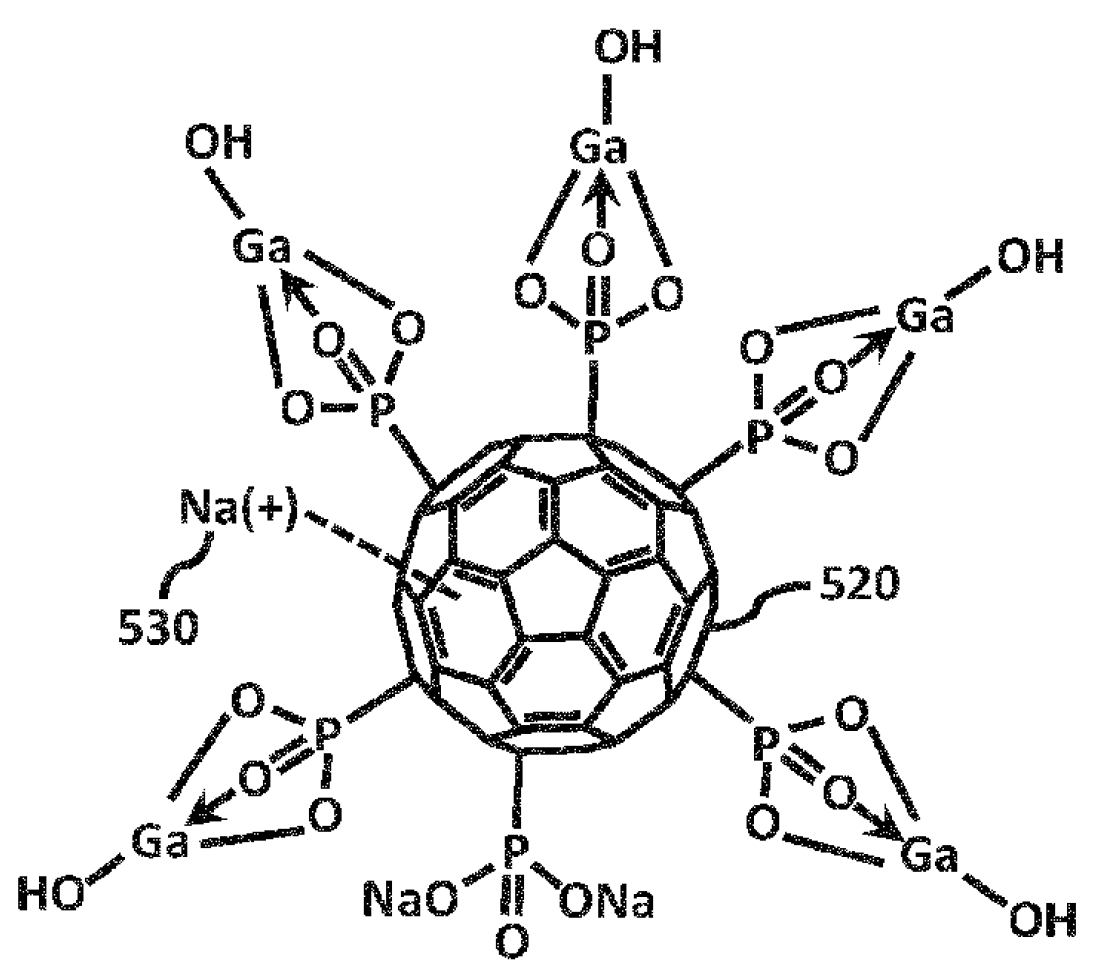
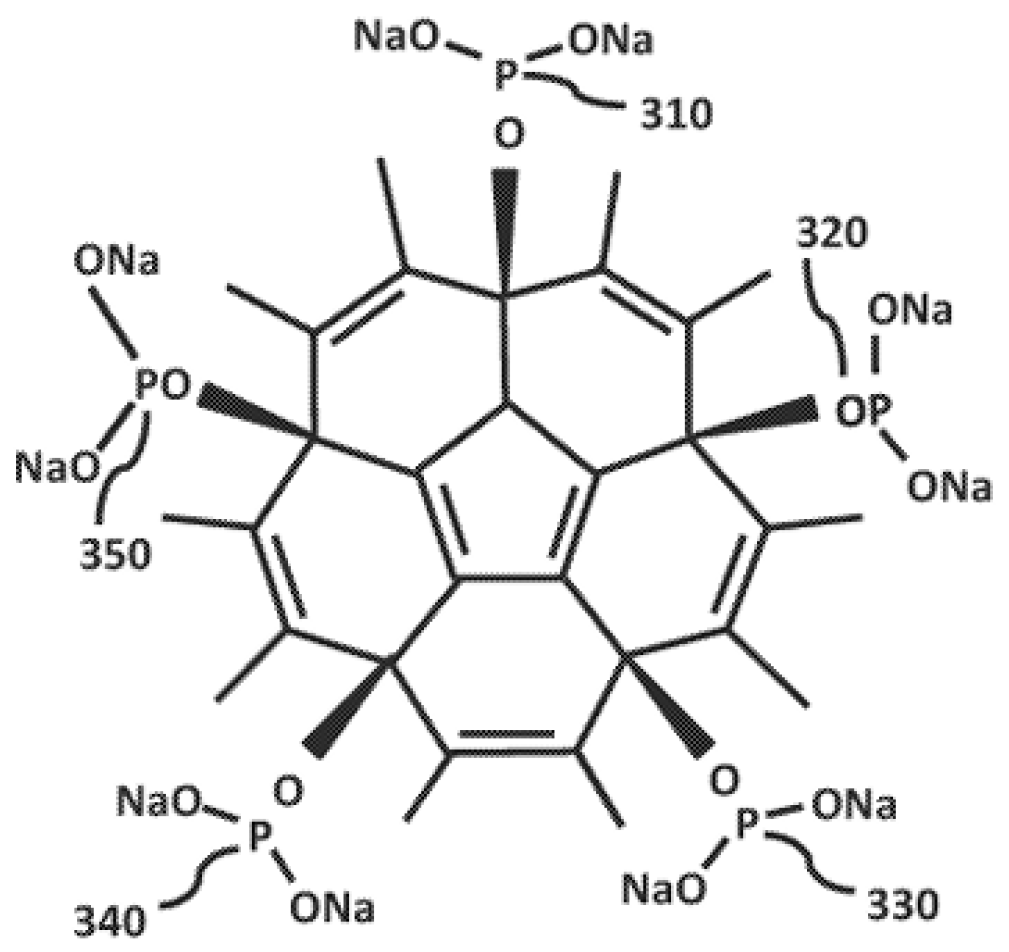
Fullerene Gallium Phosphonate Shows Antimycobacterial Effect Against Mycobacterium avium
Abstract
1. Introduction
2. Results
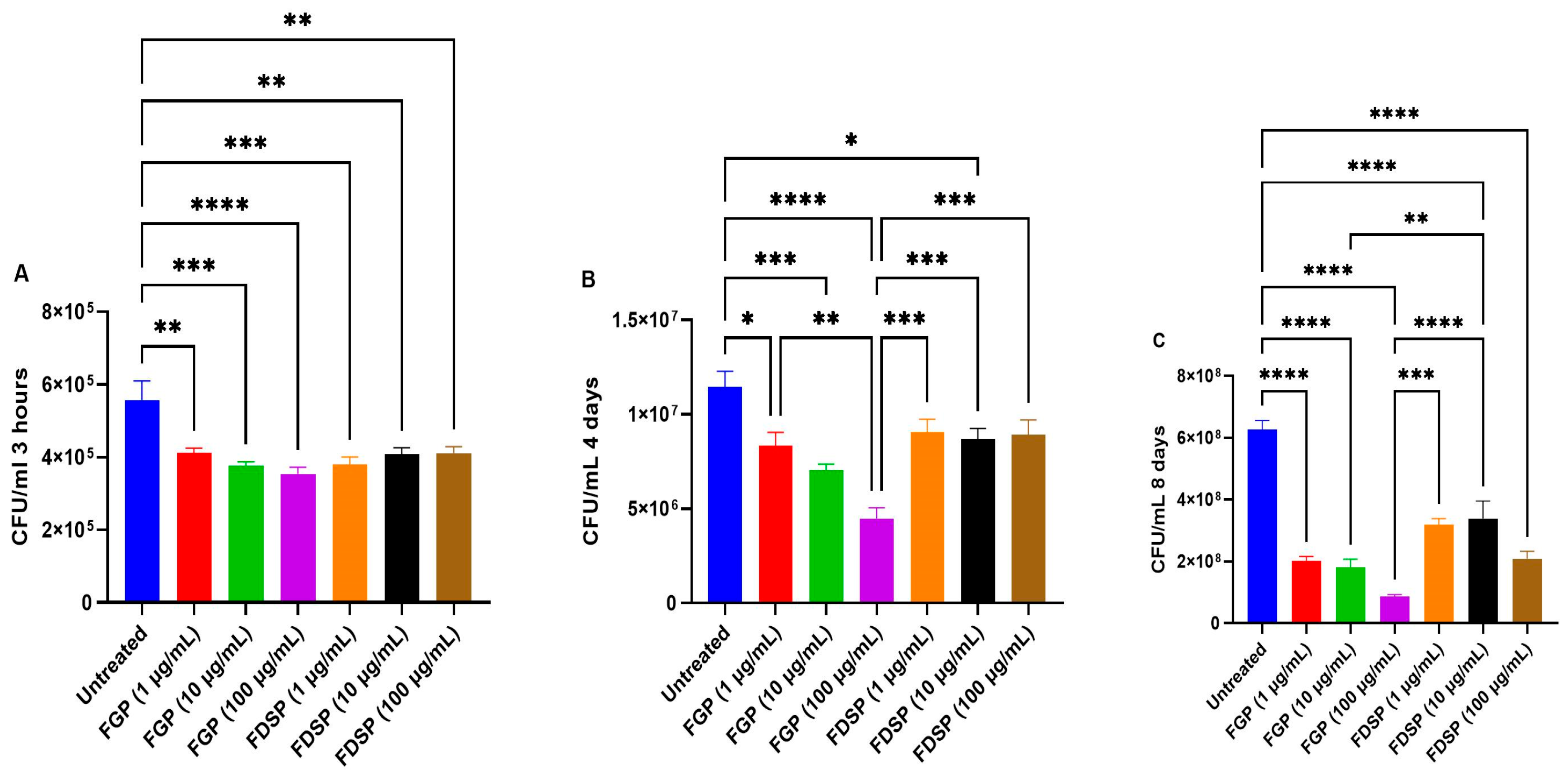
2.1. Direct Effects of FGP and FDSP on M. avium
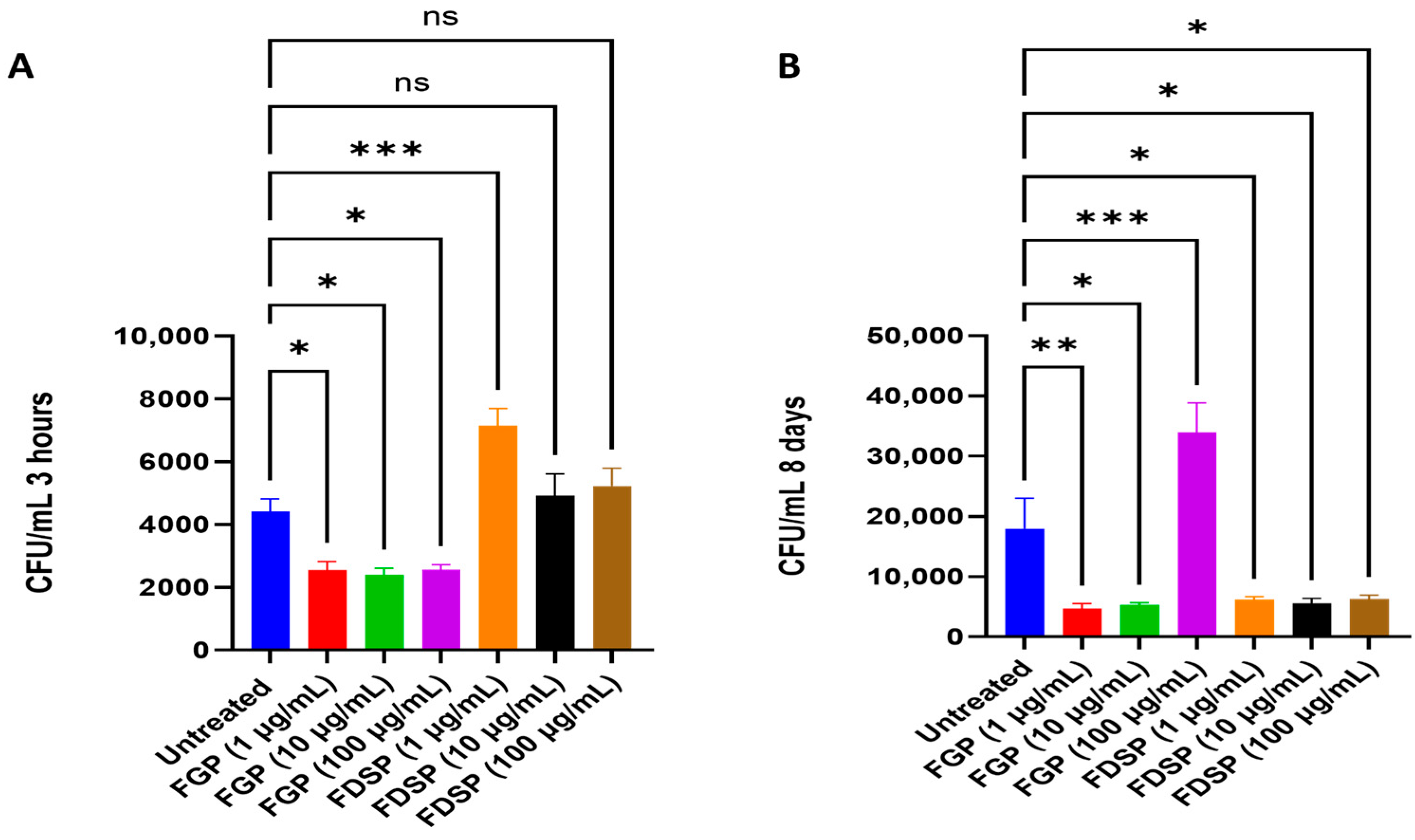
2.2. Efficacy of FGP and FDSP on Infected Host Cells
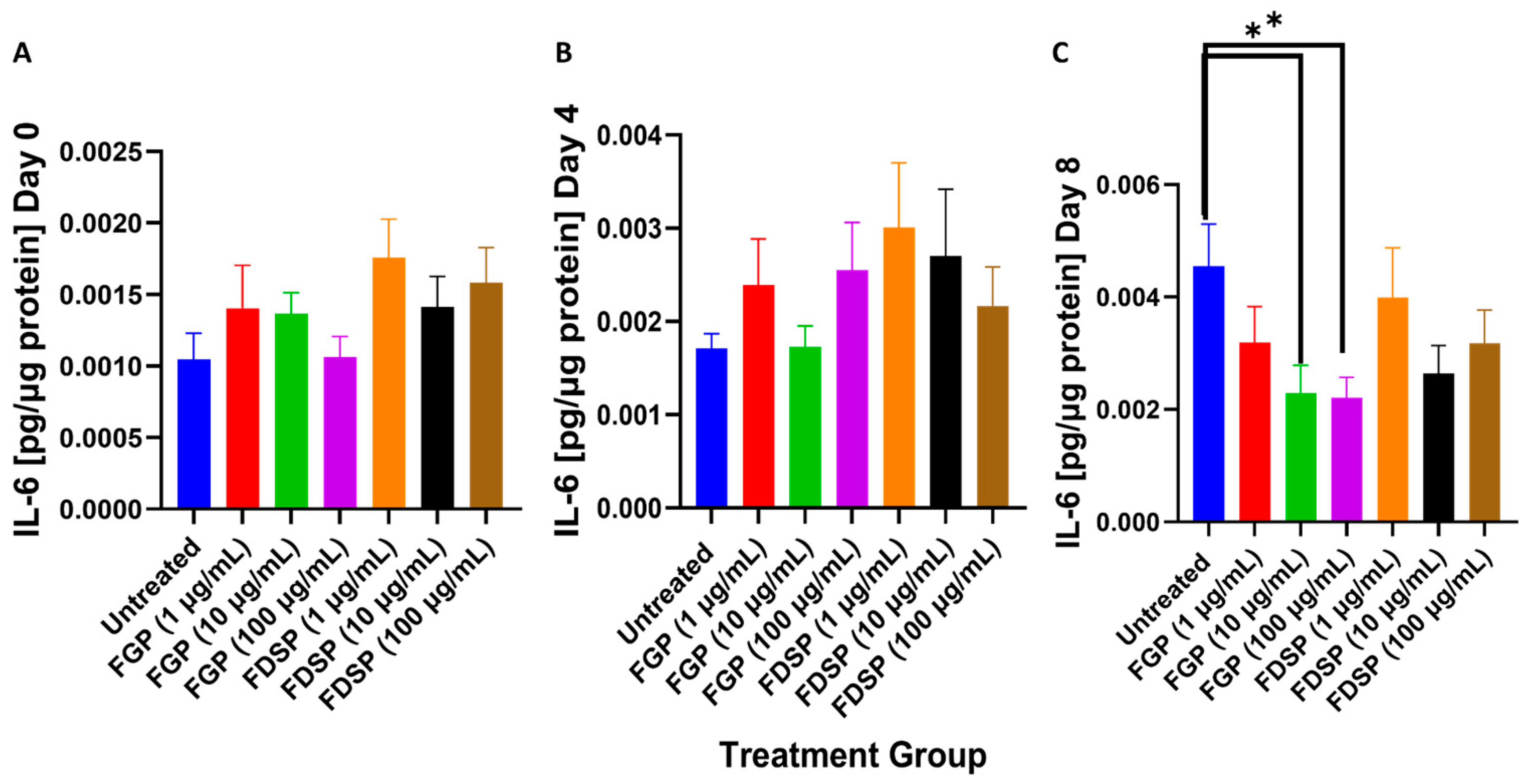
2.3. IL-6 Levels in the Supernatants of M. avium Infected THP-1 Cells with FGP/FDSP Treatment
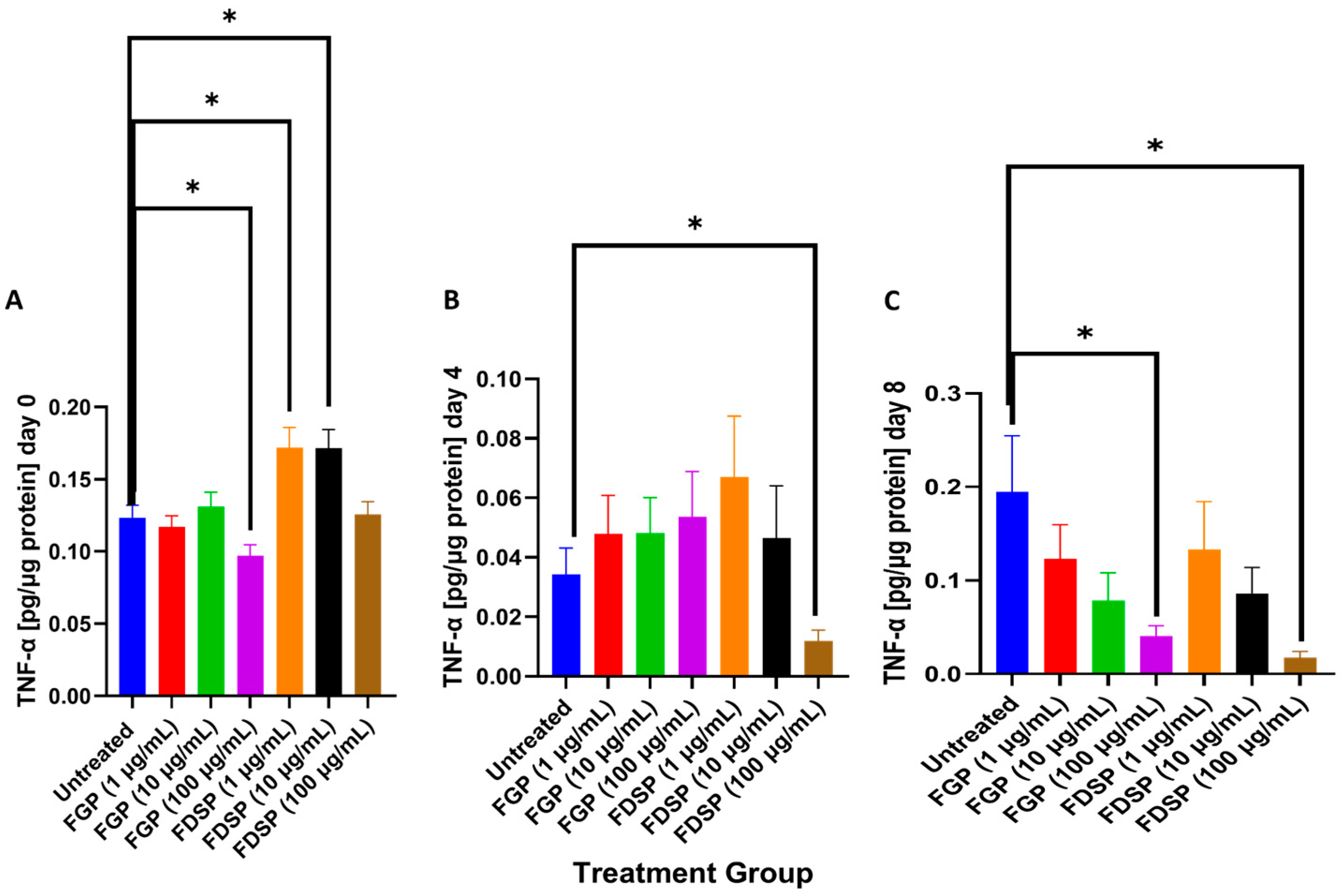
2.4. TNF-a Levels in the Supernatants of M. avium-Infected THP-1 Cells and Treated with FGP or FDSP
2.5. MDA Levels in M. avium-Infected THP-1 Cells Without Treatment (Control) or with FGP or FDSP Treatment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial Processing and Preparation
4.3. THP-1 Cell Differentiation, Infection, and Antibiotic Treatment
