor |
| SAT | Subcutaneous Adipose Tissue |
| SVF | Stromal Vascular Fraction |
| T2D | Type 2 Diabetes |
| TNFα | Tumor Necrosis Factor Alpha |
| Treg | Regulatory T Cells |
| UCP | Uncoupling Proteins (UCP1, UCP2, UCP3) |
| VAT | Visceral Adipose Tissue |
| VD | Vitamin D |
| VDR | Vitamin D Receptor |
| VDRE | Vitamin D Response Element |
| 25(OH)D | 25-hydroxyvitamin D |
| 1,25(OH)2D/1α,25(OH)2D3 | Calcitriol |
References
- Lyall, D.M.; Celis-Morales, C.; Ward, J.; Iliodromiti, S.; Anderson, J.J.; Gill, J.M.R.; Smith, D.J.; Ntuk, U.E.; Mackay, D.F.; Holmes, M.V.; et al. Association of Body Mass Index with Cardiometabolic Disease in the UK Biobank: A Mendelian Randomization Study. JAMA Cardiol. 2017, 2, 882–889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trayhurn, P. Endocrine and signalling role of adipose tissue: New perspectives on fat. Acta Physiol. Scand. 2005, 184, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Konradsen, S.; Ag, H.; Lindberg, F.; Hexeberg, S.; Jorde, R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur. J. Nutr. 2008, 47, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, J.; Broch, M.; Vilarrasa, N.; Molina, A.; Gómez, J.M.; Gutiérrez, C.; Simón, I.; Soler, J.; Richart, C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: Relationships in obesity. Obes. Res. 2004, 12, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Than, A.; He, H.L.; Chua, S.H.; Xu, D.; Sun, L.; Leow, M.K.; Chen, P. Apelin Enhances Brown Adipogenesis and Browning of White Adipocytes. J. Biol. Chem. 2015, 290, 14679–14691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bełtowski, J. Apelin and visfatin: Unique “beneficial” adipokines upregulated in obesity? Med. Sci. Monit. 2006, 12, RA112–RA119. [Google Scholar] [PubMed]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919; quiz 920. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Mahlakõiv, T.; Flamar, A.L.; Johnston, L.K.; Moriyama, S.; Putzel, G.G.; Bryce, P.J.; Artis, D. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci. Immunol. 2019, 4, eaax0416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hildreth, A.D.; Ma, F.; Wong, Y.Y.; Sun, R.; Pellegrini, M.; O’Sullivan, T.E. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat. Immunol. 2021, 22, 639–653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, X.; Qian, X.; Ding, X.; Wang, W.; Yin, X.; Zhuang, G.; Zeng, W. Eosinophils regulate intra-adipose axonal plasticity. Proc. Natl. Acad. Sci. USA 2022, 119, e2112281119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, W.; Qi, Y.; Yi, H.; Mao, C.; Meng, Q.; Wang, H.; Zheng, C. The Roles of Adipose Tissue Macrophages in Human Disease. Front. Immunol. 2022, 13, 908749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brestoff, J.R.; Kim, B.S.; Saenz, S.A.; Stine, R.R.; Monticelli, L.A.; Sonnenberg, G.F.; Thome, J.J.; Farber, D.L.; Lutfy, K.; Seale, P.; et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015, 519, 242–246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rana, B.M.J.; Jou, E.; Barlow, J.L.; Rodriguez-Rodriguez, N.; Walker, J.A.; Knox, C.; Jolin, H.E.; Hardman, C.S.; Sivasubramaniam, M.; Szeto, A.; et al. A stromal cell niche sustains ILC2-mediated type-2 conditioning in adipose tissue. J. Exp. Med. 2019, 216, 1999–2009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Yang, P.; Cui, R.; Zhang, M.; Li, H.; Qian, C.; Sheng, C.; Qu, S.; Bu, L. Eosinophils Reduce Chronic Inflammation in Adipose Tissue by Secreting Th2 Cytokines and Promoting M2 Macrophages Polarization. Int. J. Endocrinol. 2015, 2015, 565760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kunz, H.E.; Hart, C.R.; Gries, K.J.; Parvizi, M.; Laurenti, M.; Man, C.D.; Moore, N.; Zhang, X.; Ryan, Z.; Polley, E.C.; et al. Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. Am. J. Physiol.-Endocrinol. Metab. 2021, 321, E105–E121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Ciccarelli, G.; Baroni, M.G.; Cavallo, M.G. Sick fat: The good and the bad of old and new circulating markers of adipose tissue inflammation. J. Endocrinol. Investig. 2019, 42, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Divoux, A.; Tordjman, J.; Lacasa, D.; Veyrie, N.; Hugol, D.; Aissat, A.; Basdevant, A.; Guerre-Millo, M.; Poitou, C.; Zucker, J.D.; et al. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. Diabetes 2010, 59, 2817–2825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arner, P.; Bernard, S.; Salehpour, M.; Possnert, G.; Liebl, J.; Steier, P.; Buchholz, B.A.; Eriksson, M.; Arner, E.; Hauner, H.; et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 2011, 478, 110–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.-I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, C.Y.; Han, S.N. The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation. J. Lipid Atheroscler. 2021, 10, 130–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lontchi-Yimagou, E.; Kang, S.; Goyal, A.; Zhang, K.; You, J.Y.; Carey, M.; Jain, S.; Bhansali, S.; Kehlenbrink, S.; Guo, P.; et al. Insulin-sensitizing effects of vitamin D repletion mediated by adipocyte vitamin D receptor: Studies in humans and mice. Mol. Metab. 2020, 42, 101095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLOS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tushuizen, M.E.; Bunck, M.C.; Pouwels, P.J.; Bontemps, S.; van Waesberghe, J.H.T.; Schindhelm, R.K.; Mari, A.; Heine, R.J.; Diamant, M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007, 30, 2916–2921, Erratum in Diabetes Care 2008, 31, 835. [Google Scholar] [CrossRef] [PubMed]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. Diabetes 2007, 56, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Festa, A.; D’Agostino, R., Jr.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336, Erratum in Nature 2023, 619, E25. https://doi.org/10.1038/s41586-023-06285-0. [Google Scholar] [CrossRef] [PubMed]
- Verboven, K.; Wouters, K.; Gaens, K.; Hansen, D.; Bijnen, M.; Wetzels, S.; Stehouwer, C.D.; Goossens, G.H.; Schalkwijk, C.G.; Blaak, E.E.; et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci. Rep. 2018, 8, 4677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rakotoarivelo, V.; Lacraz, G.; Mayhue, M.; Brown, C.; Rottembourg, D.; Fradette, J.; Ilangumaran, S.; Menendez, A.; Langlois, M.F.; Ramanathan, S. Inflammatory Cytokine Profiles in Visceral and Subcutaneous Adipose Tissues of Obese Patients Undergoing Bariatric Surgery Reveal Lack of Correlation with Obesity or Diabetes. eBioMedicine 2018, 30, 237–247, Erratum in eBioMedicine 2018, 37, 564–568. https://doi.org/10.1016/j.ebiom.2018.10.025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, K.; Walsh, J.P.; Hunter, M.; Murray, K.; Hui, J.; Hung, J. Longitudinal associations of DXA-measured visceral adipose tissue and cardiometabolic risk in middle-to-older aged adults. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2519–2527. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Haussler, C.A.; Jurutka, P.W.; Thompson, P.D.; Hsieh, J.C.; Remus, L.S.; Selznick, S.H.; Whitfield, G.K. The vitamin D hormone and its nuclear receptor: Molecular actions and disease states. J. Endocrinol. 1997, 154 (Suppl. S3), S57–S73. [Google Scholar] [PubMed]
