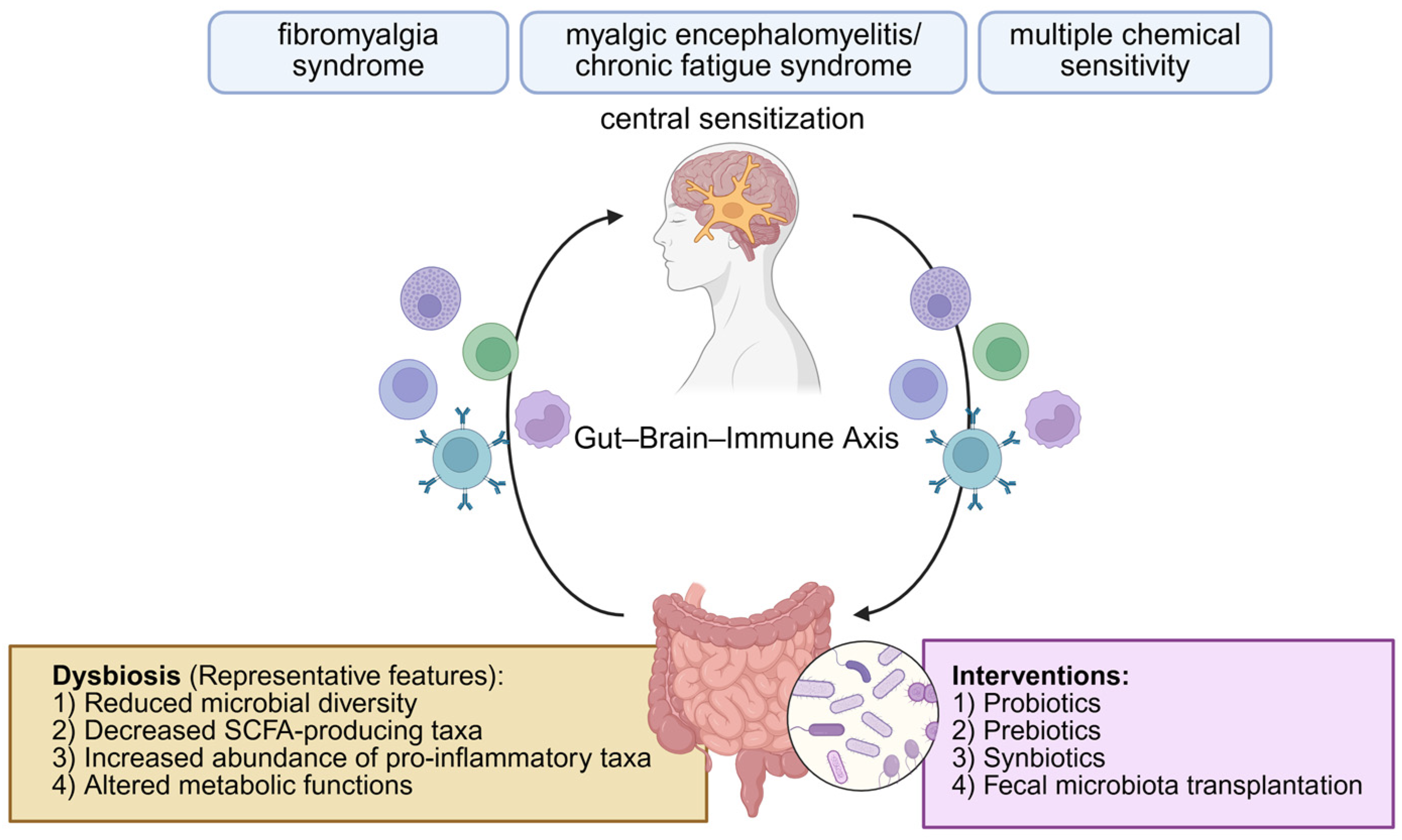
The Gut–Brain–Immune Axis in Environmental Sensitivity Illnesses: Microbiome-Centered Narrative Review of Fibromyalgia Syndrome, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, and Multiple Chemical Sensitivity
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Screening and Eligibility Criteria
- Human subjects: Clinical populations were studied, rather than animal or in vitro models.
- Target conditions: Participants had a confirmed diagnosis of FMS, ME/CFS, or MCS. Studies of overlapping but distinct syndromes (e.g., chronic pain not meeting fibromyalgia syndrome criteria) were excluded.
- Sample size: Each study arm included at least five participants.
- Microbiome analysis: Validated methodologies such as 16S rRNA gene sequencing, shotgun metagenomics, metatranscriptomics, quantitative PCR, metabolomics, or culture-based approaches were employed.
- Outcomes: At least one microbiome-related endpoint was reported, including microbial composition, alpha or beta diversity, abundance of specific taxa, or functional/metabolic pathways.
- Study design: Observational (cross-sectional, cohort, or case–control) or interventional studies were eligible.
2.3. Data Extraction
2.4. Extracted Data Included:
- Study design: Type (cross-sectional, case–control, cohort, interventional) and methodological features (e.g., multi-omic approaches, machine learning).
- Participant characteristics: Sample size, case–control distribution, diagnostic categories, demographics (age, sex distribution), and disease duration, when available.
- Microbiome analysis methods: Sequencing platforms (e.g., Illumina, 454), targeted rRNA regions (V3/V4, V4), metagenomics or metatranscriptomics, metabolomics, and immune profiling.
- Microbiome findings: Changes in microbial diversity (alpha, beta), abundance of specific taxa, functional or metabolic pathway alterations, and directionality of change (increased, decreased, divergent).
- Clinical correlations: Associations between microbiome features and clinical outcomes, including symptoms (fatigue, pain, cognitive dysfunction, psychological distress, gastrointestinal symptoms) or biomarkers (e.g., inflammatory cytokines, bile acids, short-chain fatty acids).