4.4. Cytokine Measurement
4.5. Malondialdehyde Measurement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, M.V.H.; Haas, M.K.; Kasperbauer, S.H.; de Moura, V.C.N.; Eddy, J.J.; Mitchell, J.D.; Khare, R.; Griffith, D.E.; Chan, E.D.; Daley, C.L. Nontuberculous Mycobacterial Pulmonary Disease: Patients, Principles, and Prospects. Clin. Infect. Dis. 2024, 79, e27–e47. [Google Scholar] [CrossRef]
- Daley, C.L. Mycobacterium avium Complex Disease. Microbiol Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Meier, E.; Pennington, K.; Gallo de Moraes, A.; Escalante, P. Characteristics of Mycobacterium avium complex (MAC) pulmonary disease in previously treated lung cancer patients. Respir. Med. Case Rep. 2017, 22, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, Q.; Li, X.J.; Jin, L. The applications of buckminsterfullerene C60 and derivatives in orthopaedic research. Connect. Tissue Res. 2014, 55, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sinegubova, E.O.; Kraevaya, O.A.; Volobueva, A.S.; Zhilenkov, A.V.; Shestakov, A.F.; Baykov, S.V.; Troshin, P.A.; Zarubaev, V.V. Water-Soluble Fullerene C60 Derivatives Are Effective Inhibitors of Influenza Virus Replication. Microorganisms 2023, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Zhiyentayev, T.; Huang, L.; Khalil, S.; Nasim, F.; Tegos, G.P.; Gali, H.; Jahnke, A.; Wharton, T.; Hamblin, M.R. Antimicrobial Photodynamic Therapy with Functionalized Fullerenes: Quantitative Structure-activity Relationships. J. Nanomed. Nanotechnol. 2011, 2, 1. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef]
- Bolshakova, O.; Lebedev, V.; Mikhailova, E.; Zherebyateva, O.; Aznabaeva, L.; Burdakov, V.; Kulvelis, Y.; Yevlampieva, N.; Mironov, A.; Miroshnichenko, I.; et al. Fullerenes on a Nanodiamond Platform Demonstrate Antibacterial Activity with Low Cytotoxicity. Pharmaceutics 2023, 15, 1984. [Google Scholar] [CrossRef]
