Calcium Homeostasis Machinery in the Human Uterus—A Potential Therapeutic Target in Endometrial Cancer
Abstract
1. Introduction
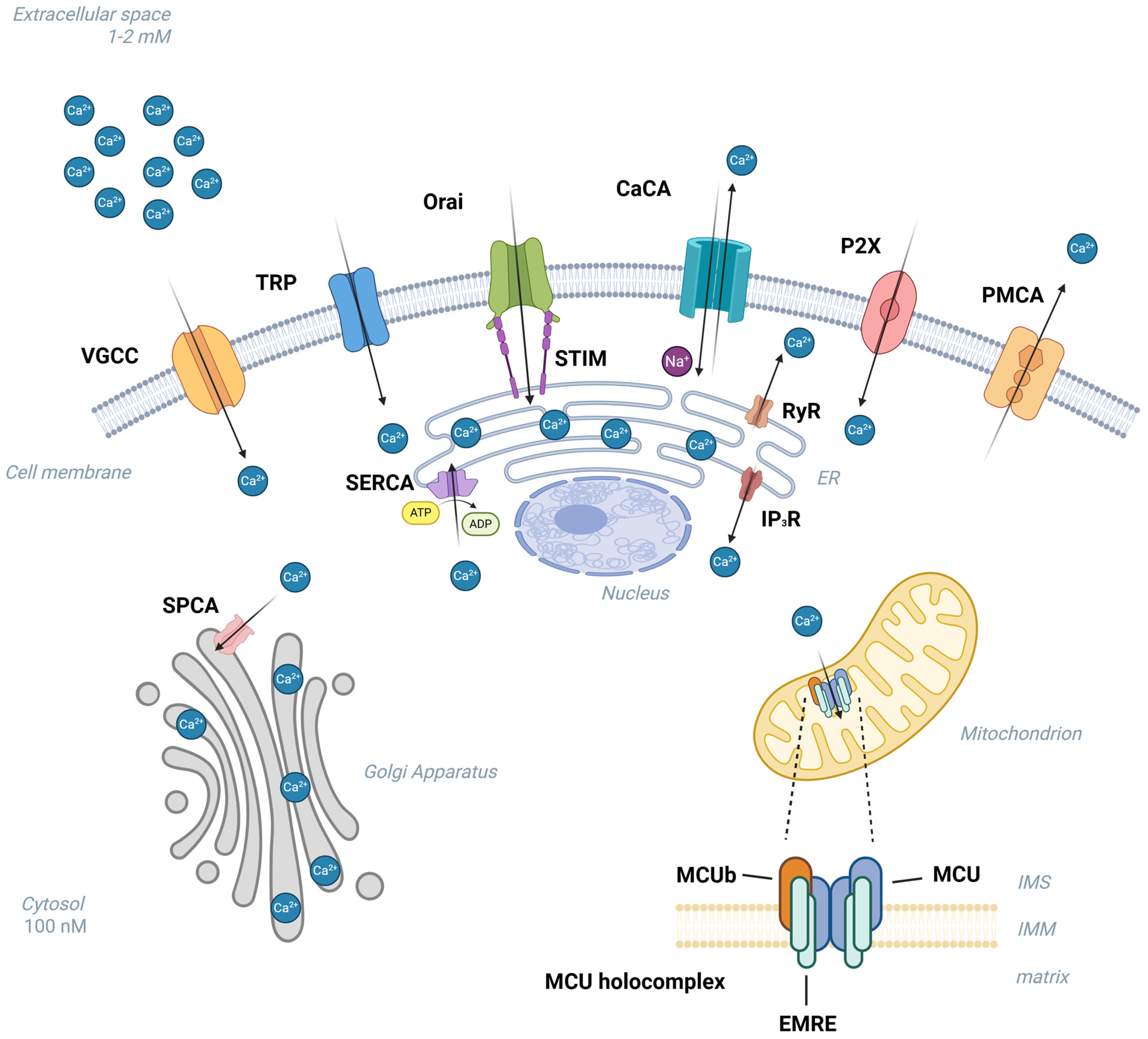
2. Calcium Homeostasis “Toolkit”
3. Deregulation of Calcium Influx
3.1. Voltage-Gated Calcium Channels (VGCCs)
3.2. Transient Receptor Potential Channels (TRPs)
3.3. Ca2+ Release-Activated Ca2+ Channels
3.4. Calcium/Cation Antiporters
3.5. Purinergic Receptors
4. Deregulation of Calcium Extrusion to the Extracellular Space or Sequestration into ER/SR—Ca2+-ATPases
5. Calcium Release from Intracellular Reservoirs (Endoplasmic/Sarcoplasmic Reticulum)
5.1. Ryanodine Receptors (RyRs)
5.2. Inositol 1,4,5-Trisphosphate Receptors (IP3Rs)
6. Mitochondrial Ca2+ Uptake
Mitochondrial Ca2+ Uniporter Complex (MCU Complex)
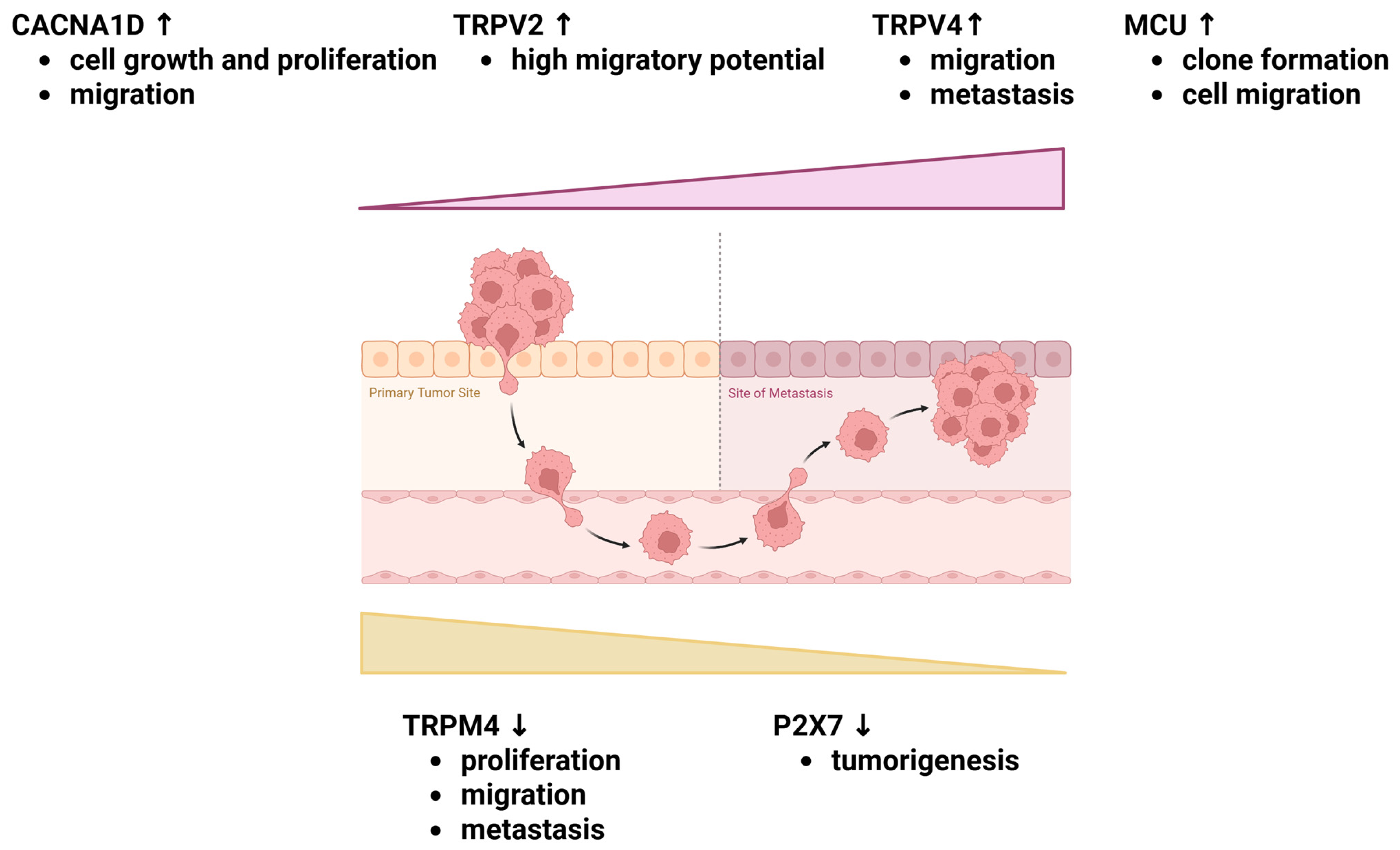
7. Potential Targets for Novel Treatment Strategies of Endometrial Cancer
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 28 July 2025).
