Ubiquitin E3 Ligases and p53 in Doxorubicin-Induced Cardiotoxicity
Abstract
1. Doxorubicin Cardiotoxicity and p53
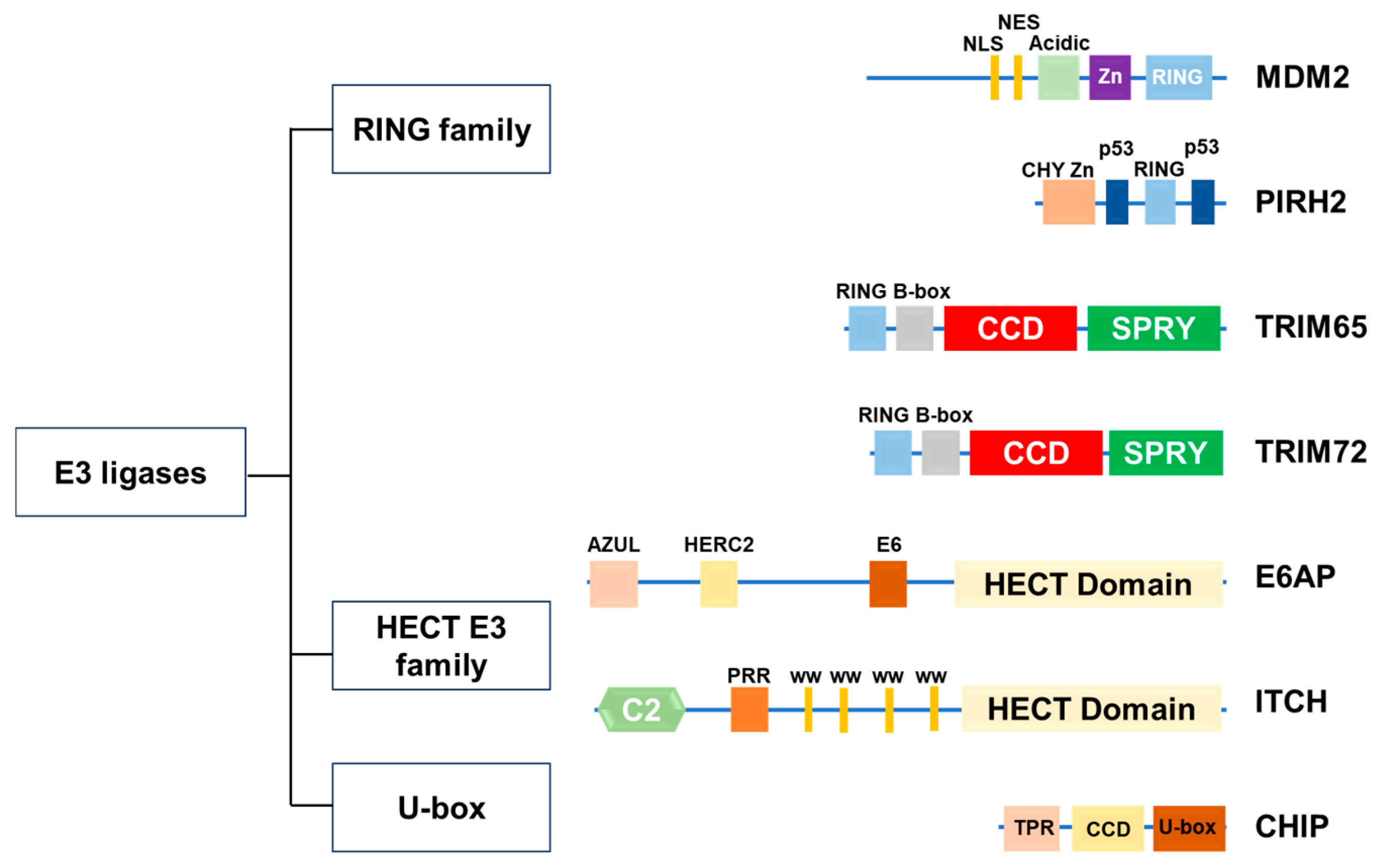
2. Ubiquitin E3 Ligase
2.1. RING-Type E3 Ligases
2.2. HECT-Type E3 Ligases
2.3. RBR-Type E3 Ligase
2.4. U-Box-Type E3 Ligase
3. Ubiquitin E3 Ligases Associated with p53 Protein Expression
3.1. MDM2
3.2. Pirh2
3.3. TRIM65
3.4. TRIM72
3.5. E6AP
3.6. ITCH
3.7. CHIP
4. The Action of Cardioprotective Drugs via Ubiquitylation
4.1. Dihydromyricetin
4.2. Licochalcone A
4.3. Resveratrol
4.4. Quercetin
4.5. Ganoderma Lucidum Polysaccharides
4.6. FGF1 Variant
4.7. Saussurea involucrata
4.8. Qishen Granule
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHIP | Carboxyl terminus of Hsp70-interacting protein |
| DIC | Doxorubicin-induced cardiotoxicity |
| E6AP | E6-associated protein |
| FGF1 | Fibroblast growth factor 1 |
| GPX4 | Glutathione peroxidase 4 |
| HECT | Homologous to E6AP C-terminus |
| MDM2 | Mouse double mutant 2 homolog |
| Nrf2 | Transcription factor NF-E2-related factor 2 |
| RBRs | RING-between-RINGs |
| RING | Really interesting new gene |
| TRIM | Tripartite motif family proteins |
| SIRT1 | Sirtuin 1 |
| SLC7A11 | Solute carrier family 7 member 11 |
References
- Renu, K.; Abilash, V.G.; Tirupathi Pichiah, P.B.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Tewey, K.M.; Rowe, T.C.; Yang, L.; Halligan, B.D.; Liu, L.F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 1984, 226, 466–468. [Google Scholar] [CrossRef]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anticancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef]
- Frias, M.A.; Lang, U.; Gerber-Wicht, C.; James, R.W. Native and reconstituted HDL protect cardiomyocytes from doxorubicin-induced apoptosis. Cardiovasc. Res. 2010, 85, 118–126. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Wu, B.B.; Leung, K.T.; Poon, E.N. Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2022, 23, 1912. [Google Scholar] [CrossRef] [PubMed]
- Linders, A.N.; Dias, I.B.; Lopez Fernandez, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A review of the pathophysiological mechanisms of doxorubicin-induced cardiotoxicity and aging. NPJ Aging 2024, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Kitakata, H.; Endo, J.; Ikura, H.; Moriyama, H.; Shirakawa, K.; Katsumata, Y.; Sano, M. Therapeutic Targets for DOX-Induced Cardiomyopathy: Role of Apoptosis vs. Ferroptosis. Int. J. Mol. Sci. 2022, 23, 1414. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef]
- Xie, L.H.; Fefelova, N.; Pamarthi, S.H.; Gwathmey, J.K. Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease. Cells 2022, 11, 2726. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Wang, G.; Ren, J. Molecular Mechanisms and Therapeutic Targeting of Ferroptosis in Doxorubicin-Induced Cardiotoxicity. JACC Basic Transl. Sci. 2024, 9, 811–826. [Google Scholar] [CrossRef]
- Men, H.; Cai, H.; Cheng, Q.; Zhou, W.; Wang, X.; Huang, S.; Zheng, Y.; Cai, L. The regulatory roles of p53 in cardiovascular health and disease. Cell Mol. Life Sci. 2021, 78, 2001–2018. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Cheng, H.; Li, H.; Zhang, Q.; He, C.; Fu, C.; Wei, Q. Targeting autophagy in doxorubicin-induced cardiotoxicity: A comprehensive review of scientific landscapes and therapeutic innovations. Ageing Res. Rev. 2025, 110, 102818. [Google Scholar] [CrossRef]
- Wallace, K.B.; Sardao, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2020, 5, e132747. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, K.M.; Bozza, W.P.; Alterovitz, W.L.; Zhang, B. Transcriptomic profiling reveals p53 as a key regulator of doxorubicin-induced cardiotoxicity. Cell Death Discov. 2019, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, S.; Otaki, Y.; Watanabe, T.; Goto, J.; Ochi, H.; Tanaka, T.; Ono, H.; Yamaguchi, R.; Sato, J.; Takahashi, H.; et al. Diacylglycerol Kinase zeta Attenuates Doxorubicin-Induced Cardiotoxicity Through p53 Degradation. J. Am. Heart Assoc. 2025, 14, e035608. [Google Scholar] [CrossRef]
- Gu, B.; Zhu, W.G. Surf the post-translational modification network of p53 regulation. Int. J. Biol. Sci. 2012, 8, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Fischbach, A.; Kruger, A.; Hampp, S.; Assmann, G.; Rank, L.; Hufnagel, M.; Stockl, M.T.; Fischer, J.M.F.; Veith, S.; Rossatti, P.; et al. The C-terminal domain of p53 orchestrates the interplay between non-covalent and covalent poly(ADP-ribosyl)ation of p53 by PARP1. Nucleic Acids Res. 2018, 46, 804–822. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Kon, N.; Gu, A.P.; Tavana, O.; Gu, W. Deciphering the acetylation code of p53 in transcription regulation and tumor suppression. Oncogene 2022, 41, 3039–3050. [Google Scholar] [CrossRef]