- Tegos, G.P.; Demidova, T.N.; Arcila-Lopez, D.; Lee, H.; Wharton, T.; Gali, H.; Hamblin, M.R. Cationic Fullerenes Are Effective and Selective Antimicrobial Photosensitizers. Chem. Biol. 2005, 12, 1127–1135. [Google Scholar] [CrossRef]
- Limantoro, C.; Das, T.; He, M.; Dirin, D.; Manos, J.; Kovalenko, M.V.; Chrzanowski, W. Synthesis of Antimicrobial Gallium Nanoparticles Using the Hot Injection Method. ACS Mater. Au 2023, 3, 310–320. [Google Scholar] [CrossRef]
- Yin, J.J.; Lao, F.; Fu, P.P.; Wamer, W.G.; Zhao, Y.; Wang, P.C.; Qiu, Y.; Sun, B.; Xing, G.; Dong, J.; et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 2008, 30, 611–621. [Google Scholar] [CrossRef]
- Ye, S.; Chen, M.; Jiang, Y.; Chen, M.; Zhou, T.; Wang, Y.; Hou, Z.; Ren, L. Polyhydroxylated fullerene attenuates oxidative stress-induced apoptosis via a fortifying Nrf2-regulated cellular antioxidant defence system. Int. J. Nanomed. 