- Baker, A.R.; McDonnell, D.P.; Hughes, M.; Crisp, T.M.; Mangelsdorf, D.J.; Haussler, M.R.; Pike, J.W.; Shine, J.; O'Malley, B.W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc. Natl. Acad. Sci. USA 1988, 85, 3294–3298. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Garrett, K.P.; Zhou, X.-Y.; Pike, J.W.; Haussler, M.R.; Dempster, D.W. Immunocytochemical localization of the 1,25-dihydroxyvitamin-D3 receptor in target cells. Endocrinology 1988, 122, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.C.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, W.E.; Sar, M.; Clark, S.A.; DeLuca, H.F. Brain target sites for 1,25-dihydroxyvitamin D3. Science 1982, 215, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Saccone, D.; Asani, F.; Bornman, L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene 2015, 561, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zella, L.A.; Kim, S.; Shevde, N.K.; Pike, J.W. Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol. Endocrinol. 2006, 20, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
- Zella, L.A.; Meyer, M.B.; Nerenz, R.D.; Lee, S.M.; Martowicz, M.L.; Pike, J.W. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol. Endocrinol. 2010, 24, 128–147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Clemens, T.L.; Parrish, J.A.; Holick, M.F. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N. Engl. J. Med. 1982, 306, 722–725. [Google Scholar] [CrossRef]
- Matsuoka, L.Y.; Ide, L.; Wortsman, J.; MacLaughlin, J.; Holick, M.F. Sunscreens suppress cutaneous vitamin D3 synthesis. J. Clin. Endocrinol. Metab. 1987, 64, 1165–1168. [Google Scholar] [CrossRef]
- Jorde, R.; Sneve, M.; Emaus, N.; Figenschau, Y.; Grimnes, G. Cross-sectional and longitudinal relation between serum 25-hydroxyvitamin D and body mass index: The Tromso study. Eur. J. Nutr. 2010, 49, 401–407. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Arai, H.; Miyamoto, K.; Taketani, Y.; Yamamoto, H.; Iemori, Y.; Morita, K.; Tonai, T.; Nishisho, T.; Mori, S.; Takeda, E. A vitamin D receptor gene polymorphism in the translation initiation codon: Effect on protein activity and relation to bone mineral density in Japanese women. J. Bone Miner. Res. 1997, 12, 915–921. [Google Scholar] [CrossRef]
- Bertoccini, L.; Sentinelli, F.; Leonetti, F.; Bailetti, D.; Capoccia, D.; Cimini, F.A.; Barchetta, I.; Incani, M.; Lenzi, A.; Cossu, E.; et al. The vitamin D receptor functional variant rs2228570 (C>T) does not associate with type 2 diabetes mellitus. Endocr. Res. 2017, 42, 331–335. [Google Scholar] [CrossRef]
- Arai, H.; Miyamoto, K.-I.; Yoshida, M.; Yamamoto, H.; Taketani, Y.; Morita, K.; Kubota, M.; Yoshida, S.; Ikeda, M.; Watabe, F.; et al. The polymorphism in the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. J. Bone Miner. Res. 2001, 16, 1256–1264. [Google Scholar] [CrossRef]
- Sentinelli, F.; Bertoccini, L.; Barchetta, I.; Capoccia, D.; Incani, M.; Pani, M.; Loche, S.; Angelico, F.; Arca, M.; Morini, S.; et al. The vitamin D receptor (VDR) gene rs11568820 variant is associated with type 2 diabetes and impaired insulin secretion in Italian adult subjects, and associates with increased cardio-metabolic risk in children. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 407–413. [Google Scholar] [CrossRef]
- Fang, Y.; van Meurs, J.B.; D'Alesio, A.; Jhamai, M.; Zhao, H.; Rivadeneira, F.; Hofman, A.; van Leeuwen, J.P.; Jehan, F.; Pols, H.A.; et al. Promoter and 3′-untranslated-region haplotypes in the vitamin D receptor gene predispose to osteoporotic fracture: The Rotterdam study. Am. J. Hum. Genet. 2005, 77, 807–823. [Google Scholar] [CrossRef]
- Mayr, C. Regulation by 3’-Untranslated Regions. Annu. Rev. Genet. 2017, 51, 171–194. [Google Scholar] [CrossRef]