- Nagasaka, M.; Miyajima, C.; Aoki, H.; Aoyama, M.; Morishita, D.; Inoue, Y.; Hayashi, H. Insights into Regulators of p53 Acetylation. Cells 2022, 11, 3825. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Wang, W.; Liu, G.; Xian, W.; McKeon, F.; Zhou, J.; Zhang, R. Targeting the p53-MDM2 pathway for neuroblastoma therapy: Rays of hope. Cancer Lett. 2021, 496, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, D. Deciphering the PTM codes of the tumor suppressor p53. J. Mol. Cell Biol. 2022, 13, 774–785. [Google Scholar] [CrossRef]
- Morimoto, T.; Fujita, M.; Kawamura, T.; Sunagawa, Y.; Takaya, T.; Wada, H.; Shimatsu, A.; Kita, T.; Hasegawa, K. Myocardial regulation of p300 and p53 by doxorubicin involves ubiquitin pathways. Circ. J. 2008, 72, 1506–1511. [Google Scholar] [CrossRef]
- Willis, M.S.; Townley-Tilson, W.H.; Kang, E.Y.; Homeister, J.W.; Patterson, C. Sent to destroy: The ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ. Res. 2010, 106, 463–478. [Google Scholar] [CrossRef]
- Toma-Fukai, S.; Shimizu, T. Structural Diversity of Ubiquitin E3 Ligase. Molecules 2021, 26, 6682. [Google Scholar] [CrossRef]
- Popovic, D.; Vucic, D.; Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014, 20, 1242–1253. [Google Scholar] [CrossRef]
- Metzger, M.B.; Hristova, V.A.; Weissman, A.M. HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 2012, 125, 531–537. [Google Scholar] [CrossRef]
- George, M.; Masamba, P.; Iwalokun, B.A.; Kappo, A.P. Zooming into the structure-function of RING finger proteins for anti-cancer therapeutic applications. Am. J. Cancer Res. 2023, 15, 2773–2789. [Google Scholar]
- Wang, Y.; Argiles-Castillo, D.; Kane, E.I.; Zhou, A.; Spratt, D.E. HECT E3 ubiquitin ligases—emerging insights into their biological roles and disease relevance. J. Cell Sci. 2020, 133, jcs228072, Erratum in: J. Cell Sci. 2020, 133, jcs258087. [Google Scholar] [CrossRef]
- Goto, J.; Otaki, Y.; Watanabe, T.; Watanabe, M. The Role of HECT-Type E3 Ligase in the Development of Cardiac Disease. Int. J. Mol. Sci. 2021, 22, 6065. [Google Scholar] [CrossRef]
- Safreena, N.; Nair, I.C.; Chandra, G. Therapeutic potential of Parkin and its regulation in Parkinson’s disease. Biochem. Pharmacol. 2024, 230, 116600. [Google Scholar] [CrossRef]
- Idrissou, M.; Marechal, A. The PRP19 Ubiquitin Ligase, Standing at the Cross-Roads of mRNA Processing and Genome Stability. Cancers 2022, 14, 878. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 ubiquitin ligases: Styles, structures and functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Momand, J.; Zambetti, G.P.; Olson, D.C.; George, D.; Levine, A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992, 69, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Huun, J.; Gansmo, L.B.; Mannsaker, B.; Iversen, G.T.; Ovrebo, J.I.; Lonning, P.E.; Knappskog, S. Impact of the MDM2 splice-variants MDM2-A, MDM2-B and MDM2-C on cytotoxic stress response in breast cancer cells. BMC Cell Biol. 2017, 18, 17. [Google Scholar] [CrossRef]
- Koo, N.; Sharma, A.K.; Narayan, S. Therapeutics Targeting p53-MDM2 Interaction to Induce Cancer Cell Death. Int. J. Mol. Sci. 2022, 23, 5005. [Google Scholar] [CrossRef]
- Chen, J.; Marechal, V.; Levine, A.J. Mapping of the p53 and mdm-2 interaction domains. Mol. Cell. Biol. 1993, 13, 4107–4114. [Google Scholar] [CrossRef]
- Shadfan, M.; Lopez-Pajares, V.; Yuan, Z.M. MDM2 and MDMX: Alone and together in regulation of p53. Transl. Cancer Res. 2012, 1, 88–89. [Google Scholar]
- Watanabe, T.; Ichikawa, A.; Saito, H.; Hotta, T. Overexpression of the MDM2 oncogene in leukemia and lymphoma. Leuk. Lymphoma 1996, 21, 391–397. [Google Scholar] [CrossRef]
- Schmitz-Drager, B.J.; Kushima, M.; Goebell, P.; Jax, T.W.; Gerharz, C.D.; Bultel, H.; Schulz, W.A.; Ebert, T.; Ackermann, R. p53 and MDM2 in the development and progression of bladder cancer. Eur. Urol. 1997, 32, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Dembla, V.; Somaiah, N.; Barata, P.; Hess, K.; Fu, S.; Janku, F.; Karp, D.D.; Naing, A.; Piha-Paul, S.A.; Subbiah, V.; et al. Prevalence of MDM2 amplification and coalterations in 523 advanced cancer patients in the MD Anderson phase 1 clinic. Oncotarget 2018, 9, 33232–33243. [Google Scholar] [CrossRef]
- Gao, C.; Xiao, G.; Piersigilli, A.; Gou, J.; Ogunwobi, O.; Bargonetti, J. Context-dependent roles of MDMX (MDM4) and MDM2 in breast cancer proliferation and circulating tumor cells. Breast Cancer Res. 2019, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Alaseem, A.M. Advancements in MDM2 inhibition: Clinical and pre-clinical investigations of combination therapeutic regimens. Saudi Pharm. J. 2023, 31, 101790. [Google Scholar] [CrossRef] [PubMed]
- Watson, I.R.; Li, B.K.; Roche, O.; Blanch, A.; Ohh, M.; Irwin, M.S. Chemotherapy induces NEDP1-mediated destabilization of MDM2. Oncogene 2010, 29, 297–304. [Google Scholar] [CrossRef]
- Pant, V.; Lozano, G. Dissecting the p53-Mdm2 feedback loop in vivo: Uncoupling the role in p53 stability and activity. Oncotarget 2014, 5, 1149–1156. [Google Scholar] [CrossRef]