- Beavis, A.L.; Smith, A.J.B.; Fader, A.N. Lifestyle Changes and the Risk of Developing Endometrial and Ovarian Cancers: Opportunities for Prevention and Management. Int. J. Womens Health 2016, 8, 151–167. [Google Scholar] [CrossRef]
- Qiu, S.; Jiang, S.; Ye, Q.; Yang, Y.; Li, X. Global Trends and Geographical Disparities in the Incidence of Uterine Cancer from 1990 to 2021. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 311, 114066. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two Pathogenetic Types of Endometrial Carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M. Environmental (Nongenetic) Factors in Gynecological Cancers: Update and Future Perspectives. Future Oncol. 2015, 11, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Gravbrot, N.; Weil, C.R.; DeCesaris, C.M.; Gaffney, D.K.; Suneja, G.; Burt, L.M. Differentiation of Survival Outcomes by Anatomic Involvement and Histology with the Revised 2023 International Federation of Gynecology and Obstetrics Staging System for Endometrial Cancer. Eur. J. Cancer 2024, 20, 113913, Correction in Eur. J. Cancer 2024, 202, 114017. [Google Scholar] [CrossRef]
- Pietras, R.J.; Szego, C.M. Endometrial Cell Calcium and Oestrogen Action. Nature 1975, 253, 357–359. [Google Scholar] [CrossRef]
- Perret, S.; Dockery, P.; Harvey, B.J. 17β-Oestradiol Stimulates Capacitative Ca2+ Entry in Human Endometrial Cells. Mol. Cell. Endocrinol. 2001, 176, 77–84. [Google Scholar] [CrossRef]
- Pohóczky, K.; Kun, J.; Szalontai, B.; Szöke, É.; Sághy, É.; Payrits, M.; Kajtár, B.; Kovács, K.; Környei, J.L.; Garai, J.; et al. Estrogen-Dependent up-Regulation of TRPA1 and TRPV1 Receptor Proteins in the Rat Endometrium. J. Mol. Endocrinol. 2016, 56, 135–149. [Google Scholar] [CrossRef]
- Yang, H.; Choi, K.C.; Hyun, S.H.; Jeung, E.B. Coexpression and Estrogen-Mediated Regulation of TRPV6 and PMCA1 in the Human Endometrium during the Menstrual Cycle. Mol. Reprod. Dev. 2011, 78, 274–282. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Jaffe, L.F. A Calcium-Based Theory of Carcinogenesis. Adv. Cancer Res. 2005, 94, 231–263. [Google Scholar] [CrossRef]
- Moe, S.M. Calcium Homeostasis in Health and in Kidney Disease. Compr. Physiol. 2016, 6, 1781–1800. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium Signalling Remodelling and Disease. Biochem. Soc. Trans. 2012, 40, 297–309. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef] [PubMed]
- Monteith, G.R.; Prevarskaya, N.; Roberts-Thomson, S.J. The Calcium-Cancer Signalling Nexus. Nat. Rev. Cancer 2017, 17, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, X.; Zhao, D.; Liu, H.; Hu, Y. Calcium Homeostasis and Cancer: Insights from Endoplasmic Reticulum-Centered Organelle Communications. Trends Cell Biol. 2022, 33, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E. Calcium Signaling: A Tale for All Seasons. Proc. Natl. Acad. Sci. USA 2002, 99, 1115–1122. [Google Scholar] [CrossRef]
- Giorgi, C.; Danese, A.; Missiroli, S.; Patergnani, S.; Pinton, P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 2018, 28, 258–273. [Google Scholar] [CrossRef]
- Garbincius, J.F.; Elrod, J.W. Mitochondrial Calcium Exchange in Physiology and Disease. Physiol. Rev. 2022, 102, 893–992. [Google Scholar] [CrossRef]
- Song, L. Calcium and Bone Metabolism Indices. Adv. Clin. Chem. 2017, 82, 1–46. [Google Scholar] [CrossRef]
- Morciano, G.; Rimessi, A.; Patergnani, S.; Vitto, V.A.M.; Danese, A.; Kahsay, A.; Palumbo, L.; Bonora, M.; Wieckowski, M.R.; Giorgi, C.; et al. Calcium Dysregulation in Heart Diseases: Targeting Calcium Channels to Achieve a Correct Calcium Homeostasis. Pharmacol. Res. 2022, 177, 106119. [Google Scholar] [CrossRef]
- Cascella, R.; Cecchi, C. Calcium Dyshomeostasis in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4914. [Google Scholar] [CrossRef]
- Zündorf, G.; Reiser, G. Calcium Dysregulation and Homeostasis of Neural Calcium in the Molecular Mechanisms of Neurodegenerative Diseases Provide Multiple Targets for Neuroprotection. Antioxid. Redox Signal. 2011, 14, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, Y.; Wang, Z.; Zhou, J.; Jia, Y.; He, X.; Zhao, L.; Dong, Y.; Fan, Y.; Yang, X.; et al. Calcium and TRPV4 Promote Metastasis by Regulating Cytoskeleton through the RhoA/ROCK1 Pathway in Endometrial Cancer. Cell Death Dis. 2020, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhou, J.; Cao, L.; Xu, Q.; Hao, J.; Zhao, L.; Wang, J. Serum Calcium Is a Novel Parameter to Assess Metabolic Syndrome in Endometrial Carcinoma. J. Gynecol. Oncol. 2019, 30, e12. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting Calcium Signaling in Cancer Therapy. Acta Pharm. Sin. B 2017, 7, 3–17. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Zhou, J. The Role of Oestrogen and Oestrogen-Calcium Axis in Endometrial Carcinoma. Gynecol. Obstet. Clin. Med. 2024, 4, e000012. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, J.; Wang, J. Calcium and Calcium-Related Proteins in Endometrial Cancer: Opportunities for Pharmacological Intervention. Int. J. Biol. Sci. 2022, 18, 1065–1078. [Google Scholar] [CrossRef]
- Shen, B.; Hao, J.; Lin, Y.; Li, X.; Yang, X.; Huang, T.; Wang, J.; Jia, Y.; Zhou, J.; Wang, J. Estrogen-Induced Extracellular Calcium Influx Promotes Endometrial Cancer Progress by Regulating Lysosomal Activity and Mitochondrial ROS. Front. Med. 2022, 9, 835700. [Google Scholar] [CrossRef]
- Chen, Y.F.; Lin, P.C.; Yeh, Y.M.; Chen, L.H.; Shen, M.R. Store-Operated Ca2+ Entry in Tumor Progression: From Molecular Mechanisms to Clinical Implications. Cancers 2019, 11, 899. [Google Scholar]