- Mocharla, H.; Butch, A.W.; Pappas, A.A.; Flick, J.T.; Weinstein, R.S.; De Togni, P.; Jilka, R.L.; Roberson, P.K.; Parfitt, A.M.; Manolagas, S.C. Quantification of vitamin D receptor mRNA by competing polymerase chain reaction in PBMC: Lack of correspondence with common allelic variants. J. Bone Miner. Res. 1997, 12, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, W.; Gombart, A.F.; Shiohara, M.; Campbell, M.; Koeffler, H. Vitamin D receptor: No evidence for allele-specific mRNA stability in cells which are heterozygous for the Taq I restriction enzyme polymorphism. Biochem. Biophys. Res. Commun. 1997, 238, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Carling, T.; Rastad, J.; Åkerström, G.; Westin, G. Vitamin D receptor (VDR) and parathyroid hormone messenger ribonucleic acid levels correspond to polymorphic VDR alleles in human parathyroid tumors. J. Clin. Endocrinol. Metab. 1998, 83, 2255–2259. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Bendik, I.; Wyss, A.; Meier, E.; Sturzenbecker, L.J.; Grippo, J.F.; Hunziker, W. Two nuclear signalling pathways for vitamin D. Nature 1993, 361, 657–660. [Google Scholar] [CrossRef]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef]
- Burke, L.J.; Baniahmad, A. Co-repressors 2000. FASEB J. 2000, 14, 1876–1888. [Google Scholar] [CrossRef]
- Polly, P.; Herdick, M.; Moehren, U.; Baniahmad, A.; Heinzel, T.; Carlberg, C. VDR-Alien: A novel, DNA-selective vitamin D(3) receptor-corepressor partnership. FASEB J. 2000, 14, 1455–1463. [Google Scholar] [CrossRef]
- Tagami, T.; Lutz, W.H.; Kumar, R.; Jameson, J.L. The interaction of the vitamin D receptor with nuclear receptor corepressors and coactivators. Biochem. Biophys. Res. Commun. 1998, 253, 358–363. [Google Scholar] [CrossRef]
- Kim, M.S.; Fujiki, R.; Murayama, A.; Kitagawa, H.; Yamaoka, K.; Yamamoto, Y.; Mihara, M.; Takeyama, K.; Kato, S. 1α,25(OH)2D3-induced transrepression by vitamin D receptor through E-box-type elements in the human parathyroid hormone gene promoter. Mol. Endocrinol. 2007, 21, 334–342. [Google Scholar] [CrossRef]
- Russell, J.; Ashok, S.; Koszewski, N.J. Vitamin D receptor interactions with the rat parathyroid hormone gene: Synergistic effects between two negative vitamin D response elements. J. Bone Miner. Res. 1999, 14, 1828–1837. [Google Scholar] [CrossRef]
- Kamei, Y.; Kawada, T.; Kazuki, R.; Ono, T.; Kato, S.; Sugimoto, E. Vitamin D receptor gene expression is up-regulated by 1,25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 1993, 193, 948–955. [Google Scholar] [CrossRef]
- Ding, C.; Gao, D.; Wilding, J.; Trayhurn, P.; Bing, C. Vitamin D signalling in adipose tissue. Br. J. Nutr. 2012, 108, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; O’Hara, A.; Bing, C. Interrogation of microarray datasets indicates that macrophage-secreted factors stimulate the expression of genes associated with vitamin D metabolism (VDR and CYP27B1) in human adipocytes. Adipobiology 2011, 3, 29–34. [Google Scholar] [CrossRef]
- Ching, S.; Kashinkunti, S.; Niehaus, M.D.; Zinser, G.M. Mammary adipocytes bioactivate 25-hydroxyvitamin D3 and signal via vitamin D3 receptor, modulating mammary epithelial cell growth. J. Cell. Biochem. 2011, 112, 3393–3405. [Google Scholar] [CrossRef]
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of vitamin D-metabolizing enzymes in human adipose tissue—The effect of obesity and diet-induced weight loss. Int. J. Obes. 2012, 37, 651–657. [Google Scholar] [CrossRef] [PubMed]
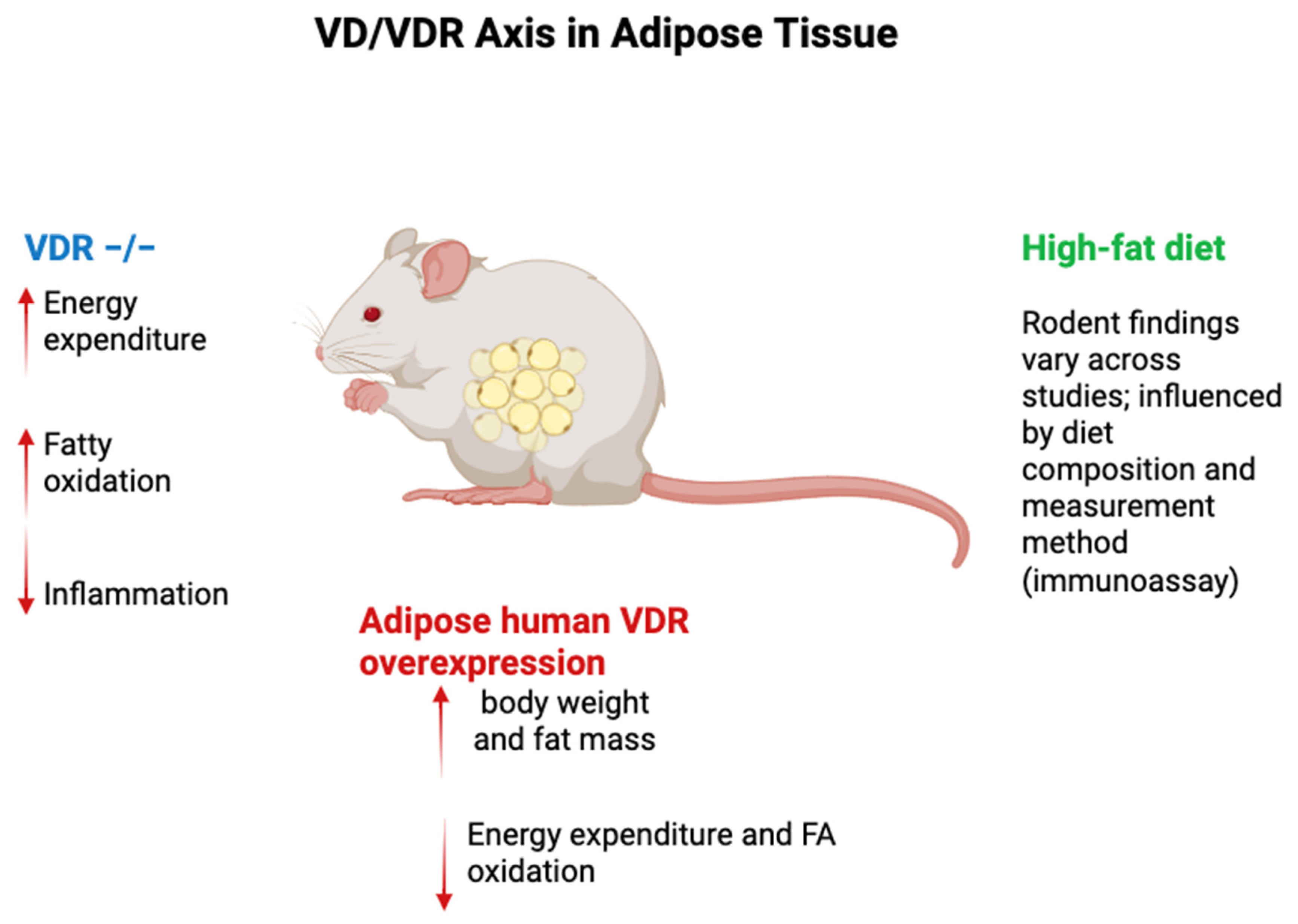
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J. Biol. Chem. 2012, 287, 42324–42332, Erratum in J. Biol. Chem. 2013, 288, 24871. [Google Scholar] [CrossRef]
- Bidulescu, A.; Morris, A.A.; Stoyanova, N.; Meng, Y.-X.; Vaccarino, V.; Quyyumi, A.A.; Gibbons, G.H. Association between vitamin D and adiponectin and its relationship with body mass index: The META-Health Study. Front. Public Health 2014, 2, 193–199. [Google Scholar] [CrossRef]