- Maehama, T.; Kawahara, K.; Nishio, M.; Suzuki, A.; Hanada, K. Nucleolar stress induces ubiquitination-independent proteasomal degradation of PICT1 protein. J. Biol. Chem. 2014, 289, 20802–20812. [Google Scholar] [CrossRef]
- Burgess, A.; Chia, K.M.; Haupt, S.; Thomas, D.; Haupt, Y.; Lim, E. Clinical Overview of MDM2/X-Targeted Therapies. Front. Oncol. 2016, 6, 7. [Google Scholar] [CrossRef]
- Sane, S.; Rezvani, K. Essential Roles of E3 Ubiquitin Ligases in p53 Regulation. Int. J. Mol. Sci. 2017, 18, 442. [Google Scholar] [CrossRef]
- Hauck, L.; Stanley-Hasnain, S.; Fung, A.; Grothe, D.; Rao, V.; Mak, T.W.; Billia, F. Cardiac-specific ablation of the E3 ubiquitin ligase Mdm2 leads to oxidative stress, broad mitochondrial deficiency and early death. PLoS ONE 2017, 12, e0189861. [Google Scholar] [CrossRef] [PubMed]
- Jean-Charles, P.Y.; Yu, S.M.; Abraham, D.; Kommaddi, R.P.; Mao, L.; Strachan, R.T.; Zhang, Z.S.; Bowles, D.E.; Brian, L.; Stiber, J.A.; et al. Mdm2 regulates cardiac contractility by inhibiting GRK2-mediated desensitization of beta-adrenergic receptor signaling. JCI Insight 2017, 2, e95998. [Google Scholar] [CrossRef]
- Shridhar, P.; Glennon, M.S.; Pal, S.; Waldron, C.J.; Chetkof, E.J.; Basak, P.; Clavere, N.G.; Banerjee, D.; Gingras, S.; Becker, J.R. MDM2 Regulation of HIF Signaling Causes Microvascular Dysfunction in Hypertrophic Cardiomyopathy. Circulation 2023, 148, 1870–1886. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Hasegawa, K.; Morimoto, T.; Iwai-Kanai, E.; Miyamoto, S.; Kawase, Y.; Ono, K.; Wada, H.; Akao, M.; Kita, T. Expression of p300 protects cardiac myocytes from apoptosis in vivo. Biochem. Biophys. Res. Commun. 2004, 315, 733–738. [Google Scholar] [CrossRef]
- Leng, R.P.; Lin, Y.; Ma, W.; Wu, H.; Lemmers, B.; Chung, S.; Parant, J.M.; Lozano, G.; Hakem, R.; Benchimol, S. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 2003, 112, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Laister, R.C.; Lemak, A.; Wu, B.; Tai, E.; Duan, S.; Lukin, J.; Sunnerhagen, M.; Srisailam, S.; Karra, M.; et al. Molecular basis of Pirh2-mediated p53 ubiquitylation. Nat. Struct. Mol. Biol. 2008, 15, 1334–1342. [Google Scholar] [CrossRef]
- Daks, A.; Fedorova, O.; Parfenyev, S.; Nevzorov, I.; Shuvalov, O.; Barlev, N.A. The Role of E3 Ligase Pirh2 in Disease. Cells 2022, 11, 1515. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, J.; Chen, L.; Cheng, H.; Song, X.; Xue, J.; Xu, R.; Ma, J.; Ge, J. AIG1 protects against doxorubicin-induced cardiomyocyte ferroptosis and cardiotoxicity by promoting ubiquitination-mediated p53 degradation. Theranostics 2025, 15, 4931–4954. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S. TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis. Trends Biochem. Sci. 2017, 42, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Wang, X.; Hu, W.; Feng, Z. Tumor suppressor p53 cross-talks with TRIM family proteins. Genes Dis. 2021, 8, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Valletti, A.; Marzano, F.; Pesole, G.; Sbisa, E.; Tullo, A. Targeting Chemoresistant Tumors: Could TRIM Proteins-p53 Axis Be a Possible Answer? Int. J. Mol. Sci. 2019, 20, 1776. [Google Scholar] [CrossRef]
- Fornage, M.; Debette, S.; Bis, J.C.; Schmidt, H.; Ikram, M.A.; Dufouil, C.; Sigurdsson, S.; Lumley, T.; DeStefano, A.L.; Fazekas, F.; et al. Genome-wide association studies of cerebral white matter lesion burden: The CHARGE consortium. Ann. Neurol. 2011, 69, 928–939. [Google Scholar] [CrossRef]
- Liu, B.; Tang, Y.; Yang, P.; Wu, C.; Huang, Y. TRIM65 in White Matter Lesions, Innate Immunity, and Tumor. Curr. Mol. Pharmacol. 2021, 14, 798–805. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Fu, B.; Berman, M.A.; Diallo, A.; Dorf, M.E. TRIM65 regulates microRNA activity by ubiquitination of TNRC6. Proc. Natl. Acad. Sci. USA 2014, 111, 6970–6975. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Zhou, Z.; Zhou, Y.; Liu, Y.; Liu, W.; Ma, X. The Magic and Mystery of TRIM65 in Diseases. Curr. Med. Chem. 2024, 32, 6460–6475. [Google Scholar] [CrossRef]
- Li, Y.; Ma, C.; Zhou, T.; Liu, Y.; Sun, L.; Yu, Z. TRIM65 negatively regulates p53 through ubiquitination. Biochem. Biophys. Res. Commun. 2016, 473, 278–282. [Google Scholar] [CrossRef]
- Wei, W.S.; Chen, X.; Guo, L.Y.; Li, X.D.; Deng, M.H.; Yuan, G.J.; He, L.Y.; Li, Y.H.; Zhang, Z.L.; Jiang, L.J.; et al. TRIM65 supports bladder urothelial carcinoma cell aggressiveness by promoting ANXA2 ubiquitination and degradation. Cancer Lett. 2018, 435, 10–22. [Google Scholar] [CrossRef]
- Yang, Y.F.; Zhang, M.F.; Tian, Q.H.; Zhang, C.Z. TRIM65 triggers beta-catenin signaling via ubiquitylation of Axin1 to promote hepatocellular carcinoma. J. Cell Sci. 2017, 130, 3108–3115. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Y.; Zhang, X.; Wu, H.; Wang, Q.; Cai, J.; Cui, Y.; Liu, H.; Lan, P.; Wang, J.; et al. Ubiquitin ligase TRIM65 promotes colorectal cancer metastasis by targeting ARHGAP35 for protein degradation. Oncogene 2019, 38, 6429–6444. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Tang, T.; Jin, T.; Ding, C.; Zhou, R.; Jiang, W. TRIM65-catalized ubiquitination is essential for MDA5-mediated antiviral innate immunity. J. Exp. Med. 2017, 214, 459–473. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Guo, F.; Lei, T.; Li, S.; Monaghan-Nichols, P.; Jiang, Z.; Xin, H.B.; Fu, M. TRIM65 E3 ligase targets VCAM-1 degradation to limit LPS-induced lung inflammation. J. Mol. Cell Biol. 2020, 12, 190–201. [Google Scholar] [CrossRef]
