Role of Nitric Oxide in the Antidepressant Effect of an Aqueous Extract of Punica granatum L.: Effects on GSH/GSSG Ratio and Lipoperoxidation in Adult Male Swiss Webster Mice
Abstract
1. Introduction
2. Results
2.1. Chromatographic Profile of P. granatum Aqueous Extract
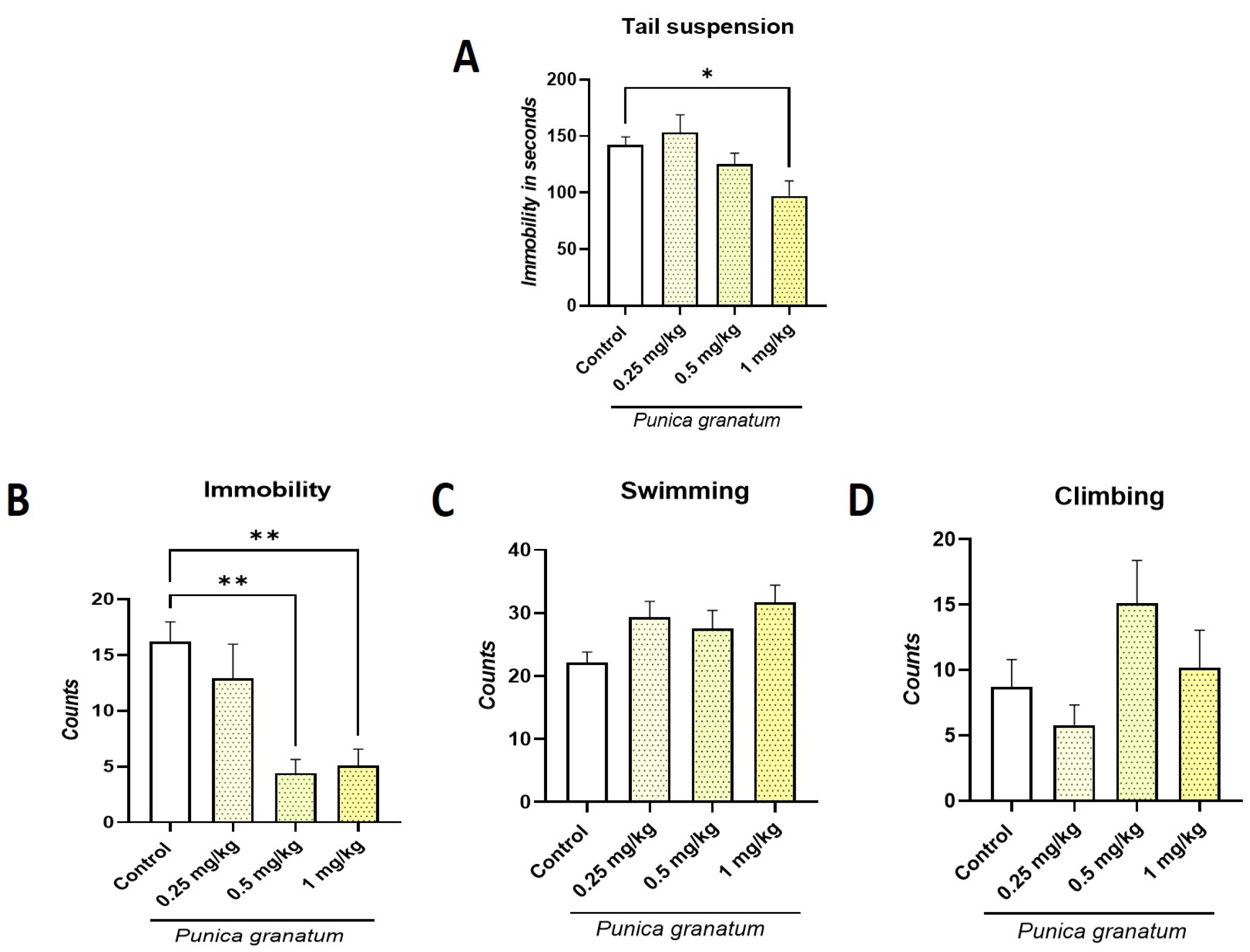
2.2. Effect of P. granatum on Tail Suspension Test (TST) and Forced Swimming Test (FST)
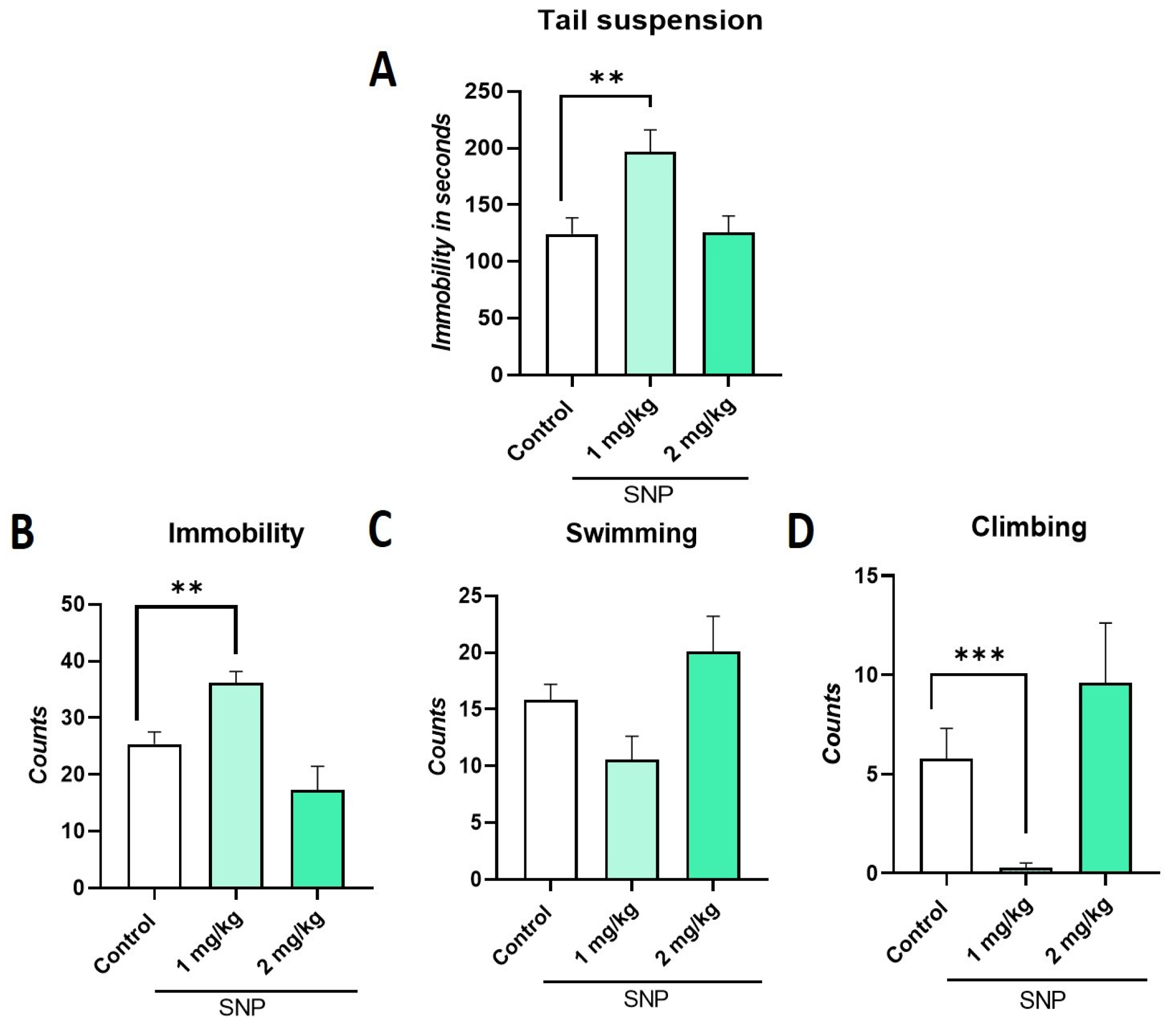
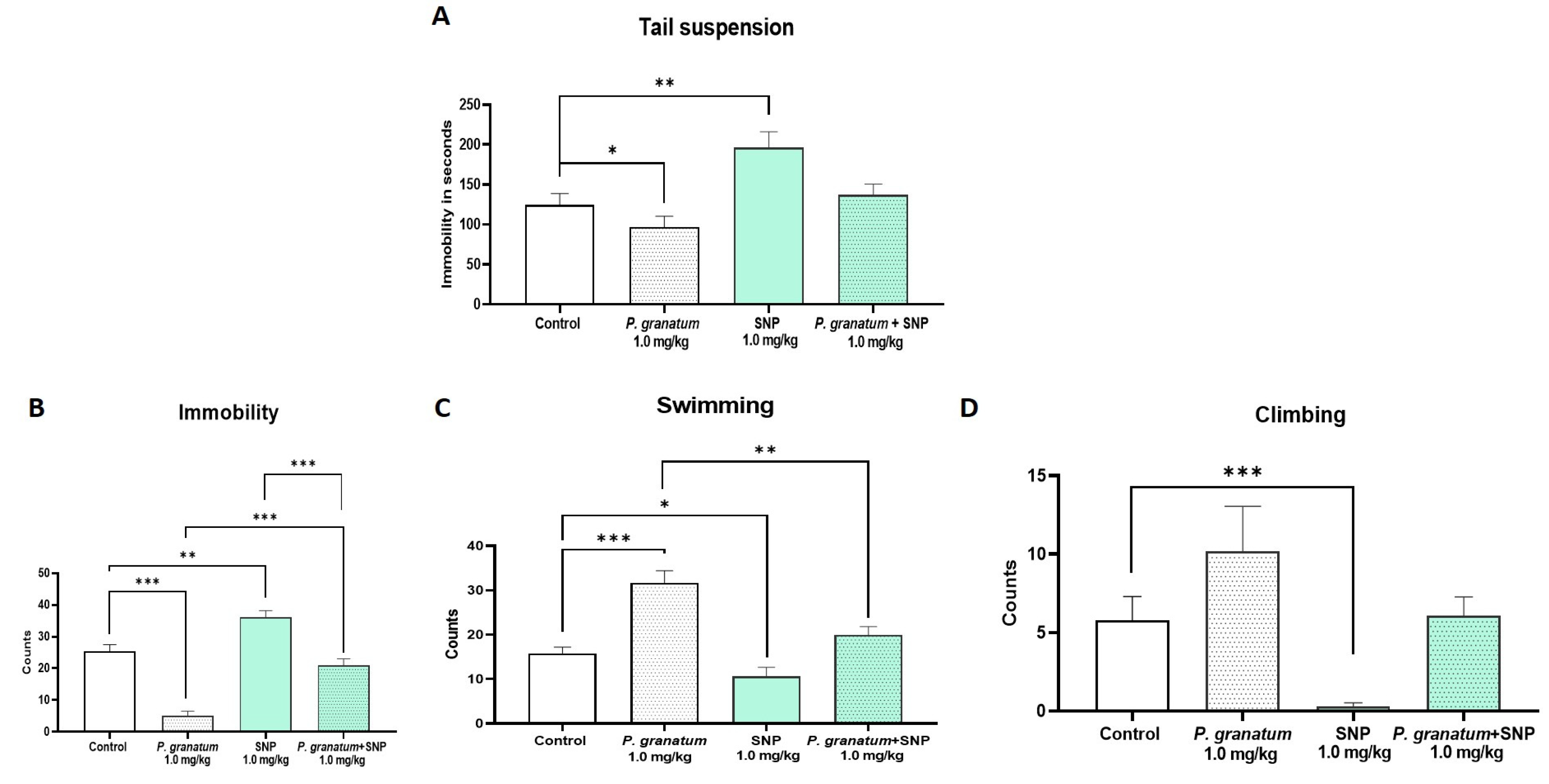
2.3. Effect of Sodium Nitroprusside (SNP), NO Donor, on TST and FST
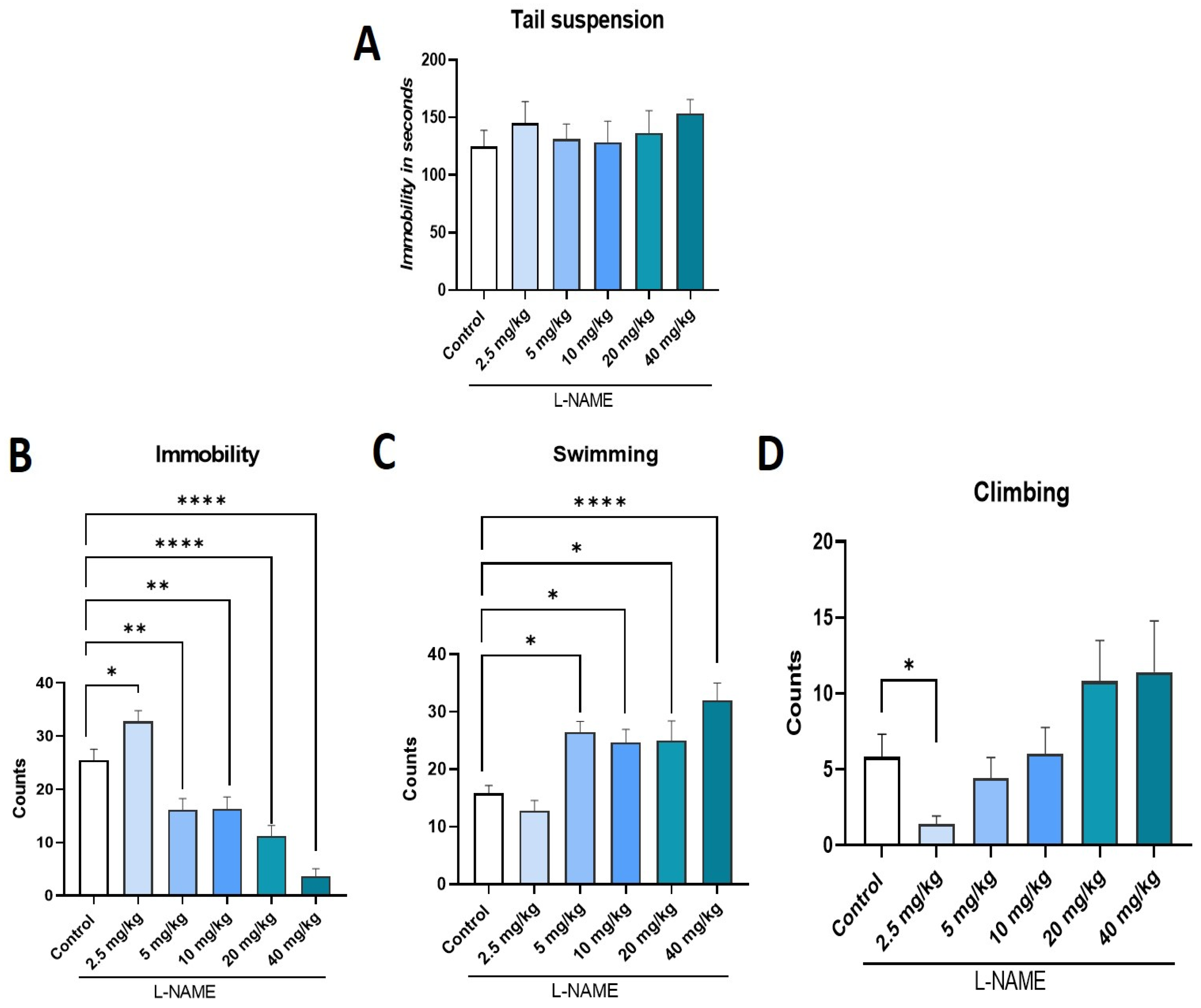
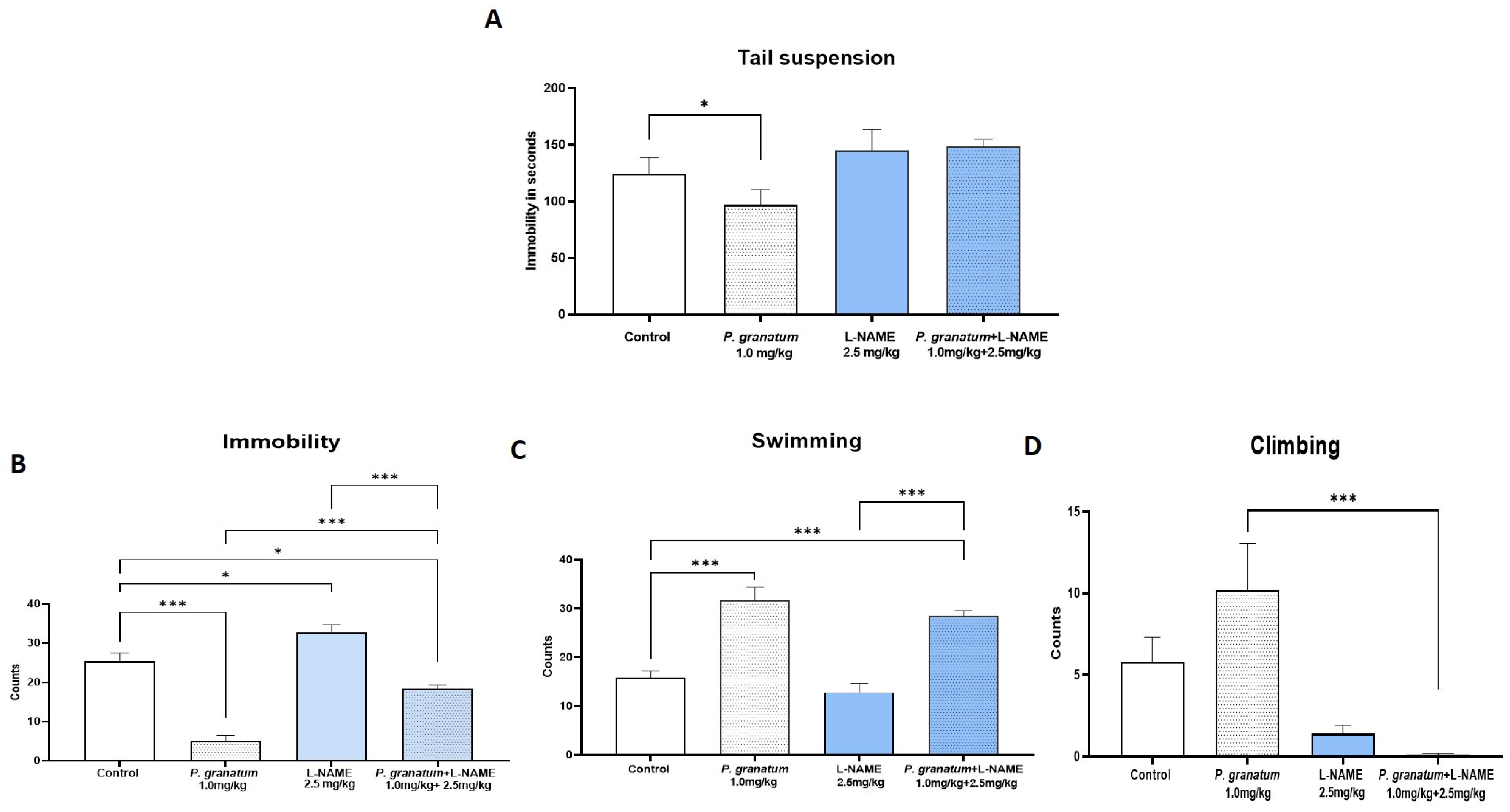
2.4. Effect of L-NAME, NOS Inhibitor, on TST and FST
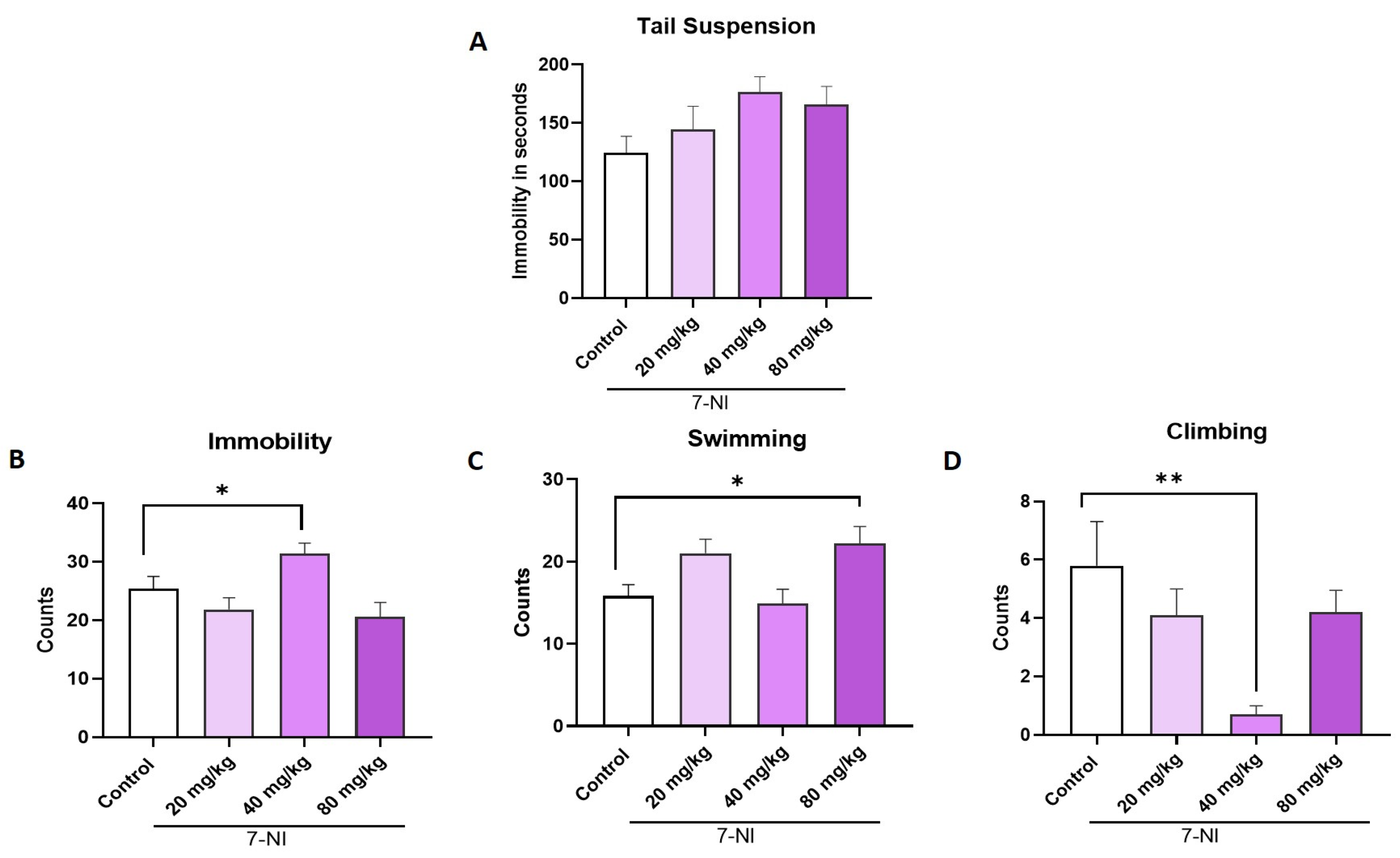