- Okumura, T.; Raja Xavier, J.P.; Pasternak, J.; Yang, Z.; Hang, C.; Nosirov, B.; Singh, Y.; Admard, J.; Brucker, S.Y.; Kommoss, S.; et al. Rel Family Transcription Factor NFAT5 Upregulates COX2 via HIF-1α Activity in Ishikawa and HEC1a Cells. Int. J. Mol. Sci. 2024, 25, 3666. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O. Calcium’s Role in Orchestrating Cancer Apoptosis: Mitochondrial-Centric Perspective. Int. J. Mol. Sci. 2023, 24, 8982. [Google Scholar] [CrossRef] [PubMed]
- Pitt, G.S.; Matsui, M.; Cao, C. Voltage-Gated Calcium Channels in Nonexcitable Tissues. Annu. Rev. Physiol. 2021, 83, 183–203. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; Yang, B.; Ding, L. The Regulatory and Modulatory Roles of TRP Family Channels in Malignant Tumors and Relevant Therapeutic Strategies. Acta Pharm. Sin. B 2022, 12, 1761–1780. [Google Scholar] [CrossRef]
- Humer, C.; Berlansky, S.; Grabmayr, H.; Sallinger, M.; Bernhard, A.; Fahrner, M.; Frischauf, I. Science CommuniCa2+tion Developing Scientific Literacy on Calcium: The Involvement of CRAC Currents in Human Health and Disease. Cells 2022, 11, 1849. [Google Scholar] [CrossRef]
- Rodrigues, T.; Piccirillo, S.; Magi, S.; Preziuso, A.; dos Santos Ramos, V.; Serfilippi, T.; Orciani, M.; Maciel Palacio Alvarez, M.; Luis dos Santos Tersariol, I.; Amoroso, S.; et al. Control of Ca2+ and Metabolic Homeostasis by the Na+/Ca2+ Exchangers (NCXs) in Health and Disease. Biochem. Pharmacol. 2022, 203, 115163. [Google Scholar] [CrossRef]
- Parys, J.B.; Bultynck, G.; Vervliet, T. IP3 Receptor Biology and Endoplasmic Reticulum Calcium Dynamics in Cancer. Prog. Mol. Subcell. Biol. 2021, 59, 215–237. [Google Scholar] [CrossRef]
- Hadiatullah, H.; He, Z.; Yuchi, Z. Structural Insight Into Ryanodine Receptor Channelopathies. Front. Pharmacol. 2022, 13, 897494. [Google Scholar] [CrossRef]
- Reggiani, C.; Marcucci, L. A Controversial Issue: Can Mitochondria Modulate Cytosolic Calcium and Contraction of Skeletal Muscle Fibers? J. Gen. Physiol. 2022, 154, e202213167, Correction in J. Gen. Physiol. 2022, 154, e20221316709092022c. [Google Scholar] [CrossRef]
- Elíes, J.; Yáñez, M.; Pereira, T.M.C.; Gil-Longo, J.; MacDougall, D.A.; Campos-Toimil, M. An Update to Calcium Binding Proteins. Adv. Exp. Med. Biol. 2020, 1131, 183–213. [Google Scholar] [CrossRef]
- Yáñez, M.; Gil-Longo, J.; Campos-Toimil, M. Calcium Binding Proteins. Adv. Exp. Med. Biol. 2012, 740, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A.; Uversky, V.N. What Is Parvalbumin For? Biomolecules 2022, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Sugino, H.; Sawada, Y. Influence of S100A2 in Human Diseases. Diagnostics 2022, 12, 1756. [Google Scholar] [CrossRef]
- Hussey, J.W.; Limpitikul, W.B.; Dick, I.E. Calmodulin Mutations in Human Disease. Channels 2023, 17, 2165278. [Google Scholar] [CrossRef]
- Ulengin-Talkish, I.; Cyert, M.S. A Cellular Atlas of Calcineurin Signaling. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119366. [Google Scholar] [CrossRef]
- Braunewell, K.H. The Darker Side of Ca2+ Signaling by Neuronal Ca2+-Sensor Proteins: From Alzheimer’s Disease to Cancer. Trends Pharmacol. Sci. 2005, 26, 345–351. [Google Scholar] [CrossRef]
- Leisner, T.M.; Freeman, T.C.; Black, J.L.; Parise, L.V. CIB1: A Small Protein with Big Ambitions. FASEB J. 2016, 30, 2640–2650. [Google Scholar] [CrossRef]
- Weisz, J.; Uversky, V.N. Zooming into the Dark Side of Human Annexin-S100 Complexes: Dynamic Alliance of Flexible Partners. Int. J. Mol. Sci. 2020, 21, 5879. [Google Scholar] [CrossRef]
- Groenendyk, J.; Wang, W.A.; Robinson, A.; Michalak, M. Calreticulin and the Heart. Cells 2022, 11, 1722. [Google Scholar] [CrossRef]
- Wang, Q.; Michalak, M. Calsequestrin. Structure, Function, and Evolution. Cell Calcium 2020, 90, 102242. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, R. Functional Pleiotropy of Calcium Binding Protein Regucalcin in Signaling and Diseases. Cell. Signal. 2023, 102, 110533. [Google Scholar] [CrossRef] [PubMed]
- Kadio, B.; Yaya, S.; Basak, A.; Djè, K.; Gomes, J.; Mesenge, C. Calcium Role in Human Carcinogenesis: A Comprehensive Analysis and Critical Review of Literature. Cancer Metastasis Rev. 2016, 35, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.S.; Houy, S.; Sørensen, J.B. C2-Domain Containing Calcium Sensors in Neuroendocrine Secretion. J. Neurochem. 2016, 139, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Busch, E.; Hohenester, E.; Timpl, R.; Paulsson, M.; Maurer, P. Calcium Affinity, Cooperativity, and Domain Interactions of Extracellular EF-Hands Present in BM-40. J. Biol. Chem. 2000, 275, 25508–25515. [Google Scholar] [CrossRef]
- Vaz, J.; Ansari, D.; Sasor, A.; Andersson, R. SPARC: A Potential Prognostic and Therapeutic Target in Pancreatic Cancer. Pancreas 2015, 44, 1024–1035. [Google Scholar] [CrossRef]
- Wang, C.K.; Ghani, H.A.; Bundock, A.; Weidmann, J.; Harvey, P.J.; Edwards, I.A.; Schroeder, C.I.; Swedberg, J.E.; Craik, D.J. Calcium-Mediated Allostery of the EGF Fold. ACS Chem. Biol. 2018, 13, 1659–1667. [Google Scholar] [CrossRef]
- Cristiani, A.; Maset, F.; De Toni, L.; Guidolin, D.; Sabbadin, D.; Strapazzon, G.; Moro, S.; De Filippis, V.; Foresta, C. Carboxylation-Dependent Conformational Changes of Human Osteocalcin. Front. Biosci. 2014, 19, 1105–1116. [Google Scholar] [CrossRef][Green Version]
- Noronha, C.; Ribeiro, A.S.; Taipa, R.; Castro, D.S.; Reis, J.; Faria, C.; Paredes, J. Cadherin Expression and EMT: A Focus on Gliomas. Biomedicines 2021, 9, 1328. [Google Scholar] [CrossRef]
- Wong, S.H.M.; Fang, C.M.; Chuah, L.H.; Leong, C.O.; Ngai, S.C. E-Cadherin: Its Dysregulation in Carcinogenesis and Clinical Implications. Crit. Rev. Oncol. Hematol. 2018, 121, 11–22. [Google Scholar] [CrossRef]
- Lepenies, B.; Johannssen, T.; Hütter, J.; Zimmermann, S. C-Type Lectins. In Glycoscience: Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 2022; pp. 675–683. Available online: https://www.ncbi.nlm.nih.gov/books/NBK579916/ (accessed on 19 October 2025).