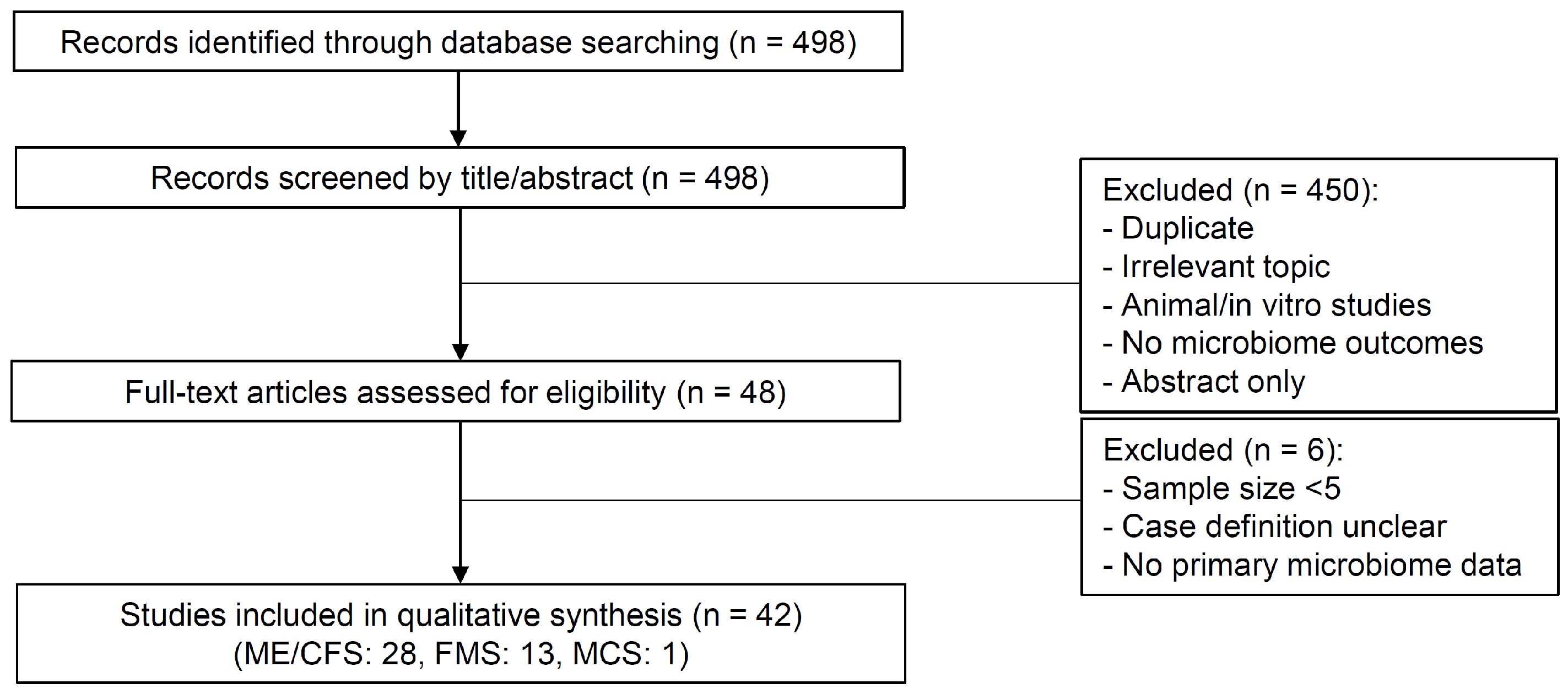
2.5. Flow of Study Selection
3. Microbial Diversity in Environmental Sensitivity Illnesses
3.1. Microbial Diversity and Clinical Relevance
3.2. Diversity Metrics: Alpha and Beta Diversity
3.3. Alpha Diversity in Environmental Sensitivity Illnesses
3.4. Beta Diversity in Environmental Sensitivity Illnesses
4. Taxonomic Shifts in the Gut Microbiome at the Phylum, Genus, and Species Levels
4.1. Increased Taxa
| Taxa (Genus/Species) | Direction of Change | Reported in | Functional/Clinical Relevance |
|---|---|---|---|
| Akkermansia muciniphila | ↑ | FMS, MCS [45,54] | Mucin degradation; excess may compromise gut barrier |
| Clostridium sensu stricto | ↑ | FMS, ME/CFS [45,48] | Includes D-lactate producers; implicated in cognitive symptoms |
| Bacteroides spp. (B. vulgatus, B. dorei, B. ovatus, B. uniformis) | ↑ | MCS [54] | Polysaccharide degradation, bile acid metabolism; overabundance may alter immune signaling |
| ↓ | FMS, ME/CFS [45,58] | ||
| Veillonella spp. | ↑ | MCS [54] | Lactate fermentation to propionate/acetate; altered SCFA balance |
| Faecalibacterium prausnitzii | ↓ | FMS, ME/CFS, MCS [45,46,50,53,54] | Keystone butyrate producer; depletion linked to impaired epithelial integrity and systemic inflammation |
| Eubacterium rectale | ↓ | FMS, ME/CFS [45,50] | Major butyrate producer; loss reduces anti-inflammatory capacity |
| Roseburia spp. | ↓ | FMS, ME/CFS [45,48] | Butyrate synthesis; depletion associated with fatigue and pain severity |
| Bifidobacterium spp. | ↓ | FMS [46] | Fiber fermentation; acetate/lactate production; reduced levels may impair immune tolerance |
4.2. Decreased Taxa
5. Functional Alterations in Host–Microbiome Metabolic Interactions
5.1. Functional Analysis in Fibromyalgia Syndrome
5.1.1. Short-Chain Fatty Acids Metabolism
| Pathway/Metabolism | Key Findings | Clinical/Functional Relevance |
|---|---|---|
| Short-chain fatty acid metabolism [45,47] | Reduced abundance of butyrate- and propionate-producing bacteria (Faecalibacterium prausnitzii, Eubacterium spp.); decreased serum SCFAs | Loss of anti-inflammatory and neuromodulatory effects; may promote systemic inflammation and pain |
| Neurotransmitter metabolism [46,62] | Altered glutamate metabolism; reduced abundance of Bifidobacterium and Eubacterium; elevated circulating glutamate | Excess glutamate implicated in central sensitization and chronic pain |
| Bile acid metabolism [63] | Altered secondary bile acids (e.g., depletion of α-muricholic acid); changes in bile acid-metabolizing bacteria | Correlated with pain intensity and fatigue; link between bile acid dysregulation and nociplastic pain |
| Energy and carbohydrate metabolism [64] | Elevated metabolites related to energy/carbohydrate metabolism; altered host- and gut-derived metabolites | Suggests disturbances in microbial–host metabolic pathways influencing fatigue and pain |
5.1.2. Neurotransmitter Metabolism
5.1.3. Bile Acid Metabolism
5.1.4. Energy Metabolism and Gut–Brain Axis Interactions
5.2. Functional Analysis in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
5.2.1. Butyrate Synthesis and Shifts in Carbohydrate and Lactate Metabolism
5.2.2. Amino Acid and Energy Metabolism Pathways
5.2.3. Lipid Biosynthesis and Vitamin B6 Pathways
5.2.4. Choline, Carnitine, and Complex Lipid Metabolism
5.2.5. Sphingolipid, Phospholipid, Purine, Cholesterol, Amino Acid, and Mitochondrial Metabolism
5.2.6. Peroxisomal Dysfunction and Lipid/Tricarboxylic Acid Cycle Abnormalities
5.3. Functional Analysis in Multiple Chemical Sensitivity
6. Studies on Microbiome-Targeted and Nutritional Approaches in Environmental Sensitivity Illnesses
6.1. Fecal Microbiota Transplantation
6.2. Probiotics and Nutritional Intervention in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
6.3. Probiotics, Prebiotics, Synbiotics, and Nutritional Intervention in Fibromyalgia Syndrome
6.4. Probiotics, Prebiotics, and Nutritional Intervention in Multiple Chemical Sensitivity
| Disease | Participants (n) | Intervention Dose/Regimen | Duration | Main Outcomes |
|---|---|---|---|---|
| Fecal microbiota transplantation (FMT) | ||||
| FMS [71] | 4 mice with FMS fecal vs. 4 with healthy fecal | Germ-free mice colonized with FMS fecal microbiota (n = 4) vs. healthy fecal microbiota (n = 4) | Single FMT | The recipient mice exhibited increased mechanical pain sensitivity and depression-like behaviors |
| ME/CFS (±IBS) [73] | 21 FMT vs. 21 oral treatment | FMT: 10 separate implants per patient (different donors); Oral treatment: Probiotics (Lactobacillus, Bifidobacterium, S. boulardii); Prebiotics (FOS, inulin, psyllium); Nutritional supplements (vitamins, minerals, omega-3); Mediterranean-style diet and lifestyle advice | 10 implants (interval not specified) | FMT group showed greater improvement in fatigue and gastrointestinal symptoms; no serious adverse events. |