- Ma, X.F.; Zhou, Y.R.; Zhou, Z.X.; Liu, H.T.; Zhou, B.B.; Deng, N.H.; Zhou, K.; Tian, Z.; Wu, Z.F.; Liu, X.Y.; et al. TRIM65 Suppresses oxLDL-induced Endothelial Inflammation by Interaction with VCAM-1 in Atherogenesis. Curr. Med. Chem. 2024, 31, 4898–4911. [Google Scholar] [CrossRef]
- Wang, X.Y.; Mao, H.W.; Guan, X.H.; Huang, Q.M.; Yu, Z.P.; Wu, J.; Tan, H.L.; Zhang, F.; Huang, X.; Deng, K.Y.; et al. TRIM65 Promotes Cervical Cancer Through Selectively Degrading p53-Mediated Inhibition of Autophagy and Apoptosis. Front. Oncol. 2022, 12, 853935. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Hu, Z.; Xie, L.; Li, Z.; Liu, H.; Li, Z.; Jiang, Z.; Huang, J.; Jiang, C.; et al. Trim65 mitigates doxorubicin-induced myocardial injury by reducing ferroptosis. Exp. Cell Res. 2025, 450, 114613. [Google Scholar] [CrossRef]
- Cai, C.; Masumiya, H.; Weisleder, N.; Matsuda, N.; Nishi, M.; Hwang, M.; Ko, J.K.; Lin, P.; Thornton, A.; Zhao, X.; et al. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 2009, 11, 56–64. [Google Scholar] [CrossRef]
- Park, S.H.; Han, J.; Jeong, B.C.; Song, J.H.; Jang, S.H.; Jeong, H.; Kim, B.H.; Ko, Y.G.; Park, Z.Y.; Lee, K.E.; et al. Structure and activation of the RING E3 ubiquitin ligase TRIM72 on the membrane. Nat. Struct. Mol. Biol. 2023, 30, 1695–1706. [Google Scholar] [CrossRef]
- Wang, Y.F.; An, Z.Y.; Li, J.W.; Dong, Z.K.; Jin, W.L. MG53/TRIM72: Multi-organ repair protein and beyond. Front. Physiol. 2024, 15, 1377025. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Peng, W.; Zhang, Y.; Lv, F.; Wu, H.K.; Guo, J.; Cao, Y.; Pi, Y.; Zhang, X.; Jin, L.; et al. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 2013, 494, 375–379. [Google Scholar] [CrossRef]
- Faleti, O.D.; Gong, Y.; Long, J.; Luo, Q.; Tan, H.; Deng, S.; Qiu, L.; Lyu, X.; Yao, J.; Wu, G. TRIM72 inhibits cell migration and epithelial-mesenchymal transition by attenuating FAK/akt signaling in colorectal cancer. Heliyon 2024, 10, e37714. [Google Scholar] [CrossRef]
- Fang, M.; Wu, H.K.; Pei, Y.; Zhang, Y.; Gao, X.; He, Y.; Chen, G.; Lv, F.; Jiang, P.; Li, Y.; et al. E3 ligase MG53 suppresses tumor growth by degrading cyclin D1. Signal Transduct. Target. Ther. 2023, 8, 263. [Google Scholar] [CrossRef]
- Ma, X.; Ma, X.; Zhu, L.; Zhao, Y.; Chen, M.; Li, T.; Lin, Y.; Ma, D.; Sun, C.; Han, L. The E3 ubiquitin ligase MG53 inhibits hepatocellular carcinoma by targeting RAC1 signaling. Oncogenesis 2022, 11, 40. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Ong, H.; Tan, T.; Park, K.H.; Bian, Z.; Zou, X.; Haggard, E.; Janssen, P.M.; Merritt, R.E.; et al. MG53 suppresses NF-kappaB activation to mitigate age-related heart failure. JCI Insight 2021, 6, e148375. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, L.; Mu, N.; Zhang, Z.; Ma, H. MG53 inhibits ferroptosis by targeting the p53/SLC7A11/GPX4 pathway to alleviate doxorubicin-induced cardiotoxicity. Free Radic. Biol. Med. 2024, 223, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Huibregtse, J.M.; Scheffner, M.; Howley, P.M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991, 10, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Huibregtse, J.M.; Vierstra, R.D.; Howley, P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993, 75, 495–505. [Google Scholar] [CrossRef]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Vande Pol, S.; Podjarny, A.; et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- Kao, W.H.; Beaudenon, S.L.; Talis, A.L.; Huibregtse, J.M.; Howley, P.M. Human papillomavirus type 16 E6 induces self-ubiquitination of the E6AP ubiquitin-protein ligase. J. Virol. 2000, 74, 6408–6417. [Google Scholar] [CrossRef]
- Crinelli, R.; Bianchi, M.; Menotta, M.; Carloni, E.; Giacomini, E.; Pennati, M.; Magnani, M. Ubiquitin over-expression promotes E6AP autodegradation and reactivation of the p53/MDM2 pathway in HeLa cells. Mol. Cell Biochem. 2008, 318, 129–145. [Google Scholar] [CrossRef]
- Ali, A.; Farooqui, S.R.; Rai, J.; Singh, J.; Kumar, V.; Mishra, R.; Banerjea, A.C. HIV-1 Nef promotes ubiquitination and proteasomal degradation of p53 tumor suppressor protein by using E6AP. Biochem. Biophys. Res. Commun. 2020, 529, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Kishino, T.; Lalande, M.; Wagstaff, J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat. Genet. 1997, 15, 70–73, Erratum in: Nat. Genet. 1997, 15, 411. [Google Scholar] [CrossRef]