- Walker, G.E.; Ricotti, R.; Roccio, M.; Moia, S.; Bellone, S.; Prodam, F.; Bona, G. Pediatric Obesity and Vitamin D Deficiency: A Proteomic Approach Identifies Multimeric Adiponectin as a Key Link between These Conditions. PLoS ONE 2014, 9, e83685. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Holick, M.F.; Fried, S.K.; Lee, M.-J. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 2012, 7, e52171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, J.; Chen, Y.; Zhu, G.; Zhao, Q.; Li, Y.C. 1,25-Dihydroxyvitamin D3 upregulates leptin expression in mouse adipose tissue. J. Endocrinol. 2012, 216, 265–271. [Google Scholar] [CrossRef]
- Vaidya, A.; Williams, J.S.; Forman, J.P. The independent association between 25-hydroxyvitamin D and adiponectin and its relation with BMI in two large cohorts: The NHS and the HPFS. Obesity 2012, 20, 186–191. [Google Scholar] [CrossRef]
- Neyestani, T.R.; Nikooyeh, B.; Alavi-Majd, H.; Shariatzadeh, N.; Kalayi, A.; Tayebinejad, N.; Heravifard, S.; Salekzamani, S.; Zahedirad, M. Improvement of Vitamin D Status via Daily Intake of Fortified Yogurt Drink Either with or without Extra Calcium Ameliorates Systemic Inflammatory Biomarkers, including Adipokines, in Subjects with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 2005–2011. [Google Scholar] [CrossRef]
- Dinca, M.; Serban, M.-C.; Sahebkar, A.; Mikhailidis, D.P.; Toth, P.P.; Martin, S.S.; Blaha, M.J.; Blüher, M.; Gurban, C.; Penson, P.; et al. Does vitamin D supplementation alter plasma adipokines concentrations? A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 107, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrián, S.; Eriksson, A.; Dunlop, T.; Mejhert, N.; Dahlman, I.; Åström, G.; Sjölin, E.; Wåhlén, K.; Carlberg, C.; Laurencikiene, J.; et al. Differential effects of 1α,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur. J. Nutr. 2011, 51, 335–342. [Google Scholar] [CrossRef]
- Sun, X.; Morris, K.L.; Zemel, M.B. Role of calcitriol and cortisol on human adipocyte proliferation and oxidative and inflammatory stress: A microarray study. J. Nutr. Nutr. 2008, 1, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N.; Song, Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol. Nutr. Food Res. 2014, 58, 1342–1348. [Google Scholar] [CrossRef]
- Li, J.; Byrne, M.E.; Chang, E.; Jiang, Y.; Donkin, S.S.; Buhman, K.K.; Burgess, J.R.; Teegarden, D. 1α,25-Dihydroxyvitamin D hydroxylase in adipocytes. J. Steroid Biochem. Mol. Biol. 2008, 112, 122–126. [Google Scholar] [CrossRef]
- Bonnet, L.; Hachemi, M.A.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Martin, J.-C.; Tourniaire, F.; Landrier, J.-F. Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice. J. Steroid Biochem. Mol. Biol. 2019, 185, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Wu, D.; Smith, D.; Meydani, S.N.; Han, S.N. Dysregulated 1,25-dihydroxyvitamin D levels in high-fat diet-induced obesity can be restored by changing to a lower-fat diet in mice. Nutr. Res. 2018, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Q.; Pan, D.X.; Hu, C.Q.; Zhu, Y.L.; Liu, X.J. Vitamin D-vitamin D receptor system down-regulates expression of uncoupling proteins in brown adipocyte through interaction with Hairless protein. Biosci. Rep. 2020, 40, BSR20194294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, Y.C. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matthews, D.G.; D’Angelo, J.; Drelich, J.; Welsh, J. Adipose-specific Vdr deletion alters body fat and enhances mammary epithelial density. J. Steroid Biochem. Mol. Biol. 2016, 164, 299–308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, E. Effects of Vitamin D Supplementation on Adipose Tissue Inflammation and NF-κB/AMPK Activation in Obese Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2022, 23, 10915. [Google Scholar] [CrossRef]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.F.; Riva, C. Vitamin