| Probiotics | ||||
| ME/CFS [77] | 15 (Control: Baseline self-control) | Probiotic yogurt (L. paracasei F19, L. acidophilus, B. lactis) 200 mL twice daily (1 × 108 CFU/mL) | 4 weeks | No significant change in fatigue or physical activity; modest improvement in selected neurocognitive measures. |
| ME/CFS [78] | 44 (Control: Baseline self-control) | Erythromycin 400 mg, twice daily (week-on); D-lactate-free probiotic 5 × 1010 CFU, twice daily (week-off); alternated weekly for 4 weeks. | 4 weeks | Reduced Streptococcus abundance; improved sleep and cognition; fatigue unchanged. |
| ME/CFS [79] | 14 (Control: Baseline self-control) | Stepwise probiotic regimen: Week 1: Enterelle® (E. faecium, S. boulardii) 2 capsules twice daily; Week 2: Bifiselle® (B. longum, B. breve, B. bifidum, B. infantis) 2 capsules twice daily; Week 3: Ramnoselle® (L. rhamnosus GG, L. acidophilus) 2 capsules twice daily; Weeks 4–8: Enterelle® 2 capsules/day + Citogenex® (L. casei, B. lactis) 2 capsules/day + Rotanelle® (B. longum AR81) 2 capsules/day. | 8 weeks | Improved Quality of life (QoL), mood, oxidative stress markers; no adverse events reported. |
| ME/CFS [80] | 22 probiotic, 17 placebo | L. casei Shirota 24 × 109 CFU/day Control: Placebo drink (identical appearance/taste, no probiotics) | 8 weeks | Reduced anxiety vs. placebo; increased Lactobacillus/Bifidobacterium. |
| FMS [86] | 23 probiotic, 21 placebo | Multi-strain probiotic (L. rhamnosus GG, L. casei, L. acidophilus, L. bulgaricus, B. breve, B. longum, S. thermophilus), 4 capsules/day | 8 weeks | Improved decision-making; limited effects on mood. |
| FMS [87] | 15 probiotic, 16 placebo | ERGYPHILUS Plus® (L. rhamnosus GG, L. casei, L. acidophilus, B. bifidum), 4 capsules per day | 8 weeks | Reduced attentional errors; no effect on memory. |
| Synbiotics | ||||
| FMS (±CFS) [88] | 15 (Control: Baseline self-control) | Synbiotic Gasteel Plus® (B. lactis CBP-001010, L. rhamnosus CNCM I-4036, B. longum ES1 + fructooligosaccharides), 1 stick/day (1 × 109 CFU) | 30 days | Improved Fibromyalgia Impact Questionnaire-Revised (FIQR), fatigue, and trait anxiety; immuno-neuroendocrine markers improved. |
| Nutritional approach | ||||
| ME/CFS [81] | 10 Crossover design | High cocoa liquor/polyphenol-rich chocolate (HCL/PR chocolate) Iso-caloric, low-polyphenol chocolate as comparator 8 weeks each with 2-week washout | 8 weeks | Improved fatigue, function, and mood with HCL/PR; control chocolate worsened outcomes. |
| ME/CFS [83] | 36 treatment, 37 placebo | coenzyme Q10 200 mg/day + nicotinamide adenine dinucleotide (NADH) 20 mg/day vs. placebo | 8 weeks | Reduced fatigue, improved oxidative stress markers; safe and well tolerated. |
| ME/CFS [84] | 14 treatment, 12 placebo | NADH 10 mg/day vs. placebo | 4 weeks | Improved fatigue and function; safe and well tolerated. |
| FMS [91] | 50 intervention, 50 control | Personalized Mediterranean diet Diet counseling Control: General balanced diet counseling | 8 weeks | Improved pain, fatigue, function, and pain-related anxiety. |
| FMS [93] | 30 (female) Crossover design | Extra virgin olive oil (EVOO), 50 mL/day Control: Refined olive oil (ROO), 50 mL/day, 3 weeks; crossover with a 2-week washout | 3 weeks | EVOO reduced RBC and ESR; differences in cortisol and Platelet Distribution Width. |
| FMS [94] | 22 intervention, 24 control | Anti-inflammatory diet (AID) plus low-FODMAP for 1 month, then AID alone for an additional 2 months (total of 3 months). Control: Habitual diet, 3 months | 3 months | Improved FIQR, pain, fatigue, gastrointestinal symptoms, sleep, QoL; biomarkers unchanged. |
| FMS [95] | 40 (Control: Baseline self-control) | Histamine-release test–guided individualized elimination diet. | 6 months | Improved digestive symptoms and weight; no effect on pain or Fibromyalgia Impact Questionnaire. |
| FMS (±CFS) [89] | 27 intervention, 27 control | SYNCHRONIZE application plus brief nutritional counseling to improve Mediterranean-diet adherence. Control: Usual care only (no app, no counseling) | 12 weeks | Improved Mediterranean-diet adherence, nutritional quality, and intake patterns; clinical outcomes not assessed. |
| FMS [90] | 181 (FMS patients, online survey) | Mediterranean diet adherence (observational, Mediterranean diet adherence assessed) | Not applicable (cross-sectional survey) | Negative correlation between Mediterranean-diet adherence and disease severity. |
| FMS [92] | 95 (female) | None (observational; Dietary Inflammatory Index, DII scores calculated) Control: None (cross-sectional, within-group comparison by DII levels) | Not applicable (cross-sectional survey) | Higher DII scores associated with lower pressure pain thresholds. |
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFU | colony-forming units |
| CoQ10 | coenzyme Q10 |
| DII | dietary inflammatory index |