- Zou, J.; Jiang, J.Y.; Yang, J.J. Molecular Basis for Modulation of Metabotropic Glutamate Receptors and Their Drug Actions by Extracellular Ca2+. Int. J. Mol. Sci. 2017, 18, 672. [Google Scholar] [CrossRef]
- Patel, B.S.; Ravix, J.; Pabelick, C.; Prakash, Y.S. Class C GPCRs in the Airway. Curr. Opin. Pharmacol. 2020, 51, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Gerbino, A.; Hofer, A.M.; Curci, S. Recent Advances in Understanding the Extracellular Calcium-Sensing Receptor. F1000Research 2016, 5, 2535. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.T.T.; Campos, M.M.; Carvalho, V.d.P.R.; da Silva Junior, C.A.; Magno, L.A.V.; de Souza, A.H.; Gomez, M.V. Current Drug Development Overview: Targeting Voltage-Gated Calcium Channels for the Treatment of Pain. Int. J. Mol. Sci. 2023, 24, 9223. [Google Scholar] [CrossRef]
- Bao, X.; Wang, J.; Wei, L. Impact of Calcium Channel Antagonists for Estrogen Action on the Endometrial Carcinoma HEC-1A Cells. Zhonghua Fu Chan Ke Za Zhi 2012, 47, 212–217. [Google Scholar]
- Hao, J.; Bao, X.; Jin, B.; Wang, X.; Mao, Z.; Li, X.; Wei, L.; Shen, D.; Wang, J.L. Ca2+ Channel Subunit α 1D Promotes Proliferation and Migration of Endometrial Cancer Cells Mediated by 17β-Estradiol via the G Protein-Coupled Estrogen Receptor. FASEB J. 2015, 29, 2883–2893. [Google Scholar] [CrossRef]
- Huang, T.; Feng, X.; Wang, J.; Zhou, J.; Wang, J. Calcium-Related Genes Predicting Outcomes and Serving as Therapeutic Targets in Endometrial Cancer. Cells 2022, 11, 3156. [Google Scholar] [CrossRef]
- De Clercq, K.; Held, K.; Van Bree, R.; Meuleman, C.; Peeraer, K.; Tomassetti, C.; Voets, T.; D’Hooghe, T.; Vriens, J. Functional Expression of Transient Receptor Potential Channels in Human Endometrial Stromal Cells during the Luteal Phase of the Menstrual Cycle. Hum. Reprod. 2015, 30, 1421–1436. [Google Scholar] [CrossRef]
- De Clercq, K.; Van Den Eynde, C.; Hennes, A.; Van Bree, R.; Voets, T.; Vriens, J. The Functional Expression of Transient Receptor Potential Channels in the Mouse Endometrium. Hum. Reprod. 2017, 32, 615–630. [Google Scholar] [CrossRef]
- Liu, L.; Lin, J.; He, H. Identification of Potential Crucial Genes Associated With the Pathogenesis and Prognosis of Endometrial Cancer. Front. Genet. 2019, 10, 373. [Google Scholar] [CrossRef]
- Marinelli, O.; Morelli, M.B.; Annibali, D.; Aguzzi, C.; Zeppa, L.; Tuyaerts, S.; Amantini, C.; Amant, F.; Ferretti, B.; Maggi, F.; et al. The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. Int. J. Mol. Sci. 2020, 21, 5409. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Schneider, S.; Yang, W.; Liu, G.; Schmidt, E.M.; Schmid, E.; Mia, S.; Brucker, S.; Stournaras, C.; Wallwiener, D.; et al. TGFβ1 and SGK1-Sensitive Store-Operated Ca2+ Entry and Orai1 Expression in Endometrial Ishikawa Cells. Mol. Hum. Reprod. 2014, 20, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Chin-Smith, E.C.; Slater, D.M.; Johnson, M.R.; Tribe, R.M. STIM and Orai Isoform Expression in Pregnant Human Myometrium: A Potential Role in Calcium Signaling during Pregnancy. Front. Physiol. 2014, 5, 169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sutovska, M.; Kocmalova, M.; Sadlonova, V.; Dokus, K.; Adamkov, M.; Luptak, J.; Franova, S. Orai1 Protein Expression and the Role of Calcium Release-Activated Calcium Channels in the Contraction of Human Term-Pregnant and Non-Pregnant Myometrium. J. Obstet. Gynaecol. Res. 2015, 41, 704–711. [Google Scholar] [CrossRef]
- Yang, H.; Kim, T.H.; Lee, H.H.; Choi, K.C.; Jeung, E.B. Distinct Expression of the Calcium Exchangers, NCKX3 and NCX1, and Their Regulation by Steroid in the Human Endometrium during the Menstrual Cycle. Reprod. Sci. 2011, 18, 577–585. [Google Scholar] [CrossRef]
- Yang, H.; Kim, T.H.; An, B.S.; Choi, K.C.; Lee, H.H.; Kim, J.M.; Jeung, E.B. Differential Expression of Calcium Transport Channels in Placenta Primary Cells and Tissues Derived from Preeclamptic Placenta. Mol. Cell. Endocrinol. 2013, 367, 21–30. [Google Scholar] [CrossRef]
- Cunha, G.R.; Sinclair, A.; Ricke, W.A.; Robboy, S.J.; Cao, M.; Baskin, L.S. Reproductive Tract Biology: Of Mice and Men. Differentiation 2019, 110, 49–63. [Google Scholar] [CrossRef]
- Tribe, R.M.; Moriarty, P.; Poston, L. Calcium Homeostatic Pathways Change with Gestation in Human Myometrium. Biol. Reprod. 2000, 63, 748–755. [Google Scholar] [CrossRef]
- Ackerman IV, W.E.; Buhimschi, C.S.; Snedden, A.; Summerfield, T.L.; Zhao, G.; Buhimschi, I.A. Molecular Signatures of Labor and Nonlabor Myometrium with Parsimonious Classification from 2 Calcium Transporter Genes. JCI Insight 2021, 6, e148425. [Google Scholar] [CrossRef]
- Martin, C.; Chapman, K.E.; Thornton, S.; Ashley, R.H. Changes in the Expression of Myometrial Ryanodine Receptor MRNAs during Human Pregnancy. Biochim. Biophys. Acta 1999, 1451, 343–352. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, Z.; Dai, H. Calcium Concentration Response to Uterine Ischemia: A Comparison of Uterine Fibroid Cells and Adjacent Normal Myometrial Cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 174, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.S.; Lamb, H.K.; Morgan, J.M.; Dunlop, W.; Gillespie, J.I. Differential Expression of Ryanodine Receptor RyR2 MRNA in the Non-Pregnant and Pregnant Human Myometrium. Biochem. J. 1997, 322 Pt 3, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; López Bernal, A.; Varney, M.; Watson, S.P. Inositol 1,4,5-Trisphosphate and Oxytocin Binding in Human Myometrium. Endocrinology 1990, 127, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakova, P.A.; Tarasova, N.V.; Volodina, M.A.; Tsvirkun, D.V.; Sukhanova, I.A.; Kurchakova, T.A.; Kan, N.E.; Medzidova, M.K.; Sukhikh, G.T.; Vysokikh, M.Y. Gestation Age-Associated Dynamics of Mitochondrial Calcium Uniporter Subunits Expression in Feto-Maternal Complex at Term and Preterm Delivery. Sci. Rep. 2019, 9, 5501. [Google Scholar] [CrossRef]
- Xiao, H.; Ma, L.; Ding, J.; Wang, H.; Bi, X.; Tan, F.; Piao, W. Mitochondrial Calcium Uniporter (MCU) That Modulates Mitochondrial Uptake and Facilitates Endometrial Cancer Progression through Interaction with VDAC1. Curr. Cancer Drug Targets 2023, 24, 354–367. [Google Scholar] [CrossRef]
- Diver, M.M.; Lin King, J.V.; Julius, D.; Cheng, Y. Sensory TRP Channels in Three Dimensions. Annu. Rev. Biochem. 2022, 91, 629–649. [Google Scholar] [CrossRef]
- Soussi, M.; Hasselsweiller, A.; Gkika, D. TRP Channels: The Neglected Culprits in Breast Cancer Chemotherapy Resistance? Membranes 2023, 13, 788. [Google Scholar] [CrossRef]
- Van den Eynde, C.; Vriens, J.; De Clercq, K. Transient Receptor Potential Channel Regulation by Growth Factors. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118950. [Google Scholar] [CrossRef]