D Supplementation Improves Adipose Tissue Inflammation and Reduces Hepatic Steatosis in Obese C57BL/6J Mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebrahimzadeh, F.; Farhangi, M.A.; Tausi, A.Z.; Mahmoudinezhad, M.; Mesgari-Abbasi, M.; Jafarzadeh, F. Vitamin D supplementation and cardiac tissue inflammation in obese rats. BMC Nutr. 2022, 8, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mallard, S.R.; Howe, A.S.; Houghton, L.A. Vitamin D status and weight loss: A systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials. Am. J. Clin. Nutr. 2016, 104, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.J.; Bendahan, D.; Bernard, M.; Martin, J.C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Benetti, E.; Mastrocola, R.; Chiazza, F.; Nigro, D.; D’Antona, G.; Bordano, V.; Fantozzi, R.; Aragno, M.; Collino, M.; Minetto, M.A. Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS ONE 2018, 13, e0189707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haghighat, N.; Sohrabi, Z.; Bagheri, R.; Akbarzadeh, M.; Esmaeilnezhad, Z.; Ashtary-Larky, D.; Barati-Boldaji, R.; Zare, M.; Amini, M.; Hosseini, S.V.; et al. A Systematic Review and Meta-Analysis of Vitamin D Status of Patients with Severe Obesity in Various Regions Worldwide. Obes. Facts 2023, 16, 519–539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, X.; Shang, G.; Wang, W.; Chen, X.; Lou, Q.; Zhai, G.; Li, D.; Du, Z.; Ye, Y.; Jin, X.; et al. Fatty Acid Oxidation in Zebrafish Adipose Tissue Is Promoted by 1α,25(OH)2D3. Cell Rep. 2017, 19, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, Y. Vitamin D Insufficiency Exacerbates Adipose Tissue Macrophage Infiltration and Decreases AMPK/SIRT1 Activity in Obese Rats. Nutrients 2017, 9, 338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, C.E.; Liu, Y. Low serum 25-hydroxyvitamin D concentrations are associated with total adiposity of children in the United States: National Health and Examination Survey 2005 to 2006. Nutr. Res. 2016, 36, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Zheng, X.; Gao, W.L.; Tao, F.; Chen, Y. Association between serum vitamin D levels and visceral adipose tissue among adolescents: A cross-sectional observational study in NHANES 2011-2015. BMC Pediatr. 2022, 22, 634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nejabat, A.; Emamat, H.; Afrashteh, S.; Jamshidi, A.; Jamali, Z.; Farhadi, A.; Talkhabi, Z.; Nabipour, I.; Larijani, B.; Spitz, J. Association of serum 25-hydroxy vitamin D status with cardiometabolic risk factors and total and regional obesity in southern Iran: Evidence from the PoCOsteo study. Sci. Rep. 2024, 14, 17983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pott-Junior, H.; Nascimento, C.M.C.; Costa-Guarisco, L.P.; Gomes, G.A.d.O.; Gramani-Say, K.; Orlandi, F.d.S.; Gratão, A.C.M.; Orlandi, A.A.d.S.; Pavarini, S.C.I.; Vasilceac, F.A.; et al. Vitamin D Deficient Older Adults Are More Prone to Have Metabolic Syndrome, but Not to a Greater Number of Metabolic Syndrome Parameters. Nutrients 2020, 12, 748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pramono, A.; Jocken, J.W.E.; Goossens, G.H.; Blaak, E.E. Vitamin D release across abdominal adipose tissue in lean and obese men: The effect of β-adrenergic stimulation. Physiol. Rep. 2019, 7, e14308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, E.G.; Lips, P.; Schoonmade, L.J.; Lanham-New, S.A.; van Schoor, N.M. Comparison of the Effect of Daily Vitamin D2 and Vitamin D3 Supplementation on Serum 25-Hydroxyvitamin D Concentration (Total 25(OH)D, 25(OH)D2, and 25(OH)D3) and Importance of Body Mass Index: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2023, 15, 100133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.-M.; Buring, J.E.; et al. Association of Body Weight with Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drincic, A.; Fuller, E.; Heaney, R.P.; Armas, L.A. 25-Hydroxyvitamin D response to graded vitamin D3 supplementation among obese adults. J. Clin. Endocrinol. Metab. 2013, 98, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