| EVOO | extra virgin olive oil |
| FIQR | fibromyalgia impact questionnaire-revised |
| FMS | fibromyalgia syndrome |
| FMT | fecal microbiota transplantation |
| HPA | hypothalamic–pituitary–adrenal axis |
| IBS | irritable bowel syndrome |
| MCS | multiple chemical sensitivity |
| ME/CFS | myalgic encephalomyelitis/chronic fatigue syndrome |
| NADH | nicotinamide adenine dinucleotide |
| QoL | quality of life |
| ROO | refined olive oil |
| SCFAs | short-chain fatty acids |
References
- Petersen, M.W.; Schröder, A.; Jørgensen, T.; Ørnbøl, E.; Meinertz Dantoft, T.; Eliasen, M.; Benros, M.E.; Fink, P. Irritable bowel, chronic widespread pain, chronic fatigue and related syndromes are prevalent and highly overlapping in the general population: DanFunD. Sci. Rep. 2020, 10, 3273. [Google Scholar] [CrossRef]
- Petersen, M.W.; Schröder, A.; Jørgensen, T.; Ørnbøl, E.; Dantoft, T.M.; Eliasen, M.; Carstensen, T.W.; Falgaard Eplov, L.; Fink, P. Prevalence of functional somatic syndromes and bodily distress syndrome in the Danish population: The DanFunD study. Scand. J. Public Health 2020, 48, 567–576. [Google Scholar] [CrossRef]
- Wiesmüller, G.A.; Hornberg, C. Environmental medical syndromes. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2017, 60, 597–604. [Google Scholar] [CrossRef]
- Molot, J.; Sears, M.; Marshall, L.M.; Bray, R.I. Neurological susceptibility to environmental exposures: Pathophysiological mechanisms in neurodegeneration and multiple chemical sensitivity. Rev. Environ. Health 2022, 37, 509–530. [Google Scholar] [CrossRef]
- Favretti, M.; Iannuccelli, C.; Di Franco, M. Pain Biomarkers in Fibromyalgia Syndrome: Current Understanding and Future Directions. Int. J. Mol. Sci. 2023, 24, 10443. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Baron, R.; Hans, G.; Dickenson, A.H. Peripheral input and its importance for central sensitization. Ann. Neurol. 2013, 74, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Aguilar, E.; Marcos-Pasero, H.; Ikonomopoulou, M.P.; Loria-Kohen, V. Food Implications in Central Sensitization Syndromes. J. Clin. Med. 2020, 9, 4106. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.B. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Semin. Arthritis Rheum. 2007, 36, 339–356. [Google Scholar] [CrossRef]
- Lim, M.; Kim, D.J.; Nascimento, T.D.; Ichesco, E.; Kaplan, C.; Harris, R.E.; DaSilva, A.F. Functional Magnetic Resonance Imaging Signal Variability Is Associated with Neuromodulation in Fibromyalgia. Neuromodulation 2023, 26, 999–1008. [Google Scholar] [CrossRef]
- Nhu, N.T.; Chen, D.Y.; Kang, J.H. Functional Connectivity and Structural Signatures of the Visual Cortical System in Fibromyalgia: A Magnetic Resonance Imaging Study. J. Rheumatol. 2023, 50, 1063–1070. [Google Scholar] [CrossRef]
- Kaur, R.; Greeley, B.; Ciok, A.; Mehta, K.; Tsai, M.; Robertson, H.; Debelic, K.; Zhang, L.X.; Nelson, T.; Boulter, T.; et al. A Multimodal Magnetic Resonance Imaging Study on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Feasibility and Clinical Correlation. Medicina 2024, 60, 1370. [Google Scholar] [CrossRef]
- Nakatomi, Y.; Mizuno, K.; Ishii, A.; Wada, Y.; Tanaka, M.; Tazawa, S.; Onoe, K.; Fukuda, S.; Kawabe, J.; Takahashi, K.; et al. Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An 11C-(R)-PK11195 PET Study. J. Nucl. Med. 2014, 55, 945–950. [Google Scholar] [CrossRef]
- Alessandrini, M.; Micarelli, A.; Chiaravalloti, A.; Bruno, E.; Danieli, R.; Pierantozzi, M.; Genovesi, G.; Öberg, J.; Pagani, M.; Schillaci, O. Involvement of Subcortical Brain Structures During Olfactory Stimulation in Multiple Chemical Sensitivity. Brain Topogr. 2016, 29, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Orriols, R.; Costa, R.; Cuberas, G.; Jacas, C.; Castell, J.; Sunyer, J. Brain dysfunction in multiple chemical sensitivity. J. Neurol. Sci. 2009, 287, 72–78. [Google Scholar] [CrossRef]
- Azuma, K.; Uchiyama, I.; Tanigawa, M.; Bamba, I.; Azuma, M.; Takano, H.; Yoshikawa, T.; Sakabe, K. Chemical intolerance: Involvement of brain function and networks after exposure to extrinsic stimuli perceived as hazardous. Environ. Health Prev. Med. 2019, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Porsche, C.; Kozik, A.J.; Lynch, S.V. Microbiome-Immune Interactions in Allergy and Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 2244–2251. [Google Scholar] [CrossRef]
- Galazzo, G.; van Best, N.; Bervoets, L.; Dapaah, I.O.; Savelkoul, P.H.; Hornef, M.W.; GI-MDH consortium; Lau, S.; Hamelmann, E.; Penders, J. Development of the Microbiota and Associations with Birth Mode, Diet, and Atopic Disorders in a Longitudinal Analysis of Stool Samples, Collected from Infancy Through Early Childhood. Gastroenterology 2020, 158, 1584–1596. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota–gut–brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Amin, N.; Liu, J.; Bonnechere, B.; MahmoudianDehkordi, S.; Arnold, M.; Batra, R.; Chiou, Y.J.; Fernandes, M.; Ikram, M.A.; Kraaij, R.; et al. Interplay of Metabolome and Gut Microbiome in Individuals with Major Depressive Disorder vs Control Individuals. JAMA Psychiatry 2023, 80, 597–609. [Google Scholar] [CrossRef]