- Hennes, A.; Held, K.; Boretto, M.; De Clercq, K.; Van Den Eynde, C.; Vanhie, A.; Van Ranst, N.; Benoit, M.; Luyten, C.; Peeraer, K.; et al. Functional Expression of the Mechanosensitive PIEZO1 Channel in Primary Endometrial Epithelial Cells and Endometrial Organoids. Sci. Rep. 2019, 9, 1779. [Google Scholar] [CrossRef]
- Persoons, E.; Hennes, A.; De Clercq, K.; Van Bree, R.; Vriens, G.; O, D.F.; Peterse, D.; Vanhie, A.; Meuleman, C.; Voets, T.; et al. Functional Expression of TRP Ion Channels in Endometrial Stromal Cells of Endometriosis Patients. Int. J. Mol. Sci. 2018, 19, 2467. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-Induced Cell Death in Endometrial Cancer Cells: Involvement of TRPV1 Receptors in Apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Barnes, C.J.; Kumar, R. Estrogen and Tamoxifen Induce Cytoskeletal Remodeling and Migration in Endometrial Cancer Cells. Endocrinology 2006, 147, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Cheng, Y.; Yang, X.; Zhou, J.Y.; Dong, Y.Y.; Shen, B.Q.; Wang, J.Q.; Zhao, L.J.; Wang, Z.Q.; Li, X.P.; et al. Decreased Expression of TRPM4 Is Associated with Unfavorable Prognosis and Aggressive Progression of Endometrial Carcinoma. Am. J. Transl. Res. 2020, 12, 3926. [Google Scholar] [PubMed]
- Berlansky, S.; Humer, C.; Sallinger, M.; Frischauf, I. More than Just Simple Interaction between STIM and Orai Proteins: Crac Channel Function Enabled by a Network of Interactions with Regulatory Proteins. Int. J. Mol. Sci. 2021, 22, 471. [Google Scholar] [CrossRef]
- Berna-Erro, A.; Sanchez-Collado, J.; Nieto-Felipe, J.; Macias-Diaz, A.; Redondo, P.C.; Smani, T.; Lopez, J.J.; Jardin, I.; Rosado, J.A. The Ca2+ Sensor STIM in Human Diseases. Biomolecules 2023, 13, 1284. [Google Scholar] [CrossRef]
- Shim, A.H.R.; Tirado-Lee, L.; Prakriya, M. Structural and Functional Mechanisms of CRAC Channel Regulation. J. Mol. Biol. 2015, 427, 77–93. [Google Scholar] [CrossRef]
- Salker, M.S.; Singh, Y.; Durairaj, R.R.P.; Yan, J.; Alauddin, M.; Zeng, N.; Steel, J.H.; Zhang, S.; Nautiyal, J.; Webster, Z.; et al. LEFTY2 Inhibits Endometrial Receptivity by Downregulating Orai1 Expression and Store-Operated Ca2+ Entry. J. Mol. Med. 2018, 96, 173–182. [Google Scholar] [CrossRef]
- Sohn, J.O.; Seong, S.Y.; Kim, H.J.; Jo, Y.M.; Lee, K.H.; Chung, M.K.; Song, H.J.; Park, K.S.; Lim, J.M. Alterations in Intracellular Ca2+ Levels in Human Endometrial Stromal Cells after Decidualization. Biochem. Biophys. Res. Commun. 2019, 515, 318–324. [Google Scholar] [CrossRef]
- Murtazina, D.A.; Chung, D.; Ulloa, A.; Bryan, E.; Galan, H.L.; Sanborn, B.M. TRPC1, STIM1, and ORAI Influence Signal-Regulated Intracellular and Endoplasmic Reticulum Calcium Dynamics in Human Myometrial Cells. Biol. Reprod. 2011, 85, 315–326. [Google Scholar] [CrossRef]
- Hassan, M.T.; Lytton, J. Potassium-Dependent Sodium-Calcium Exchanger (NCKX) Isoforms and Neuronal Function. Cell Calcium 2020, 86, 102135. [Google Scholar] [CrossRef]
- Giladi, M.; Shor, R.; Lisnyansky, M.; Khananshvili, D. Structure-Functional Basis of Ion Transport in Sodium–Calcium Exchanger (NCX) Proteins. Int. J. Mol. Sci. 2016, 17, 1949. [Google Scholar] [CrossRef]
- Rodrigues, T.; Estevez, G.N.N.; Tersariol, I.L.d.S. Na+/Ca2+ Exchangers: Unexploited Opportunities for Cancer Therapy? Biochem. Pharmacol. 2019, 163, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Chovancova, B.; Liskova, V.; Babula, P.; Krizanova, O. Role of Sodium/Calcium Exchangers in Tumors. Biomolecules 2020, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Al-Khannaq, M.; Lytton, J. Regulation of K+-Dependent Na+/Ca2+-Exchangers (NCKX). Int. J. Mol. Sci. 2022, 24, 598. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lytton, J. Molecular Cloning of a Sixth Member of the K+-Dependent Na+/Ca2+ Exchanger Gene Family, NCKX6. J. Biol. Chem. 2004, 279, 5867–5876. [Google Scholar] [CrossRef]
- Kostic, M.; Sekler, I. Functional Properties and Mode of Regulation of the Mitochondrial Na+/Ca2+ Exchanger, NCLX. Semin. Cell Dev. Biol. 2019, 94, 59–65. [Google Scholar] [CrossRef]
- Katoshevski, T.; Ben-Kasus Nissim, T.; Sekler, I. Recent Studies on NCLX in Health and Diseases. Cell Calcium 2021, 94, 102345. [Google Scholar] [CrossRef]
- Nuñez-Rios, J.D.; Ulrich, H.; Díaz-Muñoz, M.; Lameu, C.; Vázquez-Cuevas, F.G. Purinergic System in Cancer Stem Cells. Purinergic Signal. 2023, 21, 23–38. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.G.; Huang, C.; et al. From Purines to Purinergic Signalling: Molecular Functions and Human Diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef]
- Dal Ben, D.; Buccioni, M.; Lambertucci, C.; Marucci, G.; Thomas, A.; Volpini, R. Purinergic P2X Receptors: Structural Models and Analysis of Ligand-Target Interaction. Eur. J. Med. Chem. 2015, 89, 561–580. [Google Scholar] [CrossRef]
- Coddou, C.; Yan, Z.; Obsil, T.; Pablo Huidobro-Toro, J.; Stojilkovic, S.S. Activation and Regulation of Purinergic P2X Receptor Channels. Pharmacol. Rev. 2011, 63, 641–683. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, X.; Zhou, L.; Catera, D.; Rote, N.S.; Potashkin, J.; Abdul-Karim, F.W.; Gorodeski, G.I. Decreased Expression of P2X7 in Endometrial Epithelial Pre-Cancerous and Cancer Cells. Gynecol. Oncol. 2007, 106, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M. P-Type ATPases: Many More Enigmas Left to Solve. J. Biol. Chem. 2023, 299, 105352. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Carafoli, E. Calcium Pumps in Health and Disease. Physiol. Rev. 2009, 89, 1341–1378. [Google Scholar] [CrossRef]
- Calì, T.; Brini, M.; Carafoli, E. Regulation of Cell Calcium and Role of Plasma Membrane Calcium ATPases. In International Review of Cell and Molecular Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 332, pp. 259–296. [Google Scholar]
- Bergner, A.; Huber, R. Regulation of the Endoplasmic Reticulum Ca(2+)-Store in Cancer. Anticancer Agents Med. Chem. 2008, 8, 705–709. [Google Scholar] [CrossRef]
- Verkhratsky, A. Physiology and Pathophysiology of the Calcium Store in the Endoplasmic Reticulum of Neurons. Physiol. Rev. 2005, 85, 201–279. [Google Scholar] [CrossRef]
- Baba-Aissa, F.; Raeymaekers, L.; Wuytack, F.; Dode, L.; Casteels, R. Distribution and Isoform Diversity of the Organellar Ca2+ Pumps in the Brain. Mol. Chem. Neuropathol. 1998, 33, 199–208. [Google Scholar] [CrossRef]
- Vangheluwe, P.; Wuytack, F. Improving Cardiac Ca2+ Transport into the Sarcoplasmic Reticulum in Heart Failure: Lessons from the Ubiquitous SERCA2b Ca2+ Pump. Biochem. Soc. Trans. 2011, 39, 781–787. [Google Scholar] [CrossRef]