2.5. Effect of 7-NI, a Selective Inhibitor of nNOS, on the TST and FST
2.6. Determination of the Effect of the Combination of P. granatum Plus SNP in the TST and FST
2.7. Effect of the Combination of P. granatum and L-NAME in the TST and FST
2.8. Effect of the Combination of P. granatum and 7-NI in the TST and FST
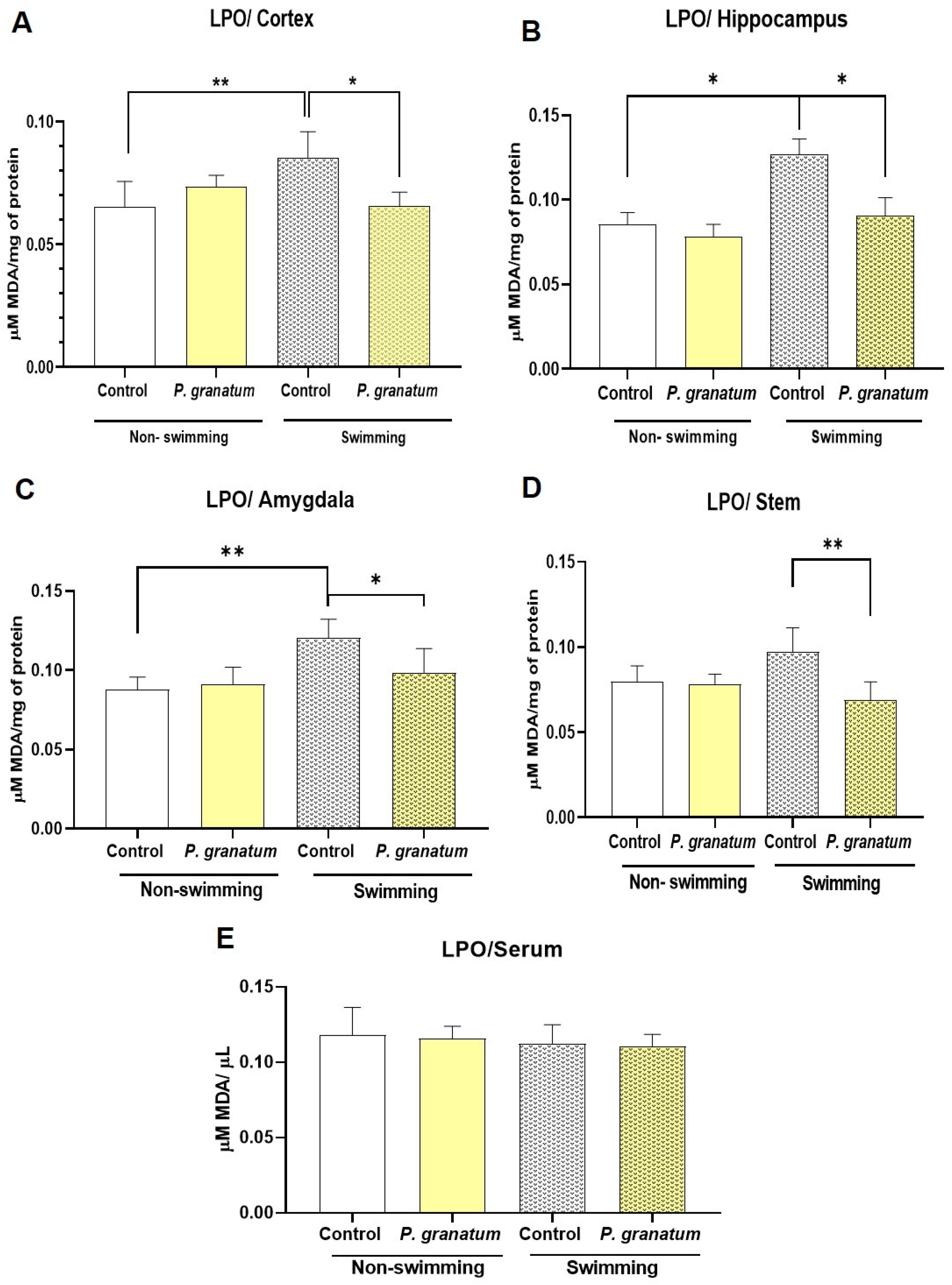
2.9. Effect of P. granatum on Lipid Peroxidation in Brain and Serum in Mice Exposed, or Not, to FST
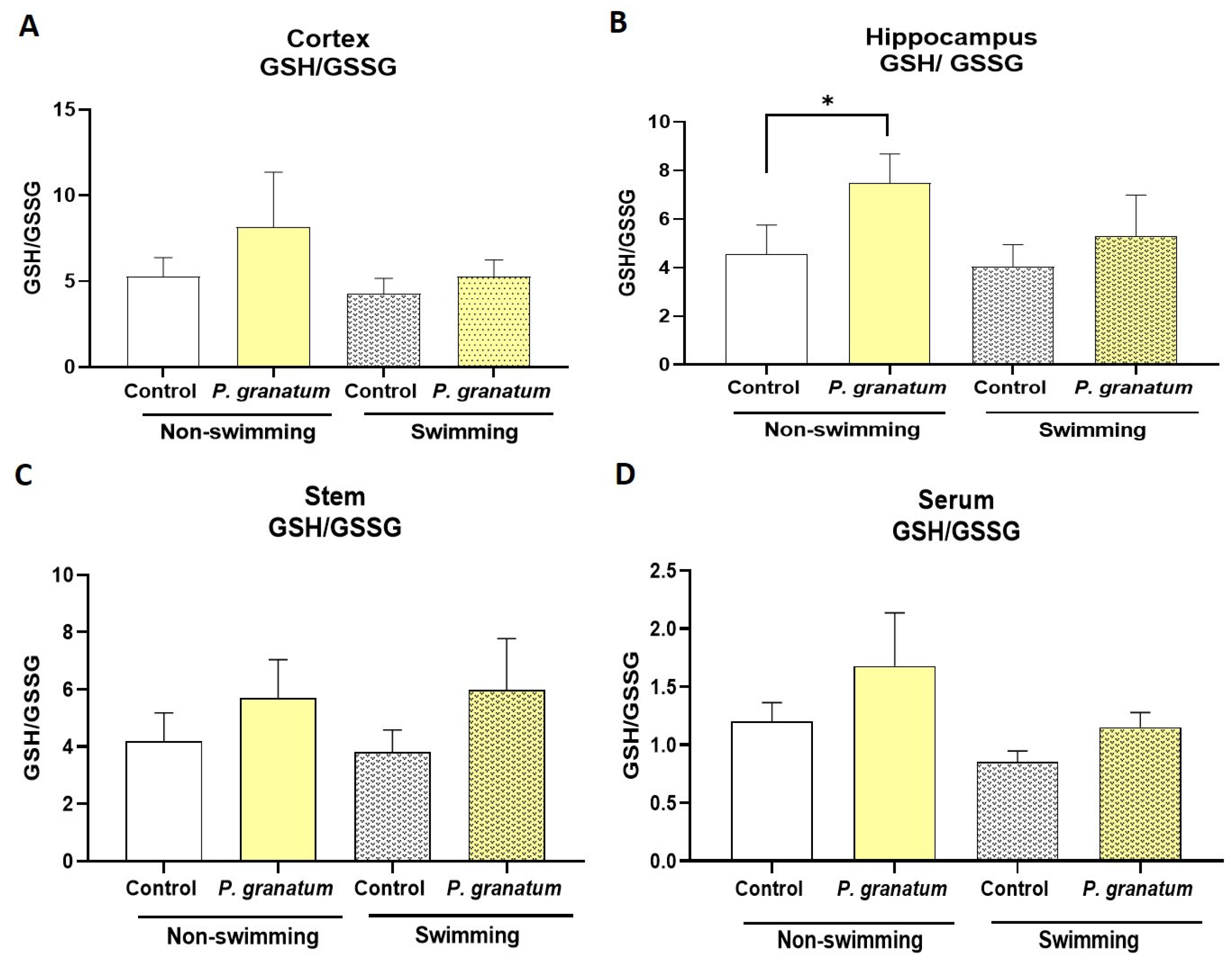
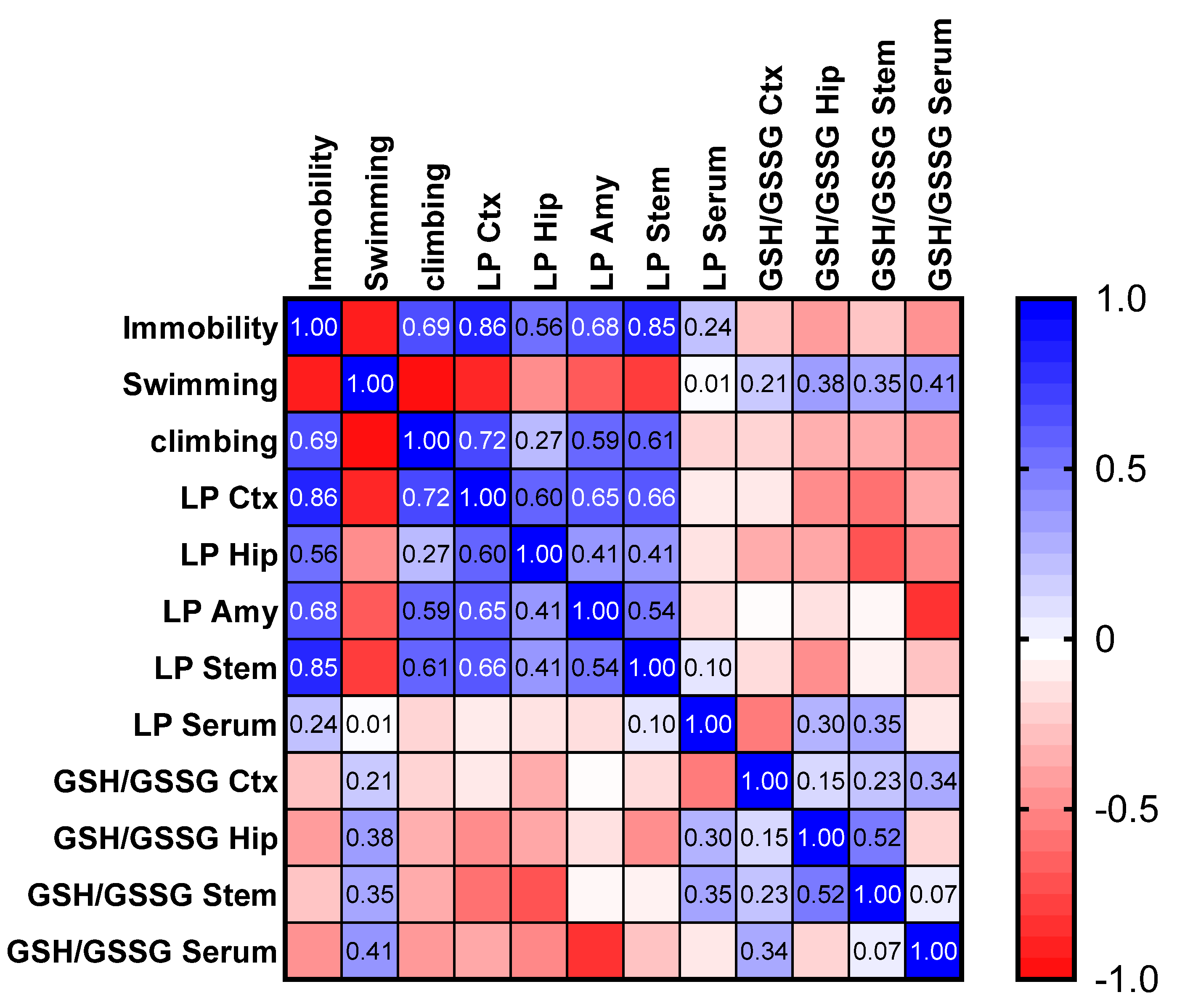
2.10. Effect of P. granatum on Redox Status in Mice Brains
2.11. Effect of P. granatum and the Different Study Treatments in the Locomotor Activity Test