- De Palma, G.; Shimbori, C.; Reed, D.E.; Yu, Y.; Rabbia, V.; Lu, J.; Jimenez-Vargas, N.; Sessenwein, J.; Lopez-Lopez, C.; Pigrau, M.; et al. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci. Transl. Med. 2022, 14, eabj1895. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Pollock, J.; Glendinning, L.; Wisedchanwet, T.; Watson, M. The Madness of Microbiome: Attempting to Find Consensus “Best Practice” for 16S Microbiome Studies. Appl. Environ. Microbiol. 2018, 84, e02627-17. [Google Scholar] [CrossRef] [PubMed]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 2017, 35, 833–844, Erratum in Nat. Biotechnol. 2017, 35, 1211. [Google Scholar] [CrossRef]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef]
- Tierney, B.T.; Yang, Z.; Luber, J.M.; Beaudin, M.; Wibowo, M.C.; Baek, C.; Mehlenbacher, E.; Patel, C.J.; Kostic, A.D. The Landscape of Genetic Content in the Gut and Oral Human Microbiome. Cell Host Microbe 2019, 26, 283–295.e8. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.E.; Scholes-Robertson, N.; Craig, J.C.; Hawley, C.M.; Howell, M.; Johnson, D.W.; Teixeira-Pinto, A.; Jaure, A.; Wong, G. Synbiotics, prebiotics and probiotics for solid organ transplant recipients. Cochrane Database Syst. Rev. 2022, 9, Cd014804. [Google Scholar]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Selma-Royo, M.; Crispi, F.; Castro-Barquero, S.; Casas, I.; Larroya, M.; Genero, M.; Paules, C.; Benitez, L.; Youssef, L.; Pascal, R.; et al. Effects of Mediterranean diet or Mindfulness Based-Stress Reduction during Pregnancy on Maternal Gut and Vaginal Microbiota. A sub-analysis of the IMPACT BCN trial. Am. J. Clin. Nutr. 2025, 122, 1121–1133. [Google Scholar] [CrossRef]
- Kortlever, T.L.; Ten Bokkel Huinink, S.; Offereins, M.; Hebblethwaite, C.; O’Brien, L.; Leeper, J.; Mulder, C.J.J.; Barrett, J.S.; Gearry, R.B. Low-FODMAP Diet Is Associated with Improved Quality of Life in IBS Patients-A Prospective Observational Study. Nutr. Clin. Pract. 2019, 34, 623–630. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef]
- Shade, A. Diversity is the question, not the answer. ISME J. 2017, 11, 1–6. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P.; Lozupone, C.A.; Nipperess, D.; Knight, R. The cladistic basis for the phylogenetic diversity (PD) measure links evolutionary features to environmental gradients and supports broad applications of microbial ecology’s “phylogenetic beta diversity” framework. Int. J. Mol. Sci. 2009, 10, 4723–4741. [Google Scholar] [CrossRef] [PubMed]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. eBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G.-T.; Kang, J. Microbial Composition and Stool Short Chain Fatty Acid Levels in Fibromyalgia. Int. J. Environ. Res. Public Health 2023, 20, 3183. [Google Scholar] [CrossRef]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef]
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front. Immunol. 2021, 12, 628741, Erratum in Front. Immunol. 2022, 13, 878196. [Google Scholar] [CrossRef]
- Guo, C.; Che, X.; Briese, T.; Ranjan, A.; Allicock, O.; Yates, R.A.; Cheng, A.; March, D.; Hornig, M.; Komaroff, A.L.; et al. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell Host Microbe 2023, 31, 288–304.e8. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Gunter, C.; Fleming, E.; Vernon, S.D.; Bateman, L.; Unutmaz, D.; Oh, J. Multi-’omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients. Cell Host Microb. 2023, 31, 273–287.e5. [Google Scholar] [CrossRef] [PubMed]
- Watai, K.; Suda, W.; Kurokawa, R.; Sekiya, K.; Hayashi, H.; Iwata, M.; Nagayama, K.; Nakamura, Y.; Hamada, Y.; Kamide, Y.; et al. Metagenomic gut microbiome analysis of Japanese patients with multiple chemical sensitivity/idiopathic environmental intolerance. BMC Microbiol. 2024, 24, 84. [Google Scholar] [CrossRef]
- Wang, K.; Wu, W.; Wang, Q.; Yang, L.; Bian, X.; Jiang, X.; Lv, L.; Yan, R.; Xia, J.; Han, S.; et al. The negative effect of Akkermansia muciniphila-mediated post-antibiotic reconstitution of the gut microbiota on the development of colitis-associated colorectal cancer in mice. Front. Microbiol. 2022, 13, 932047. [Google Scholar] [CrossRef]
- Wallis, A.; Ball, M.; McKechnie, S.; Butt, H.; Lewis, D.P.; Bruck, D. Examining clinical similarities between myalgic encephalomyelitis/chronic fatigue syndrome and D-lactic acidosis: A systematic review. J. Transl. Med. 2017, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Choi, Y.; Lee, J.-S.; Hwang, S.-J.; Gu, J.; Son, C.-G. Clinical evidence of the link between gut microbiome and myalgic encephalomyelitis/chronic fatigue syndrome: A retrospective review. Eur. J. Med. Res. 2024, 29, 148. [Google Scholar] [CrossRef]