- Boczek, T.; Sobolczyk, M.; Mackiewicz, J.; Lisek, M.; Ferenc, B.; Guo, F.; Zylinska, L. Crosstalk among Calcium ATPases: PMCA, SERCA and SPCA in Mental Diseases. Int. J. Mol. Sci. 2021, 22, 2785. [Google Scholar] [CrossRef]
- Tribe, R.M.; Moriarty, P.; Dalrymple, A.; Hassoni, A.A.; Poston, L. Interleukin-1beta Induces Calcium Transients and Enhances Basal and Store Operated Calcium Entry in Human Myometrial Smooth Muscle. Biol. Reprod. 2003, 68, 1842–1849. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Taheri, M. Maternally Expressed Gene 3 (MEG3): A Tumor Suppressor Long Non Coding RNA. Biomed. Pharmacother. 2019, 118, 109129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Klibanski, A. MEG3 Noncoding RNA: A Tumor Suppressor. J. Mol. Endocrinol. 2012, 48, R45–R53. [Google Scholar] [CrossRef] [PubMed]
- Chetry, M.; Thapa, S.; Hu, X.; Song, Y.; Zhang, J.; Zhu, H.; Zhu, X. The Role of Galectins in Tumor Progression, Treatment and Prognosis of Gynecological Cancers. J. Cancer 2018, 9, 4742–4755. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Cui, D.; Fei, X.; Lv, Y.; Lin, J. LncRNA MEG3-210 Regulates Endometrial Stromal Cells Migration, Invasion and Apoptosis through P38 MAPK and PKA/SERCA2 Signalling via Interaction with Galectin-1 in Endometriosis. Mol. Cell. Endocrinol. 2020, 513, 110870. [Google Scholar] [CrossRef]
- Vanoevelen, J.; Dode, L.; Van Baelen, K.; Fairclough, R.J.; Missiaen, L.; Raeymaekers, L.; Wuytack, F. The Secretory Pathway Ca2+/Mn2+-ATPase 2 Is a Golgi-Localized Pump with High Affinity for Ca2+ Ions. J. Biol. Chem. 2005, 280, 22800–22808. [Google Scholar] [CrossRef]
- Pestov, N.B.; Dmitriev, R.I.; Kostina, M.B.; Korneenko, T.V.; Shakhparonov, M.I.; Modyanov, N.N. Structural Evolution and Tissue-Specific Expression of Tetrapod-Specific Second Isoform of Secretory Pathway Ca2+-ATPase. Biochem. Biophys. Res. Commun. 2012, 417, 1298–1303. [Google Scholar] [CrossRef]
- Wootton, L.L.; Argent, C.C.H.; Wheatley, M.; Michelangeli, F. The Expression, Activity and Localisation of the Secretory Pathway Ca2+-ATPase (SPCA1) in Different Mammalian Tissues. Biochim. Biophys. Acta Biomembr. 2004, 1664, 189–197. [Google Scholar] [CrossRef]
- Van Baelen, K.; Dode, L.; Vanoevelen, J.; Callewaert, G.; De Smedt, H.; Missiaen, L.; Parys, J.B.; Raeymaekers, L.; Wuytack, F. The Ca2+/Mn2+ Pumps in the Golgi Apparatus. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1742, 103–112. [Google Scholar] [CrossRef]
- Xiang, M.; Mohamalawari, D.; Rao, R. A Novel Isoform of the Secretory Pathway Ca2+,Mn(2+)-ATPase, HSPCA2, Has Unusual Properties and Is Expressed in the Brain. J. Biol. Chem. 2005, 280, 11608–11614. [Google Scholar] [CrossRef]
- Dode, L.; Andersen, J.P.; Vanoevelen, J.; Raeymaekers, L.; Missiaen, L.; Vilsen, B.; Wuytack, F. Dissection of the Functional Differences between Human Secretory Pathway Ca2+/Mn2+-ATPase (SPCA) 1 and 2 Isoenzymes by Steady-State and Transient Kinetic Analyses. J. Biol. Chem. 2006, 281, 3182–3189. [Google Scholar] [CrossRef]
- Garside, V.C.; Kowalik, A.S.; Johnson, C.L.; DiRenzo, D.; Konieczny, S.F.; Pin, C.L. MIST1 Regulates the Pancreatic Acinar Cell Expression of Atp2c2, the Gene Encoding Secretory Pathway Calcium ATPase 2. Exp. Cell Res. 2010, 316, 2859–2870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, M.; Wu, C.; Song, T.; Pan, K.; Wang, Y.; Liu, Z. Structure and Transport Mechanism of the Human Calcium Pump SPCA1. Cell Res. 2023, 33, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Grice, D.M.; Faddy, H.M.; Nguyen, N.; Leitch, S.; Wang, Y.; Muend, S.; Kenny, P.A.; Sukumar, S.; Roberts-Thomson, S.J.; et al. Store-Independent Activation of Orai1 by SPCA2 in Mammary Tumors. Cell 2010, 143, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Prasad, H.; Rao, R. Secretory Pathway Ca2+ -ATPases Promote in Vitro Microcalcifications in Breast Cancer Cells. Mol. Carcinog. 2017, 56, 2474–2485. [Google Scholar] [CrossRef]
- Grice, D.M.; Vetter, I.; Faddy, H.M.; Kenny, P.A.; Roberts-Thomson, S.J.; Monteith, G.R. Golgi Calcium Pump Secretory Pathway Calcium ATPase 1 (SPCA1) Is a Key Regulator of Insulin-like Growth Factor Receptor (IGF1R) Processing in the Basal-like Breast Cancer Cell Line MDA-MB-231. J. Biol. Chem. 2010, 285, 37458–37466. [Google Scholar] [CrossRef]
- Smaardijk, S.; Chen, J.; Wuytack, F.; Vangheluwe, P. SPCA2 Couples Ca2+ Influx via Orai1 to Ca2+ Uptake into the Golgi/Secretory Pathway. Tissue Cell 2017, 49, 141–149. [Google Scholar] [CrossRef]
- Tempel, B.L.; Shilling, D.J. The Plasma Membrane Calcium ATPase and Disease. Subcell. Biochem. 2007, 45, 365–383. [Google Scholar] [CrossRef]
- Strehler, E.E. Plasma Membrane Calcium ATPases: From Generic Ca2+ Sump Pumps to Versatile Systems for Fine-Tuning Cellular Ca2+. Biochem. Biophys. Res. Commun. 2015, 460, 26–33. [Google Scholar] [CrossRef]
- Dang, D.; Rao, R. Calcium-ATPases: Gene Disorders and Dysregulation in Cancer. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 1344–1350. [Google Scholar] [CrossRef]
- Di Leva, F.; Domi, T.; Fedrizzi, L.; Lim, D.; Carafoli, E. The Plasma Membrane Ca2+ ATPase of Animal Cells: Structure, Function and Regulation. Arch. Biochem. Biophys. 2008, 476, 65–74. [Google Scholar] [CrossRef]
- Krebs, J. Structure, Function and Regulation of the Plasma Membrane Calcium Pump in Health and Disease. Int. J. Mol. Sci. 2022, 23, 1027. [Google Scholar] [CrossRef] [PubMed]
- Hegedűs, L.; Zámbó, B.; Pászty, K.; Padányi, R.; Varga, K.; Penniston, J.T.; Enyedi, Á. Molecular Diversity of Plasma Membrane Ca2+ Transporting ATPases: Their Function Under Normal and Pathological Conditions. Adv. Exp. Med. Biol. 2020, 1131, 93–129. [Google Scholar] [PubMed]
- Thomas, R.C. The Plasma Membrane Calcium ATPase (PMCA) of Neurones Is Electroneutral and Exchanges 2H+ for Each Ca2+ or Ba2+ Ion Extruded. J. Physiol. 2009, 587, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Woll, K.A.; Van Petegem, F. Calcium-Release Channels: Structure and Function of IP3 Receptors and Ryanodine Receptors. Physiol. Rev. 2022, 102, 209–268. [Google Scholar] [CrossRef]
- Van Petegem, F. Ryanodine Receptors: Structure and Function. J. Biol. Chem. 2012, 287, 31624–31632. [Google Scholar] [CrossRef]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine Receptors: Structure, Expression, Molecular Details, and Function in Calcium Release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef]
- Takeshima, H.; Nishimura, S.; Matsumoto, T.; Ishida, H.; Kangawa, K.; Minamino, N.; Matsuo, H.; Ueda, M.; Hanaoka, M.; Hirose, T.; et al. Primary Structure and Expression from Complementary DNA of Skeletal Muscle Ryanodine Receptor. Nature 1989, 339, 439–445. [Google Scholar] [CrossRef]