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents and Treatment
4.3. Preparation and Phytochemical Characterization of the P. granatum Extract
4.4. Behavioral Tests
- (a)
- Tail suspension test (TST)
- (b)
- Forced swimming test (FST)
- (c)
- Locomotor activity test
4.5. Lipid Peroxidation in the Mouse Brain
4.5.1. Protein Determination
4.5.2. Determination of GSH and GSSG in the Mouse Brain
4.6. Experimental Design
4.6.1. Experiments 1 and 2: Effects of P. granatum, SNP, L-NAME, and 7-NI Alone and in Combination on the TST and FST
4.6.2. Experiment 3: Effect of P. granatum on GSH/GSSG and Lipoperoxidation in Hippocampus, Frontal Cortex, Amygdala, Brain Stem, and Serum
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LPO | Lipid peroxidation |
| HPA | Hypothalamic–pituitary–adrenal |
| GSH | Glutation |
| ROS | Reactive oxygen species |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| nNOS | Neuronal nitric oxide synthase |
| iNOS | Inducible nitric oxide synthase |
| SSRIs | Selective serotonin reuptake inhibitors |
| 5-HT | Serotonin |
| SNP | Sodium nitroprusside |
| L-NAME | NG-Nitro-L-arginine methyl ester |
| 7-NI | 7-nitroindazole |
| TST | Tail suspension test |
| FST | Forced swimming test |
| TBA-RS | Thiobarbituric acid reactive species |
| MDA | Malondialdehyde |
References