2014, 9, 2073–2087. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Alvarez, P.J.J. Fullerene water suspension (NC60) exerts antibacterial effects via ROS-independent protein oxidation. Environ. Sci. Technol. 2008, 42, 8127–8132. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, T.; Wang, M.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: In vitro and in vivo studies. Nanomedicine 2015, 10, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Takahashi, E.; Hatakeyama, D.; Iwai, Y.; Morita, Y.; Shirayama, R.; Echigo, N.; Kido, H.; Nakamura, S.; Mashino, T.; et al. Anti-influenza activity of C60 fullerene derivatives. PLoS ONE 2013, 8, e66337. [Google Scholar] [CrossRef]
- Truong, V.K.; Hayles, A.; Bright, R.; Luu, T.Q.; Dickey, M.D.; Kalantar-Zadeh, K.; Vasilev, K. Gallium Liquid Metal: Nanotoolbox for Antimicrobial Applications. ACS Nano 2023, 17, 14406–14423. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef]
- Li, F.; Liu, F.; Huang, K.; Yang, S. Advancement of Gallium and Gallium-Based Compounds as Antimicrobial Agents. Front. Bioeng. Biotechnol. 2022, 10, 827960. [Google Scholar] [CrossRef]
- Choi, S.R.; Hassan, M.A.; Britigan, B.E.; Narayanasamy, P. Antimicrobial Activity of Gallium(III) Compounds: Pathogen-Dependent Targeting of Multiple Iron/Heme-Dependent Biological Processes. Curr. Issues Mol. Biol. 2024, 46, 9149. [Google Scholar] [CrossRef]
- Qu, C.C.; Liang, Y.T.; Wang, X.Q.; Gao, S.; He, Z.Z.; Sun, X.Y. Gallium-Based Liquid Metal Materials for Antimicrobial Applications. Bioengineering 2022, 9, 416. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Britigan, B.E.; Moran, D.M.; Narayanasamy, P. Gallium nanoparticles facilitate phagosome maturation and inhibit growth of virulent Mycobacterium tuberculosis in macrophages. PLoS ONE 2017, 12, e0177987. [Google Scholar] [CrossRef]
- Choi, S.R.; Britigan, B.E.; Narayanasamy, P. Dual Inhibition of Klebsiella pneumoniae and Pseudomonas aeruginosa Iron Metabolism Using Gallium Porphyrin and Gallium Nitrate. ACS Infect. Dis. 2019, 5, 1559–1569. [Google Scholar] [CrossRef]
- Chitambar, C.R. Medical Applications and Toxicities of Gallium Compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337. [Google Scholar] [CrossRef]
- Bonchi, C.; Imperi, F.; Minandri, F.; Visca, P.; Frangipani, E. Repurposing of gallium-based drugs for antibacterial therapy. BioFactors 2014, 40, 303–312. [Google Scholar] [CrossRef]
- Butzloff, P.U.S. Patent Application for Fullerene Gallium Phosphonate and Methods Patent Application (Application #20220339295 Issued 27 October 2022)—Justia Patents Search. Justia. Available online: https://patents.justia.com/patent/20220339295 (accessed on 30 September 2025).