- Bacha, D.S.; Rahme, M.; Al-Shaar, L.; Baddoura, R.; Halaby, G.; Singh, R.J.; Mahfoud, Z.R.; Habib, R.; Arabi, A.; Fuleihan, G.E.-H. Vitamin D3 Dose Requirement That Raises 25-Hydroxyvitamin D to Desirable Level in Overweight and Obese Elderly. J. Clin. Endocrinol. Metab. 2021, 106, e3644–e3654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ochs-Balcom, H.M.; Chennamaneni, R.; Millen, A.E.; Shields, P.G.; Marian, C.; Trevisan, M.; Freudenheim, J.L. Vitamin D receptor gene polymorphisms are associated with adiposity phenotypes. Am. J. Clin. Nutr. 2011, 93, 5–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, R.J.; Riestra, P.; Gebreab, S.Y.; Wilson, J.G.; Gaye, A.; Xu, R.; Davis, S.K. Vitamin D Receptor Gene Polymorphisms Are Associated with Abdominal Visceral Adipose Tissue Volume and Serum Adipokine Concentrations but Not with Body Mass Index or Waist Circumference in African Americans: The Jackson Heart Study. J. Nutr. 2016, 146, 1476–1482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correa-Rodríguez, M.; Carrillo-Ávila, J.A.; Schmidt-RioValle, J.; González-Jiménez, E.; Vargas, S.; Martín, J.; Rueda-Medina, B. Genetic association analysis of vitamin D receptor gene polymorphisms and obesity-related phenotypes. Gene 2018, 640, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Emond, J.A.; Flatt, S.W.; Heath, D.D.; Karanja, N.; Pakiz, B.; Sherwood, N.E.; Thomson, C.A. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity 2012, 20, 2296–2301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holt, R.; Holt, J.; Jorsal, M.J.; Sandsdal, R.M.; Jensen, S.B.K.; Byberg, S.; Juhl, C.R.; Lundgren, J.R.; Janus, C.; Stallknecht, B.M.; et al. Weight Loss Induces Changes in Vitamin D Status in Women with Obesity But Not in Men: A Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2025, 110, 2215–2224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
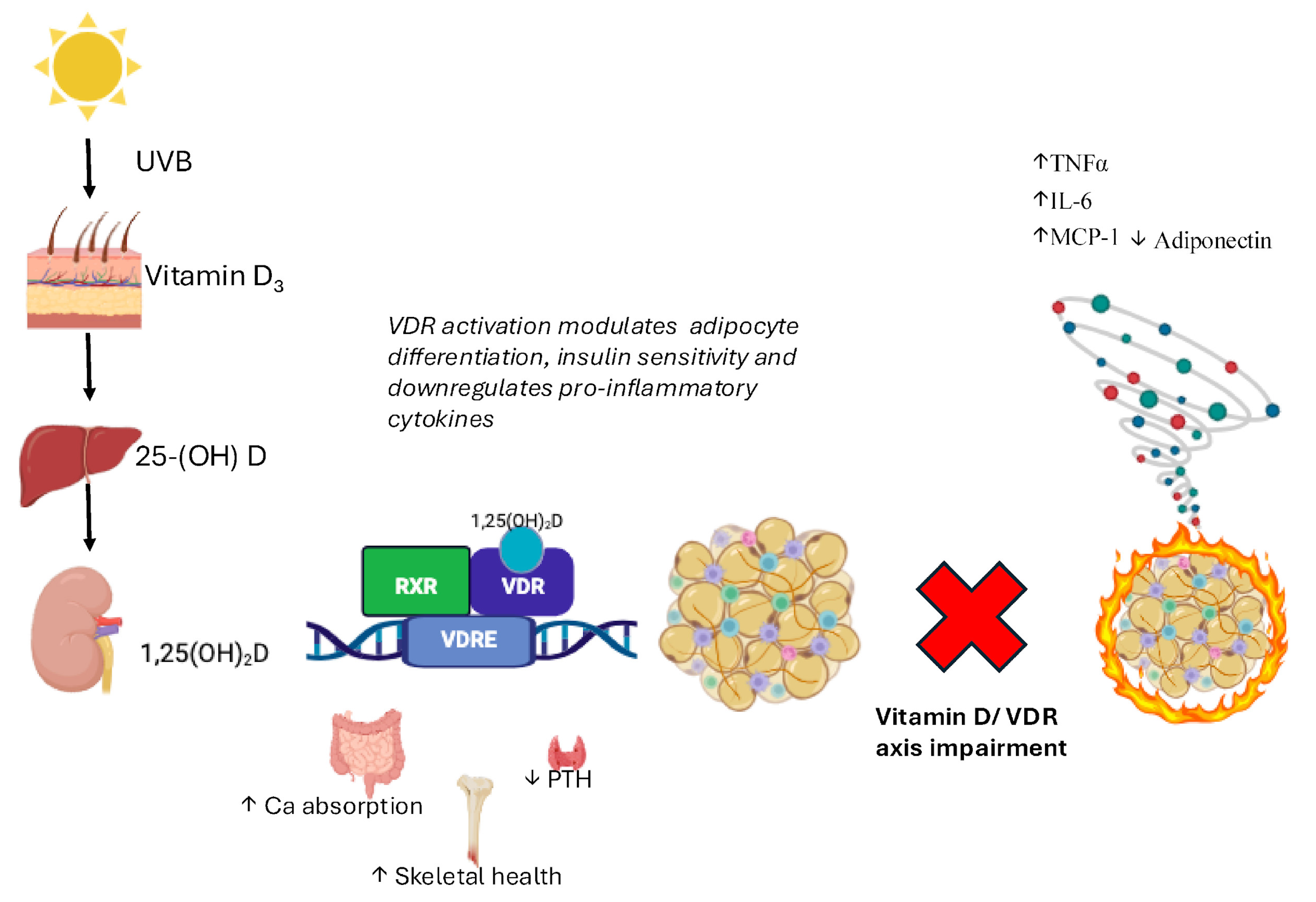
| Function | Description | Key Molecules/Cells |
|---|---|---|
| Energy Storage and Release | Storage of triglycerides and regulated lipolysis to provide energy when needed | Triglycerides, Lipolysis pathways |
| Endocrine Function | Secretion of adipokines that influence systemic metabolism, appetite, and insulin sensitivity [4,5,6] | Leptin, Adiponectin, Resistin, Apelin, Visfatin, Adipsin |
| Metabolic Regulation | Modulation of insulin sensitivity, energy expenditure, and glucose homeostasis [4,8] | Adiponectin (↑ sensitivity), Leptin, Resistin (↓ sensitivity) |
| Neuro-immune Interaction | Sympathetic innervation influences lipolysis and immune activity; immune cells secrete neurotrophic factors influencing sympathetic tone [7] | Sympathetic nerves, Neurotrophic factors, Macrophages, Eosinophils |
| Immune Cell Niche | Stromal vascular fraction (SVF) supports mesenchymal, endothelial, and immune cells, forming a regulatory microenvironment [2] | SVF, MSCs, Endothelial cells |
| Function | Description | Key Molecules/Cells |
|---|---|---|
| Inflammatory Signaling | Release of cytokines and regulation of local and systemic inflammation Balance of M1 (pro-inflammatory) and M2 (anti-inflammatory) macrophages determines inflammatory status and insulin sensitivity [12,18,24,25,30] | IL-6, TNFα, IL-10, IL-1β, IL-12, IL-23, M1: TNFα, IL-1β, M2: IL-10 |