- Yan, H.C.; Cao, X.; Das, M.; Zhu, X.H.; Gao, T.M. Behavioral animal models of depression. Neurosci. Bull. 2010, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Davinelli, S.; Drago, F.; De Lorenzo, A.; Oriani, G. Antioxidants as Antidepressants Fact or Fiction? CNS Drugs 2012, 26, 477–490. [Google Scholar] [CrossRef]
- Pathak, L.; Agrawal, Y.; Dhir, A. Natural polyphenols in the management of major depression. Expert Opin. Investig. Drugs 2013, 22, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Khawam, E.A.; Laurencic, G.; Malone, D.A. Side effects of antidepressants: An overview. Cleve. Clin. J. Med. 2006, 73, 351–353. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Girish, C.; Raj, V.; Arya, J.; Balakrishnan, S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur. J. Pharmacol. 2012, 682, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Llorach, R.; Cerón, J.J.; Espín, J.C.; Tomás-Barberán, F.A. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur. J. Nutr. 2003, 42, 18–28. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Riaz, A.K.R. Effect of Punica granatum on behavior in rats. Afr. J. Pharm. Pharmacol. 2014, 8, 1118–1126. [Google Scholar]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Rahman, S.A.; Aris, M.; Moklas, M.; Kadar, B.A.; Taufik, M.; Baharuldin, H. Antidepressant-Like Effect of Methanolic Extract of Punica granatum (Pomegranate) in Mice Model of Depression. 2015. Available online: https://www.researchgate.net/publication/281114313 (accessed on 1 May 2025).