- Kuhnle, S.; Kogel, U.; Glockzin, S.; Marquardt, A.; Ciechanover, A.; Matentzoglu, K.; Scheffner, M. Physical and functional interaction of the HECT ubiquitin-protein ligases E6AP and HERC2. J. Biol. Chem. 2011, 286, 19410–19416. [Google Scholar] [CrossRef]
- Owais, A.; Mishra, R.K.; Kiyokawa, H. The HECT E3 Ligase E6AP/UBE3A as a Therapeutic Target in Cancer and Neurological Disorders. Cancers 2020, 12, 2108. [Google Scholar] [CrossRef]
- Sakane, F.; Yamada, K.; Kanoh, H.; Yokoyama, C.; Tanabe, T. Porcine diacylglycerol kinase sequence has zinc finger and E-F hand motifs. Nature 1990, 344, 345–348. [Google Scholar] [CrossRef]
- Tanaka, T.; Okada, M.; Hozumi, Y.; Tachibana, K.; Kitanaka, C.; Hamamoto, Y.; Martelli, A.M.; Topham, M.K.; Iino, M.; Goto, K. Cytoplasmic localization of DGKzeta exerts a protective effect against p53-mediated cytotoxicity. J. Cell Sci. 2013, 126, 2785–2797. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakano, T.; Hozumi, Y.; Martelli, A.M.; Goto, K. Regulation of p53 and NF-kappaB transactivation activities by DGKzeta in catalytic activity-dependent and -independent manners. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118953. [Google Scholar] [CrossRef] [PubMed]
- Perry, W.L.; Hustad, C.M.; Swing, D.A.; O’Sullivan, T.N.; Jenkins, N.A.; Copeland, N.G. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat. Genet. 1998, 18, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Infante, P.; Lospinoso Severini, L.; Bernardi, F.; Bufalieri, F.; Di Marcotullio, L. Targeting Hedgehog Signalling through the Ubiquitylation Process: The Multiple Roles of the HECT-E3 Ligase Itch. Cells 2019, 8, 98. [Google Scholar] [CrossRef]
- Schwarz SE, R.J.a.S.M. Characterization of human hect domain family members and their interaction with UbcH5 and UbcH7. J. Biol. Chem. 1998, 273, 12148–12154. [Google Scholar] [CrossRef]
- Goto, J.; Otaki, Y.; Watanabe, T.; Kobayashi, Y.; Aono, T.; Watanabe, K.; Wanezaki, M.; Kutsuzawa, D.; Kato, S.; Tamura, H.; et al. HECT (Homologous to the E6-AP Carboxyl Terminus)-Type Ubiquitin E3 Ligase ITCH Attenuates Cardiac Hypertrophy by Suppressing the Wnt/beta-Catenin Signaling Pathway. Hypertension 2020, 76, 1868–1878. [Google Scholar] [CrossRef]
- Saito, Y.; Otaki, Y.; Watanabe, T.; Tachibana, S.; Sato, J.; Kobayashi, Y.; Aono, T.; Goto, J.; Wanezaki, M.; Kutsuzawa, D.; et al. Cardiac-specific ITCH overexpression ameliorates septic cardiomyopathy via inhibition of the NF-kappaB signaling pathway. J. Mol. Cell Cardiol. Plus 2022, 2, 100018. [Google Scholar] [CrossRef]
- Otaki, Y.; Takahashi, H.; Watanabe, T.; Funayama, A.; Netsu, S.; Honda, Y.; Narumi, T.; Kadowaki, S.; Hasegawa, H.; Honda, S.; et al. HECT-Type Ubiquitin E3 Ligase ITCH Interacts with Thioredoxin-Interacting Protein and Ameliorates Reactive Oxygen Species-Induced Cardiotoxicity. J. Am. Heart Assoc. 2016, 5, e002485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.C.; Yang, J.H.; Liu, G.H.; Yang, F.; Gong, J.L.; Jia, M.G.; Zhang, M.J.; Zhao, L.S. miR-34b/c regulates doxorubicin-induced myocardial cell injury through ITCH. Cell Cycle 2019, 18, 3263–3274. [Google Scholar] [CrossRef]
- Han, D.; Wang, Y.; Wang, Y.; Dai, X.; Zhou, T.; Chen, J.; Tao, B.; Zhang, J.; Cao, F. The Tumor-Suppressive Human Circular RNA CircITCH Sponges miR-330-5p to Ameliorate Doxorubicin-Induced Cardiotoxicity Through Upregulating SIRT6, Survivin, and SERCA2a. Circ. Res. 2020, 127, e108–e125. [Google Scholar] [CrossRef]
- Ballinger, C.A.; Connell, P.; Wu, Y.; Hu, Z.; Thompson, L.J.; Yin, L.Y.; Patterson, C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell Biol. 1999, 19, 4535–4545. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; Ballinger, C.A.; Wu, Y.X.; Dai, Q.; Cyr, D.M.; Höhfeld, J.; Patterson, C. CHIP is a U-box-dependent E3 ubiquitin ligase: Identification of Hsc70 as a target for ubiquitylation. J. Biol. Chem. 2001, 276, 42938–42944. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Tang, X. STUB1/CHIP: New insights in cancer and immunity. Biomed. Pharmacother. 2023, 165, 115190. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lou, W.; Yang, J.C.; Liu, L.; Armstrong, C.M.; Lombard, A.P.; Zhao, R.; Noel, O.D.V.; Tepper, C.G.; Chen, H.W.; et al. Proteostasis by STUB1/HSP70 complex controls sensitivity to androgen receptor targeted therapy in advanced prostate cancer. Nat. Commun. 2018, 9, 4700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, Z.W.; Mao, C.Y.; Shi, C.H.; Xu, Y.M. CHIP as a therapeutic target for neurological diseases. Cell Death Dis. 2020, 11, 727. [Google Scholar] [CrossRef]
- Paul, I.; Ghosh, M.K. The E3 ligase CHIP: Insights into its structure and regulation. BioMed Res. Int. 2014, 2014, 918183. [Google Scholar] [CrossRef]
- Ye, Z.; Needham, P.G.; Estabrooks, S.K.; Whitaker, S.K.; Garcia, B.L.; Misra, S.; Brodsky, J.L.; Camacho, C.J. Symmetry breaking during homodimeric assembly activates an E3 ubiquitin ligase. Sci. Rep. 2017, 7, 1789. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, C.; Huang, Z.; Huang, J.; Zhu, B. STUB1 exacerbates calcium oxalate-induced kidney injury by modulating reactive oxygen species-mediated cellular autophagy via regulating CFTR ubiquitination. Urolithiasis 2024, 52, 55. [Google Scholar] [CrossRef]