- Armstrong, C.W.; McGregor, N.R.; Lewis, D.P.; Butt, H.L.; Gooley, P.R. The association of fecal microbiota and fecal, blood serum and urine metabolites in myalgic encephalomyelitis/chronic fatigue syndrome. Metabolomics 2016, 13, 8. [Google Scholar] [CrossRef]
- Culp, E.J.; Goodman, A.L. Cross-feeding in the gut microbiome: Ecology and mechanisms. Cell Host Microbe 2023, 31, 485–499. [Google Scholar] [CrossRef]
- Breyner, N.M.; Michon, C.; de Sousa, C.S.; Vilas Boas, P.B.; Chain, F.; Azevedo, V.A.; Langella, P.; Chatel, J.M. Microbial Anti-Inflammatory Molecule (MAM) from Faecalibacterium prausnitzii Shows a Protective Effect on DNBS and DSS-Induced Colitis Model in Mice through Inhibition of NF-κB Pathway. Front. Microbiol. 2017, 8, 114. [Google Scholar] [CrossRef]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef]
- Harris, R.E.; Sundgren, P.C.; Craig, A.D.; Kirshenbaum, E.; Sen, A.; Napadow, V.; Clauw, D.J. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009, 60, 3146–3152. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered serum bile acid profile in fibromyalgia is associated with specific gut microbiome changes and symptom severity. Pain 2022, 164, e66–e76. [Google Scholar] [CrossRef]
- Malatji, B.G.; Mason, S.; Mienie, L.J.; Wevers, R.A.; Meyer, H.; van Reenen, M.; Reinecke, C.J. The GC-MS metabolomics signature in patients with fibromyalgia syndrome directs to dysbiosis as an aspect contributing factor of FMS pathophysiology. Metabolomics 2019, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, L.; Xu, C.; Pan, K.; Mo, M.; Duan, M.; Zhang, Y.; Xiong, H. Chronic fatigue syndrome patients have alterations in their oral microbiome composition and function. PLoS ONE 2018, 13, e0203503. [Google Scholar] [CrossRef]
- Nagy-Szakal, D.; Williams, B.L.; Mishra, N.; Che, X.; Lee, B.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; Levine, S.; Montoya, J.G.; et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2017, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Szakal, D.; Barupal, D.K.; Lee, B.; Che, X.; Williams, B.L.; Kahn, E.J.R.; Ukaigwe, J.E.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci. Rep. 2018, 8, 10056. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E5472–E5480, Erratum in Proc. Natl. Acad. Sci. USA 2017, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Brydges, C.R.; Yu, Y.; Price, A.; Joshi, S.; Roy, A.; Lee, B.; Barupal, D.K.; Cheng, A.; Palmer, D.M.; et al. Metabolomic Evidence for Peroxisomal Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Int. J. Mol. Sci. 2022, 23, 7906. [Google Scholar] [CrossRef]
- Shogbesan, O.; Poudel, D.R.; Victor, S.; Jehangir, A.; Fadahunsi, O.; Shogbesan, G.; Donato, A. A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplant for Clostridium difficile Infection in Immunocompromised Patients. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1394379. [Google Scholar] [CrossRef]
- Cai, W.; Haddad, M.; Haddad, R.; Kesten, I.; Hoffman, T.; Laan, R.; Westfall, S.; Defaye, M.; Abdullah, N.S.; Wong, C.; et al. The gut microbiota promotes pain in fibromyalgia. Neuron 2025, 113, 2161–2175.e13. [Google Scholar] [CrossRef]
- Borody, T.J.; Nowak, A.; Finlayson, S. The GI microbiome and its role in Chronic Fatigue Syndrome: A summary of bacteriotherapy. J. Australas. Coll. Nutr. Environ. Med. 2012, 31, 3–8. [Google Scholar]
- Kenyon, J.N.; Coe, S.; Izadi, H. A retrospective outcome study of 42 patients with Chronic Fatigue Syndrome, 30 of whom had Irritable Bowel Syndrome. Half were treated with oral approaches, and half were treated with Faecal Microbiome Transplantation. Hum. Microbiome J. 2019, 13, 100061. [Google Scholar] [CrossRef]
- Niedoszytko, M.; Chelminska, M.; Buss, T.; Roik, E.; Jassem, E. Drug intolerance in patients with idiopathic environmental intolerance syndrome. Int. J. Clin. Pract. 2006, 60, 1327–1329. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Nicco, C.; Paule, A.; Konturek, P.; Edeas, M. From Donor to Patient: Collection, Preparation and Cryopreservation of Fecal Samples for Fecal Microbiota Transplantation. Diseases 2020, 8, 9. [Google Scholar] [CrossRef]
- Sullivan, A.; Nord, C.E.; Evengård, B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr. J. 2009, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wallis, A.; Ball, M.; Butt, H.; Lewis, D.P.; McKechnie, S.; Paull, P.; Jaa-Kwee, A.; Bruck, D. Open-label pilot for treatment targeting gut dysbiosis in myalgic encephalomyelitis/chronic fatigue syndrome: Neuropsychological symptoms and sex comparisons. J. Transl. Med. 2018, 16, 24. [Google Scholar] [CrossRef]