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [PubMed]
- Valdés-Sustaita, B.; Estrada-Camarena, E.; González-Trujano, M.E.; López-Rubalcava, C. Estrogen receptors-β and serotonin mediate the antidepressant-like effect of an aqueous extract of pomegranate in ovariectomized rats. Neurochem. Int. 2021, 142, 104904. [Google Scholar] [CrossRef] [PubMed]
- Ly, M.; Yu, G.Z.; Mian, A.; Cramer, A.; Meysami, S.; Merrill, D.A.; Samara, A.; Eisenstein, S.A.; Hershey, T.; Babulal, G.M.; et al. Neuroinflammation: A Modifiable Pathway Linking Obesity, Alzheimer’s disease, and Depression. Am. J. Geriatr. Psychiatry 2023, 31, 853–866. [Google Scholar] [CrossRef]
- Cervantes-Anaya, N.; Azpilcueta-Morales, G.; Estrada-Camarena, E.; Ramírez Ortega, D.; Pérez de la Cruz, V.; González-Trujano, M.E.; López-Rubalcava, C. Pomegranate and Its Components, Punicalagin and Ellagic Acid, Promote Antidepressant, Antioxidant, and Free Radical-Scavenging Activity in Ovariectomized Rats. Front. Behav. Neurosci. 2022, 16, 836681. [Google Scholar] [CrossRef]
- Lana, J.V.; Rios, A.; Takeyama, R.; Santos, N.; Pires, L.; Santos, G.S.; Rodrigues, I.J.; Jeyaraman, M.; Purita, J.; Lana, J.F. Nebulized Glutathione as a Key Antioxidant for the Treatment of Oxidative Stress in Neurodegenerative Conditions. Nutrients 2024, 16, 2476. [Google Scholar] [CrossRef]
- Chiueh, C.C.; Rauhala, P. The Redox Pathway of S-Nitrosoglutathione, Glutathione and Nitric Oxide in Cell to Neuron Communications. Free Radic. Res. 1999, 31, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Kishimoto, J.; Koyama, J.; Ozawa, T. Modulatory effect of L-NAME, a specific nitric oxide synthase NOS inhibitor, on stress-induced changes in plasma adrenocorticotropic hormone Ž. ACTH and corticosterone levels in rats: Physiological significance of stress-induced NOS activation in hypothalamic-pituitary-adrenal axis. Brain Res. 1997, 776, 68–74. [Google Scholar] [PubMed]
- Dhir, A.; Kulkarni, S.K. Nitric oxide and major depression. Nitric Oxide Biol. Chem. 2011, 24, 125–131. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Salimi, N.; Soltani, A.; Amini-Khoei, H. Implication of NMDA-NO pathway in the antidepressant-like effect of ellagic acid in male mice. Neuropeptides 2019, 76, 101928. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.G.; Liang, W.L.; Wang, C.C. Anti-inflammatory effects of PunicagranatumLinne in vitro and in vivo. Food Chem. 2010, 118, 315–322. [Google Scholar] [CrossRef]
- O’Neil, M.F.; Moore, N.A. Animal models of depression: Are there any? Hum. Psychopharmacol. 2003, 18, 239–254. [Google Scholar] [CrossRef]
- Aleksandrova, S.; Alexova, R.; Dragomanova, S.; Kalfin, R.; Nicoletti, F.; Fagone, P.; Petralia, M.C.; Mangano, K.; Tancheva, L. Preventive and Therapeutic Effects of Punicagranatum L. Polyphenols in Neurological Conditions. Int. J. Mol. Sci. 2023, 24, 1856. [Google Scholar] [CrossRef]
- Detke, M.J.; Lucki, I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: The effects of water depth. Behav. Brain Res. 1996, 73, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, F.; Amini-Khoei, H.; Sureda, A.; Zarean, E.; Lorigooini, Z. Exploring the anti-depressant effects and nitric oxide modulation of quercetin: A preclinical study in Socially Isolated mice. World J. Biol. Psychiatry 2024, 25, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Sadaghiani, M.S.; Javadi-Paydar, M.; Gharedaghi, M.H.; Fard, Y.Y.; Dehpour, A.R. Antidepressant-like effect of pioglitazone in the forced swimming test in mice: The role of PPAR-gamma receptor and nitric oxide pathway. Behav. Brain Res. 2011, 224, 336–343. [Google Scholar] [CrossRef]
- Sales, A.J.; Joca, S.R.L.; Del Bel, E.; Guimarães, F.S. The antidepressant-like effect of doxycycline is associated with decreased nitric oxide metabolite levels in the prefrontal cortex. Behav. Brain Res. 2024, 458, 114764. [Google Scholar] [CrossRef]
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef]
- Vogt, M.A.; Vogel, A.S.; Pfeiffer, N.; Gass, P.; Inta, D. Role of the nitric oxide donor sodium nitroprusside in the antidepressant effect of ketamine in mice. Eur. Neuropsychopharmacol. 2015, 25, 1848–1852. [Google Scholar] [CrossRef] [PubMed]
- Orfanidou, M.A.; Lafioniatis, A.; Trevlopoulou, A.; Touzlatzi, N.; Pitsikas, N. Acute and repeated exposure with the nitric oxide (NO) donor sodium nitroprusside (SNP) differentially modulate responses in a rat model of anxiety. Nitric Oxide Biol. Chem. 2017, 69, 56–60. [Google Scholar] [CrossRef]
- Umathe, S.N.; Bhutada, P.S.; Jain, N.S.; Mundhada, Y.R.; Borkar, S.S.; Dhumal, B. Role of nitric oxide in obsessive-compulsive behavior and its involvement in the anti-compulsive effect of paroxetine in mice. Nitric Oxide Biol. Chem. 2009, 21, 140–147. [Google Scholar] [CrossRef]
- Papageorgoulis, A.; Fallon, P.; Mpalantes, N.; Papageorgouli, D.; Pitsikas, N. Repeated but not acute exposure with a low dose range of the nitric oxide (NO) donor sodium nitroprusside (SNP) induces anxiolytic-like behaviour in a dose-independent manner in two different rat models of anxiety. Nitric Oxide Biol. Chem. 2020, 99, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pałasz, A.; Menezes, I.C.; Worthington, J.J. The role of brain gaseous neurotransmitters in anxiety. Pharmacol. Rep. 2021, 73, 357–371. [Google Scholar] [CrossRef]
- Karolewicz, B.; Paul, I.A.; Antkiewicz-Michaluk, L. Effect of NOS inhibitor on forced swim test and neurotransmitters turnover in the mouse brain. Pol. J. Pharmacol. 2001, 53, 587–596. [Google Scholar]
- Zhang, J.; Huang, X.Y.; Ye, M.L.; Luo, C.X.; Wu, H.Y.; Hu, Y.; Zhou, Q.-G.; Wu, D.-L.; Zhu, L.-J.; Zhu, D.-Y. Neuronal nitric oxide synthase alteration accounts for the role of 5-HT1A receptor in modulating anxiety-related behaviors. J. Neurosci. 2010, 30, 2433–2441. [Google Scholar] [CrossRef]
- Kumar, A.; Chanana, P. Role of Nitric Oxide in Stress-Induced Anxiety: From Pathophysiology to Therapeutic Target. In Vitamins and Hormones; Academic Press Inc.: Cambridge, MA, USA, 2017; pp. 147–167. [Google Scholar]
- Czech, D.A.; Jacobson, E.B.; LeSueur-Reed, K.T.; Kazel, M.R. Putative anxiety-linked effects of the nitric oxide synthase inhibitor L-NAME in three murine exploratory behavior models. Pharmacol. Biochem. Behav. 2003, 75, 741–748. [Google Scholar] [CrossRef]