- Nadel, C.M.; Thwin, A.C.; Callahan, M.; Lee, K.; Connelly, E.; Craik, C.S.; Southworth, D.R.; Gestwicki, J.E. The E3 Ubiquitin Ligase, CHIP/STUB1, Inhibits Aggregation of Phosphorylated Proteoforms of Microtubule-associated Protein Tau (MAPT). J. Mol. Biol. 2023, 435, 168026. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Bailey, T.A.; Clubb, R.J.; Mohapatra, B.C.; Bhat, A.M.; Chakraborty, S.; Islam, N.; Mushtaq, I.; Storck, M.D.; Raja, S.M.; et al. CHIP/STUB1 Ubiquitin Ligase Functions as a Negative Regulator of ErbB2 by Promoting Its Early Post-Biosynthesis Degradation. Cancers 2021, 13, 3936. [Google Scholar] [CrossRef]
- Dong, H.; Jia, W.; Meng, W.; Zhang, R.; Qi, Z.; Chen, Z.; Xie, S.; Min, J.; Liu, L.; Shen, J. DAB2IP inhibits glucose uptake by modulating HIF-1alpha ubiquitination under hypoxia in breast cancer. Oncogenesis 2024, 13, 20. [Google Scholar] [CrossRef]
- Sisoula, C.; Trachana, V.; Patterson, C.; Gonos, E.S. CHIP-dependent p53 regulation occurs specifically during cellular senescence. Free Radic. Biol. Med. 2011, 50, 157–165. [Google Scholar] [CrossRef]
- Kumar, S.; Basu, M.; Ghosh, M.K. Chaperone-assisted E3 ligase CHIP: A double agent in cancer. Genes Dis. 2022, 9, 1521–1555. [Google Scholar] [CrossRef] [PubMed]
- Narayan, V.; Pion, E.; Landre, V.; Muller, P.; Ball, K.L. Docking-dependent ubiquitination of the interferon regulatory factor-1 tumor suppressor protein by the ubiquitin ligase CHIP. J. Biol. Chem. 2011, 286, 607–619. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Wang, Q.; Xie, R.; Landay, A.; Chen, D. The E3 ubiquitin ligase CHIP in normal cell function and in disease conditions. Ann. N. Y. Acad. Sci. 2020, 1460, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Gallardo, L.; Martin-Benito, J.; Marcilla, M.; Espadas, G.; Sabido, E.; Valpuesta, J.M. The cochaperone CHIP marks Hsp70- and Hsp90-bound substrates for degradation through a very flexible mechanism. Sci. Rep. 2019, 9, 5102. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.T.; Okada, S.; Minamino, T.; Iwanaga, K.; Liu, M.L.; Sumida, T.; Nomura, S.; Sahara, N.; Mizoroki, T.; Takashima, A.; et al. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circ. Res. 2010, 106, 1692–1702. [Google Scholar] [CrossRef]
- Xu, C.W.; Zhang, T.P.; Wang, H.X.; Yang, H.; Li, H.H. CHIP enhances angiogenesis and restores cardiac function after infarction in transgenic mice. Cell Physiol. Biochem. 2013, 31, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.H.; Le, N.T.; Shishido, T.; Chang, E.; Lee, H.; Heo, K.S.; Mickelsen, D.M.; Lu, Y.; McClain, C.; Spangenberg, T.; et al. Novel role of C terminus of Hsc70-interacting protein (CHIP) ubiquitin ligase on inhibiting cardiac apoptosis and dysfunction regulating ERK5-mediated degradation of inducible cAMP early repressor. FASEB J. 2010, 24, 4917–4928. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.P.; Zhang, Y.; Bi, H.L.; Guan, X.M.; Wang, H.X.; Wang, X.; Du, J.; Xia, Y.L.; Li, H.H. Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein. Sci. Rep. 2016, 6, 28399. [Google Scholar] [CrossRef]
- Wouters, K.A.; Kremer, L.C.; Miller, T.L.; Herman, E.H.; Lipshultz, S.E. Protecting against anthracycline-induced myocardial damage: A review of the most promising strategies. Br. J. Haematol. 2005, 131, 561–578. [Google Scholar] [CrossRef]
- Hasinoff, B.B.; Herman, E.H. Dexrazoxane: How it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc. Toxicol. 2007, 7, 140–144. [Google Scholar] [CrossRef]
- Wei, L.; Sun, X.; Qi, X.; Zhang, Y.; Li, Y.; Xu, Y. Dihydromyricetin Ameliorates Cardiac Ischemia/Reperfusion Injury through Sirt3 Activation. BioMed Res. Int. 2019, 2019, 6803943. [Google Scholar] [CrossRef]
- Xiao, H.; Xiao, Y.; Zeng, X.; Xie, H.; Wang, Z.; Guo, Y. Dihydromyricetin Improves Myocardial Functioning by Influencing Autophagy Through SNHG17/Mir-34a/SIDT2 Axis. Curr. Mol. Pharmacol. 2024, 17, e18761429374180. [Google Scholar] [CrossRef]
- Song, Q.; Liu, L.; Yu, J.; Zhang, J.; Xu, M.; Sun, L.; Luo, H.; Feng, Z.; Meng, G. Dihydromyricetin attenuated Ang II induced cardiac fibroblasts proliferation related to inhibitory of oxidative stress. Eur. J. Pharmacol. 2017, 807, 159–167. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, W.; Lin, N.; Lin, H.; Zhang, J.; Ni, T.; Meng, L.; Zhang, C.; Guo, H. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem. Pharmacol. 2020, 175, 113888. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Wang, B.; Chi, W.; Li, Z.; Zhang, M.; Shen, Y.; Liu, X.; Lu, Y.; Liu, Y. Dihydromyricetin protects against Doxorubicin-induced cardiotoxicity through activation of AMPK/mTOR pathway. Phytomedicine 2022, 99, 154027. [Google Scholar] [CrossRef]