- Otsu, K.; Willard, H.F.; Khanna, V.K.; Zorzato, F.; Green, N.M.; MacLennan, D.H. Molecular Cloning of CDNA Encoding the Ca2+ Release Channel (Ryanodine Receptor) of Rabbit Cardiac Muscle Sarcoplasmic Reticulum. J. Biol. Chem. 1990, 265, 13472–13483. [Google Scholar] [CrossRef]
- Zorzato, F.; Fujii, J.; Otsu, K.; Phillips, M.; Green, N.M.; Lai, F.A.; Meissner, G.; MacLennan, D.H. Molecular Cloning of CDNA Encoding Human and Rabbit Forms of the Ca2+ Release Channel (Ryanodine Receptor) of Skeletal Muscle Sarcoplasmic Reticulum. J. Biol. Chem. 1990, 265, 2244–2256. [Google Scholar] [CrossRef]
- Nakai, J.; Imagawa, T.; Hakamata, Y.; Shigekawa, M.; Takeshima, H.; Numa, S. Primary Structure and Functional Expression from CDNA of the Cardiac Ryanodine Receptor/Calcium Release Channel. FEBS Lett. 1990, 271, 169–177. [Google Scholar] [CrossRef]
- Hakamata, Y.; Nakai, J.; Takeshima, H.; Imoto, K. Primary Structure and Distribution of a Novel Ryanodine Receptor/Calcium Release Channel from Rabbit Brain. FEBS Lett. 1992, 312, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N. Molecular Insights into Calcium Dependent Regulation of Ryanodine Receptor Calcium Release Channels. Adv. Exp. Med. Biol. 2020, 1131, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Noble, K.; Matthew, A.; Burdyga, T.; Wray, S. A Review of Recent Insights into the Role of the Sarcoplasmic Reticulum and Ca Entry in Uterine Smooth Muscle. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, S11–S19. [Google Scholar] [CrossRef] [PubMed]
- Wray, S.; Shmygol, A. Role of the Calcium Store in Uterine Contractility. Semin. Cell Dev. Biol. 2007, 18, 315–320. [Google Scholar] [CrossRef]
- Mistry, A.M.; Saldanha, G.; Bersselaar, L.R.v.d.; Knock, G.A.; Goldberg, M.F.; Vanegas, M.I.; Fernandez-Garcia, M.A.; Treves, S.; Voermans, N.C.; Tribe, R.M.; et al. Obstetric and Gynaecological Features in Females Carrying Variants in the Skeletal Muscle Ryanodine Receptor Type 1 (RYR1) Gene: A Questionnaire Study. Neuromuscul. Disord. 2025, 49, 105335. [Google Scholar] [CrossRef]
- Barata, H.; Thompson, M.; Zielinska, W.; Han, Y.S.; Mantilla, C.B.; Prakash, Y.S.; Feitoza, S.; Sieck, G.; Chini, E.N. The Role of Cyclic-ADP-Ribose-Signaling Pathway in Oxytocin-Induced Ca2+ Transients in Human Myometrium Cells. Endocrinology 2004, 145, 881–889. [Google Scholar] [CrossRef]
- Worton, S.A.; Pritchard, H.A.T.; Greenwood, S.L.; Alakrawi, M.; Heazell, A.E.P.; Wareing, M.; Greenstein, A.; Myers, J.E. Kynurenine Relaxes Arteries of Normotensive Women and Those with Preeclampsia. Circ. Res. 2021, 128, 1679–1693. [Google Scholar] [CrossRef]
- Kusama, K.; Yoshie, M.; Tamura, K.; Imakawa, K.; Isaka, K.; Tachikawa, E. Regulatory Action of Calcium Ion on Cyclic AMP-Enhanced Expression of Implantation-Related Factors in Human Endometrial Cells. PLoS ONE 2015, 10, e0132017. [Google Scholar] [CrossRef]
- Fan, G.; Baker, M.R.; Terry, L.E.; Arige, V.; Chen, M.; Seryshev, A.B.; Baker, M.L.; Ludtke, S.J.; Yule, D.I.; Serysheva, I.I. Conformational Motions and Ligand-Binding Underlying Gating and Regulation in IP3R Channel. Nat. Commun. 2022, 13, 6942. [Google Scholar] [CrossRef]
- Baker, M.R.; Fan, G.; Serysheva, I.I. Structure of IP3R Channel: High-Resolution Insights from Cryo-EM. Curr. Opin. Struct. Biol. 2017, 46, 38–47. [Google Scholar] [CrossRef]
- Foskett, J.K.; White, C.; Cheung, K.H.; Mak, D.O.D. Inositol Trisphosphate Receptor Ca2+ Release Channels. Physiol. Rev. 2007, 87, 593–658. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Michikawa, T.; Bosanac, I.; Ikura, M.; Mikoshiba, K. Molecular Basis of the Isoform-Specific Ligand-Binding Affinity of Inositol 1,4,5-Trisphosphate Receptors. J. Biol. Chem. 2007, 282, 12755–12764. [Google Scholar] [CrossRef] [PubMed]
- Prole, D.L.; Taylor, C.W. Structure and Function of Ip3 Receptors. Cold Spring Harb. Perspect. Biol. 2019, 11, a035063. [Google Scholar] [CrossRef] [PubMed]
- Bustos, G.; Ahumada-Castro, U.; Silva-Pavez, E.; Puebla, A.; Lovy, A.; Cesar Cardenas, J. The ER-Mitochondria Ca2+ Signaling in Cancer Progression: Fueling the Monster. Int. Rev. Cell Mol. Biol. 2021, 363, 49–121. [Google Scholar] [CrossRef]
- Phaneuf, S.; Carrasco, M.P.; Europe-Finner, G.N.; Hamilton, C.H.; López Bernal, A. Multiple G Proteins and Phospholipase C Isoforms in Human Myometrial Cells: Implication for Oxytocin Action. J. Clin. Endocrinol. Metab. 1996, 81, 2098–2103. [Google Scholar] [CrossRef][Green Version]
- Morgan, J.M.; De Smedt, H.; Gillespie, J.I. Identification of Three Isoforms of the InsP3 Receptor in Human Myometrial Smooth Muscle. Pflug. Arch. 1996, 431, 697–705. [Google Scholar] [CrossRef]
- Brighton, P.J.; Fossler, M.J.; Quenby, S.; Blanks, A.M. Functionally Selective Inhibition of the Oxytocin Receptor by Retosiban in Human Myometrial Smooth Muscle. Endocrinology 2020, 161, bqz043. [Google Scholar] [CrossRef]
- Hennes, A.; Devroe, J.; De Clercq, K.; Ciprietti, M.; Held, K.; Luyten, K.; Van Ranst, N.; Maenhoudt, N.; Peeraer, K.; Vankelecom, H.; et al. Protease Secretions by the Invading Blastocyst Induce Calcium Oscillations in Endometrial Epithelial Cells via the Protease-Activated Receptor 2. Reprod. Biol. Endocrinol. 2023, 21, 37. [Google Scholar] [CrossRef]
- Borodkina, A.V.; Shatrova, A.N.; Deryabin, P.I.; Griukova, A.A.; Abushik, P.A.; Antonov, S.M.; Nikolsky, N.N.; Burova, E.B. Calcium Alterations Signal Either to Senescence or to Autophagy Induction in Stem Cells upon Oxidative Stress. Aging 2016, 8, 3400–3418. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Shi, C.; Zhang, D.; Zhang, Q.; Wang, L.; Gong, Z. Mitochondrial Calcium Uniporter Complex: Unveiling the Interplay between Its Regulators and Calcium Homeostasis. Cell. Signal. 2024, 121, 111284. [Google Scholar] [CrossRef]
- Raffaello, A.; De Stefani, D.; Sabbadin, D.; Teardo, E.; Merli, G.; Picard, A.; Checchetto, V.; Moro, S.; Szabò, I.; Rizzuto, R. The Mitochondrial Calcium Uniporter Is a Multimer That Can Include a Dominant-Negative Pore-Forming Subunit. EMBO J. 2013, 32, 2362–2376. [Google Scholar] [CrossRef]
- Sancak, Y.; Markhard, A.L.; Kitami, T.; Kovács-Bogdán, E.; Kamer, K.J.; Udeshi, N.D.; Carr, S.A.; Chaudhuri, D.; Clapham, D.E.; Li, A.A.; et al. EMRE Is an Essential Component of the Mitochondrial Calcium Uniporter Complex. Science 2013, 342, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative Genomics Identifies MCU as an Essential Component of the Mitochondrial Calcium Uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabó, I.; Rizzuto, R. A Forty-Kilodalton Protein of the Inner Membrane Is the Mitochondrial Calcium Uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, D.; Vecellio Reane, D.; Raffaello, A. Neither Too Much nor Too Little: Mitochondrial Calcium Concentration as a Balance between Physiological and Pathological Conditions. Front. Mol. Biosci. 2023, 10, 1336416. [Google Scholar] [CrossRef]