| Immune modulation | Interaction with resident immune cells (macrophages, eosinophils, ILC2s, T cells) that modulate inflammation and tissue homeostasis. Maintenance of metabolic homeostasis via ILC2-induced eosinophil activation, which promotes M2 macrophage polarization [14,24] | M1/M2 macrophages, ILC2s, ATEs, T cells M1: TNFα, IL-1β M2: IL-10 |
| Pathophysiology in obesity | Dysfunctional adipokine secretion and immune infiltration lead to chronic low-grade inflammation (“metaflammation”) and metabolic disease progression [4,5,6] | ↓ Adiponectin, ↑ Leptin/Resistin, ↑ M1 macrophages |
| Category | Details |
|---|---|
| VDR Expression | Expression nearly ubiquitous across ~250 human tissues/cell types [47]. Highest protein levels in adipose tissue, bone, kidneys, intestine [43,44]. low/absent in erythrocytes, striated muscle, Purkinje cells [49]. |
| Genome Control | >3% of the human genome under direct or indirect VDR control [48]. |
| Autoregulation (VDREs) | Highly conserved VDRE regions in two large introns and 6 kb upstream of TSS. Allow VDR to autoregulate its own expression [50,51]. |
| Promoters | Four promoters control VDR transcription, some tissue-specific, contributing to functional diversity [48]. |
| Environmental Regulators | UVB exposure increases VDR expression [53]; sunscreens decrease it [54]. Dietary vitamin D intake influences VDR levels [52]. Obesity, air pollution, and aging also modulate expression. [55,56] |
| Adipose Tissue Expression | VDR expressed in 3T3-L1 adipocytes, human pre-adipocytes, differentiated adipocytes, subcutaneous/visceral AT, and mammary adipocytes [74]. Highlights the role of vitamin D/VDR in adipose inflammation and metabolism [75]. |
| Structural Domains | VDR belongs to the nuclear receptor superfamily with the conserved DNA-binding domain (DBD) and ligand-binding domain (LBD). |
| Polymorphism rs11568820 (Cdx2) | G > A in promoter; A-allele enhances Cdx-2 transcription factor binding, increasing intestine-specific VDR transcription [66]. AA genotype linked to higher T2DM risk and impaired insulin secretion, and early life cardiometabolic alterations [59,60]. |
| Polymorphisms BsmI/ApaI/TaqI | Located in 3′-UTR; studies show conflicting effects on mRNA stability and transcript levels; potential linkage with other regulatory sequences [63,64,65]. |
| Polymorphism rs2228570 (FokI) | C > T at start codon; C-allele uses downstream ATG yielding shorter VDR (424 aa) with higher transactivation compared to long form (427 aa) [57,58]. |
| Co-regulators & nVDREs | Co-activators (CoAs) remodel chromatin and promote transcription [68]; co-repressors (CoRs) condense chromatin to repress genes [69]. Negative VDREs in some targets mediate transcriptional repression [72]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimini, F.A.; Sentinelli, F.; Oldani, A.; Barchetta, I.; Cavallo, M.G. Adipose Tissue Dysfunction and Metabolic Diseases: The Role of Vitamin D/Vitamin D Receptor Axis. Int. J. Mol. Sci. 2025, 26, 10256. https://doi.org/10.3390/ijms262110256
Cimini FA, Sentinelli F, Oldani A, Barchetta I, Cavallo MG. Adipose Tissue Dysfunction and Metabolic Diseases: The Role of Vitamin D/Vitamin D Receptor Axis. International Journal of Molecular Sciences. 2025; 26(21):10256. https://doi.org/10.3390/ijms262110256
Chicago/Turabian StyleCimini, Flavia Agata, Federica Sentinelli, Alessandro Oldani, Ilaria Barchetta, and Maria Gisella Cavallo. 2025. "Adipose Tissue Dysfunction and Metabolic Diseases: The Role of Vitamin D/Vitamin D Receptor Axis" International Journal of Molecular Sciences 26, no. 21: 10256. https://doi.org/10.3390/ijms262110256
APA StyleCimini, F. A., Sentinelli, F., Oldani, A., Barchetta, I., & Cavallo, M. G. (2025). Adipose Tissue Dysfunction and Metabolic Diseases: The Role of Vitamin D/Vitamin D Receptor Axis. International Journal of Molecular Sciences, 26(21), 10256. https://doi.org/10.3390/ijms262110256