- McKimpson, W.M.; Weinberger, J.; Czerski, L.; Zheng, M.; Crow, M.T.; Pessin, J.E.; Chua, S.C., Jr.; Kitsis, R.N. The apoptosis inhibitor ARC alleviates the ER stress response to promote beta-cell survival. Diabetes 2013, 62, 183–193. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, P.; Fu, Y.; Wang, J.; Dai, J.; Shao, J.; Yang, X.; Chang, L.; Weng, Q.; Yang, B.; et al. Dihydromyricetin prevents cardiotoxicity and enhances anticancer activity induced by adriamycin. Oncotarget 2015, 6, 3254–3267. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Chen, H.; Fan, X.; Wang, J.; Lu, L.; Yang, G.; Liu, J.; Yao, W.; Ding, F.; Ding, J.; et al. Exploring the effective components of honey-processed licorice (Glycyrrhiza uralensis Fisch.) in attenuating Doxorubicin-induced myocardial cytotoxicity by combining network pharmacology and in vitro experiments. J. Ethnopharmacol. 2024, 329, 118178. [Google Scholar] [CrossRef]
- de Freitas, K.S.; Squarisi, I.S.; Acesio, N.O.; Nicolella, H.D.; Ozelin, S.D.; Reis Santos de Melo, M.; Guissone, A.P.P.; Fernandes, G.; Silva, L.M.; da Silva Filho, A.A.; et al. Licochalcone A, a licorice flavonoid: Antioxidant, cytotoxic, genotoxic, and chemopreventive potential. J. Toxicol. Environ. Health A 2020, 83, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Luo, S.; Guo, H.; Lin, J.; Xu, S. Licochalcone A alleviates ferroptosis in doxorubicin-induced cardiotoxicity via the PI3K/AKT/MDM2/p53 pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 4247–4262. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Zhang, D.D. Resveratrol, a polyphenol phytoalexin, protects against doxorubicin-induced cardiotoxicity. J. Cell Mol. Med. 2015, 19, 2324–2328. [Google Scholar] [CrossRef]
- Hu, L.F.; Lan, H.R.; Li, X.M.; Jin, K.T. A Systematic Review of the Potential Chemoprotective Effects of Resveratrol on Doxorubicin-Induced Cardiotoxicity: Focus on the Antioxidant, Antiapoptotic, and Anti-Inflammatory Activities. Oxid. Med. Cell. Longev. 2021, 2021, 2951697. [Google Scholar] [CrossRef]
- Li, M.; Chen, D.; Shiloh, A.; Luo, J.; Nikolaev, A.Y.; Qin, J.; Gu, W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 2002, 416, 648–653. [Google Scholar] [CrossRef]
- Sin, T.K.; Tam, B.T.; Yung, B.Y.; Yip, S.P.; Chan, L.W.; Wong, C.S.; Ying, M.; Rudd, J.A.; Siu, P.M. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J. Physiol. 2015, 593, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and cancer chemoprevention. Evid. Based Complement. Altern. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef]
- Angeloni, C.; Spencer, J.P.; Leoncini, E.; Biagi, P.L.; Hrelia, S. Role of quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie 2007, 89, 73–82. [Google Scholar] [CrossRef]
- Mojzisova, G.; Sarissky, M.; Mirossay, L.; Martinka, P.; Mojzis, J. Effect of flavonoids on daunorubicin-induced toxicity in H9c2 Cardiomyoblasts. Phytother. Res. 2009, 23, 136–139. [Google Scholar] [CrossRef]
- Ismail, I.H.; Andrin, C.; McDonald, D.; Hendzel, M.J. BMI1-mediated histone ubiquitylation promotes DNA double-strand break repair. J. Cell Biol. 2010, 191, 45–60. [Google Scholar] [CrossRef]
- Ginjala, V.; Nacerddine, K.; Kulkarni, A.; Oza, J.; Hill, S.J.; Yao, M.; Citterio, E.; van Lohuizen, M.; Ganesan, S. BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair. Mol. Cell. Biol. 2011, 31, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Chen, L.; Lu, Q.; Sharma, S.; Li, L.; Morimoto, S.; Wang, G. Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br. J. Pharmacol. 2014, 171, 4440–4454. [Google Scholar] [CrossRef] [PubMed]
- Staedler, D.; Idrizi, E.; Kenzaoui, B.H.; Juillerat-Jeanneret, L. Drug combinations with quercetin: Doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother. Pharmacol. 2011, 68, 1161–1172. [Google Scholar] [CrossRef]
- Kaiserova, H.; Simunek, T.; van der Vijgh, W.J.; Bast, A.; Kvasnickova, E. Flavonoids as protectors against doxorubicin cardiotoxicity: Role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochim. Biophys. Acta 2007, 1772, 1065–1074. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genomics 2018, 50, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, J.H.; Thomasova, D.; Mulay, S.R.; Anders, H.J. Nrf2 signalling promotes ex vivo tubular epithelial cell survival and regeneration via murine double minute (MDM)-2. Nephrol. Dial. Transplant. 2013, 28, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, X.; Xiao, X.; Liu, L.F.; Zhang, L.; Lin, P.P.; Zhang, S.L.; Li, Q.S. Effects of Ganoderma lucidum polysaccharides against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2017, 95, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.B.; Hockman, D. Fgf1. Differentiation 2024, 139, 100802. [Google Scholar] [CrossRef]
- Babina, I.S.; Turner, N.C. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 2017, 17, 318–332. [Google Scholar] [CrossRef]