- Venturini, L.; Bacchi, S.; Capelli, E.; Lorusso, L.; Ricevuti, G.; Cusa, C. Modification of Immunological Parameters, Oxidative Stress Markers, Mood Symptoms, and Well-Being Status in CFS Patients after Probiotic Intake: Observations from a Pilot Study. Oxid. Med. Cell Longev. 2019, 2019, 1684198. [Google Scholar] [CrossRef]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Sathyapalan, T.; Beckett, S.; Rigby, A.S.; Mellor, D.D.; Atkin, S.L. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr. J. 2010, 9, 55. [Google Scholar] [CrossRef]
- Campagnolo, N.; Johnston, S.; Collatz, A.; Staines, D.; Marshall-Gradisnik, S. Dietary and nutrition interventions for the therapeutic treatment of chronic fatigue syndrome/myalgic encephalomyelitis: A systematic review. J. Hum. Nutr. Diet. 2017, 30, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Cordero, M.D.; Segundo, M.J.; Sáez-Francàs, N.; Calvo, N.; Román-Malo, L.; Aliste, L.; Fernández de Sevilla, T.; Alegre, J. Does oral coenzyme Q10 plus NADH supplementation improve fatigue and biochemical parameters in chronic fatigue syndrome? Antioxid Redox Signal 2015, 22, 679–685. [Google Scholar] [CrossRef]
- Forsyth, L.M.; Preuss, H.G.; MacDowell, A.L.; Chiazze, L., Jr.; Birkmayer, G.D.; Bellanti, J.A. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann. Allergy Asthma Immunol. 1999, 82, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Quickert, S.; Puta, C.; Reuken, P.A. The gastrointestinal microbiota in the development of ME/CFS: A critical view and potential perspectives. Front. Immunol. 2024, 15, 1352744. [Google Scholar] [CrossRef]
- Roman, P.; Estévez, A.F.; Miras, A.; Sánchez-Labraca, N.; Cañadas, F.; Vivas, A.B.; Cardona, D. A Pilot Randomized Controlled Trial to Explore Cognitive and Emotional Effects of Probiotics in Fibromyalgia. Sci. Rep. 2018, 8, 10965. [Google Scholar] [CrossRef]
- Cardona, D.; Roman, P.; Cañadas, F.; Sánchez-Labraca, N. The Effect of Multiprobiotics on Memory and Attention in Fibromyalgia: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 3543. [Google Scholar] [CrossRef]
- Hinchado, M.D.; Quero-Calero, C.D.; Otero, E.; Gálvez, I.; Ortega, E. Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome. Nutrients 2023, 15, 1591. [Google Scholar] [CrossRef]
- Carrasco-Querol, N.; Cabricano-Canga, L.; Bueno Hernández, N.; Martín-Borràs, C.; Gonçalves, A.Q.; Vila-Martí, A.; Ribot, B.; Solà, J.; Valls-Llobet, C.; Caballol Angelats, R.; et al. Effectiveness of the SYNCHRONIZE + Brief Intervention in Improving Mediterranean Diet Adherence, Nutritional Quality and Intake Pattern in Persons with Fibromyalgia and Chronic Fatigue Syndrome. Nutrients 2024, 17, 11. [Google Scholar] [CrossRef]
- Proietti, E.; Rapallo, F.; Molinari, E.; Mucci, V.; Marinelli, L.; Borgarelli, C.; Burlando, B.; Pisciotta, L.; Demori, I. Online Questionnaire with Fibromyalgia Patients Shows Negative Correlations between Disease Severity and Adherence to Mediterranean Diet. Nutrients 2024, 16, 1078. [Google Scholar] [CrossRef] [PubMed]
- Casini, I.; Ladisa, V.; Clemente, L.; Delussi, M.; Rostanzo, E.; Peparini, S.; Aloisi, A.M.; de Tommaso, M. A Personalized Mediterranean Diet Improves Pain and Quality of Life in Patients with Fibromyalgia. Pain Ther. 2024, 13, 609–620. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; Casas-Barragán, A.; González-Jiménez, E.; Schmidt-RioValle, J.; Molina, F.; Aguilar-Ferrándiz, M.E. Dietary Inflammatory Index Scores Are Associated with Pressure Pain Hypersensitivity in Women with Fibromyalgia. Pain Med. 2020, 21, 586–594. [Google Scholar] [CrossRef]
- Rus, A.; Molina, F.; Martínez-Ramírez, M.J.; Aguilar-Ferrándiz, M.E.; Carmona, R.; Del Moral, M.L. Effects of Olive Oil Consumption on Cardiovascular Risk Factors in Patients with Fibromyalgia. Nutrients 2020, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Bernardo, A.; de Mesquita, M.F.; Vaz-Patto, J.; Moreira, P.; Silva, M.L.; Padrão, P. An anti-inflammatory and low fermentable oligo, di, and monosaccharides and polyols diet improved patient reported outcomes in fibromyalgia: A randomized controlled trial. Front. Nutr. 2022, 9, 856216. [Google Scholar] [CrossRef]
- Gomez-Arguelles, J.M.; Caceres, O.; Blanco, M.; Maestu, C.; Martin, F. Improvement of digestive symptoms in fibromyalgia patients following a diet modification according to histamine release test—An observational study. Reumatologia 2022, 60, 209–212. [Google Scholar] [CrossRef]
- Miller, E.E.; Jayakrishnan, S.; Froio, F.; Ahmed, D.; Haq, H.; Schnurer, S.; Messina, M.V.; Barillà, B.; Saturnino, A.; Vomero, M.; et al. Mediterranean diet and its low-antigenic and anti-inflammatory properties on fibromyalgia: A systematic review. Clin. Exp. Rheumatol. 2025, 43, 970–977. [Google Scholar] [CrossRef]
- Azuma, K.; Uchiyama, I.; Kunugita, N. Potential factors affecting chronic chemical intolerance associated with constitutional predisposition or lifestyle and environment during childhood: From a six-year follow-up study. J. Psychosom. Res. 2021, 151, 110665. [Google Scholar] [CrossRef] [PubMed]