- Choi, S.R.; Switzer, B.; Britigan, B.E.; Narayanasamy, P. Gallium Porphyrin and Gallium Nitrate Synergistically Inhibit Mycobacterial Species by Targeting Different Aspects of Iron/Heme Metabolism. ACS Infect. Dis. 2020, 6, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Fréhel, C.; Offredo, C.; de Chastellier, C. The phagosomal environment protects virulent Mycobacterium avium from killing and destruction by clarithromycin. Infect. Immun. 1997, 65, 2792. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Matsuzawa, Y.; Hayakawa, S.; Irie, T.; Rikitake, H.; Tatsuno, I. Serum oxidative stress in patients with pulmonary Mycobacterium avium complex disease. Heliyon 2019, 5, e02775. [Google Scholar] [CrossRef]
- Ghassemi, M.; Andersen, B.R.; Roebuck, K.A.; Rabbi, M.F.; Plate, J.M.D.; Novak, R.M. Mycobacterium avium complex activates nuclear factor κB via induction of inflammatory cytokines. Cell. Immunol. 1999, 191, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.S.; Kim, S.Y.; Kim, E.J.; Shin, S.J.; Koh, W.J. Impaired Expression of MAPK Is Associated with the Downregulation of TNF-α, IL-6, and IL-10 in Mycobacterium abscessus Lung Disease. Tuberc. Respir. Dis. 2012, 72, 275. [Google Scholar] [CrossRef]
- Souza, C.D.; Evanson, O.A.; Weiss, D.J. Role of the MAPKERK pathway in regulation of cytokine expression by Mycobacterium avium subsp paratuberculosis–exposed bovine monocytes. Am. J. Vet. Res. 2007, 68, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Gidon, A.; Louet, C.; Røst, L.M.; Bruheim, P.; Flo, T.H. The Tumor Necrosis Factor Alpha and Interleukin 6 Auto-paracrine Signaling Loop Controls Mycobacterium avium Infection via Induction of IRF1/IRG1 in Human Primary Macrophages. mBio 2021, 12, e02121-21. [Google Scholar] [CrossRef] [PubMed]
- Heredia, D.A.; Durantini, A.M.; Durantini, J.E.; Durantini, E.N. Fullerene C60 derivatives as antimicrobial photodynamic agents. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100471. [Google Scholar] [CrossRef]
- European Patent Application EP4292656A1. Fullerene Derivatives and Uses Thereof. Available online: https://data.epo.org/publication-server/rest/v1.2/patents/EP4292656NWA1/document.html (accessed on 30 September 2025).
- International Patent Application WO2022159332A1. Fullerene Disodium Phosphonate and Related Derivatives. Available online: https://patents.google.com/patent/WO2022159332A1/en (accessed on 30 September 2025).
- ThermoFisher Scientific. Mouse IL-6 Uncoated ELISA Kit, Cat# 88-7,064-88. Available online: https://www.thermofisher.com/elisa/product/Mouse-IL-6-Uncoated-ELISA-Kit/88-7064-88 (accessed on 30 September 2025).
- ThermoFisher Scientific. Mouse TNF Alpha Uncoated ELISA Kit, Cat# 88-7,324-88. Available online: https://www.thermofisher.com/elisa/product/Mouse-TNF-alpha-Uncoated-ELISA-Kit/88-7324-88 (accessed on 30 September 2025).
- Cayman Chemical. TBARS Assay Kit, Item No. 10009055. Available online: https://www.caymanchem.com/product/10009055/tbars-assay-kit (accessed on 30 September 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Sasaninia, K.; Era, I.H.; Dhama, S.; Mohan, A.S.; Patel, A.; Nguyen, L.; Karapetyan, A.; Sy, C.; Yedgarian, N.; et al. Fullerene Gallium Phosphonate Shows Antimycobacterial Effect Against Mycobacterium avium. Int. J. Mol. Sci. 2025, 26, 9998. https://doi.org/10.3390/ijms26209998
Yoon S, Sasaninia K, Era IH, Dhama S, Mohan AS, Patel A, Nguyen L, Karapetyan A, Sy C, Yedgarian N, et al. Fullerene Gallium Phosphonate Shows Antimycobacterial Effect Against Mycobacterium avium. International Journal of Molecular Sciences. 2025; 26(20):9998. https://doi.org/10.3390/ijms26209998
Chicago/Turabian StyleYoon, Sonyeol, Kayvan Sasaninia, Iffat Hasnin Era, Sanya Dhama, Aishvaryaa Shree Mohan, Ami Patel, Lannhi Nguyen, Arshavir Karapetyan, Cristian Sy, Nickolas Yedgarian, and et al. 2025. "Fullerene Gallium Phosphonate Shows Antimycobacterial Effect Against Mycobacterium avium" International Journal of Molecular Sciences 26, no. 20: 9998. https://doi.org/10.3390/ijms26209998
APA StyleYoon, S., Sasaninia, K., Era, I. H., Dhama, S., Mohan, A. S., Patel, A., Nguyen, L., Karapetyan, A., Sy, C., Yedgarian, N., Newman, N., Bi, X., Baudry, M., Yang, P. R., & Venketaraman, V. (2025). Fullerene Gallium Phosphonate Shows Antimycobacterial Effect Against Mycobacterium avium. International Journal of Molecular Sciences, 26(20), 9998. https://doi.org/10.3390/ijms26209998