- Xiao, M.; Tang, Y.; Wang, J.; Lu, G.; Niu, J.; Wang, J.; Li, J.; Liu, Q.; Wang, Z.; Huang, Z.; et al. A new FGF1 variant protects against adriamycin-induced cardiotoxicity via modulating p53 activity. Redox Biol. 2022, 49, 102219. [Google Scholar] [CrossRef]
- Chik, W.I.; Zhu, L.; Fan, L.L.; Yi, T.; Zhu, G.Y.; Gou, X.J.; Tang, Y.N.; Xu, J.; Yeung, W.P.; Zhao, Z.Z.; et al. Saussurea involucrata: A review of the botany, phytochemistry and ethnopharmacology of a rare traditional herbal medicine. J. Ethnopharmacol. 2015, 172, 44–60. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Yu, R. Unraveling the Therapeutic Mechanism of Saussurea involucrata against Rheumatoid Arthritis: A Network Pharmacology and Molecular Modeling-Based Investigation. Nutrients 2023, 15, 4294. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Y.; Yang, M.; Xue, Y.; Zhang, X.; Guo, Y.; Li, X.; Ma, K. Cardioprotective effect of Saussurea involucrata injection against Doxorubicin-induced cardiotoxicity by network pharmacology analysis and experimental verification. Acta Biochim. Biophys. Sin. 2024, 57, 554–568. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, J.; Guo, D.; Wang, Q.; Yang, X.; Lu, W.; Sun, X.; He, H.; Li, N.; Wang, Y.; et al. Qishen Granule alleviates endoplasmic reticulum stress-induced myocardial apoptosis through IRE-1-CRYAB pathway in myocardial ischemia. J. Ethnopharmacol. 2020, 252, 112573. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Mita, Y.; Okawa, Y.; Ariyoshi, M.; Iwai-Kanai, E.; Ueyama, T.; Ikeda, K.; Ogata, T.; Matoba, S. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 2013, 4, 2308. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Wang, X.; Cao, J.; Qian, W.; Ling, G.; Tan, N.; Jiang, J.; Sun, Q.; Li, C.; et al. Qishen Granule Protects against Doxorubicin-Induced Cardiotoxicity by Coordinating MDM2-p53-Mediated Mitophagy and Mitochondrial Biogenesis. Oxid. Med. Cell. Longev. 2022, 2022, 4344677. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Rotblat, B.; Ansell, K.; Amelio, I.; Caraglia, M.; Misso, G.; Bernassola, F.; Cavasotto, C.N.; Knight, R.A.; Ciechanover, A.; et al. High throughput screening for inhibitors of the HECT ubiquitin E3 ligase ITCH identifies antidepressant drugs as regulators of autophagy. Cell Death Dis. 2014, 5, e1203. [Google Scholar] [CrossRef]
- Kanack, A.J.; Olp, M.D.; Newsom, O.J.; Scaglione, J.B.; Gooden, D.M.; McMahon, K.; Smith, B.C.; Scaglione, K.M. Chemical Regulation of the Protein Quality Control E3 Ubiquitin Ligase C-Terminus of Hsc70 Interacting Protein (CHIP). Chembiochem 2022, 23, e202100633. [Google Scholar] [CrossRef]

| E3 Ligase | Type | Experimental Model (In Vivo/In Vitro) | Downstream Effects |
|---|---|---|---|
| MDM2 | RING | Mouse myocardium /H9c2 cells | Modulation of apoptosis and autophagy/mitophagy; cardioprotection under Dox stress |
| PIRH2 | RING | Mouse myocardium /HL-1 cells | Inhibition of Dox-induced ferroptosis; improvement in cardiac function |
| TRIM65 | RING | Mouse myocardium /H9c2 cells | Suppression of Dox-induced ferroptosis; myocardial protection |
| TRIM72 | RING | Mouse myocardium /H9c2 cells, NRCM | Suppression of ferroptosis via p53/SLC7A11/GPX4 axis; cardioprotection |
| E6AP | HECT | Mouse myocardium /H9c2 cells, NRCM | Dgkζ–Hsp70 complex-mediated regulation; reduced apoptosis |
| ITCH | HECT | Cardiac-specific ITCH transgenic mice /NRCM | Degradation of TXNIP; reduced ROS production; suppression of apoptosis; improved cardiac function after Dox exposure or MI |
| CHIP | U-box | CHIP transgenic mouse myocardium /H9c2 cells, NRCM | Attenuation of Dox-induced cardiotoxicity; inhibition of apoptosis |
| Agents | Substrate | E3 Ligases/ De-Ubiquitinating Enzyme |
|---|---|---|
| Dihydromyricetin | ARC ↑ | MDM2 ↓ |
| Licochalcone A | p53 ↓ | MDM2 ↑ |
| Resveratrol | p53 ↓ | USP7 ↓ |
| Quercetin | p53 ↓ (indirect) | Bmi-1 ↑ |
| Ganoderma lucidum polysaccharides | p53 ↓ | MDM2 |
| FGF1 variant | p53 ↓ | MDM2 |
| Saussurea involucrata | p53 ↓ | MDM2 |
| Qishen granule | p53 ↓ | MDM2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachibana, S.; Otaki, Y.; Goto, J.; Watanabe, T.; Watanabe, M. Ubiquitin E3 Ligases and p53 in Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2025, 26, 10252. https://doi.org/10.3390/ijms262110252
Tachibana S, Otaki Y, Goto J, Watanabe T, Watanabe M. Ubiquitin E3 Ligases and p53 in Doxorubicin-Induced Cardiotoxicity. International Journal of Molecular Sciences. 2025; 26(21):10252. https://doi.org/10.3390/ijms262110252
Chicago/Turabian StyleTachibana, Shingo, Yoichiro Otaki, Jun Goto, Tetsu Watanabe, and Masafumi Watanabe. 2025. "Ubiquitin E3 Ligases and p53 in Doxorubicin-Induced Cardiotoxicity" International Journal of Molecular Sciences 26, no. 21: 10252. https://doi.org/10.3390/ijms262110252
APA StyleTachibana, S., Otaki, Y., Goto, J., Watanabe, T., & Watanabe, M. (2025). Ubiquitin E3 Ligases and p53 in Doxorubicin-Induced Cardiotoxicity. International Journal of Molecular Sciences, 26(21), 10252. https://doi.org/10.3390/ijms262110252