- Watai, K.; Fukutomi, Y.; Hayashi, H.; Kamide, Y.; Sekiya, K.; Taniguchi, M. Epidemiological association between multiple chemical sensitivity and birth by caesarean section: A nationwide case-control study. Environ. Health 2018, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Aguilar, E.; Marcos-Pasero, H.; de la Iglesia, R.; Espinosa-Salinas, I.; Ramírez de Molina, A.; Reglero, G.; Loria-Kohen, V. Characteristics and determinants of dietary intake and physical activity in a group of patients with multiple chemical sensitivity. Endocrinol. Diabetes Nutr. 2018, 65, 564–570. [Google Scholar] [CrossRef]
| Pathway/Metabolism | Key Findings | Clinical/Functional Relevance |
|---|---|---|
| Butyrate synthesis, Carbohydrate and lactate metabolism [50,53,58] | Depletion of bacterial butyrate synthesis modules (acetyl-CoA, pyruvate, glutarate pathways); reduced butyryl-CoA–acetate CoA-transferase genes | Reduced butyrate capacity; linked to fatigue and impaired immune regulation |
| Reduced modules for lactate utilization and polysaccharide fermentation; enrichment of lactate production and mucin degradation | Altered fermentation and lactate accumulation may contribute to fatigue and gut symptoms | |
| Amino acid and energy metabolism [58,65] | PICRUSt-based inference: altered amino acid and energy pathways | Suggests systemic effects of oral/gut dysbiosis on host metabolism |
| Lipid biosynthesis and vitamin B6 pathways [66] | Reduced unsaturated fatty acid biosynthesis; enhanced vitamin B6 biosynthesis/salvage and pyrimidine degradation | May affect host nutrient metabolism and disease pathophysiology |
| Choline, carnitine, and complex lipid metabolism [67] | Altered plasma choline, carnitine, ceramide; integrated microbiome–metabolome models improved diagnostic prediction | Links gut microbiome changes to systemic lipid metabolism and clinical phenotypes |
| Mitochondrial and peroxisomal metabolism [68] | Abnormalities in >20 pathways: reduced sphingolipid, phospholipid, purine, cholesterol metabolism; decreased plasmalogens and phosphatidylcholines; increased dicarboxylic acids | Consistent with hypometabolic syndrome and peroxisomal dysfunction |
| Pathway/Process | Key Findings | Clinical/Functional Relevance |
|---|---|---|
| Degradation of environmental pollutants [54] | Xylene and dioxin degradation pathways enriched | Indicates heightened microbial potential to metabolize exogenous toxic chemicals |
| Antimicrobial resistance [54] | Two-component system, antimicrobial resistance genes, cationic antimicrobial peptide resistance increased | Suggests increased microbial resilience to environmental stressors |
| Amino acid metabolism and biosynthesis [54] | Glycine, serine, threonine metabolism; arginine biosynthesis; aminoacyl-tRNA biosynthesis decreased | Impaired microbial capacity for amino acid metabolism, potentially affecting host–microbe interactions |
| Vitamin and cofactor metabolism [54] | Pathways related to vitamin and cofactor metabolism reduced | Suggests limited microbial contribution to essential cofactor availability |
| Neurotransmission-related pathways [54] | GABAergic and glutamatergic synapse-related pathways decreased | May contribute to impaired gut–brain axis signaling and heightened sensitivity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watai, K.; Taniguchi, M.; Azuma, K. The Gut–Brain–Immune Axis in Environmental Sensitivity Illnesses: Microbiome-Centered Narrative Review of Fibromyalgia Syndrome, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, and Multiple Chemical Sensitivity. Int. J. Mol. Sci. 2025, 26, 9997. https://doi.org/10.3390/ijms26209997
Watai K, Taniguchi M, Azuma K. The Gut–Brain–Immune Axis in Environmental Sensitivity Illnesses: Microbiome-Centered Narrative Review of Fibromyalgia Syndrome, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, and Multiple Chemical Sensitivity. International Journal of Molecular Sciences. 2025; 26(20):9997. https://doi.org/10.3390/ijms26209997
Chicago/Turabian StyleWatai, Kentaro, Masami Taniguchi, and Kenichi Azuma. 2025. "The Gut–Brain–Immune Axis in Environmental Sensitivity Illnesses: Microbiome-Centered Narrative Review of Fibromyalgia Syndrome, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, and Multiple Chemical Sensitivity" International Journal of Molecular Sciences 26, no. 20: 9997. https://doi.org/10.3390/ijms26209997
APA StyleWatai, K., Taniguchi, M., & Azuma, K. (2025). The Gut–Brain–Immune Axis in Environmental Sensitivity Illnesses: Microbiome-Centered Narrative Review of Fibromyalgia Syndrome, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, and Multiple Chemical Sensitivity. International Journal of Molecular Sciences, 26(20), 9997. https://doi.org/10.3390/ijms26209997