- Jesse, C.R.; Wilhelm, E.A.; Bortolatto, C.F.; Rocha, J.B.T.; Nogueira, C.W. Involvement of l-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effect of bis selenide in the mouse tail suspension test. Eur. J. Pharmacol. 2010, 635, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Siwek, M.; Woroń, J.; Wrzosek, A.; Gupało, J.; Chrobak, A.A. Harder, better, faster, stronger? Retrospective chart review of adverse events of interactions between adaptogens and antidepressant drugs. Front. Pharmacol. 2023, 14, 1271776. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, 29, e3638. [Google Scholar] [CrossRef]
- Packer LVitamin, E. physical exercise and tissue damage in animals. Med. Biol. 1984, 62, 105–109. [Google Scholar]
- Hara, M.; Abe, M.; Suzuki, T.; Reiter, R.J. Tissue changes in glutathione metabolism and lipid peroxidation induced by swimming are partially prevented by melatonin. Pharmacol. Toxicol. 1996, 78, 308–312. [Google Scholar] [PubMed]
- Tan, D.-X. LDCBPLCMRJ. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endo Crine J. 1993, 1, 57–60. [Google Scholar]
- Rj, R.; Melchiorri, D.; Sewerynek, E.; Barlow-Walden, L.; Chuang, J. A review of the evidence supporting melatonin’s U role as an antioxidant. J. Pineal Res. 1995, 199, 1–11. [Google Scholar]
- Rosenblat, M.; Elias, A.; Volkova, N.; Aviram, M. Monocyte-macrophage membrane possesses free radicals scavenging activity: Stimulation by polyphenols or by paraoxonase 1 (PON1). Free Radic. Res. 2013, 47, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, M.; Yu, G.; Pu, J.; Tian, K.; Tang, X.; Du, Y.; Wu, H.; Hu, J.; Luo, X.; et al. Comparative analysis of the phenolic contents and antioxidant activities of different parts of two pomegranate (Punica granatum L.) Cultivars: ‘Tunisia’ and ‘Qingpi’. Front. Plant Sci. 2013, 14, 1265018. [Google Scholar] [CrossRef] [PubMed]
- Cano-Lamadrid, M.; Marhuenda-Egea, F.C.; Hernández, F.; Rosas-Burgos, E.C.; Burgos-Hernández, A.; Carbonell-Barrachina, A.A. BiologicalActivity of Conventional and OrganicPomegranateJuices: Antioxidant and AntimutagenicPotential. Plant Foods Hum. Nutr. 2016, 71, 375–380. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Lipan, L.; Calín-Sánchez, Á.; Hernández, F.; Carbonell-Barrachina, Á.A. A Comparative Study Between Labeling and Reality: The Case of Phytochemical Composition of Commercial Pomegranate-Based Products. J. Food Sci. 2017, 82, 1820–1826. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Pellicer, F.; Mena, P.; Moreno, D.A.; García-Viguera, C. Antinociceptive and anti-inflammatory activities of a pomegranate (Punicagranatum L.) extract rich in ellagitannins. Int. J. Food Sci. Nutr. 2015, 66, 395–399. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punicagranatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1892–1906. [Google Scholar] [CrossRef]
- Clementi, M.E.; Pani, G.; Sampaolese, B.; Tringali, G. Punicalagin reduces H2O2-induced cytotoxicity and apoptosis in PC12 cells by modulating the levels of reactive oxygen species. Nutr. Neurosci. 2018, 21, 447–454. [Google Scholar] [CrossRef]
- Clementi, M.E.; Maulucci, G.; Bianchetti, G.; Pizzoferrato, M.; Sampaolese, B.; Tringali, G. Cytoprotective Effects of Punicalagin on Hydrogen-Peroxide-Mediated Oxidative Stress and Mitochondrial Dysfunction in Retinal Pigment Epithelium Cells. Antioxidants 2021, 10, 192. [Google Scholar] [CrossRef]
- Mori-Okamoto, J.; Otawara-Hamamoto, Y.; Yamato, H.; Yoshimura, H. Pomegranate extract improves a depressive state and bone properties in menopausal syndrome model ovariectomized mice. J. Ethnopharmacol. 2004, 92, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Detke, M.J.; Rickels, M.; Lucki, I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 1995, 121, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Camarena, E.; Sollozo-Dupont, I.; Islas-Preciado, D.; González-Trujano, M.E.; Carro-Juárez, M.; López-Rubalcava, C. Anxiolytic- and anxiogenic-like effects of Montanoa tomentosa (Asteraceae): Dependence on the endocrine condition. J. Ethnopharmacol. 2019, 241, 112006. [Google Scholar] [CrossRef] [PubMed]




| Test | Variable | Factor Treatment | One-Way ANOVA Values | p-Value |
|---|---|---|---|---|
| Locomotor activity | Number of crosses | Punica granatum | F(4, 45) = 7.485 | 0.0001 |
| SNP | F(3, 36) = 10.73 | <0.0001 | ||
| L-NAME | F(5, 54) = 0.8505 | ns | ||
| 7-NI | F(3, 35) = 1.207 | ns | ||
| Punica granatum + SNP | F(5, 54) = 7.211 | <0.0001 | ||
| Punica granatum + L-NAME | F(3, 36) = 1.288 | ns | ||
| Punica granatum + 7-NI | F(3, 36) = 8.726 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes-Anaya, N.; Aedo-Torrado, A.; Estrada-Camarena, E.; Pérez de la Cruz, V.; Ramírez Ortega, D.; González-Trujano, M.E.; López-Rubalcava, C. Role of Nitric Oxide in the Antidepressant Effect of an Aqueous Extract of Punica granatum L.: Effects on GSH/GSSG Ratio and Lipoperoxidation in Adult Male Swiss Webster Mice. Int. J. Mol. Sci. 2025, 26, 10255. https://doi.org/10.3390/ijms262110255
Cervantes-Anaya N, Aedo-Torrado A, Estrada-Camarena E, Pérez de la Cruz V, Ramírez Ortega D, González-Trujano ME, López-Rubalcava C. Role of Nitric Oxide in the Antidepressant Effect of an Aqueous Extract of Punica granatum L.: Effects on GSH/GSSG Ratio and Lipoperoxidation in Adult Male Swiss Webster Mice. International Journal of Molecular Sciences. 2025; 26(21):10255. https://doi.org/10.3390/ijms262110255
Chicago/Turabian StyleCervantes-Anaya, Nancy, Alexandere Aedo-Torrado, Erika Estrada-Camarena, Verónica Pérez de la Cruz, Daniela Ramírez Ortega, María Eva González-Trujano, and Carolina López-Rubalcava. 2025. "Role of Nitric Oxide in the Antidepressant Effect of an Aqueous Extract of Punica granatum L.: Effects on GSH/GSSG Ratio and Lipoperoxidation in Adult Male Swiss Webster Mice" International Journal of Molecular Sciences 26, no. 21: 10255. https://doi.org/10.3390/ijms262110255
APA StyleCervantes-Anaya, N., Aedo-Torrado, A., Estrada-Camarena, E., Pérez de la Cruz, V., Ramírez Ortega, D., González-Trujano, M. E., & López-Rubalcava, C. (2025). Role of Nitric Oxide in the Antidepressant Effect of an Aqueous Extract of Punica granatum L.: Effects on GSH/GSSG Ratio and Lipoperoxidation in Adult Male Swiss Webster Mice. International Journal of Molecular Sciences, 26(21), 10255. https://doi.org/10.3390/ijms262110255