- Garg, V.; Suzuki, J.; Paranjpe, I.; Unsulangi, T.; Boyman, L.; Milescu, L.S.; Jonathan Lederer, W.; Kirichok, Y. The Mechanism of Micu-Dependent Gating of the Mitochondrial Ca2+ Uniporter. Elife 2021, 10, e69312. [Google Scholar] [CrossRef]
- Vecellio Reane, D.; Serna, J.D.C.; Raffaello, A. Unravelling the Complexity of the Mitochondrial Ca2+ Uniporter: Regulation, Tissue Specificity, and Physiological Implications. Cell Calcium 2024, 121, 102907. [Google Scholar] [CrossRef]
- Su, M.; Zheng, S.; Liu, H.; Tang, T.S.; Hu, Y. Ca2+ Homeostasis: A Potential Target for Cancer Therapies. Biophys. Rep. 2024, 10, 283. [Google Scholar] [CrossRef]
- Devis-Jauregui, L.; Eritja, N.; Davis, M.L.; Matias-Guiu, X.; Llobet-Navàs, D. Autophagy in the Physiological Endometrium and Cancer. Autophagy 2020, 17, 1077–1095. [Google Scholar] [CrossRef]
- Zhang, L.; Au-Yeung, C.L.; Huang, C.; Yeung, T.L.; Ferri-Borgogno, S.; Lawson, B.C.; Kwan, S.Y.; Yin, Z.; Wong, S.T.; Thomas, V.; et al. Ryanodine Receptor 1-Mediated Ca2+ Signaling and Mitochondrial Reprogramming Modulate Uterine Serous Cancer Malignant Phenotypes. J. Exp. Clin. Cancer Res. 2022, 41, 242. [Google Scholar] [CrossRef]
- Colussi, D.M.; Stathopulos, P.B. The Mitochondrial Calcium Uniporter: Balancing Tumourigenic and Anti-Tumourigenic Responses. J. Physiol. 2024, 602, 3315–3339. [Google Scholar] [CrossRef]
- Hong, Z.; Mo, T.; Zhou, P.; Chen, J.; Li, X. Progress of Estrogen Receptor and Spliceosome in Endometrial Carcinoma. Front Endocrinol 2025, 16, 1586191. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, N.; Pan, H.; Xie, J.; Han, W. Development of Store-Operated Calcium Entry-Targeted Compounds in Cancer. Front. Pharmacol. 2021, 12, 688244. [Google Scholar] [CrossRef]
- Costa, B.P.; Nunes, F.B.; Noal, F.C.; Branchini, G. Ion Channels in Endometrial Cancer. Cancers 2022, 14, 4733. [Google Scholar] [CrossRef]
- Gharote, M.A.; Deshpande, A.A.; Kale, A.S. Amlodipine with Letrozole as Maintenance Post-Chemotherapy: Targeting Calcium Calcineurin Calmodulin Pathway and Aromatase Inhibitor in Disseminated Endometrioid Adenocarcinoma of the Endometrium-a Case Report. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 13903–13906. [Google Scholar] [CrossRef]

| Transporter/Channel/Pump | Tissue | Changes | Comments | Ref. |
|---|---|---|---|---|
| VGCC | ||||
| CACNA1D | atypical hyperplasia, cancerous tissue | increased | IHC; vs. benign endometrial lesions | [68] |
| CACNA2D1 | endometrial cancer (TCGA-DEG) | increased | mRNA vs. normal endometrium | [69] |
| TRP | ||||
| TRPC1/4 | endometrial stroma | mRNA | [70,71] | |
| TRPC6 | endometrial stroma | mRNA | [70,71] | |
| TRPM4 | endometrial cancer (TCGA-DEG) | decreased | lower level—poorer prognosis vs. normal endometrium | [69,72] |
| TRPM6 | endometrial epithelium | mRNA | [70,71] | |
| TRPV2 | non-endometrioid cancer | increased | IHC; shorter progression-free survival vs. normal endometrium | [73] |
| TRPV2 | endometrial stroma | mRNA | [70,71] | |
| TRPV4 | endometrial cancer | increased | IHC vs. normal endometrium | [25] |
| TRPV4 | endometrial epithelium | mRNA | [70,71] | |
| TRPV6 | endometrial epithelium | mRNA | [70,71] | |
| TRPV6 | normal endometrium | secretory phase | [10] | |
| Orai/STIM | ||||
| Orai1 | normal endometrium | increased | IHC; secretory phase vs. other phases | [74] |
| Orai2 | myometrium | mRNA | [75] | |
| Orai2 | myometrium | increased | non-pregnant vs. pregnant | [76] |
| CaCA | ||||
| NCKX3 | normal endometrium | increased | mRNA; IHC; early-, mid-proliferative phases; early-secretory phase vs. other phases | [77] |
| NCKX3 | fetal and maternal placenta | increased | mRNA; IHC; preeclamptic tissue preterm labour | [78] |
| NCKX3 | fetal and maternal placenta | decreased | mRNA; IHC; term labour | [78] |
| NCKX3 | isolated placental cells (1st trimester) | increased | mRNA; WB; 1st trimester; hypoxic conditions | |
| NCX1 | fetal and maternal placenta | increased | mRNA; IHC; preeclamptic tissue preterm labour | [78] |
| NCX1 | fetal and maternal placenta | decreased | mRNA; IHC; term labour vs. preterm birth | [78] |
| NCX1 | isolated placental cells (1st trimester) | increased | mRNA; WB; hypoxic conditions vs. normoxic conditions | [78] |
| Purinergic receptors | ||||
| P2X7 | complex hyperplasia with atypia, endometrial adenocarcinoma | decreased | mRNA; IHC; vs. normal endometrium, simple hyperplasia, complex hyperplasia | [79] |
| P-type ATPases | ||||
| SERCA2(a/b) | myometrium | increased | WB; labour vs. non-labour | [80] |
| PMCA1 | normal endometrium | increased | mRNA; proliferative phase vs. other phases | [10] |
| PMCA1 | myometrium | increased | WB; labour vs. non-labour | [80] |
| PMCA4 | myometrium | increased | mRNA; labour vs. non-labour | [81] |
| RyR | ||||
| RyR1 | myometrium | constant | mRNA; pregnancy | [82] |
| RyR1 | uterine fibroids | increased | mRNA; WB vs. non-fibroids | [83] |
| RyR2 | myometrium | increased | mRNA; pregnancy vs. nonpregnant | [84] |
| RyR3 | myometrium | constant | mRNA | [82] |
| IP3R | ||||
| IP3Rs | myometrium | constant | IP3 binding assay; regardless of pregnancy status | [85] |
| IP3R1 | uterine fibroids | increased | mRNA; WB; vs. adjacent myometrium | [83] |
| MCU complex | ||||
| MCU | placenta | increase | mRNA; WB; pregnancy vs. nonpregnant | [86] |
| MCU | endometrial cancer | increased | IHC; IF vs. normal endometrium | [87] |
| MICU1 | placenta | increase | mRNA; WB; pregnancy vs. nonpregnant | [86] |
| MCU | myometrium | increase | mRNA; preterm birth vs. term birth | [86] |
| MCUb | myometrium | increase | mRNA; preterm birth vs. term birth | [86] |
| EMRE | myometrium | increase | mRNA; preterm birth vs. term birth | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, P.K. Calcium Homeostasis Machinery in the Human Uterus—A Potential Therapeutic Target in Endometrial Cancer. Int. J. Mol. Sci. 2025, 26, 10253. https://doi.org/10.3390/ijms262110253
Zakrzewski PK. Calcium Homeostasis Machinery in the Human Uterus—A Potential Therapeutic Target in Endometrial Cancer. International Journal of Molecular Sciences. 2025; 26(21):10253. https://doi.org/10.3390/ijms262110253
Chicago/Turabian StyleZakrzewski, Piotr K. 2025. "Calcium Homeostasis Machinery in the Human Uterus—A Potential Therapeutic Target in Endometrial Cancer" International Journal of Molecular Sciences 26, no. 21: 10253. https://doi.org/10.3390/ijms262110253
APA StyleZakrzewski, P. K. (2025). Calcium Homeostasis Machinery in the Human Uterus—A Potential Therapeutic Target in Endometrial Cancer. International Journal of Molecular Sciences, 26(21), 10253. https://doi.org/10.3390/ijms262110253






